Abstract
Among genetic alterations in human cancers, mutations in the tumor suppressor p53 gene are the most common, occurring in over 50% of human cancers. The majority of p53 mutations are missense mutations and result in the accumulation of dysfunctional p53 protein in tumors. These mutants frequently have oncogenic gain-of-function activities and exacerbate malignant properties of cancer cells, such as metastasis and drug resistance. Increasing evidence reveals that stabilization of mutant p53 in tumors is crucial for its oncogenic activities, while depletion of mutant p53 attenuates malignant properties of cancer cells. Thus, mutant p53 is an attractive druggable target for cancer therapy. Different approaches have been taken to develop small-molecule compounds that specifically target mutant p53. These include compounds that restore wild-type conformation and transcriptional activity of mutant p53, induce depletion of mutant p53, inhibit downstream pathways of oncogenic mutant p53, and induce synthetic lethality to mutant p53. In this review article, we comprehensively discuss the current strategies targeting oncogenic mutant p53 in cancers, with special focus on compounds that restore wild-type p53 transcriptional activity of mutant p53 and those reducing mutant p53 levels.
Keywords: mutant p53, depletion, compounds, reactivation, cancer therapy, gain of function, dominant negative, oncogenes
Introduction
The tumor suppressor p53 exerts its biological function by regulating transcription of numerous downstream target genes involved in cell cycle arrest, apoptosis, DNA repair, senescence, and metabolism as a transcription factor (1, 2). p53 is also directly recruited to the mitochondria and induces apoptosis independent of its function as a transcription factor (3). Under unstressed physiological conditions, p53 expression is maintained at a low level, mainly by being degraded by its E3 ubiquitin ligases, MDM2, Pirh2, and COP1 (4). Once cells are exposed to genotoxic stresses, p53 is posttranslationally modified through phosphorylation and acetylation, becomes stabilized, and induces cell cycle arrest and/or cell death. When p53 activity is lost by gene deletion or mutations, normal cells lose the abilities to control their growth and death, leading to immortalization and ultimately cancer (5). The observation that over 50% of human cancers have mutations in the p53 gene indicates the indispensability of intact p53 activity for suppressing tumor development (6).
Mutations in the p53 gene occur mainly in the DNA-binding domain, the majority of which are missense mutations, resulting in loss of function as a transcription factor and accumulation of dysfunctional p53 protein in tumors (7). Mutant p53 can be categorized roughly into two types: DNA contact (class I) mutant where mutations are present on amino acids directly binding to the p53-responsive element in DNA (e.g., p53R273H and p53R280K) and conformational (class II) mutant in which mutations alter structure of p53 to abolish its DNA-binding ability (e.g., p53R175H and p53V143A) (8). Both the mutant types not only lose the transcriptional activity, but also have the dominant-negative (DN) activity by hetero-oligomerizing with wild-type p53. Moreover, mutant p53 shows oncogenic gain-of-function (GOF) activities, such as enhanced tumor progression, metastatic potential, and drug resistance, when overexpressed even in cells lacking wild-type p53 (7). These findings are supported by the fact that p53 was originally appreciated as an oncogene, since researchers unknowingly used plasmids encoding mutations in the p53 gene. Thus, mutant p53 functions as an oncogene and greatly contributes to malignant properties of cancer cells.
Disrupting specific mechanisms which cancer cells develop for their survival and growth is a rational approach to selectively kill cancer cells with minimal effects on normal cells. In this regard, mutant p53 is one of the best druggable targets, since over half of human cancers have p53 mutations, while normal cells mostly do not have mutations in the p53 gene (9). To exploit the frequent presence of mutant p53 in tumors and target mutant p53 in cancer therapy, two strategies including restoration of wild-type p53 transcriptional activity and depletion of mutant p53 have been extensively undertaken, in addition to inhibition of downstream target pathways involved in mutant p53 GOF and induction of synthetic lethality to mutant p53. Since mutant p53 is generally accumulated in tumors (10), reactivating p53 activity can efficiently induce proliferation arrest and/or cell death of cancer cell. Specifically, in the late stage of tumor development, cancer cells express only mutant p53 with loss of heterozygosity of the other wild-type p53 allele (11, 12). Such cells often have high metastatic and chemotherapy resistant properties. Hence, this p53 reactivation strategy is powerful to treat cancers expressing mutant p53. The other strategy to specifically deplete oncogenic mutant p53 in cancer cells should have minimal impact on wild-type p53, since depletion of wild-type p53 in normal and cancer cells can accelerate tumorigenesis or tumor progression. Accumulating studies suggest that knockdown of mutant p53 significantly reduces oncogenic potential of cancer cells expressing only mutant p53 (13–16), suggesting that malignant properties of cancer cells are, at least partially, dependent on the presence of mutant p53. This could be simply due to the loss of oncogenic activity of mutant p53 or possibly because cancer cells are addicted to mutant p53 for their survival and proliferation. This strategy would work even better when cancer cells retain the wild-type p53 allele with the mutant p53 allele (heterozygous), since it can also restore wild-type p53 activity which is suppressed by the DN activity of mutant p53. Thus, depletion of mutant p53 is also an effective strategy to suppress tumor progression.
In this article, toward developing precision cancer medicine, we summarize updated information about compounds which can restore wild-type p53 activity, as well as those depleting mutant p53.
Drugs/Compounds That Restore Wild-Type p53 Activity
Most p53 mutants lose their ability to bind with p53-response elements in DNA, thereby losing transcriptional activity and tumor suppressive function (17). However, the following evidence suggests that sequence-specific p53 transcriptional activities can be restored from mutant p53: (1) many p53 mutants are temperature sensitive and restore the p53 activity at the permissive temperature (18, 19), (2) synthetic peptides, CDB3 and Peptide 46 which are derived from 53BP2 and C-terminal domain of p53, respectively, restore the sequence-specific DNA binding and transcriptional activity of p53 (20, 21), and (3) insertion of second-site mutations or a N-terminal deletion in several p53 mutants restore the p53 transcriptional activities (22–24). Since the first p53-reactivating compound, CP-31398, was identified (17), investigators have made tremendous efforts to identify compounds that restore p53 transcriptional activity. Major compounds related to mutant p53 reactivation are listed in Table 1 and explained below.
Table 1.
Compounds that induce reactivation of mutant p53.
| Compound | Type of mutant | Mechanism | Reference | Structure |
|---|---|---|---|---|
| CP-31398 | V173A, S241F, R249S, R273H | Stabilize the DNA-binding core domain and induce conformational change | (17, 25–27) | 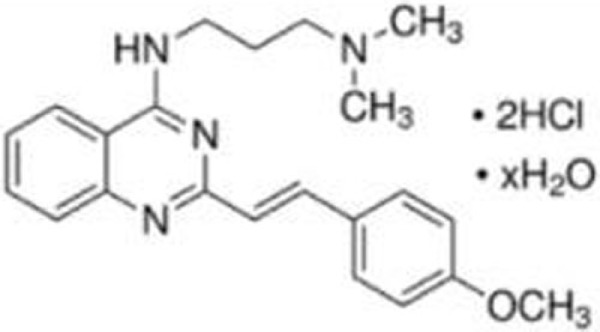 |
| STIMA-1, structural similarity to CP-31398 | R175H, R273H | Bind to the cysteine residues in the core domain and stabilize wild-type p53 conformation | (28) | 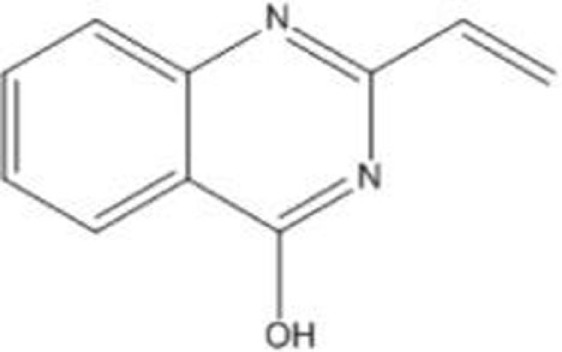 |
| PRIMA-1 and the methylated analog (APR-246/PRIMA-1MET) | R175H, R273H | Bind to thiol groups in the core domain and restore wild-type conformation | (23, 29, 30) | 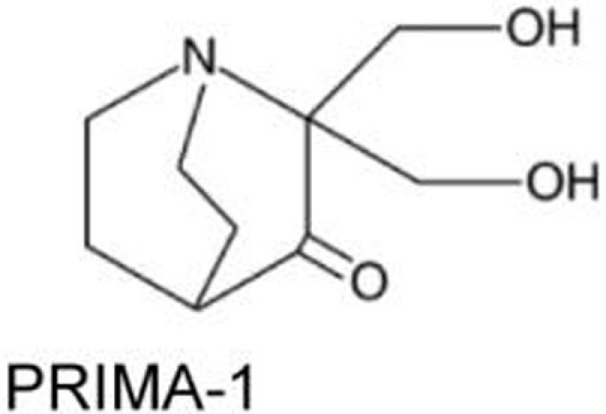 |
| MIRA-1 (NSC19630), and its analogs MIRA-2 and -3 | R175H, R248Q, R273H | Prevent unfolding of wild-type and mutant p53 and restore native wild-type p53 conformation | (31) | 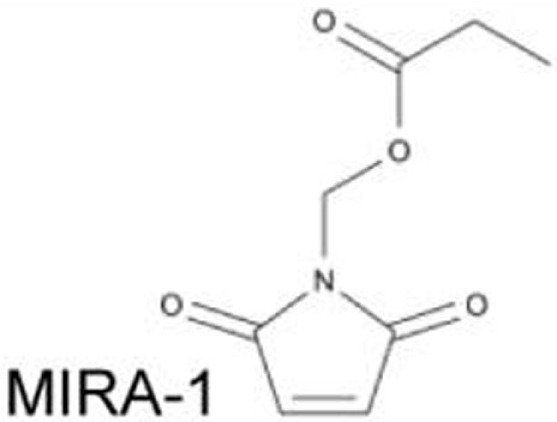 |
| RITA (NSC652287) | R175H, R248W, R273H, R280K | Restore p53 transcriptional activity and induce apoptosis | (32, 33) | 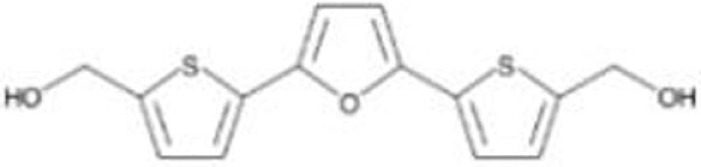 |
| NSC319726/ZMC 1 (zinc metallochaperone-1) | R175H, R172H (mouse) | Restore wild-type p53 conformation and activity with MDM2-dependent degradation | (34–36) | 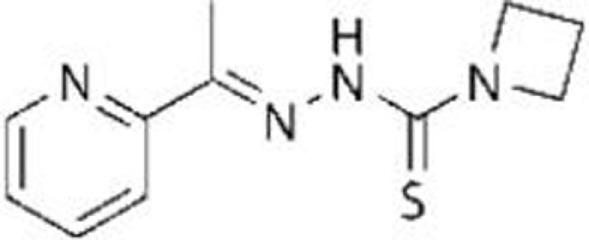 |
