Abstract
Hyperlipidemia has been extensively studied in the context of atherosclerosis, whereas the potential health consequences of the opposite extreme, hypolipidemia, remain largely uninvestigated. Circulating lipoproteins are essential carriers of insoluble lipid molecules and are increasingly recognized as innate immune effectors. Importantly, severe hypolipidemia, which may occur with trauma or critical illness, is clinically associated with bacterial pneumonia. To test the hypothesis that circulating lipoproteins are essential for optimal host innate defense in the lung, we used lipoprotein deficient mice and a mouse model of Staphylococcus aureus pneumonia in which invasive infection requires virulence factor expression controlled by the accessory gene regulator (agr) operon. Activation of agr and subsequent virulence factor expression is inhibited by apolipoprotein B, the structural protein of low density lipoprotein, which binds and sequesters the secreted agr-signaling peptide (AIP). Here we report that lipoprotein deficiency impairs early pulmonary innate defense against S. aureus quorum-sensing dependent pathogenesis. Specifically, apoB levels in the lung early post-infection are significantly reduced with lipoprotein deficiency, coinciding with impaired host control of S. aureus agr-signaling and increased agr-dependent morbidity (weight loss) and inflammation. Given that lipoproteins also inhibit LTA- and LPS-mediated inflammation, these results suggest that hypolipidemia may broadly impact post-trauma pneumonia susceptibility to both Gram positive and Gram negative pathogens. Together with previous reports demonstrating that hyperlipidemia also impairs lung innate defense, these results suggest that maintenance of normal serum lipoprotein levels is necessary for optimal host innate defense in the lung.
Introduction
Hyperlipidemia is a clinical syndrome with high circulating levels of cholesterol, triglycerides and the lipoprotein particles which carry them, including very low and low density lipoproteins (VLDL, LDL) (1). Hyperlipidemia is a risk factor for cardiovascular disease (2, 3), yet serum lipoproteins also contribute to host innate defense against infection (4, 5). Interestingly, hyperlipidemia results in impaired intrapulmonary host immunity (6), suggesting that maintenance of normal circulating cholesterol and lipoprotein levels is crucial for optimal host innate defense in the lung. Although severe hypolipidemia, which often accompanies the acute phase response (APR) following surgery or trauma (reviewed in (7-9)), has been associated with bacterial pneumonia (10-12), a critical gap in knowledge remains regarding the impact of extremely low serum lipoprotein levels on host innate defense in the lung.
Staphylococcus aureus, and methicillin-resistant S. aureus (MRSA) in particular, accounts for 20% to 40% of hospital-acquired pneumonia (HAP) cases in the USA, as well as a growing number of cases of community-acquired pneumonia (CAP) (13-15). Invasive pulmonary infection caused by S. aureus requires the expression of virulence factors controlled by the accessory gene regulator (agr) operon (16-19), which encodes a two-component quorum-sensing (QS) system for bacterial communication and coordinated gene expression (reviewed in (20, 21)). QS is facilitated by secretion of a cyclic autoinducing peptide (AIP), which binds to and activates its cognate surface receptor AgrC. This in turn leads to expression of over 200 virulence factors (22), many of which are pre- and post-transcriptionally regulated by a small RNA molecule, called RNAIII, produced by transcription from the agr P3 promoter (20, 21). Importantly, apolipoprotein B (apoB), the sole protein component of LDL lipoprotein particles, and not other serum apoproteins or associated lipids, binds and sequesters AIP, thereby inhibiting agr-signaling and limiting pathogenesis during S. aureus skin infection (23-25). However, the impact of apoB deficiency and hypolipidemia on host innate defense in the lung, and against S. aureus pneumonia in particular, has not been investigated. We hypothesized that serum lipoproteins would contribute to pulmonary host defense against S. aureus QS and agr-mediated inflammation.
Here we used a sub-lethal model of S. aureus pneumonia to demonstrate that severe hypolipidemia impairs the early host innate defense response to lung infection. Specifically, lipoprotein deficiency impairs host control of S. aureus QS in the lung, resulting in agr-dependent increases in pulmonary pro-inflammatory cytokine production and neutrophil influx. Furthermore, apoB inhibits agr-dependent inflammatory cytokine expression by human alveolar epithelial cells, supporting a role for apoB in limiting QS-dependent virulence and inflammation during human lung infection. Given that serum lipoproteins also limit inflammation via sequestration of lipotechoic acid (LTA) and lipopolysaccharide (LPS) (26-28), these studies may have broad implications for hypolipidemia in increased susceptibility to post-trauma pneumonia caused by both Gram positive and Gram negative pathogens.
Materials and Methods
Bacterial strains and growth conditions
USA300 LAC and its isogenic agr deletion mutant (USA300 LACΔagr) were provided by Dr. Frank DeLeo (Rocky Mountain Laboratories, NIAID/NIH, Hamilton, MT) and Dr. Michael Otto (NIAID/NIH, Bethesda, MD), LACΔhla was provided by Dr. Juliane Bubeck-Wardenburg (University of Chicago, Department of Microbiology, Chicago, IL) and AH1677 agr::P3-yfp (USA300 LACyfp) was provided by Dr. Alex Horswill (University of Iowa, Department of Microbiology, Carver College of Medicine). Bacteria were grown in trypticase soy broth (TSB) and early exponential phase frozen stocks were prepared as previously described (29). CFU of frozen stocks were determined by plating of serial dilutions on blood agar (BD Biosciences, Franklin Lakes, NJ).
Mouse model of S. aureus pneumonia
Animal work was carried out at the AAALAC accredited Animal Research Facility of the University of New Mexico Health Sciences Center in accordance with recommendations in the Eighth Edition of The Guide for the Care and Use of Laboratory Animals and the USA Animal Welfare Act. The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of New Mexico. Eight to twelve week old male mice (C57BL/6, Pcsk9–/– on the B6 x 129 background, and B6 x 129 wild-type) were purchased from Jackson Laboratories (Bar Harbor, ME). Mice receiving 4-aminopyrazole-(3,4-D)pyrimidine (4APP) treatment were injected i.p. with 100 µl of 5.15 mg/ml 4APP (Sigma-Aldrich, St. Louis, MO) prepared as previously described (23, 24), or buffer control at 48 and 24 hours before infection, as well as at the time of infection. Reductions in serum cholesterol were determined using Infinity Cholesterol Liquid Stable Reagent (Thermo Scientific, Middletown, VA) according to manufacturer’s directions.
The mouse model of S. aureus pneumonia was performed as previously described (17). Briefly, mice were anesthetized by isoflurane inhalation and 30 µl of sterile saline containing ~4 × 108 CFU of S. aureus was administered intranasally, followed by an additional 30 µl of saline alone. Mice were weighed at the time of infection and again prior to sacrifice. At 6 hours post-infection, mice were sacrificed by CO2 asphyxiation and blood and tissues collected. For CFU determinations, right lungs were collected in bead-beating tubes containing 2.3-mm Zirconia/Silica beads (BioSpec Products, Bartlesville, OK) in 1 ml HBSS− (Life Technologies, Grand Island, NY) with 0.2% human serum albumin (Sigma-Aldrich, St. Louis, MO), and lung tissue disrupted for 1 min using a Mini-Bead Beater-24 (Biospec). Homogenates were diluted 1:10 in PBS with 0.1% Triton X-100, sonicated, and serial dilutions plated on blood agar (BD Biosciences, Franklin Lakes, NJ). For agr::P3-YFP promoter activation assays, bacteria from diluted homogenates were pelleted by centrifugation and fixed for 10 min with 1% paraformaldehyde containing 25mM CaCl2. Promoter activation was determined by measuring mean channel fluorescence (MCF) by flow cytometry (Accuri C6, BD Accuri Cytometers, Ann Arbor, MI). For cytokine and Western blot analyses, lung homogenates were clarified by centrifugation at 12,500 × g and supernatant was stored at −80 ~C until use as described below. For transcription analyses, left lungs were harvested, immediately placed into 1.8 mL of RNAlater (Qiagen, Valencia, CA), and frozen at −80~C.
