Abstract
Despite a recent shift away from anti-insulin-like growth factor I receptor (IGF-IR) therapy, this target has been identified as a key player in the resistance mechanisms to various conventional and targeted agents, emphasizing its value as a therapy, provided that it is used in the right patient population. Molecular markers predictive of antitumor activity of IGF-IR inhibitors remain largely unidentified. The aim of this study is to evaluate the impact of insulin receptor (IR) isoforms on the antitumor efficacy of cixutumumab, a humanized monoclonal antibody (mAb) against IGF-IR, and to correlate their expression with therapeutic outcome. The data demonstrate that expression of total IR rather than individual IR isoforms inversely correlates with single agent cixutumumab efficacy in pediatric solid tumor models in vivo. Total IR, IR-A and IR-B expression adversely affects the outcome of cixutumumab in combination with chemotherapy in patient-derived xenograft (PDX) models of lung adenocarcinoma. IR-A overexpression in tumor cells confers complete resistance to cixutumumab in vitro and in vivo while IR-B results in a partial resistance. Resistance in IR-B-overexpressing cells is fully reversed by anti-IGF-II antibodies, suggesting that IGF-II is a driver of cixutumumab resistance in this setting. The present study links IR isoforms, IGF-II and cixutumumab efficacy mechanistically and identifies total IR as a biomarker predictive of intrinsic resistance to anti-IGF-IR antibody.
Implications
This study identifies total IR as a biomarker predictive of primary resistance to IGF-IR antibodies, and provides a rationale for new clinical trials enriched for patients whose tumors display low IR expression.
INTRODUCTION
For more than two decades, the insulin-like growth factor (IGF) system, which includes receptors (IGF-IR, IGF-IIR, insulin receptor), ligands (IGF-I and IGF-II) and high affinity IGF binding proteins (IGFBP1–6), has been studied with great interest in cancer biology. While this highly-regulated pathway plays a crucial role in the normal development and growth of tissues, its deregulation contributes to tumor initiation, proliferation and survival (1). Elevated circulating IGF-I levels have been associated with increased cancer risk (2). Conversely, individuals with genetic disorders resulting in low circulating levels of IGF-I and IGF-II, are resistant to cancer development (3). In vitro studies have demonstrated the importance of functional insulin-like growth factor I receptor (IGF-IR) for cell transformation induced by viral and cellular oncogenes (4). IGF-IR upregulation was observed in a variety of tumor types including prostate, breast, colon, lung cancer and melanoma (5, 6). Moreover, the IGF-IR pathway has also been implicated in the development of resistance to other antitumor modalities including radiation therapy, chemotherapeutic agents and targeted therapies (1). Therefore, targeting the IGF-IR pathway represents an attractive strategy for the treatment of various tumor types.
Over the last decade, a number of monoclonal antibodies (mAbs) and small-molecule tyrosine kinase inhibitors (TKIs) directed against IGF-IR have made their way into clinical trials (www.clinicaltrials.gov). Anti-IGF-IR mAbs including cixutumumab, a fully human monoclonal antibody against IGF-IR, are currently the most clinically-advanced molecules. Despite very promising results in preclinical and early phase clinical studies, results from phase III trials have failed to meet expectations (7). It is important to note, however, that although no significant clinical benefit was observed in the intention-to-treat (ITT) population, a distinct subset of patients seems to benefit from IGF-IR targeting (8–11). Elucidating molecular markers predictive of anti-tumor efficacy of anti-IGF-IR therapy, however, is an important and ongoing challenge. Somatic genetic aberrations are frequently the major determinants of oncogenic and pharmacological dependence in cancer (12, 13). In most tumors, however, IGF-IR pathway is not altered genetically suggesting that additional non-genomic factors may mediate sensitivity or resistance to IGF-IR targeted therapies. Intrinsic or acquired resistance to targeted agents frequently results from the activation of alternative receptor tyrosine kinases (RTKs) including ERBB, MET, FGFR and AXL family members (14–18). Insulin receptor (INSR or IR), which shares up to 70% homology with IGF-IR and is commonly expressed in neoplasms and tumor cells, might be implicated in the resistance to anti-IGF-IR therapy. Alternative splicing of INSR transcript results in two isoforms, IR-A and IR-B, which differ by the exclusion of exon 11 encoding 12 amino acids (19). While IR-B isoform binds primarily insulin, IR-A is capable of binding both insulin and IGF-II (20). IGF-II upregulation has been reported in numerous tumor types (5, 6) and frequently results from the loss of imprinting (LOI) of the IGF2 gene (21). Additionally, inactivating mutations or loss of heterozygosity of the gene encoding insulin-like growth factor II receptor (IGF-IIR), thought to act as a scavenger for IGF-II, can also contribute to increased IGF-II bioavailability (22, 23). This provides yet another alternate route for IGF-II signaling via the IR and results in mitogenic and anti-apoptotic signals in tumors. Deregulated IGF-II expression in tumors and the ability of this ligand to signal through the IR-A in addition to the IGF-IR suggest that endogenous IR expression may be an important determinant of sensitivity to IGF-IR mAbs.
In the present study, we provide evidence that IR, irrespective of isoform type, mediates primary resistance to IGF-IR targeted therapy and can be used as a potential biomarker for patient selection.
MATERIALS AND METHODS
Materials
Chemicals and materials were obtained from the following sources: CHAPS (Affymetrix, Santa Clara, CA, USA); Cisplatin (Medac GmbH, Hamburg, Germany); Linsitinib (OSI-906) (Selleckchem, Houston, TX, USA), Matrigel (BD Bioscience, Sparks Glencoe, MD, USA); Ham’s F12 Nutrient Mix (F-12 HAM), Roswell Park Memorial Institute (RPMI) 1640 medium, Improved Minimum Essential Medium (IMEM), NuPage 4–12% Bis-Tris, iBlot Gel Transfer Stacks Nitrocellulose, puromycin (Invitrogen, Carlsbad, CA, USA); plasmids Lv-105-IGF-I, Lv105-IGF-II, Lv-105-IGF-IR (GeneCopoeia, Rockville, MD, USA); recombinant human IGF-I, IGF-II (R&D Systems, Minneapolis, MN, USA); Complete® Protease Inhibitor Cocktail, PhosSTOP® Phosphatase Inhibitor Cocktail, recombinant human insulin (Roche, Indianapolis, IN, USA); Xba-I, Spe-I, Pierce Protein Assay, Spectra Multicolor Broad Range Protein Ladder (Thermo Scientific, Glen Burnie, MD, USA); CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison, WI, USA); pemetrexed disodium heptahydrate (Eli Lilly, Indianapolis, IN, USA).
Antibodies
The following antibodies were purchased from commercial sources as indicated: mouse monoclonal antibodies against Akt (#2920) and p42/p44 MAPK (Erk1/2) (#9107), rabbit monoclonal antibodies against phospho-AktS473 (#4060) and phospho-IGF-IR beta Y1135/1136 / Insulin Receptor beta Y1150/1151 (#3024), rabbit polyclonal antibody against phospho-p42/p44 MAPK T202/Y204 (Erk1/2) (#9101) (Cell Signalling, Beverly, MA, USA); mouse monoclonal antibody against IGF-IR (#MS-641-P) and Insulin Receptor (#MS-632-P) (Thermo Fisher Scientific, Fremont, CA, USA); rabbit polyclonal Insulin Receptor (#sc-711) and (#sc-7953) (Santa Cruz Biotechnology, Santa Cruz, CA, USA); mouse monoclonal antibody against IGF-II (MAB292) and goat F(ab')2 anti-mouse IgG-phycoerythrin (#F0102B) (R&D Systems, Minneapolis, MN, USA); goat polyclonal anti-mouse IRDye 680 conjugated (#926–32220) and anti-rabbit IRDye 800 conjugated (#926–32211) (LI-COR Biosciences, Lincoln, Nebraska, USA). Cixutumumab was supplied by Eli Lilly and Company. Control human IgG was purchased from Equitech-Bio Inc., Kerrville, TX, USA.
IGF-IR, IR-A, IR-B mRNA Analysis in TCGA Datasets
Isoform expression RNA-Seq data was processed level 3 data (RNA-seq V2) downloaded from TCGA Data Portal. mRNA expression levels were estimated by RSEM (http://deweylab.biostat.wisc.edu/rsem/) and then RSEM expression estimates were normalized to set the upper quartile count at 300. IGF-IR (combined expression of two major IGF-IR isoforms, uc010bon.2 and uc002bul.2), IR-A (uc002mge.1) and IR-B (uc002mgd.1) expression levels were calculated for 6943 malignant samples representing 21 tumor types.
Patient Specimens
Snap-frozen NSCLC and CRC tumor samples were provided by CureLine (San Francisco, CA, USA), Indivumed (Hamburg, Germany) and Tissue Solutions (Glasgow, UK). Specimens were collected in compliance with all applicable regulations.
Cell Cultures
Cell lines used in this study were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). A549, NCI-H1299 and MCF-7 cells were cultured in F-12 HAM, RPMI 1640 and IMEM medium, respectively, supplemented with 1X GlutaMAX supplement (Life Technologies, Grand Island, NY, USA) and 10% fetal bovine serum at 37 °C in 5% CO2 atmosphere and 95% humidity.
