Abstract
Fear conditioning is a model system used to study fear memory and its dysregulation in a variety of organisms. Newly developed tools such as optogenetics, Cre recombinase and DREADD technologies have allowed researchers to manipulate anatomically or molecularly defined cell subtypes with a high degree of temporal control and determine the effect of this manipulation on behavior. These targeted molecular techniques have opened up a new appreciation for the critical contributions different subpopulations of cells make to fear behavior and potentially to treatment of fear and anxiety disorders. Here we review progress to date across a variety of techniques to understand fear-related behavior through the manipulation of different cell subtypes within the amygdala.
Keywords: fear conditioning, fear extinction, amygdala, cell subtype manipulation
Introduction
Pavlovian Fear conditioning is a technique that has improved our understanding of the molecular mechanisms of the fear pathway. Training in Pavlovian fear conditioning involves exposing an organism to a cue conditioned stimulus (CS, usually a tone) that is temporally paired with an aversive unconditioned stimulus (US, usually a shock)(1). After training, animals demonstrate a conditioned response (CR, usually freezing- the lack of all movement except for respiration) (2-5). Within just a few trials within a single session, fear conditioning quickly creates a long lasting memory that can be manipulated with a variety of treatments at different time points around the training event (6). Fear extinction is the subsequent pairing of the CS without the US, resulting in the eventual diminution of the freezing response (1, 7, 8).
The amygdala is a central node for the processing of fear and threat and a variety of other emotions; it also is vital to fear acquisition and extinction. As shown in Figure 1 the major subnuclei of the amygdala include lateral (LA) including dorsal (Lad), ventral (LAv) and medial (LAm) subnuclei, basal (B), accessory basal (AB) and central nucleus (CeA) including lateral (CeL) and medial (CeM) subnuclei. Overwhelming evidence supports the LA as a structure critical for formation and storage of fear memory (6, 9). The term basolateral amygdala (BLA) has been used to refer to LA and B together (6). The BLA is morphologically and cytoarchitecturally very similar to the cortex consisting of glutamatergic projection cells expressing calmodulin kinase (CamK) and local circuit GABAergic cells that express neuropeptide Y (NPY), somatostatin (SOM) and vasoactive intestinal peptide (VIP), cholecystokinin (CCK), parvalbumin (PV) and calbindin (CB) (10).
Figure 1. Location of different subregions of the amygdala.
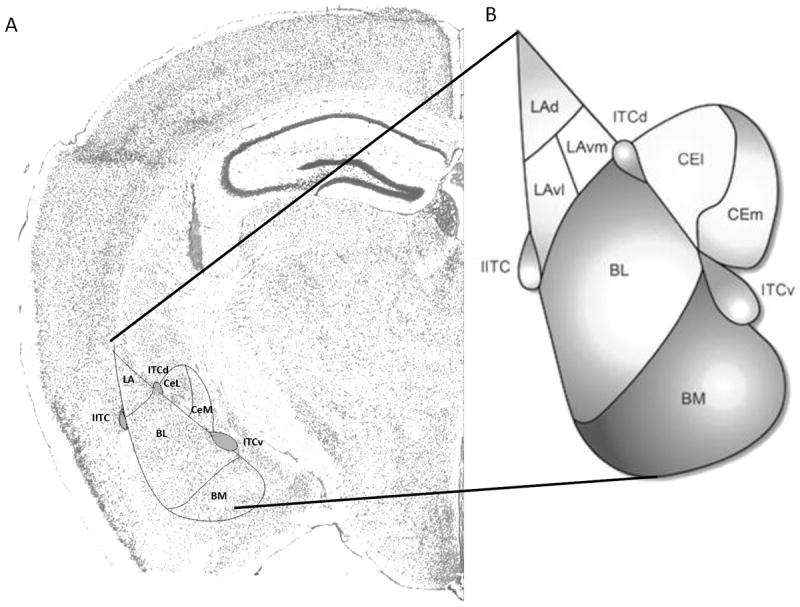
A. Nissl stain of coronal brain section from mouse (Image Credit: Allen Brain Atlas). B. Schematic diagram of amygdala complex (Adapted from Lee et al., 2013 (94). Abbreviations: Lad (Lateral amygdala dorsal), LAvm (Lateral amygdala ventromedial), LAvl (Lateral amygdala ventrolateral), ITCd (Intercalated cells dorsal), ITCv (Intercalated cells ventral), IITC (Lateral Intercalated cells), BL (Basolateral amygdala), BM (Basomedial amygdala), CEl (Central lateral amygdala), CEm (Central medial amygdala).
Studies have shown that the CeA is required for the acquisition, consolidation, and expression of fear memories (11-14) potentially working in serial with the BLA (10). The CeA is largely GABAergic (15) and can be further subdivided into central lateral (CeL) and central medial (CeM) nuclei (16). The CeL receives projections from cortical and subcortical areas (17) and projects to the CeM whereas the CeM receives direct projections from the BLA (18). GABA-producing inhibitory neurons gate expression of fear via tonic inhibition of the CeM (19-23) and go on to effect downstream structures such as the periaqueductal grey (PAG), locus coeruleus (LC) and parabrachial nuclei (PB) that direct behavioral output (6). Interestingly, neurons in the CeA express a diverse population of peptides and peptide receptors including vasopressin (VP), oxytocin (OT), corticotropin releasing factor (CRF), enkephalin (ENK), neurotensin (NT), somatostatin (SOM), dynorphin (DYN) and substance P (Subs P) among others (20-22, 24-28).
Intercalated cells (ITCs) are densely packed GABAergic neurons that form islands surrounding the BLA (29-31). ITCs may regulate the interconnectivity between amygdala subnuclei and extra-amygdala structures and are named the lateral paracapsular (lITC), the dorsal (ITCd), and the ventral (ITCv) ITC (Figure 1). They receive sensory information from the BLA and thalamus (32) and project to the CeM nucleus (32-35) which gates output of conditioned fear behavior. Work has shown that ITCs express FoxP2 (36), ENK (37, 38), and the mu opioids (38, 39), dopamine 1 (38) and GABAA α2/α3 subunit (40) containing receptors.
Many studies have assessed the role of the amygdala in fear memory using lesions, pharmacological manipulation and electrophysiological techniques (6, 10, 41-43). Until recently researchers have been limited in their ability to investigate the varying contributions, at a molecular level, of different subtypes of cells within a brain region. Work by Zirlinger and colleagues (44) used microarray analysis of the amygdala followed by in situ hybridization to demonstrate that a majority of the genes identified by microarray show expression selective to different subnuclei of the amygdala when tested using in situ hybridization. This finding suggested that these cell subtypes specific to different amygdala subnuclei may each hold specific function within the emotional regulation of behavior. To dissect the function of different cell subtypes a variety of techniques have been introduced. Along with techniques such as antisense manipulation and RNA interference, newer techniques to target and manipulate cell subtypes are being developed. One of the most revolutionary techniques, optogenetics, has offered advanced temporal precision using a combination of light presentation with genetic manipulations. Additionally, Cre/lox recombination technology for targeted deletions or inducible gene expression, DREADD (Designer Receptors Exclusively Activated by Designer Drugs) manipulations and targeted in vivo recording of amygdala cell populations have greatly increased our ability to understand the contributions of different subpopulations of neurons to fear behavior (45-48). These techniques have just begun to be utilized to give a more complete understanding of the contributions of cell specific mechanisms to fear memory. Findings from these studies highlighting fear behavior will be reviewed below (Table 1). Distribution patterns of some key cell subpopulations in the amygdala are shown in Figure 2.
Table 1.
