Abstract
The discovery of the T helper (Th) 17 lineage, involved in the protection against fungal and extracellular bacterial infections, has profoundly revolutionized our current understanding of T cell-mediated responses in autoimmune diseases, including multiple sclerosis (MS). Indeed, recent data demonstrate the pathogenic role of Th17 cells in autoimmune disorders. In particular, studies in MS and in its animal model (EAE, experimental autoimmune encephalomyelitis) have revealed a crucial role of Th17 cells in the pathogenesis of autoimmune demyelinating diseases in both mice and humans. Over the past years, several important aspects concerning Th17 cells have been elucidated, such as the factors which promote or inhibit their differentiation and the effector cytokines which mediate their responses. The identification of the features endowing Th17 cells with high pathogenicity in MS is of particular interest, and discoveries in Th17 cell biology and function could lead to the design of new strategies aimed at modulating the immune response in MS. Here, we will discuss recent advances in this field, with particular focus on the mechanisms conferring pathogenicity in MS and their potential modulation.
1. Introduction
Differentiation of naive CD4+ T cells into T helper (Th) cells with diverse effector functions is crucial for the establishment of an adaptive immune response. Until recently, only two major cell subsets, Th1 and Th2, were used to describe the different adaptive immune responses established to eradicate pathogens [1–3]. Th1 cells induce cell mediated inflammatory responses against intracellular bacteria [4–7], while Th2 cells activate a protective response against helminth infection [8]. However, persistent or uncontrolled effector T cell responses are also associated with pathological states and tissue damage: an excessive Th2 cell response is responsible for atopic diseases, such as asthma [9], and an abnormal Th1 cell response can mediate chronic inflammation and is involved in several autoimmune diseases [10, 11]. In 1998 the discovery of CD4+ T cells producing IL-17 [12] unveiled the presence of another subset of Th cells, the Th17 subset, distinct from Th1 and Th2 [13, 14], and its discovery has helped the understanding of immune responses unexplained by the Th1/Th2 paradigm, such as the response against fungi like Candida albicans [15] and extracellular bacteria such as Pseudomonas aeruginosa [16], Klebsiella pneumoniae [17], Streptococcus pneumoniae [18], and Staphylococcus aureus [19], and the development of autoimmune disorders, such as multiple sclerosis (MS), Crohn's disease, psoriasis, and rheumatoid arthritis. The pathogenic role of Th17 cells in autoimmune diseases is supported by both human studies and experiments performed in animal models. Indeed, IL-17A is highly expressed in the central nervous system (CNS) lesions and in the blood and cerebrospinal fluid (CSF) of patients with MS [20–24], in the colonic mucosa of patients with ulcerative colitis or Crohn's disease [25, 26], in the psoriatic skin [27, 28], and in the synovial tissues from rheumatoid arthritis patients [29]. Studies in murine models such as experimental autoimmune encephalomyelitis (EAE) [30], trinitrobenzene sulfuric acid- (TNBS-) induced colitis [31], and antigen or collagen-induced arthritis [32] reveal that the IL-17 pathway plays a pathogenic role in autoimmune disorders. Finally, the concept that Th17 cells are responsible for driving autoimmune inflammation was finally established when EAE, the mouse model of MS, was shown to be induced by passive transfer of IL-17-producing myelin reactive CD4 T cells [33].
In this review we discuss our current understanding of the Th17 lineage, focusing on the factors regulating their differentiation, their typical features, their pathological roles in MS, and the potential modulation of their response for therapeutic approaches.
2. Cytokine Production by Th17 Cells
IL-17 is the cytokine produced specifically by Th17 cells. IL-17A (commonly referred to as IL-17) is part of a cytokine family including IL-17B, IL-17C, IL-17D, IL-17E (also known as IL-25), and IL-17F [34]. All members of the family show some conserved regions: IL-17A and IL-17F (the only cytokines of this family produced by Th17 cells) are the most similar to a 55% homology and exert similar functions [35]; IL-25 has the sequence with lowest similarity to IL-17A (only 16%) and plays distinct roles in immunity, mainly regulating the Th2 response against helminthic parasites and allergic inflammation [36–38]. IL-17B, IL-17C, and IL-17D have been shown to induce the production of proinflammatory cytokines, but their biological function is largely unknown [39–42]. Recent studies by three different groups have highlighted the function of IL-17C in mucosal immunity and in autoimmune responses [43–45].
Within the IL-17 family of cytokines, the biological function and regulation of IL-17A and IL-17F are the best understood. Both are produced by Th17 cells and can also act as heterodimers [46]. The effective signalling of IL-17A and IL-17F requires the IL-17 receptor (IL-17R), a heteromeric complex consisting of IL-17RA and IL-17RC [47]. Although both receptors are extensively expressed in different tissues and cell types [48–50] functional studies have focused mainly on epithelial cells. Both IL-17A and IL-17F induce epithelial cells to produce granulopoietic colony stimulating factor (G-CSF), stem cell factors that regulate granulopoiesis, and CXC chemokines (CXCL1, CXCL2, CXCL5, and CXCL8) responsible for neutrophil recruitment [51–53]. IL-17A increases the expression of mucins such as MUC5AC and MUC5B in primary human bronchial epithelial cells in vitro [54]. In addition, IL-17A also induces the expression of human beta defensin-2 [55] and CCL20 in lung epithelial cells [56]. This cooperative induction of neutrophil recruitment and antimicrobial-peptide production improves epithelial-barrier integrity and may be critical for mucosal host defense against extracellular bacteria and fungi.
Moreover, several studies have documented the role of IL-17 in regulating antibody generation by plasma cells and germinal center and ectopic inducible bronchus-associated lymphoid tissue (iBALT) formation [57–59].
Altogether, these functions of IL-17 make Th17 cells potentially relevant for vaccine development: in experimental models, Th17 cells are effective in providing vaccination-induced immunity against a range of pathogens, and the identification of Th17-specific antigens for common prevalent pathogens could help formulate a serotype-independent effective vaccination strategy [60].
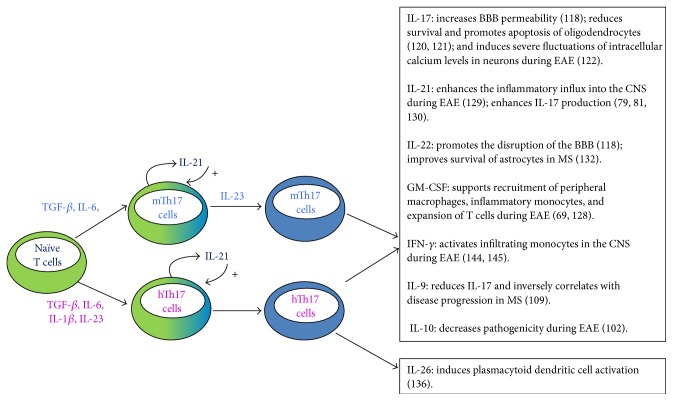
However, the Th17 cytokine profile is not restricted to IL-17 production. In fact, Th17 cells are potentially producers of a broad array of cytokines, including IL-21, IL-22, IL-26, IL-6, TNF, and in certain conditions also GM-CSF, IL-9, IL-10, and IFN-γ [61–70]. Because each of these cytokines has different functions, they collectively affect the global outcome of the Th17 response and generate different Th17 responses (Figure 1).
Figure 1.
