Abstract
Ceramide and sphingosine and their phosphorylated counterparts are recognized as “bioactive sphingolipids” and modulate membrane integrity, the activity of enzymes, or act as ligands of G protein-coupled receptors. The subcellular distribution of the bioactive sphingolipids is central to their function as the same lipid can mediate diametrically opposite effects depending on its location. To ensure that these lipids are present in the right amount and in the appropriate organelles, cells employ selective lipid transport and compartmentalize sphingolipid-metabolizing enzymes to characteristic subcellular sites. Our knowledge of key mechanisms involved in sphingolipid signaling and trafficking has increased substantially in the past decades—thanks to advances in biochemical and cell biological methods. In this review, we focus on the bioactive sphingolipids and discuss how the combination of studies in cells and in model membranes have contributed to our understanding of how they behave and function in living organisms.
Keywords: ceramide, sphingosine, ceramide 1-phosphate, sphingosine 1-phosphate, membrane, lipid trafficking
Introduction
Sphingosine was first identified and described by Johann L.W. Thudichum in the late 19th century, and since then, the class of lipids containing a sphingoid backbone has grown to over 4300 known compounds currently registered in the LIPID MAPS database (http://www.lipidmaps.org/data/structure/index.html). The sphingolipids were initially considered to be mainly structural molecules serving as building blocks of cellular membranes. In the 1980s, a role of simple sphingolipids as regulators of cell signaling started to emerge following the finding that sphingosine inhibits protein kinase C (PKC) activity1 and later the identification of the first G protein-coupled sphingosine 1-phosphate (S1P) receptor.2,3 These findings sparked an increased interest in sphingolipid research, and several specific regulatory roles of sphingolipids on proteins and cell signaling have since been reported. There is now a large body of information regarding sphingolipid trafficking and function from model membrane in in vitro and in vivo settings. Here, we review studies performed on model membranes and cells and discuss what they have taught us about the trafficking and cellular functions of ceramide, ceramide 1-phosphate (C1P), sphingosine, and S1P, which are referred to as bioactive sphingolipids.
Ceramide
Ceramide is the first sphingolipid with a bona fide sphingosine backbone to be generated in the de novo synthesis pathway and serves as a substrate for making all other sphingolipids. Ceramide drives senescence and sensitization to apoptosis, and cancer cells commonly adjust their sphingolipid metabolism such that ceramide levels are suppressed.4 Defective ceramide metabolism is also associated with various disease states and metabolic disorders, such as diabetes, atherosclerosis, and, most notably, Farber’s disease (acid ceramidase [aCERase] deficiency), underscoring the importance of ceramide as a regulator of cellular function and physiology.
Ceramide behavior in model membranes
Ceramides consist of a fatty acid linked to the amino group of a sphingosine backbone. The length and saturation of the acyl side chain have a marked effect on ceramide behavior in cells and in model membranes. Short-chain ceramides (C2 and C6) are commonly used in cell biological experiments because they are easy to deliver to cells from the solvent. However, the biophysical properties of these short-chain ceramides differ from that of the C16 and C24 ceramides that are more commonly found in cells. The critical micelle concentration for the C2 and C6 ceramides is in the low micromolar range, while the long-chain C16 ceramide is basically insoluble in an aqueous solution.5 In contrast to C16 ceramide, the short-chain ceramides may also act as detergents and solubilize phospholipid bilayers, forming mixed ceramide phospholipid micelles.5
The ability of lipids to move from one membrane leaflet to the other, either spontaneously or facilitated by transport proteins, is essential for generating and upholding specific membrane lipid profiles in cells. Spontaneous transbilayer movement of ceramide has been demonstrated in model membranes using different experimental setups. An early study by Bai and Pagano6 utilized BODIPY-labeled lipids to approximate transbilayer lipid movement and calculated the “flip-flop” halftime to be ~22 minutes, which suggested that ceramide moves much faster between the membrane leaflets than the corresponding BODIPY-labeled phospholipids and slower than the BODIPY-diacylglycerol analogs. López-Montero et al7 estimated the transmembrane movement of ceramides by studying shape changes in giant unilamellar vesicles induced by unlabeled ceramide. Using this approach, the halftime was estimated between 72 seconds (C6 ceramide) and 144 seconds (C16 ceramide). Although the measured flip-flop rate may differ by an order of magnitude depending on the methodology used for measuring, the reported relative halftime of ceramide flip-flop is commonly reported to be slower than that of diacylglycerol (DAG) and faster than that of phospholipids.
The studies of model membranes have established that long-chain ceramides increase lipid order and coalesce into “platforms” when inserted into phospholipid bilayers, measured by fluorescent probe polarization and differential scanning calorimetry.8–10 The increased lipid order in phospholipid bilayer is associated with phase separation and formation of ceramide-enriched domains.11 The coalescence of ceramide-enriched domains is dependent on acyl chain length and saturation. Very short ceramides (<C8) are miscible in dimyristoyl-phosphatidylglycerol (PC) lipid layer, longer saturated ceramides (C10–C14) form flower-shaped domains, and ceramides with C16–C18 saturated acyl chains form round- and regular-shaped micrometer-sized domains.12 In addition to acyl chain length and saturation, hydrogen bonding involving the 2NH is also crucial for efficient formation of ceramide-rich domains in fluid phosphatidylcholine bilayers.13 Ceramide-rich platforms in cells have been implicated in receptor clustering and activation at the plasma membrane and in coordinating cellular signaling events.14–17
In mammals, the most common sphingoid base is C18:1, but relatively high amounts of C20:1 and C16:1 bases can be expressed in specific cell types. The length of the sphingoid base in ceramide also affects the biophysical properties of the membranes. In a model membrane composed of palmitoyl oleoyl phosphocholine, palmitoylsphingomyelin, and cholesterol, palmitoylceramides with 16:1-, 18:1-, and 20:1-sphingoid bases induced ordered or gel phase formation, whereas the shorter C12:1-palmitoylceramide and 14:1-palmitoylceramide did not.18
The membrane-ordering effect of ceramides is also associated with increased membrane permeability to solutes. Ceramide generated by hydrolysis of sphingomyelin (SM) in vesicles induces membrane permeability and solvent leakage.19,20 A pathophysiological significance of the ceramide-induced membrane permeabilization was suggested by the finding that ceramide can induce membrane channels in mitochondria. The channels induced by ceramide in planar phospholipid membranes are up to 11 nm in diameter and stable.21 The ceramide concentration required for pore formation is within the range present in mitochondria during the induction of apoptosis,22 and ceramide can increase the permeability of mitochondrial outer membrane to cytochrome C and other small proteins.23 It has been further suggested that Bcl-2 proteins are not required for ceramide to induce channels in the mitochondrial outer membrane and that ceramide channels may be a direct mechanism for releasing proapoptotic proteins from mitochondria during apoptosis.24 Interestingly, dihydroceramide, which lacks the double bond between C4 and C5 in the sphingoid backbone, inhibits ceramide-induced channel formation in mitochondria.25 This finding is supported by the observation that ceramide but not dihydroceramide increases membrane permeability and lipid scrambling in large unilamellar vesicles.26
Model membrane studies have also been a central part of elucidating the functional properties of ceramide transfer protein (CERT), a protein that transfers ceramide from the endoplasmic reticulum (ER) to the Golgi apparatus. Using a donor–acceptor vesicle setup, CERT was found to transfer mainly ceramides with high efficiency, diacylglycerols and dihydroceramides with low efficiency, and no activity toward SM or sphingosine.27 Furthermore, CERT does not display the same transfer activity toward all ceramide species but has preference for ceramides with acyl chains of C20 or shorter.27,28
Ceramide synthesis and trafficking
In a cellular context, ceramide is the first sphingolipid with a complete sphingosine backbone to be formed in the de novo synthesis pathway29 and is the central sphingolipid from which all other sphingolipids are formed (Fig. 1). Cells utilize three pathways for generating ceramide, by removing the head group of more complex sphingolipids (eg, SM), by de novo synthesis, or via the sphingosine salvage pathway.
