Abstract
Post-traumatic migraine (PTM) (i.e., headache, nausea, light and/or noise sensitivity) is an emerging risk factor for prolonged recovery following concussion. Concussions and migraine share similar pathophysiology characterized by specific ionic imbalances in the brain. Given these similarities, patients with PTM following concussion may exhibit distinct electrophysiological patterns, although researchers have yet to examine the electrophysiological brain activation in patients with PTM following concussion. A novel approach that may help differentiate brain activation in patients with and without PTM is brain network activation (BNA) analysis. BNA involves an algorithmic analysis applied to multichannel EEG-ERP data that provides a network map of cortical activity and quantitative data during specific tasks. A prospective, repeated measures design was used to evaluate BNA (during Go/NoGo task), EEG-ERP, cognitive performance, and concussion related symptoms at 1, 2, 3, and 4-week post-injury intervals among athletes with a medically diagnosed concussion with PTM (n = 15) and without (NO-PTM) (n = 22); and age, sex, and concussion history matched controls without concussion (CONTROL) (n = 20). Participants with PTM had significantly reduced BNA compared to NO-PTM and CONTROLS for Go and NoGo components at 3 weeks and for NoGo component at 4 weeks post-injury. The PTM group also demonstrated a more prominent deviation of network activity compared to the other two groups over a longer period of time. The composite BNA algorithm may be a more sensitive measure of electrophysiological change in the brain that can augment established cognitive assessment tools for detecting impairment in individuals with PTM.
Keywords: sport-related concussion, brain network activation, electroencephalography, post-traumatic migraine
Introduction
We have only recently begun to understand the myriad risk and prognostic factors associated with adverse concussion outcomes for the 2–4 million concussions that occur each year in the US (Langlois et al. 2006). Among the risk factors that are associated with poor outcomes following concussion are age(Field et al. 2003), sex (Covassin et al. 2012), and concussion history (Guskiewicz et al. 2007). Prognostic indicators of poor outcome following concussion include post-traumatic amnesia (PTA) (Collins et al. 2003) and dizziness at the time of injury (Lau et al. 2011), as well as fogginess and slower reaction time in the first week following injury (Lau et al. 2009). Another factor that is emerging predictor for poor outcomes following concussion is post-traumatic migraine (PTM) symptoms.
Post-traumatic migraine symptoms include headache, nausea, and phono- and/or photo-sensitivity (Headache Classification Subcommittee of the International Headache 2004). The presence of PTM symptoms has been linked to poor outcomes following concussion. Kontos and colleagues (2013) reported that patients with PTM in the first week following injury were nearly 7x more likely to have a protracted recovery (i.e., > 20 days) following concussion than those without PTM. Patients with PTM were nearly 3x more likely to have protracted recovery than those with headache only (without the other PTM symptoms). Further, these researchers indicated that patients with PTM following concussion, experience more pronounced neurocognitive deficits in the first week following injury than those without PTM. Mihalk and colleagues (2005) reported similar findings indicating that athletes with PTM symptoms experience greater neurocognitive impairments compared to athletes with only posttraumatic headache or no headache following a concussion. More recently, researchers (Mihalik et al. 2013) reported that concussed patients with PTM reported elevated symptoms compared to patients with only headache only and no headache, despite demonstrating recovery within three days on balance measures (i.e., Balance Error Scoring System [BESS]) and cognitive assessment (i.e., Standardized Assessment of Concussion [SAC]) measures. Although there is growing evidence supporting PTM as a prognostic factor for prolonged symptoms, impairment, and recovery time, the underlying mechanisms for these effects are not well understood.
Concussions and migraine headaches share similar pathophysiology characterized by specific ionic imbalances in the brain, such as marked increases in extracellular potassium and intracellular calcium along with an excessive release of excitatory amino acids (Giza and Hovda 2001, 2014). These pathophysiological events result in a spreading cortical depression that culminates in the hallmark symptoms of pain, headache, nausea and photophobia. Further support of the similar pathophysiology of PTM and migraine headaches is evidenced by the effectiveness of triptan medications in managing both PTM and atraumatic migraines (Erickson 2011; Lucas 2011). The electro-physiological signature produced by the ionic imbalances can be measured through the use of electroencephalography (EEG). Given the similarities in the underlying pathophysiological mechanisms of migraine and concussion, patients with PTM following concussion may exhibit distinct electrophysiological patterns that reflect brain activation from patients without PTM. However, to date, researchers have yet to examine the electrophysiological characteristics in brain activation in patients with PTM following concussion.
A new approach that may help differentiate brain activation in patients with PTM from those without and corroborate previously reported adverse effects in patients with PTM following concussion is brain network activation (BNA) analysis (Reches et al. 2014; Shahaf et al. 2012; Reches et al. 2013). BNA is a novel algorithmic analysis method applied to multichannel EEG-ERP data that provides a network map of cortical activity and concomitant quantitative data during the performance of specific tasks. Evoked response potentials (ERP), which are a sub-component of EEG data, represent the patterns of neuroelectric activation that occur in preparation for or in response to a visual or auditory stimulus. Rather than the conventional method of ERP analysis of evaluating the individual in terms of discrete ERP values (e.g., latency, amplitude, frequency), the BNA algorithm identifies functional network dynamics and quantifies an individual’s network activity to that of a healthy normative group. Specifically, the BNA algorithm captures the dynamic integration of event-pairs of specific temporal relations, spatial locations and frequencies into a unified functional network.
BNA technology is based on a network model to depict functional connectivity. The descriptive network model approach which provides insights regarding inter-connectivity of brain networks and describes the temporal-spatial brain activation patterns has been applied to various types of data-sets including EEG, MEG and fMRI (Sporns 2014). Anatomical, physiological and clinical evidence suggests that functional networks spanning spatial and temporal scales form a critical feature of information processing in the brain (Bullmore and Sporns 2009). More specifically, the coordination of widely distributed and parallel brain activity, which is correlated with sensory processing, has been suggested to increase as a function of the synchronization of network potential oscillations (Buzsaki and Draguhn 2004; Engel et al. 2001). This synchronization has been suggested to govern the effective strength of connections between regions in the brain, providing a flexible mechanism for the generation of diverse functional networks (Fries 2005). Synchronization in brain activity may be disrupted following concussion (Slobounov et al. 2011), and may correspond to functional impairments (e.g., memory, processing speed). As such, BNA analysis may provide additional data that complement current functional assessments, inform clinical assessment and management of this injury, and identify subgroups of patients (e.g., PTM) that might be at risk for poor outcomes.
