Summary
Abnormal metabotropic glutamate receptor 5 (mGluR5) function, as a result of disrupted scaffolding with its binding partner Homer, contributes to the pathophysiology of Fragile X Syndrome, a common inherited from of intellectual disability and autism caused by mutations in Fmr1. How loss of Fmr1 disrupts mGluR5-Homer scaffolds is unknown, and little is known about the dynamic regulation of mGluR5-Homer scaffolds in wildtype neurons. Here we demonstrate that brief (minutes) elevations in neural activity cause CaMKIIα-mediated phosphorylation of long Homer proteins and dissociation from mGluR5 at synapses. In Fmr1 knockout cortex, Homers are hyperphosphorylated as a result of elevated CaMKIIα protein. Genetic or pharmacological inhibition of CaMKIIα or replacement of Homers with dephosphomimetics restores mGluR5-Homer scaffolds and multiple Fmr1 KO phenotypes, including circuit hyperexcitability and/or seizures. This work links translational control of an FMRP target mRNA, CaMKIIα, to the molecular, cellular and circuit level brain dysfunction in a complex neurodevelopmental disorder.
Introduction
Synaptic scaffolding proteins, such as those in the PSD-95, Homer, SHANK, AKAP and SAPAP families are critical for the proper organization, localization and signaling of excitatory postsynaptic receptors and thus govern the development, function and plasticity of excitatory circuits (Reviewed in (Ting et al., 2012). The importance of synaptic scaffolds to brain function and behavior is highlighted by the growing number of mutations in synaptic scaffolding proteins implicated in neuropsychiatric diseases including autism, intellectual disability, and schizophrenia (Bayes et al., 2011; Ting et al., 2012). Little is known about how mutations in synaptic scaffolds or their improper regulation contribute to brain dysfunction in these diseases. Insight into how abnormal synaptic scaffolds contribute to brain disease phenotypes comes from the mouse model of Fragile X Syndrome (FXS; Fmr1 knockout; KO), a common genetic cause of autism and intellectual disability (Giuffrida et al., 2005; Ronesi et al., 2012) . FXS is caused by loss of function mutations in Fmr1, which encodes a dendritic RNA binding protein, FMRP (Darnell and Klann, 2013). Loss of Fmr1 in animal models leads to abnormal, typically overactive, function of the postsynaptic metabotropic glutamate receptor 5 (mGluR5) which mediates many phenotypes associated with the disease (Dolen et al., 2007; Michalon et al., 2012; Ronesi et al., 2012). As a result, mGluR5 is a therapeutic target for FXS and autism. Recent work implicates a molecular mechanism for mGluR5 dysfunction in Fmr1 KO mice --dissociation of mGluR5 with its postsynaptic scaffolding protein Homer (Giuffrida et al., 2005; Ronesi et al., 2012). The Homer family of proteins bind to the intracellular C-terminal tail of group 1 mGluRs and form multi-protein signaling complexes at the postsynaptic density with mGluRs and their downstream effectors (Shiraishi-Yamaguchi and Furuichi, 2007). All Homer family members (Homer 1–3) share a common EVH1 domain at the N-terminus, which binds to mGluR1α, mGluR5, PI3 Kinase enhancer (PIKE), IP3 receptor, SHANK, ion channels and other effectors (Shiraishi-Yamaguchi and Furuichi, 2007). Homers multimerize through their coiled-coil domains to scaffold mGluRs to signaling pathways and localize mGluRs to the postsynaptic density (Hayashi et al., 2009; Shiraishi-Yamaguchi and Furuichi, 2007). An activity-dependent, short, variant of Homer, Homer1a, (H1a) lacks a coiled-coil domain, and disrupts Homer scaffolds by competing with long Homers for mGluR5 and other Homer interacting proteins. Interestingly, H1a results in constitutive, agonist-independent activity of mGluR5 (Ango et al., 2001). In Fmr1 KO forebrain mGluR5 is less associated with long Homer isoforms and more associated with H1a (Giuffrida et al., 2005). Genetic deletion of H1a restores mGluR5-Homer scaffolds and corrects multiple phenotypes in Fmr1 KO mice (Ronesi et al., 2012), including alterations in mGluR5 signaling, circuit function, and behavior. Furthermore, acute, peptide-mediated disruption of mGluR5-long Homer scaffolds in wildtype brain mimics phenotypes of the Fmr1 KO (Ronesi et al., 2012; Ronesi and Huber, 2008; Tang and Alger, 2015). Because disrupted mGluR5-Homer scaffolds contribute to disease phenotypes, understanding mechanisms that regulate mGluR5-Homer interactions and determining the cause of disrupted mGluR5-Homer scaffolds in FXS model will provide therapeutic targets for the disease.
Here we find that brief (5 min) elevations in neuronal activity rapidly dissociate mGluR5-Homer scaffolds in wildtype cortical neurons and spines. Rapid, activity-induced dissociation of mGluR5-Homer occurs independently of H1a, but is mediated by CaMKIIα phosphorylation of Homer1 and Homer2 which decreases their affinity for mGluR5. CaMKIIα, a known FMRP target mRNA, whose protein is elevated in Fmr1 KO neurons and synapses, results in hyperphosphorylation of Homers, decreased interactions with mGluR5 and disease relevant phenotypes such as seizures. This work provides knowledge of the dynamic regulation of mGluR5-Homer scaffolds in neurons, demonstrates dysfunction of this mechanism and a novel therapeutic target in FXS.
Results
Neuronal activity induces a rapid dissociation of mGluR5-Homer scaffolds that depends on CaMKIIα
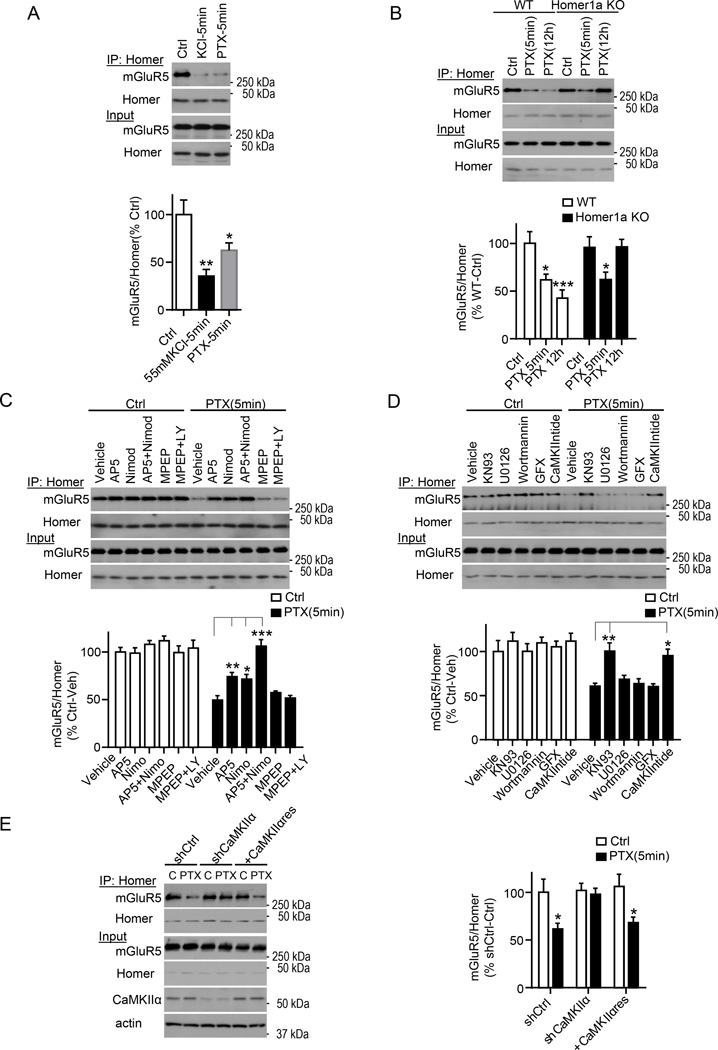
To determine if and how mGluR5-Homer scaffolds are dynamically regulated by synaptic activity we briefly (5 min) increased activity in mouse dissociated neocortical cultures (DIV 18) with either 55mM KCl or the GABAa receptor blocker picrotoxin (PTX; 50 µM). Both KCl and PTX reduced mGluR5-Homer interactions as measured by co-immunoprecipitation (co-IP) of long Homer proteins (using a pan long Homer antibody) and mGluR5 (Fig.1A; S1A). Activity-induced Homer1a (H1a), competes with long Homers for binding to mGluR5 (Xiao et al., 1998). To determine the contribution of H1a to rapid activity-induced dissociation of mGluR5-Homer, we repeated experiments in cultures of H1a knockout (KO) and wildtype (WT) littermates (Hu et al., 2010). PTX treatment of H1a KO and WT cultures for 5 min resulted in a similar dissociation of mGluR5-Homer as measured using co-IPs, suggesting a role of activity-dependent, H1a-independent mechanisms. H1a contributes to mGluR-Homer dissociation in response to chronic activity increases because 12 hr PTX dissociated mGluR5-Homer in WT neurons, but not H1a KO neurons (Fig. 1B). To determine the role of specific glutamate receptors and routes of Ca2+ influx in mGluR-Homer dissociation, we treated cultures with antagonists of NMDA receptors (50µM D-AP5) or L-type voltage-gated calcium channels (VGCCs; 5 µM nimodipine; 30 min pretreatment) which each reduced activity-induced dissociation of mGlu5-Homer, and their combined blockade completely blocked (Fig. 1C). In contrast, blockade of group 1 mGluRs (mGluR1 and 5; with 100 µM LY367385 or 10 µM MPEP; respectively) had no effect on PTX-induced mGluR5-Homer disruption.
Figure 1. Brief elevations in neural activity disrupt mGluR5-Homer scaffolds that depends on CaMKIIα, but not Homer1a.
A) Brief (5 min) treatment of dissociated neocortical neuron cultures with 55mM KCl or picrotoxin (PTX) reduces mGluR5 interactions with long Homers as assessed with coimmunoprecipitation (IP) with a pan-Homer antibody (top) n=5.
B) Brief (5 min) PTX treatment reduces mGluR5-Homer interactions assessed with co-IP in cultures prepared from wildtype (WT) or Homer1a (H1a) KO mice. In contrast, H1a is necessary for disruption of mGluR5-Homer in response to chronic (12h) PTX treatment. n=5.
C) Pharmacological blockade of NMDA receptors and L-VGCCs inhibits brief PTX-induced dissociation of mGluR5-Homer in WT cultures, as assessed by co-IP. The specific inhibitors: NMDA receptor: AP5; L-Type Voltage gated Calcium channel: nimodipine; mGluR5: MPEP; mGluR1: LY367385. n=4.
D) Pharmacological blockade of CaMKII inhibits brief PTX-induced dissociation of mGluR5-Homer in WT cultures, as assessed by co-IP. The specific inhibitors tested are: CaMKII: KN-93 and CaMKIINtide; PI3K: wortmannin; MEK: U0126; PKC: GF109203X (GFX). n=5.