A summary of recent studies targeting the amygdala that manipulate specific cell subtypes.
| Region | Name of cell type targeted | Technique used | Behavioral outcome |
|---|---|---|---|
|
| |||
| BLA | Thy1 (Jasnow et al., 2013) | Chr2 activation during fear conditioning | Disrupts fear conditioning |
| Chr2 activation during fear extinction. | Facilitates extinction | ||
|
| |||
| Parvalbumin (Wolff et al., 2014) | ChR2 activation during CS-US or US | Disrupts fear memory retention | |
| Chr2 activation during CS | Enhances fear memory retention | ||
| ARCH inhibition during US | Enhances fear memory retention | ||
|
| |||
| Somatostatin (Wolff et al., 2014) | ChR2 activation during CS | Disrupts fear learning | |
|
| |||
| CeL | GABAergic PKCδ neurons (Haubensak et al., 2010) | silencing PKCδ neurons by eliminating glutamate sensitivity during fear conditioning training and testing | Enhances freezing in high titer virus recipients |
|
| |||
| CRF GABAα1 (Gafford et al., 2012) | Transgenic disruption | Disrupts fear extinction | |
|
| |||
| CRF NR1 (Gafford et al., 2014) | Transgenic disruption | Disrupts fear acquisition/retention | |
| Virus mediated disruption in CeA | Disrupts fear acquisition/retention | ||
|
| |||
| CRF (Pitts 2009, 2011) | Pretraining infusion of antisense in CeA | Disrupts fear memory retention | |
| CRF antisense 5m, 9 hours and 24 hours after fear conditioning | Disrupts fear memory retention | ||
|
| |||
| Somatostatin (Li et al., 2013) | DREADD impairment | Impairs fear memory | |
| ChR2 activation naïve mice | Induces freezing | ||
| ARCH inhibition during fear expression | Suppresses conditioned freezing | ||
|
| |||
| CeM | Tac2 (Andero et al., 2014) | Lentiviral mediated Tac2 overexpression | Enhances fear memory consolidation |
Figure 2. Distribution patterns of key neuronal cell populations in the amygdala.
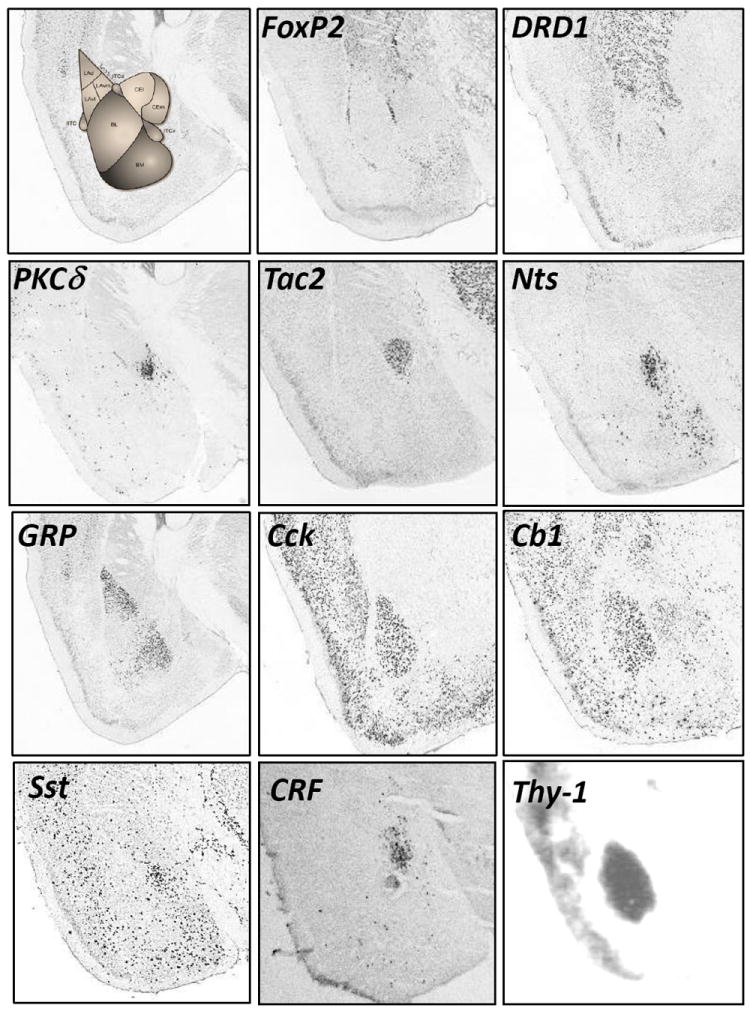
Distribution patterns of FoxP2 (Forkhead box protein P2), DRD1 (Dopamine receptor D1), PKCδ (Protein kinase C delta), Tac2 (Tachykinin 2), Nts (Neurotensin), GRP (Calcitonin Gene Related Peptide), CCK (Cholecystokinin), Cb1 (Cannabinoid receptor type 1), Sst (Somatostatin), CRF (Corticotropin Releasing Factor), Thy1 (Thy1 cell surface antigen) in the amygdala. Images from in situ hybridizations from Allen Brain Atlas as well as Ressler lab.
Basolateral Amygdala (BLA) neuronal populations
In a study conducted to test whether there are cell responses that act in opposing directions underlying fear acquisition and fear extinction, Herry and colleagues (49) demonstrated the existence of two electrophysiologically distinct fear responsive populations of neurons in awake behaving mice. One subpopulation of neurons increased firing specifically during and after fear conditioning and decreased firing during extinction training. A second population of neurons increased firing only after fear extinction. Herry and colleagues referred to these excitatory pyramidal neurons within the BLA as ‘Fear’ and ‘Extinction’ neurons, implying that the cellular activation of these cells differentially represented the memory traces of these different functions. Importantly for the behavioral relevance of these findings, firing frequency corresponded to freezing behavior. While the molecular identity of these neurons was not investigated in this study, the data highlight the critical contribution of subsets of neurons to fear behavior.
BLA pyramidal neurons
Johansen and colleagues (50) assessed whether optogenetic activation of pyramidal neurons in the LA along with presentation of a tone is sufficient to produce fear learning. When mice were trained to a tone paired with optical activation of pyramidal cells in the LA as an US, mice demonstrated fear conditioning. Importantly, this author showed that fear memory was only formed when the tone CS preceded the optical stimulation US indicating the process is associative (51). If the tone and optical stimulation were unpaired no learning occurred. Although fear conditioning occurred with LA pyramidal neuron stimulation the resulting freezing was relatively low. The authors suggest this may be due to activation of pyramidal cells en masse rather than in a cell subtype specific manner. Specifically, LA plasticity is also involved with appetitive learning making it possible that both appetitive and aversive types of neurons were stimulated. Later work by this same group (52) showed that this effect was enhanced with the addition of a beta noradrenergic receptor agonist. Specifically, presentation of a weak training protocol (3 CS tone presentations + ChR2 optical activation) coincident with an agonist of the beta adrenergic receptor resulted in enhanced freezing behavior during testing the following day. Together, these data suggest that with limited training a combination of Hebbian and neuromodulatory mechanisms triggers plasticity in the LA and behavioral associative learning.
Thy1 appears to mark the Fear-off / Extinction BLA Neurons
A later study by Jasnow and colleagues (53) used transgenic mice in which expression of ChR2 is driven by the Thy1-promoter to investigate the activities of Thy1-marked pyramidal neurons within the BLA during fear acquisition and extinction. The Thy1(H) line of Sanes and colleagues (54) appears to mark a specific population of neocortical and subcortical neurons, but no paleocortical populations. Jasnow and colleagues showed that the Thy1-marked cohort represented only about 1/3 of the excitatory neurons within the BLA. Pairing optogenetic activation of glutamatergic Thy1 neurons within the BLA with CS–US presentation during training inhibited fear consolidation, as indicated by attenuated freezing when tested the following day in the absence of optogenetic stimulation. To test the role of Thy1 containing neurons in fear extinction, BLA Thy1 neurons received optogenetic activation coincident with the CS alone presentation during fear extinction training. During the unstimulated extinction retention test the following day memory for extinction was enhanced (freezing was attenuated) compared to controls. These data suggest the BLA holds a subpopulation of glutamatergic neurons that are specifically tuned to inhibit the fear response possibly via an indirect connection with the CeM. Ongoing work is further examining whether the Thy1(h) subpopulation of BLA pyramidal neurons serves as a molecular mark of the Extinction neurons physiologically identified by Herry and colleagues. If so, it may provide a powerful way to drive specific fear inhibition or enhancement of extinction behavior that may help provide novel approaches to treating neuropsychiatric disease with unregulated fear processing.