Although PTM following concussion has gained attention from researchers, (Mihalik et al. 2013; Kontos et al. 2013) little is known about the underlying changes in brain activation that might accompany PTM. The BNA analysis may provide a method to measure electrophysiological changes in brain activation that accompany PTM following concussion. Therefore, a study examining changes in brain activation in conjunction with behavioral assessments in patients with PTM following concussion is warranted. The primary purpose of the current study was to compare the brain activation- using BNA- of patients with PTM following concussion to those without PTM and healthy controls. We hypothesized that patients with PTM would exhibit greater and prolonged network impairment as demonstrated by BNA when compared to those without PTM following a concussion, and controls. In the literature, there are mixed results regarding the effects of non-traumatic migraine on the N1 wave that might be exhibited as either reduced (Buodo et al. 2004) or enhanced amplitudes (Morlet et al. 2014) relative to healthy controls. These changes in the N1 component may be related to automated sensory processing in the early stages of perception and to the discrimination of stimulus properties (Dockree and Robertson 2011) as well as to the orienting of attention (Linden et al. 1987) and inhibitory processes (Coppola et al. 2013). Accordingly, we also expected that patients with PTM following concussion would demonstrate changes in the N1 component relative to healthy controls. A secondary purpose of this study was to compare neurocognitive impairment and symptoms of patients with and without PTM following concussion to healthy non-injured controls. We hypothesized that the patients with PTM following concussion would exhibit the most neurocognitive impairment and symptoms followed by patients without PTM, and healthy controls.
Materials and Methods
Design and Participants
A prospective, repeated measures design was used for this study. A total of 70 (58%) out of 120 eligible patients recruited from a concussion clinic were initially enrolled in the protocol. Thirteen (18.6%) enrolled participants were lost to follow-up (i.e., did not return to complete the measures in the study across all time periods), leaving 57 (81.4%) high school and college-aged athletes and students who participated in all aspects of the study. Participants were divided into one of three mutually exclusive groups: 1) concussed with PTM (PTM, n= 15), concussed without PTM (NO-PTM, n= 22), and healthy age-, sex-, and concussion history-matched controls (CONTROL, n= 20). Both the NO-PTM and PTM groups were diagnosed with a SRC by a medical professional (e.g., neuropsychologist, physician) trained in concussion, and met each of the following criteria: 1) a clear mechanism of injury (direct or indirect), 2) presence of at least one sign (e.g., loss of consciousness, amnesia) or symptom (e.g., dizziness, headache) at time of injury, and 3) total concussion symptom score (see Post-concussion Symptoms Scale description) of ≥10 at first test session. The PTM group also met the following symptom-based inclusion criteria at the first time period (1 week post-injury): headache, nausea, and photo- and/or phono-sensitivity. All participants were required to be between 14–29 years of age and have normal hearing function and normal or corrected-to-normal vision to perform the tasks in the protocol. Exclusion criteria for all participants included any one of the following: history of special education; history of neurological or psychiatric disorders; previous traumatic brain injury (TBI: Glasgow Coma Scale <13); previous brain surgery; or current use of CNS-affecting medications. In addition, CONTROL participants were excluded if they had a concussion in the previous six months or if they had a history of more than two concussions. A summary of participants’ age, sex, concussion history, and migraine history data are presented for each group in Table 1. The CONTROL group (18.35, 2.17 yrs) was significantly older than the PTM (16.45, 1.52 yrs) and NO-PTM (16.50, 2.27 yrs) groups. The groups did not differ on any other variables.
Table 1.
Statistical Summary of Age, Previous Concussions, Sex, and Migraine History among PTM, NO-PTM, and CONTROL Groups.
| PTM (n= 15) | NO-PTM (n= 22) | CONTROL (n= 20) | F | p | ||
|---|---|---|---|---|---|---|
| Age | ||||||
| M | 16.45 | 16.50 | 18.35* | 5.35 | .008 | |
| SD | 1.52 | 2.27 | 2.17 | |||
| Previous Concussions | ||||||
| M | 0.53 | 0.45 | 0.15 | 1.90 | .16 | |
| SD | 0.52 | 0.80 | 0.50 | |||
|
| ||||||
| χ2 | p | |||||
|
| ||||||
| Sex = Female | ||||||
| #, % | 7, 47% | 10, 45% | 6, 30% | 1.38 | .50 | |
| Migraine History | ||||||
| #, % | 1, 7% | 4, 18% | 1, 5% | 2.26 | .32 | |
Higher than PTM and CONCUSSED.