Generation of Stable Cell Lines that Overexpress Insulin Receptor Isoforms
Plasmids with cDNA encoding the IR-A and IR-B sequence were generated at Eli Lilly. cDNAs encoding IR isoforms were amplified with 5’TATACTAGTATGGCCACCGGGGGAAGGAG-3’ and 5’-TAATCTAGACTAAGAAGGATTGGACCGAGGC-3’ primers, digested with Spe-I and Xba-I and ligated into pLVX-IRES-puro HIV-1-based lentiviral expression vector (Clontech ®, Mountain View, CA, USA). Lentiviruses were engineered by transfecting plasmids into HEK-293T cells using reagents and protocols provided with the Lenti-X™ Lentiviral Expression Systems (Clontech ®, Mountain View, CA, USA). A549, NCI-H1299 and MCF-7 cells were transduced with the respective virus for 48 hours and then cultured in the presence of puromycin (1 µg/ml) for selection of stably transduced A549 (A549-Mock, A549-IR-A, A549-IR-B), NCI-H1299 (NCI-H1299-Mock, NCI-H1299-IR-A, NCI-H1299-IR-B) and MCF-7 (MCF-7-Mock, MCF-7-IR-A, MCF-7-IR-B) variants.
Colony Formation Assay
Colony formation assay was performed as described by Ulanet et al. (24) with some minor modifications. Tested on control (T/C) values below and above 50% were considered sensitive and resistant, respectively.
Animal Models
Experimental studies in xenograft models of pediatric solid tumors were conducted through Pediatric Preclinical Testing Program (PPTP) and have been described previously (25). In the in vivo studies including xenograft A549 model with overexpression of IR-A and IR-B isoforms and patient-derived xenograft (PDX) models of lung adenocarcinoma, 5–8-week old female nude mice (nu/nu) were used (The Jackson Laboratory, Bar Harbor, MA, USA; Harlan Laboratories, Horst, The Netherlands). To evaluate pharmacodynamics effects of cixutumumab, C57B1/6 male mice were used (Charles River Laboratories, Kingston, NY, USA). Animals were maintained under barrier conditions, and experiments were performed according to the institutional protocols (Eli Lilly, Oncotest) in compliance with National Institutes of Health Guide for the Care and Use of Laboratory Animals and German Animal Welfare Act (Tierschutzgesetz). Mice were kept on a 12-hour light/dark cycle with access to a standard laboratory chow diet and fresh water ad libitum.
To study the antitumor efficacy of cixutumumab in xenograft A549 model with overexpression of IR isoforms, mice (n=30 for each variant) were subcutaneously injected with 2 × 107 A549-Mock, A549-IR-A or A549-IR-B cells resuspended in 100% Matrigel. When tumors reached approximately 250–300 mm3, animals were randomized into two treatment groups (0.9% USP Saline and cixutumumab; n=12 for each group). USP Saline (10 uL/g of body weight (BW)) and cixutumumab (60 mg/kg BW) were administered intraperitoneally (IP) twice weekly for five weeks. To evaluate the efficacy of cixutumumab in combination with chemotherapy, nine PDX models of lung adenocarcinoma were employed. LXFA-289, LXFA-297, LXFA-526, LXFA-623, LXFA-629, LXFA-737, LXFA-749, LXFA-923, LXFA-983 models were derived from tumor specimens of patients treated at the University Hospital in Freiburg, Germany, and directly implanted into nude mice according to the procedure established at Oncotest GmbH (Freiburg, Germany). Additional information on the PDX models including clinical annotations and mutational status of genes commonly mutated in lung adenocarcinoma is provided in Supplemental Table 2. The aforementioned models were used to evaluate the anti-tumor activity of cixutumumab in monotherapy and in combination with cisplatin and pemetrexed, a current standard of care in patients with metastatic non-squamous NSCLC. Mice were randomized into four treatment group (6–7 animals per group) when tumors reach 50–250 mm3 (control, cixutumumab, pemetrexed/cisplatin, cixutumumab/pemetrexed/cisplatin) hIgG and cixutumumab were administered IP at 40 mg/kg of BW three times a week for a duration of three to four weeks. Pemetrexed and its vehicle (0.9% (w/v) NaCl) were given IP at 100 mg/kg of BW, daily except weekends, for two to four weeks. Cisplatin (3.2 mg/kg BW) and its vehicle (PBS) were administered subcutaneously once weekly for three weeks.
Tumor size was measured twice weekly via calipers. Anti-tumor activity was evaluated as maximum tumor volume inhibition compared with the vehicle control group (optimal T/C values calculated based on median values). Prior to euthanasia, tumors were carefully excised and immediately snap-frozen in liquid nitrogen for further analysis.
RNA Extraction and cDNA Preparation
Total RNA was isolated from snap-frozen tumor tissue using the TissueLyser with stainless steel beads (5 mm) and the AllPrep DNA/RNA Mini kit from QIAGEN (Valencia, CA, USA) or the MagMAX 96 Total RNA isolation kit from Life Technologies (Grand Island, NY, USA). RNA concentration was determined spectrophotometrically with the OD260 (260/280>1.9) and purity was verified with the Agilent Bioanalyzer. RNA was reverse-transcribed with a high-capacity complementary DNA (cDNA) reverse transcription kit (Life Technologies, Grand Island, NY, USA) and random primers according to the manufacturer’s instructions. cDNA was then diluted 1:5 (20 ng/uL) for quantitative real-time PCR (qPCR) use.
PCR Primers and Probe Sets
Commercial TaqMan Gene Expression assays were purchased from Life Technologies (Grand Island, NY, USA) for quantification of total IR (INSR, Assay ID: Hs00961554_m1), IGF-IR (IGF-IR, Assay ID: Hs00609566_m1), IGF-I (IGF-I, Assay ID: Hs01547656_m1), IGF-II (IGF-II, Assay ID: Hs01005963_m1) and EGFR (EGFR, Assay ID: Hs01076078_m1). Full length mRNA transcript sequences for IR-A (NM_001079817) and IR-B (NM_000208) isoforms were retrieved from the NCBI Reference Sequence database. The IR-B TaqMan Gene Expression assay was designed using the Custom TaqMan Assay Design Tool from Life Technologies. Program input specified exon 11 inclusion; final positioning of the probe spanned the exon 10/11 junction. All probes incorporated a minor groove binding (MGB) moiety and were labelled with a fluorescent dye (FAM) for detection and a non-fluorescent quencher. Custom-made primers/probe sequence for IR-B includes: probe sequence: 5’-TCCCCAGAAAAACCTC-3’, forward primer: 5’-CCTGCACAACGTGGTTTTCG-3’ and reverse primer: 5’-CGGCACCAGTGCCTGAA-3’. To confirm specificity of the IR-B probe, spiking experiments were performed utilizing plasmids containing cDNA encoding IR-A or IR-B. It was not possible to identify completely specific TaqMan probes to IR-A. IR-A expression levels were, therefore, calculated by subtracting IR-B values from total IR values.
Gene Expression Analysis
qPCR was performed in a 96-well format on 100ng of cDNA (20ng/uL) with 1uL of TaqMan Gene Expression Assay (20X) and 10uL of TaqMan Gene Expression Master Mix (2X) in a final volume of 20uL. Each sample was tested in triplicate. All assays were run on Applied Biosystems 7500 Fast detection system using standard setting (10 min incubation at 95°C, followed by 40 cycles at 95°C for 15 seconds and 60°C for 1 minute). Absolute values (mRNA copies/ng of cDNA) were derived from a standard curve generated with a serial dilution of plasmid containing the cloned sequence of the target gene. Each target standard curve demonstrated linearity with regression coefficients (r2 values) above 0.997. All assays had efficiency between 88–100% with a standard curve slope between −3.3 to −3.6. Data were extracted with 7500 Software V2.0.5 (Life Technologies, Grand Island, NY, USA).
Statistical Analysis
Results are expressed as the mean ± SEM. Student’s t-test, one way ANOVA, two-factor ANOVA, ANOVA on repeated measurement (RM-ANOVA) followed by Tukey’s post-test and mixed model were used for statistical analysis. Mixed model analysis for repeated measures was used for the evaluation of the statistical significance of tumor inhibition. The term mixed model refers to the use of both random and fixed effects in the same analysis which is ideal for analyzing “unbalanced” datasets (due to death of mice etc).