E) shRNA-mediated knockdown of endogenous CaMKIIα expression blocks brief PTX-induced dissociation of mGluR5-Homer in WT cultures, as assessed by co-IP in comparison to cultures expressing a control shRNA (shCtrl). Co-expression of a shRNA-resistant CaMKIIα (CaMKIIαres) rescues PTX-induced decreases in mGluR5-Homer. n=5.
All experiments are repeated in at least 3 independent cultures. In all figures, error bars represent SEM. *p<0.05, **p<0.01, ***p<0.001. See also Figure S1.
These results suggest a role for a rapid, Ca2+-dependent, posttranslational regulation of mGluR5-Homer and thus we tested inhibitors of Ca2+/Calmodulin-dependent protein kinase II (CaMKII; KN-93 or myristolated CaMKIINtide peptide inhibitor; 5 µM), MAP-ERK kinase (MEK), the upstream kinase of Extracellular-Regulated Kinase (ERK1/2; U0126; 10 µM), Phosphoinositide-3-kinase (PI3K; wortmannin 100nM), and Protein Kinase C (PKC; GF109203× 5 µM). Preincubation of cultures (30 min) in CaMKII inhibitors, but not inhibitors of PI3K, ERK or PKC, blocked PTX- and KCl-induced decrease in mGluR5-Homer (Fig. 1D; S1A). To test the role of a specific form of CaMKII enriched at postsynaptic densities (PSDs) (Hell, 2014), we used lentiviral-mediated expression of a shRNA for CaMKIIα (at 7 DIV) to knock down CaMKIIα protein (~75% at 16–18 DIV; shCaMKIIα; Fig. 1E). CaMKIIα knockdown did not affect mGluR5-Homer interactions under basal conditions, compared to control shRNA (shCtrl), but blocked activity-induced disruption of mGluR5-Homer. The latter was rescued by co-transfection of shCaMKIIα and a shRNA-resistant CaMKIIα cDNA (CaMKIIαres; Fig. 1E). In contrast, CaMKII activity was not required for persistent, H1a-dependent decreases in mGluR5-Homer in response to chronic PTX treatment (Fig. S1B). Similarly, inhibition of CaMKII after PTX treatment did not restore Homer-mGluR5 (Fig. S1C). These results indicate that brief periods of enhanced neuronal activity induce a rapid, CaMKIIα-dependent disruption of mGluR5-Homer scaffolds and this mechanism is distinct from a later, H1a-dependent disruption of mGluR5-Homer in response to chronic activity increases.
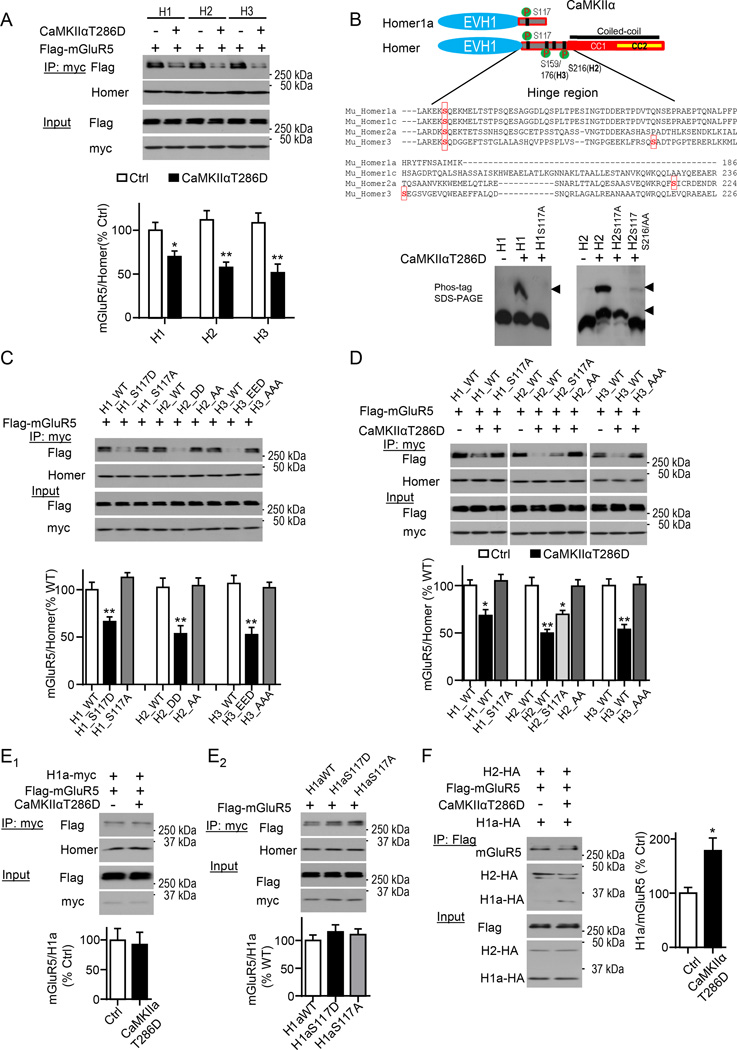
CaMKIIα phosphorylation of the “hinge region” of Homer 1, 2 and 3 reduces interactions with mGluR5
To determine if active CaMKIIα is sufficient to decrease mGluR5-Homer complexes and regulate interactions of specific Homer family members, we co-expressed a constitutively active form of CaMKIIα (T286D), myc-tagged long Homers, 1, 2 or 3 or Homer1a and Flag-tagged mGluR5a in HEK 293 cells. Active CaMKIIα (T286D) reduced interactions of mGluR5 with all long Homers by 30–40% (Fig. 2A). CaMKIIα phosphorylates Homer3 (H3), a cerebellar-enriched Homer, which reduces interactions with binding partners such as drebin and mGluR1 (Mizutani et al., 2008). Because Homer1 (H1) and Homer2; (H2) are the neocortically expressed forms of Homer, we determined if CaMKIIα phosphorylates H1 and H2 using a Phos-tag gel of lysates of HEK cells expressing T286D CaMKIIα and specific Homers (Fig. 2B; (Kinoshita et al., 2006)). CaMKIIα induced a higher molecular weight (MW), phosphorylated, band of H1 and H1a and two phosphorylated species of H2 on the Phos-tag gel which were sensitive to alkaline phosphatase (Fig. S2A). Only an N-terminal fragment of H1 (aa1–177) was phosphorylated by CaMKIIα, whereas both N-terminal (aa1–177) and C-terminal (aa180–354) fragments of H2 were phosphorylated (Fig. S2B). H1a, H1, H2 and H3 are all predicted to share a conserved CaMKIIα phosphorylation site at Ser-117 (H3; Ser-120), and another unique CaMKIIα site (S216) is present in H2 (scansite.mit.edu; Fig.2B) and conserved across species (Fig. S2H). In support of these predictions, dephosphomimetic mutations of H1 (S117A) or H2 (S117/216AA) abolished the CaMKII-dependent higher MW bands on Phos-tag gels (Fig. 2B), suggesting these sites are CaMKII-phosphorylated sites.
Figure 2. CaMKIIα phosphorylation of the “hinge” region of Homer reduces interactions with mGluR5.
A) Constitutively active CaMKIIα (T286D) decreases interactions of long Homers (H1, H2, and H3) with mGluR5. Flag-tagged mGluR5 and myc-tagged Homer1, 2 and 3 were co-expressed together with or without CaMKIIα T286D in HEK293 cells. Western blots of Flag antibody and pan-Homer antibody after co-IP with myc antibody (Top). n=4.
B) CaMKII phosphorylates H1 at S117, H2 at S117 and S216. Upper: A schematic diagram of Homer1a or long Homer 1, 2, 3 with the sequences of “hinge region” with phosphorylation sites highlighted in red color. Lower: Myc-tagged WT or dephosphomimetic mutants of H1 or H2 were co-transfected with or without CaMKIIT286D in HEK cells. Phosphorylated H1 or H2 is observed as higher MW species (arrowheads) on a Phos-tag conjugated gel and blotted for myc.
C) Phosphomimetic mutations of CaMKII phosphorylation sites of H1, H2, and H3 display reduced interactions with mGluR5. Myc-tagged WT, phosphomimetic or dephosphomimetic mutants of H1, H2, and H3 were co-transfected with Flag-tagged mGluR5 in HEK293 cells. Western blots of Flag and pan-Homer after co-IP with myc. n=4.
D) Dephosphomimetic mutations of CaMKII phosphorylation sites on H1, H2 and H3 prevent CaMKIIα-induced dissociation of mGluR5-Homer in HEK cells. Flag-tagged mGluR5 and myc-tagged WT or mutants of Homer were co-expressed with or without CaMKIIα T286D in HEK cells. Blots of Flag and pan-Homer after co-IP with myc. n=4.
E) Phosphorylation of H1a at CaMKII site does not reduce interaction with mGluR5. E1) Flag-tagged mGluR5 and myc-tagged H1a were co-expressed with or without CaMKIIα T286D in HEK cells. E2) WT, phosphomimetic and dephosphomimetic H1a mutants were co-transfected with Flag-tagged mGluR5 in HEK cells. Blots of Flag and pan-Homer after co-IP with myc. n=4.
F) CaMKIIα reduces the ratio of H2/H1a interactions with mGluR5 in HEK cells. Flag-tagged mGluR5 was co-expressed with HA-tagged H2 and H1a with or without CaMKIIα T286D. Blots of HA after co-IP with Flag. n=4.
All experiments are repeated in at least 3 independent cultures. *P<0.05, **P<0.01, ***P<0.001. See also Figure S2
To determine if these Homer phosphorylation sites affected their interactions with mGluR5 in HEK cells, we coexpressed myc-tagged phosphomimetic mutations of CaMKIIα phosphorylation sites on H1 (S117D), H2 (S117/216DD) or H3 (S120E/159E/176D) with Flag-tagged mGluR5. Phosphomimetic mutations on all Homers were sufficient to decrease interactions with mGluR5 (Fig. 2C). Conversely, dephosphomimetic mutations, to alanines, of H1, H2 or H3 at these sites had similar affinities for mGluR5 as wildtype Homers, but prevented CaMKIIα–induced decreases in mGluR5-Homer (Fig. 2C,D). Therefore, CaMKIIα activity induces phosphorylation of all long Homers at specific sites which is necessary and sufficient to decrease interactions with mGluR5 in cells.
CaMKIIα phosphorylation differentially affects binding of long and short Homer proteins to mGluR5
Although CaMKIIα induces phosphorylation of H1a, as detected on a Phos-tag gel, it does not decrease H1a interactions with mGluR5 in HEK cells (Fig. 2E; S2A). Similarly, phospho- or dephosphomimetics of H1a, S117D or S117A respectively, have no effect on mGluR5 interactions (Fig. 2E). Because H1a and long Homers compete for binding to mGluR5 (Shiraishi-Yamaguchi and Furuichi, 2007) the selective effect of CaMKIIα-dependent phosphorylation on long Homers may shift the equilibrium binding of mGluR5 from long Homers to favor H1a. In support of this idea, in HEK cells co-expressing H1a and H2, constitutively active CaMKIIα reduced mGluR5 interactions with H2, but increased mGluR5 interactions with H1a (Fig. 2F).