Differentiation of Th17 cells and their potential functions in MS. The T helper (Th) 17 cell differentiation program in mice (blue labels) and humans (purple labels) shares significant similarities. In both organisms, IL-6, TGF-β, IL-23, and IL-21 are involved in complete Th17 differentiation. Human Th17 cell differentiation requires also IL-1β. Fully differentiated Th17 cells produce specific sets of cytokines. Both murine and human Th17 cells produce IL-17, IL-21, IL-22, GM-CSF, IFN-γ, IL-9, and IL-10, with potentially relevant functions in MS pathogenesis. Human Th17 cells also produce IL-26.
3. Stimuli Required for Th17 Cell Differentiation
Human in vitro studies reveal that dendritic cells (DCs) exposed to bacteria or fungi, but not viruses, elicit strong Th17 responses [62, 80]. Among pathogen-associated molecular patterns (PAMPs), peptidoglycan (TLR2 agonist) [62, 80] and its product muramyl dipeptide (NOD2 agonist) [80] are the most potent stimuli for the production of IL-17. In mice, an alternative pattern-recognition pathway activated by fungal infection has also been described, involving the engagement of a C-type lectin receptor, dectin-1, by fungal β-glucans components of zymosan [81]. Moreover, DCs stimulated with the pure β-glucan curdlan (dectin-1 agonist) prime T cells for a much higher IL-17 production than does IFN-γ [82].
Stimulation of DC by pathogen-derived structures induces the production of cytokines which ultimately drive Th17 cell differentiation [83, 84]; these cytokines have been identified by using antigen presenting cell- (APC-) free models of T cell polarization. In mice, several reports have shown that transforming growth factor-β (TGF-β) and IL-6 [85–87] have a critical role in inducing Th17 differentiation. Although not necessary, other proinflammatory cytokines such as IL-1β, TNF, and IL-21 can enhance this differentiation [87, 88]. Finally, IL-23 induces expansion of murine Th17 cells both in vitro [87] and in vivo [89] (Figure 1).
Studies on human Th17 cell differentiation have reported a critical role for IL-1β, IL-6, IL-23, and TGF-β [65, 66]. TGF-β was also shown to act in synergy with the inflammatory mediator IL-21 in driving Th17 cell differentiation [90]. Moreover, in humans it has been demonstrated that supernatants from DCs stimulated with zymosan or β-glucan induce the development of Th17 cells with requirements for TGF-β, IL-1β, and IL-6 [91]. Collectively, these studies reveal that similar pathways regulate both human and mouse Th17 cell differentiation [92] (Figure 1).
4. Transcription Factors Required for Th17 Cell Differentiation
The key transcription factor involved in the differentiation program of Th17 cells is the retinoic acid-related orphan receptor (ROR) γt [93, 94], a member of the ROR family of nuclear receptors encoded by the RORC gene. Studies in mice [93] and humans [65, 66] revealed that RORγt controls the expression of IL-17A and IL-17F by Th17 cells. However, RORγt does not regulate all genes related to the Th17 lineage, suggesting that other transcription factors contribute to the expression of genes involved in its functional differentiation.
Although all cytokine pathways involved in Th17 cell differentiation result in the upregulation of RORγt expression, IL-6, IL-21, and IL-23 signalling pathways additionally activate STAT3, which directly binds the IL-17 and IL-21 promoters [95, 96]. Cells transduced with both RORγt and an active form of STAT3 (STAT3C) produce more IL-17 per cell, suggesting a cooperation between RORγt and STAT3 at transcriptional target sites [63]. Patients with autosomal-dominant hyper-IgE syndrome associated with dominant-negative mutations in STAT3 lack Th17 cells, revealing the importance of STAT3-dependent signals in the differentiation and/or expansion of human IL-17-producing cells [97].
IRF4 has also been reported to be essential for Th17 cell differentiation [98]. IRF4-deficient mice have an impaired RORγt induction, since IRF4 is located upstream of RORγt in the Th17 cell differentiation process [98].
Another transcription factor known to cooperate with RORγt is the basic leucine zipper transcription factor ATF like (BATF), which is upregulated in Th cells following T cell receptor (TCR) activation [99, 100] and which is essential for the maintenance of RORγt expression induced by TGF-β and IL-6 [100]. Recent studies have identified IRF4 and BATF as “pioneer factors” that bind and govern the accessibility of chromatin, enabling RORγt recruitment and binding to Th17 signature genes [101, 102].
Another member of the ROR family, RORα, also contributes to mouse Th17 cell development, and coexpression of RORα and RORγt causes synergistic increases in IL-17A, IL-17F, and IL-23R expression [103]. RORα and RORγt bind to the same retinoid response-like elements individually or as heterodimers and may be functionally redundant [104].
Retinoic acid receptors can be regulated by the ligand-dependent transcription factor aryl hydrocarbon receptor (AHR) [105, 106], whose binding to environmental pollutants such as dioxin influence Th17 cell differentiation [107, 108]. Different cellular contexts might provide distinct transcriptional partners for AHR and determine diverse outcomes of the immune response.
Another transcription factor associated with environmental conditions is the hypoxia-inducible factor 1α (HIF1α), which is a key sensor of hypoxia. HIF1α directly binds and drives transcription of RORC [109], and lack of HIF1α results in diminished Th17 development [110].
Therefore, combinatorial interactions of multiple transcription factors, including RORγt, RORα, activated STAT3, IRF4, BATF, AHR, HIF1α, and other unidentified factors regulate the genes that define the Th17 lineage. Decoding this transcriptional network will provide a better understanding of pathways involved in the differentiation of Th17 cells and may facilitate the development of strategies to manipulate the immune responses associated with these cells.
5. Immunomodulation of Th17 Responses
The conjunct action of multiple soluble factors fine-tunes and regulates the outcome of immune responses. For instance, IL-10 has an important immunomodulatory role during the Th17 response. In fact, IL-10 production by restimulated mouse Th17 cells in the presence of TGF-β and IL-6 is able to regulate Th17 cell immunopathology and reduces EAE disease severity [111]. In contrast, restimulation in the presence of IL-23 does not induce IL-10 production, conferring pathogenic functions to Th17 cells [111]. Thus, IL-10 production in Th17-polarizing conditions may be a mechanism of self-regulation of the potentially dangerous Th17 cell response. In humans, the regulation of IL-10 production in Th17 conditions is dependent on IL-1β [65, 112]. Human Th17 cell differentiation in the presence of IL-1β results in an enhancement of IL-17 and in a decrease of IL-10 production suggesting that, during the resolution of inflammation, a decrease in or a lack of IL-1β may simultaneously decrease the production of IL-17 and enhance the production of IL-10 [65, 112]. Moreover, several studies suggest that IL-10 inhibits differentiation of Th17 cells in a direct manner [113, 114]. Given the role of IL-10 as a potent negative regulator of inflammation [115, 116], its presence could be an important mechanism for controlling the Th17 response.
The cytokine IL-2 has a similar inhibitory effect on Th17 cell differentiation. In fact, addition of exogenous IL-2 reduces the proportion of murine Th17 cells differentiated from naive T cells in the presence of TGF-β and IL-6, whereas inversely blocking autocrine IL-2 by the addition of neutralizing antibodies enhances Th17 differentiation. Furthermore, IL-2-deficient mice contain a substantially greater fraction of Th17 T cells, and in vitro stimulation of T cells from these mice results in higher proportions of IL-17-producing cells [117]. In contrast, IL-2 seems to have a positive effect on IL-17 expression by human Th17 cells, and the addition of an IL-2-blocking antibody during differentiation prevents cell proliferation and IL-17 production [66].
