Abstract
In the present study, we investigated genetic and environmental effects on motor impulsivity from childhood to late adolescence using a longitudinal sample of twins from ages 9 to 18 years. Motor impulsivity was assessed using errors of commission (no-go errors) in a visual go/no-go task at 4 time points: ages 9–10, 11–13, 14–15, and 16–18 years. Significant genetic and nonshared environmental effects on motor impulsivity were found at each of the 4 waves of assessment with genetic factors explaining 22%–41% of the variance within each of the 4 waves. Phenotypically, children’s average performance improved across age (i.e., fewer no-go errors during later assessments). Multivariate biometric analyses revealed that common genetic factors influenced 12%–40% of the variance in motor impulsivity across development, whereas nonshared environmental factors common to all time points contributed to 2%–52% of the variance. Nonshared environmental influences specific to each time point also significantly influenced motor impulsivity. Overall, results demonstrated that although genetic factors were critical to motor impulsivity across development, both common and specific nonshared environmental factors played a strong role in the development of motor impulsivity across age.
Keywords: motor impulsivity, go/no-go task, twins, genetic and environmental effects
Impulsivity is a highly researched behavioral trait that has captured the interest of researchers, clinicians, and psychiatrists alike (Congdon & Canli, 2008; Evenden, 1999; Eysenck & Eysenck, 1977; Costa & McCrae, 1992; Cloninger, Svrakic, & Przybeck, 1993; Tellegen, 1982). Undeniably, impulsivity is of key importance and appears in one form or another in almost every major personality system and numerous psychiatric disorders (Whiteside & Lynam, 2001). It encompasses a wide array of traits that are only moderately related (Fischer, Smith, & Cyders, 2008; Smith, Fischer, Cyders, Annus, & Spillane, 2007; Whiteside & Lynam, 2001) and is thus considered a complex multifactorial construct with multiple manifestations (Dick et al., 2010). Several studies have shown that genetic effects are important in impulsive traits (Anokhin, Golosheykin, Grant, & Heath, 2011; Anokhin, Heath, & Meyers, 2004; Dougherty et al., 2003; Finkel & McGue, 1997; Niv, Tuvblad, Raine, Wang, & Baker, 2011; Schachar, Forget-Dubois, Dionne, Boivin, & Robaey, 2011; Young et al., 2009). However, no study has yet examined how genetic and environmental factors influences impulsivity across age and development. In the present study, we aimed to address this gap in the literature by investigating genetic and environmental effects on the development of one facet of impulsivity—motor impulsivity’ (errors of commission; Halperin, Wolf, Greenblatt, & Young, 1991; Horn, Dolan, Elliot, Deakin, & Woodruff, 2003; Saunders et al., 2008)—during a go/no-go task in 9- to 18-year-old twins studied on multiple occasions.
Impulsivity Is a Stable Trait
Several studies have investigated various forms of impulsive behaviors using either self-report surveys (Dougherty et al., 2003; Eysenck & Eysenck, 1977; Finkel & McGue, 1997; Patton, Stanford, & Barratt, 1995), which tend to be broad and encompass multiple facets of impulsivity, or laboratory tasks (Aron & Poldrack, 2005; Chamberlain & Sahakian, 2007; Evenden, 1999), which typically assess narrower forms of impulsivity. To this end, these studies have generally demonstrated that impulsive behaviors tend to be stable across development.
Heritability of Impulsivity
Numerous studies have examined the extent of genetic and environmental influences on impulsive behaviors using self-report surveys. These studies have demonstrated the heritability of impulsive traits to range between 20% and 60% (Dougherty et al., 2003; Finkel & McGue, 1997; Niv et al., 2011; Schachar et al., 2011; Young et al., 2009). Additional studies have also examined the genetic and environmental etiology of behaviors of disinhibition using laboratory-based measures, including the go/no-go task and a delayed discounting task (Anokhin et al., 2004, 2011). In a recent study investigating the heritability of response inhibition (as measured by event-related potential components N2 and P3) using a go/no-go task in 194 female twins who were 18–28 years old, it was demonstrated that 60% of the variance in N2 and P3 amplitudes was attributed to genetic factors (Anokhin et al., 2004).
To date, only a handful of studies have explored the genetic and environmental effects on laboratory-based measures of impulsive behaviors using a longitudinal twin design. For example, a recent study exploring the heritability of delayed discounting (a purported component of impulsivity) in a longitudinal sample of 12- and 14-year-old adolescent twins demonstrated that the heritability of delayed discounting was 30% and 51% (at ages 12 and 14 years, respectively). No age-specific genetic effects at age 14 years existed, suggesting that the same genetic factors may be influencing these traits at both ages (Anokhin et al., 2011). The present study expands on previous findings by investigating genetic and environmental effects on in motor impulsivity throughout the course of development from childhood (ages 9–11 years) to late adolescence (ages 16–18 years).
Motor Impulsivity and Go/No-Go Tasks
During go/no-go tasks, participants are instructed to make quick motor responses to go trials but withhold their responses on no-go trials. By including more go than no-go trials, responses are rendered prepotent. Motor impulsivity is assessed in terms of the number of inappropriate motor responses to no-go stimuli, referred to as errors of commission. The go/no-go task demonstrates reasonable test–retest reliability (Kindlon, Mezzacappa, & Earls, 1995) and requires participants to respond to the presence of a target stimulus amidst a stream of similar stimuli (for a more detailed overview of the go/no-go task, see Bezdjian, Baker, Lozano, & Raine, 2009). Because errors of commission (no-go errors) are responses that occur when no response is required, they are presumed to reflect impulsivity (Halperin et al., 1991). Disinhibited children respond too quickly and too often when they are required to wait and watch for events, as is often seen in impulsive errors (errors of commission; Corkum & Siegel, 1993). Males also commit more impulsive (no-go) errors than females do during continuous performance-type tasks (Newcorn et al., 2001). Studies have also found impulsive responding declines with age, while peaking during late childhood (Steinberg, 2010).