Procedures
EEG Data Acquisition
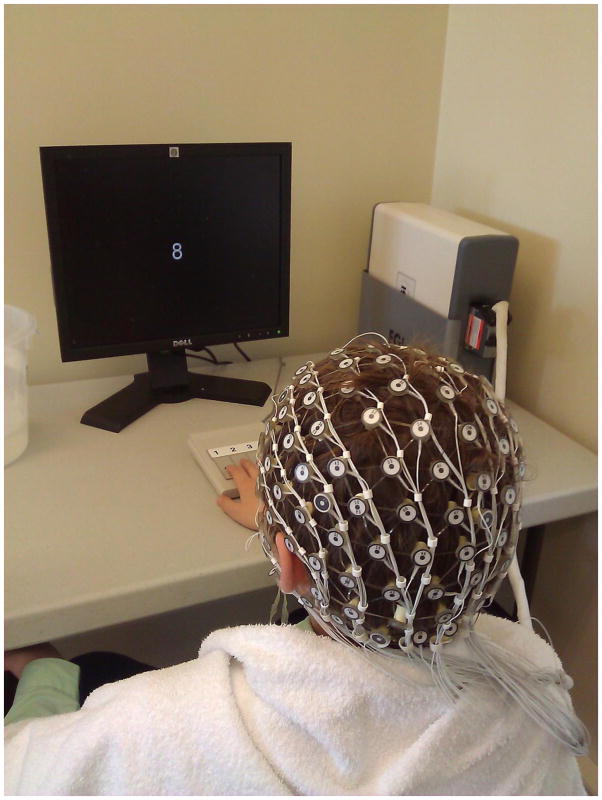
Participants sat comfortably in a quiet room with an ambient temperature of ~23°C. In each test session, participants were asked to perform a cognitive task (see below). Participants were instructed to avoid eye movements, blinking and body movements as much as possible, and to keep their gaze on the center of the screen during task performance. Nuisance components of horizontal eye movements and blinks were removed as described in Reches et al. (2013) Standard EEG recording was performed using an FDA-cleared system manufactured by Electrical Geodesics, Inc (EGI) from 128 locations by using active Ag-AgCl electrodes mounted in an elastic cap, referenced to an additional active electrode (Common Mode Sense) (see Figure 1). Channels were sampled at 256 Hz and connected to insulated Active-Two amplifiers activated by battery. Each test session required approximately 45 min including EEG set-up time.
Figure 1.
Subject performing the cognitive task while wearing the EEG net.
BNA Cognitive Task
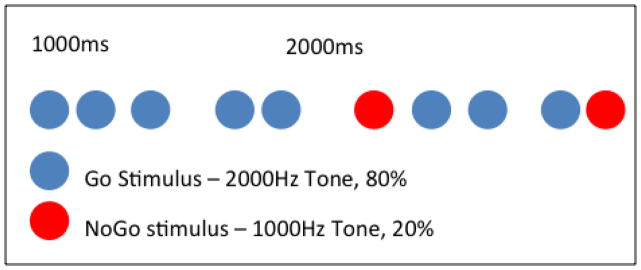
High density EEG data were collected using the EGI system described above during the performance of an Auditory Go/NoGo task. This task is used to evaluate cognitive function including response inhibition, executive function, and sustained attention (Simmonds et al. 2008). The Auditory Go/NoGo task involves measuring reaction time to an auditory cue. Participants are asked to press a response button when they hear a high beep and inhibit their response when they hear a low beep. The Go component represented 80% of the stimuli presented and required a motor response (i.e., pressing a button) (see Figure 2). The NoGo component represented 20% of stimuli presented and required an inhibitory response (i.e., no response).
Figure 2.
Schematic description of the Go/NoGo task. Go component appeared in 80% of the trials and consisted of a 2000 Hz tone. NoGo component appeared in 20% of the trials and consisted of a 1000 Hz tone. The Inter Stimulus Interval (ISI) varied randomly between 1000 ms and 2000 ms. The task included 3 blocks of 200 trials during which participants were instructed to respond to Go stimuli only.
Computerized Neurocognitive Performance
The Immediate Post-concussion Assessment and Cognitive Test (ImPACT) was used to assess neurocognitive performance. The ImPACT test is a computerized neurocognitive test comprised of three sections that include demographic/health history questionnaire, the 22-item Post-concussion Symptom Scale (PCSS- see below), and six neurocognitive test modules covering memory, attention, learning, processing speed, and reaction time. The six modules comprise four composite scores for verbal and visual memory, visual motor processing speed, and reaction time. Reliability data on the ImPACT composite scores range from .67 to .85 over a 7-day period (Iverson et al. 2003) and .62 – .85 over one year (Elbin et al. 2011).
Post-concussion Symptoms
The Post-concussion Symptom Scale (PCSS) was used to assess concussion symptoms. The PCSS assesses 22 concussion symptoms (e.g., dizziness, headache, memory) that are rated on a Likert scale of severity ranging from 0 (“no symptoms”) to 6 (“severe”). The presence of PTM symptoms (i.e., headache, nausea, and photo- and/or phono-sensitivity) was determined based on responses to the PCSS.
Human Participants and Study Procedures
The study protocol was approved by the IRB committee at the University of Pittsburgh. The researchers informed participants about the study, screened participants for inclusion/exclusion criteria, and obtained written informed consent (adult/parent) and assent (child) from each eligible participant. Participants then completed the BNA tasks as described above followed by the ImPACT and PCSS. All tests were completed under the direction of the research team in a private research laboratory testing room. Total test time was approximately 1.5–2 hours per test session. Participants completed a total of four post-injury test sessions per above at 1-week +/− 3 days time intervals.
Data Analysis
Processing of EEG Data for BNA Analysis
The EEG data recorded at each electrode location was processed with the BNA algorithm. The first step of this process involves clustering the basic time-frequency building blocks of the ERPs (i.e., waveforms) at specific location, amplitude, frequency, and timing (i.e., latency). Next, the BNA algorithm is used to locate the spatial-temporal relations between the clusters. A detailed description of this methodology is described elsewhere. (Shahaf et al. 2012) The BNA algorithm was then used to map the spatio-temporal activity patterns common to a normative database during the performance of the Go/NoGo task generating a reference BNA network (see Results). The normative database group consisted of 89 healthy participants (43 females, mean age = 19.20 [SD = 2.79]).
All participants’ EEG data were processed with the BNA algorithm, comparing them to the reference BNA network, which generates similarity scores in relation to the reference network. The subsequent BNA scores are measures of similarity with 100% being perfectly identical scores to the reference network. Specifically, we examined similarity on two aspects of the Go/NoGo tasks: 1) for the Go component we examined the absolute time network, which is the latency of an event locked to the stimulus onset; and 2) for the NoGo component we examined the relative time network, which is the time difference between the latencies of the events comprising the event-pair. These two networks were selected because they were identified by the algorithm as among the more characteristic networks for differentiating between concussed (PTM, NO-PTM) and CONTROLS in a different sample (see Kontos et al., in review).