We recently demonstrated that IL-9 has an inhibitory effect on IL-17 production by human Th17-polarized cells. Importantly, the interaction between IL-17 and IL-9 reveals a decisive mechanism regulating the pathogenic inflammation generated by Th17 cells in MS [118]. In fact, we found that IL-9 level in the CSF of MS patients inversely correlates with the progression of MS and with the levels of IL-17 observed in the CSF, indicating that inhibition of IL-17 via IL-9 could be protective in MS [118], despite the controversial results on the role of IL-9 in EAE [68, 119].
Th17 responses can also be suppressed by antigen presenting cells producing IL-27: this cytokine inhibits IL-17 production by human and mouse Th17 cells [120]. Interestingly, plasma levels of IL-27 negatively correlate with the percentage of circulating Th17 or with plasma IL-17 concentration in patients with progressive MS, suggesting that IL-27 might be involved in this disease [121].
Thus, the establishment, progression, and outcome of chronic inflammation, which underlies the pathogenesis of MS, are highly dependent on the nature of the complex network of cytokines which modulate Th17 cells and which are produced during the immune response.
6. Pathogenic Role of Th17 Cells in Multiple Sclerosis
MS is a heterogenous disease characterized by a wide variety of neurological symptoms and signs attributed to discrete areas of inflammation, demyelination, and axonal loss in the CNS [122]. Two main courses of MS exist: relapsing-remitting (RR) and primary or secondary progressive (PP and SP). RR-MS is characterized by recurrent neurologic symptoms interspersed by periods of stability, with full or partial recovery; the progressive form is characterized by gradual neurological dysfunction with or without exacerbations [123, 124].
Immunological mechanisms such as myelin destruction by specific CD8 T cells, activated microglia, invading macrophages, natural killer cells, and autoantibodies produced by B cells contribute to demyelination and axonal loss. Importantly, Th17 cells are mainly responsible for the persistent inflammation that characterizes both forms of MS [122].
Among the typical features of Th17 cells that could confer pathogenicity to MS, their abilities to enter the encephalic compartment, to penetrate the blood brain barrier (BBB), and to recruit inflammatory cells have been documented. In particular, Th17 cells express high levels of the C-C chemokine receptor 6 (CCR6) on the cell surface [62, 125] that binds the C-C chemokine ligand 20 (CCL20) constitutively expressed by the vascular endothelium of the blood-cerebrospinal barrier, thus enabling the entry of Th17 cells into the encephalic compartment through the choroid plexus [125]. Once in the brain parenchyma, Th17 cells release several proinflammatory mediators including IL-17A, responsible for the downregulation of tight junction proteins of the BBB, increasing BBB permeability, and favoring migration of both soluble inflammatory molecules and other circulating immune cells into the CNS [126]. Consistent with murine studies, IL-17A levels in the CSF of MS patients are associated with neutrophil expansion and blood brain barrier disruption, indicating that IL-17 may have similar pathogenic roles in EAE and MS [127].
It has been proposed that IL-17 also interferes with remyelinating processes, reducing survival [128], and promoting apoptosis of oligodendrocytes, the myelin-forming cells [129]. Moreover, a potential neurotoxic effect of Th17 cells has also been reported, by the induction of severe fluctuations of intracellular calcium levels in neurons during EAE [130] or through the release of granzyme B in human fetal neurons [126].
However, murine studies suggest that not only production of IL-17, but also other features of Th17 cells collectively confer encephalitogenic potential to these cells in MS. In fact, the course of EAE is unperturbed or only lightly ameliorated in IL-17 knockout mice and in wild-type mice treated with neutralizing antibodies specific for IL-17A [131, 132].
Among the proinflammatory mediators produced by Th17 cells, GM-CSF is gaining much attention in MS pathogenesis. Recent studies have suggested that this cytokine plays a fundamental role in the pathogenicity of Th17 cells in EAE [69, 70]. In fact, GM-CSF-deficient mice are resistant to EAE [69, 133] and GM-CSF production by Th17 cells is crucial for their capacity to induce EAE [70, 134], likely supporting recruitment of peripheral macrophages, inflammatory monocytes, and expansion of T cells [69, 135, 136].
Another cytokine produced by Th17 cells is IL-21, whose administration before induction of EAE enhances the inflammatory influx into the CNS as well as the severity of the disease [137], likely due to the role of IL-21 in the induction and expansion of Th17 cells [88, 90, 138]. Interestingly, IL-21 and its receptor have been detected in lymphocytes infiltrating acute and chronic active white matter MS lesions [139], underlining the role for this cytokine in CNS inflammation.
The role of IL-22 in MS is still unclear. High levels of IL-22 and of IL-22 producing cells have been detected in the serum, CSF, and peripheral blood of MS patients [140–142], and it has been demonstrated that IL-22 promotes the disruption of BBB in vitro and in vivo [126]. However, in the brain tissue IL-22 improves survival of astrocytes that express its receptor [140], thus suggesting a protective role of this cytokine in MS.
Among the cytokines produced by Th17 cells, IL-26 has never been studied in the context of brain inflammation, due to the fact that only human Th17 cells produce it, thus making the EAE model inappropriate. However, the identification of a risk locus containing IL26 and single-nucleotide polymorphisms within the IL26 gene region associated with MS [143] encourages the study of its role in MS. Interestingly, IL-26 forms complexes with bacterial DNA and self-DNA released by dying cells, thus inducing plasmacytoid dendritic cells to produce IFN-α [144], that could have important implications in the modulation of the autoimmune response.
Moreover, the potential pathogenic role of Th17 cells in MS and in other autoimmune disorders could be related to their enhanced capability to survive, self-renew, generate effector progeny, and enter the memory pool with an efficiency superior to that of Th1 cells [145], and to their resistance to activation induced cell death [146–148]. In particular, we have recently demonstrated that human Th17 cells derived from healthy donors and MS patients express lower levels of FASL compared to Th1 cells, with a consequent lower sensitivity to cell death [148]. This mechanism could explain the persistence of IL-17-producing cells in autoimmune diseases, such as MS, contributing to the chronic inflammation typical of the disease.
Interestingly, Th17 cells could play a pathogenic role in MS also by converting their phenotype into a proinflammatory Th1 profile, as demonstrated by IL-17F reporter mice, where committed Th17 cells give rise to a progeny that shifts toward enhanced IFN-γ expression, contingent upon limited or absent TGF-β [149].
Similarly, in humans there are evidences indicating that, in the presence of IL-12, Th17 cells produce also IFN-γ. These cells which produce both IL-17 and IFN-γ, called Th1/17 cells [26], together with “nonclassical Th1 cells” might contribute to disease pathogenesis through properties shared by both the Th1 and Th17 subsets [150]. Moreover, Th17 cells producing IFN-γ are enriched in myelin oligodendrocyte glycoprotein-specific T cells [151]. IFN-γ produced by these cells could strongly activate macrophages [152] whose infiltration in the CNS correlates with EAE severity [153].