Present Study
No study has yet examined the extent to which genetic and environmental factors influence motor impulsivity across development from childhood through late adolescence using the go/no-go task. Thus, with the present study, we aimed to fill this gap in the literature. On the basis of a longitudinal twin sample of male and female twins assessed on four occasions across development from ages 9 to 18 years, we examined (a) how genetic and environmental factors influence motor impulsivity within each of the four measurement time points and (b) the extent to which genetic and environmental factors influence motor impulsivity across development using an independent pathway model. In an independent pathway model, the genetic and environmental effects are of two types: general (or common) and specific. The model specifies that a general or shared latent genetic, a single shared environmental factor, and a single nonshared environmental factor together may explain the covariation common for the no-go response across the four time points. The model also specifies genetic, shared environmental, and nonshared environmental factors specific to the no-go response at each of the four time points. Thus, the model decomposes both the variance of each time point and the covariance among the four time points into their genetic and environmental sources. The strength of this model is that it may help to clarify the effects of genetic and environmental influences on impulsivity over time. For instance, over time, genetic effects may become more or less prominent with the accumulation of new environmental changes and effects. Within such a model, development may be considered a longitudinal process of incorporating new genetic and environmental influences into the no-go response. Thus, performance in Wave 1 (or Time 1) may directly influence performance at later times (Eaves, Long, & Heath, 1986). Because impulsive behaviors have been shown to be stable over time (Anokhin et al., 2011; Bezdjian, Baker, & Tuvblad, 2011; Evenden, 1999; Finkel & McGue, 1997), we expected that motor impulsivity would be influenced by genetic factors across development. At the same time, because experiences presumably influence development, we also expected that environmental effects would significantly contribute to the development of motor impulsivity. Generally, we do not expect these effects to be different between males and females.
Method
Participants
The sample comprised participants from the University of Southern California (USC) Twin Study of Risk Factors for Antisocial Behavior (RFAB). The RFAB study is a longitudinal study assessing the development of aggressive and antisocial behaviors from childhood to late adolescence using a community sample of twins. For complete study protocol details, see Baker et al., 2013. The present analyses were based on data from four waves of assessment conducted from 2000 to 2012 (when participants were 9–18 years old). The first wave of assessment was conducted in 2000–2004 when the children were 9–10 years old (N = 614 twin pairs, Mage = 9.60 years, SD = 0.59), the second wave of assessment was conducted in 2002–2006 when the children were 11–13 years old (N = 445 twin pairs, Mage = 11.79 years, SD = 0.92), the third wave of assessment was conducted in 2006–2010 when the children were 14–15 years old (N = 604 twin pairs, Mage = 14.87 years, SD = 0.87), and the fourth wave was conducted in 2008–2012 when the twins were 16–18 years old (N = 504 twin pairs, Mage = 17.28 years, SD = 0.77). The retention rate of families from wave to wave was approximately 75%–80%; however, our overall retention rate for Wave 1 participants was 86%, representing original Wave 1 families who participated in at least one subsequent wave to date. Additional families were recruited throughout the course of this longitudinal study (specifically, 166 new families were recruited at Wave 3); thus, the overall sample for the present analyses includes 1,516 twins (for a more detailed description, see Baker et al., 2013).
Zygosity was based on DNA microsatellite analysis (>7 concordant and 0 discordant markers = monozygotic [MZ]; one or more discordant markers = dizygotic [DZ]) for 87% of the same-sex twin pairs. For the remaining same-sex twin pairs, zygosity was established by questionnaire items about the twins’ physical similarity and the frequency with which people confuse them. The questionnaire was used only when DNA samples were insufficient for one or both twins. When both questionnaire and DNA results were available, there was 90% agreement between the two. For total number of participants with data on the no-go task broken down by zygosity and sex, please see Tables 1 and 2 (for information pertaining to zygosity).
Table 1.
No-Go Errors: Means, Standard Deviations, and Phenotypic Correlations Across Waves 1, 2, 3, and 4
| Wave and participants | Males
|
Females
|
Participants
|
Phenotypic correlations across waves
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | Pairs | Individuals | Wave 1 | Wave 2 | Wave 3 | |
| Wave 1 Ages 9–10 | 16.11a | 5.51 | 11.71 | 5.37 | 560 | 1,156 | — | ||
| Wave 2 Ages 11–13 | 14.71a,b | 6.43 | 8.96 | 5.69 | 179 | 366 | .53** | — | |
| Wave 3 Ages 14–15 | 12.23a,b | 6.52 | 8.08 | 5.46 | 426 | 863 | .41** | .63** | — |
| Wave 4 Ages 16–18 | 9.70a,b | 6.33 | 6.44 | 4.68 | 226 | 463 | .31** | .62** | .60** |
Note. Means and standard deviations are presented on untransformed data.
There were significant mean differences in no-go errors between males and females.
There were significant mean differences in no-go errors across waves.
p < .01.
Table 2.
Twin Correlations for Mean No-Go Errors During Waves 1–4
| Wave and participants | Males
|
Females
|
Opposite sex
|
||
|---|---|---|---|---|---|
| MZ | DZ | MZ | DZ | DZ | |
| Wave 1 (N = 560) | |||||
| Correlation | .22* | .19 | .30** | .23* | .11 |
| n | 125 | 79 | 137 | 85 | 134 |
| Wave 2 (N = 179) | |||||
| Correlation | .36* | .01 | .53** | .20 | .06 |
| n | 45 | 24 | 44 | 28 | 38 |
| Wave 3 (N = 426) | |||||
| Correlation | .21 | .01 | .30** | .08 | .07 |
| n | 86 | 68 | 94 | 77 | 101 |
| Wave 4 (N = 226) | |||||
| Correlation | .38** | .13 | .25 | .23 | .06 |
| n | 46 | 56 | 38 | 36 | 50 |
Note. Ns represent twin pairs. MZ = monozygotic twins; DZ = dizygotic twins.
p < .05.
p < .01.
Procedures
Participants were invited to USC to take part in the study, which involved an approximately six- to eight-hour laboratory assessment, divided into two parts. The first part included both behavioral interviews and neurocognitive testing, whereas the second part involved psychophysiological assessment. One twin would participate in the first part, while the other would participate in the second part, they would then switch places. In the meantime, the parent or primary caregiver, typically the biological mother (>90%), would complete all measures and interviews on one twin and then would answer items about the second twin.