RESULTS
IGF-IR, IR-A and IR-B Expression in Human Malignancies
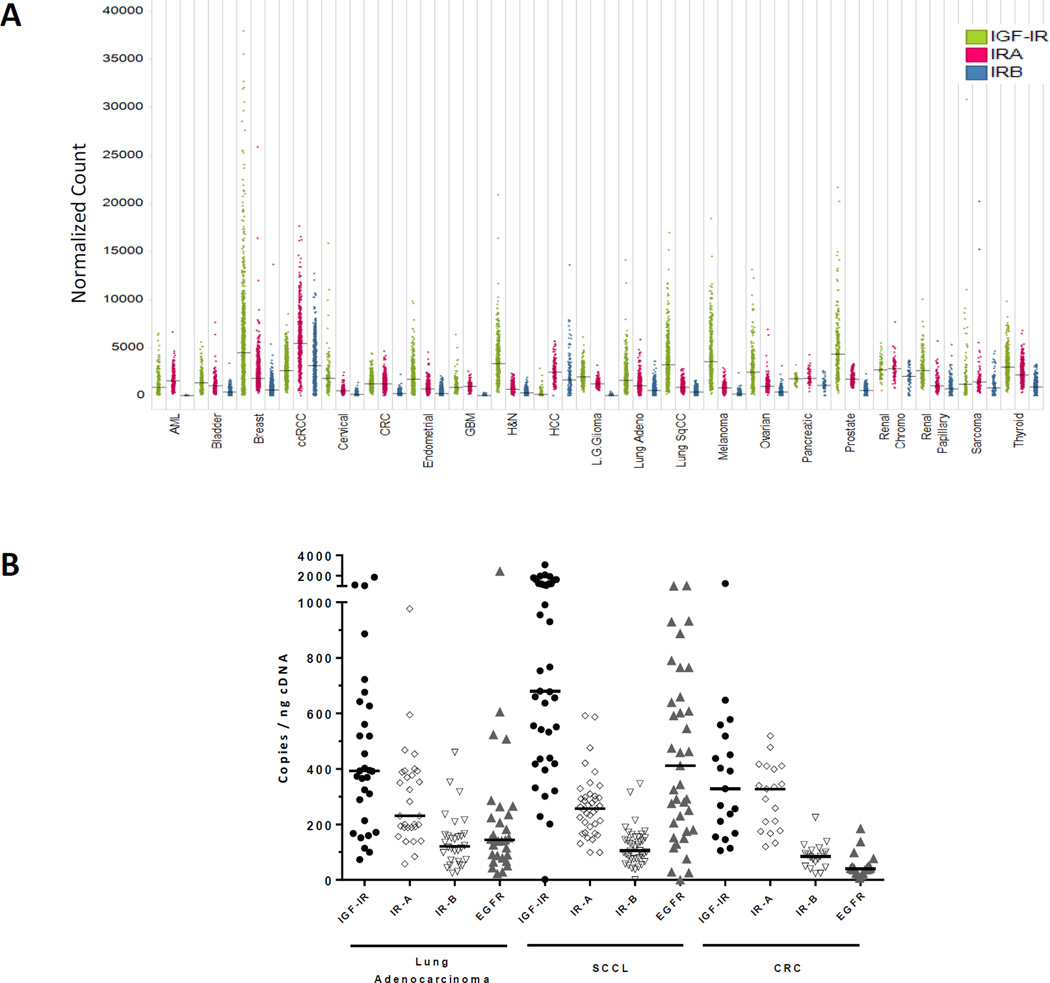
To understand the prevalence of IGF-IR, IR-A and IR-B mRNA expression in patient tumor samples, we evaluated mRNA levels of IGF-IR and IR isoforms using TCGA (The Cancer Genome Atlas) RNA-seq data from 6,943 samples representing 21 tumor types. These results suggest that expression of all three receptors significantly varies between tumor types and individual tumors (Figure 1A). High levels of IGF-IR expression were observed in breast, ovarian, prostate, head and neck, squamous lung cancer and melanoma. IR-A expression was observed in virtually all tumor types, and was particularly high in clear cell renal cell carcinoma (ccRCC). Many tumor samples also displayed significant IR-B expression, with highest levels observed in ccRCC and hepatocellular carcinoma. We further verified RNA-seq data by a more accurate qPCR analysis of clinical NSCLC and colon cancer samples. Median levels of IGF-IR transcripts were 392, 680 and 328 copies/ng cDNA in lung adenocarcinoma (LA), squamous cell carcinoma of the lung (SCCL) and colorectal carcinoma (CRC), respectively (Figure 1B). Median levels of IR-A and IR-B mRNA were 230 and 120 copies/ng cDNA in LA, 256 and 106 copies/ng cDNA in SCCL, 328 and 85 copies/ng cDNA in CRC. Furthermore, in CRC samples IR-A and IR-B expression was several fold higher compared to expression levels of EGFR, a clinically validated target in this tumor type. Taken together, these data indicate that both IR isoforms are broadly expressed in human tumors.
Figure 1.
(A) IGF-IR, IR-A and IR-B mRNA expression in major tumor indications according to the TCGA RNA-seq V2 dataset (n=6,943). Expression levels were estimated by RSEM followed by normalization of RSEM expression estimates to set the upper quartile count at 300. Green: combined expression of two major IGF-IR isoforms (uc010bon.2 and uc002bul.2); red: IR-A (uc002mge.1); blue: IR-B (uc002mgd.1). Represented are individual and median values. (B) IGF-IR, IR-A, IR-B and EGFR mRNA expression in tumor samples obtained from patients with lung adenocarcinoma (n=30), squamous cell carcinoma of the lung (n=39), colon carcinoma (n=19). Represented are individual and median values.
Total IR Expression Inversely Correlates with Antitumor Efficacy of Cixutumumab Monotherapy in Preclinical Models of Pediatric Solid Tumors
As previously demonstrated (25), single agent cixutumumab exhibited antitumor activity in vivo (T/C <50%) in 16 of 34 xenograft models of pediatric solid tumors (rhabdoid, Ewing’s sarcoma, rhabdomyosarcoma, glioblastoma, neuroblastoma, osteosarcoma) tested through PPTP.
It is important to state that cixutumumab exhibits equipotent binding to both human and mouse IGF-IR and is therefore suitable for xenograft studies in mice. We observed elevated levels of growth hormone, IGF-I and insulin in the circulation of mice treated with cixutumumab (Suppl. Figure 1). Therefore, mouse models properly mimic the pharmacodynamic changes seen in humans (Eli Lilly, data on file) (10, 11).
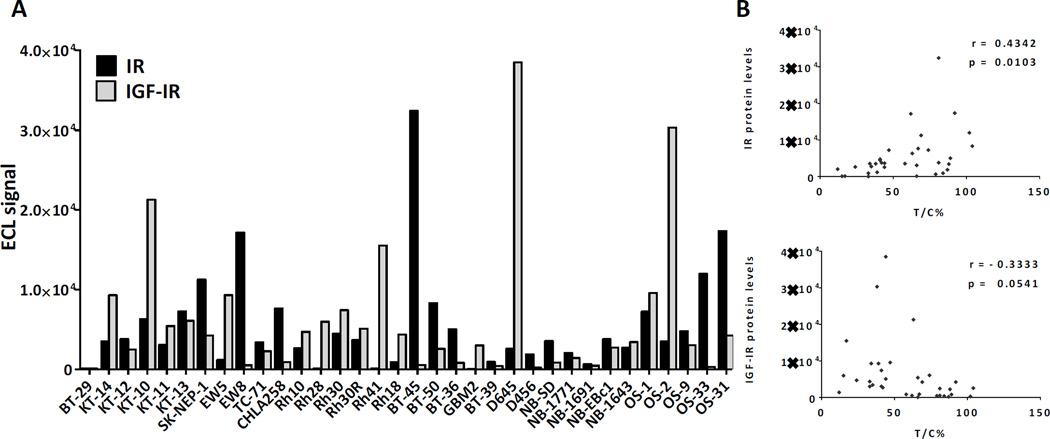
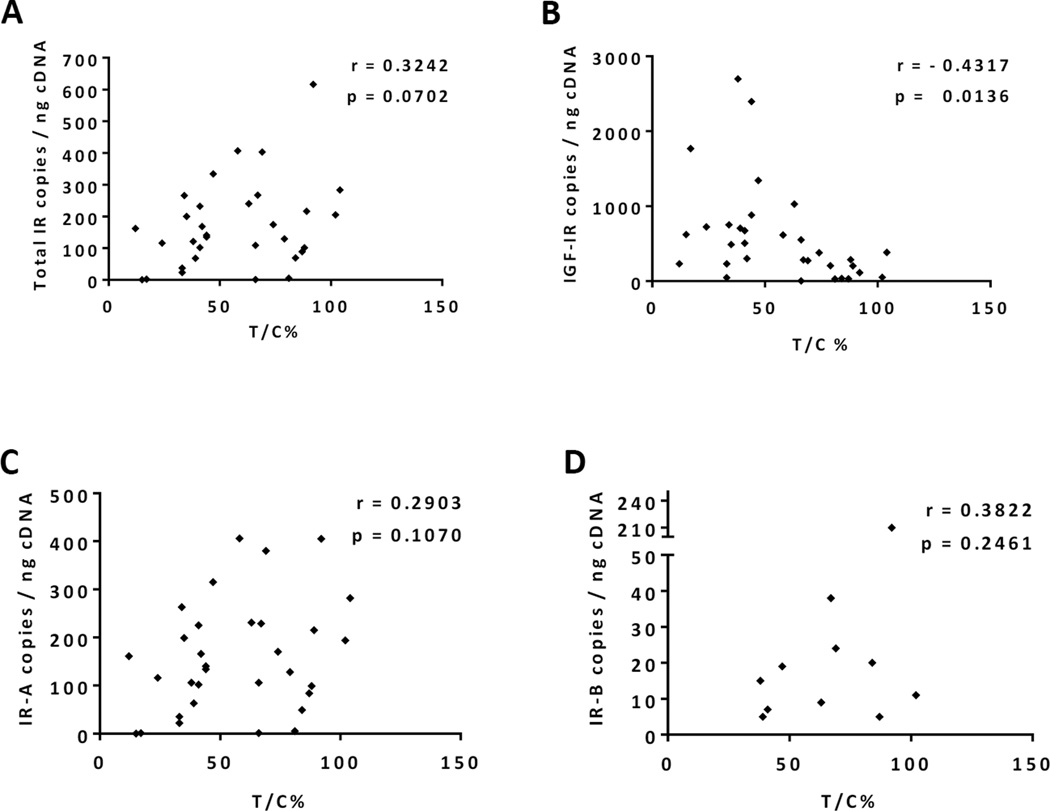
To understand if baseline IR and IGF-IR expression in tumor tissue is predictive of cixutumumab efficacy in vivo, tissue samples collected from individual tumor models were analyzed for IR and IGF-IR by ECL assay and qPCR. The amount of IR and IGF-IR protein and mRNA varied significantly across different tumor models (Figure 2). However, a significant correlation was observed between mRNA and protein levels of both IR and IGF-IR (r = 0.82 and 0.91, respectively, p < 0.0001) within individual tumor samples. The impact of the two receptors on the outcome of cixutumumab therapy, though, differed greatly. While IGF-IR expression levels tended to directly correlate with sensitivity to the antibody, high IR was associated with higher T/C values indicative of de novo or intrinsic resistance (Figures 2, 3A and 3B). Expression of IR isoforms, IR-A and IR-B, was also evaluated by qPCR analysis. While IR-A was the predominant isoform, ubiquitously and robustly expressed in various pediatric solid tumors (range: 0–406, mean 157 copies/ng cDNA), expression of IR-B was rather weak (range: 0–210, mean 12 copies/ng cDNA) and restricted to fewer tumor models. In contrast to total IR, however, expression of either IR isoform failed to correlate with cixutumumab efficacy suggesting that both IR isoforms can potentially contribute to cixutumumab resistance (Figures 3C and 3D).