A549 cell exposure to bacterial supernatant
For A549 assays, bacteria were cultured in TSB (5 mL with 10:1 air to volume ratio) for 6 hours at 37 ~C with shaking and with or without 100 nM human LDL (hLDL). Bacteria were then pelleted by centrifugation and supernatants filter sterilized by passage through 0.2 µm filters, aliquoted, and frozen at −80 ~C until use. A549 human alveolar epithelial cells were grown to 80-90% confluence in 12-well plates in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% heat-inactivated Fetal Bovine Serum (HI-FBS) and penicillin/streptomycin. Twenty-four hours prior to use, cell culture medium was changed to DMEM with 10% human lipoprotein deficient serum with penicillin/streptomycin. Sterile bacterial supernatants were thawed, diluted to working concentrations in TSB, and added to A549 cells at a 1:10 dilution in cell culture media. Post-culture hLDL was added to respective supernatants at 100 nM concentration prior to addition to A549 cells. Cells were incubated at 37 ~C for 4 hours. At 4 hours post-exposure, cell culture supernatants were collected and frozen at −80 ~C for future analysis. To collect RNA, cells were immediately lysed with Qiagen RLT buffer with 1% β-mercaptoethanol and QIAshredder spin columns (Qiagen, Valencia, CA). RNA was processed using an RNeasy Mini kit, according to the manufacturer’s Animal Cell Spin protocol (Qiagen, Valencia, CA).
Quantification of cytokines and myeloperoxidase ELISA
Cytokines were measured in cell culture supernatant or clarified supernatant from mouse lung homogenates using custom designed Milliplex Cytokine Magnetic kits according to the manufacturer’s specifications (Millipore, Billerica, MA). Cell culture supernatants were pooled from identically treated triplicates. Myeloperoxidase was also measured in clarified supernatants using the Mouse Myeloperoxidase DuoSet ELISA (R&D Systems, Minneapolis, MN) according to manufacturer’s directions.
Western blot analyses
Clarified lung homogenate supernatants were quick thawed (37~C) and protein concentrations determined by A280 absorption (Nanodrop 100 Spectrophotometer, Thermo Fisher Scientific, Wilmington, DE). For apoB Western blots, equivalent amounts of total protein were separated by SDS-PAGE on 3-8% Tris-Acetate gels (Novex Life Technologies, Grand Island, NY) prior to transfer to polyvinylidene fluoride (PVDF) membranes. Membranes were blocked with 5% non-fat milk in TBS (20mM Tris pH 7.5, 150 mM NaCl) containing 0.1% Tween-20 (TBST), then probed with rabbit anti-apoB antibody (Abcam, Cambridge, MA). Unbound antibody was removed by triplicate 5 min washes with TBST, and membranes developed using goat anti-rabbit IgG-alkaline phosphatase-conjugated secondary antibody (KPL, Gaithersburg, MD) and 1-Step nitroblue tetrazolium (NBT)/5-bromo-4-chloro-3-indolyl-phosphate (BCIP) (Thermo Scientific).
For Western blot analysis of secreted Hla in bacterial supernatants applied to A549 cells (above), equivalent amounts of total protein were separated by SDS-PAGE on 4-12% Bolt gels (Novex Life Technologies, Grand Island, NY) prior to transfer to polyvinylidene fluoride (PVDF) membranes and overnight blocking. Membranes were probed with mouse anti-Hla mAb (Integrated BioTherapeutics, Gaithersburg, MD), followed by goat anti-rabbit IgG poly-horseradish peroxidase (Thermo Fisher Scientific). Blots were developed using Thermo Pierce SuperSignal West Pico Chemiluminescent substrate (Thermo Fisher Scientific). All blots were imaged using a ProteinSimple FluorChem R instrument and quantified with AlphaView software (Protein Simple, Santa Clara, CA).
Rabbit erythrocyte lysis assay
Sterile bacterial culture supernatants prepared as described above and used for A549 cytokine induction assays were tested for hemolytic activity as previously described (30). Briefly, a 4% solution of rabbit RBCs in PBS was incubated at 37 ~C for 1 h with 2-fold serial dilutions of sterile-filtered bacterial supernatant. Lysis was determined by the A450 of the resulting RBC supernatant. Data were analyzed by nonlinear regression and are shown as the HA50 which equals 1/ the log of the dilution needed for 50% complete lysis.
Quantitative RT-PCR
For bacterial RNA isolation, bronchial alveolar lavage fluid (BALF) was collected as described below but in a 10-fold volume of RNAlater (Qiagen) and frozen at −80 ~C for later processing. After thawing, bacteria were pelleted by centrifugation, RNAlater was aspirated, and pellets were incubated with 100 µL Tris-EDTA (TE) buffer, with 20 µL/mL proteinase K (Qiagen) and 20 µg/mL of lysostaphin for 10 min. Thawed lungs were removed from RNAlater and RNA extracted using Qiazol according to manufacturer’s directions (Qiagen). RNA isolation from cell culture was performed as described above. An RNAeasy Mini kit (Qiagen) was used for RNA purification and cDNA was generated using a high-capacity cDNA RT kit with RNAse inhibitor together with random hexamer primers (Applied Biosystems, Foster City, CA). Taqman Gene Expression master mix (Applied Biosystems) and an ABI7000 Real-Timer PCR system were used for quantitative PCR. Prime Time Predesigned qPCR primers and probes (Integrated DNA Technologies, Coralville, IA) were used for transcriptional analyses of mouse hprt, il-1b, mip-2 and il-6, and for human hrpt and il-8. Primers and probes for quantification of S. aureus 16S, RNAIII and lukS were previously described (31). Gene expression was quantified using SDS RQ Manager Version 1.2.2 software (Applied Biosystems) relative to mouse or human hprt, and S. aureus 16S, as appropriate.
Cell influx analysis of bronchial alveolar lavage fluid
BALF was collected from sacrificed mice by intratracheal lavage, using three sequential washes (700 µl each) with ice cold Dulbecco’s Phosphate-Buffered Saline (2.67 mM KCl, 1.47 mM KH2PO4, 138 mM NaCl, 8.1 mM Na2HPO4) (Corning, Corning, NY). Total cell count and live/dead counts were determined by trypan blue staining using a TC 20 Automated Cell Counter System (Biorad, Hercules, CA). For immunostaining, cells were centrifuged at 800 × g for 3 min then pelleted cells resuspended in cold PBS with 0.5% BSA and 0.075% sodium azide. Cells were blocked for 30 min with 2% BSA, followed by a 1-h, 4~C incubation with anti-mouse Ly6G (neutrophils) (BioXCell, West Lebanon, NH) conjugated to AlexFluor 488 (Protein labeling kit, Molecular Probes Inc., Eugene, OR), anti-mouse CD11c-PE (alveolar macrophages) (Biolegend, San Diego, CA), or isotype controls. Cells were washed with PBS prior to analysis by flow cytometry (Accuri C6, BD Accuri Cytometers).