Examiners consisted of full- or part-time staff members with a bachelor’s degree, as well as USC graduate students and upper-class undergraduates. All examiners were rigorously trained (approximately 3–4 weeks) on the psychophysiological and neuropsychological testing procedures and in the administration of the behavioral interviews. Training included interexaminer reliability checks, videotaped monitoring to ensure strict adherence to standardized testing protocols, and supervised training sessions for all aspects of testing. A more detailed description of the study sample, design, and procedures can be found in Baker, Barton, Lozano, Raine, and Fowler (2006) and Baker et al. (2013).
Motor Impulsivity: The Go/No-Go Task
Assessment of motor impulsivity in the twins was made using the go/no-go task. The go/no-go task is a response inhibition task in which a motor response must be either executed or inhibited. During this task, participants were required to watch a sequential presentation of letters and respond to a target letter by pressing a button. A single letter (P or R) was then presented for a duration of 500 ms with an interstimulus interval of 1,500 ms. Participants were asked to press a button in response to a target letter (P or R) and withhold their response to the nontarget letter for a total of 320 trials. The ratio of targets to nontargets was 80:20. Prior to the actual task, a practice session was administered to ensure participants fully comprehended the task. Behavioral performance of the task was assessed by calculating four values in each condition: (a) correct responses to the target (go) letter (hits), (b) errors of omission (misses) of the go letter, (c) errors of commission (false alarms; i.e., responding incorrectly to the no-go letter), and (d) correct rejections of the no-go letter (Bezdjian et al., 2009). No-go errors have been demonstrated to be an indicator of impulsivity (Barkley, 1991; Halperin et al., 1991). Mean errors of commission (or no-go errors) were used to assess motor impulsivity in the present study.
The go/no-go task was presented using stimulus presentation software from the James Long Company (Long, 2005). The examiner would conduct a brief practice run of the task (10–15 trials) to ensure that the children understood the full extent of the task. Once the examiner was confident that the participant fully understood the task, he or she would reiterate the task instructions and leave the room, allowing the participant to begin the task. Participants were monitored through video and audio equipment at all times during each session. The task was run from a remote computer in an adjacent control room operated by the examiner. The duration of the entire go/no-go task was approximately 10 min, including task instructions.
Attrition Analyses
Selective attrition may bias estimates in longitudinal analyses (Heath, Madden, & Martin, 1998; Wothke, 2000; Yuan, Bentler, & Zhang, 2005). We therefore tested whether twins and their families who did not participate in the no-go task during Wave 2, Wave 3, or Wave 4 differed from those who participated, on the basis of several family and individual characteristics measured during Wave 1. Logistic regression analyses showed nonsignificant odds ratios (ORs) for socioeconomic status (OR = 0.99, 95% CI [0.98, 1.01]), twin’s gender (OR =.79, 95% CI [0.59, 1.07]), interview language (OR = 1.13, 95% CI [0.76, 1.69]), and no-go errors at Wave 1 (OR = 0.98, 95% CI [0.95, 1.00]). However, participants and nonparticipants were significantly different in ethnicity (OR = 0.70, 95% CI [0.50, 0.99]), indicating Caucasians were slightly less likely to drop out. Apart from the slight ethnic difference, those that did not participate in follow-up assessments did so in a random manner.
Design
Descriptive statistics
Descriptive statistics, including means, standard deviations, and Pearson correlations, were examined at each time point. A 2 (sex) × 4 (wave) analysis of variance (ANOVA) was conducted to examine mean differences across the four waves in males and females. A mixed-model analysis was used to take into account the dependent (paired) nature of the data.
Biometric modeling, or the classical twin design
Biometric modeling was used to perform the genetic model-fitting analyses in Mx (Neale, Boker, Xie, & Maes, 2003). A basic twin model estimates the relative influence of genetic or heritable (A), shared environmental (C), and nonshared environmental (E) components of variance for a specific phenotype of interest, in our case, the no-go (motor impulsivity) response. Shared environmental factors refer to the environmental factors (e.g., family structure, socioeconomic status, neighborhood characteristics) that produce similarities in the levels of the no-go response between twins living in the same home. Nonshared environmental factors refer to environmental influences that produce differences in levels of the no-go response between twins living in the same home. Nonshared environment also includes and considers measurement error. Heritability is the proportion of total phenotypic variance due to genetic variation. This parameter is time and population specific, and it is a population, not an individual parameter. Further, as it is assumed that MZ twins share 100% of their segregating genes and DZ twins share, on average, 50% of their segregating genes, the correlation between the genetic factors is thus fixed accordingly (MZ = 1.0, DZ = 0.5). Other assumptions present within this twin model are that there are shared environmental effects that influence both MZ and DZ twins equally; therefore, the correlation between the latent shared environmental factors is fixed at 1.0 for both sets of twins. This model also assumes that the total variance can be explained by the additive combination of separate genetic and environmental influences, which sum to 100% or 1.0 (Neale et al., 2003; Plomin, DeFries, McClearn, & McGuffin, 2001; Posthuma et al., 2003).
Univariate biometric models
Univariate biometric models were first fit to estimate the relative contributions of genetic, shared environmental, and nonshared environmental factors to the variance in impulsivity. These models included saturated covariance models to estimate the variance–covariance matrices within each of the five zygosity groups (MZ males, MZ females, DZ males, DZ females, and DZ opposite sex twins) as well as univariate biometric models to determine the magnitude and significance of genetic, shared twin environment, and nonshared environment variance within each time point. Univariate biometric models were compared with baseline saturated models, which perfectly capture the observed variances, covariances, and means for each twin and zygosity group. To test for sex differences, we compared two models. In the first model, the genetic and environmental variance components were freely estimated, and in the second model, they were constrained to be equal. A series of univariate models were first fit to investigate the (genetic and environmental) nature of (motor) impulsivity across development.