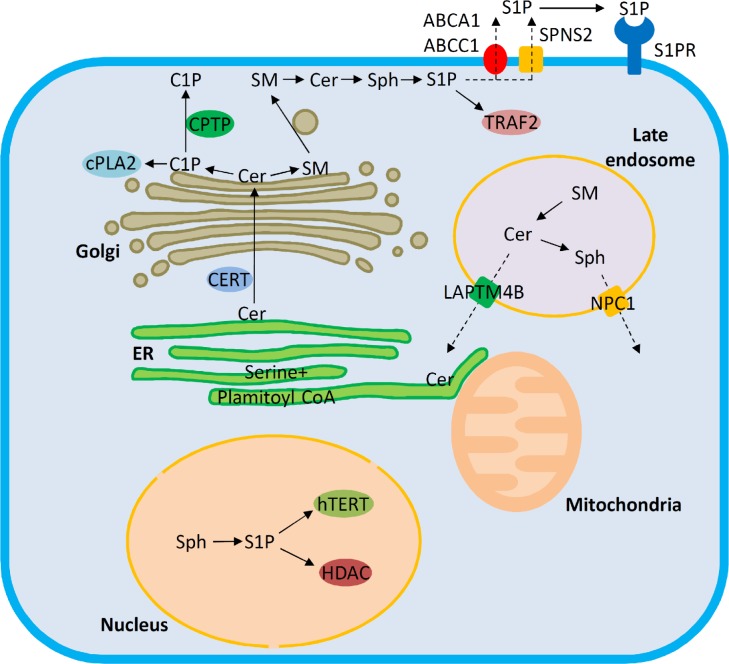
Figure 1.
Trafficking pathways and target molecules of bioactive sphingolipids. Ceramide (Cer) is synthesized de novo in the ER. From there, ceramide is transported via either vesicular or nonvesicular (ceramide transport protein, CERT) pathways to the Golgi. Ceramide transported by CERT is utilized for generating ceramide 1-phosphate (C1P) or SM. C1P and SM are transferred from the Golgi to the plasma membrane via ceramide 1-phosphate transfer protein (CPTP) and vesicular trafficking, respectively. C1P facilitates cPLA2 recruitment to cellular membranes and regulates eicosanoid production. Sphingosine 1-phosphate (S1P) generated inside the cells is subject to export facilitated by Spinster 2 (SPNS2) or ABC family transporters. The late endosomal/lysosomal compartment is a major site for generating bioactive sphingolipids by degradation of complex sphingolipids. The exit of ceramide from late endosomes is promoted by LAPTM4B, and the compartmentalization of the further degradation product sphingosine is regulated by NPC1. The endoplasmic reticulum (ER) and mitochondria are central sites for ceramide-induced apoptosis. S1P can act inside the nucleus by interaction with human telomerase reverse transcriptase (hTERT) and histone deacetylases (HDAC), on the plasma membrane via G protein-coupled S1P receptors or in the cytosol by interaction with TRAF2. Dashed arrows denote trafficking pathways that are supported by experiments in cellular settings, and solid arrows indicate that the pathways have been verified with experiments in vitro.
In cells, SM is a major source for the rapid generation of ceramide. The SM hydrolysis is carried out by sphin gomyelinases, a group of phospholipase C-like enzymes, with different pH optima and subcellular localization. The sphingomyelinase family of enzymes catalyze the removal of phosphocholine head groups from SM, generating ceramide and free phosphocholine as their catalytic products. The sphingomyelinases are divided into acid, alkaline, and neutral sphingomyelinase depending on their functional pH optima.30 Alkaline sphingomyelinase is highly expressed in the intestine and liver, secreted into the intestinal lumen, and is responsible for digesting dietary SM.31 Acid sphingomyelinase (aSMase) is mainly localized to the lumen of late endosomes/lysosomes, where it degrades endocytosed SM on intraluminal vesicles.32 aSMase also exists in a secreted form, which retains its functionality at neutral pH.33 The secreted aSMase is involved in the hydrolysis of SM on the plasma membrane upon stimulating cells with inflammatory cytokines.34 The release of aSMase during lysosomal exocytosis is a necessary component for resealing of wounded plasma membranes.35 The neutral sphingomyelinases (nSMase 1, 2, and 3) function at neutral pH, are stimulated by Mg2+ or Mn2+, and locate to the plasma membrane, Golgi and endosomes (nSMase2),36 nucleus (nSMase1),37 and ER (nSMase3).38 nSMase2 is the best studied of the nSMases, and its activity is stimulated by tumor necrosis factor (TNF),39,40 as well as cell stress, chemotherapeutic agents,41 and hydrogen peroxide.42 Ceramide generation by nSMase2 plays a central role in the budding of intraluminal vesicles destined for secretion as exosomes.43,44
The de novo synthesis of ceramide is a four-step reaction that takes place entirely in the ER. First, serine palmitoyltransferase (SPT) condensates palmitoyl-CoA and serine to produce 3-ketosphinganine. Second, 3-ketosphinganine is reduced to sphinganine, and N-acylation of sphinganine by ceramide synthases yields dihydroceramide. Finally, a double bond is introduced between C4 and C5 of dihydroceramide yielding ceramide. Reduced de novo sphingolipid synthesis is associated with hereditary sensory and autonomic neuropathy type I (HSAN I), an autosomal dominant inherited neurological disease characterized by loss of pain and temperature sensation and damaged autonomic functions.45–47 HSAN I patient lymphoblasts display >50% loss of SPT enzyme activity;48 in addition, knockout of either SPT1 or SPT2 in mice is embryonic lethal.49
In the salvage pathway, ceramide is formed by direct N-acylation of sphingosine in the ER and does not require dihydroceramide desaturase. The sphingosine utilized in this pathway comes from the degradation of more complex sphingolipids and more than half of the cellular sphingolipids are estimated to be products of this pathway.50
As a second messenger, a main recognized function of ceramide is mediating senescence and cell death. Ceramide is associated with both apoptotic and autophagic cell death, and several mechanistic explanations for ceramide-induced cell death have been provided. Ceramides with different acyl chain lengths can affect cell signaling differently. For instance, following B-cell receptor (BcR) triggering, C16-ceramide is the dominant species after 6 hours, while C24-ceramide becomes dominant 12–24 hours after BcR triggering. The C24-ceramide formation is required for the effector caspase activity.51 A study by Schiffmann et al52 showed that celecoxib induces the expression of C16:0-Cer by activating CerS6 and the salvage pathway, which contributes to the toxic effects of celecoxib. In addition, ceramides with different acyl chain lengths show different expression patterns. Koybasi et al53 found that, of the various ceramides, only C18:0-ceramide was selectively downregulated in head and neck cancer tissues. The endocytic trafficking of SM depends on its acyl chain length, with shorter chain length SM being preferentially recycled from early endosomal compartments, while longer chain length SM is targeted to later endosomees.54 Thus, part of the acyl chain-specific effects of ceramide may be due to different cellular trafficking of these lipids and their precursors.
Following its synthesis on the ER, ceramide can be transported via CERT to the Golgi27,55 for the synthesis of SM or ceramide 1-phosphate (C1P). CERT specifically extracts ceramide from phospholipid bilayers and can hold one ceramide at a time in its binding pocket.28 CERT is critically important for SM synthesis de novo and via the salvage pathway as loss of this transfer protein strongly reduces the production of SM. Downregulation of CERT causes a partial ceramide accumulation in the ER, induces ER stress, and sensitizes cancer cells to multiple cytotoxic agents.56 CERT is upregulated in many drug-resistant cell lines and in residual tumors following paclitaxel treatment of ovarian cancer, suggesting that targeting CERT might be beneficial particularly in chemotherapy-resistant cancers.56 Clinical investigations showed that CERT protein expression is an independent predictor of outcome in adjuvant chemotherapy-treated patients with breast cancer,57 by enhancing ER stress56 and autophagy.57
Ceramide synthesized de novo in the ER may also diffuse to the mitochondria58 and then take part in the regulation of apoptosis.59 The ER and associated mitochondrial membranes are a central location for ceramide-mediated functions. High ceramide in the ER may disrupt Ca2+ homeostasis and lead to ER stress-mediated apoptosis.60 The ER resident SM synthase-related protein protects cells from apoptosis by converting ceramide to ceramide phosphoethanolamine, effectively lowering ER ceramide content and reducing its access to mitochondria.61 At mitochondria, ceramide may induce mitochondrial outer membrane permeabilization (MOMP) and cytochrome C release.23 This may be caused by a direct channel formation by ceramide, as suggested by model membrane studies.21,25 Interestingly, the ceramide metabolites, such as S1P and hexadecenal, have been shown to drive mitochondrial cytochrome C release and apoptosis by interaction with the proapoptotic BAX and BAK,62 suggesting a complex relationship between sphingolipid metabolism and MOMP. In addition to promoting mitochondria-coordinated apoptosis, ceramide can regulate autophagy and autophagic cell death from within the plasma membrane. Ceramide downregulates the amino acid transporter CD98 on the plasma membrane, inducing autophagy and ultimately starving cells to death.63,64 Ceramide has also been shown to interact with LC3B-II, directing autophagolysosomes to mitochondria, resulting in lethal mitophagy.65
Considering its toxic effects, cancer cells are particularly efficient at converting ceramide to less-toxic sphingolipids, such as SM or glucosylceramide. In line with this, cancer cells have in general elevated aCERase expression compared to healthy cells.66,67
aCERase knockout embryos do not survive beyond the two-cell stage because of apoptotic death. However, treating aCERase−/− embryos with S1P allows them to progress to the four- to eight-cell stage, suggesting a role for aCERase in providing substrate for S1P synthesis in the early stages of mammalian development.68 Inhibition or downregulation of aCERase sensitizes cells to apoptosis,69–72 while inhibition of aSMase has the opposite effect.73,74 It has been unclear how ceramide that is generated in the intraendosomal compartment can activate cell death pathways that are mainly coordinated from the cytosolic side. We recently reported that ceramide is not inevitably degraded in late endosomes and may exit this compartment without being subject to hydrolysis by aCERase.75,76 LAPTM4B, a transmembrane protein associated with chemotherapy resistance, was found to interact with ceramide and facilitate its export from late endosomes.76 Interestingly, cells co-depleted of aCERase and LAPTM4B were resistant to chemotherapeutic treatment, despite having substantially higher total ceramide levels than cells depleted of aCERase alone. In the aCERase/LAPTM4B co-depleted cells, ceramide accumulated mainly in the late endosomal compartment, suggesting that it did not reach the ER or mitochondria where it promotes apoptosis and chemotherapy sensitivity.