| Chetomin (CTM) | R175H | Increase Hsp40 (DNAJB1) levels and Hsp40-p53R175H binding, restoring wild-type p53 conformation, activity, and MDM2-dependent degradation | (37) | 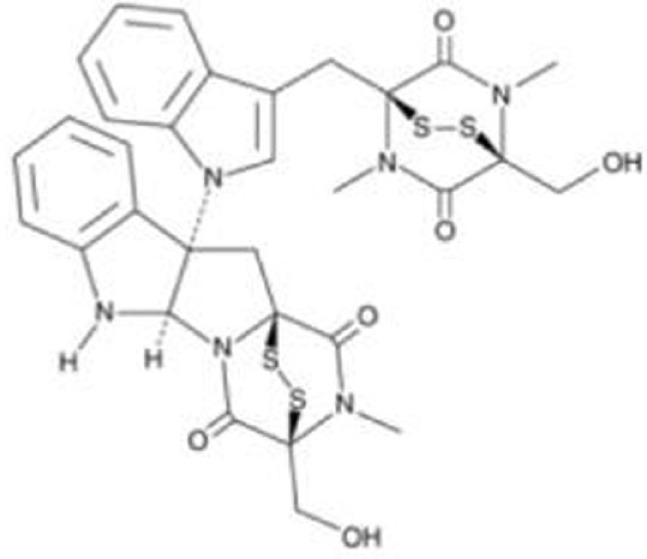 |
| PK7088 | Y220C | Bind to a p53Y220C-specific surface cavity and stabilize p53Y220C with restored wild-type p53 conformation | (38) | 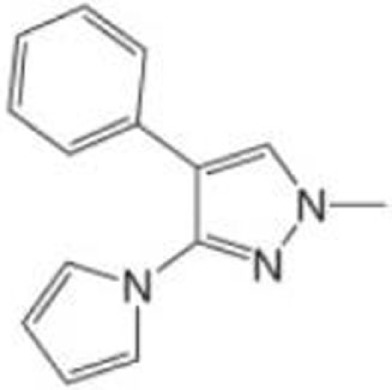 |
| Stictic acid (NSC87511) | R175H, G245S | Target cysteine 124 at the p53 core domain and restore wild-type p53 activity | (39) | 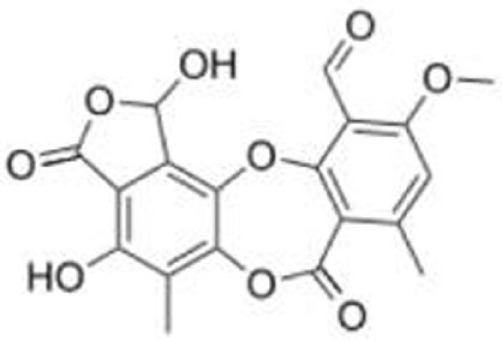 |
| p53R3 | R175H, M237I, R273H | Restore sequence-specific DNA binding and p53 transcriptional activities | (40) | 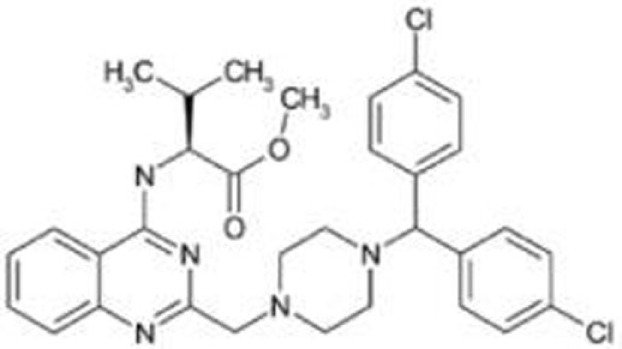 |
| SCH529074 | R175H, L194F, R248W, R249S, R273H | Restore sequence-specific DNA binding and p53 transcriptional activities | (41) | 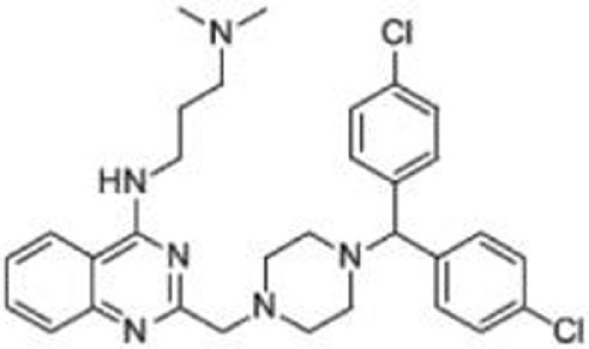 |
| WR-1065 | V272M | Restore DNA binding and transcriptional activities of p53V272M | (42–45) | 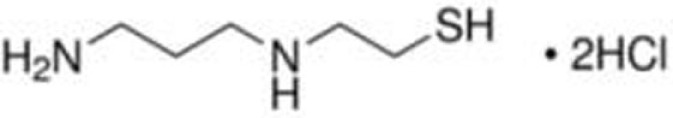 |
CP-31398
CP-31398 (styrylquinazoline) was identified through a structure-based screening as a compound which could restore native wild-type p53 conformation from a denatured conformation in the DNA-binding domain, using a conformation specific antibody PAb1620. CP-31398 leads to increase in p21 mRNA expression in Saos-2 (p53-null) cells expressing p53V173A and p53R249S mutants, and inhibits tumor growth of A375.S2 (p53R249S) and DLD1 (p53S241F) cells (17). CP-31398 increases mRNA expression of MDM2 and p21 in multiple cancer cell lines (46). CP-31398 also induces mitochondrial translocation of mutant p53R273H in A431 skin carcinoma cell line, leading to cytochrome c release and apoptosis (25). Intriguingly, CP-31398 cannot refold already misfolded mutant p53 proteins, since cycloheximide prevents the effect of CP-31398 on p53 restoration (23, 26). It also induces cell death in a p53-independent manner through free radical formation (27).
STIMA-1 (SH Group-Targeting Compound That Induces Massive Apoptosis)
STIMA-1 [2-vinylquinazolin-4-(3H)-one] was identified as one of the CP-31398 derivatives, which induced mutant p53 (p53R175H and p53R273H)-dependent growth suppression (28). Both CP-31398 and STIMA-1 bind to the cysteine residues in the core domain of mutant p53, leading to stabilization of wild-type p53 conformation and subsequent restoration of transcriptional activity (28). STIMA-1 increases the DNA-binding ability of mutant p53, resulting in upregulation mRNA expression of p21, PUMA, and BAX, and leading to mutant p53-dependent apoptosis (28).
PRIMA-1 and PRIMA-1MET/APR-246
PRIMA-1 [2,2-bis (hydroxymethyl)-3-quinuclidinone] was identified through a screening as a compound that suppressed proliferation of Saos-2 osteosarcoma cell line expressing p53R273H (Saos-2-p53R273H) with little effect on the parental Saos-2 cells. PRIMA-1 and its methylated analog PRIMA-1MET (also known as APR-246) not only enhance stability of wild-type p53 at 37°C, but also induce conformational change of p53R175H, leading to restoration of DNA-binding activity of p53R175H with increased MDM2 and p21 mRNA expression (23). Notably, PRIMA-1 refolds previously accumulated unfolded mutant p53 (23). The mechanisms underlying refolding of mutant p53 by PRIMA-1 and PRIMA-1MET involve the conversion of these compounds to products which form adducts with thiol groups in the mutant p53 core domain, leading to restoration of wild-type conformation and induction of apoptosis in tumor cells (29, 30). Several studies have successfully validated their tumor suppressive effects in mouse models of multiple types of cancer (47–50). Importantly, PRIMA-1MET is currently in clinical trials (51, 52).
MIRA-1 and Its Structural Analogs
Using the same screening strategy as PRIMA-1, MIRA-1 (NSC19630) was identified as a compound that suppressed proliferation of Saos-2-p53R273H cells (31). MIRA-1 and its structural analogs MIRA-2 and MIRA-3 from the NCI repository inhibit proliferation of cancer cells expressing p53R175H and p53R273H (31). Both MIRA-1 and MIRA-3 also induce cell death in cancer cells expressing mutant p53 (31). Furthermore, MIRA analogs prevent unfolding of wild-type and mutant p53, and also restored native wild-type p53 conformation, leading to enhanced DNA-binding activity of mutant p53 (p53R175H and p53R248Q) and increase in mRNA expression of p53 downstream target genes, MDM2 and p21, in several mutant p53-carrying cancer cell lines (31). In vivo effects of MIRA analogs have also been confirmed in mouse models (31, 53).
RITA (NSC652287)
NSC652287 [2,5-bis(5-hydroxymethyl-2-thienyl) Furan] is one of a series of thiophene derivatives and is known to inhibit tumor growth of renal cell carcinoma cells with DNA–protein cross-linking and upregulation of wild-type p53 and p21 (54, 55). NSC652287 (RITA: reactivation of p53 and induction of tumor cell apoptosis) is also identified through cell proliferation assay-based screening using isogenic cell lines of HCT116 (wild-type p53 and p53-null) as a compound that suppresses the growth of HCT116 (wild-type p53) cells in a dose-dependent manner with minimum effects on HCT116 (p53-null) cells (56). Later, NSC652287/RITA was found to suppress the growth of cancer cell lines carrying various p53 mutants (p53R175H, p53R213Q/Y234H, p53R248W, p53R248Q, p53I254D, p53R273H, and p53R280K) by restoration of p53 transcriptional activity (p21, NOXA, PUMA, and GADD45) and induction of apoptosis through upregulation of pro-apoptotic proteins and downregulation of several oncogenes or anti-apoptotic proteins (32, 33, 57). However, the exact mechanism by which RITA activates both wild-type and mutant p53 to induce apoptosis remains unclear.
NSC319726/ZMC1 (Zinc Metallochaperone-1)
Zinc is required for proper folding of p53 protein, while lack of zinc in the central core domain of p53 leads to unfolding (23, 58, 59). Also, addition of zinc to cells or its administration to mice carrying tumors are known to restore DNA-binding activity of mutant p53 (p53R175H and p53R273H) in cells and tumors, leading to tumor suppression (60). Thus, facilitating the binding of mutant p53 to zinc can be used to restore the proper folding and transcriptional activity of mutant p53 (34). Indeed, NSC319726 [zinc metallochaperone-1 (ZMC1)], a thiosemicarbazone derivative, was identified in a screen of the NCI60 panel of human tumor cell lines as a compound that exhibited selective toxicity to cells carrying p53R175H with minimum effects on cells expressing wild-type p53 and other p53 mutants (p53R248Q and p53R273H) (35). NSC319726 restores the wild-type-like conformation of mutant p53 and upregulates p53 downstream target genes (p21, PUMA, and MDM2) through increasing ROS levels (35, 36). It also reduces p53R175H levels, which is rescued by Nutlin-3a (35). Importantly, its administration induces greater toxicity in p53R172H/R172H (equivalent to human p53R175H) mice than in wild-type mice in a dose-dependent manner (35). NSC319726 also suppresses tumor growth of TOV112D (p53R175H) cells in vivo (35). Other thiosemicarbazone family members (NSC319726 and NSC328784) also preferentially reduce cell viability of p53 mutant cell lines (35).
Chetomin
Hiraki et al. (37) performed high-throughput chemical library screening using a luciferase reporter with the p53 response element of the PUMA promoter in H1299 (p53-null) cells carrying p53R175H, which identified chetomin (CTM) as a compound that increased luciferase activity dose-dependently. CTM suppresses cancer cell growth in vitro and in vivo more efficiently in cells expressing p53R175H with upregulation of MDM2, p21, and PUMA than those expressing wild-type p53 and p53R273H, as well as null for p53 (37). The effects of CTM on reduced p53R175H levels are dependent on MDM2 (37). Interestingly, CTM increases protein levels of Hsp40 (DNAJB1) and the binding of p53R175H to Hsp40, leading to restoration of wild-type p53 conformation and tumor suppression of cancer cells carrying p53R175H (37). However, CTM is also known to inhibit the hypoxia-inducible factor pathway by blocking the interaction of HIF proteins and their cofactor p300. Moreover, it suppresses in vivo tumor growth of HCT116 (wild-type p53) cells and enhances radiosensitivity of cancer cells regardless of the p53 status (61–63). Thus, CTM has other function than mutant p53 reactivation.