Multivariate biometric models
A series of multivariate models were also fit to further investigate the genetic and environmental etiology of motor impulsivity. In additional to multivariate saturated covariance models, we fit multivariate models to determine the magnitude and significance of genetic, shared twin environment, and nonshared environment variance for each time point, as well as to understand the nature of the covariance between each time point, that is, the extent to which genetic and environmental aspects may influence the shared genetic and environmental influences across all four time points. In particular, we fit a Cholesky decomposition and an independent pathway model to the data. A Cholesky decomposes the variance of motor impulsivity within each time point as well as the covariance of genetic (A), shared environmental (C) and nonshared (E) environmental factors across all time points. Thus, a Cholesky decomposition has the same number of factors in each of the A, C, and E components as the number of variables observed. An independent pathway model estimates the genetic and environmental influences common to motor impulsivity as well as the genetic and environmental influences specific to each time point. An independent pathway estimates fewer parameters compared with a Cholesky decomposition and is, therefore, a more parsimonious model.
All biometric models were fit with the structural equation program Mx (Neale et al., 2003), using a full information maximum-likelihood estimation procedure for raw data. Raw maximum likelihood yields a goodness-of-fit index called log-likelihood. The goodness of fit was also compared using a chi-square statistic. The fit of the models was also assessed with the root-mean-square error of approximation (RMSEA), with values below .05 indicating a good fit (Rigdon, 1996), as well as the Akaike information criterion (AIC; Akaike, 1987) and the Bayesian information criterion (BIC; Raftery, 1995), in which smaller values correspond to better fitting models. Prior to any analyses, no-go errors were rank standardized to account for the skewness in the data.
Results
Descriptive Statistics
Table 1 provides the means and standard deviations of no-go errors as well as the total number of participants within each wave, along with the phenotypic correlations among no-go errors across the three waves. A 2 (sex) × 4 (wave) ANOVA was conducted to examine mean differences across the four waves in males and females. A mixed-model analysis was used to take into account the dependent (paired) nature of the data. Results indicated a significant Sex × Wave interaction, t(1226) = 1.97, p =.05. Additionally, mean comparisons of no-go errors significantly decreased from one wave to the next (Waves 1–4), and males committed significantly more no-go errors, on average, than females did in each wave: In Wave 1, t(1154) = 13.76, p < .001; in Wave 2, t(364) = 9.06, p < .001; in Wave 3, t(861) = 10.16, p < .001; in Wave 4, t(460) = 6.31, p < .001.
Twin Correlations
Twin correlations for mean no-go errors (presented in Table 2) appear to be higher in MZ twins than in DZ twins for both males and females within each of the four waves in nearly all cases, suggesting that some genetic effects influenced motor impulsivity during childhood and adolescence.
Univariate Biometric Analysis
Univariate biometric models were fit to mean no-go errors separately for each of the four waves. Results indicated that a model with both genetic and nonshared environmental (AE) effects and the biometric parameters for males and females set to be equal fit the data best for all four waves (see Table 3). For example, the best fitting model for Wave 1 was Model 3 with shared environmental effects set to zero and AE parameters equated for males and females, χ2(13) = 9.82, p =.64, RMSEA < 0.01. Model 3 was the best fitting model for all four waves (see Table 3). Thus, genetic and nonshared environmental effects significantly accounted for the total variance in motor impulsivity during each wave of assessment: In Wave 1, A = 27%, E = 73%; in Wave 2, A = 41%, E = 59%; in Wave 3, A = 22%, E = 78%; in Wave 4, A = 28%, E = 72%.
Table 3.
Univariate Biometric Results for No-Go Errors
| Model | −2LL | AIC | BIC | Δχ2 | Δdf | p | RMSEA | Compared with model no. |
|---|---|---|---|---|---|---|---|---|
| Wave 1 | ||||||||
| 1. Saturated model | 3,087.93 | 795.93 | −2,117.64 | |||||
| 2. ACE (males ≠ females) | 3,095.69 | 785.69 | −2,142.52 | 7.76 | 9 | .55 | <0.01 | 1 |
| 2a. ACE (males = females) | 3,097.64 | 781.64 | −2,151.13 | 1.95 | 3 | .58 | <0.01 | 2 |
| 3. AE (males = females) Drop C | 3,097.75 | 779.75 | −2,154.27 | 0.11 | 1 | .71 | <0.01 | 2a |
| 3a. CE (males = females) Drop A | 3,099.68 | 781.69 | −2,153.30 | 2.04 | 1 | .20 | <0.01 | 2a |
| 3b. E only (males = females) Drop A, C | 3,123.61 | 803.61 | −2,144.53 | 25.97 | 2 | <.01 | 0.052 | 2a |
| A = (−0.51)2 = 0.27; 95% CI [.17, .37] | ||||||||
| E = (−0.85)2 = 0.73; 95% CI [.63, .83] | ||||||||
|
| ||||||||
| Wave 2 | ||||||||
| 1. Saturated model | 939.50 | 239.50 | −445.69 | |||||
| 2. ACE (males ≠ females) | 950.57 | 232.57 | −463.70 | 11.06 | 9 | .27 | 0.036 | 1 |
| 2a. ACE (males = females) | 952.52 | 228.52 | −470.57 | 1.95 | 3 | .58 | 0.022 | 2 |