7. Therapeutic Approaches Targeting Th17 Cells in MS
In recent years several therapies directed against Th17-related cytokines, including IL-17, IL-23, and GM-CSF, have been developed, and some are currently being tested in ongoing clinical trials (Table 1). The fully humanized antibody neutralizing IL-17A called AIN457 or Secukinumab (NCT01708603 Clinicaltrial.gov) is already approved for the first-line systemic treatment of moderate to severe plaque psoriasis [154, 155]; in MS patients, although it showed a reduction by 63% of new magnetic resonance imaging (MRI) lesions compared to placebo-treated patients, the reduction of annualized relapse rate (ARR) was not statistically significant [71]. Moreover, Secukinumab was ineffective and resulted in higher rates of adverse events, mainly infections, compared with placebo in patients with Crohn's disease [156], and the study in MS terminated early based upon development of another anti-IL-17 monoclonal antibody with better potential for treating MS patients, Ixekizumab (NCT02387801 Clinicaltrial.gov).
Table 1.
Therapeutic approaches targeting Th17 cells in MS.
| Agent | Functional role | Clinical trial identifier in MS | Clinical stage | Reference |
|---|---|---|---|---|
| Secukinumab (AIN457) | Neutralizes IL-17A | NCT01708603 | 63% reduction of new MRI lesions compared to placebo; ARR reduction not statistically significant | [71] |
|
| ||||
| Ixekizumab | Neutralizes IL-17 | Currently tested in psoriasis | [72] | |
|
| ||||
| MOR103 | Neutralizes GM-CSF | NCT01517282 | Did not show efficacy in MS | [73] |
|
| ||||
| Ustekinumab | Neutralizes the p40 subunit common to IL-12 and IL-23 | NCT00207727 | Did not show efficacy in MS | [74–76] |
|
| ||||
| Tildrakizumab | Neutralizes the p19 subunit specific of IL-23 | Currently tested in other autoimmune diseases | [72] | |
|
| ||||
| Guselkumab | Neutralizes the p19 subunit specific of IL-23 | Currently tested in other autoimmune diseases | [72] | |
|
| ||||
| AMG 139 | Neutralizes the p19 subunit specific of IL-23 | Currently tested in other autoimmune diseases | [72] | |
|
| ||||
| BI 655066 | Neutralizes the p19 subunit specific of IL-23 | Currently tested in other autoimmune diseases | [72] | |
|
| ||||
| LY3074828 | Neutralizes the p19 subunit specific of IL-23 | Currently tested in other autoimmune diseases | [72] | |
|
| ||||
| Digoxin | Interferes with RORγt | Preclinical phase | [77] | |
|
| ||||
| Ursolic acid | Interferes with RORγt | Preclinical phase | [78] | |
|
| ||||
| SR1001 | Interferes with RORα and RORγt | Preclinical phase | [79] | |
Another Th17-related cytokine that has recently gained attention, as a promising molecular target, is GM-CSF, and MOR103 (NCT01517282 Clinicaltrial.gov) a fully human monoclonal antibody against human GM-CSF was tested in clinical trials. However, although performed on a limited number of patients, the first clinical trial did not show efficacy in MS [73].
Selective targeting of single Th17 cytokines, such as IL-17 or GM-CSF, with monoclonal antibodies has not shown efficacy in MS and in other autoimmune diseases such as Crohn's disease and rheumatoid arthritis, although the involvement of Th17 cells in their pathogenesis has been widely documented. This suggests that Th17 cells' pathogenicity relies also on other factors, and thus targeting this T cell subset, inhibiting the Th17 differentiation program as a whole, may be more effective in limiting inflammation.
In this context, Ustekinumab, an antibody neutralizing the p40 subunit common to IL-12 and IL-23, was developed but the clinical trial has been completed and did not show efficacy in reducing inflammation in MS (NCT00207727 Clinicaltrial.gov) [74–76]. However, by blocking both IL-12 and IL-23 which sit upstream of the Th1 and Th17 differentiation program, respectively, this monoclonal antibody suppressed both of these responses and this could explain its low efficacy in MS, considering that interference with Th1 responses determines increased susceptibility rather than protection from EAE [157–159]. Specific inhibitors of the p19 subunit of IL-23, including Tildrakizumab, Guselkumab, AMG 139, BI 655066, and LY3074828, that have been developed and are being currently tested in other autoimmune diseases [72] could give encouraging results in MS treatment.
The RORγt transcription factor represents the ideal target for the manipulation of Th17 cell responses. Digoxin, a small molecule which binds RORγt and interferes with transcription, has been shown to inhibit Th17 cell differentiation in the mouse and to reduce EAE severity [77], and another natural product, ursolic acid, has similar effects [78]. Chemical modification of other small molecules shown to bind the ligand-binding domains of RORα and RORγt has led to the development of SR1001, which reduces human and murine Th17 cell differentiation, and suppresses the clinical severity of autoimmune disease in mice [79]. Although still in preclinical phase, the results of these studies indicate that this novel class of compounds has potential utility in the treatment of autoimmune diseases.
8. Conclusions
Over the past few years, remarkable advances in the understanding of Th responses have been reported. The discovery of the Th17 subset, of the cytokines and transcriptional factors regulating its differentiation, and of the biological functions of its effector cytokines has advanced our understanding of the role of CD4+ T cells in adaptive immunity. However, many issues remain to be addressed, especially concerning the balance between pathological and protective roles during autoimmune and infectious diseases. Advances in these points are critical for the future development of new therapeutic strategies able to modulate Th17 pathways for the treatment of MS and of diseases where Th17 cells play a pathogenic role.
Conflict of Interests
The authors declare that they have no financial conflict of interests.