Parvalbumin and Somatostatin Interneuron Populations
Using optogenetics and single unit recordings, a recent study (55) interrogated the role of the interneuron subtypes parvalbumin (PV) and somatostatin (SOM) in the BLA during fear learning. They found that PV and SOM neurons bidirectionally modulate fear acquisition. To show this in a cell subtype specific manner, the authors selectively expressed channelrhodopsin-2 (ChR2) in PV or SOM neurons in the BLA. PV cells received activation of ChR2 during pairing of the entire CS – US presentation or just during the US presentation, resulting in decreased freezing responses during the CS presentation the following day. Interestingly, PV neurons stimulated with ChR2 only during the CS resulted in increased freezing demonstrating opposing roles for PV neurons during CS and US processing. Additionally, Wolff and colleagues expressed the inhibitory opsin archaerhodopsin-3 (ARCH) in PV neurons and showed ARCH-mediated inhibition during the US presentation caused an increase in freezing during an unstimulated test of fear retrieval the following day. In contrast, manipulation of SOM containing neurons during the CS resulted in the opposite behavioral effects. These data suggest that within the BLA, PV and SOM expressing inhibitory neurons bidirectionally control the acquisition of fear conditioning.
While it is beyond the scope of this review to discuss all the identified inhibitory cell types within the BLA many of these neurons have been well characterized elsewhere (56-59). Further, a recent review gives a broad overview of the diversity of GABAergic cells within the BLA (60).
Central Amygdala (CeA) neuronal populations
Vasopressin and Oxytocin
The neuropeptides VP and OT are strongly expressed in the CeA and differentially engaged in fear and anxiety behavior. A variety of studies have shown that VP enhances and OT decreases anxiety and fear behavior (61-66). Interestingly, even though VP and OT have opposing effects on fear and anxiety behavior, application of either peptide increases neuronal excitability in a variety of different brain structures (67-69). OT is expressed in the CeL and VP is expressed in the CeM (70). Huber and colleagues (70) used different receptor agonists and antagonists to determine that OT and VP activate different neuronal subpopulations. OT neurons activated GABAergic transmission into the CeM and were restricted to CeL while VP neurons were localized to CeM and sent projections outside of the CeA. The differential connectivity of these neuropeptides within amygdala and extra-amygdala circuitry may underlie their opposing effects on fear behavior.
SOM
A study by Li and colleagues (71) investigated how a specific subset of CeL neurons respond to fear conditioning and contribute to both the learning and expression of fear. First, to test whether the CeL is essential for fear memory storage, neuronal activity was suppressed in Som-IRES-cre using chemical-genetic (72) manipulation that expresses hM4Di, an engineered inhibitory G protein–coupled receptor that suppresses neuronal activity in a Cre dependent manner. SOM activity was suppressed just prior to fear conditioning and resulted in impaired fear conditioning suggesting SOM containing neurons are critical for fear memory acquisition. Further, these authors used ChR2 to activate SOM neurons in the CeL and found they could induce freezing. To test whether SOM neurons in the CeL of fear-conditioned mice are required for the expression of learned fear, the inhibitory opsin ARCH was selectively expressed in SOM neurons. Mice were then fear conditioned and SOM neurons were inhibited during fear memory recall. Freezing behavior was attenuated in mice expressing the inhibitory opsin ARCH suggesting SOM containing neurons are necessary for conditioned fear expression. As indicated by cell specific retrograde tracing, the effects on freezing behavior caused by manipulation of SOM neurons are not occurring through the CeM because SOM neurons do not project to neurons in the CeM that project to brain areas in control of freezing (discussed further below). These findings highlight the role of SOM containing neurons in the CeL as directly modulating fear behavior.
PKCδ
Previous work has found that GABAergic neurons in the CeL gate expression of fear via tonic inhibition of the CeM (19-23), the major output of the amygdala (73). Haubensak and colleagues (20) used optogenetics to investigate a GABAergic subpopulation of PKCδ containing neurons in the CeL that may be responsible for gating CeM neurons. First Haubensak and colleagues showed that optogenetic activation of PKCδ neurons inhibits CeM output neurons that project to the PAG, a structure critical for production of freezing behavior. Further, silencing of PKCδ neurons resulted in enhanced freezing suggesting that this population of PKCδ neurons tonically inhibit CeM output neurons that gate freezing behavior.
CRF
CRF neurons are localized to the paraventricular nucleus of the hypothalamus (PVN), central nucleus of amygdala (CeA) and the bed nucleus of stria terminalis (BNST) (74-76). Dysregulation of CRF activity may underlie a number of psychiatric conditions such as PTSD (77-79). Recent studies have attempted to manipulate CRF neurons in the amygdala. Specifically, Pitts and colleagues (80, 81) used pretraining infusion of antisense against amygdala CRF neurons to test the importance of these neurons in contextual fear conditioning. No significant difference was shown during training; however when the animals were tested 48 hours later they showed significantly disrupted fear memory retention. A follow up study by the same group (81) showed post training infusion of CRF antisense into the amygdala was effective at disrupting fear memory retention when given either 5 minutes, 9 hours or 24 hours after fear conditioning, but not when given 96 hours after training. These findings highlight an extended involvement of CeA CRF neurons in contextual fear memory consolidation.
Our lab has tested the effect of inducible deletion of either the excitatory NMDA receptor, NR1 (82) or the inhibitory, GABAAα1 (83) genes specifically within the CRF neuronal populations. In these inducible genetic studies, we found different effects on fear acquisition and extinction of conditioned fear. As shown in Figure 3 we found that disruption of GABAAα1 within CRF-containing neurons did not affect fear conditioning or retention behavior but resulted in a significant and prolonged deficit in fear extinction (83). In contrast, deletion of NR1 within CRF-containing neurons leads to enhancement of fear conditioning and fear retention. A similar phenotype was seen within both transgenic mice and with mice using lentiviral mediated NR1 deletion, targeting just CRF neurons within the CeA (82). These studies report, additionally, that the GABA receptor deletion was associated with enhanced Amygdala/BNST excitation, consistent with possible increased fear after extinction. Paradoxically, the NR1 deletion mice also showed a phenotype consistent with enhanced Amygdala/BNST activation, thought to be due to compensatory increase AMPA upregulation following NR1 deletion, resulting in increased fear acquisition and retention. Altogether, our data may suggest that different CRF neuronal subpopulations (e.g. lateral CeA (CeL) vs. BNST) selectively contribute to accelerated fear acquisition or disrupted fear extinction behavior; alternatively, these effects may primarily represent the different effects of the NR1 and GABAAα1 neurotransmitter receptors within the CRH neuronal populations. Since disorders such as PTSD have been linked to enhanced fear conditioning as well as disrupted fear extinction (84-88), these data may highlight a potential mechanism for further investigation of this cell-type specific dissociation.
Figure 3. Disruption of GABAAα1 or NR1 genes within CRF neurons result in differential effects on fear acquisition and extinction.
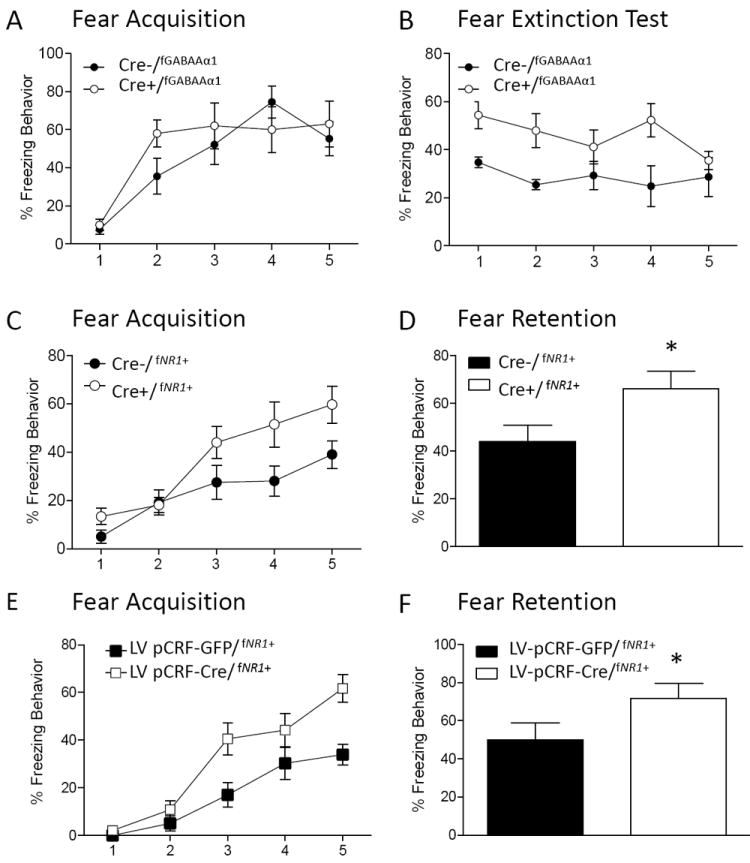
A.) CRF GABAAα1 deficient mice (Cre+/fGABAAα1) show no difference from Cre-/fGABAAα1 mice during fear conditioning. B.) However, Cre+/fGABAAα1 show significant deficits in extinction memory compared to Cre-/fGABAAα1 controls, consistent with enhanced activation of CRF neurons through disrupted GABA processing. Alternatively, disruption of CRF NR1 containing neurons (Cre+/ fNR1+) facilitates (C) fear acquisition and (D) fear retention compared to Cre-/fNR1+ controls, consistent with our data for compensatory increased glutamatergic AMPA signaling in CRF neurons. When CRF NR1 neurons were disrupted with virus infusion into the CeA (LV pCRF-CRE/ fNR1+), (E) fear acquisition and (F) fear retention were similarly significantly enhanced compared to GFP infused controls (LV pCRF-GFP/ fNR1+).
