Abstract
There has been a sharp rise in the occurrence of Candida infections and associated mortality over the last few years, due to the growing body of immunocompromised population. Limited number of currently available antifungal agents, undesirable side effects and toxicity, as well as emergence of resistant strains pose a considerable clinical challenge for the treatment of candidiasis. Therefore, molecules that derived from natural sources exhibiting considerable antifungal properties are a promising source for the development of novel anti-candidal therapy. Phenolic compounds isolated from natural sources possess antifungal properties of interest. Particularly, phenolic acids have shown promising in vitro and in vivo activity against Candida species. However, studies on their mechanism of action alone or in synergism with known antifungals are still scarce. This review attempts to discuss the potential use, proposed mechanisms of action and limitations of the phenolic acids in anti-candidal therapy.
Keywords: Candida, phenolic acids, phenolic compounds, antifungal effect, synergism
Introduction
Candida species are a major group of fungal pathogens in humans, particularly among immunocompromised and hospitalized patients (Cuellar-Cruz et al., 2012). Candida albicans inhabits various body surfaces like oral cavity, gastrointestinal tract, vagina, and skin of the healthy individuals as a commensal organism (Kleinegger et al., 1996; Huffnagle and Noverr, 2013). Host-related factors can predispose the transformation of harmless Candida into an opportunistic pathogen, causing infection or candidiasis in superficial mucous surfaces which can progress into invasive mycoses (Nett and Andes, 2006). Foregoing factors include, but not limited to immuno-suppression, prolonged treatment with wide-spectrum antibiotics and chronic diseases (Kullberg and Arendrup, 2015; Polke et al., 2015). The epidemiology of invasive candidiasis varies geographically (Morgan, 2005; Pfaller et al., 2011). It significantly increases the period of hospitalization, economic burden and mortality, especially in ICU patients or those under chemotherapy or with a history of abdominal surgery (Falagas et al., 2006; Berdal et al., 2014; Drgona et al., 2014).
Only few classes of antifungals such as polyenes, azoles, echinocandins, allylamines, and flucytosine are available for the treatment of Candida infections (Sanglard et al., 2009). However, there are various undesirable properties, most importantly the dose-related toxicity in aforementioned antifungals (Chandrasekar, 2011). Ideally, an antifungal should have null or reduced toxicity toward human cells (Wong et al., 2014). For instance, amphotericin B is a polyene available for systemic administration, but its use has been limited due to its systemic side effects such as nephrotoxicity (Odds et al., 2003). Azole antifungals have some side effects associated with gastrointestinal, hepatic, and endocrinologic disorders and interfere with oxidative drug metabolism in the liver (Joly et al., 1992).
In addition, rising drug resistance is an inevitable problem. In particular, Candida glabrata and Candida krusei show intrinsic resistance to fluconazole, the drug of choice for AIDS patients (Kanafani and Perfect, 2008; Siikala et al., 2010; Rautemaa and Ramage, 2011). Drug resistance has already been reported for recently introduced echinocandin antifungal agents (Hakki et al., 2006; Ben-Ami et al., 2011; Clancy and Nguyen, 2011; Seneviratne et al., 2011). Moreover, biofilm mode of Candida is known to be highly resistant to antifungal agents (Chandra et al., 2005; Niimi et al., 2010). Therefore, it is necessary to discover new antifungal agents or safer alternatives to improve the efficacy of treatment against Candida infections. In this regard, antifungal agents based on natural resources, such as phenolic compounds may be an alternative strategy to negate the rising antifungal drug resistance (Negri et al., 2014). This review attempts to critically analyze the possible use of phenolic acids as a therapeutic strategy against Candida infections.
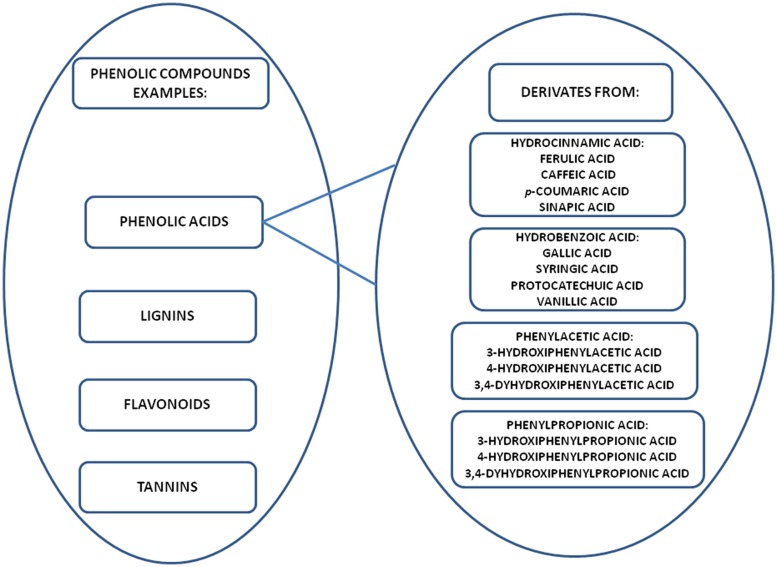
Phenolic compounds are widely found in plant foods (fruits, cereal grains, legumes, and vegetables) and beverages (tea, coffee, fruits juices, and cocoa). The most common phenolic compounds are phenolic acids (cinnamic and benzoic acids), flavonoids, proanthocyanidins, coumarins, stilbenes, lignans, and lignins (Figure 1; Cowan, 1999; Chirinos et al., 2009; Khoddami et al., 2013). The anti-Candida properties of phenolic compounds that have been widely reported in the literature include inactivation of enzyme production (Evensen and Braun, 2009) and anti-biofilm effect (Evensen and Braun, 2009; Shahzad et al., 2014).
FIGURE 1.
Examples of phenolic compounds and main derivates classified as phenolic acids.
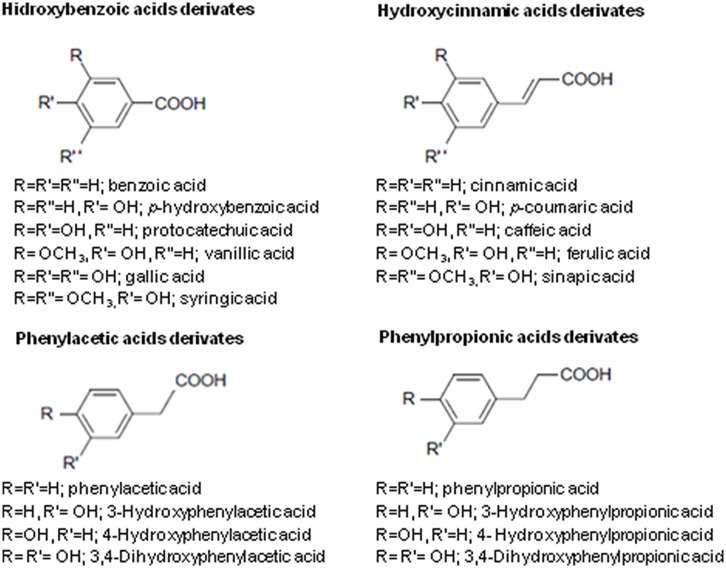
Phenolic acids are derivatives of hydrocinnamic, hydrobenzoic, phenylacetic, and phenylpropionic acids (Figures 1 and 2; Pereira et al., 2009; Cueva et al., 2010). Phenolic acids commonly exist as esters, glycosides or amides in nature, but not in their free form. The determining factor for characterization of phenolic acids is the number and the location of hydroxyl groups on the aromatic ring. Some natural sources are rich in phenolic acids and shown to possess a promising action against Candida (Table 1). In this review, we discuss the anti-candidal activity of the phenolic acid compounds, possible mechanism of actions and future directions.
FIGURE 2.
General chemical structures of the phenolic acids [based on Pereira et al. (2009) and Cueva et al. (2010)].
Table 1.