Long Homers form a dumbbell-like tetrameric structure with a pair of N-terminal globular EVH1 domains located at each end of the tetramer, separated by a hybrid of dimer and tetramer coiled-coil domains (Shiraishi-Yamaguchi and Furuichi, 2007). Long Homer proteins have a discontinuity between their C-terminal coiled-coil regions, designated CC1 and CC2; Fig. 2B). The CC1 region of Homer forms a dimer, whereas CC2 forms a dimer-tetramer hybrid coiled-coil with other long Homers (Hayashi et al., 2006). Interestingly, CaMKII phosphorylation sites are present in, what has been called, the “hinge” region between the CC1 and the EVH1 domain (Fig.2B), (Mizutani et al., 2008). Homer multimerization is necessary for co-clustering with mGluR1 (Hayashi et al., 2006) and to form higher-order complexes with Shank (Hayashi et al., 2009). Thus, CaMKII phosphorylation of the “hinge” region may reduce Homer multimerization through coiled-coil interactions and indirectly affect complexes with mGluR5 in cells. To test this idea, we cotransfected myc-tagged WT or phosphomimetic H2 together with HA-tagged WT H2 into HEK293 cells. Co-immunoprecipation results demonstrate that phosphomimetic and WT H2 did not differ in their ability to multimerize with WT H2 (Fig. S2C). Because of the proximity of the “hinge” region of Homer to CC1, we considered the possibility that CaMKII phosphorylation sites may affect dimerization of CC1 domains. Size-exclusion column chromatography and chemical cross-linking experiments with truncated versions of H2, lacking a CC2 region (H2CC1), revealed no difference in the elution profiles of a WT-H2CC1 or phosphomimetic H2CC1 (S117/216DD) multimers (Fig, S2D,E) similar to previous results with H3 (Mizutani et al., 2008). To determine whether the Homer “hinge” region regulates interactions with mGluR5, we deleted the hinge of H1 (del112–189) or H2 (del112–230), and tested their interactions with mGluR5 in HEK cells, using co-IP. The H1 and H2 hinge-deleted mutants showed greatly reduced binding with mGluR5, without affecting Homer multimerization (Fig. S2F,G). These results implicate the hinge region of Homers in the enhancement of mGluR5 interactions in cells and as an important site of regulation by CaMKIIα.
Activity-dependent CaMKIIα-dependent phosphorylation of Homer 1 and 2 reduces mGluR5 interactions in neurons
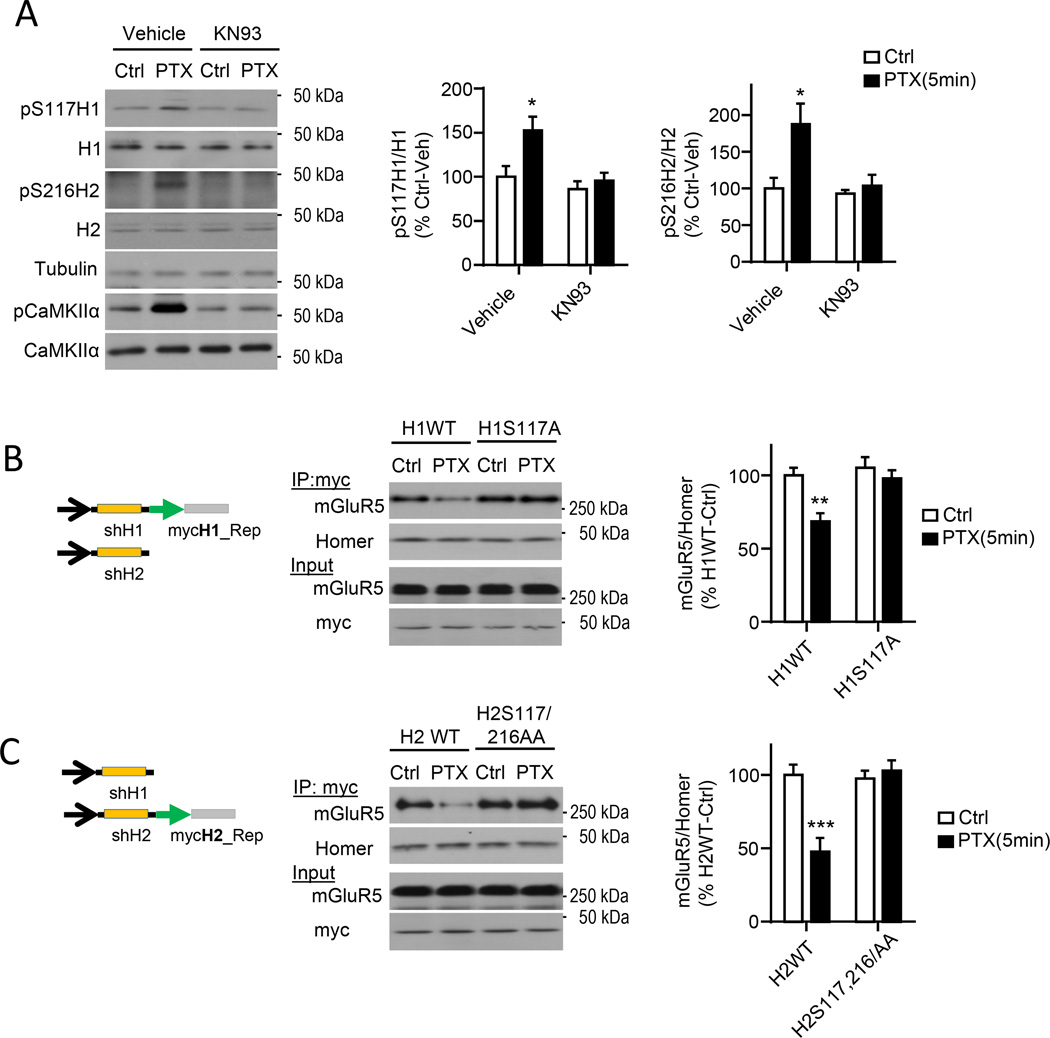
To determine whether neuronal activity induced phosphorylation of endogenous H1 and H2 at these sites, we produced phosphorylation-specific antibodies against H1 (Ser117) and H2 (Ser216; see methods). The specificity of affinity-purified anti-phospho(P)-S117_H1 and anti-PS216_H2 antibodies was assessed as described in Fig. S3A,B. A brief, 5 min, PTX treatment of dissociated cortical cultures induced H1 and H2 phosphorylation that was blocked by the CaMKII Kinase inhibitor KN93 (Fig. 3A). To determine whether Homer phosphorylation at CaMKIIα sites is required for activity-induced decreases in Homer-mGluR5 interactions in neurons, we used a lentiviral-mediated, molecular replacement strategy to knockdown endogenous H1 and H2 and replace with phosphorylation site mutants. A lentivirus expressing a bicistronic vector containing a short-hairpin (sh)RNA against H1 or H2 and a replacement cDNA encoding a myc-tagged wildtype Homer (H1mycWT or H2mycWT) or dephosphomimetics of Homer (H1mycS117A or H2mycS117/216AA) (Fig. S3C). Replacement of endogenous Homers in neurons with H1mycWT or H2mycWT and co-IP of myc revealed decreased mGluR5 interactions with H1mycWT or H2mycWT in response to PTX treatment (Fig. 3B,C). In contrast, in neurons where endogenous Homers were replaced with dephosphomimetic mutants, H1mycS117A or H2mycS117/216AA, we did not observe PTX-induced decreases in mGluR5 interactions with Homers as measured by co-IP of myc (Fig.3B,C), indicating that CaMKII phosphorylation of Homer at these sites is necessary for activity-induced dissociation of mGluR5.
Figure 3. Activity-induced disruption of mGluR5-Homer scaffolds in neurons requires Homer1 and Homer2 phosphorylation.
A) Brief PTX treatment (5 min) of cortical neurons enhances phosphorylation of H1 (S117) and H2 (S216) and is blocked by the CaMKII inhibitor KN93. n=4.
B) Knockdown of endogenous H1 and H2 and replacement with a myc-tagged dephosphomimetic (S117A) of H1 prevents activity-induced dissociation of mGluR5-Homer in cortical neurons. Left: Schematic of bicistronic knockdown and replacement lentiviral construct. Middle: Blots of mGluR5 after co-IP with myc. Right: Group data; n=4.
C) Knockdown of endogenous H1 and H2 and replacement of H2 with a myc-tagged dephosphomimetic (S117/S216AA) prevents activity-induced dissociation of mGluR5-Homer cortical neurons. Left: Schematic of bicistronic knockdown and replacement lentiviral construct. Middle: Blots of mGluR5 after co-IP with myc. Right: Group data; n=4.
All experiments are repeated in at least 3 independent cultures. *P<0.05, **P<0.01, ***P<0.001. See also Figure.S3
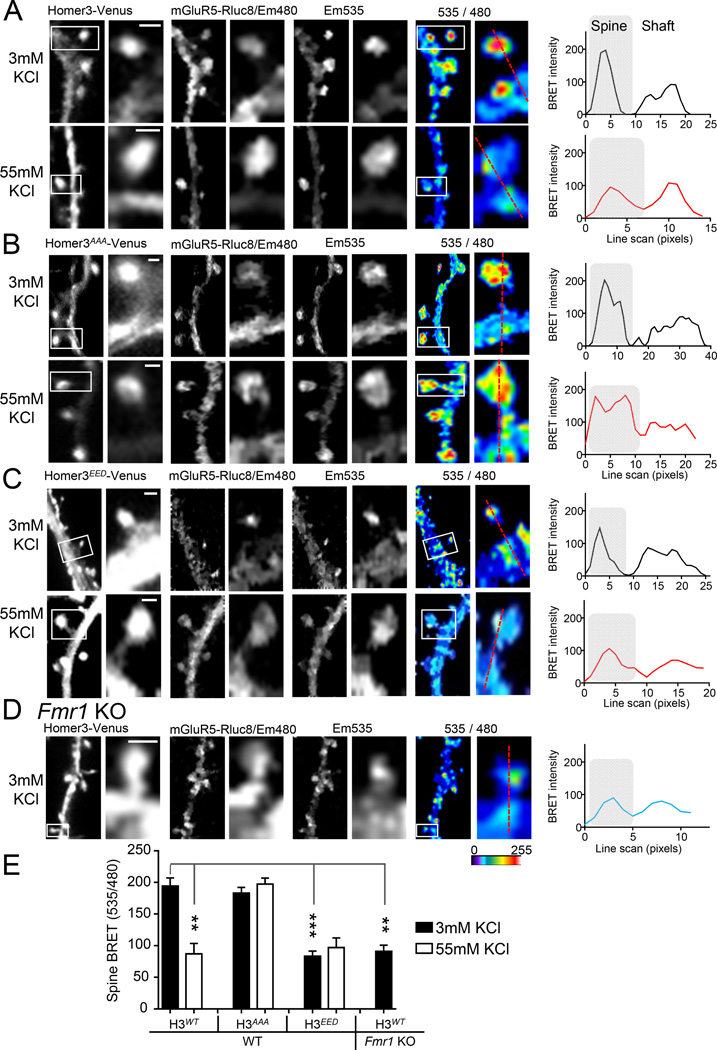
Rapid activity-induced disruption of mGluR5 -Homer in spines, as measured with bioluminescence resonance energy transfer (BRET), relies on Homer phosphorylation
To determine if neuronal activity affected mGluR5-Homer interactions at synapses we used Bioluminescence resonance energy transfer (BRET), a method that measures robust interactions of mGluR5 and Homer in dendritic spines (Moutin et al., 2012). To do this, the C-terminus of mGluR5a was fused to the energy donor Renilla luciferase (mGluR5-Rluc8) and co-expressed in neurons with H3 N-terminally fused to the acceptor Venus (H3-Venus). Direct interactions of mGluR5-Rluc8 and wildtype H3 (H3WT-Venus) in dendritic spines are detected by the enhanced BRET signal (Fig. 4A). Brief depolarization of neuron cultures (55mM KCl; 5 min) reduced the BRET in spines, reflecting a decreased interaction of mGluR5-Homer (Fig. 4A,E). The dephosphomimetic of H3 (S120A/159A/176A; H3AAA-Venus) displayed robust BRET in spines, similar to H3WT-Venus, but spine BRET was unaffected by KCl depolarization (Fig. 4B,E) indicating that phosphorylation of H3 is necessary for activity-induced dissociation of mGluR5-Homer in spines. The phosphomimetic of H3 (S120E/159E/176D; H3EED-Venus) displayed reduced BRET with mGluR5-Rluc8 in spines under basal activity conditions (3mM KCl), and was unaffected by KCl depolarization (Fig. 4C,E). These results confirm our findings with co- IPs of endogenous mGluR5-Homer and reveal the rapid dissociation of mGluR5-Homer in spines in response to activity that depends on H3 phosphorylation at CaMKII sites.
