Abstract
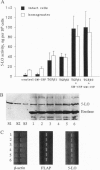
Transforming growth factor beta (TGF beta) increased the arachidonate 5-lipoxygenase (5-LO; EC 1.13.11.34) activity in HL-60 cells induced to granulocytic differentiation by dimethyl sulfoxide. The presence of a factor in human serum that caused a similar increase was recently demonstrated. Several observations indicate that the serum factor consists of isoforms of TGF beta. Heat-treated serum and TGF beta both resulted in approximately 10-fold increased 5-LO activity of HL-60 cells, antiserum to TGF beta neutralized the 5-LO-increasing activity in serum, and physical properties of the serum factor (lipophilic nature, alkaline pI, stability to heat and acid) coincided with those of TGF beta. The pattern of activity of native and heat-treated sera is compatible with activation of a latent form of TGF beta in serum. This activity was specific for TGF beta, since none of several other cytokines could increase 5-LO activity in differentiating HL-60 cells. However, granulocyte/macrophage-colony-stimulating factor (GM-CSF) and tumor necrosis factor alpha enhanced the effect of TGF beta. The most prominent effects of TGF beta, whether alone or together with GM-CSF, were observed for 5-LO activity in intact cells (10-fold or 30-fold induction, respectively). 5-LO protein levels were less affected (up to 2- or 5-fold, respectively, as judged from Western blots). There was no appreciable effect of TGF beta, or a combination of TGF beta and GM-CSF, on 5-LO mRNA expression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assoian R. K., Komoriya A., Meyers C. A., Miller D. M., Sporn M. B. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983 Jun 10;258(11):7155–7160. [PubMed] [Google Scholar]
- Bonser R. W., Siegel M. I., Chung S. M., McConnell R. T., Cuatrecasas P. Esterification of an endogenously synthesized lipoxygenase product into granulocyte cellular lipids. Biochemistry. 1981 Sep 1;20(18):5297–5301. doi: 10.1021/bi00521a032. [DOI] [PubMed] [Google Scholar]
- Breitman T. R., Collins S. J., Keene B. R. Replacement of serum by insulin and transferrin supports growth and differentiation of the human promyelocytic cell line, HL-60. Exp Cell Res. 1980 Apr;126(2):494–498. doi: 10.1016/0014-4827(80)90296-7. [DOI] [PubMed] [Google Scholar]
- Cheifetz S., Hernandez H., Laiho M., ten Dijke P., Iwata K. K., Massagué J. Distinct transforming growth factor-beta (TGF-beta) receptor subsets as determinants of cellular responsiveness to three TGF-beta isoforms. J Biol Chem. 1990 Nov 25;265(33):20533–20538. [PubMed] [Google Scholar]
- Danielpour D., Sporn M. B. Differential inhibition of transforming growth factor beta 1 and beta 2 activity by alpha 2-macroglobulin. J Biol Chem. 1990 Apr 25;265(12):6973–6977. [PubMed] [Google Scholar]
- De Benedetti F., Falk L. A., Ellingsworth L. R., Ruscetti F. W., Faltynek C. R. Synergy between transforming growth factor-beta and tumor necrosis factor-alpha in the induction of monocytic differentiation of human leukemic cell lines. Blood. 1990 Feb 1;75(3):626–632. [PubMed] [Google Scholar]
- Dixon R. A., Diehl R. E., Opas E., Rands E., Vickers P. J., Evans J. F., Gillard J. W., Miller D. K. Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature. 1990 Jan 18;343(6255):282–284. doi: 10.1038/343282a0. [DOI] [PubMed] [Google Scholar]
- Frolik C. A., Dart L. L., Meyers C. A., Smith D. M., Sporn M. B. Purification and initial characterization of a type beta transforming growth factor from human placenta. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3676–3680. doi: 10.1073/pnas.80.12.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawiger J. Platelet secretory pathways: an overview. Methods Enzymol. 1989;169:191–195. doi: 10.1016/0076-6879(89)69059-3. [DOI] [PubMed] [Google Scholar]
- Kargman S., Rouzer C. A. Studies on the regulation, biosynthesis, and activation of 5-lipoxygenase in differentiated HL60 cells. J Biol Chem. 1989 Aug 5;264(22):13313–13320. [PubMed] [Google Scholar]