Statistical Analysis
A series of repeated measures ANOVAs with Bonferroni correction for multiple comparisons and covaried for age were conducted to compare the BNA similarity scores of the PTM, NO-PTM, and CONTROL groups across the four time points. These analyses were conducted for both the absolute time network of the Go component and for the relative time network of the NoGo component of the BNA paradigm. Statistical analysis of ERPs was performed on the N1 peak as previously tested in migraneurs (Wang and Schoenen 1998; Buodo et al. 2004; Chen et al. 2007). In each one of 9 spatial locations (as defined below), the latency and amplitude of the N1 peak was entered into a mixed model analysis of variance (ANOVA), with visit (1/2/3/4) and group (PTM/NO-PTM/CONTROL) as fixed effects and Subject (nested within group) as random effect. Planned contrasts tested the difference between PTM and Control and between Non-PTM and Control in each of the visits. The Bonferroni correction was applied in order to control for multiple comparisons. The amplitude and latency values of the N1 component were calculated for the time interval of 80–200ms, and the array of recording channels was the same as in Fisher et al. (2011) The 9 spatial locations were as follows: left frontal; medial frontal; right frontal; left central; medial central; right central; left occipital parietal; medial occipital parietal; right occipital parietal. A series of repeated measures ANCOVAs (covaried for age) with Bonferroni correction for multiple comparisons were conducted to compare the neurocognitive impairment and symptom scores of the PTM, NO-PTM, and CONTROL groups across the four time points.
Results
Comparison of BNA Similarity Scores among PTM, NO-PTM, and Controls
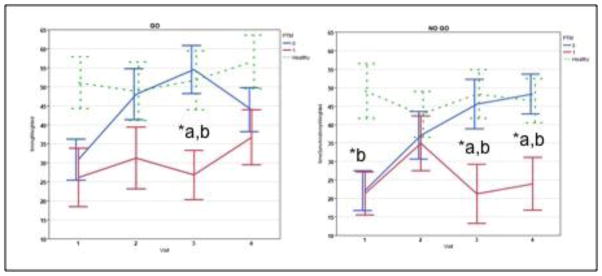
The BNA similarity scores, computed against the normative reference group, were first compared between the PTM, NO-PTM and CONTROL groups, across the four visits. The results of the ANCOVA (covaried for age) with Bonferonni correction for multiple comparisons revealed significant differences among the groups in the NoGo (Wilk’s λ= .61, F= 3.69, p=.003, η2= .22) network BNA similarity scores. Specifically, the PTM (3 weeks- M= 22.91, SD= 31.58; 4 weeks- M= 24.09, SD= 26.58) group’s NoGo network BNA similarity scores were significantly lower than those of both the NO-PTM (3 weeks- M= 44.68, SD= 29.92; 4 weeks- M= 50.49, SD= 20.92) and CONTROL (3 weeks- M= 48.07, SD= 29.34; 4 weeks- M= 44.07, SD= 25.18) group at 3 and 4 weeks post-injury (see Figure 3). Both the PTM (M= 21.47, SD= 21.71) and NO-PTM (M= 24.10, SD= 23.64) groups’ NoGo network BNA similarity scores were significantly lower than the CONTROL (M= 54.42, SD= 30.31) group at 1 week post-injury. Although the overall ANCOVA model for the Go (Wilk’s λ= .85, F= 1.07, p=<.001, η2= .08) network was not significant, subsequent post-hoc ANCOVAs for each time period revealed that the PTM (M= 21.38, SD= 31.00) group’s Go network BNA similarity scores were significantly lower than those of both the NO-PTM (M= 45.66, SD= 30.91) and CONTROL (M= 48.40, SD= 27.80) group at 3 weeks post-injury (see Figure 3).
Figure 3.
A comparison of mean GO and NO GO BNA similarity scores among PTM (red), NO-PTM (blue) and CONTROLS (dashed green) across 4 visits (1, 2, 3, and 4 week intervals post-injury).
*p <.05; a= worse than No-PTM, b= worse than control
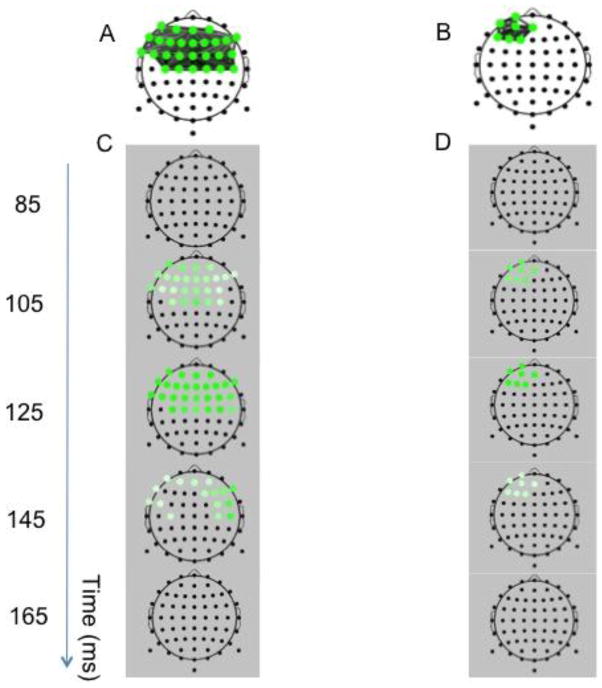
Spatial/Temporal Comparisons of BNA Reference Networks
To further investigate the observed differences among the three groups: PTM, NO-PTM and CONTROLS, a qualitative analysis of the spatiotemporal distribution of the reference networks was performed. Figures 4A and 4B display the reference networks for the Go and NoGo conditions, respectively. In the Go condition theta activity was bilaterally distributed over a relatively large area of the fronto-central scalp region, whereas in the NoGo condition theta activity was localized in a relatively small cluster of activation over mid-left anterior frontal region. Figure 4C and 4D display discrete time-frame activations depicting the temporal changes in network activation for the Go (Fig. 4C) and NoGo (Fig. 4D) conditions. Common fronto-central theta activation in response to the Go stimulus began as early as ~100 ms, peaked at ~125 ms and then waned from ~145 ms onward (Figure 4C). The group common theta activation in response to the NoGo stimulus (Figure 4B) observed over left anterior frontal regions was strongest in the ~100–125 ms interval and then began to wane from 165 ms onward (Figure 4D). This spatiotemporal pattern of results suggested that the prominent theta activation is related to the well-known N1 component in accordance with the known relationship between theta activity and the N1 wave (Kawamata et al. 2007).