Statistical analyses
Alldata were analyzed using GraphPad Prism Version 5.04 for Windows (GraphPad Software, San Diego California USA). Parametric data were analyzed using the two-tailed Student’s t-test or ANOVA with either Dunnett’s post-hoc test or Bonferroni’s multiple comparison test, and are displayed as the mean ± SEM. Non-parametric data were analyzed by the Mann-Whitney U test and displayed as the median plus 5th-95th percentiles.
Results
Lipoprotein deficiency impairs host control of S. aureus QS in the lung
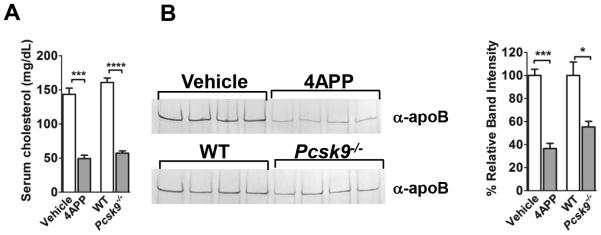
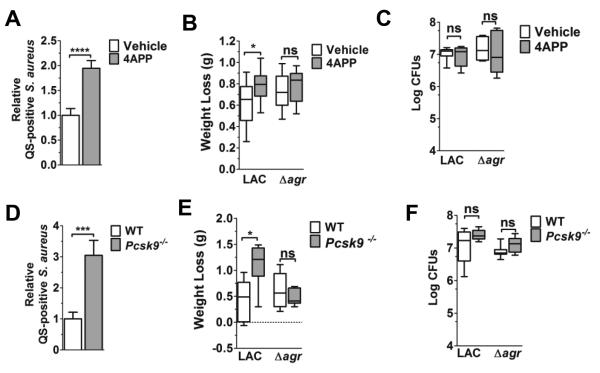
Since lipoproteins are detectable in lungs shortly after intranasal infection with S. aureus (32) and apoB on LDL binds S. aureus AIP and inhibits agr-activation (23-25), we predicted that decreased circulating lipoprotein levels would result in impaired control of S. aureus QS in the lung. To address this, we compared S. aureus agr-activation in the lungs of mice with reduced apoB levels versus controls. Given that APOB deletion is embryonic lethal (33), we used pharmacological treatment with 4-aminopyrazole-(3,4-D)pyrimidine (4APP), which inhibits lipoprotein secretion from the liver (34), and Pcsk9−’− mice, which have reduced circulating LDL levels due to overexpression of the LDL receptor (LDLR) (35), as models of apoB deficiency. Both models have an approximately 50% reduction in apoB measured in the serum (Fig. 1A,B), consistent with those of trauma patients at risk of infection (9). Using a sub-lethal challenge model (17), we intranasally infected mice with a reporter strain of the agr’ clinical isolate LAC, which expresses YFP under the control of the agr::P3 promoter (36). We measured agr-activation at six hours post-infection, at which time agr activation is detectable in the lung, as are differences in lung pathogenesis and bacterial burden between mice infected with agr+ versus Δagr isolates (16, 37). As expected, agr::P3 promoter activation was significantly increased in the lungs of both 4APP-treated and Pcsk9−/− mice compared to controls (Fig. 2A,D). In addition, bacteria in BALF collected from LAC infected 4APP-treated mice showed increased transcription of agr-regulated RNAIII and lukS-PV, the latter encoding the Panton-Valentine leukocidin (PVL) (Supplemental Fig. 1). Weight loss, used as a measure of morbidity, over the course of the six hour infection was slight, with median weight loss ranging from ~0.45 to 1.12 g and from ~1.5 to 4.8% of total body weight (Fig. 2B,E, Supplemental Fig. 2). However, 4APP-treated and Pcsk9−/− mice infected with LAC, but not with an isogenic agr mutant (LACΔagr), showed increased weight loss, compared to vehicle-treated and WT controls, respectively suggesting increased virulence in the lipoprotein deficient mice due to an inability to control agr-signaling. Importantly, differences in QS and weight loss at this time point were not the result of increased bacterial burden, as the number of CFUs in the lungs of lipoprotein deficient mice did not differ from that of controls (Fig. 2C,F). Therefore, these results suggest that serum lipoproteins contribute to host control of S. aureus QS-dependent virulence in the lungs and that this contribution is independent of host control of bacterial burden.
Figure 1. 4APP-treated and Pcsk9−/− mice show similar reductions in serum cholesterol and apoB.
(A) Quantification of mouse serum cholesterol. (B) Western blot for serum apoB (left) and apoB quantification by relative band intensity versus control (right). Data shown as mean ± SEM. N= four mice per group. *, p≤0.05; ***, p≤0.001; ****, p≤0.0001, Student’s t-test.
Figure 2. Reduction of circulating lipoproteins increases pulmonary agr-signaling and weight loss.
Vehicle- or 4APP-treated (A-C, white and gray bars, respectively) and B6 x 129 (WT) or Pcsk9−/− mice (D-F, white and gray bars, respectively) were intranasally infected with agr::P3-YFP LAC or LACΔagr (4 x 108 CFU) and analyses performed at 6 hours post-infection. (A, D) Flow cytometric quantification of S. aureus agr::P3 promoter activation in the lungs of lipoprotein deficient mice relative to controls (N=6 mice per group). Data shown as mean ± SEM. ***, p≤0.001; ****, p≤0.0001, Student’s t-test. (B, E) Weight loss (g) of infected lipoprotein deficient mice versus controls (N=7-8 mice per group from at least two independent experiments). (C, F) Bacterial burden (Log CFU) in the lung (N=6-8 mice per group from two independent experiments). (B-C, E-F) Data shown are median plus 5th-95th percentiles. ns, not significant; *, p≤0.05, **, p≤0.01, Mann-Whitney test.
Serum lipoprotein deficiency impacts post-infection lung apoB concentrations
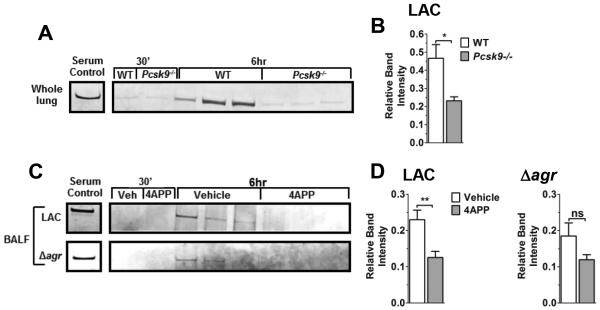
Increased agr-signaling in the lungs of S. aureus infected lipoprotein deficient mice (Fig. 2A,D) suggested that these mice would have decreased apoB present in the lungs compared to lipoprotein sufficient controls. As suggested, LAC-infected Pcsk9−/− mice showed significantly decreased apoB in the lungs at six hours post-infection compared to wild-type controls (Fig. 3A,B). Similarly, compared to vehicle-treated mice, BALF from mice treated with 4APP and infected with LAC also showed significant reductions in apoB at the six hour time point (Fig. 3C,D). In contrast, differences in apoB levels in BALF from mice infected with LACagr did not reach statistical significance. Together, these data suggest that the presence of apoB in the lung during S. aureus infection, and thus its ability to control agr-signaling in the lung, is dependent upon circulating lipoprotein concentrations.