PK7088
PK7088 [1-methyl-4-phenyl-3-(1H-pyrrol-1-yl)-1H-pyrazole] was identified as a compound that binds to a p53Y220C-specific surface cavity destabilizing this protein through protein-observed NMR screening (38, 64). PK7088 stabilizes p53Y220C and restores wild-type p53 conformation. It is biologically active in cancer cells carrying p53Y220C mutant and induces G2/M arrest of the cell cycle and apoptosis with upregulation of NOXA and p21, as well as relocation of BAX to the mitochondria (38). PK7088 and Nutlin-3a cooperatively upregulate protein expression of p21 and NOXA (38). Crystal structure of p53-Y220C core domain with PK7242, a more soluble PK7088 analog, is also presented (38).
Stictic Acid (NSC87511)
A computational analysis of p53 structural models suggests that cysteine 124 of p53 is located at the center of a transiently open binding pocket between loop L1 and sheet S3 of the p53 core domain (39). Based on the finding that additional mutation at cysteine 124 to alanine on p53R175H (p53C124A/R175H) abolished the effects of PRIMA-1 on the reactivation of p53R175H, Wassman et al. (39) performed an Ensemble-based virtual screening against this pocket and identified stictic acid as a p53 reactivation compound (39). Stictic acid stabilizes p53 in vitro and induces expression of p21 and PUMA in Saos2 (p53-null) cells expressing mutant p53 (p53R175H and p53G245S) (39).
P53R3
The p53 reactivator (P53R3) is a compound identified through a screening using an in vitro DNA-binding assay (40). P53R3 restores sequence-specific DNA binding of several p53 mutants (p53R175H, p53M237I, and p53R273H) and induces p53-dependent antiproliferative effects with increase in mRNA expression of many p53 target genes, such as p21, GADD45, BAX, PUMA, PIG3, and MDM2 (40). It should be noted that P53R3 also increases mRNA expression of several p53 target genes (p21, GADD45, PUMA, and MDM2) in cancer cells expressing wild-type p53 (40).
SCH529074
The small molecule SCH529074 was identified by a DNA-binding assay-based screening as a compound that enabled p53R273H to bind to a consensus p53 DNA-binding site (41). SCH529074 restores the PAb1620 epitope by acting as a chaperone and enhances DNA-binding activity of several p53 mutants (p53R175H, p53S241F, p53R248W, p53R249S, and p53R273H), leading to upregulation of p53 downstream target genes (p21, BAX, NOXA, cyclin G1, and PUMA), induction of proliferation arrest or apoptosis, and inhibition of in vivo tumor growth of mutant p53-expressing cell lines (41). Additionally, SCH529074 binds to DNA-binding domain of p53 and inhibits ubiquitination and degradation of wild-type p53 by MDM2 (41).
WR-1065
WR1065 is an active form of amifostine and is used to protect tissues against the damaging effects of radiation and chemotherapeutic drugs (42). WR-1065 increases wild-type p53 activity through a JNK-dependent signaling pathway, but not through genotoxic mechanisms (42–44). It is also reported that WR-1065 restores wild-type p53 conformation of a temperature-sensitive p53V272M, leading to increase in the DNA-binding activity, transactivation of several p53 target genes (p21, GADD45, and MDM2), and cell cycle arrest in G1 phase (45).
Compounds That Deplete Mutant p53
Although many p53-reactivating compounds seem to target more than one p53 mutant, it remains unclear if these compounds can reactivate all p53 mutants or specific mutant types. Also, PRIMA-1MET is the only drug currently under clinical trials. Thus, the development of p53-reactivating compounds remains challenging. Another approach to target oncogenic mutant p53 is to discover compounds that specifically deplete mutant p53 with little effect on wild-type p53. Rationale to develop mutant p53-depleting compounds is based on the following observations: (1) mutant p53 is inherently unstable, but once stabilized, it can accelerate tumor progression (10) and (2) knockdown of mutant p53 by small interference RNAs (siRNAs) or shRNAs reduces malignant properties of cancer cells (13–16), thus indicating that oncogenic potential of cancer cells are, at least partially, dependent on the presence of accumulated oncogenic mutant p53. Although the mechanisms behind stabilization or degradation of mutant p53 are not necessarily the same as those of wild-type p53 and remain elusive (5, 65), several compounds that induce mutant p53 degradation without altering wild-type p53 have been found. These compounds can be used as effective therapeutic strategies for both cancers carrying only mutant p53 and those retaining wild-type p53 with mutant p53 as mentioned in the Introduction. Thus, compounds that specifically deplete mutant p53 are valuable for cancer therapy and also for elucidating the mechanisms of stabilization or degradation of mutant p53. Major compounds which deplete mutant p53 are listed in Table 2 and explained below.
Table 2.
Compounds that deplete mutant p53.
| Compound | Type of mutant | Mechanism | Reference | Structure |
|---|---|---|---|---|
| Hsp90 inhibitors: 17-AAG, geldanamycin, ganetespib | R175H, L194F, R248Q, R273H, R280K, R172H (mouse) | Reverse the Hsp90’s function to inactivate MDM2 and CHIP | (13, 66–69) | 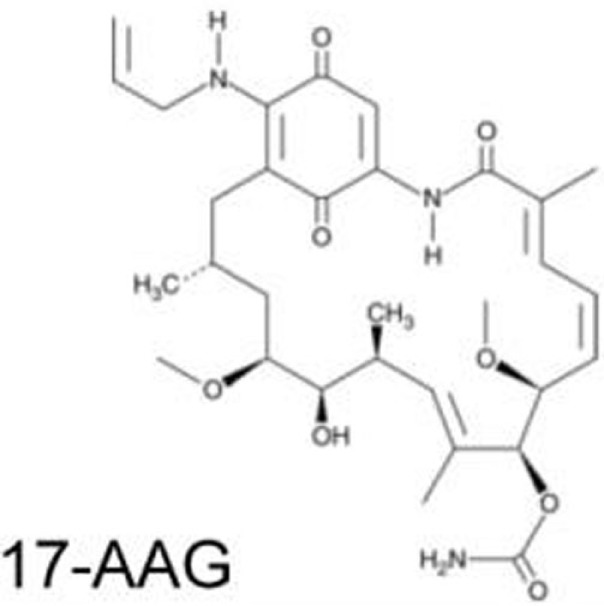 |
| HDAC inhibitors: vorinostat/SAHA, romidepsin/depsipeptide | R175H, R280K, V247F/P223L | Inhibit HDAC6 and disrupt the HDAC6/Hsp90/mutant p53 complex | (70–72) | 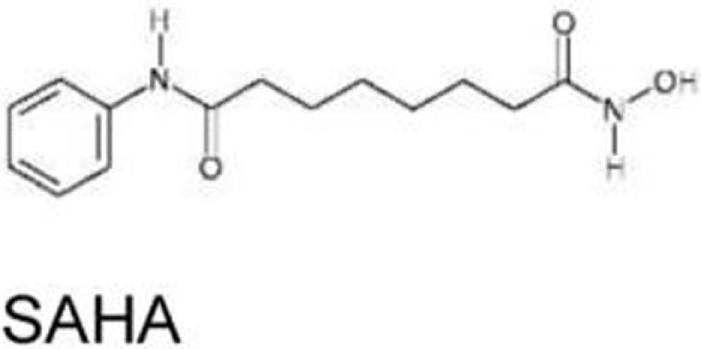 |
| Arsenic compounds | R175H, R248W, H179Y/R282W, R273H | Increase transcripts of Pirh2 and induce degradation of mutant p53 | (73, 74) | 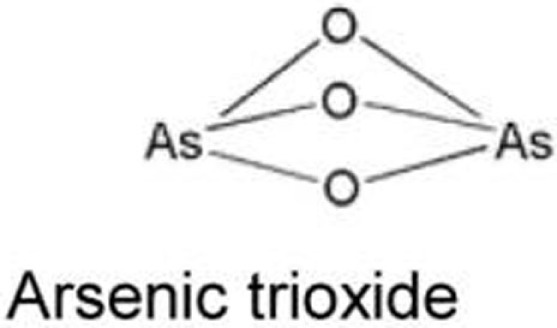 |
| Gambogic acid | R175H, G266E, R273H, R280K | Inhibit the mutant p53-Hsp90 complex and induce CHIP-dependent degradation | (75) | 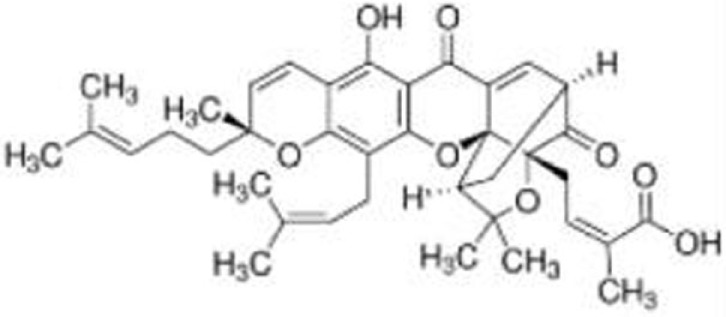 |
| Spautin-1 | R158lnF, R175H, S241F, R248Q, G266Q, R280L, R273H | Induce mutant p53 degradation via the CMA pathway activated by the suppression of macroautophagy under glucose-free and confluent conditions | (76, 77) | 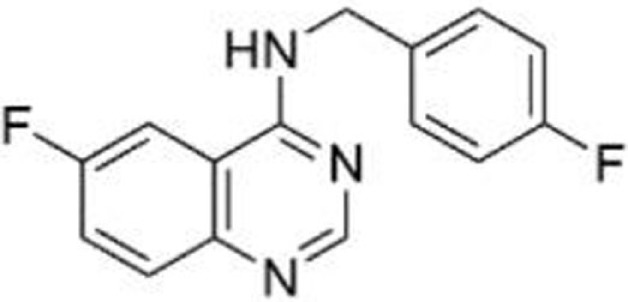 |
| YK-3-237 | V157F, M237I, R249S, R273H, R280K | Decrease mutant p53 levels through deacetylation at lysine 382 by activating SIRT1 | (78) | 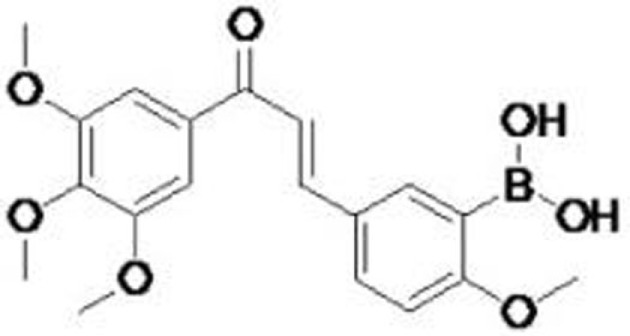 |
| NSC59984 | R175L, R175H, S241F, R273H/P309F | Induce MDM2-mediated mutant p53 degradation and activate p73 | (79) | 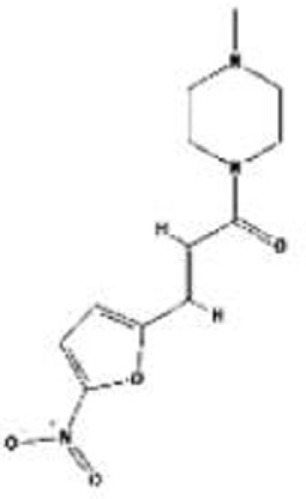 |
| Disulfiram (DSF) | R273H | Induce degradation of both wild-type p53 and p53R273H via the 26S proteasome pathway | (80) | 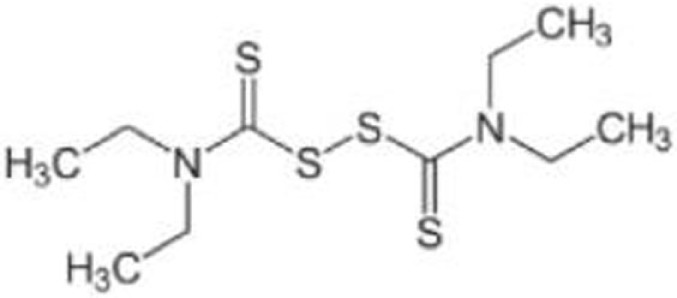 |
Hsp90 Inhibitors: Geldanamycin, 17-AAG, Ganetespib
Blocking the function of heat shock protein 90 (Hsp90) leads to depletion of several oncogenic proteins, such as Raf-1, ErbB2, and mutant p53 (66, 67), because Hsp90 contributes to accumulation of mutant p53 by inactivating p53 ubiquitin ligases, MDM2, and CHIP (68, 69). Treatment of cancer cells with 17-AAG, a Hsp90 inhibitor and an analog of geldanamycin, promotes degradation of varieties of p53 mutants (p53R175H, p53L194F, p53R273H, and p53R280K) and decreases viability of cells carrying mutant p53 (69). Importantly, another Hsp90 inhibitor, ganestespib, which is 50-fold more potent than 17-AAG in destabilizing mutant p53 with little effect on wild-type p53 levels, induces mutant p53 depletion with increased apoptosis in tumors in vivo in both p53R248Q Hupki (human p53 knock-in) and p53R172H knock-in mouse models (13). Ganetespib is currently under evaluation in clinical trials, including phase III for lung cancer (81–83).