Phenolic acids derived from plants extracts showing activity against Candida sp.
| Plant | Phenolic acids found | Type of extract | Microorganism | MIC value μg/ml | MBC value μg/ml | Reference |
|---|---|---|---|---|---|---|
| Buchenavia tomentosa | Gallic acid | Aqueous | C. albicans ATCC 18804 C. tropicalis ATCC 13803 C. krusei ATCC 6258 C. glabrata ATCC C. parapsilosis ATCC 22019 C. dubliniensis NCPF 3108 | 200–12500 | 6500 C. krusei (ATCC 6258) | Teodoro et al., 2015 |
| Rosa rugosa | Protocatechuic, gallic, and p-coumaric acids | Methanolic | C. albicans ATCC 10231 C. parapsilosis ATCC 22019 | 156 | 1250 | Nowak et al., 2014 |
| Teucrium arduini L. | Ferulic acid | Ethanolic | C. albicans ATCC 10231 | 4000 | NR | Kremer et al., 2013 |
| Potentilla sp. | Caffeic acid and ferulic acid | Acetonic and methaolic | C. albicans ATCC 10231 | 780–1560 | NR | Wang et al., 2013 |
| Dimocarpus longan Lour | Gallic acid | Spray-dried or Freeze-dried water | C. krusei ATCC 10231 C. parapsilosis ATCC 22019 C. albicans ATCC 90028 and clinical strains | 500–4000 | NR | Rangkadilok et al., 2012 |
| Ligusticum mutellina L. | Gallic, p-OH-benzoic, caffeic, p-coumaric, and ferulic acids | Methanolic | C. albicans ATCC 10231 C. parapsilosis ATCC 22019 | 1250 | 2500 | Sieniawska et al., 2013 |
| Limonium avei | Caffeic, m-coumaric, p-coumaric, ferulic, isovanillic, p-methoxybenzoic, protocatechuic, sinapinic, and vanillic acids | Ethanolic | C. albicans ATCC 10231 | 4000 | >4000 | Nostro et al., 2012 |
| Kitaibelia vitifolia | p-hydroxybenzoic, caffeic, syringic, p-coumaric, and ferulic acids | Ethanolic | C. albicans ATCC 10231 | 15.62 | NR | Maskovic et al., 2011 |
| Tamarix gallica L. | Gallic, synnapic, p-hydroxybenzoic, syringic, vanillic, p-coumaric, ferrulic, trans-2-hydroxycinnamic and trans-cinnamic acids | Hydromethanolic | C. kefyr, C. holmii, C. albicans, C. sake, C. glabrata | 2000 | NR | Ksouri et al., 2009 |
| Cirsium sp. | Caffeic, p-coumaric, ferulic, p-hidroxybenzoic, protocatechuic vanillic, and gallic acids | Aqueous | C. albicans ATCC 10231 | 780–1560 | 6250 to >50000 | Nazaruk et al., 2008 |
| Olea europaea L. | Caffeic acid | Aqueous | C. albicans CECT 1394 | 5000∗ | NR | Pereira et al., 2007 |
| Anogeissus latifolia | Gallic acid | Hydroalcoholic after maceration with ether | Candida albicans (MTCC 183) | 7.28 μg/ml | NR | Govindarajan et al., 2006 |
| Berry (Cloudberry Raspberry, Strawberry) | Hydroxycinnamic acids | Acetonic 70% | Candida albicans NCPF 3179 | 1000 μg/ml | NR | Nohynek et al., 2006 |
NR, not reported; *IC25.
Antifungal Activity of Phenolic Acids Against Candida Species
Natural extracts containing phenolic acids have demonstrated antifungal activity against Candida species (Table 1). Phenolic acid derivatives isolated from these sources such as gallic, caffeic, cinnamic, benzoic, protocatechuic, and phenylacetic acids also have antifungal activity (Table 2). However, the antifungal effect of the natural extracts may vary due to the differences in the quantity and the type of phenolic acid. In addition, the solvents used for extraction may also affect the antifungal effect. Moreover, other compounds present in natural extracts may act synergistically with phenolic acids to enhance the overall antifungal effect (Pereira et al., 2007; Nowak et al., 2014). Therefore, phenolic acids derived from different natural sources have highly variable MIC values against Candida (Table 2). Hence, a clear understanding of the composition of phenolic acids present in the natural extract is important to assess its potential as an antifungal agent (Salvador et al., 2004; Rangkadilok et al., 2012).
Table 2.
Evidences from literature regarding anti-Candida effect of phenolic acids.
| Molecule | Anti-Candida effect | Result found | Reference |
|---|---|---|---|
| Gallic acid | Planktonic cells of C. albicans (ATCC 18804), C. krusei (ATCC 6258), C. parapsilosis (ATCC 22019), C. dubliniensis (NCPF 3108), and C. glabrata (ATCC 90030) | MIC (μg/ml) respectively: 10000, 10000, 10000, 10000, 8 | Teodoro et al., 2015 |
| Planktonic cells and biofilm of C. albicans (ATCC 90028), C. glabrata (ATCC 2001), C. parapsilosis (ATCC 22019), and C. tropicalis (ATCC 750) | MIC (μg/ml) planktonic: <156 μg/ml MIC (μg/ml) biofilm respectively: 5000, 1250, 625, 625 | Alves et al., 2014 | |
| Planktonic cells (plate diffusion) | MIC (mg cm-3): 2.5 | Manayi et al., 2013 | |
| Planktonic cells of C. albicans (ATCC 10231) and C. tropicalis (ATCC 750) | MIC and MFC (μg/ml) respectively: 200, 200, 200, 100 | Gehrke et al., 2013 | |
| Planktonic cells of C. albicans (ATCC 90028) and 5 clinical strains, C. krusei (ATCC 6258), and C. parapsilosis (ATCC 20019) | MIC (μg/ml) respectively: 4000,4000,8000,4000, 16000, 16000, 8000, 4000 | Rangkadilok et al., 2012 | |
| Planktonic cells of C. albicans (ATCC 10231) and C. parapsilosis (ATCC 22019) | MIC (μg/ml) respectively: 8, 16 | Ozcelik et al., 2011 | |
| Planktonic cells of C. albicans (ATCC 90028), C. krusei (ATCC 6258), and C. parapsilosis (ATCC 22019) | MIC (μg/ml): 100 | Liu et al., 2009 | |
| Biofilm of C. albicans (not cited strain) | MIC (μg/ml): 1000 | Wang et al., 2009 | |
| Planktonic cells of C. albicans (MTCC 183) | MIC (μg/ml): 1.78 | Govindarajan et al., 2006 | |
| Planktonic cells of C. albicans (not cited strain) | Halo: 12 mm (100 μg on a sterile filter paper disk with 6 mm diameter) | Fogliani et al., 2005 | |
| Caffeic acid | Planktonic cells of C. albicans and inhibition of isocitrate lyase activity assay | MIC (μg/ml): 1000; inhibition of 91,5% of the isocitrate lyase enzyme activity | Cheah et al., 2014 |
| Planktonic cells and biofilm of C. albicans (ATCC 10231) | MIC (μg/ml): planktonic: 128; pre-formed, 4 and 24 h biofilm: 256 | De Vita et al., 2014 | |
| Planktonic cells of C. albicans (ATCC 10231) and C. parapsilosis (ATCC 22019) | MIC (μg/ml) respectively: 8, 16 | Ozcelik et al., 2011 | |
| Protocatechuic acid | Planktonic cells of C. albicans (LMP709U) | MIC and MFC (μg/ml) respectively: 156, 312 | Kuete et al., 2009 |
| Planktonic cells of C. albicans (10231) and C. tropicalis (ATCC 7349) | MIC (μg/ml) respectively: 500, 400 | Pretto et al., 2004 | |
| Phenylacetic acid | Planktonic cells (plate diffusion) of C. albicans (clinical strains) | Halo: 8–10.5 mm (20 μl of a 2000 ng/ml phenylacetic acid water solution on sterile filter paper disk with 6 mm diameter) | Mendonca Ade et al., 2009 |
| Cinnamic acid | Immunoregulatory effect on monocytes activation against C. albicans (SC 5314) | Significant reduce of C. albicans counts in 50 and 100 μg/ml | Conti et al., 2013 |
| Planktonic cells of C. albicans (ATCC 90028, ATCC 10231, PYCC 3436T) C. parapsilosis (ATCC 22019, PYCC 2545), C. glabrata (PYCC 2418T) C. tropicalis (PYCC 3097T), C. krusei (PYCC 3341), C. lusitaniae PYCC 2705T and synergism with antifungals | IC 50 (mmol l-1): 0.09 to 0.74; none synergism found | Faria et al., 2011 | |
| Benzoic acid | Planktonic cells of C. albicans (ATCC 90028, ATCC 10231, PYCC 3436T) C. parapsilosis (ATCC 22019, PYCC 2545), C. glabrata (PYCC 2418T) C. tropicalis (PYCC 3097T), C. krusei (PYCC 3341), C. lusitaniae PYCC 2705T and synergism with antifungals | IC 50 (mmol l-1): 0.05–0.73 Synergism found to C. albicans with amphotericin and itraconazole | Faria et al., 2011 |
The main Candida virulence factors are exoenzymes production, biofilm formation, adherence, and dimorphism (Vuong et al., 2004; Netea et al., 2008; Williams et al., 2011). Few studies have demonstrated the influence of phenolic acids against these factors. Anti-biofilm effect of phenolic acids against Candida sp. was reported (Wang et al., 2009; Alves et al., 2014; De Vita et al., 2014). However, the studies used only reference samples or did not cite the tested strain (Table 2). The anti-biofilm effect of these molecules should be carried out with clinical isolates in vitro and in vivo, since the ultimate goal of using these molecules is to treat candidiasis and a wider range of strains could provide more reliable results. Besides that, it also has found an influence of caffeic acid derivate against the Candida dimorphism (Sung and Lee, 2010).