Figure 3. Reduction of serum lipoproteins decreases post-infection apoB levels in the lung.
(A) Western blot analysis of apoB and (B) relative quantification of apoB versus serum control in whole lung homogenates of B6 x 129 (WT) or Pcsk9−/− mice 6 hours following intranasal infection with LAC (N=6 mice per group). (C) Western blot analysis and (D) relative quantification of apoB in BALF from vehicle- or 4APP-treated mice 6 hours following intranasal infection with LAC or LACΔagr (N=6 and 3 mice per group, respectively). Data shown are mean ± SEM. ns, not significant; *, p≤0.05, **, p≤0.01, Student’s t-test.
Lipoproteins regulate both agr-dependent and agr-independent induction of pro-inflammatory cytokines in the lung
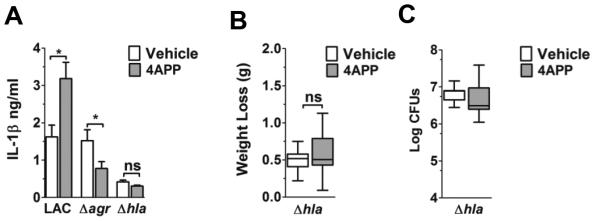
S. aureus stimulates the release of a variety of cytokines and chemokines in the lungs of infected mice, including cytokines produced in response to agr-regulated virulence factors (17, 18, 32, 38-41). In particular, expression of the agr-regulated virulence factor alpha-hemolysin (Hla), a pore-forming toxin, induces pulmonary release of the pro-inflammatory cytokine IL-1β (38, 39), suggesting there would be an agr- and Hla-dependent increase in IL-1β in the lungs of lipoprotein deficient mice compared to controls. As predicted, 4APP-treated mice infected with LAC, but not with LACΔagr or LACΔhla, had significantly increased pulmonary IL-1β levels at six hours post-infection compared to vehicle-treated mice (Fig. 4A), consistent with increased morbidity (weight loss) relative to vehicle controls only with LAC infection (Fig. 2B, 4B). Also, as seen for LAC and LACΔagr infected mice, bacterial burden did not differ between 4APP- and vehicle-treated mice infected with LACΔhla (Fig. 4C). Interestingly, IL-1β expression was reduced in lipoprotein deficient mice infected with LACΔagr (Fig. 4A), although the mechanism driving this response remains unclear. Therefore, although Hla expression in lung homogenate of LAC infected mice was below the limit of detection at six hours post-infection (Western blot data not shown), these results suggest that lipoproteins inhibit agr-dependent Hla production and associated virulence in the lungs early during S. aureus infection.
Figure 4. Lipoproteins limit Hla-dependent pulmonary IL-1β production.
(A) IL-1β levels in whole lung homogenate from vehicle- and 4APP-treated mice (white and gray bars, respectively) 6 hours following intranasal infection with the indicated isolate. Data shown are mean ± SEM. ns, not significant; *, p≤0.05, Student’s t-test. (B) Weight loss (g) of infected lipoprotein deficient mice versus controls (N=8 mice per group from two independent experiments. (C) Bacterial burden (Log CFU) in the lung. N=8 mice per group. (B-C) Data shown are median plus 5th-95th percentiles. ns, not significant, Mann-Whitney test.
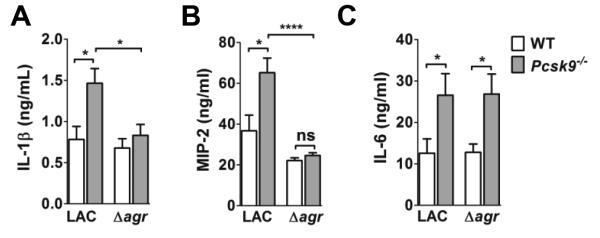
In addition to inhibiting agr-signaling, apoB on LDL also binds lipotechoic acid (LTA) from S. aureus and limits LTA-induced expression of IL-6 (28). To distinguish between agr-dependent and agr-independent effects of lipoprotein deficiency on pulmonary inflammatory cytokine production, we measured lung cytokine levels in WT and Pcsk9−/− mice intranasally infected with LAC or LACΔagr. In addition to IL-1β and IL-6, we measured production of macrophage inflammatory protein 2 (MIP-2), a neutrophil chemotactic protein, which has also been shown to be driven by agr-regulated Hla (42). At six hours post-infection, IL-1β and MIP-2 levels were significantly increased in the lungs of Pcsk9−/− mice infected with LAC, but not with LACΔagr (Fig. 5A,B), consistent with impaired host control of QS in the lungs of lipoprotein deficient mice. In contrast, compared to WT mice, IL-6 was increased in the lungs of both LAC and LACΔagr infected Pcsk9−/− mice (Fig. 5C), further supporting a role for apoB in limiting LTA-induced expression of this inflammatory cytokine (28). Therefore, these data demonstrate that apoB limits S. aureus-induced inflammatory cytokine production in the lung in both an agr-dependent and an agr-independent manner.
Figure 5. Lipoproteins limit agr-dependent and agr-independent pulmonary cytokine expression.
(A) IL-1β (ANOVA p=0.0035), (B) MIP-2 (ANOVA, p<0.0001) and (C) IL-6 levels in clarified whole lung homogenate of WT and Pcsk9−/− mice (A-C, white and gray boxes, respectively) at 6 hours following intranasal infection with the indicated isolate (N=8 mice per group). Data shown are mean ± SEM. (A-B) ns, not significant; *, p≤0.05, ****, p≤0.0001, ANOVA with Dunnett’s multiple comparison analysis. (C) *, p≤0.05, Student’s t-test.
Lipoproteins regulate agr-dependent neutrophil influx in the lung
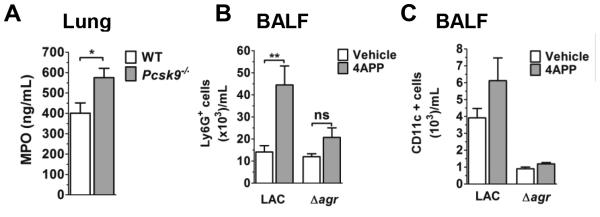
Appropriate recruitment of neutrophils during S. aureus infection is critical for bacterial clearance (43); however, excessive neutrophil recruitment can result in severe injury to sensitive lung tissues (reviewed in (44, 45)). Given that MIP-2 levels were increased in the lungs of lipoprotein deficient mice infected with LAC, but not with LACΔagr, we compared myeloperoxidase (MPO) levels, a surrogate marker of neutrophil influx, in the lungs of LAC-infected Pcsk9−/− mice versus controls. MPO levels were significantly higher in the lungs of lipoprotein deficient mice (Figure 6A), and this paralleled an increase in Ly6G+ cells in BALF from 4APP- versus vehicle-treated mice also infected with LAC (Fig. 6B). In contrast, neutrophil influx and MPO levels did not differ between 4APP- and vehicle-treated mice infected with LACΔagr (Fig. 6B) or LACΔhla (Supplemental Fig. 3), respectively. Furthermore, although macrophage levels overall were increased in BALF from LAC-infected versus LACΔagr-infected mice (Fig. 6C), macrophage presence was independent of circulating lipoprotein levels. Together, these data indicate that lipoproteins are important for early host control of agr-dependent neutrophil influx during S. aureus lung infection.
