Abstract
The bioactive sphingolipid sphingosine-1-phosphate (S1P) drives several hallmark processes of cancer, making the enzymes that synthesize S1P, i.e. sphingosine kinase 1 and 2 (SK1 and SK2), important molecular targets for cancer drug development. ABC294640 is a first-in-class SK2 small-molecule inhibitor that effectively inhibits cancer cell growth in vitro and in vivo. Given that AR and Myc are two of the most widely implicated oncogenes in prostate cancer (PCa), and that sphingolipids impact signaling by both proteins, the therapeutic potential for using ABC294640 in the treatment of PCa was evaluated. This study demonstrates that ABC294640 abrogates signaling pathways requisite for PCa growth and proliferation. Key findings validate that ABC294640 treatment of early stage and advanced PCa models downregulate Myc and AR expression and activity. This corresponds with significant inhibition of growth, proliferation, and cell cycle progression. Finally, oral administration of ABC294640 was found to dramatically impede xenograft tumor growth. Together, these pre-clinical findings support the hypotheses that SK2 activity is required for PCa function and that ABC294640 represents a new pharmacological agent for treatment of early stage and aggressive PCa.
Keywords: Androgen receptor, c-Myc, sphingosine kinase 2, castrate-resistant prostate cancer, ABC294640
Introduction
Prostate cancer (PCa) is the most commonly diagnosed non-cutaneous malignancy and the second leading cause of cancer-related deaths in US males. Patients presenting with locally confined disease have a favorable survival outlook due to surgical intervention, while patients with disseminated PCa are typically treated with androgen deprivation therapy (ADT) that is transiently effective. Recent FDA-approved therapies for advanced PCa, such as Enzalutamide, Abiraterone Acetate, Sipuleucel-T, and Radium-223 [1], have extended the therapeutic window; however, resistance still occurs and these treatments typically only provide a modest survival advantage. Therefore, novel therapeutic targets, increased therapeutic penetrance and improved overall durability of response are paramount for new treatment options.
Two prominent oncogenes driving PCa initiation, progression and resistance are androgen receptor (AR) and c-Myc (Myc). All stages of PCa are dependent on AR activity, and as such, most treatments focus on targeting this pathway. For example, ADT or direct AR antagonists are often administered for disseminated disease, which affords patients with 24–36 months of disease control. However, recurrent PCa (termed castrate-resistant (CRPC)) often results due to aberrant reactivation of AR. Myc amplification occurs frequently (~30%) in PCa and correlates with advanced disease, biochemical recurrence, and poor prognosis [2–5]. Notably, animal models of Myc-mediated PCa require functional AR for tumorigenesis [6], indicating that both AR and Myc oncogenes are essential for PCa progression. Therefore, the ability to simultaneously suppress the activities of Myc and AR could profoundly inhibit tumor cell growth and dramatically impact clinical options.
Bioactive sphingolipids are dynamic signal transducers that regulate numerous cellular processes, including host inflammation, angiogenesis, cell migration, and regulation of tumor growth [7]. As such, manipulating the ceramide/sphingosine-1-phosphate (S1P) rheostat within cancer cells is of pharmacological interest. Sphingosine kinases 1 and 2 (SK1 and SK2, respectively) catalyze phosphorylation of sphingosine to generate mitogenic S1P and are frequently overexpressed in a variety of human cancers, including Pca [8, 9]. SK1 is a cytosolic protein that translocates to the cell membrane upon activation and is necessary for tumor progression [10]. While SK1 and SK2 shares kinase homology and are 80% similar, overall, SK2 contains distinct nuclear localization and export signals, which differentiate cellular localization and biological functions from SK1 [11, 12]. SK2 also contains a pro-apoptotic BH3 domain that promotes apoptosis when overexpressed [13]; however, depletion of SK2 inhibits tumor cell proliferation and migration [14], and xenograft tumor growth [15]. More recently, isozyme-specific inhibitors have elucidated the non-redundant role of SK2 in tumor biology. In particular, ABC294640 is a first in class SK2-specific inhibitor that inactivates AKT and ERK, decreases Myc expression, elicits antiestrogenic effects on estrogen receptor-α, and promotes autophagy [16–20]. The ability of ABC294640 to inhibit AR-positive PCa proliferation and signaling pathways has not been previously investigated.
Based on these studies and the unresolved need to foster durable therapeutic options for advanced PCa, it was hypothesized that inhibiting SK2 activity with ABC294640 is a viable option for treating PCa by inhibiting several pathways implicit for disease progression. Findings described herein demonstrate that ABC294640 abrogates PCa tumor maintenance by disrupting signaling events regulated in consort by Myc and AR. Key findings demonstrate that ABC294640 treatment of ADT-sensitive cells significantly inhibits growth, proliferation and cell-cycle progression. Biochemical analyses reveal down-regulation of both Myc and AR expression, and subsequent loss of transcriptional activity. Similarly, in two models of CRPC, ABC294640 treatment down-regulates Myc and AR expression and activity, which corresponds to attenuation of cell growth and proliferation. Finally, SK2 activity is found to be requisite for ADT-sensitive cell growth in vivo, as oral administration of ABC294640 dramatically impedes tumor growth. Overall, these studies identify the SK2-specific inhibitor, ABC294640, as a potent dual modifier of oncogenic Myc and AR signaling pathways and novel therapy for PCa.
Material and Methods
Cell culture and treatments
LNCaP and C4-2 cells were maintained in improved minimum essential media (IMEM) supplemented with 5% FBS (heat-inactivated fetal bovine serum) and supplemented with 2 mmol/L of L-glutamine. 22Rv1 cells were maintained in Dulbecco’s modified Eagle’s media (DMEM) supplemented with 10% FBS. All media were supplemented with 100 units/mL penicillin-streptomycin. For hormone-deficient conditions, phenol red-free media was supplemented with charcoal dextran-treated serum (CDT). ABC294640 was synthesized as described previously [21]. Dihydrotestosterone (DHT) was used at 1 nM in ethanol for 16 hours. Okadaic acid (Santa Cruz) was treated at 10 nM for 24 hours.
Gene expression analysis
Cells were seeded at equal density in steroid-proficient (FBS) or -depleted (CDT-treated FBS) conditions and were treated as specified; RNA was isolated using TRIzol (Life Technologies) and cDNA generated using SuperScript III (Life Technologies). Quantitative PCR was conducted with primers described previously [22] and with an ABI StepOne machine and Power SYBR (Life Technologies) in accordance with the manufacturer.
Cell growth assays
To determine cell growth over time, cells were seeded at equal densities and treated with ABC294640, as indicated. Cell number was determined using trypan blue exclusion and a hemacytometer. Media and treatments were refreshed every 72 hours. To determine IC50 values, cells were seeded in 96-well plates and 24 hours later treated with 0 to 100 μM ABC29460 for 72 hours. Cell viability was determined by a standard sulforhodamine B assay. To assess clonogenic survival, cells were plated at low density and after 14 days cells were fixed in ice-cold 100% ethanol and then stained with crystal violet. Colonies with >50 cells were scored and set relative to the calculated plating efficiency for each treatment condition.