Figure 4. Brief neural activity dissociates mGluR5 from Homer in dendritic spines as revealed with Bioluminescence resonance energy transfer (BRET).
(A–D) Representative dendritic spine images (left to right) of Homer3-Venus fluorescence, mGlu5-luc emission (Em) at 480, Em535 from Homer-Venus as a result of BRET from mGluR5luc, and Em535/480 ratio. Scale bar= 1 µM. Red line indicates region of quantification of BRET of spine and dendritic shaft. Line scan is shown at right.
A. Brief depolarization (5 min; 55mM KCl) of WT neurons transfected with wildtype Homer3-Venus reduces the mGluR5-Homer BRET in spines.
B. WT neurons expressing a dephosphomimetic Homer3(AAA)-Venus display normal spine BRET under basal (3mM KCl) conditions, but no change in BRET in response to 55mM KCl.
C. WT neurons expressing a phosphomimetic Homer3(DDD)-Venus display reduced spine BRET under basal (3mM KCl) conditions, and no change in BRET in response to 55mM KCl.
D. Fmr1 KO neurons expressing Homer3-Venus show reduced BRET in spines under basal conditions.
E. Group spine BRET values from each condition. n = 3 cultures and 8–16 spines/condition.
*P<0.05, **P<0.01, ***P<0.001.
Homer proteins are hyperphosphorylated by CaMKIIα in Fragile X Syndrome mouse model neurons
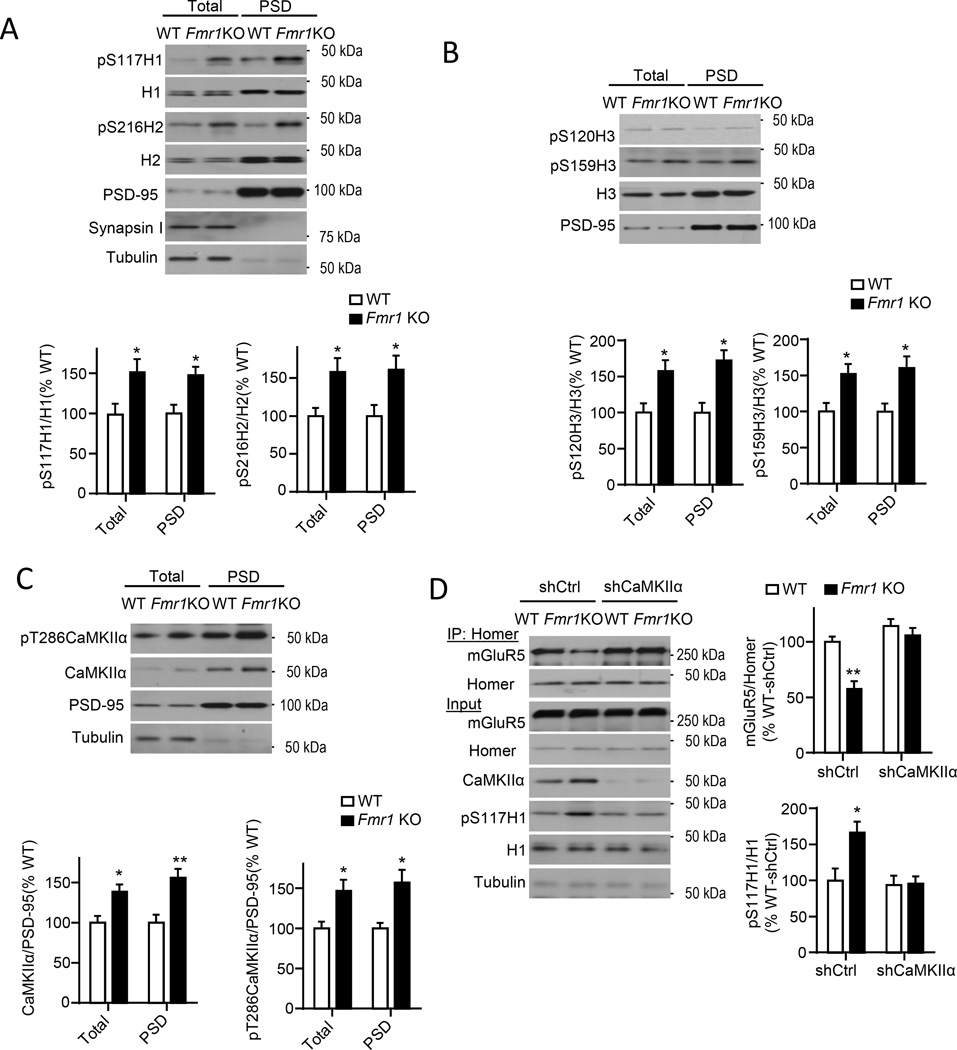
In Fmr1 KO forebrain, mGluR5 is less associated with long Homers and more associated with H1a as assessed with co-IPs (Giuffrida et al., 2005; Ronesi et al., 2012). Importantly, disrupted mGluR5-Homer scaffolds contribute to FXS disease-relevant phenotypes(Ronesi et al., 2012). Consistent with these findings, we observe reduced BRET of H3WT-Venus and mGluR5-Rluc8 in the spines of cultured Fmr1 KO neurons (Fig. 4D,E) indicating that these scaffolding changes are occurring at synapses. To determine if Homer phosphorylation may contribute to disrupted mGluR5-Homer scaffolds in Fmr1 KO brain, we blotted cortical lysates and PSD fractions from WT and Fmr1 KO littermates with phosphospecific antibodies of Homers at CaMKII sites which revealed enhanced phosphorylation of all Homer proteins (P-S117_H1, P-S216_H2, P-S120_H3 and P-S159_H3) Fmr1 KO (Fig. 5A,B).
Figure 5. Elevated CaMKIIα levels and Homer hyperphosphorylation lead to reduced mGluR5-Homer in Fmr1 KO cortex.
A) H1 (S117) and H2 (S216) phosphorylation is elevated in total lysates and PSD fractions from Fmr1 KO cortex. PSD-95, Synapsin 1 and tubulin confirm enrichment of PSDs. n = 4 mice/genotype.
B) H3 (S120; S159) phosphorylation is elevated in total lysates and PSD fractions from Fmr1 KO hippocampus. n = 4 mice/genotype.
C) Total and phosphorylated (T286) CaMKIIα levels are elevated in total lysates and PSD fractions from Fmr1 KO cortex. n = 4 mice/genotype.
D) Knockdown of CaMKIIα (shCaMKIIα) restores mGluR5-Homer interactions in cultured Fmr1 KO cortical neurons in comparison to control shRNA (shCtrl) transfected cultures. Blots of mGluR5 after co-IP with Homer. n=4 cultures/condition. *P<0.05, **P<0.01. See also Figure. S4
CaMKIIα mRNA directly interacts with FMRP (Darnell et al., 2011), and, in Fmr1 KO brain, displays increased association with polysomes suggesting enhanced translation. CaMKIIα protein levels are elevated in synaptic fractions of Fmr1 KO brain (Ronesi et al., 2012; Zalfa et al., 2003). Consistent with these data, we observed enhanced total and autophosphorylated (T286) CaMKIIα in total lysates and PSD fractions from Fmr1 KO cortices (Fig. 5C). Phosphorylated-T286 correlates with CaMKIIα kinase activity (Shonesy et al., 2014), suggesting that CaMKIIα is more active at Fmr1 KO synapses. In support of this idea, we observed enhanced phosphorylation of the NMDA receptor subunit, GluN2B, at the CaMKII site, P-S1303 (Hell, 2014). However, P-S831 of the AMPA receptor subunit, GluA1, was normal (Fig. S4A) revealing differential regulation of distinct CaMKIIα substrates.
Pharmacological or genetic reduction of CaMKIIα activity restores mGluR5-Homer scaffolds in Fmr1 KO mice
To determine if enhanced CaMKIIα levels contribute to hyperphosphorylation of Homer and reduced interactions with mGluR5 in Fmr1 KO neurons, we reduced CaMKIIα levels using lentiviral-mediated transfection of a shRNA against CaMKIIα in WT and Fmr1 KO cultured cortical neurons (Fig. 5D). Knockdown of CaMKIIα had no effect on H1S117 phosphorylation or Homer-mGluR5 interactions in WT neurons. However, in Fmr1 KO neurons, CaMKIIα knockdown restored both H1S117 phosphorylation and Homer interactions with mGluR5 to WT levels (Fig. 5D). Conversely, overexpressing CaMKIIα in WT neurons (~2-fold) similar to that observed in Fmr1 KO neurons, decreases Homer-mGluR5 (Fig. S4B).