Figure 4.
Reference BNA Networks for Go (A) and NoGo (B) stimuli. Discrete time-frame activations depicting the evolution in time (in ms) of network activation (grey background) are presented below each group network pattern (white background), respectively (Figure C and D). A colored circle at a node will receive its full hue when the timeframe coincides with the group-mean latency of the activity peak and lighter shades when away from it, the spectrum width being dependent on the spread of individual latencies.
BNA Reference Networks and the N1 Component
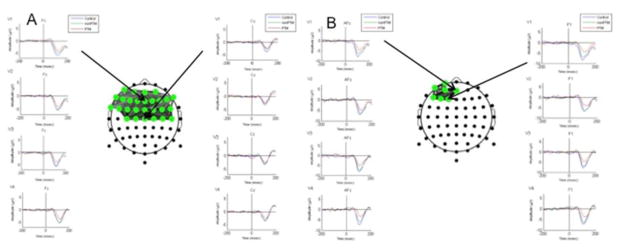
Since the differentiating activation in both the Go and NoGo networks occurred around the N1 component, the relationship between the N1 component and the BNA reference networks in both the Go and NoGo conditions was observed. To facilitate the visual inspection of these differences between groups, the reference brain networks (Figure 5A and 5B) are presented along with the grand-averaged ERP waveforms of representative electrodes, zoomed in on the time range of the N1 component in each of the four time periods, across the three groups (CONTROL, PTM, and NO-PTM).
Figure 5.
The “Go” (A) and “NoGo” (B) reference networks. The ERP waveforms grand-averaged across subjects within the N1 component range (−200 to +200 ms post-stimulus onset) are arranged in vertical columns at the sides of each network. For the Go condition the N1 component is displayed at Fz (left column) and Cz (right column). For the NoGo condition the N1 component is displayed at AFz (left column) and F1 (right column). Each vertical column of ERP waveforms displays the N1 component across the four test sessions (visits V1 through V4), respectively. Three distinct waveforms are displayed per visit for healthy controls (blue), NO-PTM (green) and PTM (red), respectively.
In the Go condition (Figure 5A) of the first post-concussive visit (top row), the N1 amplitudes of both concussed groups (PTM - red line, and NO-PTM – green line) were decreased compared to that of the CONTROL group. However, in the following post-concussive visits (rows 2–4), the N1 amplitude of the NO-PTM group gradually converged with that of the CONTROL group, whereas the N1 amplitude of the NO-PTM did not. To a lesser extent, a similar phenomenon was also observed in the latency of the peak of the N1 component. A comparable pattern of results was demonstrated in the No-Go condition (Figure 5B).
The results of the series of ANOVAs of the ERP N1 component (see Methods) revealed statistically significant results in the latency of the first visit only. More specifically, the Medial-Frontal location showed longer ERP latencies for the PTM group, compared to the Control group. This was evident both in the Go (CONTROL: M=129.80, SE=5.64; PTM: M=158.34, SE=6.70) and in the NoGo (CONTROL: M=113.20, SE=5.34; PTM: M=140.10, SE=6.36) conditions and was statistically significant after controlling for multiple comparisons (Go: F(1,133.9)=10.62, p=0.0014; NoGo: F(1,159.6)=10.49, p=0.0015).
3.4 Temporal Comparison of Neurocognitive Impairment and Symptoms among PTM, NO-PTM, and Controls
The results of a series of 3 (group- PTM, NO-PTM, CONTROL) x 4 (time) repeated measures ANCOVAs (covaried for age) with Bonferonni correction for multiple comparisons revealed several significant group x time interactions. Specifically, there were significant interactions for symptoms (Wilk’s λ= .70, F= 7.86, p<.001, η2= .35), visual memory symptoms (Wilk’s λ= .66, F= 2.97, p= .01, η2= .19), visual motor processing speed (Wilk’s λ= .73, F= 2.21, p= .05, η2=.15), and reaction time (Wilk’s λ= .68, F= 2.70, p= .02, η2=.18). The results of post-hoc comparisons indicated that both PTM and NO-PTM groups performed worse than CONTROLS on visual memory, visual processing speed, reaction time and reported more symptoms across most of the time periods (see Table 2). The PTM group reported more symptoms than the NO-PTM group up to 3 weeks post-injury.
Table 2.
A Comparison of Neurocognitive Composite and Symptom Scores across Four Time Periods among Post-traumatic Migraine (PTM), No-PTM, and Controls (N=57).
| Group | 1 Week M (SD) | 2 Weeks M (SD) | 3 Weeks M (SD) | 4 Weeks M (SD) | |
|---|---|---|---|---|---|
| Verbal Memory (%) | PTM | 80.46 | 82.23 | 89.00 | 86.31 |
| No-PTM | 75.47 | 81.24 | 81.11 | 83.76 | |
| Controls | 92.87 | 95.13 | 94.13 | 93.40 | |
|
| |||||
| Visual Memory (%) | PTM | 64.69b | 63.08b | 76.31b | 73.08b |
| No-PTM | 69.76b | 68.24b | 66.83b | 70.35b | |
| Controls | 85.27 | 79.67 | 84.27 | 84.13 | |
|
| |||||
| Visual Processing Speed (#) | PTM | 34.84b | 36.89b | 39.57b | 38.89b |
| No-PTM | 35.98b | 40.09b | 41.17b | 43.02 | |
| Controls | 46.74 | 57.09 | 47.05 | 47.87 | |
|
| |||||
| Reaction Time (sec) | PTM | 0.63b | 0.62b | 0.60b | 0.62b |
| No-PTM | 0.64b | 0.64 b | 0.59 | 0.59 | |
| Controls | 0.51 | 0.53 | 0.53 | 0.53 | |
|
| |||||
| Symptoms (#)* | PTM | 48.92a,b | 24.77 a,b | 12.23 a,b | 8.69b |
| No-PTM | 25.71b | 13.18b | 9.06b | 8.82b | |
| Controls | 1.53 | 0.58 | 1.16 | 1.00 | |
p<.05;
worse than No-PTM;
worse than Controls
Discussion
The current study compared brain network patterns and behavioral performance among individuals following a SRC with PTM to those without (i.e., NO-PTM) and healthy controls. The primary finding from this study indicated that patients with PTM following a SRC had significantly reduced BNA network scores compared to both NO-PTM and CONTROL groups for the Go and NoGo component at 3 weeks and for the NoGo component at 4 weeks post-injury. The PTM group also demonstrated a more prominent deviation of network activity compared to the other two groups over a longer period of time. These findings support our hypothesis that patients with PTM would exhibit greater and prolonged network impairment compared to those without PTM following a concussion.