| 3. AE (males = females) Drop C | 952.52 | 226.52 | −473.19 | 0 | 1 | .99 | <0.01 | 2a |
| 3a. CE (males = females) Drop A | 958.48 | 232.48 | −470.21 | 5.96 | 1 | .04 | 0.05 | 2a |
| 3b. E only (males = females) Drop A, C | 970.05 | 242.05 | −467.03 | 17.53 | 2 | <.01 | 0.08 | 2a |
| A = (−0.64)2 = 0.41; 95% CI [.23, .56] | ||||||||
| E = (−0.76)2 = 0.59, 95% CI [.44, .77] | ||||||||
|
| ||||||||
| Wave 3 | ||||||||
| 1. Saturated model | 2,383.85 | 657.85 | −1,446.13 | |||||
| 2. ACE (males ≠ females) | 2,391.96 | 647.96 | −1,469.58 | 8.11 | 9 | .52 | <0.01 | 1 |
| 2a. ACE (males = females) | 2,394.04 | 644.04 | −1,477.71 | 2.08 | 3 | .55 | <0.01 | 2 |
| 3. AE (males = females) Drop C | 2,394.04 | 642.042 | −1,480.77 | 0 | 1 | .99 | <0.01 | 2a |
| 3a. CE (males = females) Drop A | 2,397.30 | 645.30 | −1,479.14 | 3.26 | 1 | .07 | 0.01 | 2a |
| 3b. E only (males = females) Drop A, C | 2,405.21 | 651.21 | −1,478.25 | 11.17 | 2 | <.01 | 0.035 | 2a |
| A = (−0.45)2 = 0.22, 95% CI [.09, .34] | ||||||||
| E = (−0.86)2 = 0.78, 95% CI [.66, .91] | ||||||||
|
| ||||||||
| Wave 4 | ||||||||
| 1. Saturated model | 1,244.74 | 354.74 | −593.33 | |||||
| 2. ACE (males ≠ females) | 1,251.84 | 343.836 | −614.37 | 7.096 | 9 | .63 | <0.01 | 1 |
| 2a. ACE (males = females) | 1,258.708 | 644.04 | −619.131 | 6.87 | 3 | .08 | 0.03 | 2 |
| 3. AE (males = females) Drop C | 1,258.708 | 342.708 | −621.86 | 0 | 1 | .99 | 0.02 | 2a |
| 3a. CE (males = females) Drop A | 1,260.66 | 344.66 | −620.88 | 1.95 | 1 | .40 | 0.03 | 2a |
| 3b. E only (males = females) Drop A, C | 1,270.139 | 352.139 | −618.880 | 11.43 | 2 | <.01 | 0.06 | 2a |
| A = (−0.50)2 = 0.28, 95% CI [0.12, 0.42] | ||||||||
| E = (−0.81)2 = 0.72, 95% CI [0.58, 0.88] | ||||||||
Note. Univariate models were run on transformed data. −2LL = −2(log-likelihood); AIC = Akaike’s information criterion; BIC = Bayesian information criterion; χ2 = difference in log-likelihoods between nested models; Δdf = change in degrees of freedom; RMSEA = root-mean-square error of approximation; A = additive genetic influences; C = shared environmental influences; E = nonshared environmental influences. Best-fitting models and parameter estimates are indicated in bold.
Multivariate Biometric Models
A summary of the various multivariate genetic models fit to the data are provided in Table 4. Specifically, a fully saturated model (Model 1) was used as a baseline comparison for a Cholesky decomposition (Model 2) and an independent pathway model (Models 3, 3a–3c). A one-factor independent pathway model demonstrated the best fit, with shared environmental effects set to zero and the parameters for males and females set to equal each other: In Model 3c, χ2(175) = 205.54, p =.06, RMSEA = 0.031. Thus, constraining parameters to be equal for males and females did not results in a significant loss in fit (Model 3a); therefore, we constrained the sexes to be equal in all subsequent models. Additional constraints on the independent pathway model revealed that both common (Cc) and specific shared environmental effects (Cs) could be dropped without a significant loss in fit, as demonstrated by the AIC, BIC, and RMSEA. Common genetic effects (Ac) could not be dropped from the model, whereas specific genetic effects (As) could be dropped from all waves except for Wave 4 (Model 3c).
Table 4.
Fit Indices for Multivariate Genetic Models of No-Go
| Model | −2LL | AIC | BIC | Δχ2 | Δdf | p | RMSEA |
|---|---|---|---|---|---|---|---|
| 1. Saturated model (means constrained) | 7,101.16 | 1,797.16 | −5,239.96 | 33.60 | 24 | .092 | 0.047 |
| 2. Multivariate Cholesky ACE, males ≠ females | 7,262.53 | 1,702.53 | −5,583.55 | 161.38 | 128 | .024 | 0.038 |
| 2a. Multivariate Cholesky ACE, males = females | 7,297.86 | 1,677.86 | −5,665.33 | 35.33 | 30 | .23 | 0.037 |
| 3. One-factor independent pathway, males ≠ females | 7,273.36 | 1,689.36 | −5,617.91 | 172.21 | 140 | .033 | 0.036 |
| 3a. One-factor independent pathway, males = females | 7,304.84 | 1,672.84 | −5,681.73 | 31.48 | 24 | .14 | 0.037 |
| 3b. One-factor independent pathway, males = females, drop all Cs | 7,305.05 | 1,657.05 | −5,708.13 | .21 | 8 | .99 | 0.032 |
| 3c. One-factor independent pathway, males = females, drop all Cs and specific As (Waves 1–3) | 7,306.69 | 1,652.69 | −5,717.26 | 1.65 | 9 | .99 | 0.031 |
Note. −2LL = −2(log-likelihood); AIC = Akaike’s information criterion; BIC = Bayesian information criterion; χ2 = difference in log-likelihoods between models; Δdf = change in degrees of freedom; RMSEA = root-mean-square error of approximation; A = additive genetic influences; C = shared environmental influences; E = nonshared environmental influences. Best-fitting model is presented in bold.
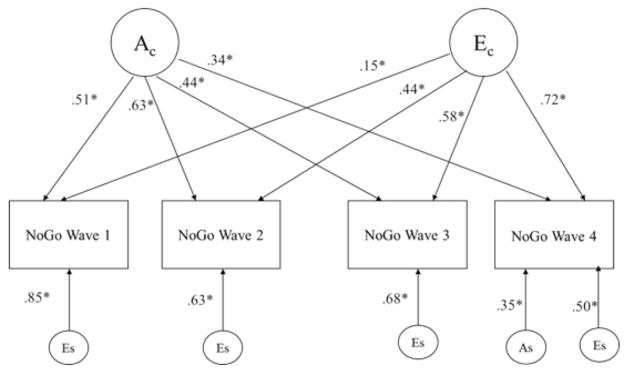
The squared standardized parameter estimates from the one-factor AE independent pathway model are presented in Figure 1. All parameters shown were significant at p < .05, illustrating overlapping genetic and nonshared environmental effects on motor impulsivity across development. Specific nonshared environmental effects also significantly influenced motor impulsivity across development, suggesting that unique experiences specific to each time point were important in motor impulsivity.