Flow cytometry
Cells were treated and seeded in hormone proficient or depleted conditions, as indicated, and labeled with BrdU (Invitrogen) 2 hours prior to harvest. Cells were fixed in 100% ethanol, stained with FITC-conjugated anti-BrdU antibody (BD Biosciences) and processed using FACS Calibur (BD Biosciences).
Western blot analysis and proteasome degradation
Cells were lysed in buffer containing 25 mM Tris-HCl, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS (G Biosciences). Total cell lysates were resolved by 10% or 4–12% gradient SDS-PAGE, transferred to PVDF and immunoblotted with the following antibodies: AR-N20, Myc, pAKT S473, total AKT (Cell Signal), and HRP-GAPDH (Genetex). For analyzing proteasomal contribution, cells were treated with 10 μM MG132 (Enzo) for 24 hours.
In vivo analysis
All procedures involving mice were performed in accordance with Pennsylvania State University IACUC protocols. 7 week old male NCR-nude mice (Charles Rivers) were subcutaneously injected in the flank with 3×106 cells in 100 μl total saline/Matrigel (BD Biosciences). When tumors reached ~100–150 mm3 mice were administered 50mg/kg ABC294640 or Vehicle (46% PEG400/47% Saline/7% EtOH) daily. Tumor volume was measured with calipers.
Statistical Analysis
All results were analyzed using the 2-tailed Student’s t test (adjusted for variance) or Mann-Whitney test. For all analyses, P < 0.05 was deemed significant.
Results
ABC294640 decreases cell survival of androgen deprivation therapy-sensitive prostate cancer cells
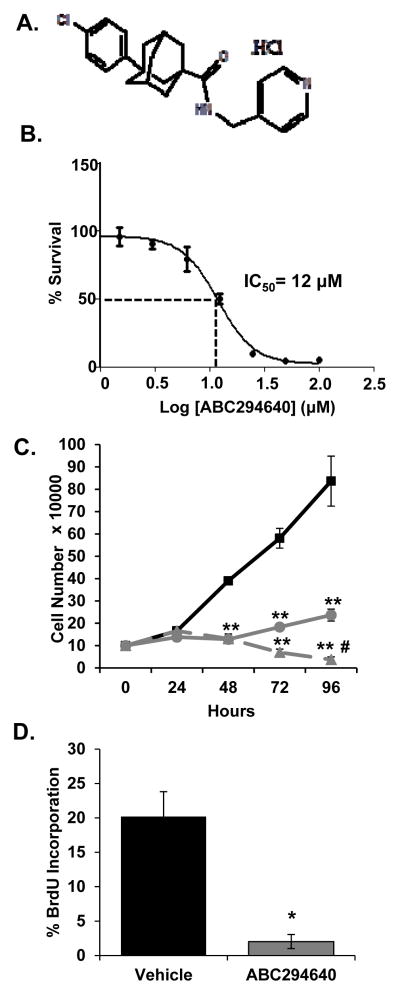
ABC294640 (Fig. 1A) is a first in class, sphingosine kinase 2 inhibitor that selectively inhibits SK2 enzymatic activity but has no effect on SK1 [23]. Previous studies demonstrated the ability of ABC294640 to inhibit cell proliferation of various tumor cell types, including androgen receptor (AR)-negative DU145 and PC3 PCa cells [23, 24]. To determine the effects of ABC294640 on the proliferation of AR-positive, ADT-sensitive LNCaP cells, sulforhodamine B (SRB) and trypan blue exclusion assays were employed. ABC294640 inhibited cell proliferation with an IC50 of 12 μM (Fig. 1B). Based on the IC50 value, cells were treated with 10 μM and 20 μM ABC294640 to further examine cell growth. Treatments resulted in significantly decreased cell numbers, compared to vehicle treatment, in a dose-dependent manner (Fig. 1C). Furthermore, ABC294640 treatment of LNCaP cells decreased the rate of cell cycle progression, as evident through a 90% decrease in BrdU incorporation, relative to control (Fig. 1D). Therefore, based on cell viability, cell proliferation and cell cycle progression analyses, SK2 activity is required for ADT-sensitive PCa cell proliferation.
Figure 1. ABC294640 decreases LNCaP cell growth in a dose-dependent manner.
A, Chemical structure of ABC294640 (3-(4-chlorophenyl)-adamantane-1-carboxylic acid (pyridin-4-ylmethyl)amide), hydrochloride salt. B, LNCaP cells were treated with the indicated concentration of ABC294640 for 72 hours and cell survival was quantified by the SRB assay. C, LNCaP cells were cultured in hormone-proficient media and treated with 10 μM (●), 20 μM (▲) ABC294640 or vehicle (■) for 96 hours. Cell growth was determined by trypan blue exclusion cell counting. D, LNCaP cells cultured as in C were treated with 20 μM ABC294640 for 72 hours and analyzed for BrdU incorporation. * p < 0.05 and **p < 0.01 relative to vehicle; #p < 0.01 relative to 10 μM.
SK2 inhibition abrogates multiple proliferative pathways in prostate cancer cells
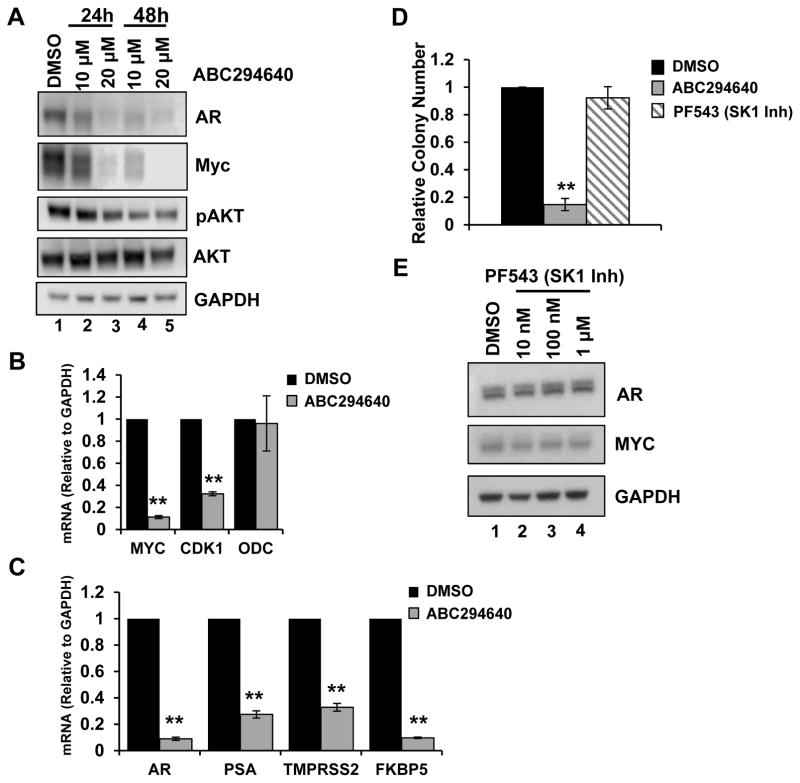
Sphingosine-1-phosphate activates multiple mitogenic signaling cascades that drive tumor growth [7]. For example, ABC294640 attenuates S1P-mediated AKT activation, which is one mechanism for cell growth suppression [17]. To understand whether additional mechanisms exist by which ABC294640 inhibits early-stage AR-positive PCa cell growth, further biochemical analyses were performed on LNCaP cells. Consistent with previous reports, ABC294640 decreased AKT phosphorylation in a time- and dose-dependent manner (Fig. 2A), suggesting that ABC294640 inhibits known S1P-mediated pathways in PCa. Myc expression is often deregulated in PCa progression and promotes aggressive phenotypes and resistance to therapies [4, 25, 26]. Previous reports demonstrate that ABC294640 downregulates Myc expression in hematologic cancers [18, 19]; however, this has not been previously assessed in epithelial tumor cells. Therefore, Myc expression was analyzed in LNCaP cells following ABC294640 treatment (10 μM and 20 μM for 24 and 48 hours). Similar to results in hematologic cancers, Myc protein levels were significantly decreased at doses that inhibit cell growth (Fig. 2A). To determine if Myc transcriptional activity was affected by ABC294640 treatment, levels of Myc transcript and the expression of Myc target genes CDK1 and ornithine decarboxylase (ODC) were examined by qRT-PCR. Myc mRNA and CDK1 expression were significantly decreased in response to ABC294640 treatment, although ODC was not altered in response to the drug, demonstrating a degree of selectivity for Myc perturbation (Fig. 2B). Therefore, based on these data, ABC294640 treatment of ADT-sensitive PCa inhibits AKT and Myc activity in PCa.