The BNA patterns that differentiated between the NO-PTM and PTM groups for the Go and NoGo stimuli captured the theta band activity evolving in time within the interval of the N1 component (Figures 4 and 5). Previous findings showed that the NoGo task involves theta power changes that are associated with inhibitory cognitive processes localized to midline frontal regions (Brier et al. 2010). Theta oscillations are related to the N1 component (Kawamata et al. 2007) and task difficulty/complexity has been shown to affect the peak latency of the theta power (Brier et al. 2010). Therefore, it is reasonable to assume that N1 latency differences between PTM and NO-PTM groups might be related to different processing impairments in the context of the Go/NoGo task. Visual inspection of the N1 wave of the PTM group demonstrated prolonged latencies across the four test sessions compared to the NO-PTM group (Figure 5). Although a statistically significant difference was observed in the first post-concussive visit only when performing a standard ERP analysis on the N1 peak, the BNA analysis supported a significantly prolonged network impairment of the PTM group over time, particularly in the 3- and 4-week post-injury time periods. This difference may be related to the composite nature of the BNA algorithm, which may provide a more sensitive measure of electrophysiological change in the brain following concussion.
The prolonged N1 latency in the Go condition reported in the PTM group in the current study is contrary to previous research showing shorter latency of N1 evoked by standard stimuli in migraine patients (Wang and Schoenen 1998). On the other hand, the visually observed smaller N1 amplitude in the PTM group in the current study is in line with findings reported for children with atraumatic migraine (Buodo et al. 2004), which could be related to an excessive prevalence of inhibition found in migraineurs (Coppola et al. 2011). However, this finding stands in contrast to an enhanced N1 orienting component associated with adult migraineurs (Morlet et al. 2014) which might signify enhanced activation of attention-related frontal networks (Linden et al. 1987). Thus, findings associated with the N1 component in the current study merit further investigation in order to examine more thoroughly the possible effects of age, brain injury, migraine history, and task on the attributes of the N1 wave
Nevertheless, despite the conflicting evidence mentioned above, the behavior of the N1 wave is considered to be related to early processing deficits in brain injury patients and is associated with impairments in automated sensory processing in the early stages of perception as well as with the discrimination of stimulus properties (Broglio et al. 2009; Dockree and Robertson 2011). Overall, our findings are in concordance with previously reported findings that support the utility of EEG in measuring neurophysiological changes in the brain following concussion (Baillargeon et al. 2012; Broglio et al. 2009; Gosselin et al. 2012; Lavoie et al. 2004; Theriault et al. 2009)). Moreover, our findings include support for the role of BNA and EEG findings to augment current behavioral assessments for SRC.
Previously, researchers have indicated that individuals with PTM following SRC experience prolonged recovery and more symptoms and cognitive impairment following injury than those without PTM (Kontos et al. 2013; Mihalik et al. 2013). The current findings partially support previous research in that the PTM group reported more symptoms than the NO-PTM and CONTROL groups up to 3 weeks post-injury. However, the PTM and NO-PTM groups did not differ significantly in cognitive impairment at any post-injury point, though non-significant trends for worse performance in the PTM groups were evident throughout the time points. Previous research has supported greater cognitive impairment in the PTM group at 1 week post-injury when compared to baseline data (Kontos et al., 2013). The smaller sample size in the current study together with a lack of baseline data may explain the lack of findings for cognitive impairment. The groups were also significantly different in BNA network similarity scores at 3 and 4 weeks post-injury. Together, these findings indicate that BNA may provide additional sensitivity to augment established cognitive assessment tools for detecting impairment in individuals with PTM. It is important to note that both the PTM and NO-PTM groups performed worse than CONTROLS on visual memory and visual processing speed up to 3 weeks post-injury that suggests cognitive impairments related to SRC may linger longer (3+ weeks) on average than previously thought (i.e., 7–14 days; McCrory et al.,(McCrory et al. 2013)).
The current study was limited by several factors. The control group and normative comparison group were older than the PTM and NO-PTM groups. Although we controlled for age in our group comparisons, it still may have influenced the findings. The BNA and behavioral data were collected at post-injury intervals only. As a result, intra-individual comparisons of post-injury to healthy baseline data were not possible. The time since injury to first testing session for participants varied between 4 and 10 days. Consequently, participants at time 1 could have been in the acute (i.e., <7 days) or sub-acute (i.e., 8 days-3 months) phase of concussion, which may have influenced results. The current study did not conduct long-term follow-up BNA or behavioral testing beyond 1 month to assess potential residual effects of concussion and examine recovery in participants protracted concussions. Researchers should examine the residual effects of changes in BNA beyond 1 month post-injury in light of recovery status. In so doing, the findings could help inform clinical decision making for patients with chronic (3+ months) recovery trajectories following SRC.