Histone Deacetylase Inhibitors: Vorinostat/SAHA, Romidepsin/Depsipeptide
The effects of histone deacetylase inhibitors (HDACi) on mutant p53 (p53R175H, p53R280K, and p53V274F/P223L) were first reported by Blagosklonny et al. (70). Later, two mechanisms describing the inhibition of HDAC8-mediated mutant p53 transcription (84) and mutant p53 destabilization through inhibition of HDAC6 by HDACi are proposed (71). Specifically, suberoylanilide hydroxamic acid (SAHA, also known as vorinostat), a FDA-approved HDACi that inhibits class I, II, and IV HDACs, induces degradation of mutant p53 by inhibiting HDAC6 activity, an essential positive regulator of Hsp90, and subsequent disruption of the HDAC6/Hsp90/mutant p53 complex, leading to mutant p53 ubiquitination by MDM2 and CHIP (71, 72). SAHA shows higher cytotoxic effects on cancer cells carrying mutant p53 than those having wild type or null for p53 (72). SAHA also sensitizes cancer cells to a topoisomerase inhibitor camptothecin in a mutant p53-dependent manner (71).
Arsenic Compounds
Arsenic trioxide (ATO), which is used to treat patients with acute promyelocytic leukemia (APL), binds to thiol groups in cysteine residues and induces degradation of proteins, such as promyelocytic leukemia protein (PML) and PML-retinoic acid receptor α (PML-RARα) fusion protein (85). It also activates wild-type p53 and upregulates p53 downstream target genes with induction of apoptosis (86). Yan et al. (73) asked the possibility of using arsenic compounds to target mutant p53 for degradation and found that ATO or sodium arsenite induced proteasomal-dependent degradation of several p53 mutants (p53R175H, p53H179Y/R282W, p53R248W, and p53R273H). ATO also increases transcripts of an E3 ubiquitin ligase Pirh2, leading to ubiquitination and degradation of several mutant p53 (74). However, it should be noted that arsenic compounds have carcinogenic effects and are known to induce several types of cancer (87).
Gambogic Acid
Gambogic acid (GA), a natural product derived from Garcinia hanburyi tree, induces apoptosis and inhibits tumor growth in vivo by upregulating wild-type p53 at protein levels (88). On the other hand, GA induces nuclear exports of mutant p53 (p53R175H, p53G266E, p53R273H, and p53R280K) for degradation by CHIP ubiquitin ligase (75). GA prevents the mutant p53-Hsp90 complex formation but enhances the mutant p53-Hsp70 interaction (75). Biologically, GA reduces viability of mutant p53-expressing cancer cells and also increases cytotoxic effects of several chemotherapy drugs in human breast cancer MDA-MB-435 (p53G266E) cell line (75).
Spautin-1
Spautin-1 is a derivative of MBCQ (4-((3,4-methylenedioxybenzyl)amino)-6-chloroquinazoline) which was identified as a small molecule that inhibited autophagy through an imaging-based screen using LC3-GFP as a marker (89). When cancer cells are placed in the nutrient-deficient environment, cells start autophagy to generate an alternative energy source to survive by degrading cellular proteins and organelles in the lysosome. Spautin-1 inhibits ubiquitin-specific peptidases, USP10 and USP13, and promotes the degradation of Vps34-PI3 kinase complexes, key regulators of autophagy, leading to inhibition of autophagy (89). Since USP10 also deubiquitinates wild-type p53, Spautin-1 promotes degradation of wild-type p53 (89, 90). Moreover, suppression of macroautophagy by Spautin-1 under glucose-free and confluent conditions is found to induce degradation of several p53 mutants (p53R158InF, p53R175H, p53R248Q, p53S241F, p53G266E, p53R280L, and p53R273H) through the chaperone-mediated autophagy (CMA) pathway (76). Spautin-1 also induces cell death under non-proliferating condition only when cancer cells express mutant p53. This effect of Spautin-1 is independent of MDM2 and the ubiquitin–proteasome pathway, but is dependent on nuclear export of mutant p53 and the presence of Hsc70 (76, 77).
YK-3-237
YK-3-237 was originally identified as a compound that showed antiproliferative effects in different cancer cell lines, but its mechanism of action was unknown (91). Yi et al. (78) investigated the effects of this compound on the proliferation of breast cancer cell lines carrying different p53 status and found that YK-3-237 preferentially inhibited the proliferation of breast cancer cell lines carrying mutant p53. YK-3-237 also decreased the levels of mutant p53 (p53V157F, p53M237I, p53R249S, p53R273H, and p53R280K) through reduction in acetylation at lysine 382 (K382) of mutant p53, a target site of a NAD+-dependent protein deacetylase SIRT1 (also known as sirtuin 1) (78). Indeed, YK-3-237 activates SIRT1 enzyme activity (78). Furthermore, treatment of triple-negative breast cancer cell lines with YK-3-237 results in induction of apoptosis and G2/M arrest of the cell cycle with increase in mRNA expression of NOXA and PUMA (78). However, the underlying mechanism remains unclear.
NSC59984
The small molecule NSC59984 was recently identified as a compound that increased luciferase activity in SW480 (p53R273H/P309S) and DLD-1 (p53S241F) cells carrying a p53-responsive luciferase reporter construct (PG13) (79). NSC59984 increases mRNA and protein levels of several p53 targets, such as p21, PUMA, and NOXA, with increase in apoptosis. It should be noted that NSC59984 also induces apoptosis in cancer cells having wild type and null for p53, suggesting it has p53-independent effects on cell death (79). Moreover, NSC59984 induces degradation of several p53 mutants (p53R175L, p53R175H, p53S241F, and p53R273H/P309S) through MDM2-mediated ubiquitination, whereas it rather increases wild-type p53 levels (79). Importantly, the effects of NSC59984 on p53 target gene expression and apoptosis are caused by activation of p73, rather than conformational changes of mutant p53 (79). In in vivo xenograft mouse models, NSC59984 suppresses tumor growth of DLD-1 in a p73-dependent manner (79).
Disulfiram
Disulfiram (DSF) is used for the treatment of chronic alcoholism by inhibiting acetaldehyde dehydrogenase. DSF has also been under clinical trials for some types of cancer including glioblastoma multiforme and metastatic non-small cell lung cancer (92, 93). DSF and its metabolites which are strong ROS inducers contain a reactive disulfide bond and readily mediate thiol-conjugating reactions, leading to S-glutathionylation of cysteine residues in proteins (94, 95). Protein S-glutathionylation in response to oxidative stress can affect function and stability of target proteins (80, 96). Interestingly, p53 is found to be S-glutathionylated at cysteine residues 124 and 141 (97, 98). Paranjpe et al. (80) reported that DSF and its derivative copper-chelated disulfiram (CuDSF) induced degradation of both wild-type p53 and p53R273H through the 26S proteasome pathway. However, DSF-induced protein degradation is not p53-specific, because it also induces degradation of other redox-regulated proteins, such as NF-κB subunit p50 and UBE1 (80).
Other Strategies to Target Mutant p53 and Its Related GOF Activity
Compounds That Induce Readthrough of Premature Termination Codons
About 8% of all the p53 mutations in human cancers are nonsense mutations, which results in the presence of premature termination codons (PTCs) (99). PTC leads to the mRNA degradation by the nonsense-mediated mRNA decay pathway or potential production of truncated proteins. However, aminoglycoside antibiotics (gentamicin, G418, and amikamicin) bind with the ribosomal RNA and promote readthrough of PTCs, leading to partial restoration of full-length protein production (100). Specifically, gentamicin, G418, and NMDI14 induce production of full-length functional p53 from p53Q192X, p53R213X, and p53E298X, leading to apoptosis induction in cancer cells carrying nonsense p53 mutations (101, 102).
Knockdown with Small Interference RNA
Knocking down specific protein expression by siRNAs or shRNAs can be a specific and potent strategy to target cancers if methodologies of efficient in vivo delivery are established (103). Indeed, downregulation of mutant p53 in T47D (p53L194F), MDA-MB-231 (p53R280K), and MDA-MB-468 (p53R273H) breast cancer cell lines induces PARP-dependent apoptosis (104, 105). In DU145 (p53P223L/V274F) prostate cancer cell line, downregulation of p53P223L/V274F by siRNA induces cell cycle arrest at G1 and G2/M phases, as well as apoptosis in a PI3K/Akt-dependent manner (106). Also, silencing of mutant p53 in 5637 (p53R280T) and T24 (in-frame deletion of Y126) bladder cancer cell lines by p53 siRNA induces G2 arrest of the cell cycle and apoptosis, and increases sensitivity to cisplatin (107). Thus, accumulated studies reveal that knockdown of mutant p53 by siRNAs or shRNAs reduces malignant properties of cancer cells. However, siRNAs for p53 used in aforementioned publications can also knockdown wild-type p53. Matinez et al. (108) first developed a p53R248W-specific siRNA which did not affect wild-type p53. Recently, our laboratory also developed siRNAs specific for p53R175H and p53R273H, which did not alter wild-type p53 expression levels (unpublished). We successfully showed reactivation of wild-type p53 and reduced cell proliferation and migration, following transfection of these mutant-specific siRNAs in genetically engineered p53 heterozygous cancer cell lines (HCT116+/R248W, SW48+/R273H) (unpublished). Thus, the extraordinary sequence specificity of siRNA makes it an attractive tool for targeted cancer therapies.
Compounds That Affect Downstream Targets of Mutant p53
Another way to target oncogenic activity of mutant p53 is to reactivate tumor suppressive pathways that are inhibited by mutant p53 or to inhibit tumor-promoting pathways that are activated by mutant p53.
Reactivate transcriptional activity (RETRA) was identified as a compound that increased β-galactosidase activity in A431 (p53R273H) human epidermal carcinoma cells expressing a p53-resonsive promoter-driven β-galactosidase construct, through high-throughput screening of a chemical library comprising of 46,250 compounds (109). RETRA increases β-galactosidase activity only in cancer cells carrying mutant p53 (p53R248Q, p53R280L, and p53G266E) with increased mRNA expression of p21 and PUMA, but fails to do so in cells wild type or null for p53. Interestingly, the effects of RETRA are nullified by knockdown of p73, but not knockdown of p53 and p63 (109). Indeed, RETRA inhibits the binding of p53R273H with p73. RETRA also reduces A431 cell viability in a p73-dependent manner and reduces tumor growth in a xenograft model (109).