Tachykinin (Tac2) within the CeA medial nucleus (CeM)
Tac2 is highly expressed in the CeA with no expression in the basolateral amygdala (BLA) or lateral amygdala (LA). Specifically within the CeA, the highest expression of Tac2 occurs in the CeM, with less expression in the CeL (89, 90). Andero and colleagues (89) initially discovered that expression of Tac2 within the CeA was dynamically altered during the consolidation of fear memory. They then showed that overexpression of Tac2 in the CeA significantly enhanced fear memory consolidation. When the Tac2 gene was silenced during fear learning using Designer Receptors Exclusively Activated by Designer Drugs (DREADD) technology, 30 minutes prior to fear conditioning, no effect was seen on fear behavior. However, the following day when fear memory was tested, the mice that had Tac2 silenced during fear conditioning showed impaired fear memory consolidation. These data suggest that the Tac2 neuronal subpopulation within CeM may represent a critical output pathway from the CeM to downstream, subcortical target nuclei representing the process of fear expression. It has long been known that the CeM nucleus of the amygdala was a final output station to the brainstem fear expression network, but specific populations of cells within CeM which could be genetically and pharmacologically targeted were previously unknown.
Intercalated cells (ITCs)
Work indicates that ITC populations are differentially recruited during fear acquisition (31) and fear extinction (31, 91, 92). ITC neurons receive sensory information from the BLA and thalamus (32) and BLA inputs to ITC neurons have been shown to express NMDA-dependent LTD and LTP (93, 94). ITC neurons project information to the CeM nucleus which gates output of conditioned fear behavior (32-35). Little is known about the contributions of different ITC cell subtypes to fear behavior. One study attempted to use a known cellular marker of ITCs to directly manipulate their role in fear extinction (95). The authors took advantage of the high expression level of mu opioid receptors (MORs) in ITC neurons and administered a targeted toxin directed at mu opioids receptors in ITCv neurons after fear extinction. When extinction memory was tested, ITCv lesioned rats showed impaired extinction expression suggesting ITCs were engaged in fear extinction consolidation. ITCv neurons have been shown to receive excitatory inputs from BLA after training in extinction (96). Altogether these findings suggest that a population of ITCv neurons, potentially those expressing MORs, inhibits CeM neurons during normal retention and expression of fear extinction memories.
Amygdala Projections
Recent work has also highlighted the importance of distinct subpopulations of amygdala projections to other structures and their importance in control of a variety of amygdala mediated behaviors. A recent study by (97) used optogenetic manipulation of glutamatergic fibers emanating from the BLA to the nucleus accumbens (NAc) to show that these fibers modulated rewarding behavior. Similar optogenetic stimulation of glutamatergic fibers between medial prefrontal cortext (mPFC) and the NAc did not result in changes in reward seeking behavior. Additionally, these authors showed that reward seeking behavior was attenuated when optogenetic inhibition was applied to glutamatergic fibers between BLA and NAc further supporting the critical nature of these fibers in reward related behavior. Another recent study (98) showed that a subpopulation of neurons projecting from the basal amygdala (BA) to the prelimbic (PL) area show increased expression of the immediate early gene FOS after fear conditioning compared to BA inputs into infralimbic (IL) areas that showed increased FOS expression after fear extinction. Further, inhibition of the pathway from BA to PL increased extinction memory while inhibition of the pathway from BA to IL disrupted fear extinction suggesting the relative activity difference between these pathways contributes to extinction memory strength. A different study (99) found that optogenetically inhibiting BLA to anterodorsal BNST (adBNST) projections enhanced measures of anxiety while stimulating those projections decreased anxiety. Further testing revealed projections from adBNST to lateral hypothalamus (LH) mediated different aspects of individual measures of decreased anxiety. Specifically the adBNST projection to 1) lateral hypothalamus (LH) decreased avoidance of open spaces (2) parabrachial nucleus (PBN) contributed to decreased respiratory rate and (3) the ventral tegmental area (VTA) contributed to increased place preference (positive valence) of an environment. A final study (100) of showed that optogenetic manipulation of BLA to ventral hippocampus (VH) synapses modulates anxiety. Specifically, inhibition of BLA axon terminals with NpHR in VH decreases anxiety. Activation of these same projections with ChR2 increases anxiety behaviors.
The CeL has been shown to have projections that bypass the CeM and innervate a variety of downstream structures (101-105) suggesting the CeL may directly influence fear behavior via these long range projections. Using retrograde tracing and immunolabeling a study by Penzo and colleagues (106) showed that a subset of SOM containing neurons in the CeL send long range projections directly to the midbrain periaqueductal gray (PAG) and paraventricular nucleus of the thalamus (PVN). These authors infused the CeL of SOM-Cre with a Cre-dependent virus which selectively expressed ChR2 in SOM CeL neurons. Optical stimulation of axons originating from the SOM CeL neurons reliably inhibited the ventrolateral PAG. Further, when slices from mice that were fear conditioned were tested compared to controls by recording miniature excitatory postsynaptic potentials (mEPSPs) they found that fear conditioning increased mEPSC frequency in projections between the CeL to PAG or CeL to PVN. Overall these findings implicate the projections between CeL and PAG as a secondary pathway outside of the projections from the CeM which can drive fear expression via PAG output.
A follow up study by Penzo and colleagues (107) showed that the projections from the CeL to the PVN regulate fear learning. First, the authors found fear conditioning and retrieval increased expression of c-Fos in the posterior PVN. The authors infused a retrograde tracer into the BLA and CeL and revealed dense, mostly non overlapping, projections to the CeL and BLA from posterior PVN. The authors further targeted posterior PVN projections to CeL with a DREADD virus that effectively allowed suppression of the CeL to posterior PVN pathway with infusion of a DREADD activating drug (clozapine-N-oxide) during fear conditioning or retrieval. Both fear conditioning and fear retrieval were significantly disrupted with disruption of the CeL to posterior PVN pathway. The authors further found the posterior PVN neurons preferentially innervated SOM containing neurons compared to PKCδ containing neurons and that BDNF-TrkB signaling mediates communication within this pathway and promotes SOM containing neuronal activation within the CeL.
Conclusion
The amygdala complex is made up of numerous subnuclei, each of which have a variety of different excitatory, inhibitory and peptidergic cell populations (Figure 4). The selectivity of their expression within these subnuclei may suggest they underlie specific aspects of amygdala - mediated emotion regulation. Cell subpopulations within the BLA, CeA, ITC and even projections from the amygdala to other structures have shown specific roles in fear memory regulation. For example, simply activating LA pyramidal cells after presentation of a CS tone results in fear conditioning and this effect can be enhanced with LA application of an agonist of the beta adrenergic receptor. Other work targeted Thy1 pyramidal cells in the BLA and showed that optogenetic activation of glutamatergic Thy1 neurons within the BLA during training inhibited fear consolidation. The same activation given during fear extinction facilitated extinction of fear. This subpopulation of BLA neurons may be specifically tuned to inhibit the fear response. PV and SOM interneurons within the BLA have also been investigated and have been shown to play opposing roles in fear memory formation. Specifically, optogenetic activation of SOM neurons or inhibition of PV neurons impairs fear learning whereas activation of PV neurons or inhibition of SOM neurons enhances learning. The relative activity of PV and SOM neurons determines the activation level of effected principal neurons and ultimately may determine whether the effected principal neuron is recruited to the memory trace. Work in the CeA focused on neuropeptides has shown that OT, VP, SOM, Tac2 and CRF are strongly engaged during fear memory. OT, SOM and CRF are localized within the CeL which has been shown to either utilize GABAergic projections to the CeM to modulate fear behavior output or directly project to downstream structures. The GABAergic projections into the CeM have been proposed to be GABAergic PKCδ neurons in the CeL that modulate the CeM through tonic inhibition. Tac2 and VP containing neurons are mostly localized to the CeM from which they send projections to downstream structures and further modulate fear acquisition and extinction. Studies have also begun investigating projections of specific cell subtypes from the BLA or CeA. These studies have shown these projections strongly contribute to different aspects of amygdala mediated behavior and they also broaden our understanding of extra amygdala contributions to fear behavior.