References
- 1.Coffman R. L. Origins of the T(H)1-T(H)2 model: a personal perspective. Nature Immunology. 2006;7(6):539–541. doi: 10.1038/ni0606-539. [DOI] [PubMed] [Google Scholar]
- 2.Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. Journal of Immunology. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 3.Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annual Review of Immunology. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 4.Scanga C. A., Aliberti J., Jankovic D., et al. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. Journal of Immunology. 2002;168(12):5997–6001. doi: 10.4049/jimmunol.168.12.5997. [DOI] [PubMed] [Google Scholar]
- 5.Seki E., Tsutsui H., Tsuji N. M., et al. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. Journal of Immunology. 2002;169(7):3863–3868. doi: 10.4049/jimmunol.169.7.3863. [DOI] [PubMed] [Google Scholar]
- 6.Sacks D., Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nature Reviews Immunology. 2002;2(11):845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 7.Fremond C. M., Yeremeev V., Nicolle D. M., Jacobs M., Quesniaux V. F., Ryffel B. Fatal Mycobacterium tuberculosis infection despite adaptive immune response in the absence of MyD88. The Journal of Clinical Investigation. 2004;114(12):1790–1799. doi: 10.1172/jci200421027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anthony R. M., Rutitzky L. I., Urban J. F., Stadecker M. J., Gause W. C. Protective immune mechanisms in helminth infection. Nature Reviews Immunology. 2007;7(12):975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohn L., Elias J. A., Chupp G. L. Asthma: mechanisms of disease persistence and progression. Annual Review of Immunology. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 10.Bouma G., Strober W. The immunological and genetic basis of inflammatory bowel disease. Nature Reviews Immunology. 2003;3(7):521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 11.Lowes M. A., Bowcock A. M., Krueger J. G. Pathogenesis and therapy of psoriasis. Nature. 2007;445(7130):866–873. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 12.Jovanovic D. V., Di Battista J. A., Martel-Pelletier J., et al. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-β and TNF-α, by human macrophages. Journal of Immunology. 1998;160(7):3513–3521. [PubMed] [Google Scholar]
- 13.Park H., Li Z., Yang X. O., et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature Immunology. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrington L. E., Hatton R. D., Mangan P. R., et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nature Immunology. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 15.Huang W., Na L., Fidel P. L., Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. Journal of Infectious Diseases. 2004;190(3):624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 16.Liu J., Feng Y., Yang K., et al. Early production of IL-17 protects against acute pulmonary Pseudomonas aeruginosa infection in mice. FEMS Immunology and Medical Microbiology. 2011;61(2):179–188. doi: 10.1111/j.1574-695x.2010.00764.x. [DOI] [PubMed] [Google Scholar]
- 17.Ye P., Garvey P. B., Zhang P., et al. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. American Journal of Respiratory Cell and Molecular Biology. 2001;25(3):335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 18.Lu Y.-J., Gross J., Bogaert D., et al. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathogens. 2008;4(9) doi: 10.1371/journal.ppat.1000159.e1000159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montgomery C. P., Daniels M., Zhao F., Alegre M.-L., Chong A. S., Daum R. S. Protective immunity against recurrent Staphylococcus aureus skin infection requires antibody and interleukin-17A. Infection and Immunity. 2014;82(5):2125–2134. doi: 10.1128/iai.01491-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lock C., Hermans G., Pedotti R., et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nature Medicine. 2002;8(5):500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 21.Tzartos J. S., Friese M. A., Craner M. J., et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. The American Journal of Pathology. 2008;172(1):146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Durelli L., Barbero P., Cucci A., et al. Neutralizing antibodies in multiple sclerosis patients treated with 375 μg interferon-β-1b. Expert Opinion on Biological Therapy. 2009;9(4):387–397. doi: 10.1517/14712590902762781. [DOI] [PubMed] [Google Scholar]
- 23.Brucklacher-Waldert V., Stuerner K., Kolster M., Wolthausen J., Tolosa E. Phenotypical and functional characterization of T helper 17 cells in multiple sclerosis. Brain. 2009;132(12):3329–3341. doi: 10.1093/brain/awp289. [DOI] [PubMed] [Google Scholar]
- 24.Durelli L., Conti L., Clerico M., et al. T-helper 17 cells expand in multiple sclerosis and are inhibited by interferon-β . Annals of Neurology. 2009;65(5):499–509. doi: 10.1002/ana.21652. [DOI] [PubMed] [Google Scholar]
- 25.Fujino S., Andoh A., Bamba S., et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52(1):65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Annunziato F., Cosmi L., Santarlasci V., et al. Phenotypic and functional features of human Th17 cells. Journal of Experimental Medicine. 2007;204(8):1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teunissen M. B. M., Koomen C. W., de Waal Malefyt R., Wierenga E. A., Bos J. D. Interleukin-17 and interferon-γ synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. The Journal of Investigative Dermatology. 1998;111(4):645–649. doi: 10.1046/j.1523-1747.1998.00347.x. [DOI] [PubMed] [Google Scholar]
- 28.Wilson N. J., Boniface K., Chan J. R., et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nature Immunology. 2007;8(9):950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 29.Kotake S., Udagawa N., Takahashi N., et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. The Journal of Clinical Investigation. 1999;103(9):1345–1352. doi: 10.1172/jci5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cua D. J., Sherlock J., Chen Y., et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421(6924):744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z., Zheng M., Bindas J., Schwarzenberger P., Kolls J. K. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflammatory Bowel Diseases. 2006;12(5):382–388. doi: 10.1097/01.mib.0000218764.06959.91. [DOI] [PubMed] [Google Scholar]
- 32.Lubberts E., Koenders M. I., Oppers-Walgreen B., et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis and Rheumatism. 2004;50(2):650–659. doi: 10.1002/art.20001. [DOI] [PubMed] [Google Scholar]
- 33.Langrish C. L., Chen Y., Blumenschein W. M., et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. The Journal of Experimental Medicine. 2005;201(2):233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin W., Dong C. IL-17 cytokines in immunity and inflammation. Emerging Microbes & Infections. 2013;2(9, article e60) doi: 10.1038/emi.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hymowitz S. G., Filvaroff E. H., Yin J., et al. IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. The EMBO Journal. 2001;20(19):5332–5341. doi: 10.1093/emboj/20.19.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fallon P. G., Ballantyne S. J., Mangan N. E., et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. The Journal of Experimental Medicine. 2006;203(4):1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Owyang A. M., Zaph C., Wilson E. H., et al. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. The Journal of Experimental Medicine. 2006;203(4):843–849. doi: 10.1084/jem.20051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fort M. M., Cheung J., Yen D., et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15(6):985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 39.Yamaguchi Y., Fujio K., Shoda H., et al. IL-17B and IL-17C are associated with TNF-α production and contribute to the exacerbation of inflammatory arthritis. Journal of Immunology. 2007;179(10):7128–7136. doi: 10.4049/jimmunol.179.10.7128. [DOI] [PubMed] [Google Scholar]
- 40.Wu Q., Martin R. J., Rino J. G., Breed R., Torres R. M., Chu H. W. IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes and Infection. 2007;9(1):78–86. doi: 10.1016/j.micinf.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H., Chen J., Huang A., et al. Cloning and characterization of IL-17B and IL-17C, two new members of the IL-17 cytokine family. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(2):773–778. doi: 10.1073/pnas.97.2.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Starnes T., Broxmeyer H. E., Robertson M. J., Hromas R. Cutting edge: IL-17D, a novel member of the IL-17 family, stimulates cytokine production and inhibits hemopoiesis. Journal of Immunology. 2002;169(2):642–646. doi: 10.4049/jimmunol.169.2.642. [DOI] [PubMed] [Google Scholar]