Ceramide-1-Phosphate
C1P has received relatively little attention compared to the other bioactive sphingolipids. C1P is involved in inflammatory signaling and immune cell trafficking. The recently identified ceramide 1-phosphate transfer protein (CPTP) is highly conserved between species and selective for C1P, which suggests that this quantitatively minor sphingolipid has an important physiological function.
C1P in model membranes
By using a recombinant sphingomyelinase D, which transforms SM to C1P, Stock et al77 studied the effect of in situ generated C1P on model membranes. Their data showed that C1P altered the lateral structure of membranes, inducing liquid-disordered/solid-ordered domains. C1P reduces the temperature for lamellar-to-hexagonal phase transition in a mixture with phosphoethanolamine, suggesting that C1P promotes negative membrane curvature.78 Interestingly, C1P, in contrast to ceramide, was reported to not segregate into lateral lipid domains in phosphatidylcholine bilayers.79 Since the domain-forming properties of ceramide are important for receptor clustering and activation, it is conceivable that cell signaling events may be regulated by fine-tuning the relative amounts of ceramide versus C1P in membranes.
The head group of C1P has been reported to have a strong affinity for calcium ions,80 and C1P and Ca2+ both modulate the plasma membrane localization and activation of cytosolic phospholipase A2 (cPLA2).81–83 Recently, a novel CPTP (previously GLTPD1) was identified. CPTP is highly specific for C1P transfer and shows no activity toward phosphatidic acid, ceramide, or complex sphingolipids.84 In the same study, CPTP was shown to play a central role in regulating cPLA2 activity and eicosanoid signaling.
C1P synthesis, localization, and function
In mammalian cells, C1P is produced in the trans-Golgi by ceramide kinase (CERK).85,86 CERK phosphorylates ceramides with acyl chain lengths from C12 to C24 with equal efficiency in vitro.87 In cells on the other hand, ≤C20 ceramides are preferentially used for generating C1P, and downregulation of CERT efficiently reduces cellular C1P production.86 This suggests that C1P synthesis by CERK is dependent on CERT-mediated ceramide transfer and that CERT preferentially transfers shorter chain ceramides in vivo as well as in vitro.27,86,87
CERK is not essential for embryonic development as CERK−/− mice are fully viably developed without gross phenotypic changes.88 CERK-deficient mice show a significant upregulation of ceramide and downregulation of dihydroceramides in serum. The main phenotypes observed in CERK-deficient mice were defects in neutrophil homeostasis and the regulation of innate immunity. Interestingly, the cPLA2 pathways were not impaired in macrophages from CERK-deficient mice, suggesting that C1P might be compensated for by other lipid mediators. Alternatively, there may be other mechanisms generating C1P than CERK, which is suggested by the finding that C1P is present at normal levels in the brain of CERK knockout mice.88
C1P stimulates arachidonic acid release in A549 cells,89 activates cPLA2, and enhances its interaction with phosphatidylcholine.90 The C1P/cPLA2α interaction is required for the recruitment of cPLA2α to intracellular membranes in response to inflammatory agonists.81 A crucial role of proper subcellular C1P localization was shown with the discovery of the CPTP. Cells depleted of CPTP had elevated levels of C1P in the trans-Golgi and reduced levels in the plasma membrane. This alteration in C1P distribution was associated with a marked increase in the release of arachidonic acid and eicosanoid generation.84
C1P has also been shown to stimulate cell growth and DNA synthesis,91 upregulate cellular levels of proliferation cell nuclear antigen,92 and stimulate macrophage proliferation through activation of the PI3-kinase/Akt, JNK, and ERK1/2 pathways.93 C1P can inhibit aSMase in bone marrow-derived macrophages, thereby blocking caspase activation and promoting survival.94 Migration of immune cells from the circulation to the infected or damaged site is central to the inflammation response. C1P has also been shown to promote migration of macrophages in a pertussis toxin-sensitive manner, implicating the involvement of a G protein-coupled receptor (GPCR).95
Sphingosine
Sphingosine is the simplest sphingolipid and is in cells generated mainly by hydrolysis of ceramide. High levels of sphingosine associate with sensitization to apoptosis and cell senescence. There are pathological states that have been in part attributed to altered levels of sphingosine. For example, patients suffering from cystic fibrosis have lowered levels of sphingosine in the airways, which makes them more susceptible to bacterial infections,96 and lysosomal storage phenotypes may be aggravated by sphingosine.97
Sphingosine behavior in model membranes
One important feature of sphingosine is the amino group in the C2 position, which carries a net positive charge when protonated at low pH.98 As a consequence, sphingosine is membrane permeable at basic but not at acidic pH. Determination of the pKa for protonation of the amino group has yielded results ranging from 6.6 in Triton X-100 micelles99 to 9.1 in a mixture with dipalmitoylphosphatidylserine.100 The large variations in the measured pKa is likely due to differences in sphingosine hydrogen bonding in the different mixtures.99 Reported critical micelle-forming concentrations of sphingosine are in the micromolar range, and determinations vary from 1 µM to 112 µM and are highly pH dependent.99,101,102 Sphingosine can increase the permeability of both model membranes and erythrocyte ghost membranes.101 Sphingosine-induced channels in planar phospholipid membranes have an estimated diameter of <2 nm and had a short lifetime,103 whereas ceramide-induced channels that have a longer lifetime and estimated diameters of over 10 nm.103 In line with the respective membrane pore sizes they induce, it has been shown that ceramide, but not sphingosine, can induce the release of cytochrome C from isolated mitochondria.103
Sphingosine localization and trafficking in cells
Sphingosine accumulates in the acidic compartments of cells due to its free amino group, which is protonated and positively charged at acidic pH. The protonated sphingosine can act as a detergent and may contribute to the apoptosis-promoting effect of sphingosine by mediating lysosomal destabilization. This type of behavior has been demonstrated under physiological conditions, where TNFα-induced sphingosine was shown to mediate lysosomal membrane permeabilization and cell death.104 Sphingosine is also known to potently inhibit PKC by displacing the activating lipid diacylglycerol from PKC,1 which contributes to sphingosine-induced cell death.
Cells from patients with Niemann-Pick’s type C or other lysosomal storage diseases often display substantial accumulation of sphingosine in acidic compartments.97,105,106 It has been shown that sphingosine is an early accumulant in a drug-induced Niemann-Pick’s type C and that sphingosine accentuates the disease phenotype.97 The reason for this sphingosine accumulation is not entirely clear but could result from defective sphingosine export from endosomes or could be due to an expanded acidic compartment that can accommodate higher amounts of sphingosine. In favor of the latter model, we have found that NPC1-depleted cells take up a larger amount of sphingosine than control cells but do not have an impaired metabolism of sphingosine to more complex sphingolipids.75 An enlarged acidic compartment is common in lysosomal storage disorders, and the use of fluorescent probes trapped in the acidic compartment by protonation of a primary amine has been suggested as a general indicator of lysosomal storage defects.107
Sphingosine 1-Phosphate
In cells, S1P is the bioactive sphingolipid that has been studied the most, but it has been subject to relatively few studies in model membranes.