Figure 2.
Insulin receptor (IR) protein levels negatively correlate with antitumor efficacy of cixutumumab monotherapy in preclinical models of pediatric solid tumors in vivo. (A) Total IR and IGF-IR protein levels in tumor lysates were measured by Meso Scale Discovery Electrochemiluminescence assay. (B) Correlation analysis of IR and IGF-IR protein levels and antitumor efficacy of cixutumumab expressed as T/C% values. Correlation coefficient (Pearson’s r) and p-value (p) are indicated.
Figure 3.
Expression of total IR rather than individual isoforms correlates with antitumor efficacy of cixutumumab monotherapy in preclinical models of pediatric solid tumors. Correlation analysis of total IR (A), total IGF-IR (B), IR-A (C) and IR-B (D) mRNA expression and antitumor efficacy of cixutumumab expressed as T/C% values. Correlation coefficient (Pearson’s r) and p-value (p) are indicated.
It is known that IGF-I primarily stimulates IGF-IR whereas IGF-II is capable of activating both IGF-IR and IR-A. We aimed to elucidate which of the two ligands is expressed in pediatric solid tumors. As demonstrated by qPCR, IGF-I expression was barely detectable in most samples (range: 0–1,100, mean 74 copies/ng cDNA) while IGF-II was expressed at markedly high levels (range: 0–107,723, mean 23,657 copies/ng cDNA) in a significant number of tumor models (Suppl. Table 1). It is therefore plausible that in cixutumumab-treated tumors with high IR expression, IGF-II may be capable of promoting tumorigenesis, bypassing the IGF-IR.
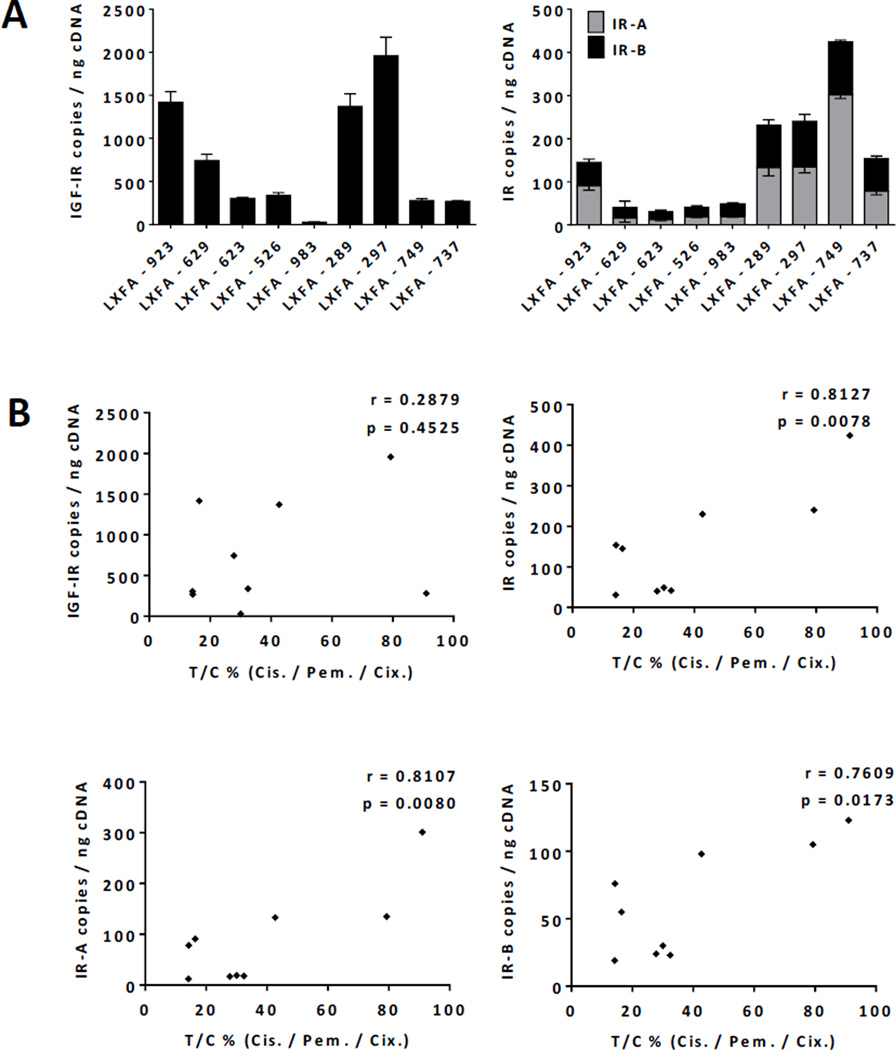
Total IR, IR-A and IR-B Expression Correlates with Anti-Tumor Efficacy of Cixutumumab in Combination with Cisplatin/Pemetrexed in Patient-Derived Xenograft Models of NSCLC
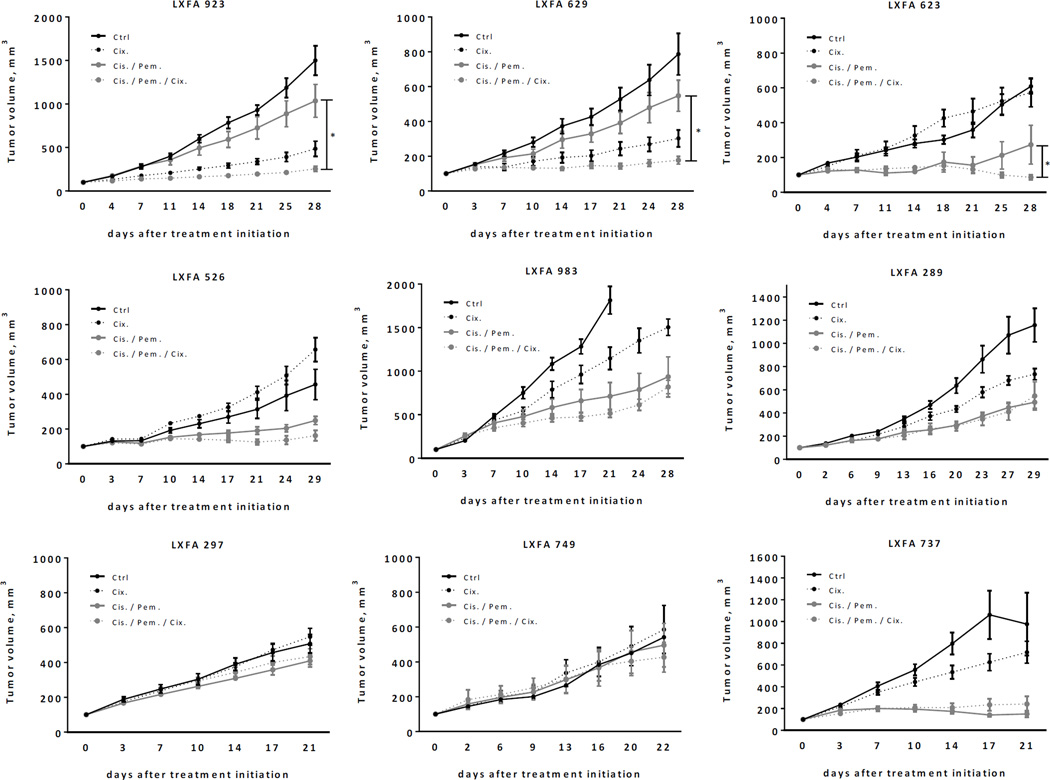
Clinical data indicate that IGF-IR antibodies display rather weak single agent activity in epithelial tumors, and in most trials they were used in combination with other antitumor agents including chemotherapy. We therefore tried to understand if baseline IGF-IR and IR expression was associated with the antitumor efficacy of cixutumumab used in combination with pemetrexed and cisplatin, which is a standard of care in NSCLC with non-squamous histology. When combined with chemotherapy, cixutumumab displayed significantly improved efficacy over chemotherapy alone in 3 out 9 patient-derived models of lung adenocarcinoma (LA) (Figure 4, Suppl. Table 2). To identify predictors of cixutumumab sensitivity or resistance, individual LA models were analyzed for baseline levels of total IGF-IR, total IR, IR-A, IR-B, IGF-I and IGF-II expression by qPCR (Figure 5A and Suppl. Figure 2). In contrast to pediatric solid tumors, IGF-IR expression was not predictive of the efficacy of the IGF-IR mAb in LA models. Expression of total IR and, more importantly, IR-A and IR-B was associated with the outcome of cixutumumab therapy (Figure 5B). Of note, although IR-A was the predominant isoform (range: 12–301, mean 89 copies/ng cDNA), IR-B expression (range: 19–123, mean 61 copies/ng cDNA) was significantly higher in LA compared to the aforementioned pediatric solid tumors. In addition, IGF-I expression was negligible while IGF-II was expressed (range: 1–2,302, mean 490 copies/ng cDNA) in most LA models at various levels (Suppl. Figure 2). These results further support our initial observation suggesting that (1) IGF-II rather than IGF-I is expressed in most tumors and (2) both IR isoforms seem to contribute to de novo cixutumumab resistance.