Figure 4. Schematic diagram of Select Neuronal Populations in Amygdala.
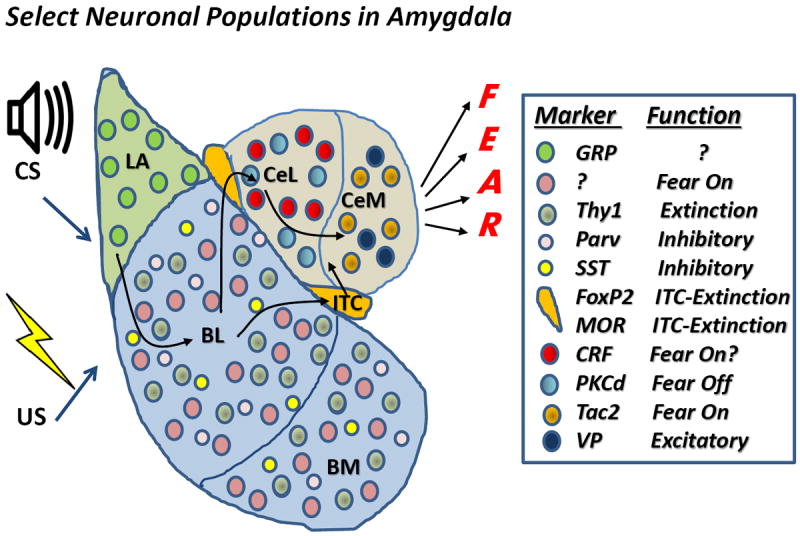
Shown are a number of different neuronal populations that have been identified via cellular markers at the mRNA or protein level, or via their function through physiological, optogenetic, and chemogenetic manipulations. Although the list is neither comprehensive or fully understood, we have attempted to outline some of the more intriguing populations discussed in this review, outlining their location within the different subdivisions of the amygdala on the left and their subcellular marker and function if known, based on the reviewed literature on the right. Abbreviations: CS-conditioned stimulus; US-unconditioned stimulus; LA- Lateral Amygdala; BL-Basolateral amygdala; BM-Basomedial amygdala; ITC-Intercalated neurons of the amygdala; CeL-Centrolateral amygdala; CeM-Centromedial amygdala; GRP-Gastrin Releasing Peptide109; Parv-Parvalbumin; SST-somatostatin; MOR-mu opiate receptor; CRF-corticotropin releasing factor; PKCd – protein kinase C, delta; Tac2 – tachykinin 2; VP – vasopressin.
Recent work showing the robust effects of manipulating neuronal subpopulations on fear and anxiety behavior highlights the importance of increased technological innovation towards understanding the contributions and changes underlying cell subpopulations in fear memory. New techniques to understand genetic changes within distinct cell populations such as translating ribosome affinity purification (TRAP) which combines cell type–specific expression of a transgene with purification and analysis of ribosomes that are being translated (108) will be crucial to our ability to learn about and manipulate these subpopulations of neurons.
By understanding the specific roles of individual, molecularly defined populations of neurons, the field is moving towards providing targeted, rationally designed approaches to both manipulate specific neuronal populations for research purposes, but also in the service of identifying novel cell circuit-based approaches to treating and preventing fear-related disorders in neuropsychiatry.
Highlights.
The amygdala is made of many subnuclei and cell types.
A detailed understanding of the cell-type structure is critical.
Fear disorders such as PTSD, Panic, and Phobia are related to amygdala dysfunction.
We review the CRF and other amygdala peptides in anxiety, fear and extinction.
Acknowledgments
Funding for work in the Ressler lab was provided by the National Institutes of Mental Health (MH096764 to K.J.R.; F32MH090785 to G.M.G.), and the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pavlov IP. In: Conditioned reflexes : an investigation of the physiological activity of the cerebral cortex. Anrep GV, editor. London: Oxford University Press, Humphrey Milford; 1927. [Google Scholar]
- 2.Bolles RC. Species-specific defense reactions and avoidance learning. Psychological Review. 1970;77(1):32–48. doi: 10.1037/h0028589. First Author & Affiliation: Bolles, Robert C. [DOI] [Google Scholar]
- 3.Blanchard RJ, Blanchard DC. Crouching as an index of fear. Journal of comparative and physiological psychology. 1969;67(3):370–5. doi: 10.1037/h0026779. Epub 1969/03/01. [DOI] [PubMed] [Google Scholar]
- 4.Fanselow MS. Conditioned and unconditional components of post-shock freezing. The Pavlovian journal of biological science. 1980;15(4):177–82. doi: 10.1007/BF03001163. Epub 1980/10/01. [DOI] [PubMed] [Google Scholar]
- 5.Abiri D, Douglas CE, Calakos KC, Barbayannis G, Roberts A, Bauer EP. Fear extinction learning can be impaired or enhanced by modulation of the CRF system in the basolateral nucleus of the amygdala. Behav Brain Res. 2014;271:234–9. doi: 10.1016/j.bbr.2014.06.021. Epub 2014/06/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LeDoux JE. Emotion circuits in the brain. Annual review of neuroscience. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. Epub 2000/06/09. [DOI] [PubMed] [Google Scholar]
- 7.Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12(2):120–50. doi: 10.1038/sj.mp.4001939. Epub 2006/12/13. [DOI] [PubMed] [Google Scholar]
- 8.Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychological bulletin. 1993;114(1):80–99. doi: 10.1037/0033-2909.114.1.80. Epub 1993/07/01. [DOI] [PubMed] [Google Scholar]
- 9.Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147(3):509–24. doi: 10.1016/j.cell.2011.10.009. Epub 2011/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiological reviews. 2010;90(2):419–63. doi: 10.1152/physrev.00037.2009. Epub 2010/04/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26(48):12387–96. doi: 10.1523/jneurosci.4316-06.2006. Epub 2006/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimmerman JM, Maren S. NMDA receptor antagonism in the basolateral but not central amygdala blocks the extinction of Pavlovian fear conditioning in rats. Eur J Neurosci. 2010;31(9):1664–70. doi: 10.1111/j.1460-9568.2010.07223.x. Epub 2010/06/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goosens KA, Maren S. Pretraining NMDA receptor blockade in the basolateral complex, but not the central nucleus, of the amygdala prevents savings of conditional fear. Behavioral neuroscience. 2003;117(4):738–50. doi: 10.1037/0735-7044.117.4.738. Epub 2003/08/23. [DOI] [PubMed] [Google Scholar]
- 14.Campeau S, Davis M. Involvement of the central nucleus and basolateral complex of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci. 1995;15(3 Pt 2):2301–11. doi: 10.1523/JNEUROSCI.15-03-02301.1995. Epub 1995/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun N, Cassell MD. Intrinsic GABAergic neurons in the rat central extended amygdala. J Comp Neurol. 1993;330(3):381–404. doi: 10.1002/cne.903300308. Epub 1993/04/15. [DOI] [PubMed] [Google Scholar]
- 16.McDonald AJ. Cytoarchitecture of the central amygdaloid nucleus of the rat. J Comp Neurol. 1982;208(4):401–18. doi: 10.1002/cne.902080409. Epub 1982/07/10. [DOI] [PubMed] [Google Scholar]
- 17.McDonald AJ, Shammah-Lagnado SJ, Shi C, Davis M. Cortical afferents to the extended amygdala. Ann N Y Acad Sci. 1999;877:309–38. doi: 10.1111/j.1749-6632.1999.tb09275.x. Epub 1999/07/23. [DOI] [PubMed] [Google Scholar]
- 18.Savander V, Go CG, Ledoux JE, Pitkanen A. Intrinsic connections of the rat amygdaloid complex: projections originating in the accessory basal nucleus. J Comp Neurol. 1996;374(2):291–313. doi: 10.1002/(SICI)1096-9861(19961014)374:2<291∷AID-CNE10>3.0.CO;2-Y. Epub 1996/10/14. [DOI] [PubMed] [Google Scholar]
- 19.Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62(6):757–71. doi: 10.1016/j.neuron.2009.05.026. Epub 2009/06/27. [DOI] [PubMed] [Google Scholar]
- 20.Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468(7321):270–6. doi: 10.1038/nature09553. Epub 2010/11/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cassell MD, Freedman LJ, Shi C. The intrinsic organization of the central extended amygdala. Ann N Y Acad Sci. 1999;877:217–41. doi: 10.1111/j.1749-6632.1999.tb09270.x. Epub 1999/07/23. [DOI] [PubMed] [Google Scholar]
- 22.Cassell MD, Gray TS. Morphology of peptide-immunoreactive neurons in the rat central nucleus of the amygdala. J Comp Neurol. 1989;281(2):320–33. doi: 10.1002/cne.902810212. Epub 1989/03/08. [DOI] [PubMed] [Google Scholar]