However, several studies described effect on Candida virulence factors of some others phenolic molecules. For instance, bisbibenzyl stimulates the synthesis of farnesol, an inhibitor of hyphae formation, via upregulation of Dpp3 gene (Zhang et al., 2011). Hence, bisbibenzyl may reduce C. albicans hyphal formation and affect biofilm formation. Moreover, anti-hyphae effect in C. albicans was also found following the treatment with epigallocatechin-gallate (Han, 2007), licochalcone A, gladribin (Messier and Grenier, 2011), and thymol (Braga et al., 2007). Additionally, eugenol reduces germ tube formation in C. albicans (Pinto et al., 2009). Beyond that, several studies have shown anti-biofilm (Messier et al., 2011; Alves et al., 2014; Rane et al., 2014; Shahzad et al., 2014) and anti-adhesive (Feldman et al., 2012; Rane et al., 2014; Shahzad et al., 2014) activities of phenolics against Candida.
The number of studies on other phenolic molecules on Candida virulence factors with interesting results inspires a carefully investigation of phenolic acids influence on these factors.
Mechanism of Action, Biological Pathways, and Synergism with Antifungal Agents of Phenolic Acids Against Candida
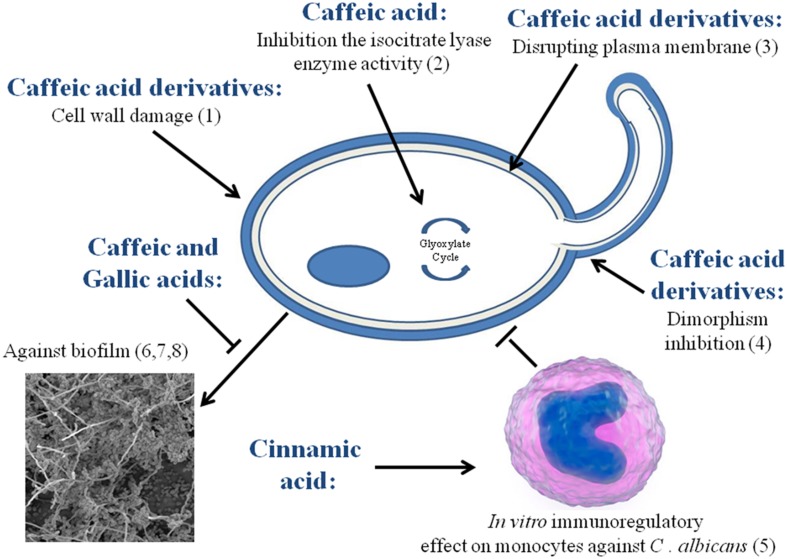
In order to obtain some insights on the antifungal activity of phenolic acids, herein we compare the existing data along the lines of mechanism of action, synergy with known antifungal agents and others biological pathways (Figure 3).
FIGURE 3.
Described mechanisms of action and biological pathways of some phenolic acids against Candida. (1) Ma et al. (2010); (2) Cheah et al. (2014); (3,4) Sung and Lee (2010); (5) Conti et al. (2013); (6) Alves et al. (2014); (7) De Vita et al. (2014); (8) Wang et al. (2009).
Mechanisms of Action and Biological Pathways
Phenolic acids such as ferulic and gallic acids are known to affect the cell membrane of Gram-positive and Gram-negative bacteria leading to a change in cell surface hydrophobicity and charge, ultimately causing leakage of cytoplasmic content (Borges et al., 2013). A similar effect has been suggested for the caffeic acid derivative on Candida cytoplasmatic membrane (Sung and Lee, 2010). Furthermore, a possible effect on the C. albicans cell wall has been shown for caffeic acid derivatives which may interfere with 1,3-β-glucan synthase (Ma et al., 2010).
It is noteworthy that polyene antifungals also cause pouring of cellular contents through direct binding to ergosterol, distorting the membrane function. Also, azole antifungal agents inhibit biosynthesis of ergosterol (Vanden Bossche et al., 2004). No study on the effect of phenolic acid on the ergosterol composition or biosynthesis could be detected.
Mode of action of several others phenolic compounds provide some clues to deduce the mechanism of phenolic acids. For instance, isoquercetin (Yun et al., 2015), curcumin (Lee and Lee, 2014), and lariciresinol (Pinto et al., 2009) can damage the C. albicans cell membrane. On the other hand, eugenol and methyleugenol cause considerable reduction in the ergosterol biosynthesis in Candida and subsequently affecting the cell membrane (Ahmad et al., 2010b). Similar effect has been observed with epigallocatechin-3-gallate (Navarro-Martinez et al., 2006), thymol and carvacrol (Ahmad et al., 2011). Besides, cardanol demonstrated chitin-binding ability in C. albicans cell wall (Mahata et al., 2014).
Few studies have found about others biological pathways of phenolic acids against Candida. Exemplifying, an in vitro immunoregulatory effect on monocytes against C. albicans by cinnamic acid (Conti et al., 2013) and a inhibition of C. albicans isocitrate lyase enzyme activity after treatment with caffeic acid (Cheah et al., 2014) was reported. However, several studies have suggested that the other biological pathways and cellular targets of others phenolic compounds may be different from that of existing antifungal agents. Some phenolic compounds have shown to induce apoptotic mechanisms in Candida, thereby contributing to their antifungal activity (Zore et al., 2011). For instance, eugenol inhibits the cell cycle at G1, S, and G2-M phases in C. albicans and consequently induces apoptosis. Another phenolic compound, curcumin also induces apoptosis in C. albicans, by increasing the reactive oxygen species (ROS) and induction of CaMCA1 gene expression (Cao et al., 2009). On the contrary, baicalein increases ROS causing perturbation in mitochondrial homeostasis in C. krusei without inducing apoptosis (Kang et al., 2010). Methyl chavicol seemed to induce aptotosis in C. albicans although the exact pathway is still not clear (Khan et al., 2014). Blocking effect of thymol, carvacrol (Ahmad et al., 2013) and baicalein (Huang et al., 2008) on the drug transporter pumps in Candida has been demonstrated using rhodamine 6G dye. Inhibition of efflux transporters results in accumulation of antifungal compounds inside the cell making Candida highly susceptible to the antifungal agent (Huang et al., 2008). These helpful anti-Candida biological pathways observed for phenolic molecules, mainly on the drug transporters pumps may contribute to elucidate the possible effects of phenolic acids against Candida.
Another aspect to be considered is that previous studies reported that some Candida species were able to metabolize phenolic acids (Middelhoven et al., 1992; Middelhoven, 1993). C. parapsilosis was able to grow in the presence of some phenolic acids after 3 days of cultivation. On the other hand, C. tropicalis was unable to grow in the presence of phenolic acids even after 14 days of cultivation (Middelhoven, 1993). These evidences should be better investigated in the future. Further studies are warranted to obtain a deeper understanding of the mechanism of action and others biological pathways of phenolic acids on Candida cells.
Synergism with Existing Antifungal Agents
Apart from rising antifungal resistance, there are other important limitations in the existing antifungal agents, such as inadequate spectrum of activity, poor bioavailability, small tolerance index, interactions with other drugs, inadequate pharmacokinetic profile, and considerable toxic effects (Lewis and Graybill, 2008; Pfaller et al., 2010). Although phytochemicals remain an important source for the discovery of new antifungal agents, micro-plate based in vitro screening assays have not shown higher effectiveness of plant extracts when compared to the existing antifungal agents with higher efficacy (Newman and Cragg, 2012). Hence, in general, plant extracts with higher minimum inhibitory concentrations (MICs) such as 1000 μg/ml are considered ineffective (Morales et al., 2008).
Therefore, some studies have explored the possibility of synergistic activity of phenolic acids and existing antifungal agents in order to maximize the antifungal effect. It is a good strategy to study the synergistic effect when MIC values of phenolic acids against Candida are highly variable (Rauha et al., 2000; Kalinowska et al., 2014). Synergistic effect of benzoic acid with amphotericin B and itraconazole against C. albicans has been reported in literature (Faria et al., 2011; Table 3). However, mechanism of this synergistic effect of phenolic acids and conventional antifungal agents is poorly understood. Therefore, it is important to examine similar synergistic effects shown by others phenolic compounds and conventional antifungal agents in order to obtain some insight.
Table 3.
Synergism of phenolic compounds with traditional antifungals in their action against Candida albicans.
∗Ineffectiveness antifungal effect of phenolic alone; ∗∗phenolic acid; ∗∗∗performed on biofilm formation; †resistant strain; ‡resistant and susceptible strains.