S1P in model membranes
S1P is fairly soluble in water compared to the other bioactive sphingolipids discussed here. S1P has a critical micelle-forming concentration of 12–14 µM and is likely present solubilized in the cytosol at physiologically relevant concentrations.99,108 This property of S1P may be critical for some of its described effects on soluble proteins, eg, binding to histone deacetylase (HDAC) and inhibiting its activity109 or to human telomerase reverse transcriptase causing its stabilization.110 S1P lowers the gel-to-fluid transition temperature of glycerophospholipid membranes and increases the phase transition temperature of dielaidoylphosphatidylethanolamine from lamellar to inverted hexagonal.108 S1P is present in cells in low concentrations, and its main effects are mediated via receptor activation or by interacting with proteins and modulating their function. Due to its low abundance, it is unlikely that S1P will have any major global effects on membrane integrity in cells.
S1P localization and trafficking in cells
In cells, S1P is produced by phosphorylation of sphingosine by the sphingosine kinases SK1 or SK2. S1P can also be generated by autotaxin acting on sphingosylphosphorylcholine, but the contribution of this pathway in vivo is uncertain.111 SK1 is a cytosolic protein and is recruited to the plasma membrane in response to, eg, growth stimulating factors such as epidermal growth factor (EGF) or phorbol 12-myristate 13-acetate.112,113 SK1 is associated with increased cell proliferation and stimulates the cell transition from G1- to S-phase.114 SK2, on the other hand, is mainly associated with generating S1P in the ER, and this enzyme contains a nuclear localization signal that regulates its subcellular localization dependent on PKD-mediated phosphorylation.115 Interestingly, SK2 expression causes cells to arrest in G1/S.116 The opposite effects of SK1 and SK2 on G1/S transition are dependent on enzyme localization, and targeting SK1 to the nucleus can mimic the DNA synthesis inhibitory effect of SK2.116
S1P has been shown to regulate cellular functions both via intra- and extracellular mechanisms. Extracellular S1P acts on a set of GPCRs (S1P1–S1P5) to regulate several cell processes, including cell survival and migration. Intracellular S1P, on the other hand, was initially shown to mediate calcium release from intracellular stores,117 but a target ion channel has not been unequivocally identified. The most well-defined intracellular action of S1P so far is the regulation of gene transcription by binding to HDAC and inhibiting its activity.109 Both intra- and extracellular S1P have been associated with inflammation signaling pathways. Intracellular S1P can bind directly to TRAF2 and thereby regulate NF-kappaB activition.118 NF-kappaB activation may also be induced by extracellular S1P acting on GPCRs,119,120 suggesting that S1P acts upon several stages within the same signaling cascade. In the macrophage-like cell line RAW 264.7, the SK1/S1P axis was required for inflammatory signaling induced by TNFα but not by lipipolysaccharide (LPS).121 However, the involvement of S1P signaling in inflammatory pathways may be cell type specific or context dependent since SK1/SK2 double knockout macrophages from mice display normal inflammatory response upon treatment with LPS or TNFα.122 Much effort has been made to resolve the mechanisms of how S1P reaches its site of action from the site of synthesis. Due to its charged polar head group and relatively high solubility in water, S1P is unlikely to undergo spontaneous flip-flop movement in membranes and will be restricted to the compartment in which it is synthesized in the absence of transporters. Therefore, in order to act on S1P receptors from outside the cells, S1P needs to be either generated extracellularly or exported after intracellular synthesis. Both of these modes have been observed in cells. Vascular endothelial cells constitutively secrete SK1 in large enough quantities to generate physiologically relevant levels of extracellular S1P.123,124 SK1 secretion may also be regulated, as shown in monocytic U937 cells, which export cellular SK1 to the medium upon treatment with oxidized and immunocomplexed low-density lipoprotein (LDL) particles.125 Several studies have established that intracellularly generated S1P can also be exported to the extracellular milieu and act on S1P receptors in an auto/paracrine fashion.112,119 Cells depleted of the adenosine triphosphate (ATP)-binding cassette family transporters ABCC1/multidrug resistance-associated protein 1126 and ABCA1/cholesterol efflux regulatory protein127 have defective S1P release, suggesting that these transporters are involved in the release of S1P to the extracellular milieu. Interestingly, S1P is present in the circulation in sub-micromolar concentrations128,129 mainly in complex with high-density lipo-protein (HDL) particles,130 suggesting that the S1P export may be coupled with ABCA1-dependent lipoprotein formation.127
Recently, a novel S1P transporter was identified by Kawahara et al131 from a zebrafish mutant with a cardia bifida phenotype resembling that of zebrafish lacking S1P2 receptor. The defective protein responsible for the phenotype was identified as Spns2.131 The human ortholog is a 549 aa protein with 11 predicted transmembrane helices. Spns2 does not contain an ATP-binding cassette and belongs to the major facilitator superfamily (MFS) and facilitates transmembrane movement down a chemiosmotic gradient. Spns2 activity is not strictly toward S1P but may also transport closely related molecules such as dihydro-S1P, phyto-S1P, and the S1P analog FTY720-P.132 Following the initial observation by the Kawahara et al, several publications in human cell models have confirmed the importance of Spns2 in regulating auto/paracrine S1P signaling.133–136 Mice deficient in Spns2 display early onset hearing loss and eye defects,137 highlighting the importance of S1P trafficking in a physiological setting. Interestingly, while mice deficient in Spns2 display a marked decrease in lymph S1P, there were no significant changes in plasma levels of S1P.138 Spns2-deficient red blood cells were able to phosphorylate sphingosine and secrete S1P similar to wild-type cells, suggesting that Spns2 may selectively supply S1P to lymph.138 The substrate-binding site in the MFS family of proteins is in the central pore formed by the transmembrane helices and does not have direct contact with the surrounding lipid bilayer.139 This suggests that the S1P, which is subject to transport by Spns2, is primarily the solubilized pool of S1P (estimated to be in the micromolar range108) rather than the membrane-bound pool. The ligand binding to G protein coupled S1P receptors on the other hand takes place from within the membrane. The crystal structure of type 1 S1P receptor in complex with a sphingolipid mimic suggests that the agonist-binding pocket is inaccessible from the extracellular medium and that S1P reaches the binding site from within the extracellular membrane leaflet via an opening between helices I and VII of the GPCR.140
Conclusion
Many sphingolipid metabolic pathways and their related diseases were elucidated using pure chemistry and biochemical methods in the early and middle 20th century. In the past decades, much of the research has shifted toward cell biological studies that have brought a new dimension to the field. Applying cell biology to the study of sphingolipids has its own set of challenges. Simple sphingolipids are rapidly converted into multiple metabolic products, and due to compartment-specific effects, it is critical to ensure that sphingolipid probes are targeted properly in cells. The literature contains many examples of studies that elegantly combine model membranes with cell or in vivo studies to illustrate sphingolipid behavior and function in living organisms.
Footnotes
ACADEMIC EDITOR: Tim Levine, Editor in Chief
PEER REVIEW: Two peer reviewers contributed to the peer review report. Reviewers’ reports totaled 559 words, excluding any confidential comments to the academic editor.
