Abstract
Background
Monoclonal B-cell lymphocytosis is classified as ‘high-count or clinical’ monoclonal B-cell lymphocytosis and ‘low-count or population’ monoclonal B-cell lymphocytosis. Previously, 167 first-degree relatives pertaining to sporadic (non-familial) chronic lymphocytic leukemia families were studied and the presence of seven monoclonal B-cell lymphocytosis individuals was reported.
Objective
The aim of this report is to describe the outcomes of five of the original monoclonal B-cell lymphocytosis individuals.
Methods
Flow cytometry analysis was performed on mononuclear cells previously isolated from peripheral blood samples. A strategy of sequential gating designed to identify the population of CD19+/CD5+ B-lymphocytes was used and, subsequently, the monoclonal B-cell lymphocytosis cells were characterized by the CD20weak/CD79bweak/negative phenotype.
Results
The monoclonal B-cell lymphocytosis clone showed consistent stability over time with little variations in size. After a median follow-up of 7.6 years, none of the five monoclonal B-cell lymphocytosis individuals progressed to chronic lymphocytic leukemia or other B-cell lymphoproliferative disease.
Conclusions
The data of this study suggest that chronic lymphocytic leukemia-like monoclonal B-cell lymphocytosis detected in the context of sporadic chronic lymphocytic leukemia families is not prone to clinical evolution and could be just a sign of immune senescence.
Keywords: Monoclonal B-cell lymphocytosis, Chronic lymphocytic leukemia, Flow cytometry, B cells, First-degree relatives
Introduction
Monoclonal B-cell lymphocytosis (MBL) is defined as the presence of low levels of circulating abnormal B-cells in the peripheral blood of apparently healthy subjects, identified by means of immunophenotypic characterization performed by flow cytometry. Phenotypically, MBL presents as chronic lymphocytic leukemia (CLL)-like MBL, atypical MBL and non-CLL MBL. Moreover, MBL is classified into two categories: ‘high-count or clinical MBL’, in which individuals have absolute lymphocytosis, but with B-cell counts lower than 5 × 109 cells/L and ‘low-count or population MBL’ cases found by screening individuals without lymphocytosis.1
Notwithstanding the same phenotype, high-count and low-count CLL-like MBL are biologically different entities regarding specific immunoglobulin heavy chain variable (IGHV) genes, the size of B-cell compartment, the prevalence in general population and, mainly, the risk of evolution to CLL requiring treatment. Thus, high-count CLL-like MBL is really a pre-malignant condition characterized by the risk of progression to overt CLL in 1–5% of cases per year.2 On the other hand, the main study on low-count CLL-like MBL published so far3 showed that the condition persists in approximately 90% of subjects, with some cases regressing over time.
Previously, a total of 167 first-degree relatives of sporadic (non-familial) CLL patients pertaining to 42 families (sporadic CLL families) were studied by the current authors and the presence of seven MBL individuals in this particular epidemiological setting was reported.4 Thus, taking advantage of the previous findings, this study describes the outcomes and the size of B-cell clones in this very particular group of subjects.
Methods
The following criteria were used to diagnose MBL: (a) a disease-specific immunophenotype or an overall kappa/lambda ratio >3:1 or <0.3:1, (b) stable monoclonal B-cell population over a three-month period, (c) absence of lymphadenopathy, organomegaly, and autoimmune or infectious diseases, and (d) B-lymphocyte counts < 5 × 109/L.5
Flow cytometry studies were performed as previously described.4 Briefly, mononuclear cells were isolated from peripheral blood samples by Ficoll Hypaque (Sigma–Aldrich, St Louis, MO, USA) density gradient centrifugation. The diluted blood sample (1:1) was carefully layered on Ficoll Hypaque (5 mL) previously added to a centrifuge tube. The sample was centrifuged at 400 × g for 30–40 min. Using a clean Pasteur pipette, the mononuclear cells were transferred to a clean centrifuge tube. The final concentration was adjusted to 1.0 × 106 cells per tube. Antibodies for the following antigens were used: CD5 (APC), CD19 (PerCP-Cy5.5), CD20 (FITC), CD79b (PE), anti-κ (FITC), anti-λ (PE), polyclonal anti-κ (FITC), and polyclonal anti-λ (PE). All monoclonal antibodies were purchased from Becton Dickinson (BD, San Jose, CA, USA) except polyclonal anti-κ and anti-λ (Dako Denmark A/S, USA). All samples were analyzed using a FACSCalibur flow cytometer (BD, San Jose, CA, USA). A total of 300,000 events per tube were acquired.