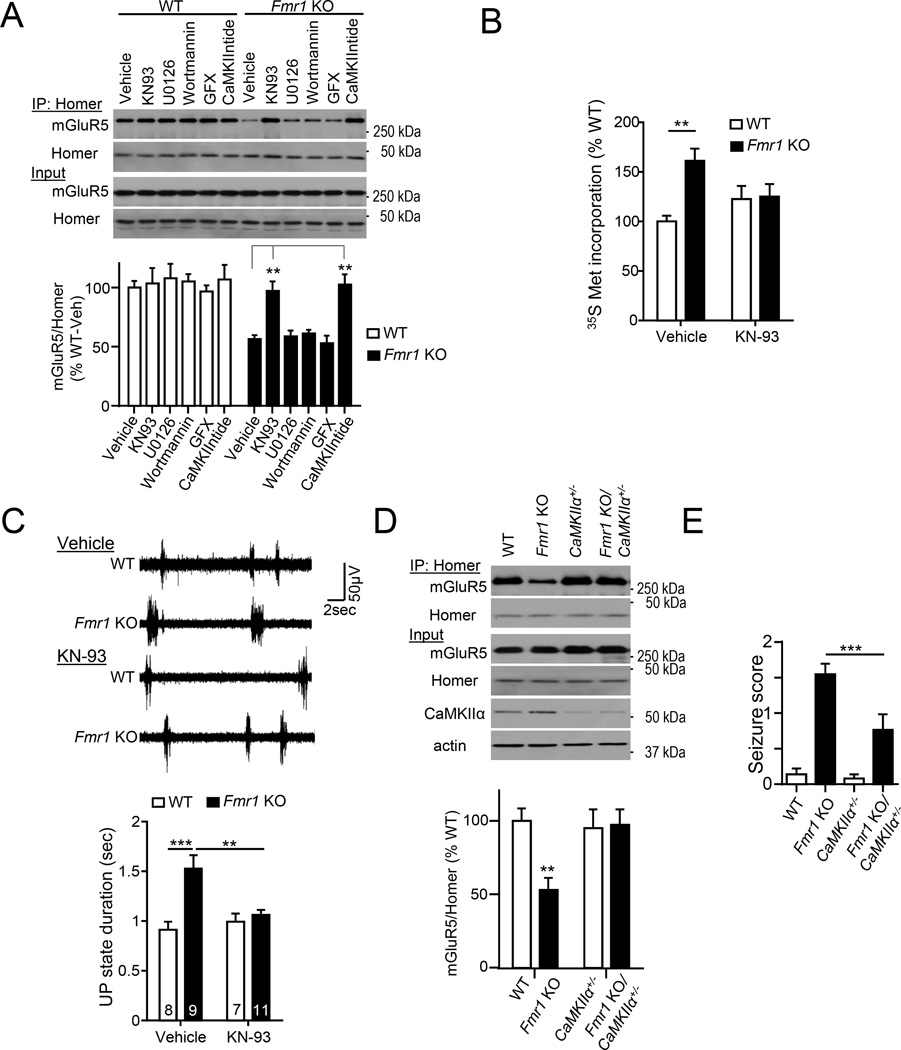
To test a causal role for CaMKIIα activity in altered mGluR5-Homer scaffolds, Fmr1 KO neocortical (Fig. 6A) or hippocampal (Fig. S5A) cultures were pretreated with CaMKII inhibitors, KN-93 or myr-CaMKIINtide (5 µM; 60 min;) which restored mGluR5-long Homer interactions to that observed in WT cultures. Consistent with data shown above (Fig. 1D), had no effect on mGluR5-Homer WT cultures (Fig. 6A). In addition to CaMKII, H3 is phosphorylated by Akt (T36, S38, S52)(Huang et al., 2008) and ERK (S141)(Mizutani et al., 2008) and PKC phosphorylates mGluR5 (Mao et al., 2008). Furthermore, the Akt and ERK pathways are hyperactive in Fmr1 KO neurons (Gross et al., 2010; Osterweil et al., 2013) To determine if these kinases contribute to altered mGluR5-Homer interactions, inhibitors ERK (U0126; 10 µM), or Akt (wortmannin; 100nM), or PKC (GF109203× 5µM) were applied to WT and Fmr1 KO cultures (Fig. 6A). Inhibition of ERK, PI3K or PKC had no effect on Homer-mGluR5 interactions in either genotype (Fig. 6A) suggesting a selective role for CaMKII activity in disruption of mGluR5-Homer in Fmr1 KO neurons. Although mGluR5-Homer scaffolds are decreased in Fmr1 KO neurons, this does not prevent PTX induced (5 min or 12 hr) disruption of mGluR5-Homer (Fig. S5B).
Figure 6. Genetic or pharmacological reduction in CaMKIIα activity rescues mGluR5-Homer scaffolds and other phenotypes associated with Fragile X Syndrome.
A) Inhibition of CaMKII activity (KN93 or CaMKIINtide) restores mGluR5-long Homer interaction in Fmr1 KO neocortical neurons. Inhibitors of other kinases (ERK, PI3K or PKC) have no effect. Blots of mGluR5 after co-IP with Homer. n=4 cultures/genotype.
B) Acute inhibition of CaMKII activity by KN93 treatment rescues enhanced basal translation rates in Fmr1 KO hippocampal slices as measured by 35S Met incorporation into total protein. n = 8 mice/ genotype.
C) Acute Inhibition of CaMKII activity by KN93 rescues prolonged neocortical UP states in Fmr1 KO neocortical slices. Left: Representative traces of UP states from each condition. Scale bar = 50 µV/ 2sec.
D) Genetic reduction of CaMKIIα in Fmr1 KO/CaMKIIα+/− mice rescues mGluR5-Homer interaction in Fmr1 KO mice. The front cortex tissue lysates were from WT/WT, Fmr1 KO/WT, WT/CaMKIIα+/- and Fmr1 KO/CaMKIIα+/− mice. Western blots of mGluR5 after coimmunoprecipitation (IP) with Homer antibody (top). Input was shown on the bottom, antibody as indicated. n = 4 mice/genotype
E) Cross Fmr1 KO mice and CaMKIIα+/− mice rescues audiogenic seizures. Fmr1 KO mice had an increased seizure score, the audiogenic seizure score was reduced in Fmr1 KO/CaMKIIα+/− mice (n = 35, 37, 28 and 21 mice for WT/WT, Fmr1 KO/WT, WT/CaMKIIα+/− and Fmr1 KO/CaMKIIα+/−, respectively).
*P<0.05, **P<0.01, ***P<0.001. See also Figure.S5
Pharmacological or genetic reduction of CaMKIIα activity rescues phenotypes in Fmr1 KO mice
To determine if CaMKIIα hyperactivity contributes to disease-related phenotypes in Fmr1 KO mice, we examined the effects of pharmacological and genetic reduction in CaMKIIα activity. Enhanced basal protein synthesis rates are commonly observed in several brain regions of Fmr1 KO mice, as well as individuals with Fragile X Syndrome (Qin et al., 2013). Pharmacological antagonism of mGluR5 and genetic deletion of H1a restore protein synthesis rates in Fmr1 KO mice suggesting that mGluR5 constitutive activity, as a result of reduced Homer binding, drives protein synthesis rates through stimulation of signaling pathways to translation factors (Osterweil et al., 2010; Ronesi et al., 2012). In support of this idea, treatment of acute hippocampal slices from WT or Fmr1 KO mice with KN-93 (60 min; 10µM) normalized basal protein synthesis rates between genotypes as measured by incorporation of 35S Met/Cys into total protein (Osterweil et al., 2010; Ronesi et al., 2012) (Fig. 6B). Elevated activity of the ERK and mTORC1 signaling pathways have been implicated in the enhanced protein synthesis rates in Fmr1 KO (Gross et al., 2010; Osterweil et al., 2010). KN-93 treatment did not affect basal phosphorylation of ERK1/2 (Thr202/Tyr204) or mTORC1 (S2448)(Fig. S5D), suggesting that CaMKIIα inhibition and intact mGluR5-Homer scaffolds affect protein synthesis rates likely downstream of ERK and mTORC1.
Another prevalent symptom of Fragile X Syndrome is sensory circuit hyperexcitability which may contribute to the observed behavioral sensory hypersensitivity and seizures (Kidd et al., 2014). Fmr1 KO mice recapitulate this phenotype and display sensory-induced behavioral hypersensitivity, seizures and hyperexcitability of sensory neocortical circuits (Rotschafer and Razak, 2014). We have observed hyperexcitability of sensory neocortical circuits in Fmr1 KO mice, as prolonged, spontaneous persistent network activity, or UP states in acute slices of somatosensory, barrel cortex, as well as in vivo in anesthetized Fmr1 KO mice (Hays et al., 2011) (Fig. 6C). Pharmacological or genetic reduction of mGluR5 activity or H1a deletion rescues UP state duration in Fmr1 KO mice to WT levels suggesting that disrupted mGluR5 hyperactivity as a result of disrupted Homer scaffolds mediates circuit hyperexcitability(Hays et al., 2011; Ronesi et al., 2012). In support of this model, pharmacological inhibition of CaMKII in Fmr1 KO cortical acute slices (10 µM KN-93; 60 min) restored both mGluR5-Homer interactions and UP state duration to WT levels (Fig. 6C; Fig. S5C). KN-93 had no effect on UP state duration in WT slices indicating that the inhibitor is not generally reducing slice excitability.
To determine if a reduction in CaMKIIα levels corrects behaviorally relevant hyperexcitability, or seizures, Fmr1 KO mice were crossed with mice heterozygous for CaMKIIα (CaMKIIα+/−) (Silva et al., 1992). Audiogenic seizures, mGluR5-Homer interactions and Homer phosphorylation were measured in littermates of all four genotypes (WT, Fmr1 KO, CaMKIIα+/−, Fmr1 KO/CaMKIIα+/−). Using co-IP of pan-Homer and mGluR5 from cortical lysates of all four genotypes we observed a decrease in mGluR5 association with Homer in Fmr1 KO, that was restored in the Fmr1 KO/CaMKIIα+/− (Fig. 6D). CaMKIIα+/− did not affect mGluR5-Homer as compared to WT mice. Fmr1 KO/CaMKIIα+/− had restored H1 phosphorylation levels in comparison to Fmr1 KO (Fig. S5E). As previously described, Fmr1 KO mice displayed an increased incidence and severity of audiogenic seizures in comparison to WT mice and this is quantified by seizure score (Fig. 6E; Table S1). CaMKIIα+/− and WT mice both had similar, low seizure scores. Importantly, Fmr1 KO/CaMKIIα+/− mice had reduced seizure score in comparison to Fmr1 KO (p< 0.003; Chi-Square), but were higher than WT littermates. In summary, genetic reduction of CaMKIIα completely restored mGluR5-Homer interactions in Fmr1 KO cortex, and reduced seizures. A similar, partial rescue of seizures was observed with mGluR5 and H1a deletion (Dolen et al., 2007) (Ronesi et al., 2012), suggesting mGluR5-Homer-independent mechanisms also contribute to audiogenic seizures.