Figure 4.
Growth curves of patient-derived lung adenocarcinoma xenograft tumors in response to cixutumumab, cisplatin/pemetrexed or cixutumumab/cisplatin/pemetrexed. For the evaluation of the statistical significance of tumor inhibition, the mixed model analysis for repeated measures was used. For comparisons between the triple combination therapies and the respective monotherapy or dual therapy, the test results are given for the day on which the optimal T/C value of the triple combination therapy group was recorded. *Statistically significant difference between cisplatin/pemetrexed or cixutumumab/cisplatin/pemetrexed groups (p ≤ 0.05).
Figure 5.
(A) Total IGF-IR, IR-A and IR-B mRNA expression in patient-derived xenograft models of lung adenocarcinoma (n=3 per tumor model). Represented are mean values +/− SEM. (B) Correlation analysis of total IGF-IR, total IR, IR-A and IR-B mRNA expression and antitumor efficacy of cixutumumab/cisplatin/pemetrexed expressed as T/C% values. Correlation coefficient (Pearson’s r) and p-value (p) are indicated.
Two Insulin Receptor Isoforms Confer Resistance to Cixutumumab in NSCLC and Breast Cancer Models
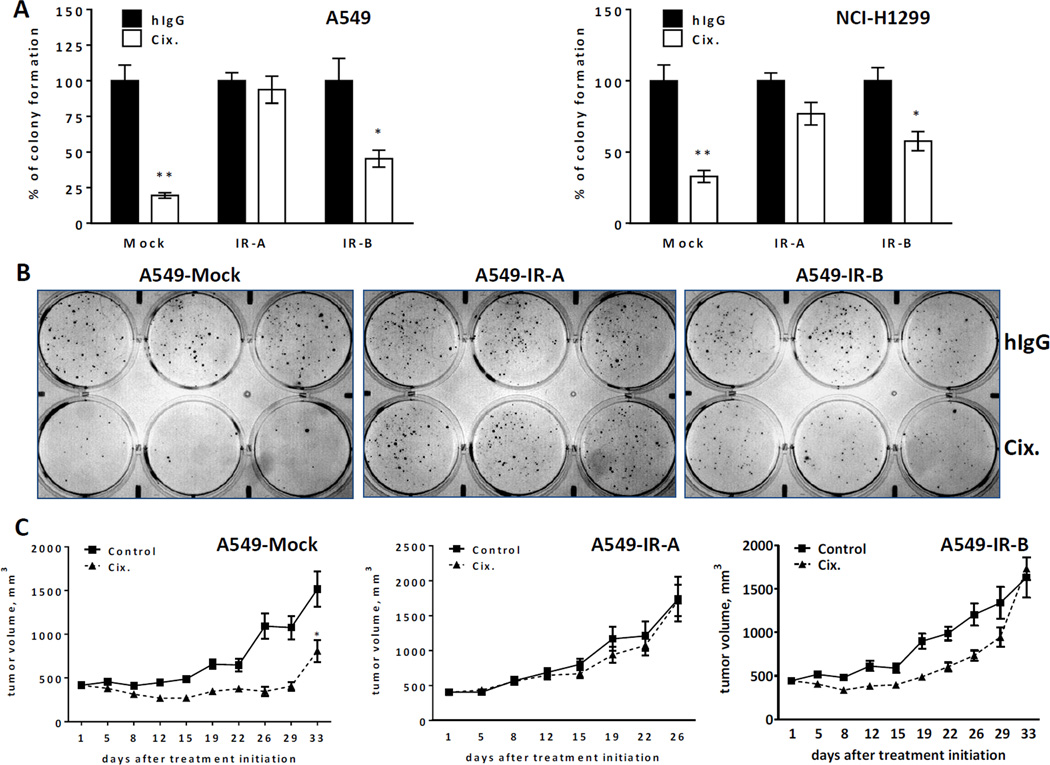
To understand if there is a mechanistic link between individual IR isoforms and cixutumumab resistance, we developed stably transduced variants of NSCLC and breast cancer cell lines (A549, NCI-H1299 and MCF-7) that overexpress either IR-A or IR-B. A549, NCI-H1299 and MCF-7 cells display sensitivity to cixutumumab when tested in vitro in anchorage-independent colony formation assay or anchorage-dependent cell viability assay. Furthermore, A549 cells exhibit sensitivity to cixutumumab in nude mice. IR expression was markedly increased in IR-A and IR-B-overexpressing A549, NCI-H1299 and MCF-7 cells as demonstrated by qPCR, western blot and/or flow cytometry. Cell surface IGF-IR expression, however, did not differ significantly between A549-Mock and A549-IR-overexpressing variants (Suppl. Figure 3A). Furthermore, A549-Mock cells were responsive to stimulation with IGF-I, IGF-II and, to a lesser extent, insulin as exemplified by increased phosphorylation of Akt and/or IGF-IR/IR. ERK1/2 phosphorylation was not significantly altered in mock-transduced A549 cells. While IGF-I-induced signal transduction was not very different in mock- and IR-overexpressing cells, insulin potently stimulated downstream signaling in both IR-overexpressing variants as demonstrated by increased phosphorylation of IGF-IR/IR, Akt and ERK1/2. In addition, treatment of A549-IR-A and A549-IR-B cells with IGF-II also resulted in increased phosphorylation of IGF-IR/IR and ERK1/2 which was barely seen in A549-Mock cells (Suppl. Figure 3B). These results are in agreement with previously published data indicating that both insulin and IGF-II are capable of binding to IR, although IGF-II affinity for IR-B is several fold lower compared to IR-A (26, 27). The efficacy of cixutumumab was then tested in A549 and NCI-H1299 variants using colony formation assay (Figure 6A–B). As expected, in A549-Mock and NCI-H1299-Mock variants, the antibody inhibited colony formation by 70–80% (T/C 19.5% and 32.8%, respectively). However, IR-A overexpression fully abolished this effect (T/C 93.8% and 76.8% for A549 and NCI-H1299 cells, respectively). Ectopic expression of IR-B resulted in a partial cixutumumab resistance (T/C 45.4% and 57.6% for A549 and NCI-H1299 cells, respectively). Moreover, the efficacy of cixutumumab was tested in MCF-7 variants under anchorage dependent conditions. In line with soft agar assay data, the mock-transfected line exhibited cixutumumab sensitivity (maximum inhibition 60.4%), while the IR-A overexpressing cells were completely resistant (maximum inhibition 0%) and the IR-B overexpressing cells were partially resistant (maximum inhibition 26.9%) (Suppl. Figure 4). To corroborate these findings in vivo, mice bearing A549-Mock, A549-IR-A or A549-IR-B xenograft tumors were treated with cixutumumab for up to 33 days (Figure 6C). A549-IR-A tumors grew more rapidly compared to A549-Mock and A549-IR-B xenografts (RM-ANOVA followed by Tukey multiple comparison test; p<0.01). More importantly, treatment with cixutumumab resulted in a statistically significant (p=0.0014) tumor growth inhibition in A549-Mock model only. Consistent with our in vitro findings, overexpression of IR-A and, to a lesser extent, IR-B led to cixutumumab resistance.
Figure 6.
Two IR isoforms confer resistance to cixutumumab in vitro and in vivo. (A) Clonogenic assay with A549 and NCI-H1299 cells, with or without IR-A or IR-B overexpression, in the presence or absence of cixutumumab. Represented are mean values +/− SEM, expressed as percentage of colony formation relative to hIgG-treated cells. *p < 0.05, **p < 0.01 (two tail Student’s t-test). (B) Representative images of clonogenic assay in A549 cells. (C) Antitumor efficacy of cixutumumab in mice bearing xenograft A549 tumors that overexpress empty vector, IR-A or IR-B models. RM ANOVA was used to compare tumor growth between treatment groups. p-values ≤ 0.05 indicate statistical significance (*) of tumor inhibition.