Figure 2. ABC294640 abrogates multiple proliferative pathways in PCa cells.
A, LNCaP cells were cultured in hormone-proficient media and treated with 10 μM, 20 μM ABC294640 or vehicle for 24 or 48 hours, as indicated. Cell lysates were immunoblotted with Myc, AR, pAKT, AKT and GAPDH antibodies. B, LNCaP cells were cultured in androgen-depleted media and mRNA transcript levels of Myc, CDK1, and ODC were analyzed by qRT-PCR. C, LNCaP cells were treated as in B and mRNA transcripts of AR, PSA, TMPRSS2, and FKBP5 were analyzed by qRT-PCR. D, LNCaP cells were plated at low density and treated with 10 μM ABC294640, 100 nM PF543 or vehicle for 2 weeks. Cell colonies with fixed, stained with crystal violet and colonies with 50 or greater cells were counted. **p < 0.01 compared to DMSO and PF543 conditions. E, LNCaP cells were treated with 10 nM PF543, 100 nM PF543, 1 μM PF543 or vehicle for 72 hours. Cell lysates were immunoblotted with AR, Myc, and GAPDH antibodies. **p < 0.01.
AR-positive, ADT-sensitive PCa cells represent early stage disease as androgen signaling is required for cell growth and proliferation [27]. Since SKI-II, a dual SK1/SK2 inhibitor, was found to modulate AR expression [28], it was hypothesized that ABC294640-mediated SK2 inhibition could disrupt AR signaling and contribute to growth suppression. To address this, AR protein levels in LNCaP cells treated with ABC294640 were examined and found to be decreased by 24 hours and remained depleted throughout ABC294640 treatment (Fig. 2A). Next, AR transcriptional activity was measured by qRT-PCR for three clinically-relevant AR target genes, PSA/KLK3, TMPRSS2, and FKBP5, and found to be decreased by 73%, 67%, and 90%, respectively, relative to control. Surprisingly, AR transcript was also decreased following ABC294640 treatment (Fig. 2C). These results demonstrate that in consort with AKT and Myc deregulation, ABC294640 treatment also disrupts AR activity, which is indispensable for early stage PCa.
To determine if the ABC294640-mediated decreases in AR and Myc expression are specifically due to inhibition of SK2 activity, LNCaP cells were depleted of SK2 by siRNA and AR and Myc levels were evaluated. siRNA-mediated SK2 depletion reduced mRNA expression 60%, which corresponded with a loss of Myc protein but not mRNA expression (Figure S1). These results are consistent with previous validations of ABC294640-mediated Myc depletion with siRNA approaches that demonstrate SK2 siRNA decreases Myc protein but not mRNA [18, 19]. Surprisingly, siRNA-mediated SK2 depletion did not alter AR mRNA or protein expression (Figure S1). This suggests that ABC294640 could be directly impacting AR activity or that Myc mRNA depletion following treatment is required for AR inhibition.
PF543 potently inhibits SK1 activity (Ki = 6 nM), blocks S1P production, but, interestingly, has no effect on cancer cell proliferation [29]. Therefore, the ability of PF543 to influence cell growth and/or AR and Myc was analyzed. Based on colony formation assay, 10 μM ABC294640 significantly decreased clonogenic cell growth, while 100 nM PF543 treatment resulted in no change in cell survival, compared to vehicle (Fig. 2D). Additionally, treatment of cells with PF543 did not decrease expression of Myc or AR protein levels, relative to control (Fig. 2E). Therefore, these data indicate that ABC294640 selectively alters cell proliferation and expression of integral PCa oncogenes, which is not observed by SK1 modulation.
ABC294640 inhibits CRPC signaling and growth
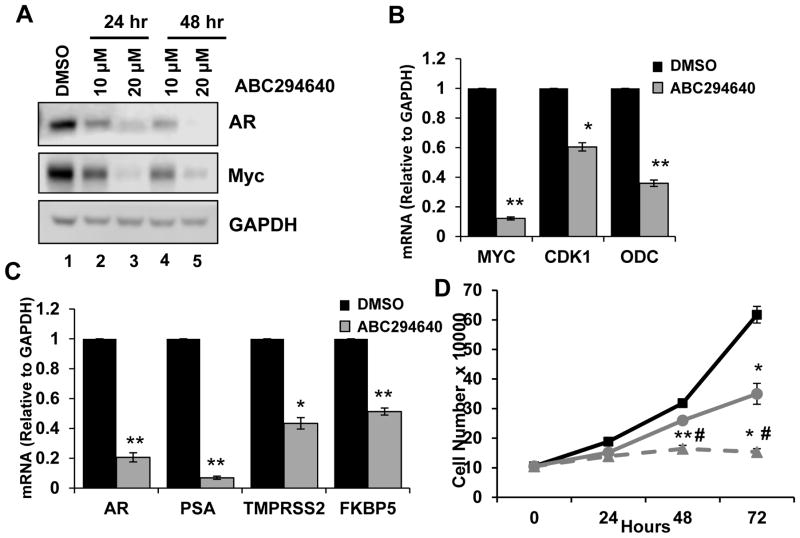
Advanced PCa is initially managed by blocking the AR signaling axis, which typically progresses to incurable CRPC due to aberrant AR reactivation. Therefore, CRPC cell models were employed to determine if ABC294640 treatment impacts Myc and AR signaling within this incurable disease state. To begin, CPRC C4-2 cells, which are metastatic derivatives of LNCaP cells, were treated with ABC294640 and protein levels of Myc and AR were analyzed. Consistent with LNCaP cells, ABC294640 treatment of C4-2 cells decreased Myc protein expression at all doses and time points (Fig. 3A). Next, the consequence of ABC294640 treatment on Myc transcriptional output was determined by qPCR. Myc gene expression in C4-2 cells was decreased 80%, relative to control, and expression of Myc target genes CDK1 and ODC were significantly decreased 40% and 65%, respectively, relative to control (Fig. 3B). Therefore, consistent with LNCaP cells, ABC294640 appreciably disrupts Myc signaling in a disease model where Myc promotes aggressiveness.