Figure 1.
Best fitting one-factor independent pathway model (parameter estimates for females set to equal males). Ac = common genetic effects; Ec = common nonshared environmental effects; Es = specific nonshared environment effects; As = specific genetic effects. All parameters shown were significant at p < .05.
Although shared etiology in motor impulsivity is evident, time-specific influences (both genetic and nonshared environmental, particularly in Wave 4) on motor impulsivity also played an important role. Summing the squared genetic paths for each time point yields the heritability estimate, while summing the squared nonshared environmental paths yields the influence of nonshared environmental effects on motor impulsivity. To illustrate an example and demonstrate the heritability and nonshared environmental influences presented in Figure 1, for Wave 1, heritability (h2) or common A = (Ac) = (0.51)2 yielded a heritability estimate of 26%, whereas common and specific nonshared environment (e2) = Ec (0.15)2 = 0.02 + Es (0.85)2 = 0.72 yielded a total estimate of 74% (2% + 72%), respectively. Thus, 26% of the total variance in the go/no-go task during Wave 1 was influenced by genetic factors, whereas 74% of the total variance was explained by non-shared environment. For Wave 2, heritability (common A) = Ac = (0.63)2 explained approximately 40% of the variance, while common and specific nonshared environment Ec = (0.44)2 + Es = (0.63)2 together explained approximately 60% of the variance in impulsive responding. For Wave 3, heritability = (0.44)2, which accounted for approximately 20% of the variance and common and specific nonshared environment = Ec = (0.58)2 + Es = (0.68)2 together explained 80% of the variance in impulsive responding. Finally, for Wave 4, heritability (both common and specific A) Ac = (0.34)2 = 0.12 + As = (0.35)2 = 0.12 together accounted for 24% of the variance, while nonshared environment = Ec = (0.72)2 + Es = (0.50)2 accounted for 76% of the variance in impulsive responding during the go/no-go task.
Thus, common genetic effects (Ac) explained 12%– 40% of the variance in motor impulsivity across the four time points, whereas common nonshared environmental effects (Ec) explained 2%–52% of the variance in motor impulsivity (see Figure 1). Nonshared environmental effects specific to each time point (Es) explained 25%–72% of the variance found in motor impulsivity, whereas specific genetic effects (As) significantly explained 12% of the variance in motor impulsivity, but only in Wave 4. As demonstrated by these results, Ac seems to be exerting less influence over time, whereas Ec is exerting greater influence over time but Es decreases in magnitude across the four time points.
Discussion
The present study provides one of the first reports from a large, longitudinal twin study to examine genetic and environmental effects on motor impulsivity (no-go errors) across age, in 9- to 18-year-old twins. Phenotypic analyses of mean levels revealed that no-go errors decreased across age in both males and females (see Table 1). That is, as the twins grew older, their performance on the go/no-go task improved, leading to fewer overall no-go errors. This steady decline in impulsive responding falls in line with previous research demonstrating a decline in impulsive responding after preadolescence (generally said to be around ages 9–12 years), conceivably due to the maturation of the prefrontal cortex (Steinberg, 2010).
Consistent with previous findings, biometrical univariate modeling results indicated that genetic and nonshared environmental effects significantly influenced motor impulsivity throughout the course of development, whereas shared environmental influences did not play a significant role. These results fall in line with previous studies that have investigated the heritability of personality traits, including impulsivity (Bezdjian et al., 2011; Finkel & McGue, 1997). An additional study examining the genetic and environmental influences on the P300 (a purported measure of prefrontal inhibition) in a sample of young female twins during a go/no-go task found the heritability of response inhibition on the go/no-go task to be 60% with no significant contributions from shared environmental effects (Anokhin et al., 2004). These within-wave analyses highlight the importance of both genetic and (non-shared) environmental effects in no-go errors across development, with some suggestion that these relative effects may vary only slightly from one occasion to the next.
Our results further demonstrated that genetic effects significantly influenced motor impulsivity across development. Non-shared environmental effects also significantly influenced the development of motor impulsivity across all time points through both common and specific (unique) environmental factors. Specifically, common genetic influences exerted less influence over time, whereas the common nonshared environmental influences exerted greater influence over time. Also, time-specific nonshared environmental influences, which include measurement error, decreased in magnitude across development. Thus, in the present model, genetic influences on the no-go response seemed to be decreasing while common nonshared environmental influences seemed to be increasing with age and time. These findings may be due to newly accumulating environmental effects over time (see Eaves et al., 1986).
Consistent with cross-sectional univariate biometric analyses, no effects of shared environment were found in multivariate biometric analyses for motor impulsivity across development. Thus, both genetic and nonshared environmental effects may contribute to the development of impulse control (or disinhibited or impulsive behaviors). Further, our finding that common genetic influences exerted less influence over time whereas the common nonshared environmental influences exerted greater influence over time may partly be explained by the fact that children become more autonomous during adolescence and more actively seek out their environment. This accumulation of individual environmental influences across adolescence may include influences from peers and other unique experiences during this critical period of development. Prior research has shown that risk taking is higher during adolescence than childhood or adulthood, as evidenced by age differences in a wide range of risk factors for antisocial behaviors, including criminal behavior, reckless driving, unprotected sex, and binge drinking (Moffitt, Caspi, Rutter, & Silva, 2001; Steinberg, 2010). Studies have long described adolescents as being prone to risk taking and engaging in impulsive behaviors as exemplified by drug use, unintentional injuries (especially car accidents), and unprotected sexual activity (Arnett, 1992). Our results illustrate that adolescent impulsivity (as measured by motor impulsivity) is a risk factor for antisocial behavior and is not a uniform phenomenon but that unique experiences play an important role in the emergence of such behavior during adolescence.