Figure 6. Lipoproteins limit agr-dependent neutrophil influx.
(A) MPO levels in whole lung homogenate of WT and Pcsk9−/− at 6 hours following intranasal infection with the indicated isolate (N=8 mice per group). (B) Ly6G+ and (C) CD11c+ cells in BALF from vehicle- and 4APP-treated mice at 6 hours post-infection. N=6 (LAC) and 3 (LACΔagr) mice per group. Data shown are mean ± SEM. ns, not significant; *, p≤0.05. **, p≤0.01, Student’s t-test.
ApoB limits agr-dependent cytokine expression by human alveolar epithelial cells
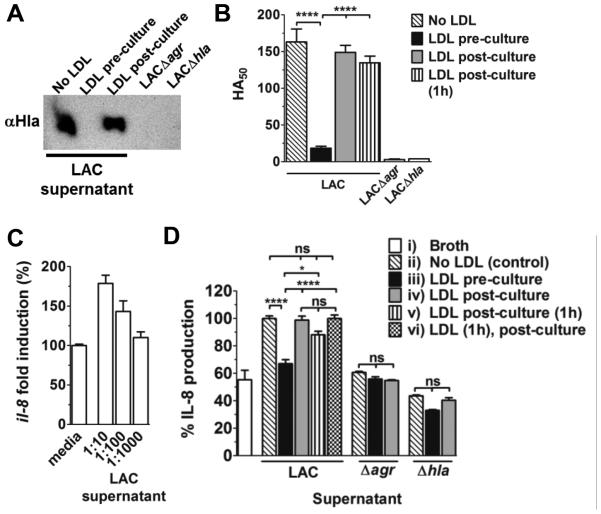
Murine MIP-2 is a functional homologue of human IL-8, and Hla strongly induces IL-8 release by human alveolar epithelial cells at toxin concentrations below those causing cell lysis (46). To demonstrate that lipoproteins protect against early inflammatory cytokine production in the lung via inhibition of agr activation, rather than by direct effects on lung cells or sequestration of agr-regulated virulence factors (28, 47, 48), we measured IL-8 expression by human alveolar epithelial cells exposed to sterile bacterial supernatant from LAC cultured in the presence versus the absence of LDL. Consistent with previous reports (23, 24), LDL limited accumulation of Hla in LAC culture supernatant (Fig. 7A), and thus hemolytic activity (Fig. 7B), whereas LDL incubated with sterile supernatant post-culture, and either used immediately or after a one hour co-incubation, did not inhibit hemolysis. Using A549 cells as a model for human alveolar epithelial cells, LAC supernatant induced il-8 transcription in a dose-dependent manner (Fig. 7C). Furthermore, compared to IL-8 release following exposure to LAC supernatant, IL-8 production by A549 cells was significantly reduced when exposed to supernatant from LAC cultured in the presence of LDL (Fig. 7D). This reduction in IL-8 was not due to direct effects of LDL on the A549 cells as LDL incubated with cells for one hour prior to the addition of LAC supernatant did not inhibit IL-8 production. Also, while there was a small reduction in IL-8 levels when LDL was co-incubated with LAC supernatant for one hour prior to addition to the A549 cells, this reduction did not reach statistical significance. This suggests that the impact of LDL on IL-8 expression in these assays is mainly independent of sequestration of virulence factors or pathogen associated molecular patterns (PAMPs) within the supernatant (28, 47, 48). Importantly, IL-8 production did not significantly differ between cells exposed to supernatant from LAC cultured with LDL and supernatant from cultures of LACΔagr or LACΔhla. Therefore, these results support a mechanism whereby apoB limits S. aureus QS in the lung and protects the host against excessive inflammatory cytokine production in response to agr-regulated virulence factors.
Figure 7. Serum lipoproteins limit agr-dependent cytokine expression by human alveolar epithelial cells.
(A) Western blot analysis and (B) functional assay of Hla in 6 hour sterile supernatant from LAC cultured ± LDL (100 nM), LAC supernatant with LDL (100nM) added post-culture, post-culture supernatant incubated with LDL (100nM) for 1h, or supernatant from LACΔagr or LACΔhla. Data shown are mean ± SEM. ANOVA (p<0.0001) with Dunnett’s multiple comparison post-hoc analysis. ****, p≤0.0001. (C) Transcription of il-8 relative to hprt by A549 cells following 4 hours incubation with LAC sterile supernatant at the indicated dilutions, and (D) IL-8 release after 4 hours exposure to 1:10 dilutions of the following: i) broth control, ii) supernatant from LAC cultured in the absence of LDL, iii) LAC cultured in the presence of LDL (100nM), iv) LAC supernatant with LDL added post-culture, v) post-culture supernatant incubated with LDL for 1h or vi) LDL incubated with cells for 1h followed by addition of post-culture supernatant. Data shown are mean percent (± SEM) of IL-8 production relative to the no LDL (ii) control. ANOVA (p<0.0001) with Bonferroni’s multiple comparison post-hoc analysis. ns, not significant; *, p≤0.05; ****, p≤0.0001.
Discussion
Considerable attention has been given to the role of hyperlipidemia in cardiovascular disease, such that the terms dyslipidemia and hyperlipidemia are often used synonymously (49). This illustrates the limited consideration given to the potential health consequences of the other dyslipidemia, severe hypolipidemia and lipoprotein deficiency, often experienced post-trauma and by other critically ill patients (7-9). Since hypolipidemia has been clinically associated with bacterial pneumonia (10-12), we sought to determine the impact of extremely low circulating lipoprotein levels on host innate defense in the lung. Using a sub-lethal mouse model of S. aureus pneumonia, we demonstrate that lipoprotein deficiency impairs early pulmonary innate defense against bacterial pathogenesis. Specifically, apoB is present in the lung early post-infection and its levels decrease significantly with lipoprotein deficiency. This decrease coincides with impaired host control of S. aureus agr-signaling, as well as increased agr-dependent morbidity and inflammation, in alignment with the role of apoB in controlling S. aureus QS-dependent virulence in the skin (23-25). In addition, lipoprotein deficiency results in an agr-independent increase in pulmonary IL-6 expression, consistent with the ability of apoB to bind LTA and limit LTA-mediated inflammation (28). Given that hyperlipidemia also impairs lung innate defense mechanisms (6), these results strongly suggest that maintenance of normal serum cholesterol and lipoprotein levels is necessary for optimal host innate defense in the lung.
The contribution of host lipoproteins to pulmonary innate immunity is not surprising considering their previously described host defense contributions, together with their demonstrated uptake by lung capillary endothelium (50) and carriage on infiltrating leukocytes (51). For example, circulating lipoproteins, including LDL and high density lipoprotein (HDL), non-specifically bind and sequester LPS, thus limiting endotoxin induced toxicity and lethality (26, 27, 52). In addition, apoB from LDL and apolipoproteins A1 and A2 from HDL bind soluble LTA and inhibit LTA-mediated cytokine release from both human and murine cells (28). The most pronounced inhibition comes from LDL which dose-dependently inhibits LTA-mediated IL-6 expression by human PBMCs. In support of this finding, here we show that lipoprotein deficiency results in significantly increased IL-6 levels in the lungs of S. aureus infected mice independent of agr status. Although in vivo validation of the role of lipoproteins in controlling LTA-mediated inflammation in the lung will require comparison of lipoprotein-sufficient versus-deficient mice following pulmonary administration of LTA or infection with LTA-deficient S. aureus mutants (53, 54), our data and that of others suggests that hypolipidemia may broadly impact post-trauma pneumonia susceptibility to both Gram positive and Gram negative pathogens.