- Kehrl J. H., Wakefield L. M., Roberts A. B., Jakowlew S., Alvarez-Mon M., Derynck R., Sporn M. B., Fauci A. S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986 May 1;163(5):1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J. R., Jacobsen S. E., Sill K. T., Ellingsworth L. R., Ruscetti F. W. Stimulation of granulopoiesis by transforming growth factor beta: synergy with granulocyte/macrophage-colony-stimulating factor. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7190–7194. doi: 10.1073/pnas.88.16.7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J. R., Sing G. K., Ellingsworth L. R., Ruscetti F. W. Transforming growth factor beta: possible roles in the regulation of normal and leukemic hematopoietic cell growth. J Cell Biochem. 1989 Feb;39(2):175–184. doi: 10.1002/jcb.240390209. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawrence D. A. Identification and activation of latent transforming growth factor beta. Methods Enzymol. 1991;198:327–336. doi: 10.1016/0076-6879(91)98033-3. [DOI] [PubMed] [Google Scholar]
- Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- Miller D. K., Gillard J. W., Vickers P. J., Sadowski S., Léveillé C., Mancini J. A., Charleson P., Dixon R. A., Ford-Hutchinson A. W., Fortin R. Identification and isolation of a membrane protein necessary for leukotriene production. Nature. 1990 Jan 18;343(6255):278–281. doi: 10.1038/343278a0. [DOI] [PubMed] [Google Scholar]
- Miyazono K., Hellman U., Wernstedt C., Heldin C. H. Latent high molecular weight complex of transforming growth factor beta 1. Purification from human platelets and structural characterization. J Biol Chem. 1988 May 5;263(13):6407–6415. [PubMed] [Google Scholar]
- O'Connor-McCourt M. D., Wakefield L. M. Latent transforming growth factor-beta in serum. A specific complex with alpha 2-macroglobulin. J Biol Chem. 1987 Oct 15;262(29):14090–14099. [PubMed] [Google Scholar]
- Ogawa Y., Seyedin S. M. Purification of transforming growth factors beta 1 and beta 2 from bovine bone and cell culture assays. Methods Enzymol. 1991;198:317–327. doi: 10.1016/0076-6879(91)98032-2. [DOI] [PubMed] [Google Scholar]
- Ohta M., Greenberger J. S., Anklesaria P., Bassols A., Massagué J. Two forms of transforming growth factor-beta distinguished by multipotential haematopoietic progenitor cells. Nature. 1987 Oct 8;329(6139):539–541. doi: 10.1038/329539a0. [DOI] [PubMed] [Google Scholar]
- Reid G. K., Kargman S., Vickers P. J., Mancini J. A., Léveillé C., Ethier D., Miller D. K., Gillard J. W., Dixon R. A., Evans J. F. Correlation between expression of 5-lipoxygenase-activating protein, 5-lipoxygenase, and cellular leukotriene synthesis. J Biol Chem. 1990 Nov 15;265(32):19818–19823. [PubMed] [Google Scholar]
- Samuelsson B., Dahlén S. E., Lindgren J. A., Rouzer C. A., Serhan C. N. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987 Sep 4;237(4819):1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- Segarini P. R., Ziman J. M., Kane C. J., Dasch J. R. Two novel patterns of transforming growth factor beta (TGF-beta) binding to cell surface proteins are dependent upon the binding of TGF-beta 1 and indicate a mechanism of positive cooperativity. J Biol Chem. 1992 Jan 15;267(2):1048–1053. [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., de Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-beta. J Cell Biol. 1987 Sep;105(3):1039–1045. doi: 10.1083/jcb.105.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhilber D., Herrmann T., Roth H. J. Separation of lipoxins and leukotrienes from human granulocytes by high-performance liquid chromatography with a Radial-Pak cartridge after extraction with an octadecyl reversed-phase column. J Chromatogr. 1989 Sep 1;493(2):361–366. doi: 10.1016/s0378-4347(00)82742-5. [DOI] [PubMed] [Google Scholar]
- Wakefield L. M., Smith D. M., Flanders K. C., Sporn M. B. Latent transforming growth factor-beta from human platelets. A high molecular weight complex containing precursor sequences. J Biol Chem. 1988 Jun 5;263(16):7646–7654. [PubMed] [Google Scholar]
- de Larco J. E., Todaro G. J. Growth factors from murine sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4001–4005. doi: 10.1073/pnas.75.8.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]