Limitations
A few limitations in the present study must be kept in mind when considering these findings. First, we examined motor impulsivity using one laboratory task, based on the go/no-go paradigm. However, research has shown that laboratory tasks provide reliable results when examining behavioral traits predominantly because they can used repeatedly.
A second limitation is associated with the smaller number of participants particularly within our Waves 2 and 4 samples. This is in part due to attrition but also due to the fact that many families opted to participate through mail surveys rather than in the laboratory. Thus, we do not have laboratory data (i.e., go/no-go) on many participating families. To reiterate, logistic regression analyses for attrition demonstrated that there were no significant differences between participating families and nonparticipating families on socioeconomic status, twin’s gender, interview language, or no-go errors. However, participants and nonparticipants were significantly different on ethnicity, indicating that Caucasians were slightly less likely to drop out. Apart from the slight ethnic difference, families who did not participate in follow-up assessments did so in a random manner. Thus, attrition of our sample had no significant bearing on our go/no-go findings. Regardless, the results obtained in the present study were significant and consistent with previous findings (particularly heritability estimates of 41% for Wave 2 assessments are close to what others have reported for impulsive behaviors; Anokhin et al., 2011; Finkel & McGue, 1997).
Conclusions
In the present study, we examined one component of the multifaceted impulsivity construct, namely, motor impulsivity. Results provide evidence suggesting that common genetic effects influence motor impulsivity in childhood, and these effects remain important throughout adolescence. Additionally, nonshared environmental effects were also greatly important across development and contributed both to common factors as well as to unique specific factors. This may be attributable to the accumulation of unique experiences across childhood and adolescence, highlighting the importance of learning and individual environmental factors in the development of impulse control across development. Future research is needed to understand how these effects might change in later adult development, for example, after brain maturation is complete and individuals become established as independent functioning adults.
Acknowledgments
This study was funded by National Institute of Mental Health Grant R01 MH58354. Serena Bezdjian was supported by National Institute on Drug Abuse Grant T32 DA07313. Adrian Raine was supported by National Institute of Mental Health Independent Scientist Award K02 MH01114-08. We thank the Southern California Twin Project staff for their assistance in collecting data, and we especially thank the twins and their families for their participation.
Contributor Information
Serena Bezdjian, University of Southern California and Department of Defense Center—Monterey Bay, Seaside, California.
Catherine Tuvblad, University of Southern California.
Pan Wang, University of Southern California.
Adrian Raine, University of Pennsylvania.
Laura A. Baker, University of Southern California
References
- Akaike AC. Factor analysis and AIC. Psychometrika. 1987;52:317–332. doi: 10.1007/BF02294359. [DOI] [Google Scholar]
- Anokhin AP, Golosheykin S, Grant JD, Heath AC. Heritability of delay discounting in adolescence: A longitudinal twin study. Behavior Genetics. 2011;41:175–183. doi: 10.1007/s10519-010-9384-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokhin AP, Heath AC, Meyers E. Genetics, prefrontal cortex, and cognitive control: A twin study of event-related brain potentials in a response inhibition task. Neuroscience Letters. 2004;368:314–318. doi: 10.1016/j.neulet.2004.07.036. [DOI] [PubMed] [Google Scholar]
- Arnett J. Reckless behavior in adolescence: A developmental perspective. Developmental Review. 1992;12:339–373. doi: 10.1016/0273-2297(92)90013-R. [DOI] [Google Scholar]
- Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: Relevance for genetic research in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:1285–1292. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Baker LA, Barton M, Lozano DI, Raine A, Fowler JH. The Southern California Twin Register at the University of Southern California: II. Twin Research and Human Genetics. 2006;9:933–940. doi: 10.1375/twin.9.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker LA, Tuvblad C, Wang P, Gomez K, Bezdjian S, Niv S, Raine A. The Southern California Twin Register at the University of Southern California: III. Twin Research and Human Genetics. 2013;16:336–343. doi: 10.1017/thg.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA. The ecological validity of laboratory and analogue assessments of ADHD symptoms. Journal of Abnormal Child Psychology. 1991;19:149–178. doi: 10.1007/BF00909976. [DOI] [PubMed] [Google Scholar]
- Bezdjian S, Baker LA, Lozano DI, Raine A. Assessing inattention and impulsivity in children during the go/nogo task. British Journal of Developmental Psychology. 2009;27:365–383. doi: 10.1348/026151008X314919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezdjian S, Baker LA, Tuvblad C. Genetic and environmental influences on impulsivity: A meta-analysis. Clinical Psychology Review. 2011;31:1209–1223. doi: 10.1016/j.cpr.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SR, Sahakian BJ. The neuropsychiatry of impulsivity. Current Opinion in Psychiatry. 2007;20:255–261. doi: 10.1097/YCO.0b013e3280ba4989. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Svrakic D, Przybeck T. A psychobiological model of temperament and character. Archives of General Psychiatry. 1993;50:975–990. doi: 10.1001/archpsyc.1993.01820240059008. [DOI] [PubMed] [Google Scholar]
- Congdon E, Canli T. A neurogenetic approach to impulsivity. Journal of Personality. 2008;76:1447–1484. doi: 10.1111/j.1467-6494.2008.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkum PV, Siegel LS. Is the continuous performance task a valuable tool to use with children with attention-deficit-hyperactivity disorder? Journal of Child Psychology and Psychiatry. 1993;34:1217–1239. doi: 10.1111/j.1469-7610.1993.tb01784.x. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Revised NEO Personality Inventory: Professional manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O’Malley SS, Sher K. Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addiction Biology. 2010;15:217–226. doi: 10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Bjork JM, Moeller RG, Harper RA, March DM, Mathias CW, Swann AC. Familial transmission of continuous performance test behavior: Attentional and impulsive response characteristics. Journal of General Psychology. 2003;130:5–21. doi: 10.1080/00221300309601271. [DOI] [PubMed] [Google Scholar]