- 23.Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468(7321):277–82. doi: 10.1038/nature09559. Epub 2010/11/12. [DOI] [PubMed] [Google Scholar]
- 24.Cassell MD, Gray TS, Kiss JZ. Neuronal architecture in the rat central nucleus of the amygdala: a cytological, hodological, and immunocytochemical study. J Comp Neurol. 1986;246(4):478–99. doi: 10.1002/cne.902460406. [DOI] [PubMed] [Google Scholar]
- 25.McDonald AJ. Coexistence of somatostatin with neuropeptide Y, but not with cholecystokinin or vasoactive intestinal peptide, in neurons of the rat amygdala. Brain Res. 1989;500(1-2):37–45. doi: 10.1016/0006-8993(89)90297-7. Epub 1989/10/23. [DOI] [PubMed] [Google Scholar]
- 26.Veening JG, Swanson LW, Sawchenko PE. The organization of projections from the central nucleus of the amygdala to brainstem sites involved in central autonomic regulation: a combined retrograde transport-immunohistochemical study. Brain Res. 1984;303(2):337–57. doi: 10.1016/0006-8993(84)91220-4. Epub 1984/06/15. [DOI] [PubMed] [Google Scholar]
- 27.Merchenthaler I, Maderdrut JL, Cianchetta P, Shughrue P, Bronstein D. In situ hybridization histochemical localization of prodynorphin messenger RNA in the central nervous system of the rat. J Comp Neurol. 1997;384(2):211–32. Epub 1997/07/28. [PubMed] [Google Scholar]
- 28.Veinante P, Freund-Mercier MJ. Distribution of oxytocin- and vasopressin-binding sites in the rat extended amygdala: a histoautoradiographic study. J Comp Neurol. 1997;383(3):305–25. Epub 1997/07/07. [PubMed] [Google Scholar]
- 29.Millhouse OE. The intercalated cells of the amygdala. J Comp Neurol. 1986;247(2):246–71. doi: 10.1002/cne.902470209. Epub 1986/05/08. [DOI] [PubMed] [Google Scholar]
- 30.McDonald AJ, Augustine JR. Localization of GABA-like immunoreactivity in the monkey amygdala. Neuroscience. 1993;52(2):281–94. doi: 10.1016/0306-4522(93)90156-a. Epub 1993/01/01. [DOI] [PubMed] [Google Scholar]
- 31.Busti D, Geracitano R, Whittle N, Dalezios Y, Manko M, Kaufmann W, et al. Different fear states engage distinct networks within the intercalated cell clusters of the amygdala. J Neurosci. 2011;31(13):5131–44. doi: 10.1523/jneurosci.6100-10.2011. Epub 2011/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Royer S, Martina M, Pare D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J Neurosci. 1999;19(23):10575–83. doi: 10.1523/JNEUROSCI.19-23-10575.1999. Epub 1999/11/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vertes RP, Hoover WB. Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. J Comp Neurol. 2008;508(2):212–37. doi: 10.1002/cne.21679. Epub 2008/03/04. [DOI] [PubMed] [Google Scholar]
- 34.Pare D, Smith Y. The intercalated cell masses project to the central and medial nuclei of the amygdala in cats. Neuroscience. 1993;57(4):1077–90. doi: 10.1016/0306-4522(93)90050-p. Epub 1993/12/01. [DOI] [PubMed] [Google Scholar]
- 35.Geracitano R, Kaufmann WA, Szabo G, Ferraguti F, Capogna M. Synaptic heterogeneity between mouse paracapsular intercalated neurons of the amygdala. The Journal of physiology. 2007;585(Pt 1):117–34. doi: 10.1113/jphysiol.2007.142570. Epub 2007/10/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaoru T, Liu FC, Ishida M, Oishi T, Hayashi M, Kitagawa M, et al. Molecular characterization of the intercalated cell masses of the amygdala: implications for the relationship with the striatum. Neuroscience. 2010;166(1):220–30. doi: 10.1016/j.neuroscience.2009.12.004. Epub 2009/12/17. [DOI] [PubMed] [Google Scholar]
- 37.Poulin JF, Castonguay-Lebel Z, Laforest S, Drolet G. Enkephalin co-expression with classic neurotransmitters in the amygdaloid complex of the rat. J Comp Neurol. 2008;506(6):943–59. doi: 10.1002/cne.21587. Epub 2007/12/19. [DOI] [PubMed] [Google Scholar]
- 38.Jacobsen KX, Hoistad M, Staines WA, Fuxe K. The distribution of dopamine D1 receptor and muopioid receptor 1 receptor immunoreactivities in the amygdala and interstitial nucleus of the posterior limb of the anterior commissure: relationships to tyrosine hydroxylase and opioid peptide terminal systems. Neuroscience. 2006;141(4):2007–18. doi: 10.1016/j.neuroscience.2006.05.054. Epub 2006/07/06. [DOI] [PubMed] [Google Scholar]
- 39.Poulin JF, Chevalier B, Laforest S, Drolet G. Enkephalinergic afferents of the centromedial amygdala in the rat. J Comp Neurol. 2006;496(6):859–76. doi: 10.1002/cne.20956. Epub 2006/04/22. [DOI] [PubMed] [Google Scholar]
- 40.Geracitano R, Fischer D, Kasugai Y, Ferraguti F, Capogna M. Functional expression of the GABA(A) receptor alpha2 and alpha3 subunits at synapses between intercalated medial paracapsular neurons of mouse amygdala. Frontiers in neural circuits. 2012;6:32. doi: 10.3389/fncir.2012.00032. Epub 2012/06/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annual review of psychology. 2005;56:207–34. doi: 10.1146/annurev.psych.56.091103.070213. Epub 2005/02/16. [DOI] [PubMed] [Google Scholar]
- 42.Maren S, Quirk GJ. Neuronal signalling of fear memory. Nature reviews Neuroscience. 2004;5(11):844–52. doi: 10.1038/nrn1535. Epub 2004/10/22. [DOI] [PubMed] [Google Scholar]
- 43.Davis M. The role of the amygdala in fear and anxiety. Annual review of neuroscience. 1992;15:353–75. doi: 10.1146/annurev.ne.15.030192.002033. Epub 1992/01/01. [DOI] [PubMed] [Google Scholar]
- 44.Zirlinger M, Kreiman G, Anderson DJ. Amygdala-enriched genes identified by microarray technology are restricted to specific amygdaloid subnuclei. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(9):5270–5. doi: 10.1073/pnas.091094698. Epub 2001/04/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johansen JP, Wolff SB, Luthi A, LeDoux JE. Controlling the elements: an optogenetic approach to understanding the neural circuits of fear. Biol Psychiatry. 2012;71(12):1053–60. doi: 10.1016/j.biopsych.2011.10.023. Epub 2011/12/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chhatwal JP, Hammack SE, Jasnow AM, Rainnie DG, Ressler KJ. Identification of cell-type-specific promoters within the brain using lentiviral vectors. Gene therapy. 2007;14(7):575–83. doi: 10.1038/sj.gt.3302898. Epub 2007/01/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bouabe H, Okkenhaug K. Gene targeting in mice: a review. Methods in molecular biology (Clifton, NJ) 2013;1064:315–36. doi: 10.1007/978-1-62703-601-6_23. Epub 2013/09/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thiel G, Kaufmann A, Rossler OG. G-protein-coupled designer receptors - new chemical-genetic tools for signal transduction research. Biological chemistry. 2013;394(12):1615–22. doi: 10.1515/hsz-2013-0164. Epub 2013/07/31. [DOI] [PubMed] [Google Scholar]
- 49.Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454(7204):600–6. doi: 10.1038/nature07166. Epub 2008/07/11. [DOI] [PubMed] [Google Scholar]
- 50.Johansen JP, Hamanaka H, Monfils MH, Behnia R, Deisseroth K, Blair HT, et al. Optical activation of lateral amygdala pyramidal cells instructs associative fear learning. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(28):12692–7. doi: 10.1073/pnas.1002418107. Epub 2010/07/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rescorla RA. Probability of shock in the presence and absence of CS in fear conditioning. Journal of comparative and physiological psychology. 1968;66(1):1–5. doi: 10.1037/h0025984. Epub 1968/08/01. [DOI] [PubMed] [Google Scholar]