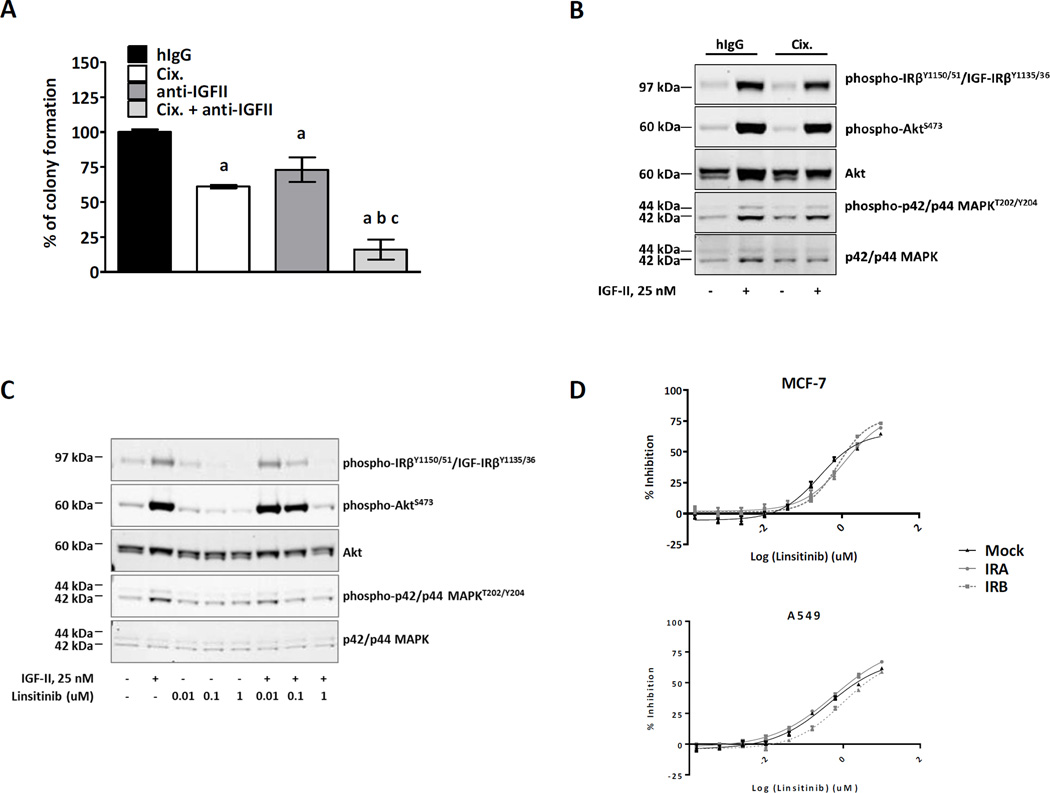
IGF-II Mediates Resistance to Cixutumumab in NSCLC Cells with IR-B Overexpression
A previous study has shown that IGF-II acting though IR-A could be accountable for the limited efficacy of anti-IGF-IR therapy (24). To understand if IR-B-mediated resistance to cixutumumab is driven by IGF-II, we examined the effect of cixutumumab in the presence or absence of neutralizing anti-IGF-II mAb (clone 75015) in A549-IR-B cells. Both cixutumumab and anti-IGF-II mAb only partially inhibited clonogenic potential of A549 cells with ectopic IR-B overexpression. Combination of the two antibodies, however, completely reversed drug-resistant phenotype suggesting that IGF-II is implicated in the development of cixutumumab resistance in tumor cells with of IR-B overexpression (Figure 7A). We also confirmed that treatment with IGF-II at the concentration capable of binding to IR-B (>20 nM) (28) resulted in marked phosphorylation of IR, Akt and ERK1/2 in cixutumumab-pretreated A549-IR-B cells (Figure 7B). A similar approach was employed with linsitinib, a dual IGF-IR/IR inhibitor. In contrast to cixutumumab, linsitinib abrogated IGF-II-mediated Akt and ERK1/2 phosphorylation in A549-IR-B cells (Figure 7C). Furthermore, linsitinib was equipotent in inhibiting A549 and MCF-7 tumor cells irrespective of insulin receptor isoform expression (Figure 7D). Collectively, these data indicate that IGF-II is capable of transducing downstream signal via IR-B in tumor cells with antibody-mediated blockade of IGF-IR.
Figure 7.
Insulin-like growth factor II (IGF-II) mediates resistance to cixutumumab in tumor cells over-expressing IR-B. Clonogenic assay in A549 cells that overexpress IR-B (A) in the presence or absence of cixutumumab and/or anti-IGF-II mAb. Represented are mean values +/− SEM, expressed as percentage of colony formation relative to control treated cells. Statistical significance was determined by one-way ANOVA followed by Tukey’s posthoc analysis. Statistically significant difference vs control treated cells (a), cixutumumab-treated cells (b), anti-IGF-II treated cells (c). (B, C) Western blot analysis of signal transduction in A549-IR-B cells treated with IGF-II (25 nM), in the presence or absence of cixutumumab and linsitinib. Serum-starved cells were pretreated with cixutumumab (100 nM) or linsitinib (0.01, 0.1 or 1 uM) for 15 min and 2 h, respectively, followed by incubation with rhIGF-II (25 nM) for 10 minutes. Proteins (15–20 ug) extracted from cultured cells were size-fractionated by SDS-PAGE and immunoblotted with anti-phospho-IRβY1150/51/IGF-IRβY1135/36, anti-phospho-AktS473 and anti-phospho-p42/p44 MAPKT202/Y204 antibodies. Total level of proteins was demonstrated by immunoblotting with antibodies directed against total Akt and p42/p44 MAPK. (D) MCF-7 and A549 cells with or without IR-A or IRB overexpression were treated with increasing concentrations of linsitinib (0.00015-10 uM) for 72 h, and tumor cell viability was quantified by CellTiter-Glo assay. The results are expressed as % of inhibition of tumor cell viability.
DISCUSSION
IGF-IR mAbs have been tested in multiple clinical trials (www.clinicaltrials.gov). While overall results in intention-to-treat population were generally negative, in some clinical trials subsets of patients seem to benefit from IGF-IR therapy (8–11). Robust molecular markers predictive of the efficacy of this class of agents remain largely unidentified.
The objective of our study was to explore the role of the insulin receptor as a potential negative predictive biomarker for IGF-IR targeted therapy. To our knowledge, this study is the first report demonstrating the adverse impact of both IR isoforms on the efficacy of IGF-IR mAb, cixutumumab.
The role of insulin signaling in cancer biology has recently received a considerable amount of attention. Based on epidemiological studies, obesity and type 2 diabetes (T2D) were found to be associated with increased cancer risk and mortality (29). Compensatory hyperinsulinemia and/or insulin therapy utilized to treat T2D can activate the IR signaling pathway in tumor cells leading to enhanced tumorigenesis (30). Moreover, a number of studies described the role of IR in different tumor types (28). A compensatory mechanism between IGF-IR and closely related IR has been demonstrated: genetic, shRNA-mediated or pharmacological inactivation of IGF-IR can result in IR upregulation (31–34). In line with these observations, we found that baseline IR expression in tumors could negatively affect the efficacy of anti-IGF-IR therapy. In our proof-of-concept experiments, we demonstrated that ectopic IR expression is indeed sufficient to alleviate the effect of cixutumumab in otherwise sensitive cell lines. We also analyzed IR expression levels in a large panel of tumor models and noted a negative correlation between IR and the efficacy of cixutumumab.
In previously published studies, the role of individual IR isoforms as a potential mechanism of intrinsic resistance to IGF-IR targeting had not been dissected, although studies in Ewing’s sarcoma cell lines with almost exclusive expression of IR-A (~100% of total IR) support a role of this isoform in the development of drug resistance (34). The role of IR-B in this process, however, remained poorly understood.
In normal tissue, the two IR isoforms mediate very distinct functions. IR-B, which primarily is expressed in the liver, muscle and fat tissue, regulates metabolic functions while IR-A, highly expressed in various tissues during prenatal life, mediates proliferative and antiapoptotic effects (20). It is currently believed that IR-A rather than IR-B is implicated in tumor growth since it is frequently upregulated in tumor tissue (28) and has a greater affinity for IGF-II, a growth factor also known to be markedly overexpressed in some tumors as result of loss of imprinting (35). However, several lines of evidence also indicate that expression levels of the B isoform in tumor cells are quite significant, representing up to 50% of the total IR pool in breast, lung and colorectal tumors (28). We therefore aimed to understand the roles of the two IR isoforms in the resistance to IGF-IR mAbs. Our results demonstrate that ectopic expression of either IR-A or IR-B in tumor cells resulted in the resistance to cixutumumab in vitro and in vivo, with IR-A inducing a stronger effect compared to IR-B. Expression of either isoform indicated an inverse effect on the antitumor activity of IGF-IR mAb in patient-derived models of lung adenocarcinoma, further supporting the role of the “metabolic” B isoform as an important determinant of cixutumumab efficacy.