- Eaves LJ, Long J, Heath AC. A theory of developmental change in quantitative phenotypes applied to cognitive development. Behavior Genetics. 1986;16:143–162. doi: 10.1007/BF01065484. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology. 1999;146:348–361. doi: 10.1007/PL00005481. [DOI] [PubMed] [Google Scholar]
- Eysenck SB, Eysenck HJ. The place of impulsiveness in a dimensional system of personality description. British Journal of Social and Clinical Psychology. 1977;16:57–68. doi: 10.1111/j.2044-8260.1977.tb01003.x. [DOI] [PubMed] [Google Scholar]
- Finkel D, McGue M. Sex differences and nonadditivity in heritability of the Multidimensional Personality Questionnaire scales. Journal of Personality and Social Psychology. 1997;72:929–938. doi: 10.1037/0022-3514.72.4.929. [DOI] [PubMed] [Google Scholar]
- Fischer S, Smith GT, Cyders MA. Another look at impulsivity: A meta-analytic review comparing specific dispositions to rash action in their relationship to bulimic symptoms. Clinical Psychology Review. 2008;28:1413–1425. doi: 10.1016/j.cpr.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin JM, Wolf LE, Greenblatt ER, Young G. Subtype analysis of commission errors on the continuous performance test in children. Developmental Neuropsychology. 1991;7:207–217. doi: 10.1080/87565649109540488. [DOI] [Google Scholar]
- Heath AC, Madden PA, Martin NG. Assessing the effects of cooperation bias and attrition in behavioral genetic research using data-weighting. Behavior Genetics. 1998;28:415– 427. doi: 10.1023/A:1021633127604. [DOI] [PubMed] [Google Scholar]
- Horn NR, Dolan M, Elliot R, Deakin JF, Woodruff PW. Response inhibition and impulsivity: An fMRI study. Neuropsychologia. 2003;41:1959–1966. doi: 10.1016/S0028-3932(03)00077-0. [DOI] [PubMed] [Google Scholar]
- Kindlon D, Mezzacappa E, Earls F. Psychometric properties of impulsivity measures: Temporal stability, validity, and factor structure. Journal of Child Psychology and Psychiatry. 1995;36:645–661. doi: 10.1111/j.1469-7610.1995.tb02319.x. [DOI] [PubMed] [Google Scholar]
- Long J. STIM stimulus presentation software manual. Caroga Lake, NY: James Long; 2005. [Google Scholar]
- Moffitt TE, Caspi A, Rutter M, Silva PA. Sex differences in antisocial behavior: Conduct disorder, delinquency, and violence in the Dunedin Longitudinal Study. Cambridge, United Kingdom: Cambridge University Press; 2001. [DOI] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes H. Mx: Statistical modeling. Richmond, VA: Department of Psychiatry, Medical College of Virginia; 2003. [Google Scholar]
- Newcorn JH, Halperin JM, Jensen PS, Abikoff HB, Arnold E, Cantwell DP. Symptom profiles in children with ADHD: Effects of comorbidity and gender. Journal of the American Academy of Child & Adolescent Psychiatry. 2001;40:137–146. doi: 10.1097/00004583-200102000-00008. [DOI] [PubMed] [Google Scholar]
- Niv S, Tuvblad C, Raine A, Wang P, Baker LA. Heritability and longitudinal stability of impulsivity in adolescence. Behavior Genetics. 2012;42:378–392. doi: 10.1007/s10519-011-9518-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt E. Factor structure of the Barratt Impulsiveness Scale. Journal of Clinical Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::AID-JCLP2270510607>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, McClearn GE, McGuffin P. Behavioral genetics. New York, NY: Worth; 2001. [Google Scholar]
- Posthuma D, Beem AL, de Geus EJC, van Baal CM, von Hjelmborg JB, Iachine I, Boomsma DI. Theory and practice in quantitative genetics. Twin Research and Human Genetics. 2003;6:361–376. doi: 10.1375/136905203770326367. [DOI] [PubMed] [Google Scholar]
- Raftery AE. Bayesian model selection in social research. Sociological Methodology. 1995;25:111–163. doi: 10.2307/271063. [DOI] [Google Scholar]
- Rigdon EE. CFI versus RMSEA: A comparison of two fit indexes for structural equation modeling. Structural Equation Modeling: A Multidisciplinary Journal. 1996;3:369–379. doi: 10.1080/10705519609540052. [DOI] [Google Scholar]
- Saunders B, Farag N, Vincent AS, Collins FL, Jr, Sorocco KH, Lovallo WR. Impulsive errors on a go-nogo reaction time task: Disinhibitory traits in relation to a family history of alcoholism. Alcoholism: Clinical and Experimental Research. 2008;32:888–894. doi: 10.1111/j.1530-0277.2008.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachar RJ, Forget-Dubois N, Dionne G, Boivin M, Robaey P. Heritability of response inhibition in children. Journal of the International Neuropsychological Society. 2011;17:238–247. doi: 10.1017/S1355617710001463. [DOI] [PubMed] [Google Scholar]
- Smith GT, Fischer S, Cyders MA, Annus AM, Spillane NS. On the validity of discriminating among impulsivity-like traits. Assessment. 2007;14:155–170. doi: 10.1177/1073191106295527. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A dual systems model of adolescent risk-taking. Developmental Psychobiology. 2010;52:216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- Tellegen A. Brief manual for the Multidimensional Personality Questionnaire. Minneapolis: University of Minnesota Press; 1982. [Google Scholar]
- Whiteside SP, Lynam DR. The five factor model and impulsivity: Using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30:669–689. doi: 10.1016/S0191-8869(00)00064-7. [DOI] [Google Scholar]
- Wothke W. Longitudinal and multigroup modeling with missing data. In: Little TD, Schnabel KU, Baumert J, editors. Modeling longitudinal and multiple group data: Practical issues, applied approaches, and specific examples. Mahwah, NJ: Erlbaum; 2000. pp. 219–240. [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, Hewitt JK. Behavioral disinhibition: Liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. Journal of Abnormal Psychology. 2009;118:117–130. doi: 10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan KH, Bentler PM, Zhang W. The effect of skewness and kurtosis on mean and covariance structure analysis: The univariate case and its multivariate implication. Sociological Methods & Research. 2005;34:240–258. doi: 10.1177/0049124105280200. [DOI] [Google Scholar]