Statins are a class of compounds that inhibit 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase in the mevalonate pathway and have been used in the clinic to treat hypercholesterolemia. HMG-CoA reductase is the rate-limiting step in cholesterol synthesis and also regulates prenylation/lipidation (farnesylation and geranyl-geranylation) of proteins. Prenylation facilitates attachment of target proteins to cell membranes which are involved in cellular adhesion, migration, and proliferation signaling (e.g., Rho, Rac, Cdc42, Ras) (110). Interestingly, knockdown of p53R273H significantly reduces mRNA expression of multiple enzymes involved in the mevalonate pathway (16). Both knockdown of mutant p53 and inhibition of protein prenylation by statins or other compounds result in impaired growth of breast cancer cells in 3D culture (16). Mechanistically, mutant p53 binds to and activates SREBP, crucial transcription factors that regulate transcription of several enzymes involved in the mevalonate pathway, leading to enhanced prenylation of proteins associated with cancer progression and activation of prenylated proteins in breast cancer cells; hence, inhibition of protein prenylation by statins leads to reduced malignancy of human breast cancer cells (16). Importantly, the presence of p53 mutation correlates with high expression of sterol biosynthesis genes in human breast tumors (16). Additionally, since nuclear localization and activation of the YAP and TAZ proto-oncogenes are regulated by prenylation and activation of Rho GTPases, statins could also suppress progression of mutant p53-expressing tumors by inhibiting YAP/TAZ activation by reducing protein prenylation of Rho GTPases, which is promoted by SREBP and its cofactor mutant p53 (111).
Compounds That Induce Synthetic Lethality
Synthetic lethality is generally used for the condition where a mutation in a gene is not lethal by itself, but its combination with a drug or other gene mutations leads to cell death (112). Since over 50% of human cancers have mutations in the p53 gene, p53 mutations become attractive targets for inducing synthetic lethality in tumors. In this regard, compounds that induce synthetic lethality to mutant p53 should selectively kill cancer cells expressing mutant p53 without affecting normal cells carrying wild-type p53. One compound that induces synthetic lethality to mutant p53 is UCN01, a protein kinase C inhibitor and a potent blocker of G2/M checkpoint of the cell cycle. Treatment of CA46 (p53R248Q) and HT29 (p53R273H) with UNO01 abrogates γ-irradiation-induced G2/M arrest of the cell cycle and increases cytotoxicity (113). UCN01 also enhances cisplatin-induced cell death in MCF7-expressing human papillomavirus type-16 E6 (MCF7/E6) with little effect on parental MCF7 cells having functional p53 (113). Another compound that is synthetic lethal to mutant p53 is BI-2536, an inhibiter of polo-like kinase 1 (PLK1), an enzyme that controls G2/M checkpoint. Inhibition of PLK1 by BI-2536 significantly enhances cytotoxic effect of ionizing radiation in DLD-1 (p53S241F) and p53R248W-overexpressing HCT116 cells, but it does not do so in parental HCT116 (wild-type p53) cells (114). Similar effects are observed with PD0166285, an inhibitor of Wee1 kinase that regulates G2/M checkpoint. PD0166285 sensitizes cancer cells (HT29: p53R273H and E6-overexpressing PA-1: wild-type p53) to radiation-induced death, whereas this effect is not detected in parental PA-1 cells (115). However, the observed synthetic lethality to mutant p53 is likely not dependent on oncogenic GOF activity of mutant p53, but rather dependent on loss of wild-type p53 activity. Identification of synthetic lethality to mutant p53 alone, but not p53-null, could improve our understanding of oncogenic GOF activity of mutant p53 and contribute to the development of new compounds that target cancer cells carrying mutant p53.
Summary and Future Directions
Accumulated evidence has proven that small-molecule compounds can restore the transcriptional activity of mutant p53 or specifically deplete mutant p53. These compounds are expected to efficiently inhibit tumor growth with minimal effects on normal tissues. Several compounds listed in Tables 1 and 2 are already in clinical trials. Within p53 reactivators, PRIMA-1MET (also known as APR-246) is the only drug under clinical trials (51, 52). On the other hand, amongst the compounds that deplete mutant p53, Hsp90 inhibitors (81–83), HDAC inhibitors (116–118), ATO (119–121), and DSF (92, 93, 122) are under clinical trials for cancer therapy. However, it is not completely understood whether these p53 reactivators and mutant p53-depleting compounds have mechanisms of action on proteins or pathways other than mutant p53, if they have an impact on all p53 mutants or specific mutant types and their underlying mechanisms. Also, it would be important to determine any synergistic or additive effects of these compounds with conventional chemotherapy drugs on cancer cell survival and proliferation. Further studies to solve these concerns would help improving the efficacy and specificity of these compounds.
In order to better understand the mechanisms of action of compounds that target mutant p53, it is important to determine whether they can directly bind with mutant p53 or proteins involved in the process of mutant p53 reactivation or depletion. There are several in vitro methodologies to determine compound–protein interaction, including Biacore assays, mass spectrometry-based approaches, and drug affinity responsive target stability (DARTS) assays (123–125). On the other hand, in vivo analysis is limited. Recently, Jafari et al. (126) reported a cell-based drug–protein interaction assay, called cellular thermal shift assay (CETSA). Investigating interactions between a compound and a specific protein in cells would significantly improve our understanding of how efficiently and specifically the compound alters intracellular activity.
Biological effects of compounds that restore p53 activity are robust, since mutant p53 is usually accumulated in cancer cells, and hence these compounds have ample substrates to restore the p53 activity. On the other hand, compounds that deplete mutant p53 may not be as robust as those restoring the p53 activity; however, as mentioned in Section “Introduction,” survival and growth of cancer cells are frequently dependent on mutant p53 (oncogene addiction) (13, 14). Thus, simply depleting mutant p53 in tumors is likely sufficient to reduce tumor malignancy. Especially, when tumor cells retain the wild-type p53 allele, compounds depleting mutant p53 alone may be even more effective, since they could reactivate wild-type p53 by releasing from DN activity of mutant p53. These mutant p53-depleting compounds could also be used for prevention of tumorigenesis when patients inherently carry mutant p53, such as in the case of human tumor-prone disease Li–Fraumeni syndrome (LFS). Over 70% of LFS patients have p53 mutations in their germlines (127), but mutant p53 is not always stable and accumulates mainly in tumors (10). A compound that depletes only accumulated mutant p53 in tumors could reduce risk of tumor development in LFS patients. The approach of mutant p53-specific knockdown by siRNAs or DNA oligomers could cause similar outcomes to those by mutant p53-depleting compounds. However, the major hurdle of this approach is efficient delivery of these oligomers to all cancer cells, which need to be addressed prior to their consideration for clinical trials (103).
Other than compounds summarized in this review article, compounds that target inhibitors of p53, such as MDM2 and MDM4, are also powerful to reactivate p53 and are summarized in other review articles (128, 129). Also, induction of synthetic lethality for mutant p53 is another specific approach for cancer cells expressing mutant p53 (128, 130–132). Inhibitors of proteins associated with G2/M arrest of the cell cycle and mitotic checkpoint appear to cause mitotic catastrophe in cancer cells lacking wild-type p53 activity.
In summary, discovering efficient and safe compounds that specifically target mutant p53 remains challenging. Hence, it is crucial to further understand how mutant p53 induces oncogenic function, to elucidate the exact mechanisms of mutant p53 stabilization or degradation in tumors, and to identify mutant p53-specific downstream signaling pathways or binding partners. The battle with cancer is unexpectedly taking longer, with cancers being wily enough to escape from current available treatments and develop novel ways of surviving. Given that several mutant p53-targeting drugs are already undergoing clinical trials, the goal toward establishment of therapies to cure mutant p53-carrying cancers may be just on the horizon.
Author Contributions
AP and TI wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We apologize to our colleagues for the exclusion of other important contributions to the field and their references, mainly due to space limitations. We thank Swathi V. Iyer, Atul Ranjan, and Kaustav Bera for helpful discussions and editing the manuscript.
Funding
This work was supported by the NIH 1-R01-CA174735-01A1 (TI) and 5-P30-CA168524-04 (PI: Roy A. Jensen) KU Cancer Center grants.