Figure 3. ABC294640 negatively regulates CRPC signaling and proliferation.
A, C4-2 cells were cultured in hormone-proficient media and treated with 10 μM, 20 μM ABC294640 or vehicle for 24 and 48 hours. Cell lysates were immunoblotted with AR, Myc, and GAPDH. B, C4-2 cells were cultured in hormone-depleted media and treated with 20 μM ABC294640 or vehicle for 72 hours and mRNA transcripts levels of Myc, CDK1, and ODC were analyzed by qRT-PCR. C, C42 cells were cultured as in B and mRNA transcripts levels of AR, PSA, TMPRSS2, FKBP5 were analyzed by qRT-PCR. D, C4-2 cells were cultured in hormone-proficient media and treated with 10 μM (●), 20 μM (▲) ABC294640 or vehicle (■) for 72 hours. Cell growth was determined by trypan blue exclusion cell counting. *p < 0.05, **p < 0.01.
CRPC cells, despite survival in the absence of androgens, still require AR signaling for survival [30]. Therefore, the ability to negatively regulate AR in CRPC through ABC294640 treatment would directly address an important clinical need. Similar to the results in LNCaP cells, AR protein was depleted in C4-2 cells following ABC294640 treatment in a dose- and time-dependent manner (Fig. 3A). Furthermore, AR mRNA levels were decreased 80%, compared to control, following ABC294640 treatment, and AR transcriptional output was negatively regulated based on 94%, 57%, and 49% decreases in the levels of PSA/KLK3, TMPRSS2 and FKBP5, respectively (Fig. 3C). Lastly, cell proliferation was measured to determine the biological consequence of ABC294640-mediated Myc and AR deregulation. Cell proliferation was significantly inhibited by ABC294640 treatment in a dose-dependent fashion, which was consistent with decreased AR/Myc expression (Fig. 3D; compare circle and triangle to square). These data illustrate the potential efficacy and utility of ABC294640 as a targeted therapy for aggressive PCa by modulating AR and Myc signaling, both of which are implicated in disease progression.
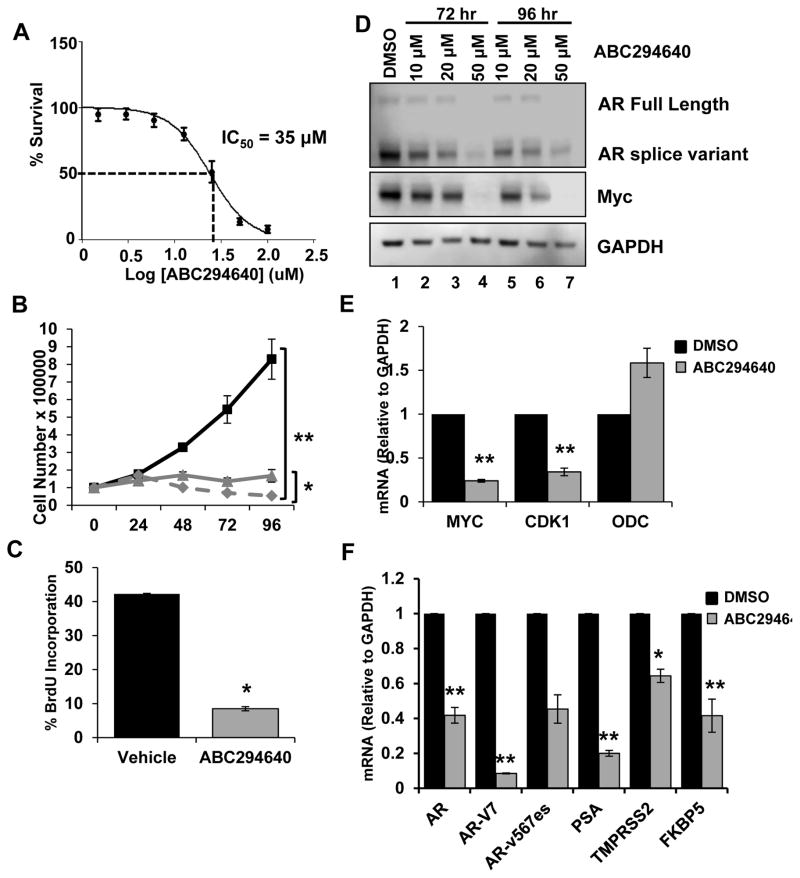
Numerous mechanisms contribute to AR dysregulation in CRPC, including AR mutations, cofactor perturbation, and intracrine androgen production [30]. Additionally, AR splice variants (AR-SVs), which lack the ligand-binding domain and are constitutively active, drive castration-resistance, correlate with decreased survival and resistance to therapy, and are clinically detectable [31–33]. To determine if ABC294640 can abrogate signaling within a model expressing AR-SVs, 22Rv1 cells were employed. The IC50 for ABC294640-induced cytotoxicity was determined to be 35 μM, which is higher than that of LNCaP cells and suggests that AR-SV expression may promote resistance to ABC294640 (Fig. 4A). Despite decreased sensitivity to drug treatment, ABC294640 doses of 20 μM and 40 μM were sufficient to significantly inhibit cell proliferation (Fig. 4B). ABC294640 treatment also significantly inhibited cell cycle progression, based on reduced BrdU incorporation, relative to control (Fig. 4C). Therefore, similar to the results described for early stage PCa cells, ABC294640 treatment is sufficient to attenuate CRPC biological output. Next, the biochemical consequences of ABC294640 on AR-SV containing cells were assessed. ABC294640 promoted downregulation of Myc, full-length AR, and AR-SVs, however, a higher dose and longer treatment was required compared with the other PCa cell lines (Fig. 4D, compare lane 1 with lanes 2,3 and 5,6). Depletion of both AR species is significant given reports that AR-SVs require full-length AR to function [34]. Finally, to investigate the impact of ABC294640 on transcriptional activity of Myc and AR, qPCR was performed on 22Rv1 cells treated with ABC294640 in the castrate setting. Myc gene expression was significantly decreased, which coincided with decreased expression of CDK1, but not ODC (Fig. 4E). Likewise, AR gene expression was decreased by 60% following ABC294640 treatment, and two well characterized AR-SVs, AR-V7 and AR-v567es, were down-regulated 92% and 55%, respectively, relative to control (Fig. 4F). The loss of AR protein and mRNA expression resulted in significant inhibition of transcriptional output as demonstrated by significantly decreased mRNA levels of PSA, TMPRSS2, and FKBP5 (Fig. 4F). Based on these data, ABC294640 significantly inhibits CRPC cell growth, oncogene expression and activity, and abrogates constitutively-active mitogenic signaling, and as such, represents a potential therapy for CRPC.