Along with sequestration of AIP, lipoproteins have other roles in host defense against S. aureus pathogenesis. Specifically, LDL has been reported to bind and partially inactivate Hla (48), while the phenol soluble modulins (PSMs) (22, 55, 56), which attract and lyse neutrophils, are also bound by lipoproteins, in particular by HDL (47). In this regard, the data reported here indicate that LDL limits IL-8 production by human alveolar epithelial cells by inhibiting expression and secretion of agr-regulated virulence factors. This is supported by our findings that (i) A549 cells produce IL-8 when exposed to LAC sterile supernatant, but not when exposed to sterile supernatant from LACΔagr or LACΔhla, (ii) that inclusion of LDL during LAC culture limits both Hla accumulation in the supernatant (23, 24) and the ability of the resulting supernatant to induce IL-8 release by A549 cells and (iii) that the addition of LDL to directly to LAC supernatant does not inhibit IL-8 release by A549 cells. Although these data suggest that LDL primarily functions by prevention of virulence factor expression in this system, lipoproteins may also directly inhibit virulence factor function in the lung. Here, pulmonary administration of LAC supernatant, purified Hla or PSMs to serum lipoprotein-sufficient versus -deficient mice would further clarify this issue. Regardless, our findings and those of others (6, 26-28, 47-49) strongly suggest that lipoproteins likely contribute in a variety of both direct and indirect means to pulmonary host innate defense.
In addition to the negative effects of lipoprotein deficiency on pulmonary innate immunity reported here, severe hypolipidemia may also impair the innate defense function of lung surfactant (reviewed in (57)). Lung surfactant is comprised of a mixture of lipids, phospholipids and proteins, and maintenance of the appropriate ratios of these components is required for functional surfactant self-assembly (58-60). Of particular importance to surfactant-mediated host innate defense are the surfactant-associated proteins, SP-A and SP-D, which act as opsonins to promote bacterial clearance (61-65). Since lipoproteins contribute to the regulation of surfactant cholesterol metabolism (66), it is unclear whether severe hypolipidemia results in impaired surfactant assembly and negatively impacts bacterial clearance by altering the distribution or otherwise impairing the antibacterial functions of SP-A and SP-D. While we saw no evidence of impaired pulmonary bacterial clearance in lipoprotein deficient mice at the early post-infection time point investigated here, the potential impact of severe hypolipidemia on pulmonary bacterial clearance warrants further investigation.
By focusing on early control of S. aureus QS in the lung, we have demonstrated one mechanism by which severe hypolipidemia impairs pulmonary host innate defense. Although a significant gap in knowledge remains regarding the full impact of hypolipidemia on infection susceptibility, disease progression and survival during pneumonia caused by S. aureus and other microbial pathogens, the work reported here regarding hypolipidemia, together with the work of others focused on hyperlipidemia (6), represent important advances in understanding the impact of both extremes of dyslipidemia on pulmonary host innate defense. Furthermore, these studies point to the potential clinical impact of severe hypolipidemia on pulmonary innate defense in critically ill patients.
Supplementary Material
Acknowledgments
We thank Dr. Pavan Muttil and Dr. Jon Femling for critical review of the manuscript, and Dr. Hattie Gresham for many helpful discussions.
This work was supported by a National Institute of Allergy and Infectious Diseases, National Institutes of Health (RO1AI091917) (PRH), and by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (MO).
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Elmehdawi R. Hypolipidemia: a word of caution. Libyan J. Med. 2008;3:84–90. doi: 10.4176/071221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daniels TF, Killinger KM, Michal JJ, W. RW, Jr, Jiang Z. Lipoproteins, cholesterol homeostasis and cardiac health. Int. J. Biol. Sci. 2009;5:474–488. doi: 10.7150/ijbs.5.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collaboration TERF. Major Lipids, Apolipoproteins and Risk of Vascular Disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han R. Plasma lipoproteins are important components of the immune system. Microbiol. Immunol. 2010;54:246–253. doi: 10.1111/j.1348-0421.2010.00203.x. [DOI] [PubMed] [Google Scholar]
- 5.Feingold KR, Grunfeld C. Lipids: a key player in the battle between the host and microorganisms. J. Lipid Res. 2012;53:2487–2489. doi: 10.1194/jlr.E033407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madenspacher JH, Draper DW, Smoak KA, Li H, Griffiths GL, Suratt BT, Wilson MD, Rudel LL, Fessler MB. Dyslipidemia induces opposing effects on intrapulmonary and extrapulmonary host defense through divergent TLR response phenotypes. J Immunol. 2010;185:1660–1669. doi: 10.4049/jimmunol.0903501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR, Grunfeld C. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J. Lipid Res. 2004;45:1169–1196. doi: 10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Vyroubal P, Chiarla C, Giovannini I, Hyspler R, Ticha A, Hrnciarikova D, Zadak Z. Hypocholesterolemia in clinically serious conditions–review. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub. 2008;152:181–189. doi: 10.5507/bp.2008.029. [DOI] [PubMed] [Google Scholar]
- 9.Femling JK, West SD, Hauswald EK, Gresham HD, Hall PR. Nosocomial infections after severe trauma are associated with lower apolipoproteins B and AII. The Journal of Trauma and Acute Care Surgery. 2013;74:1067–1073. doi: 10.1097/TA.0b013e3182826be0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gruber M, Christ-Crain M, Stolz D, Keller U, Muller C, Bingisser R, Tamm M, Mueller B, Schuetz P. Prognostic impact of plasma lipids in patients with lower respiratory tract infections - an observational study. Swiss Med. Wkly. 2009;139:166–172. doi: 10.4414/smw.2009.12494. [DOI] [PubMed] [Google Scholar]
- 11.Sankaran RT, Mattana J, Pollack S, Bhat P, Ahuja T, Patel A, Singhal PC. Laboratory abnormalities in patients with bacterial pneumonia. Chest. 1997;111:595–600. doi: 10.1378/chest.111.3.595. [DOI] [PubMed] [Google Scholar]
- 12.Chien YF, Chen CY, Hsu CL, Chen KY, Yu CJ. Decreased serum level of lipoprotein cholesterol is a poor prognostic factor for patients with severe community-acquired pneumonia that required intensive care unit admission. J. Crit. Care. 2015;30:506–510. doi: 10.1016/j.jcrc.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Rubinstein E, Kollef MH, Nathwani D. Pneumonia caused by methicillin-resistant Staphylococcus aureus. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008;46(Suppl 5):S378–385. doi: 10.1086/533594. [DOI] [PubMed] [Google Scholar]