FUNDING: The authors are supported by the Academy of Finland, The Livoch Hälsa Foundation, University of Helsinki Research Fund Grant, and the Ruth and Nils-Erik Stenbäck Foundation. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Jointly developed the structure and wrote the paper: TB and KZ. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Hannun YA, Loomis CR, Merrill AH, Bell RM. Sphingosine inhibition of protein kinase C activity and of phorbol dibutyrate binding in vitro and in human platelets. J Biol Chem. 1986;261(27):12604–12609. [PubMed] [Google Scholar]
- 2.Goodemote KA, Mattie ME, Berger A, Spiegel S. Involvement of a pertussis toxin-sensitive G protein in the mitogenic signaling pathways of sphingosine 1-phosphate. J Biol Chem. 1995;270(17):10272–10277. doi: 10.1074/jbc.270.17.10272. [DOI] [PubMed] [Google Scholar]
- 3.Lee MJ, Van Brocklyn JR, Thangada S, et al. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279(5356):1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- 4.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4(8):604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 5.Sot J, Goñi FM, Alonso A. Molecular associations and surface-active properties of short- and long-N-acyl chain ceramides. Biochim Biophys Acta. 2005;1711(1):12–19. doi: 10.1016/j.bbamem.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Bai J, Pagano RE. Measurement of spontaneous transfer and transbilayer movement of BODIPY-labeled lipids in lipid vesicles. Biochemistry. 1997;36(29):8840–8848. doi: 10.1021/bi970145r. [DOI] [PubMed] [Google Scholar]
- 7.López-Montero I, Rodriguez N, Cribier S, Pohl A, Vélez M, Devaux PF. Rapid transbilayer movement of ceramides in phospholipid vesicles and in human erythrocytes. J Biol Chem. 2005;280(27):25811–25819. doi: 10.1074/jbc.M412052200. [DOI] [PubMed] [Google Scholar]
- 8.Silva L, de Almeida RF, Fedorov A, Matos AP, Prieto M. Ceramide-platform formation and -induced biophysical changes in a fluid phospholipid membrane. Mol Membr Biol. 2006;23(2):137–148. doi: 10.1080/09687860500439474. [DOI] [PubMed] [Google Scholar]
- 9.Holopainen JM, Lehtonen JY, Kinnunen PK. Lipid microdomains in dimyristoylphosphatidylcholine–ceramide liposomes. Chem Phys Lipids. 1997;88(1):1–13. doi: 10.1016/s0009-3084(97)00040-6. [DOI] [PubMed] [Google Scholar]
- 10.Huang HW, Goldberg EM, Zidovetzki R. Ceramide induces structural defects into phosphatidylcholine bilayers and activates phospholipase A2. Biochem Biophys Res Commun. 1996;220(3):834–838. doi: 10.1006/bbrc.1996.0490. [DOI] [PubMed] [Google Scholar]
- 11.Veiga MP, Arrondo JL, Goñi FM, Alonso A. Ceramides in phospholipid membranes: effects on bilayer stability and transition to nonlamellar phases. Biophys J. 1999;76(1 pt 1):342–350. doi: 10.1016/S0006-3495(99)77201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karttunen M, Haataja MP, Säily M, Vattulainen I, Holopainen JM. Lipid domain morphologies in phosphatidylcholine-ceramide monolayers. Langmuir. 2009;25(8):4595–4600. doi: 10.1021/la803377s. [DOI] [PubMed] [Google Scholar]
- 13.Ekman P, Maula T, Yamaguchi S, et al. Formation of an ordered phase by ceramides and diacylglycerols in a fluid phosphatidylcholine bilayer—correlation with structure and hydrogen bonding capacity. Biochim Biophys Acta. 2015;1848(10 pt A):2111–2117. doi: 10.1016/j.bbamem.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 14.Grassme H, Jekle A, Riehle A, et al. CD95 signaling via ceramide-rich membrane rafts. J Biol Chem. 2001;276(23):20589–20596. doi: 10.1074/jbc.M101207200. [DOI] [PubMed] [Google Scholar]
- 15.Grassmé H, Cremesti A, Kolesnick R, Gulbins E. Ceramide-mediated clustering is required for CD95-DISC formation. Oncogene. 2003;22(35):5457–5470. doi: 10.1038/sj.onc.1206540. [DOI] [PubMed] [Google Scholar]
- 16.Grassmé H, Bock J, Kun J, Gulbins E. Clustering of CD40 ligand is required to form a functional contact with CD40. J Biol Chem. 2002;277(33):30289–30299. doi: 10.1074/jbc.M200494200. [DOI] [PubMed] [Google Scholar]
- 17.Grassme H, Jendrossek V, Bock J, Riehle A, Gulbins E. Ceramide-rich membrane rafts mediate CD40 clustering. J Immunol. 2002;168(1):298–307. doi: 10.4049/jimmunol.168.1.298. [DOI] [PubMed] [Google Scholar]
- 18.Maula T, Artetxe I, Grandell P-M, Slotte JP. Importance of the sphingoid base length for the membrane properties of ceramides. Biophys J. 2012;103(9):1870–1879. doi: 10.1016/j.bpj.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruiz-Argüello MB, Basáñez G, Goñi FM, Alonso A. Different effects of enzyme-generated ceramides and diacylglycerols in phospholipid membrane fusion and leakage. J Biol Chem. 1996;271(43):26616–26621. doi: 10.1074/jbc.271.43.26616. [DOI] [PubMed] [Google Scholar]
- 20.Montes LR, Ruiz-Argüello MB, Goñi FM, Alonso A. Membrane restructuring via ceramide results in enhanced solute efflux. J Biol Chem. 2002;277(14):11788–11794. doi: 10.1074/jbc.M111568200. [DOI] [PubMed] [Google Scholar]
- 21.Siskind LJ, Colombini M. The lipids C2- and C16-ceramide form large stable channels. Implications for apoptosis. J Biol Chem. 2000;275(49):38640–38644. doi: 10.1074/jbc.C000587200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siskind LJ, Kolesnick RN, Colombini M. Ceramide forms channels in mitochondrial outer membranes at physiologically relevant concentrations. Mitochondrion. 2006;6(3):118–125. doi: 10.1016/j.mito.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siskind LJ, Kolesnick RN, Colombini M. Ceramide channels increase the permeability of the mitochondrial outer membrane to small proteins. J Biol Chem. 2002;277(30):26796–26803. doi: 10.1074/jbc.M200754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siskind LJ, Feinstein L, Yu T, et al. Anti-apoptotic Bcl-2 family proteins disassemble ceramide channels. J Biol Chem. 2008;283(11):6622–6630. doi: 10.1074/jbc.M706115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stiban J, Fistere D, Colombini M. Dihydroceramide hinders ceramide channel formation: implications on apoptosis. Apoptosis. 2006;11(5):773–780. doi: 10.1007/s10495-006-5882-8. [DOI] [PubMed] [Google Scholar]
- 26.Contreras F-X, Basañez G, Alonso A, Herrmann A, Goñi FM. Asymmetric addition of ceramides but not dihydroceramides promotes transbilayer (flip-flop) lipid motion in membranes. Biophys J. 2005;88(1):348–359. doi: 10.1529/biophysj.104.050690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumagai K, Yasuda S, Okemoto K, Nishijima M, Kobayashi S, Hanada K. CERT mediates intermembrane transfer of various molecular species of cerami-des. J Biol Chem. 2005;280(8):6488–6495. doi: 10.1074/jbc.M409290200. [DOI] [PubMed] [Google Scholar]
- 28.Kudo N, Kumagai K, Tomishige N, et al. Structural basis for specific lipid recognition by CERT responsible for nonvesicular trafficking of ceramide. Proc Natl Acad Sci U S A. 2008;105(2):488–493. doi: 10.1073/pnas.0709191105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartke N, Hannun YA. Bioactive sphingolipids: metabolism and function. J Lipid Res. 2009;50(suppl):S91–S96. doi: 10.1194/jlr.R800080-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gault CR, Obeid LM, Hannun YA. An overview of sphingolipid metabolism: from synthesis to breakdown. Adv Exp Med Biol. 2010;688:1–23. doi: 10.1007/978-1-4419-6741-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duan RD, Cheng Y, Hansen G, et al. Purification, localization, and expression of human intestinal alkaline sphingomyelinase. J Lipid Res. 2003;44(6):1241–1250. doi: 10.1194/jlr.M300037-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Andhoff BKS, Sandhoff K. My journey into the world of sphingolipids and sphingolipidoses. Proc Jpn Acad Ser B Phys Biol Sci. 2012;88(10):554–582. doi: 10.2183/pjab.88.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schissel SL. Secretory sphingomyelinase, a product of the acid sphingomyelinase gene, can hydrolyze atherogenic lipoproteins at neutral pH. Implications for atherosclerotic lesion development. J Biol Chem. 1998;273(5):2738–2746. doi: 10.1074/jbc.273.5.2738. [DOI] [PubMed] [Google Scholar]