- 43.Ramirez-Carrozzi V., Sambandam A., Luis E., et al. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nature Immunology. 2011;12(12):1159–1166. doi: 10.1038/ni.2156. [DOI] [PubMed] [Google Scholar]
- 44.Song X., Zhu S., Shi P., et al. IL-17RE is the functional receptor for IL-17C and mediates mucosal immunity to infection with intestinal pathogens. Nature Immunology. 2011;12(12):1151–1158. doi: 10.1038/ni.2155. [DOI] [PubMed] [Google Scholar]
- 45.Chang S. H., Reynolds J. M., Pappu B. P., Chen G., Martinez G. J., Dong C. Interleukin-17C promotes Th17 cell responses and autoimmune disease via interleukin-17 receptor E. Immunity. 2011;35(4):611–621. doi: 10.1016/j.immuni.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang S. C., Long A. J., Bennett F., et al. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. Journal of Immunology. 2007;179(11):7791–7799. doi: 10.4049/jimmunol.179.11.7791. [DOI] [PubMed] [Google Scholar]
- 47.Toy D., Kugler D., Wolfson M., et al. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. Journal of Immunology. 2006;177(1):36–39. doi: 10.4049/jimmunol.177.1.36. [DOI] [PubMed] [Google Scholar]
- 48.Haudenschild D., Moseley T., Rose L., Reddi A. H. Soluble and transmembrane isoforms of novel interleukin-17 receptor-like protein by RNA splicing and expression in prostate cancer. The Journal of Biological Chemistry. 2002;277(6):4309–4316. doi: 10.1074/jbc.m109372200. [DOI] [PubMed] [Google Scholar]
- 49.Yao Z., Painter S. L., Fanslow W. C., et al. Human IL-17: a novel cytokine derived from T cells. Journal of Immunology. 1995;155(12):5483–5486. [PubMed] [Google Scholar]
- 50.Moseley T. A., Haudenschild D. R., Rose L., Reddi A. H. Interleukin-17 family and IL-17 receptors. Cytokine & Growth Factor Reviews. 2003;14(2):155–174. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 51.Fossiez F., Djossou O., Chomarat P., et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. The Journal of Experimental Medicine. 1996;183(6):2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones C. E., Chan K. Interleukin-17 stimulates the expression of interleukin-8, growth-related oncogene-alpha, and granulocyte-colony-stimulating factor by human airway epithelial cells. American Journal of Respiratory Cell and Molecular Biology. 2002;26(6):748–753. doi: 10.1165/ajrcmb.26.6.4757. [DOI] [PubMed] [Google Scholar]
- 53.Laan M., Cui Z.-H., Hoshino H., et al. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. Journal of Immunology. 1999;162(4):2347–2352. [PubMed] [Google Scholar]
- 54.Chen Y., Thai P., Zhao Y.-H., Ho Y.-S., DeSouza M. M., Wu R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. Journal of Biological Chemistry. 2003;278(19):17036–17043. doi: 10.1074/jbc.m210429200. [DOI] [PubMed] [Google Scholar]
- 55.Kao C.-Y., Chen Y., Thai P., et al. IL-17 markedly up-regulates β-defensin-2 expression in human airway epithelium via JAK and NF-κB signaling pathways. Journal of Immunology. 2004;173(5):3482–3491. doi: 10.4049/jimmunol.173.5.3482. [DOI] [PubMed] [Google Scholar]
- 56.Huang F., Kao C.-Y., Wachi S., Thai P., Ryu J., Wu R. Requirement for both JAK-mediated PI3K signaling and ACT1/TRAF6/TAK1-dependent NF-κB activation by IL-17A in enhancing cytokine expression in human airway epithelial cells. Journal of Immunology. 2007;179(10):6504–6513. doi: 10.4049/jimmunol.179.10.6504. [DOI] [PubMed] [Google Scholar]
- 57.Hsu H.-C., Yang P., Wang J., et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nature Immunology. 2008;9(2):166–175. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 58.Doreau A., Belot A., Bastid J., et al. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nature Immunology. 2009;10(7):778–785. doi: 10.1038/ni.1741. [DOI] [PubMed] [Google Scholar]
- 59.Mitsdoerffer M., Lee Y., Jäger A., et al. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(32):14292–14297. doi: 10.1073/pnas.1009234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar P., Chen K., Kolls J. K. Th17 cell based vaccines in mucosal immunity. Current Opinion in Immunology. 2013;25(3):373–380. doi: 10.1016/j.coi.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liang S. C., Tan X.-Y., Luxenberg D. P., et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. The Journal of Experimental Medicine. 2006;203(10):2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Acosta-Rodriguez E. V., Napolitani G., Lanzavecchia A., Sallusto F. Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17-producing human T helper cells. Nature Immunology. 2007;8(9):942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 63.Zhou L., Ivanov I. I., Spolski R., et al. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nature Immunology. 2007;8(9):967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 64.Laurence A., O'Shea J. J. TH-17 differentiation: of mice and men. Nature Immunology. 2007;8(9):903–905. doi: 10.1038/ni0907-903. [DOI] [PubMed] [Google Scholar]
- 65.Volpe E., Servant N., Zollinger R., et al. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human TH-17 responses. Nature Immunology. 2008;9(6):650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 66.Manel N., Unutmaz D., Littman D. R. The differentiation of human TH-17 cells requires transforming growth factor-β and induction of the nuclear receptor RORγt. Nature Immunology. 2008;9(6):641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Volpe E., Touzot M., Servant N., et al. Multiparametric analysis of cytokine-driven human Th17 differentiation reveals a differential regulation of IL-17 and IL-22 production. Blood. 2009;114(17):3610–3614. doi: 10.1182/blood-2009-05-223768. [DOI] [PubMed] [Google Scholar]
- 68.Elyaman W., Bradshaw E. M., Uyttenhove C., et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(31):12885–12890. doi: 10.1073/pnas.0812530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Codarri L., Gyülvészii G., Tosevski V., et al. RORγ3t drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nature Immunology. 2011;12(6):560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 70.El-Behi M., Ciric B., Dai H., et al. The encephalitogenicity of TH17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nature Immunology. 2011;12(6):568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miossec P., Kolls J. K. Targeting IL-17 and TH17 cells in chronic inflammation. Nature Reviews Drug Discovery. 2012;11(10):763–776. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- 72.Bartlett H. S., Million R. P. Targeting the IL-17–TH17 pathway. Nature Reviews Drug Discovery. 2015;14(1):11–12. doi: 10.1038/nrd4518. [DOI] [PubMed] [Google Scholar]
- 73.Constantinescu C. S., Asher A., Fryze W., et al. Randomized phase 1b trial of MOR103, a human antibody to GM-CSF, in multiple sclerosis. Neurology: Neuroimmunology & Neuroinflammation. 2015;2(4):e117–e117. doi: 10.1212/nxi.0000000000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Segal B. M., Constantinescu C. S., Raychaudhuri A., Kim L., Fidelus-Gort R., Kasper L. H. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. The Lancet Neurology. 2008;7(9):796–804. doi: 10.1016/s1474-4422(08)70173-x. [DOI] [PubMed] [Google Scholar]
- 75.Vollmer T. L., Wynn D. R., Alam S. M., Valdes J. A phase 2, 24-week, randomized, placebo-controlled, double-blind study examining the efficacy and safety of an anti-interleukin-12 and -23 monoclonal antibody in patients with relapsing-remitting or secondary progressive multiple sclerosis. Multiple Sclerosis. 2011;17(2):181–191. doi: 10.1177/1352458510384496. [DOI] [PubMed] [Google Scholar]
- 76.Martin R. Neutralisation of IL12 p40 or IL23 p40 does not block inflammation in multiple sclerosis. The Lancet Neurology. 2008;7(9):765–766. doi: 10.1016/s1474-4422(08)70174-1. [DOI] [PubMed] [Google Scholar]