Earlier publications reported different ligand specificity for the two IR isoforms. Insulin is known to bind to both IR-A and IR-B. IGF-II binds to IR-A with an affinity similar to insulin and seems to be also capable of binding to IR-B with several fold lower affinity (36, 37). Our in vitro experiments showed that treatment with physiological concentrations of IGF-II induced phosphorylation of IR in both A549 IR-overexpressing cell lines, with downstream PI3K/Akt and MAPK pathway activation. This effect was more prominent in A549-IR-A cells, but was also quite substantial in A549-IR-B cells. Moreover, neutralization of IGF-II in culture media conditioned by A549-IR-B cells was able to restore tumor sensitivity to cixutumumab, suggesting that IR-B-driven resistance to anti-IGF-IR mAb is indeed mediated by IGF-II. Interestingly, A549-IR-B xenografts exhibit sensitivity to cixutumumab initially but became fully resistant to the antibody four weeks after treatment initiation. Given that in mice IGF-II expression levels are negligible after birth (35), it is tempting to speculate that IR-B-mediated resistance to IGF-IR mAb can only be observed if large amounts of IGF-II are produced within IR-B-expressing tumor tissue. Since IGF-IR and IR can form hybrid receptors (1), it is also possible that resistance mediated by both IR isoforms is at least in part caused by IGF-II-mediated signaling via hybrid receptors, as a result of ligand binding to the IR arm. However, since anti-IGF-IR antibodies, including cixutumumab, have been shown to be very proficient at receptor internalization and degradation (38), hybrid receptors are likely effectively neutralized, and therefore not present in abundance on tumor cells following treatment with anti-IGF-IR mAbs. More than any other component of the IGF-IR signaling pathway, IGF-II is frequently deregulated in cancer. It is important to note that mouse may not be the most appropriate species to model tumor resistance to cixutumumab in vivo. In contrast to humans, mice have very low levels of circulating IGF-II. However, in human tumors, IGF-II is suggested to act in an autocrine/paracrine rather than endocrine manner. Loss of IGF2 gene imprinting and IGF-IIR downregulation are two fairly common phenomena that increase the bioavailability of IGF-II in the vicinity of IGF-IR and IR on tumor cells (39). Moreover, IGF2 gene is markedly overexpressed in colorectal tumors, and recent studies reported a substantial prevalence of IGF2 amplification in this tumor type (40).
IGF-II targeting may thus represent an interesting alternative to the anti-IGF-IR blockade, with the advantage of reducing signaling via IR. Of note, some pharmaceutical companies have already initiated the development of therapeutic mAbs against IGFs. BI-836845 and MEDI-573 are fully human mAbs currently in preclinical and clinical development, respectively, which neutralize both IGF-I and IGF-II and are efficacious in experimental tumor models (41, 42). In a phase I trial, MEDI-573 demonstrated acceptable safety profile and tolerability with no significant perturbations of glucose homeostasis. The antitumor activity of MEDI-573 was modest (0% responses, 33% stable disease). These safety and efficacy findings should be interpreted with caution as no changes in circulating growth hormone were observed suggesting that negative feedback regulation of growth hormone by IGFs was not altered during treatment with MEDI-573 (43).
IGF-IR TKIs are known to block both IGF-IR and IR tyrosine kinase activity (44), and IGF-II/IR-mediated tumor growth could be suppressed by this class of agents, as demonstrated by the present study. However, the antitumor efficacy of IGF-IR TKIs may require an optimal exposure and tissue distribution which might be difficult to achieve in patients due to dose limiting toxicities associated with IR inhibition and development of insulin resistance and hyperglycemia in up to 40% of treated patients (44).
Based on the current findings, we argue that discovery of suitable biomarkers to guide patient selection should become a priority to enable successful IGF-IR targeted therapy. Previous work based on expression profiling in sarcoma and neuroblastoma identified potential predictors of intrinsic and acquired resistance to IGF-IR inhibition. Higher levels of IGF-I, IGF-II and IGF-IR were linked to a better response to IGF-IR TKI whereas IGFBP-3 and IGFBP-6 overexpression were observed in resistant models (45). Similar studies in breast and colorectal cancer indicate that IGF-IR expression together with adaptor proteins IRS-1 and IRS-2 or IGF-II expression could be linked to sensitivity to IGF-IR mAb treatment (46, 47). In addition, tumor cells can overcome IGF-IR inhibition via an alternative receptor tyrosine kinase such as EGFR (45, 48). Our work further extends previously disclosed data by demonstrating that that insulin receptor mediates resistance to IGF-IR antibodies used in monotherapy or in combination with cytotoxic agents in a number of various indications including lung adenocarcinoma and breast carcinoma. This study also provides ample evidence that both IR isoforms play an important role in the sensitivity to IGF-IR targeted therapies, sheds a new light on the role of IR-B in tumor biology and provides a scientific rationale for a clinical trial with a preselection of patients based on the IR expression levels of the tumor.
Furthermore, while many pharmaceutical companies discontinued their IGF-IR programs in Oncology, there is growing evidence that IGF-IR pathway is implicated in the development of resistance to novel targeted agents (ALK inhibitors, next generation EGFR TKIs) (49, 50). It is therefore possible that there might be a need to reinvigorate the development of IGF-IR antibodies as more targeted agents will be approved for treatment of human malignancies.
Supplementary Material
Acknowledgements
This work was funded in part by the National Cancer Institute grants [NO1-CM-42216 and CA165995].
We also apologize to those colleagues whose publications were not cited owing to space limitations.
Footnotes
Conflict of Interest:
The following authors are employees of Eli Lilly and Company: A. Forest, M. Amatulli, D.L. Ludwig, C.B. Damoci, Y. Wang, C.A. Burns, G.P. Donoho, M.C. Prewett, D. Surguladze, J.T. DeLigio, R. Novosiadly. No potential conflicts of interest were disclosed by the other authors.
Reference List
- 1.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12:159–169. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- 2.Renehan AG, Zwahlen M, Minder C, O'Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 3.Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, et al. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3:70ra13. doi: 10.1126/scitranslmed.3001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baserga R. The IGF-I receptor in cancer research. Exp Cell Res. 1999;253:1–6. doi: 10.1006/excr.1999.4667. [DOI] [PubMed] [Google Scholar]
- 5.Khandwala HM, McCutcheon IE, Flyvbjerg A, Friend KE. The effects of insulin-like growth factors on tumorigenesis and neoplastic growth. Endocr Rev. 2000;21:215–244. doi: 10.1210/edrv.21.3.0399. [DOI] [PubMed] [Google Scholar]
- 6.Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- 7.Yee D. Insulin-like growth factor receptor inhibitors: baby or the bathwater? J Natl Cancer Inst. 2012;104:975–981. doi: 10.1093/jnci/djs258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naing A, LoRusso P, Fu S, Hong DS, Anderson P, Benjamin RS, et al. Insulin growth factor-receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with refractory Ewing's sarcoma family tumors. Clin Cancer Res. 2012;18:2625–2631. doi: 10.1158/1078-0432.CCR-12-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weigel B, Malempati S, Reid JM, Voss SD, Cho SY, Chen HX, et al. Phase 2 trial of cixutumumab in children, adolescents, and young adults with refractory solid tumors: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2014;61:452–456. doi: 10.1002/pbc.24605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajan A, Carter CA, Berman A, Cao L, Kelly RJ, Thomas A, et al. Cixutumumab for patients with recurrent or refractory advanced thymic epithelial tumours: a multicentre, open-label, phase 2 trial. Lancet Oncol. 2014;15:191–200. doi: 10.1016/S1470-2045(13)70596-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz GK, Tap WD, Qin LX, Livingston MB, Undevia SD, Chmielowski B, et al. Cixutumumab and temsirolimus for patients with bone and soft-tissue sarcoma: a multicentre, open-label, phase 2 trial. Lancet Oncol. 2013;14:371–382. doi: 10.1016/S1470-2045(13)70049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinstein IB. Cancer Addiction to oncogenes--the Achilles heal of cancer. Science. 2002;297:63–64. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- 13.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yonesaka K, Zejnullahu K, Okamoto I, Satoh T, Cappuzzo F, Souglakos J, et al. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci Transl Med. 2011;3:99ra86. doi: 10.1126/scitranslmed.3002442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 16.Harbinski F, Craig VJ, Sanghavi S, Jeffery D, Liu L, Sheppard KA, et al. Rescue screens with secreted proteins reveal compensatory potential of receptor tyrosine kinases in driving cancer growth. Cancer Discov. 2012;2:948–959. doi: 10.1158/2159-8290.CD-12-0237. [DOI] [PubMed] [Google Scholar]