Replacement with Homer dephosphomimetics rescues mGluR5-Homer interactions and circuit hyperexcitability in cultured Fmr1 KO cortical neurons
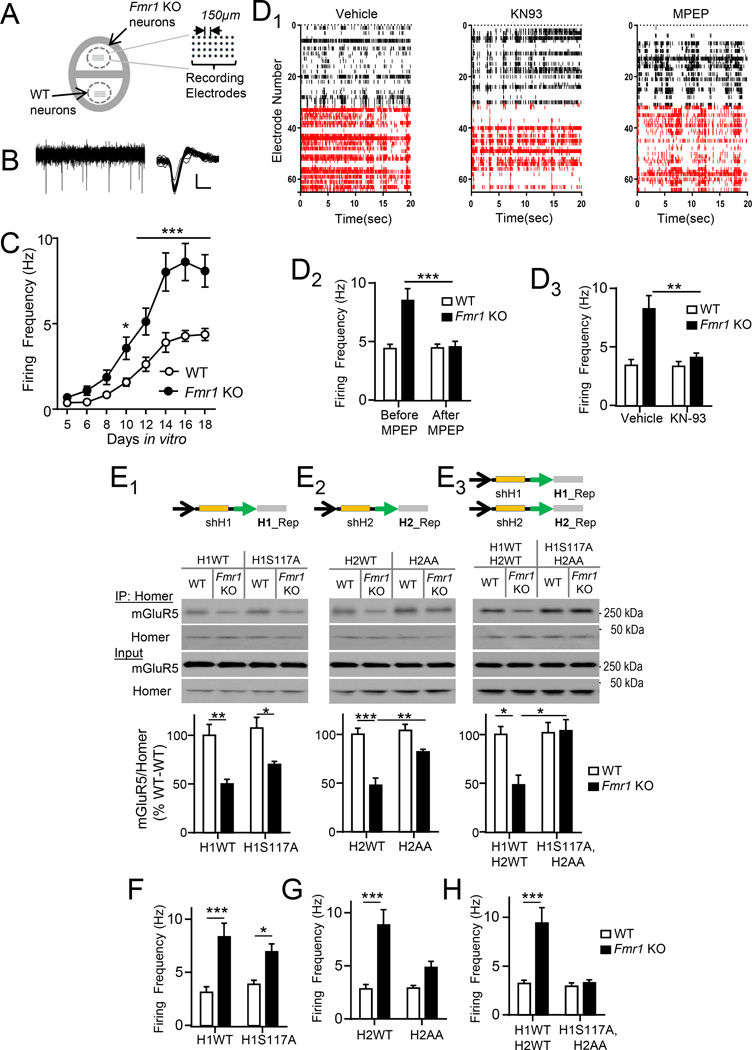
To test a specific role for Homer phosphorylation in circuit hyperexcitability, we established circuit excitability in cultured neocortical neurons. Dissociated neocortical neurons from WT or Fmr1 KO littermates were grown in dual-chamber culture dishes imbedded with a 64 microelectrode array (MED64.com; Fig. 7A). Development of functional circuits in WT and Fmr1 KO cultures was measured over 18 days in vitro (DIV) as an increase in the spontaneous action potential frequency (Fig. 7B,C). As early as 10 DIV, Fmr1 KO cultures were hyperexcitable, as measured by average firing frequency, and this hyperexcitability persisted until at least 18 DIV (Fig. 7B). To establish if the mechanisms of hyperexcitability of Fmr1 KO cultured neurons were similar to acute slices, we tested the mGluR5 negative allosteric modulator (NAM), MPEP (Hays et al., 2011) and CaMKII inhibitors. MPEP treatment (10 µM; 2 hrs at 18 DIV) reduced action potential firing frequency in Fmr1 KO cultures, but had no effect in WT cultures and thus equalized firing frequency across genotypes (Fig. 7D1,2). Similarly, application of the CaMKII inhibitor, KN93 (5 µM; 2 hrs) decreased activity in Fmr1 KO neurons compared to vehicle treatment (Fig. 7D1,3).
Figure 7. Replacement of endogenous Homer with Homer dephosphomimics rescues mGluR5-Homer interactions and circuit hyperexcitability in cultured Fmr1 KO neocortical neurons.
A) Schematic of MED64 dual recording chamber (adapted from med64.com).
B) Representative recording from a single electrode in a 32-electrode array from dissociated Fmr1 KO cortical cultures. Right: Sorted and aligned action potentials. Scale bars: 10 µV/200 or 1 msec.
C) Development of circuit hyperexcitability of Fmr1 KO cortical cultures as measured by average firing frequency vs. days in vitro (DIV). n=5 cultures/genotype.
D) Circuit hyperexcitability in Fmr1 KO neurons was rescued by pharmacological blockade of mGluR5 and CaMKII inhibitor. D1) Raster plots of multi-unit activity recorded from WT and Fmr1 KO cultures recorded at 18 DIV. Each line represents a single spike detected in a given channel during 20 seconds of a recording. Vehicle, MPEP (10µM) or KN93 (5 µM) for 2 hour prior to recording. D2–D3) Average firing frequency per electrode from WT and Fmr1 KO cultures. n=5 cultures/genotype.
E) Replacement of endogenous H1 and H2 with dephosphomimetics rescues mGluR5-Homer interactions in Fmr1 KO neocortical neurons. (E1–E3) Upper: Schematic of bicistronic lentiviral constructs expressing a shRNA against endogenous H1 and/or H2 and replacement with dephosphomimetics. Lower: Blots and group data of mGluR5 after co-IP with Homer. n=4 cultures/genotype.
F) Replacement of endogenous H1 with dephosphomimetic H1S117A is not sufficient to rescue circuit hyperexcitability in Fmr1 KO neurons. Cultures were transfected with lentivirus schematized in E1. n=5 cultures/genotype.
G) Replacement of endogenous H2 with dephosphomimetic H2S117/216AA reduces circuit hyperexcitability in Fmr1 KO cultures. Cultures were transfected with lentivirus schematized in E2. n=5 cultures/genotype.
H) Replacement of endogenous H1 and H2 with dephosphomimetics normalizes circuit hyperexcitability in Fmr1 KO neurons. Cultures were transfected with lentivirus schematized in E3. n=5 cultures/genotype. *P<0.05, **P<0.01, ***P<0.001. See also Figure.S6
We hypothesized that hyperphosphorylation of Homer at CaMKII sites leads to decreased mGluR5-Homer interactions and circuit hyperexcitability in Fmr1 KO neurons. To test this hypothesis, we again utilized the lentivirus-mediated molecular replacement approach to knock down endogenous H1 and/or H2 in cultured cortical WT or Fmr1 KO neurons and replace with either a myc-tagged wild type (WT) or dephosphomimetic of Homer at CaMKII sites (Fig. 7E). To assess the effects of the Homer molecular replacement, co-IPs of myc-Homer were used to measure mGluR5 interactions and firing frequency was measured using MED64 microelectrode arrays at 8–10 days post-transfection. Knockdown and replacement of H1 with H1S117A was insufficient to restore Homer-mGluR5 interactions or hyperexcitability in Fmr1 KO neurons in comparison to H1WT expressing cultures (Fig. 7E1; F). In contrast, knockdown and replacement of H2 with H2S117/216AA enhanced Homer-mGluR5 interactions and reduced hyperexcitability of Fmr1 KO neurons (Fig. 7E2, G). Knockdown and replacement of both H1 and H2 with their respective dephosphomimetics completely restored Homer-mGluR5 interactions and normalized circuit excitability in Fmr1 KO neurons (Fig. 7E3, H). MPEP treatment of the H1H2 dephosphomimetic replacement cultures (2 hours) did not further decrease firing frequency (Fig. S6A) suggesting that mGluR5 and Homer phosphorylation function in a common mechanism for circuit hyperexcitability. The results indicate that phosphorylation of H1 and H2 at CaMKII sites is necessary for the disrupted mGluR5-Homer scaffolds and circuit hyperexcitability observed in Fmr1 KO neurons.
DISCUSSION
The Homer family of scaffolding proteins are critical regulators of mGluR5 function because they scaffold mGluR5 to the PSD, mediate signaling to specific effectors as well as promote the timing and localization of mGluR5 signaling. However, little has been known about the dynamic regulation of the mGluR5-Homer scaffolds, and how misregulation of Homer scaffolds contributes to brain disease. Here we identified a rapid (<5 min), activity-triggered dissociation of mGluR5 from its Homer scaffold at spines which is mediated by CaMKIIα-dependent phosphorylation of long Homers. Such a dissociation would be expected to have multiple consequences on mGluR5 signaling and function at spines (Ronesi and Huber, 2008; Shiraishi-Yamaguchi and Furuichi, 2007). Importantly, we demonstrate CaMKIIα-dependent hyperphosphorylation of Homer at a basal state in neocortical neurons of the mouse model of FXS. This work eludicates a molecular mechanism by which loss of FMRP-mediated translational suppression of the specific target mRNA, CaMKIIα, leads to abnormal mGluR5 function and disease-relevant phenotypes.
CaMKIIα phosphorylation of Homer mediates a rapid activity-dependent disruption of mGluR5-Homer
Our data, taken together with previous work (Mizutani et al., 2008; Okabe et al., 2001), reveal a rapid activity-dependent disruption of mGluR5 from long Homer scaffolds mediated by Ca2+ influx through NMDA receptors and L-type Ca2+ channels, CaMKIIα activation and phosphorylation of H1 and H2. CaMKIIα, in its inactive state, binds directly to the C-terminal tail of mGluR5 which, upon Ca2+ influx into the synapse, make it well positioned to phosphorylate Homer and rapidly regulate the Homer scaffolds (Jin et al., 2013). The fast CaMKIIα-dependent phosphorylation of long Homers is not necessary for chronic activity-induced, H1a–mediated, disruption of mGluR5-Homer and thus may represent a distinct, and rapid, means to regulate mGluR5 function at synapses. Pharmacological or genetic reduction of CaMKIIα in WT cultures or in vivo did not enhance mGluR5-Homer interactions, suggesting that under basal, or low, activity conditions, CaMKIIα phosphorylation of Homer is negligible and/or interactions with mGluR5 are saturated. In support of this idea, basal phosphorylation of H1 and H2 at CaMKIIα sites is low in WT cultures and PTX treatment activates CaMKIIα, increases H1 and H2 phosphorylation and results in a CaMKIIα-dependent disruption of mGluR5-Homer scaffolds.
CaMKII activity alters the balance of long Homer/H1a interactions with mGluR5
CaMKIIα phosphorylates all forms of Homer we tested (H1, H2, H3 and H1a), but only regulates interactions between mGluR5 and long Homers. CaMKII phosphorylation of H3 reduces affinity for C-terminal peptides of drebrin and mGluR1 in in vitro binding assays (Mizutani et al., 2008). Additionally, purified CaMKII reduces Homer3-SHANK1 complexes in vitro (Hayashi et al., 2009). These results strongly suggest that the phosphorylation of H1 and H2, as well as the decreased interactions with mGluR5, observed upon CaMKII activation in cells are due to direct CaMKIIα mediated phosphorylation of Homer. In support of this assertion, CaMKII phosphorylation site mutants of Homer either mimic (phosphomimetics) or block (dephosphomimetics) activity and CaMKII-dependent disruption of mGluR5-Homer interactions. Previous results also suggest that activity-dependent, CaMKII-mediated phosphorylation of Homer reduces interactions with multiple binding partners and is a key mechanism for activity-dependent remodeling of Homer scaffolds (Hayashi et al., 2009; Mizutani et al., 2008).