References
- 1.Lane D, Levine A. p53 Research: the past thirty years and the next thirty years. Cold Spring Harb Perspect Biol (2010) 2:a000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levav-Cohen Y, Goldberg Z, Tan KH, Alsheich-Bartok O, Zuckerman V, Haupt S, et al. The p53-Mdm2 loop: a critical juncture of stress response. Subcell Biochem (2014) 85:161–86. 10.1007/978-94-017-9211-0_9 [DOI] [PubMed] [Google Scholar]
- 3.Vaseva AV, Moll UM. The mitochondrial p53 pathway. Biochim Biophys Acta (2009) 1787:414–20. 10.1016/j.bbabio.2008.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pant V, Lozano G. Limiting the power of p53 through the ubiquitin proteasome pathway. Genes Dev (2014) 28:1739–51. 10.1101/gad.247452.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muller PA, Vousden KH. p53 mutations in cancer. Nat Cell Biol (2013) 15:2–8. 10.1038/ncb2641 [DOI] [PubMed] [Google Scholar]
- 6.Rivlin N, Brosh R, Oren M, Rotter V. Mutations in the p53 tumor suppressor gene: important milestones at the various steps of tumorigenesis. Genes Cancer (2011) 2:466–74. 10.1177/1947601911408889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivlin N, Koifman G, Rotter V. p53 orchestrates between normal differentiation and cancer. Semin Cancer Biol (2015) 32:10–7. 10.1016/j.semcancer.2013.12.006 [DOI] [PubMed] [Google Scholar]
- 8.Joerger AC, Fersht AR. Structural biology of the tumor suppressor p53. Annu Rev Biochem (2008) 77:557–82. 10.1146/annurev.biochem.77.060806.091238 [DOI] [PubMed] [Google Scholar]
- 9.Goldstein I, Marcel V, Olivier M, Oren M, Rotter V, Hainaut P. Understanding wild-type and mutant p53 activities in human cancer: new landmarks on the way to targeted therapies. Cancer Gene Ther (2011) 18:2–11. 10.1038/cgt.2010.63 [DOI] [PubMed] [Google Scholar]
- 10.Terzian T, Suh YA, Iwakuma T, Post SM, Neumann M, Lang GA, et al. The inherent instability of mutant p53 is alleviated by Mdm2 or p16INK4a loss. Genes Dev (2008) 22:1337–44. 10.1101/gad.1662908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavenee WK, Scrable HJ, James CD. Molecular genetics of human cancer predisposition and progression. Mutat Res (1991) 247:199–202. 10.1016/0027-5107(91)90015-G [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez MV, Pello MF, Lopez-Larrea C, Suarez C, Menendez MJ, Coto E. Loss of heterozygosity and mutation analysis of the p16 (9p21) and p53 (17p13) genes in squamous cell carcinoma of the head and neck. Clin Cancer Res (1995) 1:1043–9. [PubMed] [Google Scholar]
- 13.Alexandrova EM, Yallowitz AR, Li D, Xu S, Schulz R, Proia DA, et al. Improving survival by exploiting tumour dependence on stabilized mutant p53 for treatment. Nature (2015) 523:352–6. 10.1038/nature14430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masciarelli S, Fontemaggi G, Di Agostino S, Donzelli S, Carcarino E, Strano S, et al. Gain-of-function mutant p53 downregulates miR-223 contributing to chemoresistance of cultured tumor cells. Oncogene (2014) 33:1601–8. 10.1038/onc.2013.106 [DOI] [PubMed] [Google Scholar]
- 15.Weisz L, Oren M, Rotter V. Transcription regulation by mutant p53. Oncogene (2007) 26:2202–11. 10.1038/sj.onc.1210294 [DOI] [PubMed] [Google Scholar]
- 16.Freed-Pastor WA, Mizuno H, Zhao X, Langerod A, Moon SH, Rodriguez-Barrueco R, et al. Mutant p53 disrupts mammary tissue architecture via the mevalonate pathway. Cell (2012) 148:244–58. 10.1016/j.cell.2011.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster BA, Coffey HA, Morin MJ, Rastinejad F. Pharmacological rescue of mutant p53 conformation and function. Science (1999) 286:2507–10. 10.1126/science.286.5449.2507 [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, Guo XY, Hu GY, Liu WB, Shay JW, Deisseroth AB. A temperature-sensitive mutant of human p53. EMBO J (1994) 13:2535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selivanova G, Kawasaki T, Ryabchenko L, Wiman KG. Reactivation of mutant p53: a new strategy for cancer therapy. Semin Cancer Biol (1998) 8:369–78. 10.1006/scbi.1998.0099 [DOI] [PubMed] [Google Scholar]
- 20.Friedler A, Hansson LO, Veprintsev DB, Freund SM, Rippin TM, Nikolova PV, et al. A peptide that binds and stabilizes p53 core domain: chaperone strategy for rescue of oncogenic mutants. Proc Natl Acad Sci U S A (2002) 99:937–42. 10.1073/pnas.241629998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selivanova G, Iotsova V, Okan I, Fritsche M, Strom M, Groner B, et al. Restoration of the growth suppression function of mutant p53 by a synthetic peptide derived from the p53 C-terminal domain. Nat Med (1997) 3:632–8. 10.1038/nm0697-632 [DOI] [PubMed] [Google Scholar]
- 22.Nikolova PV, Wong KB, DeDecker B, Henckel J, Fersht AR. Mechanism of rescue of common p53 cancer mutations by second-site suppressor mutations. EMBO J (2000) 19:370–8. 10.1093/emboj/19.3.370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bykov VJ, Issaeva N, Shilov A, Hultcrantz M, Pugacheva E, Chumakov P, et al. Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nat Med (2002) 8:282–8. 10.1038/nm0302-282 [DOI] [PubMed] [Google Scholar]
- 24.Liu WL, Midgley C, Stephen C, Saville M, Lane DP. Biological significance of a small highly conserved region in the N terminus of the p53 tumour suppressor protein. J Mol Biol (2001) 313:711–31. 10.1006/jmbi.2001.5082 [DOI] [PubMed] [Google Scholar]
- 25.Tang X, Zhu Y, Han L, Kim AL, Kopelovich L, Bickers DR, et al. CP-31398 restores mutant p53 tumor suppressor function and inhibits UVB-induced skin carcinogenesis in mice. J Clin Invest (2007) 117:3753–64. 10.1172/JCI32481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanner S, Barberis A. CP-31398, a putative p53-stabilizing molecule tested in mammalian cells and in yeast for its effects on p53 transcriptional activity. J Negat Results Biomed (2004) 3:5. 10.1186/1477-5751-3-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rippin TM, Bykov VJ, Freund SM, Selivanova G, Wiman KG, Fersht AR. Characterization of the p53-rescue drug CP-31398 in vitro and in living cells. Oncogene (2002) 21:2119–29. 10.1038/sj.onc.1205362 [DOI] [PubMed] [Google Scholar]
- 28.Zache N, Lambert JM, Rokaeus N, Shen J, Hainaut P, Bergman J, et al. Mutant p53 targeting by the low molecular weight compound STIMA-1. Mol Oncol (2008) 2:70–80. 10.1016/j.molonc.2008.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lambert JM, Gorzov P, Veprintsev DB, Soderqvist M, Segerback D, Bergman J, et al. PRIMA-1 reactivates mutant p53 by covalent binding to the core domain. Cancer Cell (2009) 15:376–88. 10.1016/j.ccr.2009.03.003 [DOI] [PubMed] [Google Scholar]
- 30.Lambert JM, Moshfegh A, Hainaut P, Wiman KG, Bykov VJ. Mutant p53 reactivation by PRIMA-1MET induces multiple signaling pathways converging on apoptosis. Oncogene (2010) 29:1329–38. 10.1038/onc.2009.425 [DOI] [PubMed] [Google Scholar]
- 31.Bykov VJ, Issaeva N, Zache N, Shilov A, Hultcrantz M, Bergman J, et al. Reactivation of mutant p53 and induction of apoptosis in human tumor cells by maleimide analogs. J Biol Chem (2005) 280:30384–91. 10.1074/jbc.M501664200 [DOI] [PubMed] [Google Scholar]
- 32.Zhao CY, Grinkevich VV, Nikulenkov F, Bao W, Selivanova G. Rescue of the apoptotic-inducing function of mutant p53 by small molecule RITA. Cell Cycle (2010) 9:1847–55. 10.4161/cc.9.9.11545 [DOI] [PubMed] [Google Scholar]
- 33.Burmakin M, Shi Y, Hedstrom E, Kogner P, Selivanova G. Dual targeting of wild-type and mutant p53 by small molecule RITA results in the inhibition of N-Myc and key survival oncogenes and kills neuroblastoma cells in vivo and in vitro. Clin Cancer Res (2013) 19:5092–103. 10.1158/1078-0432.CCR-12-2211 [DOI] [PubMed] [Google Scholar]
- 34.Blanden AR, Yu X, Wolfe AJ, Gilleran JA, Augeri DJ, O’Dell RS, et al. Synthetic metallochaperone ZMC1 rescues mutant p53 conformation by transporting zinc into cells as an ionophore. Mol Pharmacol (2015) 87:825–31. 10.1124/mol.114.097550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu X, Vazquez A, Levine AJ, Carpizo DR. Allele-specific p53 mutant reactivation. Cancer Cell (2012) 21:614–25. 10.1016/j.ccr.2012.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu X, Blanden AR, Narayanan S, Jayakumar L, Lubin D, Augeri D, et al. Small molecule restoration of wildtype structure and function of mutant p53 using a novel zinc-metallochaperone based mechanism. Oncotarget (2014) 5:8879–92. 10.18632/oncotarget.2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hiraki M, Hwang SY, Cao S, Ramadhar TR, Byun S, Yoon KW, et al. Small-molecule reactivation of mutant p53 to wild-type-like p53 through the p53-Hsp40 regulatory axis. Chem Biol (2015) 22:1206–16. 10.1016/j.chembiol.2015.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X, Wilcken R, Joerger AC, Chuckowree IS, Amin J, Spencer J, et al. Small molecule induced reactivation of mutant p53 in cancer cells. Nucleic Acids Res (2013) 41:6034–44. 10.1093/nar/gkt305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wassman CD, Baronio R, Demir O, Wallentine BD, Chen CK, Hall LV, et al. Computational identification of a transiently open L1/S3 pocket for reactivation of mutant p53. Nat Commun (2013) 4:1407. 10.1038/ncomms2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinmann L, Wischhusen J, Demma MJ, Naumann U, Roth P, Dasmahapatra B, et al. A novel p53 rescue compound induces p53-dependent growth arrest and sensitises glioma cells to Apo2L/TRAIL-induced apoptosis. Cell Death Differ (2008) 15:718–29. 10.1038/sj.cdd.4402301 [DOI] [PubMed] [Google Scholar]
- 41.Demma M, Maxwell E, Ramos R, Liang L, Li C, Hesk D, et al. SCH529074, a small molecule activator of mutant p53, which binds p53 DNA binding domain (DBD), restores growth-suppressive function to mutant p53 and interrupts HDM2-mediated ubiquitination of wild type p53. J Biol Chem (2010) 285:10198–212. 10.1074/jbc.M109.083469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pluquet O, North S, Richard MJ, Hainaut P. Activation of p53 by the cytoprotective aminothiol WR1065: DNA-damage-independent pathway and redox-dependent modulation of p53 DNA-binding activity. Biochem Pharmacol (2003) 65:1129–37. 10.1016/S0006-2952(02)01655-6 [DOI] [PubMed] [Google Scholar]
- 43.Pluquet O, North S, Bhoumik A, Dimas K, Ronai Z, Hainaut P. The cytoprotective aminothiol WR1065 activates p53 through a non-genotoxic signaling pathway involving c-Jun N-terminal kinase. J Biol Chem (2003) 278:11879–87. 10.1074/jbc.M207396200 [DOI] [PubMed] [Google Scholar]
- 44.Shen H, Chen ZJ, Zilfou JT, Hopper E, Murphy M, Tew KD. Binding of the aminothiol WR-1065 to transcription factors influences cellular response to anticancer drugs. J Pharmacol Exp Ther (2001) 297:1067–73. [PubMed] [Google Scholar]
- 45.North S, Pluquet O, Maurici D, El-Ghissassi F, Hainaut P. Restoration of wild-type conformation and activity of a temperature-sensitive mutant of p53 (p53(V272M)) by the cytoprotective aminothiol WR1065 in the esophageal cancer cell line TE-1. Mol Carcinog (2002) 33:181–8. 10.1002/mc.10038 [DOI] [PubMed] [Google Scholar]