A promising synergism between phenolic compounds and fluconazole against resistant strains of Candida tropicalis was described recently (da Silva et al., 2014). Several other studies have also demonstrated a significant synergism between other known antifungals and phenolic compounds against C. albicans (Table 3). Some studies suggested that the synergism is due to the induction of apoptosis by an increase in the production of ROS. Hence, it was found that amphotericin B together with baicalein or curcumin increases the production of ROS (Sharma et al., 2010; Fu et al., 2011). A similar effect has been observed with fluconazole and curcumin (Sharma et al., 2010).
Another hypothesis for the aforementioned synergism is the association between folic acid cycle and ergosterol biosynthesis pathways of C. albicans. Hence, epigallocatechin-gallate, a phenolic compound was demonstrated to have a synergistic antifungal effect on Candida when combined with itraconazole or ketoconazole (Navarro-Martinez et al., 2006). Azoles directly inhibit the ergosterol biosynthesis while epigallocatechin-gallate has an antifolatic effect that indirectly affects the ergosterol biosynthesis. Epigallocatechin-gallate causes a depletion of the enzyme S-adenosylmethionine which in turn affects the enzyme Sterol C24 methyltransferase. Hence, lower production of C24 methyltransferase negatively affects the ergosterol biosynthesis. Direct and indirect effects on ergosterol biosynthesis explain the synergism between epigallocatechin-gallate and azoles (Navarro-Martinez et al., 2006).
Another study has shown that phenolic compounds such as thymol and carvacrol significantly decrease the expression levels of virulence genes CDR1 and MDR1 in fluconazole-resistant C. albicans (Ahmad et al., 2013). An in vivo study on systemic candidiasis in mice demonstrated that following the treatment with honokiol and fluconazole, the survival rate was 100% while a monotherapy showed only a survival rate of 80% to fluconazole and 20% to honokiol, respectively. Furthermore, the synergism of these two compounds led to a notable reduction in C. albicans counts in mouse kidneys compared with the fluconazole treatment alone (Jin et al., 2010). Similarly, mice treated with epigallocatechin-gallate and amphotericin B survived approximately 24 and 30 days longer when compared to the groups treated only with epigallocatechin-gallate or amphotericin B, respectively (Han, 2007). Considering the foregoing evidence obtained for other phenolic compounds, it is likely that potential of synergism exists between known antifungal agents and phenolic acids and this possibility needs to be examined in future.
Safety of the Phenolic Acids In vitro and In vivo
An ‘ideal’ antifungal agent for Candida infections should not have side effects or toxicity (Chapman et al., 2008; Wong et al., 2014). However, in reality, all the antifungals currently in use have some side effects on gastrointestinal tract, liver and kidney (Wingard et al., 1999; Bates et al., 2001). Therefore, practically one would expect to have some dose-related side effects from any new antifungal agent. It is imperative to understand this limitation in order to appreciate promising qualities of the drug under investigation. DNA-damaging effect of phenolic acids has been observed in p53R cell lines treated with gallic acid (Hossain et al., 2014). Moreover, in vivo hepatotoxicity was observed in rats when given a diet supplemented with more than 200 mg/kg/day of gallic acid (Galati et al., 2006). In addition, hematological disorders, as well as liver and kidney weight increase were observed in rats fed with 0.6–5% of gallic acid daily for 13 weeks (Niho et al., 2001).
A potential carcinogenicity was observed on the fore-stomach of rats when fed with a powdered diet containing 0.4% of caffeic acid for up to 28 weeks (Hirose et al., 1998). The clastogenic power of caffeic and cinnamic acids have been described in vitro (Maistro et al., 2011). Subcronic administration of protocatechuic acid (0.1% in drinking water) for 60 days has shown a possible liver and kidney toxicity in mice (Nakamura et al., 2001). Sodium benzoate and sodium phenylacetate have been used in the treatment of acute hyperammonaemia and are derived from benzoic acid and phenylacetic acid respectively. Inappropriate doses of both substances may cause plasma acidosis, hypotension, cerebral edema and other neurotoxical effects, sometimes even death of patients (Kaufman, 1989; Praphanphoj et al., 2000). Phenylacetic acid can also affect the osteoblastic functions in vitro and increase cell proliferation in the alveolar region (Kaufmann et al., 2005; Yano et al., 2007). Sodium and potassium benzoates could be clastogenic, mutagenic and cytotoxic to human lymphocytes in vitro (Zengin et al., 2011). Therefore, is imperative to examine the dose-related toxicity of phenolic acids in a series of comprehensive in vitro, in vivo and clinical studies before administration as an antifungal agent.
Conclusion
Phenolic acids demonstrate considerable antifungal properties against Candida. Previous studies have shown phenolic acid compounds possess considerable anti-adhesion, anti-biofilm effects, and inhibitory activity on morphogenesis and exoenzyme production of Candida species. However, hitherto no clear mechanism of action of phenolic acids on Candida cells and virulence factors has been described compared to the existing antifungal agents. Interestingly, there is substantial evidence of the synergistic effect of phenolic acids and existing antifungal agents which may become a promising anti-candidal strategy. However, more studies are in demand for a conclusive statement regarding their role. Therefore, we propose that more comprehensive studies are mandatory to obtain evidence regarding the suitability of the use of phenolic acids as a successful antifungal agent in future.
Author Contributions
GT conceived, designed, did the literature review, provided and wrote the manuscript. KE assisted in the preparation, design, final review, and co-wrote the manuscript. CK-I and CS conceived, designed, assisted in the literature and final review, and co-wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful to Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, #/2013/0037-1 and #2012/16805-5) for financial support and fellowship to GT, to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for sandwich Ph.D. fellowship to GT, and to National University of Singapore for providing Ph.D. fellowship to KE. GT’s Ph.D. internship at NUS was supported by NUS-Start-up grant (R-221-000-064-133) to CS.
References
- Ahmad A., Khan A., Akhtar F., Yousuf S., Xess I., Khan L. A., et al. (2011). Fungicidal activity of thymol and carvacrol by disrupting ergosterol biosynthesis and membrane integrity against Candida. Eur. J. Clin. Microbiol. Infect. Dis. 30 41–50. 10.1007/s10096-010-1050-8 [DOI] [PubMed] [Google Scholar]
- Ahmad A., Khan A., Khan L. A., Manzoor N. (2010a). In vitro synergy of eugenol and methyleugenol with fluconazole against clinical Candida isolates. J. Med. Microbiol. 59 1178–1184. 10.1099/jmm.0.020693-0 [DOI] [PubMed] [Google Scholar]
- Ahmad A., Khan A., Manzoor N., Khan L. A. (2010b). Evolution of ergosterol biosynthesis inhibitors as fungicidal against Candida. Microb. Pathog. 48 35–41. 10.1016/j.micpath.2009.10.001 [DOI] [PubMed] [Google Scholar]
- Ahmad A., Khan A., Manzoor N. (2013). Reversal of efflux mediated antifungal resistance underlies synergistic activity of two monoterpenes with fluconazole. Eur. J. Pharm. Sci. 48 80–86. 10.1016/j.ejps.2012.09.016 [DOI] [PubMed] [Google Scholar]
- Alves C. T., Ferreira I. C., Barros L., Silva S., Azeredo J., Henriques M. (2014). Antifungal activity of phenolic compounds identified in flowers from North Eastern Portugal against Candida species. Future Microbiol. 9 139–146. 10.2217/fmb.13.147 [DOI] [PubMed] [Google Scholar]