- 52.Johansen JP, Diaz-Mataix L, Hamanaka H, Ozawa T, Ycu E, Koivumaa J, et al. Hebbian and neuromodulatory mechanisms interact to trigger associative memory formation. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(51):E5584–92. doi: 10.1073/pnas.1421304111. Epub 2014/12/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jasnow AM, Ehrlich DE, Choi DC, Dabrowska J, Bowers ME, McCullough KM, et al. Thy1-expressing neurons in the basolateral amygdala may mediate fear inhibition. J Neurosci. 2013;33(25):10396–404. doi: 10.1523/jneurosci.5539-12.2013. Epub 2013/06/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28(1):41–51. doi: 10.1016/s0896-6273(00)00084-2. Epub 2000/11/22. [DOI] [PubMed] [Google Scholar]
- 55.Wolff SB, Grundemann J, Tovote P, Krabbe S, Jacobson GA, Muller C, et al. Amygdala interneuron subtypes control fear learning through disinhibition. Nature. 2014;509(7501):453–8. doi: 10.1038/nature13258. Epub 2014/05/13. [DOI] [PubMed] [Google Scholar]
- 56.Muller JF, Mascagni F, McDonald AJ. Coupled networks of parvalbumin-immunoreactive interneurons in the rat basolateral amygdala. J Neurosci. 2005;25(32):7366–76. doi: 10.1523/jneurosci.0899-05.2005. Epub 2005/08/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mascagni F, McDonald AJ. Immunohistochemical characterization of cholecystokinin containing neurons in the rat basolateral amygdala. Brain Res. 2003;976(2):171–84. doi: 10.1016/s0006-8993(03)02625-8. Epub 2003/05/24. [DOI] [PubMed] [Google Scholar]
- 58.Muller JF, Mascagni F, McDonald AJ. Synaptic connections of distinct interneuronal subpopulations in the rat basolateral amygdalar nucleus. J Comp Neurol. 2003;456(3):217–36. doi: 10.1002/cne.10435. Epub 2003/01/16. [DOI] [PubMed] [Google Scholar]
- 59.McDonald AJ, Mascagni F. Immunohistochemical characterization of somatostatin containing interneurons in the rat basolateral amygdala. Brain Res. 2002;943(2):237–44. doi: 10.1016/s0006-8993(02)02650-1. Epub 2002/07/09. [DOI] [PubMed] [Google Scholar]
- 60.Capogna M. GABAergic cell type diversity in the basolateral amygdala. Current opinion in neurobiology. 2014;26:110–6. doi: 10.1016/j.conb.2014.01.006. Epub 2014/02/04. [DOI] [PubMed] [Google Scholar]
- 61.Griebel G, Simiand J, Serradeil-Le Gal C, Wagnon sJ, Pascal M, Scatton B, et al. Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(9):6370–5. doi: 10.1073/pnas.092012099. Epub 2002/04/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004;29(3):483–93. doi: 10.1038/sj.npp.1300360. Epub 2003/12/03. [DOI] [PubMed] [Google Scholar]
- 63.Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Frontiers in neuroendocrinology. 2004;25(3-4):150–76. doi: 10.1016/j.yfrne.2004.05.001. Epub 2004/12/14. [DOI] [PubMed] [Google Scholar]
- 64.Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stressinduced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138(7):2829–34. doi: 10.1210/endo.138.7.5255. Epub 1997/07/01. [DOI] [PubMed] [Google Scholar]
- 65.Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nature genetics. 2000;25(3):284–8. doi: 10.1038/77040. Epub 2000/07/11. [DOI] [PubMed] [Google Scholar]
- 66.Bale TL. Sensitivity to stress: dysregulation of CRF pathways and disease development. Horm Behav. 2005;48(1):1–10. doi: 10.1016/j.yhbeh.2005.01.009. Epub 2005/05/28. [DOI] [PubMed] [Google Scholar]
- 67.Raggenbass M. Vasopressin- and oxytocin-induced activity in the central nervous system: electrophysiological studies using in-vitro systems. Progress in neurobiology. 2001;64(3):307–26. doi: 10.1016/s0301-0082(00)00064-2. Epub 2001/03/10. [DOI] [PubMed] [Google Scholar]
- 68.Lu YF, Moriwaki A, Tomizawa K, Onuma H, Cai XH, Matsui H. Effects of vasopressin and involvement of receptor subtypes in the rat central amygdaloid nucleus in vitro. Brain Res. 1997;768(1-2):266–72. doi: 10.1016/s0006-8993(97)00655-0. Epub 1997/11/22. [DOI] [PubMed] [Google Scholar]
- 69.Condes-Lara M, Veinante P, Rabai M, Freund-Mercier MJ. Correlation between oxytocin neuronal sensitivity and oxytocin-binding sites in the amygdala of the rat: electrophysiological and histoautoradiographic study. Brain Res. 1994;637(1-2):277–86. doi: 10.1016/0006-8993(94)91245-9. Epub 1994/02/21. [DOI] [PubMed] [Google Scholar]
- 70.Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308(5719):245–8. doi: 10.1126/science.1105636. Epub 2005/04/12. [DOI] [PubMed] [Google Scholar]
- 71.Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, Li B. Experience-dependent modification of a central amygdala fear circuit. Nature neuroscience. 2013;16(3):332–9. doi: 10.1038/nn.3322. Epub 2013/01/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dong S, Allen JA, Farrell M, Roth BL. A chemical-genetic approach for precise spatio-temporal control of cellular signaling. Molecular bioSystems. 2010;6(8):1376–80. doi: 10.1039/c002568m. Epub 2010/06/10. [DOI] [PubMed] [Google Scholar]
- 73.LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8(7):2517–29. doi: 10.1523/JNEUROSCI.08-07-02517.1988. Epub 1988/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Merchenthaler I. Corticotropin releasing factor (CRF)-like immunoreactivity in the rat central nervous system. Extrahypothalamic distribution. Peptides. 1984;5(Suppl 1):53–69. doi: 10.1016/0196-9781(84)90265-1. Epub 1984/01/01. [DOI] [PubMed] [Google Scholar]
- 75.Palkovits M, Brownstein MJ, Vale W. Distribution of corticotropin-releasing factor in rat brain. Fed Proc. 1985;44(1 Pt 2):215–9. Epub 1985/01/01. [PubMed] [Google Scholar]
- 76.Cummings S, Elde R, Ells J, Lindall A. Corticotropin-releasing factor immunoreactivity is widely distributed within the central nervous system of the rat: an immunohistochemical study. J Neurosci. 1983;3(7):1355–68. doi: 10.1523/JNEUROSCI.03-07-01355.1983. Epub 1983/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160(1):1–12. doi: 10.1677/joe.0.1600001. Epub 1998/12/17. [DOI] [PubMed] [Google Scholar]
- 78.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annual review of pharmacology and toxicology. 2004;44:525–57. doi: 10.1146/annurev.pharmtox.44.101802.121410. Epub 2004/01/28. [DOI] [PubMed] [Google Scholar]
- 79.Ronan PJ, Summers CH. Molecular signaling and translational significance of the corticotropin releasing factor system. Progress in molecular biology and translational science. 2011;98:235–92. doi: 10.1016/b978-0-12-385506-0.00006-5. Epub 2011/01/05. [DOI] [PubMed] [Google Scholar]