- 46.Wischhusen J, Naumann U, Ohgaki H, Rastinejad F, Weller M. CP-31398, a novel p53-stabilizing agent, induces p53-dependent and p53-independent glioma cell death. Oncogene (2003) 22:8233–45. 10.1038/sj.onc.1207198 [DOI] [PubMed] [Google Scholar]
- 47.Rao CV, Patlolla JM, Qian L, Zhang Y, Brewer M, Mohammed A, et al. Chemopreventive effects of the p53-modulating agents CP-31398 and Prima-1 in tobacco carcinogen-induced lung tumorigenesis in A/J mice. Neoplasia (2013) 15:1018–27. 10.1593/neo.131256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zandi R, Selivanova G, Christensen CL, Gerds TA, Willumsen BM, Poulsen HS. PRIMA-1Met/APR-246 induces apoptosis and tumor growth delay in small cell lung cancer expressing mutant p53. Clin Cancer Res (2011) 17:2830–41. 10.1158/1078-0432.CCR-10-3168 [DOI] [PubMed] [Google Scholar]
- 49.Liang Y, Besch-Williford C, Hyder SM. PRIMA-1 inhibits growth of breast cancer cells by re-activating mutant p53 protein. Int J Oncol (2009) 35:1015–23. 10.3892/ijo_00000416 [DOI] [PubMed] [Google Scholar]
- 50.Zache N, Lambert JM, Wiman KG, Bykov VJ. PRIMA-1MET inhibits growth of mouse tumors carrying mutant p53. Cell Oncol (2008) 30:411–8. 10.3233/CLO-2008-0440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bykov VJ, Wiman KG. Mutant p53 reactivation by small molecules makes its way to the clinic. FEBS Lett (2014) 588:2622–7. 10.1016/j.febslet.2014.04.017 [DOI] [PubMed] [Google Scholar]
- 52.Duffy MJ, Synnott NC, McGowan PM, Crown J, O‘Connor D, Gallagher WM. p53 as a target for the treatment of cancer. Cancer Treat Rev (2014) 40:1153–60. 10.1016/j.ctrv.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 53.Saha MN, Chen Y, Chen MH, Chen G, Chang H. Small molecule MIRA-1 induces in vitro and in vivo anti-myeloma activity and synergizes with current anti-myeloma agents. Br J Cancer (2014) 110:2224–31. 10.1038/bjc.2014.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rivera MI, Stinson SF, Vistica DT, Jorden JL, Kenney S, Sausville EA. Selective toxicity of the tricyclic thiophene NSC 652287 in renal carcinoma cell lines: differential accumulation and metabolism. Biochem Pharmacol (1999) 57:1283–95. 10.1016/S0006-2952(99)00046-5 [DOI] [PubMed] [Google Scholar]
- 55.Nieves-Neira W, Rivera MI, Kohlhagen G, Hursey ML, Pourquier P, Sausville EA, et al. DNA protein cross-links produced by NSC 652287, a novel thiophene derivative active against human renal cancer cells. Mol Pharmacol (1999) 56:478–84. [DOI] [PubMed] [Google Scholar]
- 56.Issaeva N, Bozko P, Enge M, Protopopova M, Verhoef LG, Masucci M, et al. Small molecule RITA binds to p53, blocks p53-HDM-2 interaction and activates p53 function in tumors. Nat Med (2004) 10:1321–8. 10.1038/nm1146 [DOI] [PubMed] [Google Scholar]
- 57.Grinkevich VV, Nikulenkov F, Shi Y, Enge M, Bao W, Maljukova A, et al. Ablation of key oncogenic pathways by RITA-reactivated p53 is required for efficient apoptosis. Cancer Cell (2009) 15:441–53. 10.1016/j.ccr.2009.03.021 [DOI] [PubMed] [Google Scholar]
- 58.Margalit O, Simon AJ, Yakubov E, Puca R, Yosepovich A, Avivi C, et al. Zinc supplementation augments in vivo antitumor effect of chemotherapy by restoring p53 function. Int J cancer (2012) 131:E562–8. 10.1002/ijc.26441 [DOI] [PubMed] [Google Scholar]
- 59.Pintus SS, Ivanisenko NV, Demenkov PS, Ivanisenko TV, Ramachandran S, Kolchanov NA, et al. The substitutions G245C and G245D in the Zn(2+)-binding pocket of the p53 protein result in differences of conformational flexibility of the DNA-binding domain. J Biomol Struct Dyn (2013) 31:78–86. 10.1080/07391102.2012.691364 [DOI] [PubMed] [Google Scholar]
- 60.Puca R, Nardinocchi L, Porru M, Simon AJ, Rechavi G, Leonetti C, et al. Restoring p53 active conformation by zinc increases the response of mutant p53 tumor cells to anticancer drugs. Cell Cycle (2011) 10:1679–89. 10.4161/cc.10.10.15642 [DOI] [PubMed] [Google Scholar]
- 61.Kung AL, Zabludoff SD, France DS, Freedman SJ, Tanner EA, Vieira A, et al. Small molecule blockade of transcriptional coactivation of the hypoxia-inducible factor pathway. Cancer Cell (2004) 6:33–43. 10.1016/j.ccr.2004.06.009 [DOI] [PubMed] [Google Scholar]
- 62.Staab A, Loeffler J, Said HM, Diehlmann D, Katzer A, Beyer M, et al. Effects of HIF-1 inhibition by chetomin on hypoxia-related transcription and radiosensitivity in HT 1080 human fibrosarcoma cells. BMC Cancer (2007) 7:213. 10.1186/1471-2407-7-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kessler J, Hahnel A, Wichmann H, Rot S, Kappler M, Bache M, et al. HIF-1alpha inhibition by siRNA or chetomin in human malignant glioma cells: effects on hypoxic radioresistance and monitoring via CA9 expression. BMC Cancer (2010) 10:605. 10.1186/1471-2407-10-605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boeckler FM, Joerger AC, Jaggi G, Rutherford TJ, Veprintsev DB, Fersht AR. Targeted rescue of a destabilized mutant of p53 by an in silico screened drug. Proc Natl Acad Sci U S A (2008) 105:10360–5. 10.1073/pnas.0805326105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lukashchuk N, Vousden KH. Ubiquitination and degradation of mutant p53. Mol Cell Biol (2007) 27:8284–95. 10.1128/MCB.00050-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blagosklonny MV, Toretsky J, Neckers L. Geldanamycin selectively destabilizes and conformationally alters mutated p53. Oncogene (1995) 11:933–9. [PubMed] [Google Scholar]
- 67.Egorin MJ, Rosen DM, Wolff JH, Callery PS, Musser SM, Eiseman JL. Metabolism of 17-(allylamino)-17-demethoxygeldanamycin (NSC 330507) by murine and human hepatic preparations. Cancer Res (1998) 58:2385–96. [PubMed] [Google Scholar]
- 68.Wang C, Chen J. Phosphorylation and hsp90 binding mediate heat shock stabilization of p53. J Biol Chem (2003) 278:2066–71. 10.1074/jbc.M206697200 [DOI] [PubMed] [Google Scholar]
- 69.Li D, Marchenko ND, Schulz R, Fischer V, Velasco-Hernandez T, Talos F, et al. Functional inactivation of endogenous MDM2 and CHIP by HSP90 causes aberrant stabilization of mutant p53 in human cancer cells. Mol Cancer Res (2011) 9:577–88. 10.1158/1541-7786.MCR-10-0534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blagosklonny MV. Why therapeutic response may not prolong the life of a cancer patient: selection for oncogenic resistance. Cell Cycle (2005) 4:1693–8. 10.4161/cc.4.12.2259 [DOI] [PubMed] [Google Scholar]
- 71.Li D, Marchenko ND, Moll UM. SAHA shows preferential cytotoxicity in mutant p53 cancer cells by destabilizing mutant p53 through inhibition of the HDAC6-Hsp90 chaperone axis. Cell Death Differ (2011) 18:1904–13. 10.1038/cdd.2011.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marks PA. Discovery and development of SAHA as an anticancer agent. Oncogene (2007) 26:1351–6. 10.1038/sj.onc.1210204 [DOI] [PubMed] [Google Scholar]
- 73.Yan W, Zhang Y, Zhang J, Liu S, Cho SJ, Chen X. Mutant p53 protein is targeted by arsenic for degradation and plays a role in arsenic-mediated growth suppression. J Biol Chem (2011) 286:17478–86. 10.1074/jbc.M111.231639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yan W, Jung YS, Zhang Y, Chen X. Arsenic trioxide reactivates proteasome-dependent degradation of mutant p53 protein in cancer cells in part via enhanced expression of Pirh2 E3 ligase. PLoS One (2014) 9:e103497. 10.1371/journal.pone.0103497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang J, Zhao Q, Qi Q, Gu HY, Rong JJ, Mu R, et al. Gambogic acid-induced degradation of mutant p53 is mediated by proteasome and related to CHIP. J Cell Biochem (2011) 112:509–19. 10.1002/jcb.22941 [DOI] [PubMed] [Google Scholar]
- 76.Vakifahmetoglu-Norberg H, Kim M, Xia HG, Iwanicki MP, Ofengeim D, Coloff JL, et al. Chaperone-mediated autophagy degrades mutant p53. Genes Dev (2013) 27:1718–30. 10.1101/gad.220897.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vakifahmetoglu-Norberg H, Yuan J. A degradative detour for mutant TP53. Autophagy (2013) 9:2158–60. 10.4161/auto.26338 [DOI] [PubMed] [Google Scholar]
- 78.Yi YW, Kang HJ, Kim HJ, Kong Y, Brown ML, Bae I. Targeting mutant p53 by a SIRT1 activator YK-3-237 inhibits the proliferation of triple-negative breast cancer cells. Oncotarget (2013) 4:984–94. 10.18632/oncotarget.1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang S, Zhou L, Hong B, van den Heuvel AP, Prabhu VV, Warfel NA, et al. Small-molecule NSC59984 restores p53 pathway signaling and antitumor effects against colorectal cancer via p73 activation and degradation of mutant p53. Cancer Res (2015) 75:3842–52. 10.1158/0008-5472.CAN-13-1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paranjpe A, Srivenugopal KS. Degradation of NF-kappaB, p53 and other regulatory redox-sensitive proteins by thiol-conjugating and -nitrosylating drugs in human tumor cells. Carcinogenesis (2013) 34:990–1000. 10.1093/carcin/bgt032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ramalingam S, Goss G, Rosell R, Schmid-Bindert G, Zaric B, Andric Z, et al. A randomized phase II study of ganetespib, a heat shock protein 90 inhibitor, in combination with docetaxel in second-line therapy of advanced non-small cell lung cancer (GALAXY-1). Ann Oncol (2015) 26:1741–8. 10.1093/annonc/mdv220 [DOI] [PubMed] [Google Scholar]
- 82.Goyal L, Wadlow RC, Blaszkowsky LS, Wolpin BM, Abrams TA, McCleary NJ, et al. A phase I and pharmacokinetic study of ganetespib (STA-9090) in advanced hepatocellular carcinoma. Invest New Drugs (2015) 33:128–37. 10.1007/s10637-014-0164-8 [DOI] [PubMed] [Google Scholar]
- 83.Jhaveri K, Chandarlapaty S, Lake D, Gilewski T, Robson M, Goldfarb S, et al. A phase II open-label study of ganetespib, a novel heat shock protein 90 inhibitor for patients with metastatic breast cancer. Clin Breast Cancer (2014) 14:154–60. 10.1016/j.clbc.2013.12.012 [DOI] [PubMed] [Google Scholar]
- 84.Yan W, Liu S, Xu E, Zhang J, Zhang Y, Chen X, et al. Histone deacetylase inhibitors suppress mutant p53 transcription via histone deacetylase 8. Oncogene (2013) 32:599–609. 10.1038/onc.2012.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Beauchamp EM, Uren A. A new era for an ancient drug: arsenic trioxide and Hedgehog signaling. Vitam Horm (2012) 88:333–54. 10.1016/B978-0-12-394622-5.00015-8 [DOI] [PubMed] [Google Scholar]
- 86.Jiang W, Wang XW, Unger T, Forgues M, Kim JW, Hussain SP, et al. Cooperation of tumor-derived HBx mutants and p53-249(ser) mutant in regulating cell proliferation, anchorage-independent growth and aneuploidy in a telomerase-immortalized normal human hepatocyte-derived cell line. Int J Cancer (2010) 127:1011–20. 10.1002/ijc.25118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hughes MF, Beck BD, Chen Y, Lewis AS, Thomas DJ. Arsenic exposure and toxicology: a historical perspective. Toxicol Sci (2011) 123:305–32. 10.1093/toxsci/kfr184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gu H, Wang X, Rao S, Wang J, Zhao J, Ren FL, et al. Gambogic acid mediates apoptosis as a p53 inducer through down-regulation of mdm2 in wild-type p53-expressing cancer cells. Mol Cancer Ther (2008) 7:3298–305. 10.1158/1535-7163.MCT-08-0212 [DOI] [PubMed] [Google Scholar]