- 77.Huh J. R., Leung M. W. L., Huang P., et al. Digoxin and its derivatives suppress T H17 cell differentiation by antagonizing RORγ3t activity. Nature. 2011;472(7344):486–490. doi: 10.1038/nature09978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu T., Wang X., Zhong B., Nurieva R. I., Ding S., Dong C. Ursolic acid suppresses interleukin-17 (IL-17) production by selectively antagonizing the function of RORγt protein. The Journal of Biological Chemistry. 2011;286(26):22707–22710. doi: 10.1074/jbc.c111.250407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Solt L. A., Kumar N., Nuhant P., et al. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature. 2011;472(7344):491–494. doi: 10.1038/nature10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van Beelen A. J., Zelinkova Z., Taanman-Kueter E. W., et al. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27(4):660–669. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 81.Rogers N. C., Slack E. C., Edwards A. D., et al. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity. 2005;22:507–517. doi: 10.1016/j.immuni.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 82.LeibundGut-Landmann S., Gross O., Robinson M. J., et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nature Immunology. 2007;8(6):630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 83.Kapsenberg M. L. Dendritic-cell control of pathogen-driven T-cell polarization. Nature Reviews Immunology. 2003;3(12):984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 84.Reis e Sousa C. Activation of dendritic cells: translating innate into adaptive immunity. Current Opinion in Immunology. 2004;16(1):21–25. doi: 10.1016/j.coi.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 85.Bettelli E., Carrier Y., Gao W., et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 86.Mangan P. R., Harrington L. E., O'Quinn D. B., et al. Transforming growth factor-β induces development of the TH17 lineage. Nature. 2006;441(7090):231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 87.Veldhoen M., Hocking R. J., Atkins C. J., Locksley R. M., Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 88.Nurieva R., Yang X. O., Martinez G., et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448(7152):480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 89.Veldhoen M., Hocking R. J., Flavell R. A., Stockinger B. Signals mediated by transforming growth factor-β initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nature Immunology. 2006;7(11):1151–1156. doi: 10.1038/ni1391. [DOI] [PubMed] [Google Scholar]
- 90.Yang L., Anderson D. E., Baecher-Allan C., et al. IL-21 and TGF-β are required for differentiation of human TH17 cells. Nature. 2008;454(7202):350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gerosa F., Baldani-Guerra B., Lyakh L. A., et al. Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells. The Journal of Experimental Medicine. 2008;205(6):1447–1461. doi: 10.1084/jem.20071450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Soumelis V., Volpe E. TH17 differentiation, a complex process in mouse and man. Medecine Sciences. 2008;24(11):925–927. doi: 10.1051/medsci/20082411925. [DOI] [PubMed] [Google Scholar]
- 93.Ivanov I. I., McKenzie B. S., Zhou L., et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 94.Ivanov I. I., Zhou L., Littman D. R. Transcriptional regulation of Th17 cell differentiation. Seminars in Immunology. 2007;19(6):409–417. doi: 10.1016/j.smim.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen Z., Laurence A., Kanno Y., et al. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(21):8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wei L., Laurence A., Elias K. M., O'Shea J. J. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. The Journal of Biological Chemistry. 2007;282(48):34605–34610. doi: 10.1074/jbc.m705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.de Beaucoudtey L., Puel A., Filipe-Santos O., et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. The Journal of Experimental Medicine. 2008;205(7):1543–1550. doi: 10.1084/jem.20080321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brüstle A., Heink S., Huber M., et al. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nature Immunology. 2007;8(9):958–966. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 99.Ise W., Kohyama M., Schraml B. U., et al. The transcription factor BATF controls the global regulators of class-switch recombination in both B cells and T cells. Nature Immunology. 2011;12(6):536–543. doi: 10.1038/ni.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schraml B. U., Hildner K., Ise W., et al. The AP-1 transcription factor Batf controls TH17 differentiation. Nature. 2009;460(7253):405–409. doi: 10.1038/nature08114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lohoff M., Mittrücker H.-W., Prechtl S., et al. Dysregulated T helper cell differentiation in the absence of interferon regulatory factor 4. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(18):11808–11812. doi: 10.1073/pnas.182425099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ciofani M., Madar A., Galan C., et al. A validated regulatory network for Th17 cell specification. Cell. 2012;151(2):289–303. doi: 10.1016/j.cell.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang X. O., Pappu B. P., Nurieva R., et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28(1):29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sundrud M. S., Rao A. Regulation of T helper 17 differentiation by orphan nuclear receptors: it's not just RORγt anymore. Immunity. 2008;28(1):5–7. doi: 10.1016/j.immuni.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 105.Murphy K. A., Villano C. M., Dorn R., White L. A. Interaction between the aryl hydrocarbon receptor and retinoic acid pathways increases matrix metalloproteinase-1 expression in keratinocytes. The Journal of Biological Chemistry. 2004;279(24):25284–25293. doi: 10.1074/jbc.m402168200. [DOI] [PubMed] [Google Scholar]
- 106.Mucida D., Park Y., Kim G., et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317(5835):256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 107.Veldhoen M., Hirota K., Westendorf A. M., et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453(7191):106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 108.Quintana F. J., Basso A. S., Iglesias A. H., et al. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453(7191):65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 109.Dang E. V., Barbi J., Yang H.-Y., et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146(5):772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shi L. Z., Wang R., Huang G., et al. HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. The Journal of Experimental Medicine. 2011;208(7):1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McGeachy M. J., Bak-Jensen K. S., Chen Y., et al. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell-mediated pathology. Nature Immunology. 2007;8(12):1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 112.Zielinski C. E., Mele F., Aschenbrenner D., et al. Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β . Nature. 2012;484(7395):514–518. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
- 113.Gu Y., Yang J., Ouyang X., et al. Interleukin 10 suppresses Th17 cytokines secreted by macrophages and T cells. European Journal of Immunology. 2008;38(7):1807–1813. doi: 10.1002/eji.200838331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li M. O., Flavell R. A. Contextual regulation of inflammation: a duet by transforming growth factor-beta and interleukin-10. Immunity. 2008;28(4):468–476. doi: 10.1016/j.immuni.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 115.Moore K. W., de Waal Malefyt R., Coffman R. L., O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annual Review of Immunology. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 116.Jankovic D., Kugler D. G., Sher A. IL-10 production by CD4+ effector T cells: a mechanism for self-regulation. Mucosal Immunology. 2010;3(3):239–246. doi: 10.1038/mi.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Laurence A., Tato C. M., Davidson T. S., et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26(3):371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 118.Ruocco G., Rossi S., Motta C., et al. T helper 9 cells induced by plasmacytoid dendritic cells regulate interleukin-17 in multiple sclerosis. Clinical Science. 2015;129(4):291–303. doi: 10.1042/cs20140608. [DOI] [PubMed] [Google Scholar]
- 119.Nowak E. C., Weaver C. T., Turner H., et al. IL-9 as a mediator of Th17-driven inflammatory disease. The Journal of Experimental Medicine. 2009;206(8):1653–1660. doi: 10.1084/jem.20090246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Diveu C., McGeachy M. J., Boniface K., et al. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. Journal of Immunology. 2009;182(9):5748–5756. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- 121.Tang S.-C., Fan X.-H., Pan Q.-M., Sun Q.-S., Liu Y. Decreased expression of IL-27 and its correlation with Th1 and Th17 cells in progressive multiple sclerosis. Journal of the Neurological Sciences. 2015;348(1-2):174–180. doi: 10.1016/j.jns.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 122.Milo R., Miller A. Revised diagnostic criteria of multiple sclerosis. Autoimmunity Reviews. 2014;13(4-5):518–524. doi: 10.1016/j.autrev.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 123.Hawkes C. H., Giovannoni G. The McDonald criteria for multiple sclerosis: time for clarification. Multiple Sclerosis. 2010;16(5):566–575. doi: 10.1177/1352458510362441. [DOI] [PubMed] [Google Scholar]