- 34.Jenkins RW, Canals D, Idkowiak-Baldys J, et al. Regulated secretion of acid sphingomyelinase: implications for selectivity of ceramide formation. J Biol Chem. 2010;285(46):35706–35718. doi: 10.1074/jbc.M110.125609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tam C, Idone V, Devlin C, et al. Exocytosis of acid sphingomyelinase by wounded cells promotes endocytosis and plasma membrane repair. J Cell Biol. 2010;189(6):1027–1038. doi: 10.1083/jcb.201003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hofmann K, Tomiuk S, Wolff G, Stoffel W. Cloning and characterization of the mammalian neutral sphingomyelinase. Proc Natl Acad Sci U S A. 2000;97(11):5895–5900. doi: 10.1073/pnas.97.11.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mizutani Y, Tamiya-Koizumi K, Nakamura N, Kobayashi M, Hirabayashi Y, Yoshida S. Nuclear localization of neutral sphingomyelinase 1: biochemical and immunocytochemical analyses. J Cell Sci. 2001;114(pt 20):3727–3736. doi: 10.1242/jcs.114.20.3727. [DOI] [PubMed] [Google Scholar]
- 38.Krut O, Wiegmann K, Kashkar H, Yazdanpanah B, Krönke M. Novel tumor necrosis factor-responsive mammalian neutral sphingomyelinase-3 is a C-tail-anchored protein. J Biol Chem. 2006;281(19):13784–13793. doi: 10.1074/jbc.M511306200. [DOI] [PubMed] [Google Scholar]
- 39.Clarke CJ, Guthrie JM, Hannun YA. Regulation of neutral sphingomyelinase-2 (nSMase2) by tumor necrosis factor-alpha involves protein kinase C-delta in lung epithelial cells. Mol Pharmacol. 2008;74(4):1022–1032. doi: 10.1124/mol.108.046250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clarke CJ, Truong T-G, Hannun YA. Role for neutral sphingomyelinase-2 in tumor necrosis factor alpha-stimulated expression of vascular cell adhesion molecule-1 (VCAM) and intercellular adhesion molecule-1 (ICAM) in lung epithelial cells: p38 MAPK is an upstream regulator of nSMase2. J Biol Chem. 2007;282(2):1384–1396. doi: 10.1074/jbc.M609216200. [DOI] [PubMed] [Google Scholar]
- 41.Ito H, Murakami M, Furuhata A, et al. Transcriptional regulation of neutral sphingomyelinase 2 gene expression of a human breast cancer cell line, MCF-7, induced by the anti-cancer drug, daunorubicin. Biochim Biophys Acta. 2009;1789(11–12):681–690. doi: 10.1016/j.bbagrm.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 42.Levy M, Castillo SS, Goldkorn T. nSMase2 activation and trafficking are modulated by oxidative stress to induce apoptosis. Biochem Biophys Res Commun. 2006;344(3):900–905. doi: 10.1016/j.bbrc.2006.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trajkovic K, Hsu C, Chiantia S, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319(5867):1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 44.Guo BB, Bellingham SA, Hill AF. The neutral sphingomyelinase pathway regulates packaging of the prion protein into exosomes. J Biol Chem. 2015;290(6):3455–3467. doi: 10.1074/jbc.M114.605253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Auer-Grumbach M. Hereditary sensory neuropathy type I. Orphanet J Rare Dis. 2008;3(Hsn I):7. doi: 10.1186/1750-1172-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Houlden H, King R, Blake J, et al. Clinical, pathological and genetic characterization of hereditary sensory and autonomic neuropathy type 1 (HSAN I) Brain. 2006;129(pt 2):411–425. doi: 10.1093/brain/awh712. [DOI] [PubMed] [Google Scholar]
- 47.Dawkins JL, Hulme DJ, Brahmbhatt SB, Auer-grumbach M, Nicholson GA. Mutations in SPTLC1, encoding serine palmitoyltransferase, long chain base subunit-1, cause hereditary sensory neuropathy type I. Nat Genet. 2001;27:309–312. doi: 10.1038/85879. [DOI] [PubMed] [Google Scholar]
- 48.Bejaoui K, Wu C, Scheffler MD, et al. SPTLC1 is mutated in hereditary sensory neuropathy, type 1. Nat Genet. 2001;27(3):261–262. doi: 10.1038/85817. [DOI] [PubMed] [Google Scholar]
- 49.McCampbell A, Truong D, Broom DC, et al. Mutant SPTLC1 dominantly inhibits serine palmitoyltransferase activity in vivo and confers an age-dependent neuropathy. Hum Mol Genet. 2005;14(22):3507–3521. doi: 10.1093/hmg/ddi380. [DOI] [PubMed] [Google Scholar]
- 50.Gillard BK, Clement RG, Marcus DM. Variations among cell lines in the synthesis of sphingolipids in de novo and recycling pathways. Glycobiology. 1998;8(9):885–890. doi: 10.1093/glycob/8.9.885. [DOI] [PubMed] [Google Scholar]
- 51.Kroesen BJ, Jacobs S, Pettus BJ, et al. BcR-induced apoptosis involves differential regulation of C16 and C24-ceramide formation and sphingolipid-dependent activation of the proteasome. J Biol Chem. 2003;278(17):14723–14731. doi: 10.1074/jbc.M210756200. [DOI] [PubMed] [Google Scholar]
- 52.Schiffmann S, Ziebell S, Sandner J, et al. Activation of ceramide synthase 6 by celecoxib leads to a selective induction of C16:0-ceramide. Biochem Pharmacol. 2010;80(11):1632–1640. doi: 10.1016/j.bcp.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 53.Koybasi S, Senkal CE, Sundararaj K, et al. Defects in cell growth regulation by C18:0-ceramide and longevity assurance gene 1 in human head and neck squamous cell carcinomas. J Biol Chem. 2004;279(43):44311–44319. doi: 10.1074/jbc.M406920200. [DOI] [PubMed] [Google Scholar]
- 54.Koivusalo M, Jansen M, Somerharju P, Ikonen E. Endocytic trafficking of sphingomyelin depends on its acyl chain length. Mol Biol Cell. 2007;18(12):5113–5123. doi: 10.1091/mbc.E07-04-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanada K, Kumagai K, Yasuda S, et al. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 2003;426(6968):803–809. doi: 10.1038/nature02188. [DOI] [PubMed] [Google Scholar]
- 56.Swanton C, Marani M, Pardo O, et al. Regulators of mitotic arrest and ceramide metabolism are determinants of sensitivity to paclitaxel and other chemotherapeutic drugs. Cancer Cell. 2007;11(6):498–512. doi: 10.1016/j.ccr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 57.Lee AJ, Roylance R, Sander J, et al. CERT depletion predicts chemotherapy benefit and mediates cytotoxic and polyploid-specific cancer cell death through autophagy induction. J Pathol. 2012;226:482–494. doi: 10.1002/path.2998. [DOI] [PubMed] [Google Scholar]
- 58.Stiban J, Caputo L, Colombini M. Ceramide synthesis in the endoplasmic reticulum can permeabilize mitochondria to proapoptotic proteins. J Lipid Res. 2008;49(3):625–634. doi: 10.1194/jlr.M700480-JLR200. [DOI] [PubMed] [Google Scholar]
- 59.Siskind LJ. Mitochondral ceramide and the induction of apoptosis. J Bioenerg Biomembr. 2005;37(3):143–153. doi: 10.1007/s10863-005-6567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu Z, Xia Y, Li B, et al. Induction of ER stress-mediated apoptosis by ceramide via disruption of ER Ca 2 + homeostasis in human adenoid cystic carcinoma cells. Cell Biosci. 2014;4:71. doi: 10.1186/2045-3701-4-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tafesse FG, Vacaru AM, Bosma EF, et al. Sphingomyelin synthase-related protein SMSr is a suppressor of ceramide-induced mitochondrial apoptosis. J Cell Sci. 2014;127(pt 2):445–454. doi: 10.1242/jcs.138933. [DOI] [PubMed] [Google Scholar]