- Andrews F. A., Beggs W. H., Sarosi G. A. (1977). Influence of antioxidants on the bioactivity of amphotericin B. Antimicrob. Agents Chemother. 11 615–618. 10.1128/AAC.11.4.615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D. W., Su L., Yu D. T., Chertow G. M., Seger D. L., Gomes D. R., et al. (2001). Correlates of acute renal failure in patients receiving parenteral amphotericin B. Kidney Int. 60 1452–1459. 10.1046/j.1523-1755.2001.00948.x [DOI] [PubMed] [Google Scholar]
- Beggs W. H., Andrews F. A., Sarosi G. A. (1978). Synergistic action of amphotericin B and antioxidants against certain opportunistic yeast pathogens. Antimicrob. Agents Chemother. 13 266–270. 10.1128/AAC.13.2.266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ami R., Garcia-Effron G., Lewis R. E., Gamarra S., Leventakos K., Perlin D. S., et al. (2011). Fitness and virulence costs of Candida albicans FKS1 hot spot mutations associated with echinocandin resistance. J. Infect. Dis. 204 626–635. 10.1093/infdis/jir351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdal J. E., Haagensen R., Ranheim T., Bjornholt J. V. (2014). Nosocomial candidemia; risk factors and prognosis revisited; 11 years experience from a Norwegian secondary hospital. PLoS ONE 9:e103916 10.1371/journal.pone.0103916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges A., Ferreira C., Saavedra M. J., Simoes M. (2013). Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 19 256–265. 10.1089/mdr.2012.0244 [DOI] [PubMed] [Google Scholar]
- Braga P. C., Alfieri M., Culici M., Dal Sasso M. (2007). Inhibitory activity of thymol against the formation and viability of Candida albicans hyphae. Mycoses 50 502–506. 10.1111/j.1439-0507.2007.01412.x [DOI] [PubMed] [Google Scholar]
- Cao Y., Huang S., Dai B., Zhu Z., Lu H., Dong L., et al. (2009). Candida albicans cells lacking CaMCA1-encoded metacaspase show resistance to oxidative stress-induced death and change in energy metabolism. Fungal Genet. Biol. 46 183–189. 10.1016/j.fgb.2008.11.001 [DOI] [PubMed] [Google Scholar]
- Chandra J., Zhou G., Ghannoum M. A. (2005). Fungal biofilms and antimycotics. Curr. Drug Targets 6 887–894. 10.2174/138945005774912762 [DOI] [PubMed] [Google Scholar]
- Chandrasekar P. (2011). Management of invasive fungal infections: a role for polyenes. J. Antimicrob. Chemother. 66 457–465. 10.1093/jac/dkq479 [DOI] [PubMed] [Google Scholar]
- Chapman S. W., Sullivan D. C., Cleary J. D. (2008). In search of the holy grail of antifungal therapy. Trans. Am. Clin. Climatol. Assoc. 119 197–215; discussion 215–196. [PMC free article] [PubMed] [Google Scholar]
- Cheah H. L., Lim V., Sandai D. (2014). Inhibitors of the glyoxylate cycle enzyme ICL1 in Candida albicans for potential use as antifungal agents. PLoS ONE 9:e95951 10.1371/journal.pone.0095951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirinos R., Betalleluz-Pallardel I., Huamán A., Arbizu C., Pedreschi R., Campos D. (2009). HPLC-DAD characterisation of phenolic compounds from Andean oca (Oxalis tuberosa Mol.) tubers and their contribution to the antioxidant capacity. Food Chem. 113 1243–1251. 10.1016/j.foodchem.2008.08.015 [DOI] [Google Scholar]
- Clancy C. J., Nguyen M. H. (2011). At what cost echinocandin resistance? J. Infect. Dis. 204 499–501. 10.1093/infdis/jir355 [DOI] [PubMed] [Google Scholar]
- Conti B. J., Bufalo M. C., Golim Mde A., Bankova V., Sforcin J. M. (2013). Cinnamic Acid is partially involved in propolis immunomodulatory action on human monocytes. Evid. Based Complement. Alternat. Med. 2013 109864 10.1155/2013/109864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan M. M. (1999). Plant products as antimicrobial agents. Clin. Microbiol. Rev. 12 564–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuellar-Cruz M., Vega-Gonzalez A., Mendoza-Novelo B., Lopez-Romero E., Ruiz-Baca E., Quintanar-Escorza M. A., et al. (2012). The effect of biomaterials and antifungals on biofilm formation by Candida species: a review. Eur. J. Clin. Microbiol. Infect. Dis. 31 2513–2527. 10.1007/s10096-012-1634-6 [DOI] [PubMed] [Google Scholar]
- Cueva C., Moreno-Arribas M. V., Martin-Alvarez P. J., Bills G., Vicente M. F., Basilio A., et al. (2010). Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 161 372–382. 10.1016/j.resmic.2010.04.006 [DOI] [PubMed] [Google Scholar]
- da Silva C. R., De Andrade Neto J. B., De Sousa Campos R., Figueiredo N. S., Sampaio L. S., Magalhaes H. I., et al. (2014). Synergistic effect of the flavonoid catechin, quercetin, or epigallocatechin gallate with fluconazole induces apoptosis in Candida tropicalis resistant to fluconazole. Antimicrob. Agents Chemother. 58 1468–1478. 10.1128/aac.00651-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Auria F. D., Tecca M., Strippoli R., Simonetti N. (2001). In vitro activity of propyl gallate-azole drug combination against fluconazole- and itraconazole-resistant Candida albicans strains. Lett. Appl. Microbiol. 32 220–223. 10.1046/j.1472-765X.2001.00893.x [DOI] [PubMed] [Google Scholar]
- De Vita D., Friggeri L., D’auria F. D., Pandolfi F., Piccoli F., Panella S., et al. (2014). Activity of caffeic acid derivatives against Candida albicans biofilm. Bioorg. Med. Chem. Lett. 24 1502–1505. 10.1016/j.bmcl.2014.02.005 [DOI] [PubMed] [Google Scholar]
- Drgona L., Khachatryan A., Stephens J., Charbonneau C., Kantecki M., Haider S., et al. (2014). Clinical and economic burden of invasive fungal diseases in Europe: focus on pre-emptive and empirical treatment of Aspergillus and Candida species. Eur. J. Clin. Microbiol. Infect. Dis. 33 7–21. 10.1007/s10096-013-1944-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo E. H., Cortez D. A., Ueda-Nakamura T., Nakamura C. V., Dias Filho B. P. (2010). Potent antifungal activity of extracts and pure compound isolated from pomegranate peels and synergism with fluconazole against Candida albicans. Res. Microbiol. 161 534–540. 10.1016/j.resmic.2010.05.002 [DOI] [PubMed] [Google Scholar]
- Evensen N. A., Braun P. C. (2009). The effects of tea polyphenols on Candida albicans: inhibition of biofilm formation and proteasome inactivation. Can. J. Microbiol. 55 1033–1039. 10.1139/w09-058 [DOI] [PubMed] [Google Scholar]
- Falagas M. E., Apostolou K. E., Pappas V. D. (2006). Attributable mortality of candidemia: a systematic review of matched cohort and case-control studies. Eur. J. Clin. Microbiol. Infect. Dis. 25 419–425. 10.1007/s10096-006-0159-2 [DOI] [PubMed] [Google Scholar]
- Faria N. C., Kim J. H., Goncalves L. A., Martins Mde L., Chan K. L., Campbell B. C. (2011). Enhanced activity of antifungal drugs using natural phenolics against yeast strains of Candida and Cryptococcus. Lett. Appl. Microbiol. 52 506–513. 10.1111/j.1472-765X.2011.03032.x [DOI] [PubMed] [Google Scholar]
- Feldman M., Tanabe S., Howell A., Grenier D. (2012). Cranberry proanthocyanidins inhibit the adherence properties of Candida albicans and cytokine secretion by oral epithelial cells. BMC Complement. Altern. Med. 12:6 10.1186/1472-6882-12-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogliani B., Raharivelomanana P., Bianchini J. P., Bouraima-Madjebi S., Hnawia E. (2005). Bioactive ellagitannins from Cunonia macrophylla, an endemic Cunoniaceae from New Caledonia. Phytochemistry 66 241–247. 10.1016/j.phytochem.2004.11.016 [DOI] [PubMed] [Google Scholar]
- Fu Z., Lu H., Zhu Z., Yan L., Jiang Y., Cao Y. (2011). Combination of baicalein and Amphotericin B accelerates Candida albicans apoptosis. Biol. Pharm. Bull. 34 214–218. 10.1248/bpb.34.214 [DOI] [PubMed] [Google Scholar]
- Galati G., Lin A., Sultan A. M., O’brien P. J. (2006). Cellular and in vivo hepatotoxicity caused by green tea phenolic acids and catechins. Free Radic. Biol. Med. 40 570–580. 10.1016/j.freeradbiomed.2005.09.014 [DOI] [PubMed] [Google Scholar]