- 17.Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E, et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature. 2012;487:505–509. doi: 10.1038/nature11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Z, Lee JC, Lin L, Olivas V, Au V, LaFramboise T, et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet. 2012;44:852–860. doi: 10.1038/ng.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mosthaf L, Grako K, Dull TJ, Coussens L, Ullrich A, McClain DA. Functionally distinct insulin receptors generated by tissue-specific alternative splicing. EMBO J. 1990;9:2409–2413. doi: 10.1002/j.1460-2075.1990.tb07416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belfiore A, Malaguarnera R. Insulin receptor and cancer. Endocr Relat Cancer. 2011;18:R125–R147. doi: 10.1530/ERC-11-0074. [DOI] [PubMed] [Google Scholar]
- 21.Cui H, Horon IL, Ohlsson R, Hamilton SR, Feinberg AP. Loss of imprinting in normal tissue of colorectal cancer patients with microsatellite instability. Nat Med. 1998;4:1276–1280. doi: 10.1038/3260. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh P, Dahms NM, Kornfeld S. Mannose 6-phosphate receptors: new twists in the tale. Nat Rev Mol Cell Biol. 2003;4:202–212. doi: 10.1038/nrm1050. [DOI] [PubMed] [Google Scholar]
- 23.Scott CD, Firth SM. The role of the M6P/IGF-II receptor in cancer: tumor suppression or garbage disposal? Horm Metab Res. 2004;36:261–271. doi: 10.1055/s-2004-814477. [DOI] [PubMed] [Google Scholar]
- 24.Ulanet DB, Ludwig DL, Kahn CR, Hanahan D. Insulin receptor functionally enhances multistage tumor progression and conveys intrinsic resistance to IGF-1R targeted therapy. Proc Natl Acad Sci U S A. 2010;107:10791–10798. doi: 10.1073/pnas.0914076107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houghton PJ, Morton CL, Gorlick R, Kolb EA, Keir ST, Reynolds CP, et al. Initial testing of a monoclonal antibody (IMC-A12) against IGF-1R by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2010;54:921–926. doi: 10.1002/pbc.22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benyoucef S, Surinya KH, Hadaschik D, Siddle K. Characterization of insulin/IGF hybrid receptors: contributions of the insulin receptor L2 and Fn1 domains and the alternatively spliced exon 11 sequence to ligand binding and receptor activation. Biochem J. 2007;403:603–613. doi: 10.1042/BJ20061709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frasca F, Pandini G, Scalia P, Sciacca L, Mineo R, Costantino A, et al. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol Cell Biol. 1999;19:3278–3288. doi: 10.1128/mcb.19.5.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev. 2009;30:586–623. doi: 10.1210/er.2008-0047. [DOI] [PubMed] [Google Scholar]
- 29.Ferguson RD, Gallagher EJ, Scheinman EJ, Damouni R, LeRoith D. The epidemiology and molecular mechanisms linking obesity, diabetes, and cancer. Vitam Horm. 2013;93:51–98. doi: 10.1016/B978-0-12-416673-8.00010-1. [DOI] [PubMed] [Google Scholar]
- 30.Novosyadlyy R, Lann DE, Vijayakumar A, Rowzee A, Lazzarino DA, Fierz Y, et al. Insulin-mediated acceleration of breast cancer development and progression in a nonobese model of type 2 diabetes. Cancer Res. 2010;70:741–751. doi: 10.1158/0008-5472.CAN-09-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang H, Pelzer AM, Kiang DT, Yee D. Down-regulation of type I insulin-like growth factor receptor increases sensitivity of breast cancer cells to insulin. Cancer Res. 2007;67:391–397. doi: 10.1158/0008-5472.CAN-06-1712. [DOI] [PubMed] [Google Scholar]
- 32.Buck E, Gokhale PC, Koujak S, Brown E, Eyzaguirre A, Tao N, et al. Compensatory insulin receptor (IR) activation on inhibition of insulin-like growth factor-1 receptor (IGF-1R): rationale for cotargeting IGF-1R and IR in cancer. Mol Cancer Ther. 2010;9:2652–2664. doi: 10.1158/1535-7163.MCT-10-0318. [DOI] [PubMed] [Google Scholar]
- 33.Dinchuk JE, Cao C, Huang F, Reeves KA, Wang J, Myers F, et al. Insulin receptor (IR) pathway hyperactivity in IGF-IR null cells and suppression of downstream growth signaling using the dual IGF-IR/IR inhibitor, BMS-754807. Endocrinology. 2010;151:4123–4132. doi: 10.1210/en.2010-0032. [DOI] [PubMed] [Google Scholar]
- 34.Garofalo C, Manara MC, Nicoletti G, Marino MT, Lollini PL, Astolfi A, et al. Efficacy of and resistance to anti-IGF-1R therapies in Ewing's sarcoma is dependent on insulin receptor signaling. Oncogene. 2011;30:2730–2740. doi: 10.1038/onc.2010.640. [DOI] [PubMed] [Google Scholar]
- 35.Bergman D, Halje M, Nordin M, Engstrom W. Insulin-like growth factor 2 in development and disease: a mini-review. Gerontology. 2013;59:240–249. doi: 10.1159/000343995. [DOI] [PubMed] [Google Scholar]
- 36.Denley A, Bonython ER, Booker GW, Cosgrove LJ, Forbes BE, Ward CW, et al. Structural determinants for high-affinity binding of insulin-like growth factor II to insulin receptor (IR)-A, the exon 11 minus isoform of the IR. Mol Endocrinol. 2004;18:2502–2512. doi: 10.1210/me.2004-0183. [DOI] [PubMed] [Google Scholar]
- 37.Benyoucef S, Surinya KH, Hadaschik D, Siddle K. Characterization of insulin/IGF hybrid receptors: contributions of the insulin receptor L2 and Fn1 domains and the alternatively spliced exon 11 sequence to ligand binding and receptor activation. Biochem J. 2007;403:603–613. doi: 10.1042/BJ20061709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burtrum D, Zhu Z, Lu D, Anderson DM, Prewett M, Pereira DS, et al. A fully human monoclonal antibody to the insulin-like growth factor I receptor blocks ligand-dependent signaling and inhibits human tumor growth in vivo. Cancer Res. 2003;63:8912–8921. [PubMed] [Google Scholar]
- 39.Ferguson-Smith AC. Genomic imprinting: the emergence of an epigenetic paradigm. Nat Rev Genet. 2011;12:565–575. doi: 10.1038/nrg3032. [DOI] [PubMed] [Google Scholar]
- 40.Seshagiri S, Stawiski EW, Durinck S, Modrusan Z, Storm EE, Conboy CB, et al. Recurrent R-spondin fusions in colon cancer. Nature. 2012;488:660–664. doi: 10.1038/nature11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao J, Chesebrough JW, Cartlidge SA, Ricketts SA, Incognito L, Veldman-Jones M, et al. Dual IGF-I/II-neutralizing antibody MEDI-573 potently inhibits IGF signaling and tumor growth. Cancer Res. 2011;71:1029–1040. doi: 10.1158/0008-5472.CAN-10-2274. [DOI] [PubMed] [Google Scholar]
- 42.Friedbichler K, Hofmann MH, Kroez M, Ostermann E, Lamche HR, Koessl C, et al. Pharmacodynamic and antineoplastic activity of BI 836845, a fully human IGF ligand neutralizing antibody, and mechanistic rationale for combination with rapamycin. Mol Cancer Ther. 2013 doi: 10.1158/1535-7163.MCT-13-0598. [DOI] [PubMed] [Google Scholar]
- 43.Haluska P, Menefee M, Plimack ER, Rosenberg J, Northfelt D, LaVallee T, et al. Phase I dose-escalation study of MEDI-573, a bispecific, antiligand monoclonal antibody against IGFI and IGFII, in patients with advanced solid tumors. Clin Cancer Res. 2014;20:4747–4757. doi: 10.1158/1078-0432.CCR-14-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puzanov I, Lindsay CR, Goff LW, Sosman JA, Gilbert J, Berlin J, et al. A Phase I Study of Continuous Oral Dosing of OSI-906, a Dual Inhibitor of Insulin-Like Growth Factor-1 and Insulin Receptors in Patients with Advanced Solid Tumors. Clin Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-14-0303. [DOI] [PubMed] [Google Scholar]
- 45.Huang F, Greer A, Hurlburt W, Han X, Hafezi R, Wittenberg GM, et al. The mechanisms of differential sensitivity to an insulin-like growth factor-1 receptor inhibitor (BMS-536924) and rationale for combining with EGFR/HER2 inhibitors. Cancer Res. 2009;69:161–170. doi: 10.1158/0008-5472.CAN-08-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mukohara T, Shimada H, Ogasawara N, Wanikawa R, Shimomura M, Nakatsura T, et al. Sensitivity of breast cancer cell lines to the novel insulin-like growth factor-1 receptor (IGF-1R) inhibitor NVP-AEW541 is dependent on the level of IRS-1 expression. Cancer Lett. 2009;282:14–24. doi: 10.1016/j.canlet.2009.02.056. [DOI] [PubMed] [Google Scholar]
- 47.Zha J, O'Brien C, Savage H, Huw LY, Zhong F, Berry L, et al. Molecular predictors of response to a humanized anti-insulin-like growth factor-I receptor monoclonal antibody in breast and colorectal cancer. Mol Cancer Ther. 2009;8:2110–2121. doi: 10.1158/1535-7163.MCT-09-0381. [DOI] [PubMed] [Google Scholar]
- 48.Haluska P, Carboni JM, TenEyck C, Attar RM, Hou X, Yu C, et al. HER receptor signaling confers resistance to the insulin-like growth factor-I receptor inhibitor, BMS-536924. Mol Cancer Ther. 2008;7:2589–2598. doi: 10.1158/1535-7163.MCT-08-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lovly CM, McDonald NT, Chen H, Ortiz-Cuaran S, Heukamp LC, Yan Y, et al. Rationale for co-targeting IGF-1R and ALK in ALK fusion-positive lung cancer. Nat Med. 2014;20:1027–1034. doi: 10.1038/nm.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cortot AB, Repellin CE, Shimamura T, Capelletti M, Zejnullahu K, Ercan D, et al. Resistance to irreversible EGF receptor tyrosine kinase inhibitors through a multistep mechanism involving the IGF1R pathway. Cancer Res. 2013;73:834–843. doi: 10.1158/0008-5472.CAN-12-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.