- 62.Chipuk JE, McStay GP, Bharti A, et al. Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell. 2012;148(5):988–1000. doi: 10.1016/j.cell.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee YG, Lee J, Cho JY. Cell-permeable ceramides act as novel regulators of U937 cell-cell adhesion mediated by CD29, CD98, and CD147. Immunobiology. 2010;215(4):294–303. doi: 10.1016/j.imbio.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 64.Guenther GG, Peralta ER, Rosales KR, Wong SY, Siskind LJ, Edinger AL. Ceramide starves cells to death by downregulating nutrient transporter proteins. Proc Natl Acad Sci U S A. 2008;105(45):17402–17407. doi: 10.1073/pnas.0802781105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sentelle RD, Senkal CE, Jiang W, et al. Ceramide targets autophagosomes to mitochondria and induces lethal mitophagy. Nat Chem Biol. 2012;8(10):831–838. doi: 10.1038/nchembio.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seelan RS, Qian C, Yokomizo A, Bostwick DG, Smith DI, Liu W. Human acid ceramidase is overexpressed but not mutated in prostate cancer. Genes Chromosomes Cancer. 2000;29(2):137–146. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1018>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 67.Shah MV, Zhang R, Irby R, et al. Molecular profiling of LGL leukemia reveals role of sphingolipid signaling in survival of cytotoxic lymphocytes. Blood. 2008;112(3):770–781. doi: 10.1182/blood-2007-11-121871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eliyahu E, Park J-H, Shtraizent N, He X, Schuchman EH. Acid ceramidase is a novel factor required for early embryo survival. FASEB J. 2007;21(7):1403–1409. doi: 10.1096/fj.06-7016com. [DOI] [PubMed] [Google Scholar]
- 69.Liu X, Elojeimy S, El-Zawahry AM, et al. Modulation of ceramide metabolism enhances viral protein apoptin’s cytotoxicity in prostate cancer. Mol Ther. 2006;14(5):637–646. doi: 10.1016/j.ymthe.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 70.Holman DH, Turner LS, El-Zawahry A, et al. Lysosomotropic acid ceramidase inhibitor induces apoptosis in prostate cancer cells. Cancer Chemother Pharmacol. 2008;61(2):231–242. doi: 10.1007/s00280-007-0465-0. [DOI] [PubMed] [Google Scholar]
- 71.Bedia C, Casas J, Andrieu-Abadie N, Fabriàs G, Levade T. Acid ceramidase expression modulates the sensitivity of A375 melanoma cells to dacarbazine. J Biol Chem. 2011;286(32):28200–28209. doi: 10.1074/jbc.M110.216382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morales A, París R, Villanueva A, Llacuna L, García-Ruiz C, Fernández-Checa JC. Pharmacological inhibition or small interfering RNA targeting acid ceramidase sensitizes hepatoma cells to chemotherapy and reduces tumor growth in vivo. Oncogene. 2007;26(6):905–916. doi: 10.1038/sj.onc.1209834. [DOI] [PubMed] [Google Scholar]
- 73.Jun G, Canquan Z, Renli Z. RNA interference targeted on SMPD1 protect human granulose cells from apoptosis. Fertil Steril. 2007;88:S337. [Google Scholar]
- 74.Claus RA, Bunck AC, Bockmeyer CL, et al. Role of increased sphingomyelinase activity in apoptosis and organ failure of patients with severe sepsis. FASEB J. 2005;19(12):1719–1721. doi: 10.1096/fj.04-2842fje. [DOI] [PubMed] [Google Scholar]
- 75.Blom T, Li Z, Bittman R, Somerharju P, Ikonen E. Tracking sphingosine metabolism and transport in sphingolipidoses: NPC1 deficiency as a test case. Traffic. 2012;13(9):1234–1243. doi: 10.1111/j.1600-0854.2012.01379.x. [DOI] [PubMed] [Google Scholar]
- 76.Blom T, Li S, Dichlberger A, et al. LAPTM4B facilitates late endosomal ceramide export to control cell death pathways. Nat Chem Biol. 2015;11(10):799–806. doi: 10.1038/nchembio.1889. [DOI] [PubMed] [Google Scholar]
- 77.Stock RP, Brewer J, Wagner K, et al. Sphingomyelinase D activity in model membranes: structural effects of in situ generation of ceramide-1-phosphate. PLoS One. 2012;7(4):e36003. doi: 10.1371/journal.pone.0036003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kooijman EE, Sot J, Montes LR, et al. Membrane organization and ionization behavior of the minor but crucial lipid ceramide-1-phosphate. Biophys J. 2008;94(11):4320–4330. doi: 10.1529/biophysj.107.121046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morrow MR, Helle A, Perry J, Vattulainen I, Wiedmer SK, Holopainen JM. Ceramide-1-phosphate, in contrast to ceramide, is not segregated into lateral lipid domains in phosphatidylcholine bilayers. Biophys J. 2009;96(6):2216–2226. doi: 10.1016/j.bpj.2008.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kooijman EE, Vaknin D, Bu W, et al. Structure of ceramide-1-phosphate at the air-water solution interface in the absence and presence of Ca2+ Biophys J. 2009;96(6):2204–2215. doi: 10.1016/j.bpj.2008.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lamour NF, Subramanian P, Wijesinghe DS, Stahelin RV, Bonventre JV, Chalfant CE. Ceramide 1-phosphate is required for the translocation of group IVA cytosolic phospholipase A2 and prostaglandin synthesis. J Biol Chem. 2009;284(39):26897–26907. doi: 10.1074/jbc.M109.001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stahelin RV, Subramanian P, Vora M, Cho W, Chalfant CE. Ceramide-1-phosphate binds group IVA cytosolic phospholipase a2 via a novel site in the C2 domain. J Biol Chem. 2007;282(28):20467–20474. doi: 10.1074/jbc.M701396200. [DOI] [PubMed] [Google Scholar]
- 83.Ward KE, Bhardwaj N, Vora M, Chalfant CE, Lu H, Stahelin RV. The molecular basis of ceramide-1-phosphate recognition by C2 domains. J Lipid Res. 2013;54(3):636–648. doi: 10.1194/jlr.M031088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Simanshu DK, Kamlekar RK, Wijesinghe DS, et al. Non-vesicular trafficking by a ceramide-1-phosphate transfer protein regulates eicosanoids. Nature. 2013;500(7463):463–467. doi: 10.1038/nature12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sugiura M, Kono K, Liu H, et al. Ceramide kinase, a novel lipid kinase molecular cloning and functional characterization. J Biol Chem. 2002;277(26):23294–23300. doi: 10.1074/jbc.M201535200. [DOI] [PubMed] [Google Scholar]
- 86.Lamour NF, Stahelin RV, Wijesinghe DS, et al. Ceramide kinase uses ceramide provided by ceramide transport protein: localization to organelles of eicosanoid synthesis. J Lipid Res. 2007;48(6):1293–1304. doi: 10.1194/jlr.M700083-JLR200. [DOI] [PubMed] [Google Scholar]
- 87.Wijesinghe DS, Massiello A, Subramanian P, Szulc Z, Bielawska A, Chalfant CE. Substrate specificity of human ceramide kinase. J Lipid Res. 2005;46(12):2706–2716. doi: 10.1194/jlr.M500313-JLR200. [DOI] [PubMed] [Google Scholar]
- 88.Graf C, Zemann B, Rovina P, et al. Neutropenia with impaired immune response to Streptococcus pneumoniae in ceramide kinase-deficient mice. J Immunol. 2008;180(5):3457–3466. doi: 10.4049/jimmunol.180.5.3457. [DOI] [PubMed] [Google Scholar]
- 89.Pettus BJ, Bielawska A, Spiegel S, Roddy P, Hannun YA, Chalfant CE. Ceramide kinase mediates cytokine- and calcium ionophore-induced arachidonic acid release. J Biol Chem. 2003;278(40):38206–38213. doi: 10.1074/jbc.M304816200. [DOI] [PubMed] [Google Scholar]
- 90.Subramanian P, Stahelin RV, Szulc Z, Bielawska A, Cho W, Chalfant CE. Ceramide 1-phosphate acts as a positive allosteric activator of group IVA cytosolic phospholipase A2 alpha and enhances the interaction of the enzyme with phosphatidylcholine. J Biol Chem. 2005;280(18):17601–17607. doi: 10.1074/jbc.M414173200. [DOI] [PubMed] [Google Scholar]
- 91.Gangoiti P, Bernacchioni C, Donati C, et al. Ceramide 1-phosphate stimulates proliferation of C2C12 myoblasts. Biochimie. 2012;94(3):597–607. doi: 10.1016/j.biochi.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gomez-Muñoz A, Frago LM, Alvarez L, Varela-Nieto I. Stimulation of DNA synthesis by natural ceramide 1-phosphate. Biochem J. 1997;325(pt 2):435–440. doi: 10.1042/bj3250435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gangoiti P, Granado MH, Wang SW, Kong JY, Steinbrecher UP, Gómez-Muñoz A. Ceramide 1-phosphate stimulates macrophage proliferation through activation of the PI3-kinase/PKB, JNK and ERK1/2 pathways. Cell Signal. 2008;20(4):726–736. doi: 10.1016/j.cellsig.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 94.Gómez-Muñoz A, Kong JY, Salh B, Steinbrecher UP. Ceramide-1-phosphate blocks apoptosis through inhibition of acid sphingomyelinase in macrophages. J Lipid Res. 2004;45(1):99–105. doi: 10.1194/jlr.M300158-JLR200. [DOI] [PubMed] [Google Scholar]