- Gehrke I. T., Neto A. T., Pedroso M., Mostardeiro C. P., Da Cruz I. B., Silva U. F., et al. (2013). Antimicrobial activity of Schinus lentiscifolius (Anacardiaceae). J. Ethnopharmacol. 148 486–491. 10.1016/j.jep.2013.04.043 [DOI] [PubMed] [Google Scholar]
- Govindarajan R., Vijayakumar M., Singh M., Rao Ch V., Shirwaikar A., Rawat A. K., et al. (2006). Antiulcer and antimicrobial activity of Anogeissus latifolia. J. Ethnopharmacol. 106 57–61. 10.1016/j.jep.2005.12.002 [DOI] [PubMed] [Google Scholar]
- Guo N., Liu J., Wu X., Bi X., Meng R., Wang X., et al. (2009). Antifungal activity of thymol against clinical isolates of fluconazole-sensitive and -resistant Candida albicans. J. Med. Microbiol. 58 1074–1079. 10.1099/jmm.0.008052-0 [DOI] [PubMed] [Google Scholar]
- Hakki M., Staab J. F., Marr K. A. (2006). Emergence of a Candida krusei isolate with reduced susceptibility to caspofungin during therapy. Antimicrob. Agents Chemother. 50 2522–2524. 10.1128/aac.00148-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y. (2007). Synergic anticandidal effect of epigallocatechin-O-gallate combined with amphotericin B in a murine model of disseminated candidiasis and its anticandidal mechanism. Biol. Pharm. Bull. 30 1693–1696. 10.1248/bpb.30.1693 [DOI] [PubMed] [Google Scholar]
- Hirasawa M., Takada K. (2004). Multiple effects of green tea catechin on the antifungal activity of antimycotics against Candida albicans. J. Antimicrob. Chemother. 53 225–229. 10.1093/jac/dkh046 [DOI] [PubMed] [Google Scholar]
- Hirose M., Takesada Y., Tanaka H., Tamano S., Kato T., Shirai T. (1998). Carcinogenicity of antioxidants BHA, caffeic acid, sesamol, 4-methoxyphenol and catechol at low doses, either alone or in combination, and modulation of their effects in a rat medium-term multi-organ carcinogenesis model. Carcinogenesis 19 207–212. 10.1093/carcin/19.1.207 [DOI] [PubMed] [Google Scholar]
- Hossain M. Z., Patel K., Kern S. E. (2014). Salivary alpha-amylase, serum albumin, and myoglobin protect against DNA-damaging activities of ingested dietary agents in vitro. Food Chem. Toxicol. 70 114–119. 10.1016/j.fct.2014.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Cao Y. Y., Dai B. D., Sun X. R., Zhu Z. Y., Cao Y. B., et al. (2008). In vitro synergism of fluconazole and baicalein against clinical isolates of Candida albicans resistant to fluconazole. Biol. Pharm. Bull. 31 2234–2236. 10.1248/bpb.31.2234 [DOI] [PubMed] [Google Scholar]
- Huffnagle G. B., Noverr M. C. (2013). The emerging world of the fungal microbiome. Trends Microbiol. 21 334–341. 10.1016/j.tim.2013.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J., Guo N., Zhang J., Ding Y., Tang X., Liang J., et al. (2010). The synergy of honokiol and fluconazole against clinical isolates of azole-resistant Candida albicans. Lett. Appl. Microbiol. 51 351–357. 10.1111/j.1472-765X.2010.02900.x [DOI] [PubMed] [Google Scholar]
- Joly V., Bolard J., Yeni P. (1992). In vitro models for studying toxicity of antifungal agents. Antimicrob. Agents Chemother. 36 1799–1804. 10.1128/AAC.36.9.1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinowska M., Piekut J., Bruss A., Follet C., Sienkiewicz-Gromiuk J., Swislocka R., et al. (2014). Spectroscopic (FT-IR, FT-Raman, 1H, 13C NMR, UV/VIS), thermogravimetric and antimicrobial studies of Ca(II), Mn(II), Cu(II), Zn(II) and Cd(II) complexes of ferulic acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 122 631–638. 10.1016/j.saa.2013.11.089 [DOI] [PubMed] [Google Scholar]
- Kanafani Z. A., Perfect J. R. (2008). Antimicrobial resistance: resistance to antifungal agents: mechanisms and clinical impact. Clin. Infect. Dis. 46 120–128. 10.1086/524071 [DOI] [PubMed] [Google Scholar]
- Kang K., Fong W. P., Tsang P. W. (2010). Antifungal activity of baicalein against Candida krusei does not involve apoptosis. Mycopathologia 170 391–396. 10.1007/s11046-010-9341-2 [DOI] [PubMed] [Google Scholar]
- Kaufman S. (1989). An evaluation of the possible neurotoxicity of metabolites of phenylalanine. J. Pediatr. 114 895–900. 10.1016/S0022-3476(89)80161-1 [DOI] [PubMed] [Google Scholar]
- Kaufmann W., Mellert W., Van Ravenzwaay B., Landsiedel R., Poole A. (2005). Effects of styrene and its metabolites on different lung compartments of the mouse–cell proliferation and histomorphology. Regul. Toxicol. Pharmacol. 42 24–36. 10.1016/j.yrtph.2005.01.002 [DOI] [PubMed] [Google Scholar]
- Khan A., Ahmad A., Khan L. A., Manzoor N. (2014). Ocimum sanctum (L.) essential oil and its lead molecules induce apoptosis in Candida albicans. Res. Microbiol. 165 411–419. 10.1016/j.resmic.2014.05.031 [DOI] [PubMed] [Google Scholar]
- Khan M. S., Ahmad I. (2012). Antibiofilm activity of certain phytocompounds and their synergy with fluconazole against Candida albicans biofilms. J. Antimicrob. Chemother. 67 618–621. 10.1093/jac/dkr512 [DOI] [PubMed] [Google Scholar]
- Khoddami A., Wilkes M. A., Roberts T. H. (2013). Techniques for analysis of plant phenolic compounds. Molecules 18 2328–2375. 10.3390/molecules18022328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinegger C. L., Lockhart S. R., Vargas K., Soll D. R. (1996). Frequency, intensity, species, and strains of oral Candida vary as a function of host age. J. Clin. Microbiol. 34 2246–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer D., Kosir I. J., Kosalec I., Koncic M. Z., Potocnik T., Cerenak A., et al. (2013). Investigation of chemical compounds, antioxidant and antimicrobial properties of Teucrium arduini L. (lamiaceae). Curr. Drug Targets 14 1006–1014. 10.2174/1389450111314090009 [DOI] [PubMed] [Google Scholar]
- Ksouri R., Falleh H., Megdiche W., Trabelsi N., Mhamdi B., Chaieb K., et al. (2009). Antioxidant and antimicrobial activities of the edible medicinal halophyte Tamarix gallica L. and related polyphenolic constituents. Food Chem. Toxicol. 47 2083–2091. 10.1016/j.fct.2009.05.040 [DOI] [PubMed] [Google Scholar]
- Kuete V., Nana F., Ngameni B., Mbaveng A. T., Keumedjio F., Ngadjui B. T. (2009). Antimicrobial activity of the crude extract, fractions and compounds from stem bark of Ficus ovata (Moraceae). J. Ethnopharmacol. 124 556–561. 10.1016/j.jep.2009.05.003 [DOI] [PubMed] [Google Scholar]
- Kullberg B. J., Arendrup M. C. (2015). Invasive candidiasis. N. Engl. J. Med. 373 1445–1456. 10.1056/NEJMra1315399 [DOI] [PubMed] [Google Scholar]
- Lee W., Lee D. G. (2014). An antifungal mechanism of curcumin lies in membrane-targeted action within Candida albicans. IUBMB Life 66 780–785. 10.1002/iub.1326 [DOI] [PubMed] [Google Scholar]
- Lewis J. S., II, Graybill J. R. (2008). Fungicidal versus fungistatic: what’s in a word? Expert Opin. Pharmacother. 9 927–935. 10.1517/14656566.9.6.927 [DOI] [PubMed] [Google Scholar]
- Liu M., Katerere D. R., Gray A. I., Seidel V. (2009). Phytochemical and antifungal studies on Terminalia mollis and Terminalia brachystemma. Fitoterapia 80 369–373. 10.1016/j.fitote.2009.05.006 [DOI] [PubMed] [Google Scholar]
- Liu W., Li L. P., Zhang J. D., Li Q., Shen H., Chen S. M., et al. (2014). Synergistic antifungal effect of glabridin and fluconazole. PLoS ONE 9:e103442 10.1371/journal.pone.0103442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C. M., Abe T., Komiyama T., Wang W., Hattori M., Daneshtalab M. (2010). Synthesis, anti-fungal and 1,3-beta-D-glucan synthase inhibitory activities of caffeic and quinic acid derivatives. Bioorg. Med. Chem. 18 7009–7014. 10.1016/j.bmc.2010.08.022 [DOI] [PubMed] [Google Scholar]
- Mahata D., Mandal S. M., Bharti R., Gupta V. K., Mandal M., Nag A., et al. (2014). Self-assembled cardanol azo derivatives as antifungal agent with chitin-binding ability. Int. J. Biol. Macromol. 69 5–11. 10.1016/j.ijbiomac.2014.05.017 [DOI] [PubMed] [Google Scholar]