Conclusion
The findings of this study suggest that individuals with PTM following SRC exhibit reduced BNA network scores compared to both NO-PTM and CONTROL groups at 3 and 4 weeks post-injury. In conclusion, the current findings provide preliminary support for the use of BNA as a temporal-spatial measure of brain activity to augment current cognitive and symptom-based assessments of individuals with PTM following SRC. Further research that employs pre- to post-injury BNA assessments and additional behavioral measures such as vestibular, oculomotor, and balance are warranted. In addition, researchers should examine potential sex and age differences in both BNA and behavioral outcomes in individuals with PTM following SRC.
Acknowledgments
Funding: This study was funded by ElMindA, Ltd. through a research contract with the University of Pittsburgh.
Footnotes
Conflict of Interest: Authors Reches, Dickman, Laufer, Geva, and Shacham are employed by ElMindA, Ltd. With regard to the study data, Drs. Reches, Dickman, Laufer, Geva, and Shacham were not involved in data collection, entry, or analysis; they were only involved in blinded data transformation using the BNA algorithm and data interpretation of the BNA and EEG-ERP findings. Author Collins owns a 10% share in ImPACT Applications, Inc. With regard to the study data, Dr. Collins was not involved in data collection, entry or analysis; he was only involved in data interpretation. Authors Kontos, Elbin, and DeWolf declare no conflict of interest.
Compliance with Ethical Standards:
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- Baillargeon A, Lassonde M, Leclerc S, Ellemberg D. Neuropsychological and neurophysiological assessment of sport concussion in children, adolescents and adults. Brain Injury. 2012;26(3):211–220. doi: 10.3109/02699052.2012.654590. [DOI] [PubMed] [Google Scholar]
- Brier MR, Ferree TC, Maguire MJ, Moore P, Spence J, Tillman GD, et al. Frontal theta and alpha power and coherence changes are modulated by semantic complexity in Go/NoGo tasks. International Journal of Psychophysiology. 2010;78(3):215–224. doi: 10.1016/j.ijpsycho.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Broglio SP, Pontifex MB, O’Connor P, Hillman CH. The persistent effects of concussion on neuroelectric indices of attention. Journal of Neurotrauma. 2009;26(9):1463–1470. doi: 10.1089/neu.2008-0766. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nature Reviews: Neuroscience. 2009;10(3):186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Buodo G, Palomba D, Sarlo M, Naccarella C, Battistella PA. Auditory event-related potentials and reaction times in migraine children. Cephalalgia. 2004;24(7):554–563. doi: 10.1111/j.1468-2982.2003.00716.x. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304(5679):1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Chen W, Shen X, Liu X, Luo B, Liu Y, Yu R, et al. Passive paradigm single-tone elicited ERPs in tension-type headaches and migraine. Cephalalgia. 2007;27(2):139–144. doi: 10.1111/j.1468-2982.2006.01256.x. [DOI] [PubMed] [Google Scholar]
- Collins MW, Iverson GL, Lovell MR, McKeag DB, Norwig J, Maroon J. On-field predictors of neuropsychological and symptom deficit following sports-related concussion. Clinical Journal of Sport Medicine. 2003;13(4):222–229. doi: 10.1097/00042752-200307000-00005. [DOI] [PubMed] [Google Scholar]
- Coppola G, Cremers J, Gerard P, Pierelli F, Schoenen J. Effects of light deprivation on visual evoked potentials in migraine without aura. BMC Neurology. 2011;11:91. doi: 10.1186/1471-2377-11-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola G, Parisi V, Di Lorenzo C, Serrao M, Magis D, Schoenen J, et al. Lateral inhibition in visual cortex of migraine patients between attacks. Journal of Headache and Pain. 2013;14(1):20. doi: 10.1186/1129-2377-14-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covassin T, Elbin RJ, Harris W, Parker T, Kontos A. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. American Journal of Sports Medicine. 2012;40(6):1303–1312. doi: 10.1177/0363546512444554. [DOI] [PubMed] [Google Scholar]
- Dockree PM, Robertson IH. Electrophysiological markers of cognitive deficits in traumatic brain injury: a review. International Journal of Psychophysiology. 2011;82(1):53–60. doi: 10.1016/j.ijpsycho.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Elbin RJ, Schatz P, Covassin T. One-year test-retest reliability of the online version of ImPACT in high school athletes. American Journal of Sports Medicine. 2011;39(11):2319–2324. doi: 10.1177/0363546511417173. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nature Reviews: Neuroscience. 2001;2(10):704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Erickson JC. Treatment outcomes of chronic post-traumatic headaches after mild head trauma in US soldiers: an observational study. Headache. 2011;51(6):932–944. doi: 10.1111/j.1526-4610.2011.01909.x. [DOI] [PubMed] [Google Scholar]
- Field M, Collins MW, Lovell MR, Maroon J. Does age play a role in recovery from sports-related concussion? A comparison of high school and collegiate athletes. Journal of Pediatrics. 2003;142(5):546–553. doi: 10.1067/mpd.2003.190. [DOI] [PubMed] [Google Scholar]
- Fisher T, Aharon-Peretz J, Pratt H. Dis-regulation of response inhibition in adult Attention Deficit Hyperactivity Disorder (ADHD): an ERP study. Clinical Neurophysiology. 2011;122(12):2390–2399. doi: 10.1016/j.clinph.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9(10):474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Giza CC, Hovda DA. The Neurometabolic Cascade of Concussion. J Athl Train. 2001;36(3):228–235. [PMC free article] [PubMed] [Google Scholar]