- 89.Liu J, Xia H, Kim M, Xu L, Li Y, Zhang L, et al. Beclin1 controls the levels of p53 by regulating the deubiquitination activity of USP10 and USP13. Cell (2011) 147:223–34. 10.1016/j.cell.2011.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yuan J, Luo K, Zhang L, Cheville JC, Lou Z. USP10 regulates p53 localization and stability by deubiquitinating p53. Cell (2010) 140:384–96. 10.1016/j.cell.2009.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kong Y, Wang K, Edler MC, Hamel E, Mooberry SL, Paige MA, et al. A boronic acid chalcone analog of combretastatin A-4 as a potent anti-proliferation agent. Bioorg Med Chem (2010) 18:971–7. 10.1016/j.bmc.2009.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kona FR, Buac D, Burger A M. Disulfiram, and disulfiram derivatives as novel potential anticancer drugs targeting the ubiquitin-proteasome system in both preclinical and clinical studies. Curr Cancer Drug Targets (2011) 11:338–46. 10.2174/156800911794519798 [DOI] [PubMed] [Google Scholar]
- 93.Nechushtan H, Hamamreh Y, Nidal S, Gotfried M, Baron A, Shalev YI, et al. A phase IIb trial assessing the addition of disulfiram to chemotherapy for the treatment of metastatic non-small cell lung cancer. Oncologist (2015) 20:366–7. 10.1634/theoncologist.2014-0424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Petersen EN. The pharmacology and toxicology of disulfiram and its metabolites. Acta Psychiatr Scand Suppl (1992) 369:7–13. 10.1111/j.1600-0447.1992.tb03309.x [DOI] [PubMed] [Google Scholar]
- 95.Mathieu C, Duval R, Xu X, Rodrigues-Lima F, Dupret JM. Effects of pesticide chemicals on the activity of metabolic enzymes: focus on thiocarbamates. Expert Opin Drug Metab Toxicol (2015) 11:81–94. 10.1517/17425255.2015.975691 [DOI] [PubMed] [Google Scholar]
- 96.Davies KJ. Degradation of oxidized proteins by the 20S proteasome. Biochimie (2001) 83:301–10. 10.1016/S0300-9084(01)01250-0 [DOI] [PubMed] [Google Scholar]
- 97.Velu CS, Niture SK, Doneanu CE, Pattabiraman N, Srivenugopal KS. Human p53 is inhibited by glutathionylation of cysteines present in the proximal DNA-binding domain during oxidative stress. Biochemistry (2007) 46:7765–80. 10.1021/bi700425y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yusuf MA, Chuang T, Bhat GJ, Srivenugopal KS. Cys-141 glutathionylation of human p53: studies using specific polyclonal antibodies in cancer samples and cell lines. Free Radic Biol Med (2010) 49:908–17. 10.1016/j.freeradbiomed.2010.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol (2010) 2:a001008. 10.1101/cshperspect.a001008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Micale L, Augello B, Maffeo C, Selicorni A, Zucchetti F, Fusco C, et al. Molecular analysis, pathogenic mechanisms, and readthrough therapy on a large cohort of Kabuki syndrome patients. Hum Mutat (2014) 35:841–50. 10.1002/humu.22547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Floquet C, Deforges J, Rousset JP, Bidou L. Rescue of non-sense mutated p53 tumor suppressor gene by aminoglycosides. Nucleic Acids Res (2011) 39:3350–62. 10.1093/nar/gkq1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Martin L, Grigoryan A, Wang D, Wang J, Breda L, Rivella S, et al. Identification and characterization of small molecules that inhibit nonsense-mediated RNA decay and suppress nonsense p53 mutations. Cancer Res (2014) 74:3104–13. 10.1158/0008-5472.CAN-13-2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mater (2013) 12:967–77. 10.1038/nmat3765 [DOI] [PubMed] [Google Scholar]
- 104.Lim LY, Vidnovic N, Ellisen LW, Leong CO. Mutant p53 mediates survival of breast cancer cells. Br J Cancer (2009) 101:1606–12. 10.1038/sj.bjc.6605335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hui L, Zheng Y, Yan Y, Bargonetti J, Foster DA. Mutant p53 in MDA-MB-231 breast cancer cells is stabilized by elevated phospholipase D activity and contributes to survival signals generated by phospholipase D. Oncogene (2006) 25:7305–10. 10.1038/sj.onc.1209735 [DOI] [PubMed] [Google Scholar]
- 106.Zhu H, Mao Q, Lin Y, Yang K, Xie L. RNA interference targeting mutant p53 inhibits growth and induces apoptosis in DU145 human prostate cancer cells. Med Oncol (2011) 28(Suppl 1):S381–7. 10.1007/s12032-010-9679-9 [DOI] [PubMed] [Google Scholar]
- 107.Zhu HB, Yang K, Xie YQ, Lin YW, Mao QQ, Xie LP. Silencing of mutant p53 by siRNA induces cell cycle arrest and apoptosis in human bladder cancer cells. World J Surg Oncol (2013) 11:22. 10.1186/1477-7819-11-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Martinez LA, Naguibneva I, Lehrmann H, Vervisch A, Tchenio T, Lozano G, et al. Synthetic small inhibiting RNAs: efficient tools to inactivate oncogenic mutations and restore p53 pathways. Proc Natl Acad Sci U S A (2002) 99:14849–54. 10.1073/pnas.222406899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kravchenko JE, Ilyinskaya GV, Komarov PG, Agapova LS, Kochetkov DV, Strom E, et al. Small-molecule RETRA suppresses mutant p53-bearing cancer cells through a p73-dependent salvage pathway. Proc Natl Acad Sci U S A (2008) 105:6302–7. 10.1073/pnas.0802091105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shimoyama S. Statins are logical candidates for overcoming limitations of targeting therapies on malignancy: their potential application to gastrointestinal cancers. Cancer Chemother Pharmacol (2011) 67:729–39. 10.1007/s00280-011-1583-2 [DOI] [PubMed] [Google Scholar]
- 111.Sorrentino G, Ruggeri N, Specchia V, Cordenonsi M, Mano M, Dupont S, et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol (2014) 16:357–66. 10.1038/ncb2936 [DOI] [PubMed] [Google Scholar]
- 112.Kaelin WG, Jr. The concept of synthetic lethality in the context of anticancer therapy. Nat Rev Cancer (2005) 5:689–98. 10.1038/nrc1691 [DOI] [PubMed] [Google Scholar]
- 113.Wang Q, Fan S, Eastman A, Worland PJ, Sausville EA, O’Connor PM. UCN-01: a potent abrogator of G2 checkpoint function in cancer cells with disrupted p53. J Natl Cancer Inst (1996) 88:956–65. 10.1093/jnci/88.14.956 [DOI] [PubMed] [Google Scholar]
- 114.Sur S, Pagliarini R, Bunz F, Rago C, Diaz LA, Jr, Kinzler KW, et al. A panel of isogenic human cancer cells suggests a therapeutic approach for cancers with inactivated p53. Proc Natl Acad Sci U S A (2009) 106:3964–9. 10.1073/pnas.0813333106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang Y, Li J, Booher RN, Kraker A, Lawrence T, Leopold WR, et al. Radiosensitization of p53 mutant cells by PD0166285, a novel G(2) checkpoint abrogator. Cancer Res (2001) 61:8211–7. [PubMed] [Google Scholar]
- 116.Reguart N, Rosell R, Cardenal F, Cardona AF, Isla D, Palmero R, et al. Phase I/II trial of vorinostat (SAHA) and erlotinib for non-small cell lung cancer (NSCLC) patients with epidermal growth factor receptor (EGFR) mutations after erlotinib progression. Lung Cancer (2014) 84:161–7. 10.1016/j.lungcan.2014.02.011 [DOI] [PubMed] [Google Scholar]
- 117.Krug LM, Kindler HL, Calvert H, Manegold C, Tsao AS, Fennell D, et al. Vorinostat in patients with advanced malignant pleural mesothelioma who have progressed on previous chemotherapy (VANTAGE-014): a phase 3, double-blind, randomised, placebo-controlled trial. Lancet Oncol (2015) 16:447–56. 10.1016/S1470-2045(15)70056-2 [DOI] [PubMed] [Google Scholar]
- 118.Yoo C, Ryu MH, Na YS, Ryoo BY, Lee CW, Maeng J, et al. Phase I and pharmacodynamic study of vorinostat combined with capecitabine and cisplatin as first-line chemotherapy in advanced gastric cancer. Invest New Drugs (2014) 32:271–8. 10.1007/s10637-013-9983-2 [DOI] [PubMed] [Google Scholar]
- 119.Yanada M, Tsuzuki M, Fujita H, Fujimaki K, Fujisawa S, Sunami K, et al. Phase 2 study of arsenic trioxide followed by autologous hematopoietic cell transplantation for relapsed acute promyelocytic leukemia. Blood (2013) 121:3095–102. 10.1182/blood-2012-11-466862 [DOI] [PubMed] [Google Scholar]
- 120.Efficace F, Mandelli F, Avvisati G, Cottone F, Ferrara F, Di Bona E, et al. Randomized phase III trial of retinoic acid and arsenic trioxide versus retinoic acid and chemotherapy in patients with acute promyelocytic leukemia: health-related quality-of-life outcomes. J Clin Oncol (2014) 32:3406–12. 10.1200/JCO.2014.55.3453 [DOI] [PubMed] [Google Scholar]
- 121.Qu FL, Hao XZ, Qin SK, Liu JW, Sui GJ, Chen Q, et al. [Multicenter phase II clinical trial of arsenic trioxide injection in the treatment of primary hepatocarcinoma]. Zhonghua Zhong Liu Za Zhi (2011) 33:697–701. [PubMed] [Google Scholar]
- 122.Verma S, Stewart DJ, Maroun JA, Nair RC. A randomized phase II study of cisplatin alone versus cisplatin plus disulfiram. Am J Clin Oncol (1990) 13:119–24. 10.1097/00000421-199004000-00007 [DOI] [PubMed] [Google Scholar]
- 123.Karlsson R, Kullman-Magnusson M, Hamalainen MD, Remaeus A, Andersson K, Borg P, et al. Biosensor analysis of drug-target interactions: direct and competitive binding assays for investigation of interactions between thrombin and thrombin inhibitors. Anal Biochem (2000) 278:1–13. 10.1006/abio.1999.4406 [DOI] [PubMed] [Google Scholar]
- 124.Lamos SM, Krusemark CJ, McGee CJ, Scalf M, Smith LM, Belshaw PJ. Mixed isotope photoaffinity reagents for identification of small-molecule targets by mass spectrometry. Angew Chem Int Ed Engl (2006) 45:4329–33. 10.1002/anie.200600743 [DOI] [PubMed] [Google Scholar]
- 125.Lomenick B, Hao R, Jonai N, Chin RM, Aghajan M, Warburton S, et al. Target identification using drug affinity responsive target stability (DARTS). Proc Natl Acad Sci U S A (2009) 106:21984–9. 10.1073/pnas.0910040106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jafari R, Almqvist H, Axelsson H, Ignatushchenko M, Lundback T, Nordlund P, et al. The cellular thermal shift assay for evaluating drug target interactions in cells. Nat Protoc (2014) 9:2100–22. 10.1038/nprot.2014.138 [DOI] [PubMed] [Google Scholar]
- 127.Lammens CR, Bleiker EM, Aaronson NK, Wagner A, Sijmons RH, Ausems MG, et al. Regular surveillance for Li-Fraumeni syndrome: advice, adherence and perceived benefits. Fam Cancer (2010) 9:647–54. 10.1007/s10689-010-9368-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang Z, Sun Y. Targeting p53 for novel anticancer therapy. Transl Oncol (2010) 3:1–12. 10.1593/tlo.09250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Khoo KH, Verma CS, Lane DP. Drugging the p53 pathway: understanding the route to clinical efficacy. Nat Rev Drug Discov (2014) 13:217–36. 10.1038/nrd4236 [DOI] [PubMed] [Google Scholar]
- 130.Tongyang L, Haiqiang G, Meiyan Z, Yingze H, Shuting J, Ying L, et al. [Synthetic lethal genes to mutant p53]. Yi Chuan (2015) 37:321–6. 10.16288/j.yczz.14-277 [DOI] [PubMed] [Google Scholar]
- 131.Shaheen M, Allen C, Nickoloff JA, Hromas R. Synthetic lethality: exploiting the addiction of cancer to DNA repair. Blood (2011) 117:6074–82. 10.1182/blood-2011-01-313734 [DOI] [PubMed] [Google Scholar]
- 132.Wang X, Simon R. Identification of potential synthetic lethal genes to p53 using a computational biology approach. BMC Med Genomics (2013) 6:30. 10.1186/1755-8794-6-30 [DOI] [PMC free article] [PubMed] [Google Scholar]