Figure 4. ABC294640 inhibits AR-SV CRPC cell signaling and growth.
A, 22Rv1 cells were treated with the indicated concentrations of ABC294640 for 72 hours, and cell survival was quantified by the SRB assay. B, 22Rv1 cells were cultured in hormone-proficient media and treated with 20 μM (▲), 40μM ABC294640 (◆) or vehicle (■) over 96 hours. Cell growth was determined by trypan blue exclusion. C, 22Rv1 cells cultured as in B were treated with 40 μM ABC294640 for 72 hours and analyzed for BrdU incorporation. D, 22Rv1 cells were cultured in hormone-proficient media and treated with 10 μM, 20 μM, 50 μM ABC294640 or vehicle for 72 or 96 hours. Cell lysates were immunoblotted with AR (detecting full length and splice variant), Myc, and GAPDH. E, 22Rv1 cells were cultured in hormone-depleted media and treated with 30 μM ABC294640 for 72 hours. mRNA transcripts levels of Myc, CDK1 and ODC were analyzed by qRT-PCR. F, 22Rv1 cells were cultured as in E and mRNA transcript levels of AR, AR-V7, AR-v567es, PSA, TMPRSS2, FKBP5 were analyzed by qRT-PCR. *p < 0.05, **p < 0.01.
Proteasomal degradation plays a role in AR/Myc protein depletion
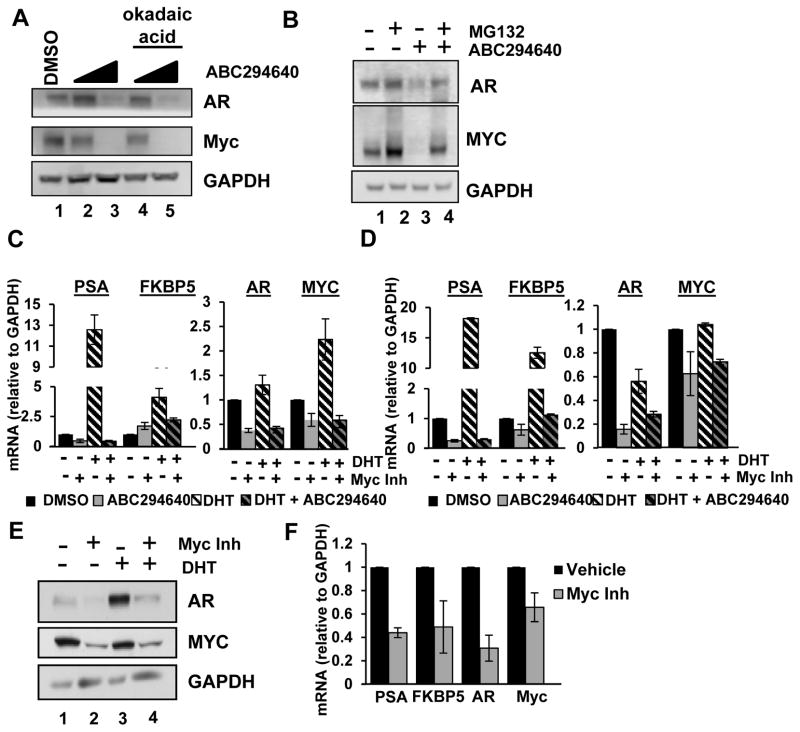
These studies demonstrate that ABC294640 decreases Myc and AR expression and signaling in multiple PCa models. This is consistent with a report that SK2 regulates Myc mRNA and protein by altering HDAC activity [19]. Alternatively, effects of ABC294640 may be mediated by ceramide accumulation following SK2 inhibition since ceramide is reported to activate protein phosphatase 2A (PP2A), resulting in decreased Myc expression, which is reversible by PP2A inhibitors [35]. To determine if ABC294640 decreases Myc through the ceramide-PP2A pathway, LNCaP cells were treated with vehicle, ABC294640 alone, or ABC294640 in combination with PP2A inhibitor, okadaic acid. ABC294640 treatment resulted in decreased Myc and AR protein expression (Fig. 5A, compare lane 1 with 2 and 3); however, okadaic acid treatment did not alter the effects of ABC294640 (lanes 4 and 5). This indicates that Myc depletion is not due to PP2A activation by ceramide accumulation. Next, the role of the proteasome was explored because Myc and AR are degraded by the ubiquitin-proteasome pathway and proteasome inhibitors can in some cases rescue SK inhibitor-induced Myc depletion [18, 28]. Treatment of LNCaP cells with the proteasome inhibitor, MG132, alone increased the steady state levels of AR and Myc (Fig. 5B, compare lanes 1 and 2); whereas, ABC294640 decreased AR and Myc protein levels (compare lanes 1 and 3). Importantly, MG132 rescued ABC294640-mediated depletion of both AR and Myc (compare lanes 3 and 4). Combined, these data indicate that ABC294640-mediated down-regulation of Myc and AR occurs at both the gene expression level and the protein level, which is mediated, at least in part, through the proteasome.
Figure 5. Myc inhibition downregulates AR activity and expression.
A, LNCaP cells were treated with 10 μM or 20 μM ABC294640 or vehicle for 48 hours with or without 10 nM okadaic acid for 24 hours. Cell lysates were immunoblotted with AR, Myc, and GAPDH antibodies. B, LNCaP cells were cultured in hormone-proficient media and treated with 10 μM MG132, 20 μM ABC294640, MG132 plus ABC294640 combined or vehicle for 24 hours. Cell lysates were immunoblotted with AR, Myc and GAPDH antibodies. C, LNCaP cells were cultured in androgen-deprived media and stimulated with 1 nM DHT or vehicle for 16 hours and/or 50 μM Myc inhibitor (10058-F4) for 20 hours. mRNA transcript levels of AR target genes PSA and FKBP5 (left) or AR and Myc (right) were analyzed by qRT-PCR. D, C4-2 cells were treated as in A and mRNA transcript levels of PSA, FKBP5, AR and Myc were analyzed by qRT-PCR. E, C4-2 cells were treated as in A and cell lysates were immunoblotted with AR, Myc and GAPDH antibodies. F, 22Rv1 cells were cultured in androgen-deprived media and treated with 50 μM Myc inhibitor (10058-F4) or Vehicle for 20 hours. mRNA transcript levels of PSA, FKBP5, AR and Myc were analyzed by qRT-PCR. All experiments were performed at least twice.