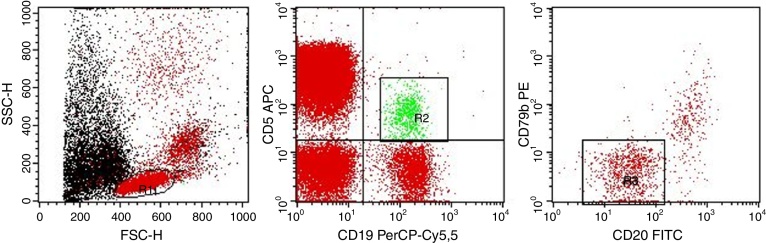
Flow cytometry analysis was performed as follows: after the identification of the whole lymphocyte population (R1 gate) the sequential gating strategy was designed to specifically identify the population of CD19+/CD5+ B-lymphocytes (R2 gate) and, subsequently, the MBL cells were characterized for the CD20weak/CD79bweak/negative phenotype (R3 gate) (Figure 1). The percentage of MBL cells was calculated based on the population of CD19+/CD5+ B-lymphocytes in peripheral blood.
Figure 1.
Gating strategy. Left dot-plot: R1 gate (red) represents the total lymphocytes. Central dot-plot: R2 gate (green) represents the CD19+/CD5+ B-lymphocytes. Right dot-plot: R3 gate (red) represents the MBL cells (CD20weak/CD79bweak/negative).
As previously described, MBL was detected in seven first-degree relatives from five families in a total of 167 subjects studied.4 Five of these seven MBL individuals were followed up. Two individuals refused clinical surveillance. The other five individuals were followed up at Universidade de São Paulo (USP) in Ribeirão Preto with annual medical consultations including history, physical exam and complete blood count. All MBL subjects had no lymphocytosis at diagnosis and were initially classified as ‘low-count MBL’, although these individuals did not come from the general population (see Discussion section).
Results
The clinical and laboratorial characteristics of the seven originally described MBL individuals are shown in Table 1. After a median follow-up of 7.6 years (range: 6.6–8.1 years) no individual progressed to CLL or other chronic B-cell lymphoproliferative disease, since all of them persisted with a B-cell count lower than 5 × 109 cells/L.
Table 1.
Clinical and laboratorial characteristics of monoclonal B-cell lymphocytosis individuals at diagnosis.
| Name | Age/gender | Leucocytes (×109/L) | Lymphocytes (×109/L) | MBL immunophenotype | κ:λ ratio | Extended phenotyping | PCR for IGHV |
|---|---|---|---|---|---|---|---|
| P1 | 46/F | 5.4 | 1.8 | CD5+/CD20+bright/CD79b+dim | 0.08:1 | CD11a+bright/CD23+/CD38−/CD49c−/CD49d+/CD54+/FMC7+ | Monoclonal |
| P2 | 75/M | 5.6 | 1.7 | CD5+/CD20+dim/CD79b+dim | 0.25:1 | CD11a+bright/CD23+/CD38−/CD49c+/CD49d+/CD54+/FMC7− | Negative |
| P3 | 72/M | 4.5 | 1.3 | CD5+/CD20+dim/CD79b+dim | 1.28:1 | CD11a+dim/CD23+/CD38+/CD49c+/CD49d+/CD54+/FMC7− | Monoclonal |
| P4 | 53/M | 8.1 | 1.2 | CD5+/CD20+dim/CD79b+dim | 1.12:1 | CD11a+dim/CD23+/CD38−/CD49c+/CD49d−/CD54+/FMC7− | Monoclonal |
| P5 | 62/M | 9.1 | 1.8 | CD5+/CD20+dim/CD79b+dim | 1.35:1 | CD11a+dim/CD23+/CD38−/CD49c+/CD49d−/CD54+/FMC7− | Monoclonal |
| P6 | 75/M | 5.1 | 1.1 | CD5+/CD20+dim/CD79b+dim | 4.35:1 | Not Performed | Not performed |
| P7 | 61/F | 8.4 | 4.0 | CD5+/CD20+dim/CD79b+dim | 4.94:1 | CD11a+dim/CD23+/CD38−/CD49c+/CD49d−/CD54+/FMC7− | Negative |
MBL: monoclonal B-cell lymphocytosis; PCR: polymerase chain reaction; IGHV: immunoglobulin heavy chain variable region genes; F: female; M: male.