- Giza CC, Hovda DA. The new neurometabolic cascade of concussion. Neurosurgery. 2014;75(Suppl 4):S24–33. doi: 10.1227/NEU.0000000000000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin N, Bottari C, Chen JK, Huntgeburth SC, De Beaumont L, Petrides M, et al. Evaluating the cognitive consequences of mild traumatic brain injury and concussion by using electrophysiology. Neurosurgical Focus. 2012;33(6):E7, 1–7. doi: 10.3171/2012.10.FOCUS12253. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Harding HP, Jr, Matthews A, et al. Recurrent concussion and risk of depression in retired professional football players. Medicine and Science in Sports and Exercise. 2007;39(6):903–909. doi: 10.1249/mss.0b013e3180383da5. [DOI] [PubMed] [Google Scholar]
- Headache Classification Subcommittee of the International Headache S. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- Iverson GL, Lovell MR, Collins MW. Interpreting change on ImPACT following sport concussion. Clinical Neuropsychologist. 2003;17(4):460–467. doi: 10.1076/clin.17.4.460.27934. [DOI] [PubMed] [Google Scholar]
- Kawamata M, Kirino E, Inoue R, Arai H. Event-related desynchronization of frontal-midline theta rhythm during preconscious auditory oddball processing. Clinical EEG and Neuroscience. 2007;38(4):193–202. doi: 10.1177/155005940703800403. [DOI] [PubMed] [Google Scholar]
- Kontos AP, Elbin RJ, Lau B, Simensky S, Freund B, French J, et al. Posttraumatic migraine as a predictor of recovery and cognitive impairment after sport-related concussion. American Journal of Sports Medicine. 2013;41(7):1497–1504. doi: 10.1177/0363546513488751. [DOI] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. Journal of Head Trauma Rehabilitation. 2006;21(5):375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- Lau BC, Kontos AP, Collins MW, Mucha A, Lovell MR. Which on-field signs/symptoms predict protracted recovery from sport-related concussion among high school football players? American Journal of Sports Medicine. 2011;39(11):2311–2318. doi: 10.1177/0363546511410655. [DOI] [PubMed] [Google Scholar]
- Lau BC, Lovell MR, Collins MW, Pardini J. Neurocognitive and symptom predictors of recovery in high school athletes. Clinical Journal of Sport Medicine. 2009;19(3):216–221. doi: 10.1097/JSM.0b013e31819d6edb. [DOI] [PubMed] [Google Scholar]
- Lavoie ME, Dupuis F, Johnston KM, Leclerc S, Lassonde M. Visual p300 effects beyond symptoms in concussed college athletes. Journal of Clinical and Experimental Neuropsychology. 2004;26(1):55–73. doi: 10.1076/jcen.26.1.55.23936. [DOI] [PubMed] [Google Scholar]
- Linden RD, Picton TW, Hamel G, Campbell KB. Human auditory steady-state evoked potentials during selective attention. Electroencephalography and Clinical Neurophysiology. 1987;66(2):145–159. doi: 10.1016/0013-4694(87)90184-2. [DOI] [PubMed] [Google Scholar]
- Lucas S. Headache management in concussion and mild traumatic brain injury. PM R. 2011;3(10 Suppl 2):S406–412. doi: 10.1016/j.pmrj.2011.07.016. [DOI] [PubMed] [Google Scholar]
- McCrory P, Meeuwisse WH, Aubry M, Cantu B, Dvorak J, Echemendia RJ, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Journal of the American College of Surgeons. 2013;216(5):e55–71. doi: 10.1016/j.jamcollsurg.2013.02.020. [DOI] [PubMed] [Google Scholar]
- Mihalik JP, Register-Mihalik J, Kerr ZY, Marshall SW, McCrea MC, Guskiewicz KM. Recovery of posttraumatic migraine characteristics in patients after mild traumatic brain injury. American Journal of Sports Medicine. 2013;41(7):1490–1496. doi: 10.1177/0363546513487982. [DOI] [PubMed] [Google Scholar]
- Mihalik JP, Stump JE, Collins MW, Lovell MR, Field M, Maroon JC. Posttraumatic migraine characteristics in athletes following sports-related concussion. Journal of Neurosurgery. 2005;102(5):850–855. doi: 10.3171/jns.2005.102.5.0850. [DOI] [PubMed] [Google Scholar]
- Morlet D, Demarquay G, Brudon F, Fischer C, Caclin A. Attention orienting dysfunction with preserved automatic auditory change detection in migraine. Clinical Neurophysiology. 2014;125(3):500–511. doi: 10.1016/j.clinph.2013.05.032. [DOI] [PubMed] [Google Scholar]
- Reches A, Laufer I, Ziv K, Cukierman G, McEvoy K, Ettinger M, et al. Network dynamics predict improvement in working memory performance following donepezil administration in healthy young adults. Neuroimage. 2013;88C:228–241. doi: 10.1016/j.neuroimage.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reches A, Levy-Cooperman N, Laufer I, Shani-Hershkovitch R, Ziv K, Kerem D, et al. Brain Network Activation (BNA) reveals scopolamine-induced impairment of visual working memory. Journal of Molecular Neuroscience. 2014;54(1):59–70. doi: 10.1007/s12031-014-0250-6. [DOI] [PubMed] [Google Scholar]
- Shahaf G, Reches A, Pinchuk N, Fisher T, Ben Bashat G, Kanter A, et al. Introducing a novel approach of network oriented analysis of ERPs, demonstrated on adult attention deficit hyperactivity disorder. Clinical Neurophysiology. 2012;123(8):1568–1580. doi: 10.1016/j.clinph.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46(1):224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobounov SM, Gay M, Zhang K, Johnson B, Pennell D, Sebastianelli W, et al. Alteration of brain functional network at rest and in response to YMCA physical stress test in concussed athletes: RsFMRI study. Neuroimage. 2011;55(4):1716–1727. doi: 10.1016/j.neuroimage.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O. Contributions and challenges for network models in cognitive neuroscience. Nature Neuroscience. 2014;17(5):652–660. doi: 10.1038/nn.3690. [DOI] [PubMed] [Google Scholar]
- Theriault M, De Beaumont L, Gosselin N, Filipinni M, Lassonde M. Electrophysiological abnormalities in well functioning multiple concussed athletes. Brain Injury. 2009;23(11):899–906. doi: 10.1080/02699050903283189. [DOI] [PubMed] [Google Scholar]
- Wang W, Schoenen J. Interictal potentiation of passive “oddball” auditory event-related potentials in migraine. Cephalalgia. 1998;18(5):261–265. doi: 10.1046/j.1468-2982.1998.1805261.x. discussion 241. [DOI] [PubMed] [Google Scholar]