Myc inhibitor recapitulates ABC294640 treatment
Disrupting Myc/Max binding with small molecule inhibitors, such as 10058-F4, effectively decreases Myc expression and transcriptional activity, thereby promoting apoptosis and growth inhibition, and enhancing chemosensitivity [36–38]. To determine if 10058-F4-mediated Myc inhibition impinges on AR signaling, thus phenocopying ABC294640 treatment and representing a mechanism for Myc and AR inactivation, cells were cultured in androgen-deprived media and stimulated with dihydrotestosterone (DHT) and/or 10058-F4. As shown, DHT stimulation increased PSA, FKBP5 and Myc transcripts, and 10058-F4 decreased ligand-independent PSA expression and DHT-induced PSA and FKBP5 (Fig 5C). Myc mRNA expression was decreased by 10058-F4, which coincided with reduced AR mRNA. Similar results were observed in C4-2 cells, where 10058-F4 inhibited ligand independent and DHT-stimulated AR target gene expression (Fig. 5D, left), and decreased AR and Myc transcript (5D, right). Western blot analyses of C4-2 cells following 10058-F4 treatment indicated that Myc expression was decreased in the absence or presence of DHT (Fig. 5E, compare lane 1 to 2 and 3 to 4). Interestingly, AR expression, which is stabilized through DHT-mediated receptor dimerization [39], was decreased by 10058-F4 in both androgen-depleted and DHT-stimulated conditions (compare lanes 1 to 2 and 3 to 4). Finally, the effect of Myc inhibition on AR activity in AR-SV containing cells was determined. Myc inhibitor treatment decreased AR activity, AR transcript levels, and Myc transcript (Fig. 5F). Overall, similar to ABC294640, Myc inhibition decreases Myc and AR transcript levels, and illustrates the differences following transient SK2 depletion. Based on these results, modulation of AR signaling by ABC294640 is mimicked by direct inhibition of Myc and is it feasible to hypothesize that ABC294640 modulates AR signaling through a Myc-mediated mechanism.
Decreased in vivo tumor growth
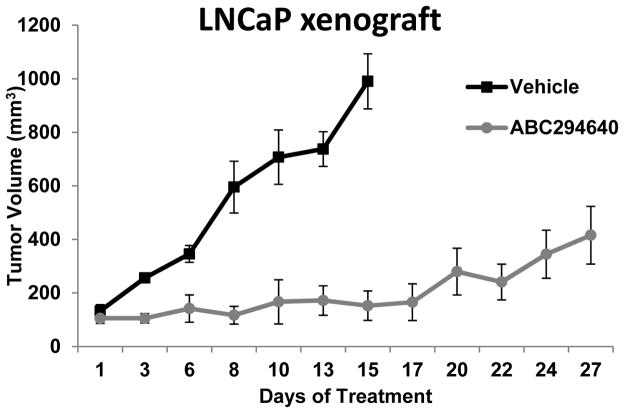
Because ABC294640 was determined to significantly decrease key mediators of prostate cancer progression and diminish cellular proliferation in vitro, the ability of ABC294640 to attenuate in vivo PCa tumor growth was evaluated. LNCaP tumor cells were subcutaneously implanted into male nude mice and tumors were randomized into Vehicle (46% PEG400/47% Saline/7% EtOH) or ABC294640 treatment groups upon tumor measurement of ~100mm3. Animals received 50 mg/kg ABC294640 daily by oral gavage (or equivalent vehicle volume) and tumors were measured by calipers 3 times per week. While tumors of vehicle-treated animals progressively grew, requiring sacrifice of the animals after 15 days, ABC294640 treatment resulted in pronounced and sustained suppression of tumor growth (Fig. 6). Overall, these findings support the compelling hypothesis that inhibition of SK2 by the bioavailable inhibitor ABC294640 would effectively impede PCa tumor growth by simultaneously inhibiting two key oncogenic signaling pathways required for disease progression.
Figure 6. ABC294640 inhibits tumor growth in vivo.
Nude mice were injected with 3 × 106 LNCaP cell in flank. When tumors reached 100-150 mm3, mice were randomized into vehicle (46% PEG400 / 47% Saline / 7% EtOH, (■) or 50 mg/kg/day ABC294640 (●) oral gavage treatment groups.
Discussion
The bioactive sphingolipid S1P drives several hallmark processes of cancer, making the enzymes that synthesize S1P, i.e. SK1 and SK2, important new targets for cancer drug development. ABC294640 is a first-in-class SK2 inhibitor that effectively inhibits cancer cell growth in vitro and in vivo, and has recently completed phase 1 clinical testing in patients with advanced solid tumors (NCT01488513). Previous studies highlight the ability of ABC294640 to modulate sphingolipid metabolism and downregulate mitogenic AKT and MAPK pathways. This study presents novel data that ABC294640 abrogates several additional signaling pathways fundamental to PCa growth and proliferation. Key findings demonstrate that ABC294640 treatment of ADT-sensitive PCa cells significantly inhibits growth, proliferation, and cell-cycle progression, which is accompanied by inactivation of the AKT, AR, and Myc signaling pathways. In particular, Myc and AR expression levels and subsequent transcriptional activity are both significantly decreased following ABC294640 treatment. Similarly, within two models of CRPC, ABC294640 treatment is sufficient to downregulate Myc and AR expression and activity, including constitutively active AR splice variants, which corresponds with significant attenuation of cell growth and proliferation. Finally, oral administration of ABC294640 was found to dramatically impede xenograft tumor growth. Together, these results support the hypotheses that SK2 activity is required for PCa function and that ABC294640 represents a new pharmacological agent for treatment of early stage and castrate-resistant PCa.
Multiple mechanisms to modify Myc expression
ABC294640 inhibits cell growth in several tumor types, including hematological acute lymphoblastic leukemia (ALL), multiple myeloma, and KHSV-associated primary lymphoma, and solid tumors of the ovaries, breast, liver and prostate [16–19, 40, 41]. Within many of these cell models ABC294640 treatment downregulates Myc protein and/or mRNA expression. Multiple mechanisms exist to explain how ABC294640 treatment may decrease Myc. In one case, SK2 regulates histone deacetylase (HDAC) in the nucleus, with nuclear S1P inhibiting HDAC activity and promoting accumulation of histone H3 lysine 9 acetylation (H3K9ac) at MYC promoter elements, which often represents sites of active transcription [19, 42]. ABC294640 treatment decreases nuclear S1P, gene-specific H3K9ac and MYC transcription, demonstrating the ability of ABC294640 to reverse SK2-mediated gene expression. In other models, ABC294640 promotes cell apoptosis and downregulates Myc protein in a pS6-mediated fashion that is rescued by MG132 and implicates a role for the proteasome [18]. Finally, ceramide has been reported to decrease Myc protein expression in PCa cells by a protein phosphatase 2A-dependent mechanism [35]. However, our data argue this mechanism is not active in PCa cells since inhibition of PP2A did not attenuate ABC294640-induced down-regulation of Myc or AR. Our studies do suggest there are multiple mechanisms by which ABC294640 impacts Myc and AR expression since treatment decreased mRNA and enhanced proteasomal degradation of both proteins.