- Maistro E. L., Angeli J. P., Andrade S. F., Mantovani M. S. (2011). In vitro genotoxicity assessment of caffeic, cinnamic and ferulic acids. Genet. Mol. Res. 10 1130–1140. 10.4238/vol10-2gmr1278 [DOI] [PubMed] [Google Scholar]
- Manayi A., Saeidnia S., Faramarzi M. A., Samadi N., Jafari S., Vazirian M., et al. (2013). A comparative study of anti-Candida activity and phenolic contents of the calluses from Lythrum salicaria L. in different treatments. Appl. Biochem. Biotechnol. 170 176–184. 10.1007/s12010-013-0185-3 [DOI] [PubMed] [Google Scholar]
- Maskovic P., Solujic S., Mihailovic V., Mladenovic M., Cvijovic M., Mladenovic J., et al. (2011). Phenolic compounds and biological activity of Kitaibelia vitifolia. J. Med. Food 14 1617–1623. 10.1089/jmf.2011.0013 [DOI] [PubMed] [Google Scholar]
- Mendonca Ade L., Da Silva C. E., De Mesquita F. L., Campos Rda S., Do Nascimento R. R., Ximenes E. C., et al. (2009). Antimicrobial activities of components of the glandular secretions of leaf cutting ants of the genus Atta. Antonie Van Leeuwenhoek 95 295–303. 10.1007/s10482-009-9312-0 [DOI] [PubMed] [Google Scholar]
- Messier C., Epifano F., Genovese S., Grenier D. (2011). Inhibition of Candida albicans biofilm formation and yeast-hyphal transition by 4-hydroxycordoin. Phytomedicine 18 380–383. 10.1016/j.phymed.2011.01.013 [DOI] [PubMed] [Google Scholar]
- Messier C., Grenier D. (2011). Effect of licorice compounds licochalcone A, glabridin and glycyrrhizic acid on growth and virulence properties of Candida albicans. Mycoses 54 e801–e806. 10.1111/j.1439-0507.2011.02028.x [DOI] [PubMed] [Google Scholar]
- Middelhoven W. J. (1993). Catabolism of benzene compounds by ascomycetous and basidiomycetous yeasts and yeastlike fungi. A literature review and an experimental approach. Antonie Van Leeuwenhoek 63 125–144. 10.1007/BF00872388 [DOI] [PubMed] [Google Scholar]
- Middelhoven W. J., Coenen A., Kraakman B., Sollewijn Gelpke M. D. (1992). Degradation of some phenols and hydroxybenzoates by the imperfect ascomycetous yeasts Candida parapsilosis and Arxula adeninivorans: evidence for an operative gentisate pathway. Antonie Van Leeuwenhoek 62 181–187. 10.1007/BF00582578 [DOI] [PubMed] [Google Scholar]
- Morales G., Paredes A., Sierra P., Loyola L. A. (2008). Antimicrobial activity of three baccharis species used in the traditional medicine of Northern Chile. Molecules 13 790–794. 10.3390/molecules13040790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. (2005). Global trends in candidemia: review of reports from 1995-2005. Curr. Infect. Dis. Rep. 7 429–439. 10.1007/s11908-005-0044-7 [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Torikai K., Ohigashi H. (2001). Toxic dose of a simple phenolic antioxidant, protocatechuic acid, attenuates the glutathione level in ICR mouse liver and kidney. J. Agric. Food Chem. 49 5674–5678. 10.1021/jf0106594 [DOI] [PubMed] [Google Scholar]
- Navarro-Martinez M. D., Garcia-Canovas F., Rodriguez-Lopez J. N. (2006). Tea polyphenol epigallocatechin-3-gallate inhibits ergosterol synthesis by disturbing folic acid metabolism in Candida albicans. J. Antimicrob. Chemother. 57 1083–1092. 10.1093/jac/dkl124 [DOI] [PubMed] [Google Scholar]
- Nazaruk J., Czechowska S. K., Markiewicz R., Borawska M. H. (2008). Polyphenolic compounds and in vitro antimicrobial and antioxidant activity of aqueous extracts from leaves of some Cirsium species. Nat. Prod. Res. 22 1583–1588. 10.1080/14786410701825053 [DOI] [PubMed] [Google Scholar]
- Negri M., Salci T. P., Shinobu-Mesquita C. S., Capoci I. R., Svidzinski T. I., Kioshima E. S. (2014). Early state research on antifungal natural products. Molecules 19 2925–2956. 10.3390/molecules19032925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea M. G., Brown G. D., Kullberg B. J., Gow N. A. (2008). An integrated model of the recognition of Candida albicans by the innate immune system. Nat. Rev. Microbiol. 6 67–78. 10.1038/nrmicro1815 [DOI] [PubMed] [Google Scholar]
- Nett J., Andes D. (2006). Candida albicans biofilm development, modeling a host-pathogen interaction. Curr. Opin. Microbiol. 9 340–345. 10.1016/j.mib.2006.06.007 [DOI] [PubMed] [Google Scholar]
- Newman D. J., Cragg G. M. (2012). Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 75 311–335. 10.1021/np200906s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niho N., Shibutani M., Tamura T., Toyoda K., Uneyama C., Takahashi N., et al. (2001). Subchronic toxicity study of gallic acid by oral administration in F344 rats. Food Chem. Toxicol. 39 1063–1070. 10.1016/S0278-6915(01)00054-0 [DOI] [PubMed] [Google Scholar]
- Niimi M., Firth N. A., Cannon R. D. (2010). Antifungal drug resistance of oral fungi. Odontology 98 15–25. 10.1007/s10266-009-0118-3 [DOI] [PubMed] [Google Scholar]
- Nohynek L. J., Alakomi H. L., Kahkonen M. P., Heinonen M., Helander I. M., Oksman-Caldentey K. M., et al. (2006). Berry phenolics: antimicrobial properties and mechanisms of action against severe human pathogens. Nutr. Cancer 54 18–32. 10.1207/s15327914nc5401_4 [DOI] [PubMed] [Google Scholar]
- Nostro A., Filocamo A., Giovannini A., Catania S., Costa C., Marino A., et al. (2012). Antimicrobial activity and phenolic content of natural site and micropropagated Limonium avei (De Not.) Brullo & Erben plant extracts. Nat. Prod. Res. 26 2132–2136. 10.1080/14786419.2011.628669 [DOI] [PubMed] [Google Scholar]
- Nowak R., Olech M., Pecio L., Oleszek W., Los R., Malm A., et al. (2014). Cytotoxic, antioxidant, antimicrobial properties and chemical composition of rose petals. J. Sci. Food Agric. 94 560–567. 10.1002/jsfa.6294 [DOI] [PubMed] [Google Scholar]
- Odds F. C., Brown A. J., Gow N. A. (2003). Antifungal agents: mechanisms of action. Trends Microbiol. 11 272–279. 10.1016/S0966-842X(03)00117-3 [DOI] [PubMed] [Google Scholar]
- Ozcelik B., Kartal M., Orhan I. (2011). Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 49 396–402. 10.3109/13880209.2010.519390 [DOI] [PubMed] [Google Scholar]
- Pereira A. P., Ferreira I. C., Marcelino F., Valentao P., Andrade P. B., Seabra R., et al. (2007). Phenolic compounds and antimicrobial activity of olive (Olea europaea L. Cv. Cobrancosa) leaves. Molecules 12 1153–1162. 10.3390/12051153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira D., Valentão P., Pereira J., Andrade P. (2009). Phenolics: from chemistry to biology. Molecules 14 2202–2211. 10.3390/molecules14062202 [DOI] [Google Scholar]
- Pfaller M. A., Castanheira M., Messer S. A., Moet G. J., Jones R. N. (2010). Variation in Candida spp. distribution and antifungal resistance rates among bloodstream infection isolates by patient age: report from the SENTRY Antimicrobial Surveillance Program (2008-2009). Diagn. Microbiol. Infect. Dis. 68 278–283. 10.1016/j.diagmicrobio.2010.06.015 [DOI] [PubMed] [Google Scholar]
- Pfaller M. A., Moet G. J., Messer S. A., Jones R. N., Castanheira M. (2011). Candida bloodstream infections: comparison of species distributions and antifungal resistance patterns in community-onset and nosocomial isolates in the SENTRY Antimicrobial Surveillance Program, 2008-2009. Antimicrob. Agents Chemother. 55 561–566. 10.1128/aac.01079-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto E., Vale-Silva L., Cavaleiro C., Salgueiro L. (2009). Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. J. Med. Microbiol. 58 1454–1462. 10.1099/jmm.0.010538-0 [DOI] [PubMed] [Google Scholar]
- Polke M., Hube B., Jacobsen I. D. (2015). Candida survival strategies. Adv. Appl. Microbiol. 91 139–235. 10.1016/bs.aambs.2014.12.002 [DOI] [PubMed] [Google Scholar]
- Praphanphoj V., Boyadjiev S. A., Waber L. J., Brusilow S. W., Geraghty M. T. (2000). Three cases of intravenous sodium benzoate and sodium phenylacetate toxicity occurring in the treatment of acute hyperammonaemia. J. Inherit. Metab. Dis. 23 129–136. 10.1023/A:1005661631281 [DOI] [PubMed] [Google Scholar]