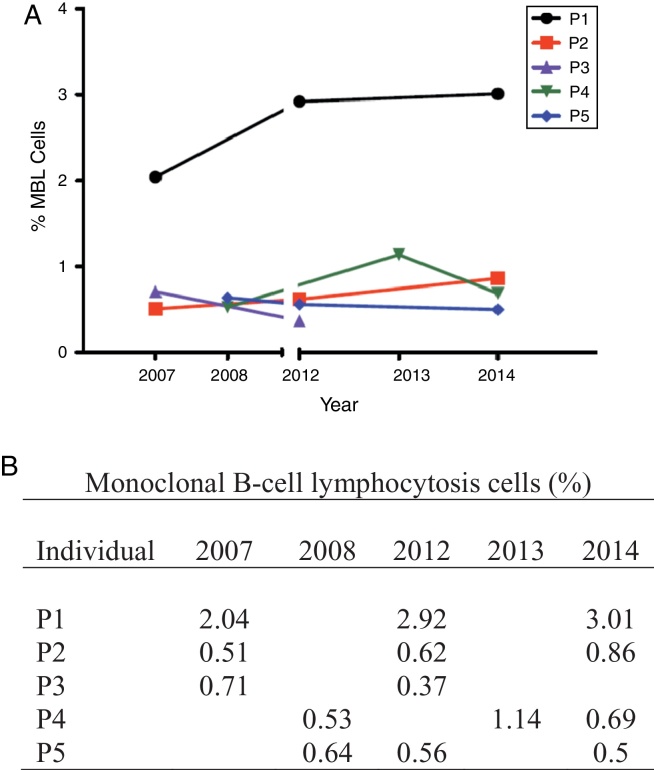
The MBL clone showed consistent stability over time, with little variations in size (Figure 2). It is worth noting that only one patient (P1 – Figure 2) had a little increase of clone size. At diagnosis in 2006, this individual presented with a monoclonal population (κ/λ = 0.08:1) characterized by an atypical MBL phenotype (CD5+/CD20bright). At the last follow-up in 2014, flow cytometry analysis showed that the monoclonal λ+ population persisted without an expressive increase.
Figure 2.
(A) Monoclonal B-cell lymphocytosis clone size variation. (B) Percentage of monoclonal B-cell lymphocytosis cells of each individual (P1, P2, P3, P4, P5) over time.
Discussion
CLL-like MBL is classified into two subgroups: ‘high-count or clinical MBL’, usually diagnosed in a clinical setting, and ‘low-count or population MBL’, found by screening healthy individuals in the general population.6 It is noteworthy that our group of subjects, from an epidemiological perspective, does not pertain either to clinical or population MBL, because they came from specific sporadic (non-familial) CLL families. However, with regard to the size of the abnormal B-cell compartment, our group is somehow related to low-count CLL-like MBL. The results of this study recall those of Fazi et al.3 that showed the persistency of low count CLL-like MBL without clinical progression, though their study was conducted in a different population, i.e., healthy individuals diagnosed in an Italian rural valley community. Furthermore, the Leeds group reevaluated 42 MBL cases diagnosed in hospital outpatients after a median follow-up of 4.3 years. The absolute lymphocyte count was normal in 38/42, with 4/42 developing lymphocytosis, of which three were diagnosed with high-count CLL-like MBL.2
As stated by Ghia et al.,7 probably the most important question concerning population MBL is whether, given enough time, all these cases will become clinical MBL and, subsequently, every clinical MBL will become overt CLL. This question has yet more relevance with regard to MBL cases diagnosed inside sporadic CLL families. In fact, the best epidemiological data confirm genetic susceptibility to CLL, as first-degree relatives of CLL cases were found to be at significantly increased risk for CLL [relative risk (RR) = 7.5; 95% confidence interval (95% CI): 3.63–15.56].8 Thus, the data of this study, though limited due to sample size, but with a median follow-up of 7.6 years, suggest that ‘sporadic CLL family-MBL’ is not prone to clinical evolution and that these abnormal B-cell expansions could be just a sign of immune senescence. Thus, clonal expansions among some subsets of T cells, as commonly seen in the elderly, closely resemble CLL-like MBL in healthy individuals, with the frequency of which being age-related.9, 10
Conclusions
CLL-like MBL detected in the context of sporadic CLL families is apparently stable over time, similar to other low-count MBL cases diagnosed in population-screening surveys. This entity is probably not a pre-leukemic condition and could just be a signal of immune repertoire restrictions occurring with age in healthy individuals.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors thank Aglair B. Garcia and Denise A.P. Gallo for the technical assistance.