Myc/AR crosstalk
Myc and AR regulate expression of numerous genes involved in cell growth and proliferation, are required for PCa progression, and are commonly perturbed in aggressive disease. Interestingly, it is not clearly understood how these molecules impinge on signaling of one another. For example, AR suppresses Myc expression in normal prostate epithelial cells but increases Myc in cancer cells [43]. Myc overexpression confers castrate-resistant cell growth independent of AR signaling while Myc inhibition impairs ADT-sensitive and CRPC cell growth [44, 45]. Early studies indicate that Myc binding to AR is required for AR autoregulation [46]. In AR-positive molecular apocrine breast cancer, AR and Myc are involved in a feed-forward mechanism whereby AR activates Myc and Myc reinforces AR signaling by binding to AR target genes [47]. Furthermore, treatment of CRPC cells with JQ1, a bromodomain inhibitor that disrupts Myc transcriptional activity, decreases Myc protein and AR transcriptional activity, but does not alter AR protein [48]. JQ1 inhibits CRPC tumor cell growth, but the mechanism is downstream of AR, is Myc-independent, and involves blocking AR recruitment into transcriptional complexes with other bromodomain proteins. Data presented here demonstrate the ability of ABC294640 to down-regulate Myc and AR signaling. Treatment with 10058-F4 Myc inhibitor similarly decreases Myc expression and attenuates AR expression and activity, suggesting that within this model of PCa, Myc expression and/or activity is requisite for productive AR signaling.
Targeting oncogene addiction
PCa cells are addicted to the oncogenic signaling network perpetuated by AR, which underscores the significance of therapies targeting this pathway. The effectiveness of ABC294640 treatment in ADT-sensitive and CRPC cells is likely mediated, ultimately, by loss of AR signaling. Downregulation of both Myc and AR by ABC294640 may provide a unique opportunity for PCa therapy. Furthermore, determining which cells are addicted to Myc oncogenic networks could uncover tumor subtypes that are sensitive to the anticancer activity of ABC294640. For example, ALL and multiple myeloma patient samples overexpress Myc and Myc gene signatures, and are sensitive to ABC294640 treatment [18, 19]. In mouse models of ALL, a small percentage of tumors recur following Myc suppression and these tumors are still addicted to Myc because subsequent depletion effectively results in tumor regression [49]. In breast cancer, Myc silencing causes tumor regression, however, heterogeneity within tumor samples allows for escape from the oncogene dependence [50, 51]. Therefore, in addition to disrupting oncogenic signaling of Myc and AR in PCa, targeting tumors that are Myc addicted could be a feasible therapeutic option with widespread ramifications.
Oncogene addiction could also help explain the ability of ABC294640 to inhibit AR-negative PCa cells. DU145 and PC-3 are both growth-inhibited, with IC50 values approximately 2-fold higher than LNCaP but similar to aggressive 22Rv1 cells [20, 23]. PC3 and DU145 PCa cells express constitutively active AKT and ERK [52], respectively, and PC3 expresses elevated levels of Myc [44]. Therefore, ABC294640 treatment should be efficacious in blocking cell growth despite the absence of AR expression. Overall, ABC294640 represents a potent pharmacological agent that effectively attenuates AR-positive and Myc-dependent PCa cells.
Supplementary Material
Implications.
Sphingosine kinase inhibition disrupts multiple oncogenic signaling pathways that are deregulated in prostate cancer.
Acknowledgments
Funding
This work was supported by a grant from the Pennsylvania Department of Health (SAP# 4100059191 for RSS, SNK, CDS), a Prostate Cancer Foundation Young Investigator award (to MJS) and a Prostate Cancer Foundation Mazzone Challenge award (to KEK).
We would like to thank Ryan Smith, Cecelia Green, Lynn Maines and Yan Zhuang for comments on the project.
Footnotes
Conflict of Interest
The following authors are employed by Apogee Biotechnology Corporation (RSS, CDS, SNK) or own equity in Apogee Biotechnology Corporation (CDS).
References
- 1.Patel NK, et al. Advanced prostate cancer - patient survival and potential impact of enzalutamide and other emerging therapies. Ther Clin Risk Manag. 2014;10:651–64. doi: 10.2147/TCRM.S57509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hawksworth D, et al. Overexpression of C-MYC oncogene in prostate cancer predicts biochemical recurrence. Prostate Cancer Prostatic Dis. 2010;13(4):311–5. doi: 10.1038/pcan.2010.31. [DOI] [PubMed] [Google Scholar]
- 3.Sato K, et al. Clinical significance of alterations of chromosome 8 in high-grade, advanced, nonmetastatic prostate carcinoma. J Natl Cancer Inst. 1999;91(18):1574–80. doi: 10.1093/jnci/91.18.1574. [DOI] [PubMed] [Google Scholar]
- 4.Taylor BS, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18(1):11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fromont G, et al. 8q24 amplification is associated with Myc expression and prostate cancer progression and is an independent predictor of recurrence after radical prostatectomy. Hum Pathol. 2013;44(8):1617–23. doi: 10.1016/j.humpath.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Ellwood-Yen K, et al. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell. 2003;4(3):223–38. doi: 10.1016/s1535-6108(03)00197-1. [DOI] [PubMed] [Google Scholar]
- 7.Fyrst H, Saba JD. An update on sphingosine-1-phosphate and other sphingolipid mediators. Nat Chem Biol. 2010;6(7):489–97. doi: 10.1038/nchembio.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.French KJ, et al. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res. 2003;63(18):5962–9. [PubMed] [Google Scholar]
- 9.Neubauer HA, Pitson SM. Roles, regulation and inhibitors of sphingosine kinase 2. FEBS J. 2013;280(21):5317–36. doi: 10.1111/febs.12314. [DOI] [PubMed] [Google Scholar]
- 10.Kunkel GT, et al. Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat Rev Drug Discov. 2013;12(9):688–702. doi: 10.1038/nrd4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Igarashi N, et al. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J Biol Chem. 2003;278(47):46832–9. doi: 10.1074/jbc.M306577200. [DOI] [PubMed] [Google Scholar]
- 12.Liu H, et al. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J Biol Chem. 2000;275(26):19513–20. doi: 10.1074/jbc.M002759200. [DOI] [PubMed] [Google Scholar]
- 13.Liu H, et al. Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. J Biol Chem. 2003;278(41):40330–6. doi: 10.1074/jbc.M304455200. [DOI] [PubMed] [Google Scholar]
- 14.Gao P, Smith CD. Ablation of sphingosine kinase-2 inhibits tumor cell proliferation and migration. Mol Cancer Res. 2011;9(11):1509–19. doi: 10.1158/1541-7786.MCR-11-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weigert A, et al. Sphingosine kinase 2 deficient tumor xenografts show impaired growth and fail to polarize macrophages towards an anti-inflammatory phenotype. Int J Cancer. 2009;125(9):2114–21. doi: 10.1002/ijc.24594. [DOI] [PubMed] [Google Scholar]
- 16.Beljanski V, et al. Combined anticancer effects of sphingosine kinase inhibitors and sorafenib. Invest New Drugs. 2011;29(6):1132–42. doi: 10.1007/s10637-010-9452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antoon JW, et al. Antiestrogenic effects of the novel sphingosine kinase-2 inhibitor ABC294640. Endocrinology. 2010;151(11):5124–35. doi: 10.1210/en.2010-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kummetha Venkata J, et al. Inhibition of sphingosine kinase 2 down-regulates the expression of c-Myc and Mcl-1 and induces apoptosis in multiple myeloma. Blood. 2014 doi: 10.1182/blood-2014-03-559385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallington-Beddoe CT, et al. Sphingosine kinase 2 promotes acute lymphoblastic leukemia by enhancing MYC expression. Cancer Res. 2014;74(10):2803–15. doi: 10.1158/0008-5472.CAN-13-2732. [DOI] [PubMed] [Google Scholar]