- Pretto J. B., Cechinel-Filho V., Noldin V. F., Sartori M. R., Isaias D. E., Cruz A. B. (2004). Antimicrobial activity of fractions and compounds from Calophyllum brasiliense (Clusiaceae/Guttiferae). Z. Naturforsch. C 59 657–662. 10.1515/znc-2004-9-1009 [DOI] [PubMed] [Google Scholar]
- Rane H. S., Bernardo S. M., Howell A. B., Lee S. A. (2014). Cranberry-derived proanthocyanidins prevent formation of Candida albicans biofilms in artificial urine through biofilm– and adherence-specific mechanisms. J. Antimicrob. Chemother. 69 428–436. 10.1093/jac/dkt398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangkadilok N., Tongchusak S., Boonhok R., Chaiyaroj S. C., Junyaprasert V. B., Buajeeb W., et al. (2012). In vitro antifungal activities of longan (Dimocarpus longan Lour.) seed extract. Fitoterapia 83 545–553. 10.1016/j.fitote.2011.12.023 [DOI] [PubMed] [Google Scholar]
- Rauha J. P., Remes S., Heinonen M., Hopia A., Kahkonen M., Kujala T., et al. (2000). Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int. J. Food Microbiol. 56 3–12. 10.1016/S0168-1605(00)00218-X [DOI] [PubMed] [Google Scholar]
- Rautemaa R., Ramage G. (2011). Oral candidosis–clinical challenges of a biofilm disease. Crit. Rev. Microbiol. 37 328–336. 10.3109/1040841x.2011.585606 [DOI] [PubMed] [Google Scholar]
- Salvador M. J., Zucchi O. L. A. D., Candido R. C., Ito I. Y., Dias D. A. (2004). in vitro antimicrobial activity of crude extracts and isolated constituents of Alternanthera maritima. Pharm. Biol. 42 138–148. 10.1080/13880200490511954 [DOI] [Google Scholar]
- Sanglard D., Coste A., Ferrari S. (2009). Antifungal drug resistance mechanisms in fungal pathogens from the perspective of transcriptional gene regulation. FEMS Yeast Res. 9 1029–1050. 10.1111/j.1567-1364.2009.00578.x [DOI] [PubMed] [Google Scholar]
- Seneviratne C. J., Wong S. S., Yuen K. Y., Meurman J. H., Parnanen P., Vaara M., et al. (2011). Antifungal susceptibility and virulence attributes of bloodstream isolates of Candida from Hong Kong and Finland. Mycopathologia 172 389–395. 10.1007/s11046-011-9444-4 [DOI] [PubMed] [Google Scholar]
- Shahzad M., Sherry L., Rajendran R., Edwards C. A., Combet E., Ramage G. (2014). Utilising polyphenols for the clinical management of Candida albicans biofilms. Int. J. Antimicrob. Agents 44 269–273. 10.1016/j.ijantimicag.2014.05.017 [DOI] [PubMed] [Google Scholar]
- Sharma M., Manoharlal R., Puri N., Prasad R. (2010). Antifungal curcumin induces reactive oxygen species and triggers an early apoptosis but prevents hyphae development by targeting the global repressor TUP1 in Candida albicans. Biosci. Rep. 30 391–404. 10.1042/bsr20090151 [DOI] [PubMed] [Google Scholar]
- Sieniawska E., Baj T., Los R., Skalicka-Wozniak K., Malm A., Glowniak K. (2013). Phenolic acids content, antioxidant and antimicrobial activity of Ligusticum mutellina L. Nat. Prod. Res. 27 1108–1110. 10.1080/14786419.2012.698413 [DOI] [PubMed] [Google Scholar]
- Siikala E., Rautemaa R., Richardson M., Saxen H., Bowyer P., Sanglard D. (2010). Persistent Candida albicans colonization and molecular mechanisms of azole resistance in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) patients. J. Antimicrob. Chemother. 65 2505–2513. 10.1093/jac/dkq354 [DOI] [PubMed] [Google Scholar]
- Simonetti G., Simonetti N., Villa A. (2003). Increase of activity of tioconazole against resistant microorganisms by the addition of butylated hydroxyanisole. Int. J. Antimicrob. Agents 22 439–443. 10.1016/S0924-8579(03)00120-1 [DOI] [PubMed] [Google Scholar]
- Simonetti G., Villa A., Simonetti N. (2002). Enhanced contact activity of fluconazole in association with antioxidants against fluconazole-resistant organisms. J. Antimicrob. Chemother. 50 257–259. 10.1093/jac/dkf102 [DOI] [PubMed] [Google Scholar]
- Strippoli V., Dauria F. D., Tecca M., Callari A., Simonetti G. (2000). Propyl gallate increases in vitro antifungal imidazole activity against Candida albicans. Int. J. Antimicrob. Agents 16 73–76. 10.1016/S0924-8579(00)00200-4 [DOI] [PubMed] [Google Scholar]
- Sung W. S., Lee D. G. (2010). Antifungal action of chlorogenic acid against pathogenic fungi, mediated by membrane disruption. Pure Appl. Chem. 82 219–226. 10.1351/PAC-CON-09-01-08 [DOI] [Google Scholar]
- Teodoro G. R., Brighenti F. L., Delbem A. C., Delbem A. C., Khouri S., Gontijo A. V., et al. (2015). Antifungal activity of extracts and isolated compounds from Buchenavia tomentosa on Candida albicans and non-albicans. Future Microbiol. 10 917–927. 10.2217/fmb.15.20 [DOI] [PubMed] [Google Scholar]
- Vanden Bossche H., Ausma J., Bohets H., Vermuyten K., Willemsens G., Marichal P., et al. (2004). The novel azole R126638 is a selective inhibitor of ergosterol synthesis in Candida albicans, Trichophyton spp., and Microsporum canis. Antimicrob. Agents Chemother. 48 3272–3278. 10.1128/aac.48.9.3272-3278.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong C., Kocianova S., Voyich J. M., Yao Y., Fischer E. R., Deleo F. R., et al. (2004). A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence. J. Biol. Chem. 279 54881–54886. 10.1074/jbc.M411374200 [DOI] [PubMed] [Google Scholar]
- Wang C., Cheng H., Guan Y., Wang Y., Yun Y. (2009). [In vitro activity of gallic acid against Candida albicans biofilms]. Zhongguo Zhong Yao Za Zhi 34 1137–1140. [PubMed] [Google Scholar]
- Wang S. S., Wang D. M., Pu W. J., Li D. W. (2013). Phytochemical profiles, antioxidant and antimicrobial activities of three Potentilla species. BMC Complement. Altern. Med. 13:321 10.1186/1472-6882-13-321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. W., Kuriyama T., Silva S., Malic S., Lewis M. A. (2011). Candida biofilms and oral candidosis: treatment and prevention. Periodontol. 2000 55 250–265. 10.1111/j.1600-0757.2009.00338.x [DOI] [PubMed] [Google Scholar]
- Wingard J. R., Kubilis P., Lee L., Yee G., White M., Walshe L., et al. (1999). Clinical significance of nephrotoxicity in patients treated with amphotericin B for suspected or proven aspergillosis. Clin. Infect. Dis. 29 1402–1407. 10.1086/313498 [DOI] [PubMed] [Google Scholar]
- Wong S. S., Samaranayake L. P., Seneviratne C. J. (2014). In pursuit of the ideal antifungal agent for Candida infections: high-throughput screening of small molecules. Drug Discov. Today 19 1721–1730. 10.1016/j.drudis.2014.06.009 [DOI] [PubMed] [Google Scholar]
- Yano S., Yamaguchi T., Kanazawa I., Ogawa N., Hayashi K., Yamauchi M., et al. (2007). The uraemic toxin phenylacetic acid inhibits osteoblastic proliferation and differentiation: an implication for the pathogenesis of low turnover bone in chronic renal failure. Nephrol. Dial. Transplant. 22 3160–3165. 10.1093/ndt/gfm455 [DOI] [PubMed] [Google Scholar]
- Yun J., Lee H., Ko H. J., Woo E. R., Lee D. G. (2015). Fungicidal effect of isoquercitrin via inducing membrane disturbance. Biochim. Biophys. Acta 1848 695–701. 10.1016/j.bbamem.2014.11.019 [DOI] [PubMed] [Google Scholar]
- Zengin N., Yuzbasioglu D., Unal F., Yilmaz S., Aksoy H. (2011). The evaluation of the genotoxicity of two food preservatives: sodium benzoate and potassium benzoate. Food Chem. Toxicol. 49 763–769. 10.1016/j.fct.2010.11.040 [DOI] [PubMed] [Google Scholar]
- Zhang L., Chang W., Sun B., Groh M., Speicher A., Lou H. (2011). Bisbibenzyls, a new type of antifungal agent, inhibit morphogenesis switch and biofilm formation through upregulation of DPP3 in Candida albicans. PLoS ONE 6:e28953 10.1371/journal.pone.0028953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zore G. B., Thakre A. D., Jadhav S., Karuppayil S. M. (2011). Terpenoids inhibit Candida albicans growth by affecting membrane integrity and arrest of cell cycle. Phytomedicine 18 1181–1190. 10.1016/j.phymed.2011.03.008 [DOI] [PubMed] [Google Scholar]