- 124.Koch M., Kingwell E., Rieckmann P., Tremlett H. The natural history of primary progressive multiple sclerosis. Neurology. 2009;73(23):1996–2002. doi: 10.1212/wnl.0b013e3181c5b47f. [DOI] [PubMed] [Google Scholar]
- 125.Reboldi A., Coisne C., Baumjohann D., et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nature Immunology. 2009;10(5):514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 126.Kebir H., Kreymborg K., Ifergan I., et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nature Medicine. 2007;13(10):1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kostic M., Stojanovic I., Marjanovic G., Zivkovic N., Cvetanovic A. Deleterious versus protective autoimmunity in multiple sclerosis. Cellular Immunology. 2015;296(2):122–132. doi: 10.1016/j.cellimm.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 128.Kang Z., Wang C., Zepp J., et al. Act1 mediates IL-17-induced EAE pathogenesis selectively in NG2+ glial cells. Nature Neuroscience. 2013;16(10):1401–1408. doi: 10.1038/nn.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Paintlia M. K., Paintlia A. S., Singh A. K., Singh I. Synergistic activity of interleukin-17 and tumor necrosis factor-α enhances oxidative stress-mediated oligodendrocyte apoptosis. Journal of Neurochemistry. 2011;116(4):508–521. doi: 10.1111/j.1471-4159.2010.07136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Siffrin V., Radbruch H., Glumm R., et al. In vivo imaging of partially reversible th17 cell-induced neuronal dysfunction in the course of encephalomyelitis. Immunity. 2010;33(3):424–436. doi: 10.1016/j.immuni.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 131.Komiyama Y., Nakae S., Matsuki T., et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. Journal of Immunology. 2006;177(1):566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 132.Hofstetter H. H., Ibrahim S. M., Koczan D., et al. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cellular Immunology. 2005;237(2):123–130. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 133.Baecher-Allan C., Wolf E., Hafter D. A. MHC class II expression identifies functionally distinct human regulatory T cells. Journal of Immunology. 2006;176(8):4622–4631. doi: 10.4049/jimmunol.176.8.4622. [DOI] [PubMed] [Google Scholar]
- 134.Huehn J., Hamann A. Homing to suppress: address codes for Treg migration. Trends in Immunology. 2005;26(12):632–636. doi: 10.1016/j.it.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 135.Haas J., Fritzsching B., Trübswetter P., et al. Prevalence of newly generated naive regulatory T cells (Treg) is critical for Treg suppressive function and determines Treg dysfunction in multiple sclerosis. Journal of Immunology. 2007;179(2):1322–1330. doi: 10.4049/jimmunol.179.2.1322. [DOI] [PubMed] [Google Scholar]
- 136.Croxford A., Lanzinger M., Hartmann F., et al. The cytokine GM-CSF drives the inflammatory signature of CCR2+ monocytes and licenses autoimmunity. Immunity. 2015;43(3):502–514. doi: 10.1016/j.immuni.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 137.Vollmer T. L., Liu R., Price M., Rhodes S., La Cava A., Shi F.-D. Differential effects of IL-21 during initiation and progression of autoimmunity against neuroantigen. Journal of Immunology. 2005;174(5):2696–2701. doi: 10.4049/jimmunol.174.5.2696. [DOI] [PubMed] [Google Scholar]
- 138.Korn T., Bettelli E., Gao W., et al. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature. 2007;448(7152):484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tzartos J. S., Craner M. J., Friese M. A., et al. IL-21 and IL-21 receptor expression in lymphocytes and neurons in multiple sclerosis brain. The American Journal of Pathology. 2011;178(2):794–802. doi: 10.1016/j.ajpath.2010.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Perriard G., Mathias A., Enz L., et al. Interleukin-22 is increased in multiple sclerosis patients and targets astrocytes. Journal of Neuroinflammation. 2015;12, article 19 doi: 10.1186/s12974-015-0335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xu W., li R., Dai Y., et al. IL-22 secreting CD4+ T cells in the patients with neuromyelitis optica and multiple sclerosis. Journal of Neuroimmunology. 2013;261(1-2):87–91. doi: 10.1016/j.jneuroim.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 142.Rolla S., Bardina V., De Mercanti S., et al. Th22 cells are expanded in multiple sclerosis and are resistant to IFN-β . Journal of Leukocyte Biology. 2014;96(6):1155–1164. doi: 10.1189/jlb.5a0813-463rr. [DOI] [PubMed] [Google Scholar]
- 143.Goris A., Marrosu M. G., Vandenbroeck K. Novel polymorphisms in the IL-10 related AK155 gene (chromosome 12q15) Genes and Immunity. 2001;2(5):284–286. doi: 10.1038/sj.gene.6363772. [DOI] [PubMed] [Google Scholar]
- 144.Meller S., Di Domizio J., Voo K. S., et al. TH17 cells promote microbial killing and innate immune sensing of DNA via interleukin 26. Nature Immunology. 2015;16(9):970–979. doi: 10.1038/ni.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Muranski P., Borman Z. A., Kerkar S. P., et al. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity. 2011;35(6):972–985. doi: 10.1016/j.immuni.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shi G., Ramaswamy M., Vistica B. P., et al. Unlike Th1, Th17 cells mediate sustained autoimmune inflammation and are highly resistant to restimulation-induced cell death. Journal of Immunology. 2009;183(11):7547–7556. doi: 10.4049/jimmunol.0900519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yu Y., Iclozan C., Yamazaki T., et al. Abundant c-Fas-associated death domain-like interleukin-1-converting enzyme inhibitory protein expression determines resistance of T helper 17 cells to activation-induced cell death. Blood. 2009;114(5):1026–1028. doi: 10.1182/blood-2009-03-210153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cencioni M. T., Santini S., Ruocco G., et al. FAS-ligand regulates differential activation-induced cell death of human T-helper 1 and 17 cells in healthy donors and multiple sclerosis patients. Cell Death & Disease. 2015;6 doi: 10.1038/cddis.2015.100.e1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lee Y. K., Turner H., Maynard C. L., et al. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30(1):92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Maggi L., Capone M., Giudici F., et al. CD4+CD161+ T lymphocytes infiltrate crohn's disease-associated perianal fistulas and are reduced by anti-TNF-α local therapy. International Archives of Allergy and Immunology. 2013;161(1):81–86. doi: 10.1159/000343467. [DOI] [PubMed] [Google Scholar]
- 151.Duhen R., Glatigny S., Arbelaez C. A., Blair T. C., Oukka M., Bettelli E. Cutting edge: the pathogenicity of IFN-γ-producing Th17 cells is independent of T-bet. Journal of Immunology. 2013;190(9):4478–4482. doi: 10.4049/jimmunol.1203172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Adams D. O. Molecular interactions in macrophage activation. Immunology Today. 1989;10(2):33–35. doi: 10.1016/0167-5699(89)90298-3. [DOI] [PubMed] [Google Scholar]
- 153.Ajami B., Bennett J. L., Krieger C., McNagny K. M., Rossi F. M. V. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nature Neuroscience. 2011;14(9):1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- 154.Garnock-Jones K. P. Secukinumab: a review in moderate to severe plaque psoriasis. American Journal of Clinical Dermatology. 2015;16(4):323–330. doi: 10.1007/s40257-015-0143-7. [DOI] [PubMed] [Google Scholar]
- 155.McInnes I. B., Mease P. J., Kirkham B., et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet. 2015;386(9999):1137–1146. doi: 10.1016/s0140-6736(15)61134-5. [DOI] [PubMed] [Google Scholar]
- 156.Hueber W., Sands B. E., Lewitzky S., et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: unexpected results of a randomised, double-blindplacebo-controlled trial. Gut. 2012;61(12):1693–1700. doi: 10.1136/gutjnl-2011-301668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ferber I. A., Brocke S., Taylor-Edwards C., et al. Mice with a disrupted IFN-γ gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) Journal of Immunology. 1996;156(1):5–7. [PubMed] [Google Scholar]
- 158.Bettelli E., Sullivan B., Szabo S. J., Sobel R. A., Glimcher L. H., Kuchroo V. K. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. The Journal of Experimental Medicine. 2004;200(1):79–87. doi: 10.1084/jem.20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gran B., Zhang G.-X., Yu S., et al. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. Journal of Immunology. 2002;169(12):7104–7110. doi: 10.4049/jimmunol.169.12.7104. [DOI] [PubMed] [Google Scholar]