- 20.Gestaut MM, et al. Inhibition of sphingosine kinase-2 ablates androgen resistant prostate cancer proliferation and survival. Pharmacol Rep. 2014;66(1):174–8. doi: 10.1016/j.pharep.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 21.Maines LW, et al. Suppression of ulcerative colitis in mice by orally available inhibitors of sphingosine kinase. Dig Dis Sci. 2008;53(4):997–1012. doi: 10.1007/s10620-007-0133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schrecengost RS, et al. USP22 regulates oncogenic signaling pathways to drive lethal cancer progression. Cancer Res. 2014;74(1):272–86. doi: 10.1158/0008-5472.CAN-13-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.French KJ, et al. Pharmacology and antitumor activity of ABC294640, a selective inhibitor of sphingosine kinase-2. J Pharmacol Exp Ther. 2010;333(1):129–39. doi: 10.1124/jpet.109.163444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beljanski V, Knaak C, Smith CD. A novel sphingosine kinase inhibitor induces autophagy in tumor cells. J Pharmacol Exp Ther. 2010;333(2):454–64. doi: 10.1124/jpet.109.163337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koh CM, et al. MYC and Prostate Cancer. Genes Cancer. 2010;1(6):617–28. doi: 10.1177/1947601910379132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nupponen NN, et al. Genetic alterations in hormone-refractory recurrent prostate carcinomas. Am J Pathol. 1998;153(1):141–8. doi: 10.1016/S0002-9440(10)65554-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schrecengost R, Knudsen KE. Molecular pathogenesis and progression of prostate cancer. Semin Oncol. 2013;40(3):244–58. doi: 10.1053/j.seminoncol.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tonelli F, et al. The sphingosine kinase inhibitor 2-(p-hyroxyanilino)-4-(p-chlorophenyl)thiazole reduces androgen receptor expression via an oxidative stress-dependent mechanism. Br J Pharmacol. 2013;168(6):1497–505. doi: 10.1111/bph.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schnute ME, et al. Modulation of cellular S1P levels with a novel, potent and specific inhibitor of sphingosine kinase-1. Biochem J. 2012;444(1):79–88. doi: 10.1042/BJ20111929. [DOI] [PubMed] [Google Scholar]
- 30.Knudsen KE, Penning TM. Partners in crime: deregulation of AR activity and androgen synthesis in prostate cancer. Trends Endocrinol Metab. 2010;21(5):315–24. doi: 10.1016/j.tem.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antonarakis ES, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371(11):1028–38. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ware KE, et al. Biologic and clinical significance of androgen receptor variants in castration resistant prostate cancer. Endocr Relat Cancer. 2014;21(4):T87–T103. doi: 10.1530/ERC-13-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dehm SM, et al. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68(13):5469–77. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watson PA, et al. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc Natl Acad Sci U S A. 2010;107(39):16759–65. doi: 10.1073/pnas.1012443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mukhopadhyay A, et al. Targeting inhibitor 2 of protein phosphatase 2A as a therapeutic strategy for prostate cancer treatment. Cancer Biol Ther. 2013;14(10):962–72. doi: 10.4161/cbt.25943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin CP, et al. Small-molecule c-Myc inhibitor, 10058-F4, inhibits proliferation, downregulates human telomerase reverse transcriptase and enhances chemosensitivity in human hepatocellular carcinoma cells. Anticancer Drugs. 2007;18(2):161–70. doi: 10.1097/CAD.0b013e3280109424. [DOI] [PubMed] [Google Scholar]
- 37.Yin X, et al. Low molecular weight inhibitors of Myc-Max interaction and function. Oncogene. 2003;22(40):6151–9. doi: 10.1038/sj.onc.1206641. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, et al. Evaluation of the antitumor effects of c-Myc-Max heterodimerization inhibitor 100258-F4 in ovarian cancer cells. J Transl Med. 2014;12:226. doi: 10.1186/s12967-014-0226-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kemppainen JA, et al. Androgen receptor phosphorylation, turnover, nuclear transport, and transcriptional activation. Specificity for steroids and antihormones. J Biol Chem. 1992;267(2):968–74. [PubMed] [Google Scholar]
- 40.Qin Z, et al. Targeting sphingosine kinase induces apoptosis and tumor regression for KSHV-associated primary effusion lymphoma. Mol Cancer Ther. 2014;13(1):154–64. doi: 10.1158/1535-7163.MCT-13-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White MD, et al. Targeting ovarian cancer and chemoresistance through selective inhibition of sphingosine kinase-2 with ABC294640. Anticancer Res. 2013;33(9):3573–9. [PubMed] [Google Scholar]
- 42.Hait NC, et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325(5945):1254–7. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vander Griend DJ, I, Litvinov V, Isaacs JT. Conversion of androgen receptor signaling from a growth suppressor in normal prostate epithelial cells to an oncogene in prostate cancer cells involves a gain of function in c-Myc regulation. Int J Biol Sci. 2014;10(6):627–42. doi: 10.7150/ijbs.8756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernard D, et al. Myc confers androgen-independent prostate cancer cell growth. J Clin Invest. 2003;112(11):1724–31. doi: 10.1172/JCI19035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao L, et al. Androgen receptor promotes ligand-independent prostate cancer progression through c-Myc upregulation. PLoS One. 2013;8(5):e63563. doi: 10.1371/journal.pone.0063563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grad JM, et al. Multiple androgen response elements and a Myc consensus site in the androgen receptor (AR) coding region are involved in androgen-mediated up-regulation of AR messenger RNA. Mol Endocrinol. 1999;13(11):1896–911. doi: 10.1210/mend.13.11.0369. [DOI] [PubMed] [Google Scholar]
- 47.Ni M, et al. Amplitude modulation of androgen signaling by c-MYC. Genes Dev. 2013 doi: 10.1101/gad.209569.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Asangani IA, et al. Therapeutic targeting of BET bromodomain proteins in castration-resistant prostate cancer. Nature. 2014;510(7504):278–82. doi: 10.1038/nature13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choi PS, et al. Lymphomas that recur after MYC suppression continue to exhibit oncogene addiction. Proc Natl Acad Sci U S A. 2011;108(42):17432–7. doi: 10.1073/pnas.1107303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.D’Cruz CM, et al. c-MYC induces mammary tumorigenesis by means of a preferred pathway involving spontaneous Kras2 mutations. Nat Med. 2001;7(2):235–9. doi: 10.1038/84691. [DOI] [PubMed] [Google Scholar]
- 51.Leung JY, et al. Heterogeneity in MYC-induced mammary tumors contributes to escape from oncogene dependence. Oncogene. 2012;31(20):2545–54. doi: 10.1038/onc.2011.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uzgare AR, Isaacs JT. Enhanced redundancy in Akt and mitogen-activated protein kinase-induced survival of malignant versus normal prostate epithelial cells. Cancer Res. 2004;64(17):6190–9. doi: 10.1158/0008-5472.CAN-04-0968. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.