- 80.Pitts MW, Todorovic C, Blank T, Takahashi LK. The central nucleus of the amygdala and corticotropin-releasing factor: insights into contextual fear memory. J Neurosci. 2009;29(22):7379–88. doi: 10.1523/jneurosci.0740-09.2009. Epub 2009/06/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pitts MW, Takahashi LK. The central amygdala nucleus via corticotropin-releasing factor is necessary for time-limited consolidation processing but not storage of contextual fear memory. Neurobiology of learning and memory. 2011;95(1):86–91. doi: 10.1016/j.nlm.2010.11.006. Epub 2010/11/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gafford G, Jasnow AM, Ressler KJ. Grin1 receptor deletion within CRF neurons enhances fear memory. PloS one. 2014;9(10):e111009. doi: 10.1371/journal.pone.0111009. Epub 2014/10/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gafford GM, Guo JD, Flandreau EI, Hazra R, Rainnie DG, Ressler KJ. Cell-type specific deletion of GABA(A)alpha1 in corticotropin-releasing factor-containing neurons enhances anxiety and disrupts fear extinction. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(40):16330–5. doi: 10.1073/pnas.1119261109. Epub 2012/09/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behaviour research and therapy. 2007;45(9):2019–33. doi: 10.1016/j.brat.2007.02.012. Epub 2007/04/20. [DOI] [PubMed] [Google Scholar]
- 85.Glover EM, Phifer JE, Crain DF, Norrholm SD, Davis M, Bradley B, et al. Tools for translational neuroscience: PTSD is associated with heightened fear responses using acoustic startle but not skin conductance measures. Depression and anxiety. 2011;28(12):1058–66. doi: 10.1002/da.20880. Epub 2011/09/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, et al. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behaviour research and therapy. 2005;43(11):1391–424. doi: 10.1016/j.brat.2004.10.007. Epub 2005/05/12. [DOI] [PubMed] [Google Scholar]
- 87.Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in traumaexposed individuals with and without posttraumatic stress disorder. Journal of abnormal psychology. 2000;109(2):290–8. Epub 2000/07/15. [PubMed] [Google Scholar]
- 88.VanElzakker MB, Dahlgren MK, Davis FC, Dubois S, Shin LM. From Pavlov to PTSD: the extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiology of learning and memory. 2014;113:3–18. doi: 10.1016/j.nlm.2013.11.014. Epub 2013/12/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Andero R, Dias BG, Ressler KJ. A Role for Tac2, NkB, and Nk3 Receptor in Normal and Dysregulated Fear Memory Consolidation. Neuron. 2014 doi: 10.1016/j.neuron.2014.05.028. Epub 2014/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mar L, Yang FC, Ma Q. Genetic marking and characterization of Tac2-expressing neurons in the central and peripheral nervous system. Molecular brain. 2012;5:3. doi: 10.1186/1756-6606-5-3. Epub 2012/01/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson RM, Saksida LM, et al. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J Neurosci. 2008;28(32):8074–85. doi: 10.1523/jneurosci.4904-07.2008. Epub 2008/08/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Whittle N, Hauschild M, Lubec G, Holmes A, Singewald N. Rescue of impaired fear extinction and normalization of cortico-amygdala circuit dysfunction in a genetic mouse model by dietary zinc restriction. J Neurosci. 2010;30(41):13586–96. doi: 10.1523/jneurosci.0849-10.2010. Epub 2010/10/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Royer S, Pare D. Bidirectional synaptic plasticity in intercalated amygdala neurons and the extinction of conditioned fear responses. Neuroscience. 2002;115(2):455–62. doi: 10.1016/s0306-4522(02)00455-4. Epub 2002/11/08. [DOI] [PubMed] [Google Scholar]
- 94.Royer S, Pare D. Conservation of total synaptic weight through balanced synaptic depression and potentiation. Nature. 2003;422(6931):518–22. doi: 10.1038/nature01530. Epub 2003/04/04. [DOI] [PubMed] [Google Scholar]
- 95.Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454(7204):642–5. doi: 10.1038/nature07167. Epub 2008/07/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Amano T, Unal CT, Pare D. Synaptic correlates of fear extinction in the amygdala. Nature neuroscience. 2010;13(4):489–94. doi: 10.1038/nn.2499. Epub 2010/03/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475(7356):377–80. doi: 10.1038/nature10194. Epub 2011/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Senn V, Wolff SB, Herry C, Grenier F, Ehrlich I, Grundemann J, et al. Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron. 2014;81(2):428–37. doi: 10.1016/j.neuron.2013.11.006. Epub 2014/01/28. [DOI] [PubMed] [Google Scholar]
- 99.Kim SY, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS, et al. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature. 2013;496(7444):219–23. doi: 10.1038/nature12018. Epub 2013/03/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Felix-Ortiz AC, Beyeler A, Seo C, Leppla CA, Wildes CP, Tye KM. BLA to vHPC inputs modulate anxietyrelated behaviors. Neuron. 2013;79(4):658–64. doi: 10.1016/j.neuron.2013.06.016. Epub 2013/08/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moga MM, Gray TS. Evidence for corticotropin-releasing factor, neurotensin, and somatostatin in the neural pathway from the central nucleus of the amygdala to the parabrachial nucleus. J Comp Neurol. 1985;241(3):275–84. doi: 10.1002/cne.902410304. Epub 1985/11/15. [DOI] [PubMed] [Google Scholar]
- 102.Moga MM, Herbert H, Hurley KM, Yasui Y, Gray TS, Saper CB. Organization of cortical, basal forebrain, and hypothalamic afferents to the parabrachial nucleus in the rat. J Comp Neurol. 1990;295(4):624–61. doi: 10.1002/cne.902950408. Epub 1990/05/22. [DOI] [PubMed] [Google Scholar]
- 103.Sun N, Roberts L, Cassell MD. Rat central amygdaloid nucleus projections to the bed nucleus of the stria terminalis. Brain Res Bull. 1991;27(5):651–62. doi: 10.1016/0361-9230(91)90041-h. Epub 1991/11/01. [DOI] [PubMed] [Google Scholar]
- 104.Petrovich GD, Swanson LW. Projections from the lateral part of the central amygdalar nucleus to the postulated fear conditioning circuit. Brain Res. 1997;763(2):247–54. doi: 10.1016/s0006-8993(96)01361-3. Epub 1997/07/25. [DOI] [PubMed] [Google Scholar]
- 105.Bourgeais L, Gauriau C, Bernard JF. Projections from the nociceptive area of the central nucleus of the amygdala to the forebrain: a PHA-L study in the rat. Eur J Neurosci. 2001;14(2):229–55. doi: 10.1046/j.0953-816x.2001.01640.x. Epub 2001/09/13. [DOI] [PubMed] [Google Scholar]
- 106.Penzo MA, Robert V, Li B. Fear conditioning potentiates synaptic transmission onto long-range projection neurons in the lateral subdivision of central amygdala. J Neurosci. 2014;34(7):2432–7. doi: 10.1523/jneurosci.4166-13.2014. Epub 2014/02/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Penzo MA, Robert V, Tucciarone J, De Bundel D, Wang M, Van Aelst L, et al. The paraventricular thalamus controls a central amygdala fear circuit. Nature. 2015;519(7544):455–9. doi: 10.1038/nature13978. Epub 2015/01/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Heiman M, Schaefer A, Gong S, Peterson JD, Day M, Ramsey KE, et al. A translational profiling approach for the molecular characterization of CNS cell types. Cell. 2008;135(4):738–48. doi: 10.1016/j.cell.2008.10.028. Epub 2008/11/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shumyatsky GP, Tsvetkov E, Malleret G, Vronskaya S, Hatton M, Hampton L, Battey JF, Dulac C, Kandel ER, Bolshakov VY. Identification of a signaling network in lateral nucleus of amygdala important for inhibiting memory specifically related to learned fear. Cell. 2002 Dec 13;111(6):905–18. doi: 10.1016/s0092-8674(02)01116-9. [DOI] [PubMed] [Google Scholar]