- 14.Nakou A, Woodhead M, Torres A. MRSA as a cause of community-acquired pneumonia. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2009;34:1013–1014. doi: 10.1183/09031936.00120009. [DOI] [PubMed] [Google Scholar]
- 15.Defres S, Marwick C, Nathwani D. MRSA as a cause of lung infection including airway infection, community-acquired pneumonia and hospital-acquired pneumonia. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2009;34:1470–1476. doi: 10.1183/09031936.00122309. [DOI] [PubMed] [Google Scholar]
- 16.Montgomery CP, Boyle-Vavra S, Daum RS. Importance of the global regulators agr and SaeRS in the pathogenesis of CA-MRSA USA300 infection. PLoS One. 2010;5:e15177. doi: 10.1371/journal.pone.0015177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bubeck Wardenburg J, Patel RJ, Schneewind O. Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect. Immun. 2007;75:1040–1044. doi: 10.1128/IAI.01313-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heyer G, Saba S, Adamo R, Rush W, Soong G, Cheung A, Prince A. Staphylococcus aureus agr and sarA functions are required for invasive infection but not inflammatory responses in the lung. Infect. Immun. 2002;70:127–133. doi: 10.1128/IAI.70.1.127-133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parker D, Prince A. Immunopathogenesis of Staphylococcus aureus pulmonary infection. Semin. Immunopathol. 2012;34:281–297. doi: 10.1007/s00281-011-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thoendel M, Kavanaugh JS, Flack CE, Horswill AR. Peptide signaling in the staphylococci. Chem. Rev. 2011;111:117–151. doi: 10.1021/cr100370n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novick RP, Geisinger E. Quorum sensing in staphylococci. Annu. Rev. Genet. 2008;42:541–564. doi: 10.1146/annurev.genet.42.110807.091640. [DOI] [PubMed] [Google Scholar]
- 22.Cheung GY, Wang R, Khan BA, Sturdevant DE, Otto M. Role of the accessory gene regulator agr in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. Infect. Immun. 2011;79:1927–1935. doi: 10.1128/IAI.00046-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peterson MM, Mack JL, Hall PR, Alsup AA, Alexander SM, Sully EK, Sawires YS, Cheung AL, Otto M, Gresham HD. Apolipoprotein B Is an innate barrier against invasive Staphylococcus aureus infection. Cell Host Microbe. 2008;4:555–566. doi: 10.1016/j.chom.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall PR, Elmore BO, Spang CH, Alexander SM, Manifold-Wheeler BC, Castleman MJ, Daly SM, Peterson MM, Sully EK, Femling JK, Otto M, Horswill AR, Timmins GS, Gresham HD. Nox2 Modification of LDL Is Essential for Optimal Apolipoprotein B-mediated Control of agr Type III Staphylococcus aureus Quorum-sensing. PLoS Path. 2013;9 doi: 10.1371/journal.ppat.1003166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elmore BO, Triplett KD, Hall PR. Apolipoprotein B48, the Structural Component of Chylomicrons, Is Sufficient to Antagonize Staphylococcus aureus Quorum-Sensing. PLoS One. 2015;10:e0125027. doi: 10.1371/journal.pone.0125027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris HW, Grunfeld C, Feingold KR, Rapp JH. Human very low density lipoproteins and chylomicrons can protect against endotoxin-induced death in mice. J. Clin. Invest. 1990;86:696–702. doi: 10.1172/JCI114765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Read TE, Harris HW, Grunfeld C, Feingold KR, Kane JP, Rapp JH. The protective effect of serum lipoproteins against bacterial lipopolysaccharide. Eur. Heart J. 1993;14(Suppl K):125–129. [PubMed] [Google Scholar]
- 28.Sigel S, Bunk S, Meergans T, Doninger B, Stich K, Stulnig T, Derfler K, Hoffmann J, Deininger S, Aulock S. v., Knapp S. Apolipoprotein B100 is a suppressor of Staphylococcus aureus-induced innate immune responses in humans and mice. Eur. J. Immunol. 2012;42:2983–2989. doi: 10.1002/eji.201242564. [DOI] [PubMed] [Google Scholar]
- 29.Rothfork JM, Timmins GS, Harris MN, Chen X, Lusis AJ, Otto M, Cheung AL, Gresham HD. Inactivation of a bacterial virulence pheromone by phagocyte-derived oxidants: new role for the NADPH oxidase in host defense. Proc. Natl. Acad. Sci. U. S. A. 2004;101:13867–13872. doi: 10.1073/pnas.0402996101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernheimer AW. Assay of hemolytic toxins. Methods Enzymol. 1988;165:213–217. doi: 10.1016/s0076-6879(88)65033-6. [DOI] [PubMed] [Google Scholar]
- 31.Sully EK, Malachowa N, Elmore BO, Alexander SM, Femling JK, Gray BM, DeLeo FR, Otto M, Cheung AL, Edwards BS, Sklar LA, Horswill AR, Hall PR, Gresham HD. Selective chemical inhibition of agr quorum sensing in Staphylococcus aureus promotes host defense with minimal impact on resistance. PLoS Pathog. 2014;10:e1004174. doi: 10.1371/journal.ppat.1004174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ventura CL, Higdon R, Hohmann L, Martin D, Kolker E, Liggitt HD, Skerrett SJ, Rubens CE. Staphylococcus aureus elicits marked alterations in the airway proteome during early pneumonia. Infect. Immun. 2008;76:5862–5872. doi: 10.1128/IAI.00865-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farese RV, Jr., Ruland SL, Flynn LM, Stokowski RP, Young SG. Knockout of the mouse apolipoprotein B gene results in embryonic lethality in homozygotes and protection against diet-induced hypercholesterolemia in heterozygotes. Proc. Natl. Acad. Sci. U. S. A. 1995;92:1774–1778. doi: 10.1073/pnas.92.5.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mounkes LC, Zhong W, Silva H. V. d., Handumrongkul C, Desai B, Tse E, Taylor JM, Debs RJ. Evaluation of the role of lipoprotein metabolism genes in systemic cationic liposome-mediated gene transfer in vivo. Hum. Gene Ther. 2001;12:1939–1954. doi: 10.1089/104303401753204526. [DOI] [PubMed] [Google Scholar]
- 35.Rashid S, Curtis DE, Garuti R, Anderson NN, Bashmakov Y, Ho YK, Hammer RE, Moon YA, Horton JD. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proc. Natl. Acad. Sci. U. S. A. 2005;102:5374–5379. doi: 10.1073/pnas.0501652102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malone CL, Boles BR, Lauderdale KJ, Thoendel M, Kavanaugh JS, Horswill AR. Fluorescent reporters for Staphylococcus aureus. J. Microbiol. Methods. 2009;77:251–260. doi: 10.1016/j.mimet.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaffin DO, Taylor D, Skerrett SJ, Rubens CE. Changes in the Staphylococcus aureus transcriptome during early adaptation to the lung. PLoS One. 2012;7:e41329. doi: 10.1371/journal.pone.0041329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kebaier C, Chamberland RR, Allen IC, Gao X, Broglie PM, Hall JD, Jania C, Doerschuk CM, Tilley SL, Duncan JA. Staphylococcus aureus alpha-hemolysin mediates virulence in a murine model of severe pneumonia through activation of the NLRP3 inflammasome. J Infect Dis. 2012;205:807–817. doi: 10.1093/infdis/jir846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bubeck Wardenburg J, Schneewind O. Vaccine protection against Staphylococcus aureus pneumonia. J. Exp. Med. 2008;205:287–294. doi: 10.1084/jem.20072208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ragle BE, Wardenburg JB. Anti-alpha-hemolysin monoclonal antibodies mediate protection against Staphylococcus aureus pneumonia. Infect. Immun. 2009;77:2712–2718. doi: 10.1128/IAI.00115-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bubeck Wardenburg J, Williams WA, Missiakas D. Host defenses against Staphylococcus aureus infection require recognition of bacterial lipoproteins. Proc. Natl. Acad. Sci. U. S. A. 2006;103:13831–13836. doi: 10.1073/pnas.0603072103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bartlett AH, Foster TJ, Hayashida A, Park PW. Alpha-toxin facilitates the generation of CXC chemokine gradients and stimulates neutrophil homing in Staphylococcus aureus pneumonia. J Infect Dis. 2008;198:1529–1535. doi: 10.1086/592758. [DOI] [PubMed] [Google Scholar]