References
- 1.Matos D.M., Falcão R.P. Monoclonal B-cell lymphocytosis: a brief review for general clinicians. Sao Paulo Med J. 2011;129(3):171–175. doi: 10.1590/S1516-31802011000300008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rawstron A.C. Monoclonal B-cell lymphocytosis – what does it really mean? Curr Hematol Malig Rep. 2013;8(1):52–59. doi: 10.1007/s11899-012-0144-z. [DOI] [PubMed] [Google Scholar]
- 3.Fazi C., Scarfò L., Pecciarini L., Cottini F., Dagklis A., Janus A. General population low-count CLL-like MBL persists over time without clinical progression, although carrying the same cytogenetic abnormalities of CLL. Blood. 2011;118(25):6618–6625. doi: 10.1182/blood-2011-05-357251. [DOI] [PubMed] [Google Scholar]
- 4.Matos D.M., Ismael S.J., Scrideli C.A., de Oliveira F.M., Rego E.M., Falcão R.P. Monoclonal B-cell lymphocytosis in first-degree relatives of patients with sporadic (non-familial) chronic lymphocytic leukaemia. Br J Haematol. 2009;147(3):339–346. doi: 10.1111/j.1365-2141.2009.07861.x. [DOI] [PubMed] [Google Scholar]
- 5.Marti G.E., Rawstron A.C., Ghia P., Hillmen P., Houlston R.S., Kay N. Diagnostic criteria for monoclonal B-cell lymphocytosis. Br J Haematol. 2005;130(3):325–332. doi: 10.1111/j.1365-2141.2005.05550.x. [DOI] [PubMed] [Google Scholar]
- 6.Dagklis A., Fazi C., Sala C., Cantarelli V., Scielzo C., Massacane R. The immunoglobulin gene repertoire of low-count CLL-like MBL is different from CLL: diagnostic implications for clinical monitoring. Blood. 2009;114(1):26–32. doi: 10.1182/blood-2008-09-176933. [DOI] [PubMed] [Google Scholar]
- 7.Ghia P., Caligaris-Cappio F. Monoclonal B-cell lymphocytosis: right track or red herring? Blood. 2012;119(19):4358–4362. doi: 10.1182/blood-2012-01-404681. [DOI] [PubMed] [Google Scholar]
- 8.Goldin L.R., Caporaso N.E. Family studies in chronic lymphocytic leukaemia and other lymphoproliferative tumours. Br J Haematol. 2007;139(5):774–779. doi: 10.1111/j.1365-2141.2007.06810.x. [DOI] [PubMed] [Google Scholar]
- 9.Vardi A., Dagklis A., Scarfò L., Jelinek D., Newton D., Bennett F. Immunogenetics shows that not all MBL are equal: the larger the clone, the more similar to CLL. Blood. 2013;121(22):4521–4528. doi: 10.1182/blood-2012-12-471698. [DOI] [PubMed] [Google Scholar]
- 10.Ghia P., Melchers F., Rolink A.G. Age-dependent changes in B lymphocyte development in man and mouse. Exp Gerontol. 2000;35(2):159–165. doi: 10.1016/s0531-5565(99)00095-9. [DOI] [PubMed] [Google Scholar]