Many CaMKII phosphorylation sites on Homers (H1,H2_S117; H3_S120 H3) occur within the linker or “hinge” region C-terminal to the EVH1 domain (aa111–180 in H1) (Irie et al., 2002; Mizutani et al., 2008). Deletion of the hinge region of H1 or H2 reduces mGluR5 interactions without affecting Homer multimerization, suggesting that the hinge stabilizes mGluR5-EVH1 binding. Surprisingly, CaMKII phosphorylates H1a in HEK cells, but does not decrease interactions with mGluR5, revealing a requirement for the C-terminal coiled-coil domains or Homer multimerization in regulation of mGluR5 binding. However, neither CaMKII activation, nor phosphorylation site mutants of H2 affected Homer dimerization or interactions between coiled-coil domains, as observed for H3 (Mizutani et al., 2008). A proline-rich motif (or P-motif; 138-SPLTP-142) in the hinge region of H1 interacts with the EVH1 domain of neighboring Homer molecules (Irie et al., 2002). The P-motif and mGluR1/5 binding sites within the EVH1 domain partially overlap. Therefore, CaMKII phosphorylation of the hinge region may affect P-motif binding to the EVH1 domain of neighboring Homers and destabilize mGluR5-EVH1 interactions. Such a mechanism would confer specific regulation of Homer proteins within a multimeric scaffold and may explain how CaMKIIα and FMRP affect mGluR5-interactions with long Homers, but not H1a (Giuffrida et al., 2005; Ronesi et al., 2012). The novel CaMKII phosphorylation site in H2 (S216) occurs within the C-terminal coiled-coil domain, but does not affect H2 multimerization. Interestingly, S216 resides in a region of H2 that interacts with the small GTPase Cdc42; which regulates localization of H2 in spines (Shiraishi-Yamaguchi et al., 2009). Phosphorylation of S216 may indirectly affect mGluR5 EVH1 binding through regulation of Cdc42 interactions and/or H2 localization in cells.
CaMKII-mediated hyperphosphorylation of Homer contributes to mGluR5 dysfunction and phenotypes in Fmr1 KO mice
mGluR5 hyperactivity is causally associated with the phenotypes of FXS in animal models (Dolen et al., 2007; Michalon et al., 2012), but the molecular mechanisms by which loss of FMRP leads to abnormal mGluR5 function has been elusive. Our new data herein, provide a molecular mechanism by which FMRP regulates mGluR5 function, through CaMKIIα-dependent phosphorylation of Homer. CaMKIIα mRNA is present in dendrites and directly interacts with FMRP (Darnell et al., 2011). Dendritic CaMKIIα mRNA levels are normal in Fmr1 KO neurons (Steward et al., 1998), but more CaMKIIα mRNA is associated with synaptic polyribosomes and CaMKIIα protein levels are elevated (Ronesi et al., 2012; Zalfa et al., 2003). These data together with our own suggest that loss of FMRP-mediated translational suppression of CaMKIIα in Fmr1 KO neurons leads to enhanced total and active CaMKIIα levels at synapses, hyperphosphorylation of long Homers and decreased affinity for mGluR5. Revealing the importance of CaMKIIα levels in regulation of Homer, knockdown of CaMKIIα restores normal mGluR5-Homer in Fmr1 KO and overexpression of CaMKIIα in WT neurons is sufficient to reduce their interactions. When associated with H1a, mGluR5 displays constitutive activity or signaling in the absence of glutamate (Ango et al., 2001). Such enhanced constitutive mGluR5 activity in Fmr1 KO neurons may underlie the sensitivity of protein synthesis rates and circuit hyperexcitability to mGluR5 NAMs (Hays et al., 2011; Michalon et al., 2012; Osterweil et al., 2010). Because Homer phosphorylation reduces its affinity with other interacting proteins such as drebin, mGluR1 (Mizutani et al., 2008), and SHANK (Hayashi et al., 2009) there may be a general disruption of Homer scaffolds in Fmr1 KO neurons that alters synaptic structure and function in addition to mGluR5. Therefore, treatment strategies targeting CaMKIIα, would be expected to restore Homer scaffolds with its many binding partners and may be have added therapeutic value towards FXS in comparison to specific mGluR5 compounds.
CaMKII-mediated hyperphosphorylation of Homer leads to circuit hyperexcitability in FXS
Here we implicate CaMKIIα regulation of mGluR5-Homer scaffolds in a well-established and FXS-relevant phenotype of sensory circuit hyperexcitability and seizures (Rotschafer and Razak, 2014). Pharmacological or genetic reduction of CaMKIIα or molecular replacement of Homers with dephosphomimetics at CaMKII sites corrects and restores normal circuit hyperexcitability in neocortical cultured neurons, UP state duration in acute slices and/or audiogenic seizures in vivo. This work, together with previous studies manipulating mGluR5 or Homer scaffolds (Dolen et al., 2007; Hays et al., 2011; Ronesi et al., 2012; Tang and Alger, 2015) support a model where disrupted mGluR5-Homer scaffolds, as a result of CaMKIIα hyperphosphorylation of H1 and H2, result in hyper- or constitutively active mGluR5 activity, neocortical hyperexcitability and seizures. The cellular and synaptic locus where these misregulated scaffolds function in circuit hyperexcitability is unknown. Deletion of FMRP in excitatory, but not inhibitory neurons, is sufficient to recapitulate prolonged UP states and pharmacologically-isolated excitatory circuits in Fmr1 KO neocortex are hyperexcitable (Hays, 2011 #31). These results suggest that disrupted mGluR5-Homer in excitatory neurons contributes to the circuit excitability. Homers scaffold mGluR5 with other effectors such as NMDA receptors, the endocannabinoid synthesizing enzyme, DAG lipaseα, ion channels as well as signaling pathways that modulate ion channel function, including intracellular Ca2+ (Shiraishi-Yamaguchi and Furuichi, 2007). Disrupted Homer scaffolds in Fmr1 KO neurons may result in constitutive and/or abnormal mGluR5 signaling to ion channels and/or synaptic function. Recent findings implicated two candidate mechanisms in hyperexcitability of Fmr1 KO cortical circuits; reduced activity voltage- and Ca(2+)-activated K(+) (BK) channels in layer 5 dendrites (Zhang et al., 2014) and an imbalance of mGluR5 and endocannabinoid regulation of inhibitory and excitatory synaptic transmission in cortical neurons (Jung et al., 2012; Tang and Alger, 2015; Zhang and Alger, 2010). Both BK channel and endocannabinoid function is regulated by Homers (Ango et al., 2001; Tang and Alger, 2015).
CaMKIIα, synaptic scaffolds and neurodevelopmental disorders
CaMKIIα is one of the most strongly implicated brain protein kinase in cognition, learning and memory in animals (Hell, 2014). To our knowledge, our findings are the first to implicate hyperactivity of CaMKIIα in a mouse model of human cognitive disease and we also identify a relevant CaMKIIα substrate, Homer. The mouse model of Angelman Syndrome (AS) (Ube3A maternal deletion) expresses reduced CaMKIIα activity stemming from enhanced phosphorylation of an inhibitory site (T305) on CaMKIIα that contributes to the behavioral phenotypes in the AS mice (van Woerden et al., 2007). However, the relevant CaMKIIα substrate in AS is unknown. Interestingly, AS mouse model neurons have increased mGluR5-Homer interactions and altered mGluR5 synaptic function (Pignatelli et al., 2014) suggesting that an imbalance of CaMKII levels or activity can alter Homer scaffolds and lead to mGluR5 dysfunction and autism-relevant phenotypes. Consistent with this notion, a de novo missense mutation in CaMKIIα was recently identified in whole exome sequencing data from autism families (Iossifov et al., 2014). Autism-associated mutations are also found in Homer, Shank1,2,3, and other proteins in the Homer-Shank synaptic scaffold, such as SAPAPs, and Neuroligins (De Rubeis et al., 2014; Delorme et al., 2013; Kelleher et al., 2012). Interestingly, loss of function mutations in SHANK, SAPAP3 and Neuroligin 3 result in abnormal mGluR1 or mGluR5 function (D'Antoni et al., 2014) suggesting that destabilization and/or abnormal activity-dependent remodeling of mGluR1/5-Homer scaffolds may be a common synaptic etiology among distinct genetic causes of ASD.
Experimental Procedures
Animals
Congenic Fmr1 KO mice were bred on the C57/BL6J background from the UT Southwestern mouse breeding core facility. See Supplemental Experimental Procedures.
Immunoprecipitation and Western Blotting
For immunoprecipitation, the brain tissues or dissociated neocortical or hippocampal cultured neurons were lysed with Co-IP buffer (50mMTris, pH 7.4, 120mMNaCl, 50 mM NaF, and 1% Triton X-100). See Supplemental Experimental Procedures.
BRET measurements
Single cell BRET imaging in cultured hippocampal neurons were performed according to previous protocols (Moutin et al., 2012). See Supplemental Experimental Procedures.
Multielectrode Array Recordings of neuronal cultures
Multielectrode Array Recordings of neuronal cultures were performed and analyzed as described (Bateup et al., 2013). See Supplemental Experimental Procedures.