- 43.Rigby KM, DeLeo FR. Neutrophils in innate host defense against Staphylococcus aureus infections. Semin. Immunopathol. 2012;34:237–259. doi: 10.1007/s00281-011-0295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol. Med. 2011;17:293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Craig A, Mai J, Cai S, Jeyaseelan S. Neutrophil recruitment to the lungs during bacterial pneumonia. Infect. Immun. 2009;77:568–575. doi: 10.1128/IAI.00832-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rose F, Dahlem G, Guthmann B, Grimminger F, Maus U, Hanze J, Duemmer N, Grandel U, Seeger W, Ghofrani HA. Mediator generation and signaling events in alveolar epithelial cells attacked by S. aureus alpha-toxin. Am. J. Physiol. Lung Cell Mol. Physiol. 2002;282:L207–214. doi: 10.1152/ajplung.00156.2001. [DOI] [PubMed] [Google Scholar]
- 47.Surewaard BG, Nijland R, Spaan AN, Kruijtzer JA, de Haas CJ, van Strijp JA. Inactivation of staphylococcal phenol soluble modulins by serum lipoprotein particles. PLoS Pathog. 2012;8:e1002606. doi: 10.1371/journal.ppat.1002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhakdi S, Tranum-Jensen J, Utermann G, Fussle R. Binding and partial inactivation of Staphylococcus aureus alpha-toxin by human plasma low density lipoprotein. J Biol Chem. 1983;258:5899–5904. [PubMed] [Google Scholar]
- 49.Gowdy KM, Fessler MB. Emerging roles for cholesterol and lipoproteins in lung disease. Pulm. Pharmacol. Ther. 2013;26:430–437. doi: 10.1016/j.pupt.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nistor A, Simionescu M. Uptake of low density lipoproteins by the hamster lung. Isourceteractions with capillary endothelium. Am. Rev. Respir. Dis. 1986;134:1266–1272. doi: 10.1164/arrd.1986.134.6.1266. [DOI] [PubMed] [Google Scholar]
- 51.Alipour A, van Oostrom AJ, Izraeljan A, Verseyden C, Collins JM, Frayn KN, Plokker TW, Elte JW, Castro Cabezas M. Leukocyte activation by triglyceride-rich lipoproteins. Arterioscler. Thromb. Vasc. Biol. 2008;28:792–797. doi: 10.1161/ATVBAHA.107.159749. [DOI] [PubMed] [Google Scholar]
- 52.Feingold KR, Funk JL, Moser AH, Shigenaga JK, Rapp JH, Grunfeld C. Role for circulating lipoproteins in protection from endotoxin toxicity. Infect. Immun. 1995;63:2041–2046. doi: 10.1128/iai.63.5.2041-2046.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oku Y, Kurokawa K, Matsuo M, Yamada S, Lee BL, Sekimizu K. Pleiotropic roles of polyglycerolphosphate synthase of lipoteichoic acid in growth of Staphylococcus aureus cells. J. Bacteriol. 2009;191:141–151. doi: 10.1128/JB.01221-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fedtke I, Mader D, Kohler T, Moll H, Nicholson G, Biswas R, Henseler K, Gotz F, Zahringer U, Peschel A. A Staphylococcus aureus ypfP mutant with strongly reduced lipoteichoic acid (LTA) content: LTA governs bacterial surface properties and autolysin activity. Mol. Microbiol. 2007;65:1078–1091. doi: 10.1111/j.1365-2958.2007.05854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang R, Braughton KR, Kretschmer D, Bach THL, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, Deleo FR, Otto M. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 2007;13:1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 56.Queck SY, Jameson-Lee M, Villaruz AE, Bach THL, Khan BA, Sturdevant DE, Ricklefs SM, Li M, Otto M. RNAIII-Independent Target Gene Control by the agr Quorum-Sensing System: Insight into the Evolution of Virulence Regulation in Staphylococcus aureus. Mol. Cell. 2008;32:150–158. doi: 10.1016/j.molcel.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chroneos ZC, Sever-Chroneos Z, Shepherd VL. Pulmonary surfactant: an immunological perspective. Cell. Physiol. Biochem. 2010;25:13–26. doi: 10.1159/000272047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leonenko Z, Gill S, Baoukina S, Monticelli L, Doehner J, Gunasekara L, Felderer F, Rodenstein M, Eng LM, Amrein M. An elevated level of cholesterol impairs self-assembly of pulmonary surfactant into a functional film. Biophys. J. 2007;93:674–683. doi: 10.1529/biophysj.107.106310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nahak P, Nag K, Hillier A, Devraj R, Thompson DW, Manna K, Makino K, Ohshima H, Nakahara H, Shibata O, Panda AK. Effect of serum, cholesterol and low density lipoprotein on the functionality and structure of lung surfactant films. Journal of Oleo Science. 2014;63:1333–1349. doi: 10.5650/jos.ess14071. [DOI] [PubMed] [Google Scholar]
- 60.Suzuki Y, Tabata R. Selective reduction of phosphatidylglycerol and phosphatidylcholine in pulmonary surfactant by 4-aminopyrazolo(3,4d)pyrimidine in the rat. J. Lipid Res. 1980;21:1090–1096. [PubMed] [Google Scholar]
- 61.Ariki S, Nishitani C, Kuroki Y. Diverse functions of pulmonary collectins in host defense of the lung. J. Biomed. Biotechnol. 20122012:532071. doi: 10.1155/2012/532071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCormack FX, Whitsett JA. The pulmonary collectins, SP-A and SP-D, orchestrate innate immunity in the lung. J. Clin. Invest. 2002;109:707–712. doi: 10.1172/JCI15293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hartshorn KL, Crouch E, White MR, Colamussi ML, Kakkanatt A, Tauber B, Shepherd V, Sastry KN. Pulmonary surfactant proteins A and D enhance neutrophil uptake of bacteria. Am. J. Physiol. 1998;274:L958–969. doi: 10.1152/ajplung.1998.274.6.L958. [DOI] [PubMed] [Google Scholar]
- 64.Sever-Chroneos Z, Krupa A, Davis J, Hasan M, Yang CH, Szeliga J, Herrmann M, Hussain M, Geisbrecht BV, Kobzik L, Chroneos ZC. Surfactant protein A (SP-A)-mediated clearance of Staphylococcus aureus involves binding of SP-A to the staphylococcal adhesin eap and the macrophage receptors SP-A receptor 210 and scavenger receptor class A. J Biol Chem. 2011;286:4854–4870. doi: 10.1074/jbc.M110.125567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kantyka T, Pyrc K, Gruca M, Smagur J, Plaza K, Guzik K, Zeglen S, Ochman M, Potempa J. Staphylococcus aureus proteases degrade lung surfactant protein A potentially impairing innate immunity of the lung. J. Innate Immun. 2013;5:251–260. doi: 10.1159/000345417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hass MA, Longmore WJ. Regulation of lung surfactant cholesterol metabolism by serum lipopoteins. Lipids. 1980;15:401–406. doi: 10.1007/BF02534063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.