Statistics
Data plotted in the figures represent the mean ± S.E.M. All Statistical tests were performed with GraphPad Prism6. See the Supplemental Experimental Procedures.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by the grants from the National Institutes of Health R01-NS045711 (KMH), Training Grant 1T32NS069562 (KC); FRAXA Research Foundation (WG); and Agence Nationale de la Recherche [ANR-13-JSV4-0005-01, SYNcity], Région Languedoc-Roussillon (Chercheur d’Avenir) and European Research Community (ERC consolidator, VERTICALcity) (JP); We would like to thank Dr. Gemma Molinaro, Nicole Cabalo and Maria Diosdado for technical assistance, Drs. Joseph Albanesi, Neal Waxham, Paul Worley and members of the Huber lab for technical advice and helpful discussions. We thank Dr. James Bibb for use of the MED64 recording system.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ango F, Prezeau L, Muller T, Tu JC, Xiao B, Worley PF, Pin JP, Bockaert J, Fagni L. Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein Homer. Nature. 2001;411:962–965. doi: 10.1038/35082096. [DOI] [PubMed] [Google Scholar]
- Bateup HS, Johnson CA, Denefrio CL, Saulnier JL, Kornacker K, Sabatini BL. Excitatory/inhibitory synaptic imbalance leads to hippocampal hyperexcitability in mouse models of tuberous sclerosis. Neuron. 2013;78:510–522. doi: 10.1016/j.neuron.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayes A, van de Lagemaat LN, Collins MO, Croning MD, Whittle IR, Choudhary JS, Grant SG. Characterization of the proteome, diseases and evolution of the human postsynaptic density. Nat Neurosci. 2011;14:19–21. doi: 10.1038/nn.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Antoni S, Spatuzza M, Bonaccorso CM, Musumeci SA, Ciranna L, Nicoletti F, Huber KM, Catania MV. Dysregulation of group-I metabotropic glutamate (mGlu) receptor mediated signalling in disorders associated with Intellectual Disability and Autism. Neuroscience and biobehavioral reviews. 2014 doi: 10.1016/j.neubiorev.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, Klann E. The translation of translational control by FMRP: therapeutic targets for FXS. Nat Neurosci. 2013;16:1530–1536. doi: 10.1038/nn.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW, et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146:247–261. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rubeis S, He X, Goldberg AP, Poultney CS, Samocha K, Cicek AE, Kou Y, Liu L, Fromer M, Walker S, et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 2014;515:209–215. doi: 10.1038/nature13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme R, Ey E, Toro R, Leboyer M, Gillberg C, Bourgeron T. Progress toward treatments for synaptic defects in autism. Nature medicine. 2013;19:685–694. doi: 10.1038/nm.3193. [DOI] [PubMed] [Google Scholar]
- Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffrida R, Musumeci S, D'Antoni S, Bonaccorso CM, Giuffrida-Stella AM, Oostra BA, Catania MV. A reduced number of metabotropic glutamate subtype 5 receptors are associated with constitutive homer proteins in a mouse model of fragile X syndrome. J Neurosci. 2005;25:8908–8916. doi: 10.1523/JNEUROSCI.0932-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Nakamoto M, Yao X, Chan CB, Yim SY, Ye K, Warren ST, Bassell GJ. Excess phosphoinositide 3-kinase subunit synthesis and activity as a novel therapeutic target in fragile X syndrome. J Neurosci. 2010;30:10624–10638. doi: 10.1523/JNEUROSCI.0402-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi MK, Ames HM, Hayashi Y. Tetrameric hub structure of postsynaptic scaffolding protein homer. J Neurosci. 2006;26:8492–8501. doi: 10.1523/JNEUROSCI.2731-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi MK, Tang C, Verpelli C, Narayanan R, Stearns MH, Xu RM, Li H, Sala C, Hayashi Y. The postsynaptic density proteins Homer and Shank form a polymeric network structure. Cell. 2009;137:159–171. doi: 10.1016/j.cell.2009.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays SA, Huber KM, Gibson JR. Altered Neocortical Rhythmic Activity States in Fmr1 KO Mice Are Due to Enhanced mGluR5 Signaling and Involve Changes in Excitatory Circuitry. J Neurosci. 2011;31:14223–14234. doi: 10.1523/JNEUROSCI.3157-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell JW. CaMKII: claiming center stage in postsynaptic function and organization. Neuron. 2014;81:249–265. doi: 10.1016/j.neuron.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JH, Park JM, Park S, Xiao B, Dehoff MH, Kim S, Hayashi T, Schwarz MK, Huganir RL, Seeburg PH, et al. Homeostatic Scaling Requires Group I mGluR Activation Mediated by Homer1a. Neuron. 2010;68:1128–1142. doi: 10.1016/j.neuron.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GN, Huso DL, Bouyain S, Tu J, McCorkell KA, May MJ, Zhu Y, Lutz M, Collins S, Dehoff M, et al. NFAT binding and regulation of T cell activation by the cytoplasmic scaffolding Homer proteins. Science. 2008;319:476–481. doi: 10.1126/science.1151227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iossifov I, O'Roak BJ, Sanders SJ, Ronemus M, Krumm N, Levy D, Stessman HA, Witherspoon KT, Vives L, Patterson KE, et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515:216–221. doi: 10.1038/nature13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie K, Nakatsu T, Mitsuoka K, Miyazawa A, Sobue K, Hiroaki Y, Doi T, Fujiyoshi Y, Kato H. Crystal structure of the Homer 1 family conserved region reveals the interaction between the EVH1 domain and own proline-rich motif. J Mol Biol. 2002;318:1117–1126. doi: 10.1016/S0022-2836(02)00170-5. [DOI] [PubMed] [Google Scholar]
- Jin DZ, Guo ML, Xue B, Mao LM, Wang JQ. Differential regulation of CaMKIIαlpha interactions with mGluR5 and NMDA receptors by Ca(2+) in neurons. J Neurochem. 2013;127:620–631. doi: 10.1111/jnc.12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KM, Sepers M, Henstridge CM, Lassalle O, Neuhofer D, Martin H, Ginger M, Frick A, DiPatrizio NV, Mackie K, et al. Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nature communications. 2012;3:1080. doi: 10.1038/ncomms2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Geigenmuller U, Hovhannisyan H, Trautman E, Pinard R, Rathmell B, Carpenter R, Margulies D. High-throughput sequencing of mGluR signaling pathway genes reveals enrichment of rare variants in autism. PloS one. 2012;7:e35003. doi: 10.1371/journal.pone.0035003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd SA, Lachiewicz A, Barbouth D, Blitz RK, Delahunty C, McBrien D, Visootsak J, Berry-Kravis E. Fragile X syndrome: a review of associated medical problems. Pediatrics. 2014;134:995–1005. doi: 10.1542/peds.2013-4301. [DOI] [PubMed] [Google Scholar]
- Kinoshita E, Kinoshita-Kikuta E, Takiyama K, Koike T. Phosphate-binding tag, a new tool to visualize phosphorylated proteins. Molecular & cellular proteomics : MCP. 2006;5:749–757. doi: 10.1074/mcp.T500024-MCP200. [DOI] [PubMed] [Google Scholar]
- Mao LM, Liu XY, Zhang GC, Chu XP, Fibuch EE, Wang LS, Liu Z, Wang JQ. Phosphorylation of group I metabotropic glutamate receptors (mGluR1/5) in vitro and in vivo. Neuropharmacology. 2008;55:403–408. doi: 10.1016/j.neuropharm.2008.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalon A, Sidorov M, Ballard TM, Ozmen L, Spooren W, Wettstein JG, Jaeschke G, Bear MF, Lindemann L. Chronic pharmacological mGlu5 inhibition corrects fragile X in adult mice. Neuron. 2012;74:49–56. doi: 10.1016/j.neuron.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani A, Kuroda Y, Futatsugi A, Furuichi T, Mikoshiba K. Phosphorylation of Homer3 by calcium/calmodulin-dependent kinase II regulates a coupling state of its target molecules in Purkinje cells. J Neurosci. 2008;28:5369–5382. doi: 10.1523/JNEUROSCI.4738-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutin E, Raynaud F, Roger J, Pellegrino E, Homburger V, Bertaso F, Ollendorff V, Bockaert J, Fagni L, Perroy J. Dynamic remodeling of scaffold interactions in dendritic spines controls synaptic excitability. The Journal of cell biology. 2012;198:251–263. doi: 10.1083/jcb.201110101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe S, Urushido T, Konno D, Okado H, Sobue K. Rapid redistribution of the postsynaptic density protein PSD-Zip45 (Homer 1c) and its differential regulation by NMDA receptors and calcium channels. J Neurosci. 2001;21:9561–9571. doi: 10.1523/JNEUROSCI.21-24-09561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterweil EK, Chuang SC, Chubykin AA, Sidorov M, Bianchi R, Wong RK, Bear MF. Lovastatin corrects excess protein synthesis and prevents epileptogenesis in a mouse model of fragile X syndrome. Neuron. 2013;77:243–250. doi: 10.1016/j.neuron.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterweil EK, Krueger DD, Reinhold K, Bear MF. Hypersensitivity to mGluR5 and ERK1/2 Leads to Excessive Protein Synthesis in the Hippocampus of a Mouse Model of Fragile X Syndrome. J Neurosci. 2010;30:15616–15627. doi: 10.1523/JNEUROSCI.3888-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatelli M, Piccinin S, Molinaro G, Di Menna L, Riozzi B, Cannella M, Motolese M, Vetere G, Catania MV, Battaglia G, et al. Changes in mGlu5 receptor-dependent synaptic plasticity and coupling to homer proteins in the hippocampus of Ube3A hemizygous mice modeling angelman syndrome. J Neurosci. 2014;34:4558–4566. doi: 10.1523/JNEUROSCI.1846-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin M, Schmidt KC, Zametkin AJ, Bishu S, Horowitz LM, Burlin TV, Xia Z, Huang T, Quezado ZM, Smith CB. Altered cerebral protein synthesis in fragile X syndrome: studies in human subjects and knockout mice. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013;33:499–507. doi: 10.1038/jcbfm.2012.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronesi JA, Collins KA, Hays SA, Tsai NP, Guo W, Birnbaum SG, Hu JH, Worley PF, Gibson JR, Huber KM. Disrupted Homer scaffolds mediate abnormal mGluR5 function in a mouse model of fragile X syndrome. Nat Neurosci. 2012;15:431–440. doi: 10.1038/nn.3033. S431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronesi JA, Huber KM. Homer interactions are necessary for metabotropic glutamate receptor-induced long-term depression and translational activation. J Neurosci. 2008;28:543–547. doi: 10.1523/JNEUROSCI.5019-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotschafer SE, Razak KA. Auditory processing in fragile x syndrome. Frontiers in cellular neuroscience. 2014;8:19. doi: 10.3389/fncel.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi-Yamaguchi Y, Furuichi T. The Homer family proteins. Genome Biol. 2007;8:206. doi: 10.1186/gb-2007-8-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi-Yamaguchi Y, Sato Y, Sakai R, Mizutani A, Knopfel T, Mori N, Mikoshiba K, Furuichi T. Interaction of Cupidin/Homer2 with two actin cytoskeletal regulators, Cdc42 small GTPase and Drebrin, in dendritic spines. BMC neuroscience. 2009;10:25. doi: 10.1186/1471-2202-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonesy BC, Jalan-Sakrikar N, Cavener VS, Colbran RJ. CaMKII: a molecular substrate for synaptic plasticity and memory. Prog Mol Biol Transl Sci. 2014;122:61–87. doi: 10.1016/B978-0-12-420170-5.00003-9. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Stevens CF, Tonegawa S, Wang Y. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992;257:201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- Steward O, Bakker CE, Willems PJ, Oostra BA. No evidence for disruption of normal patterns of mRNA localization in dendrites or dendritic transport of recently synthesized mRNA in FMR1 knockout mice, a model for human fragile-X mental retardation syndrome. Neuroreport. 1998;9:477–481. doi: 10.1097/00001756-199802160-00022. [DOI] [PubMed] [Google Scholar]
- Tang AH, Alger BE. Homer protein-metabotropic glutamate receptor binding regulates endocannabinoid signaling and affects hyperexcitability in a mouse model of fragile x syndrome. J Neurosci. 2015;35:3938–3945. doi: 10.1523/JNEUROSCI.4499-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting JT, Peca J, Feng G. Functional consequences of mutations in postsynaptic scaffolding proteins and relevance to psychiatric disorders. Annual review of neuroscience. 2012;35:49–71. doi: 10.1146/annurev-neuro-062111-150442. [DOI] [PubMed] [Google Scholar]
- van Woerden GM, Harris KD, Hojjati MR, Gustin RM, Qiu S, de Avila Freire R, Jiang YH, Elgersma Y, Weeber EJ. Rescue of neurological deficits in a mouse model for Angelman syndrome by reduction of alphaCaMKII inhibitory phosphorylation. Nat Neurosci. 2007;10:280–282. doi: 10.1038/nn1845. [DOI] [PubMed] [Google Scholar]
- Xiao B, Tu JC, Petralia RS, Yuan JP, Doan A, Breder CD, Ruggiero A, Lanahan AA, Wenthold RJ, Worley PF. Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron. 1998;21:707–716. doi: 10.1016/s0896-6273(00)80588-7. [DOI] [PubMed] [Google Scholar]
- Zalfa F, Giorgi M, Primerano B, Moro A, Di Penta A, Reis S, Oostra B, Bagni C. The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell. 2003;112:317–327. doi: 10.1016/s0092-8674(03)00079-5. [DOI] [PubMed] [Google Scholar]
- Zhang L, Alger BE. Enhanced endocannabinoid signaling elevates neuronal excitability in fragile X syndrome. J Neurosci. 2010;30:5724–5729. doi: 10.1523/JNEUROSCI.0795-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bonnan A, Bony G, Ferezou I, Pietropaolo S, Ginger M, Sans N, Rossier J, Oostra B, LeMasson G, et al. Dendritic channelopathies contribute to neocortical and sensory hyperexcitability in Fmr1(−/y) mice. Nat Neurosci. 2014;17:1701–1709. doi: 10.1038/nn.3864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.