- 95.Granado MH, Gangoiti P, Ouro A, et al. Ceramide 1-phosphate (C1P) promotes cell migration involvement of a specific C1P receptor. Cell Signal. 2009;21(3):405–412. doi: 10.1016/j.cellsig.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 96.Pewzner-Jung Y, Tavakoli Tabazavareh S, Grassmé H, et al. Sphingoid long chain bases prevent lung infection by Pseudomonas aeruginosa. EMBO Mol Med. 2014;6(9):1205–1214. doi: 10.15252/emmm.201404075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lloyd-Evans E, Morgan AJ, He X, et al. Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat Med. 2008;14(11):1247–1255. doi: 10.1038/nm.1876. [DOI] [PubMed] [Google Scholar]
- 98.Kågedal K, Zhao M, Svensson I, Brunk UT. Sphingosine-induced apoptosis is dependent on lysosomal proteases. Biochem J. 2001;359(pt 2):335–343. doi: 10.1042/0264-6021:3590335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sasaki H, Arai H, Cocco MJ, White SH. pH dependence of sphingosine aggregation. Biophys J. 2009;96(7):2727–2733. doi: 10.1016/j.bpj.2008.12.3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.López-García F, Micol V, Villalaín J, Gómez-Fernández JC. Interaction of sphingosine and stearylamine with phosphatidylserine as studied by DSC and NMR. Biochim Biophys Acta. 1993;1153(1):1–8. doi: 10.1016/0005-2736(93)90269-6. [DOI] [PubMed] [Google Scholar]
- 101.Contreras F-X, Sot J, Alonso A, Goñi FM. Sphingosine increases the permeability of model and cell membranes. Biophys J. 2006;90(11):4085–4092. doi: 10.1529/biophysj.105.076471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Deguchi H, Yegneswaran S, Griffin JH. Sphingolipids as bioactive regulators of thrombin generation. J Biol Chem. 2004;279(13):12036–12042. doi: 10.1074/jbc.M302531200. [DOI] [PubMed] [Google Scholar]
- 103.Siskind LJ, Fluss S, Bui M, Colombini M. Sphingosine forms channels in membranes that differ greatly from those formed by ceramide. J Bioenerg Biomembr. 2005;37(4):227–236. doi: 10.1007/s10863-005-6632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ullio C, Casas J, Brunk UT, et al. Sphingosine mediates TNFα-induced lysosomal membrane permeabilization and ensuing programmed cell death in hepatoma cells. J Lipid Res. 2012;53(6):1134–1143. doi: 10.1194/jlr.M022384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lloyd-Evans E, Platt FM. Lipids on trial: the search for the offending metabolite in Niemann-Pick type C disease. Traffic. 2010;11(4):419–428. doi: 10.1111/j.1600-0854.2010.01032.x. [DOI] [PubMed] [Google Scholar]
- 106.Rodriguez-Lafrasse C, Rousson R, Pentchev PG, Louisot P, Vanier MT. Free sphingoid bases in tissues from patients with type C Niemann-Pick disease and other lysosomal storage disorders. Biochim Biophys Acta. 1994;1226(2):138–144. doi: 10.1016/0925-4439(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 107.te Vruchte D, Speak AO, Wallom KL, et al. Relative acidic compartment volume as a lysosomal storage disorder-associated biomarker. J Clin Invest. 2014;124(3):1320–1328. doi: 10.1172/JCI72835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.García-Pacios M, Collado MI, Busto JV, et al. Sphingosine-1-phosphate as an amphipathic metabolite: its properties in aqueous and membrane environments. Biophys J. 2009;97(5):1398–1407. doi: 10.1016/j.bpj.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hait NC, Allegood J, Maceyka M, et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325(5945):1254–1257. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Panneer Selvam S, De Palma RM, Oaks JJ, et al. Binding of the sphingolipid S1P to hTERT stabilizes telomerase at the nuclear periphery by allosterically mimicking protein phosphorylation. Sci Signal. 2015;8(381):ra58. doi: 10.1126/scisignal.aaa4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ohkawa R, Kurano M, Mishima Y, et al. Possible involvement of sphingomyelin in the regulation of the plasma sphingosine 1-phosphate level in human subjects. Clin Biochem. 2015;48(10–11):690–697. doi: 10.1016/j.clinbiochem.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 112.Johnson KR, Becker KP, Facchinetti MM, Hannun YA, Obeid LM. PKC-dependent activation of sphingosine kinase 1 and translocation to the plasma membrane. Extracellular release of sphingosine-1-phosphate induced by phorbol 12-myristate 13-acetate (PMA) J Biol Chem. 2002;277(38):35257–35262. doi: 10.1074/jbc.M203033200. [DOI] [PubMed] [Google Scholar]
- 113.Paugh BS, Paugh SW, Bryan L, et al. EGF regulates plasminogen activator inhibitor-1 (PAI-1) by a pathway involving c-Src, PKCdelta, and sphingosine kinase 1 in glioblastoma cells. FASEB J. 2008;22(2):455–465. doi: 10.1096/fj.07-8276com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Olivera A. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J Cell Biol. 1999;147(3):545–558. doi: 10.1083/jcb.147.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ding G, Sonoda H, Yu H, et al. Protein kinase D-mediated phosphorylation and nuclear export of sphingosine kinase 2. J Biol Chem. 2007;282(37):27493–27502. doi: 10.1074/jbc.M701641200. [DOI] [PubMed] [Google Scholar]
- 116.Igarashi N, Okada T, Hayashi S, Fujita T, Jahangeer S, Nakamura S. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J Biol Chem. 2003;278(47):46832–46839. doi: 10.1074/jbc.M306577200. [DOI] [PubMed] [Google Scholar]
- 117.Ghosh TK, Bian J, Gill DL. Intracellular calcium release mediated by sphingosine derivatives generated in cells. Science. 1990;248(4963):1653–1656. doi: 10.1126/science.2163543. [DOI] [PubMed] [Google Scholar]
- 118.Alvarez SE, Harikumar KB, Hait NC, et al. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465(7301):1084–1088. doi: 10.1038/nature09128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Blom T, Bergelin N, Meinander A, et al. An autocrine sphingosine-1-phosphate signaling loop enhances NF-kappaB-activation and survival. BMC Cell Biol. 2010;11(1):45. doi: 10.1186/1471-2121-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Siehler S, Wang Y, Fan X, Windh RT, Manning DR. Sphingosine 1-phosphate activates nuclear factor-kappa B through Edg receptors. Activation through Edg-3 and Edg-5, but not Edg-1, in human embryonic kidney 293 cells. J Biol Chem. 2001;276(52):48733–48739. doi: 10.1074/jbc.M011072200. [DOI] [PubMed] [Google Scholar]
- 121.Hammad SM, Crellin HG, Wu BX, Melton J, Anelli V, Obeid LM. Dual and distinct roles for sphingosine kinase 1 and sphingosine 1 phosphate in the response to inflammatory stimuli in RAW macrophages. Prostaglandins Other Lipid Mediat. 2008;85(3–4):107–114. doi: 10.1016/j.prostaglandins.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xiong Y, Lee HJ, Mariko B, et al. Sphingosine kinases are not required for inflammatory responses in macrophages. J Biol Chem. 2013;288(45):32563–32573. doi: 10.1074/jbc.M113.483750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Venkataraman K, Thangada S, Michaud J, et al. Extracellular export of sphingosine kinase-1a contributes to the vascular S1P gradient. Biochem J. 2006;397(3):461–471. doi: 10.1042/BJ20060251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ancellin N, Colmont C, Su J, et al. Extracellular export of sphingosine kinase-1 enzyme. Sphingosine 1-phosphate generation and the induction of angiogenic vascular maturation. J Biol Chem. 2002;277(8):6667–6675. doi: 10.1074/jbc.M102841200. [DOI] [PubMed] [Google Scholar]
- 125.Hammad SM, Taha TA, Nareika A, Johnson KR, Lopes-Virella MF, Obeid LM. Oxidized LDL immune complexes induce release of sphingosine kinase in human U937 monocytic cells. Prostaglandins Other Lipid Mediat. 2006;79(1–2):126–140. doi: 10.1016/j.prostaglandins.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 126.Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci U S A. 2006;103(44):16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sato K, Malchinkhuu E, Horiuchi Y, et al. Critical role of ABCA1 transporter in sphingosine 1-phosphate release from astrocytes. J Neurochem. 2007;103(6):2610–2619. doi: 10.1111/j.1471-4159.2007.04958.x. [DOI] [PubMed] [Google Scholar]
- 128.Murata N, Sato K, Kon J, Tomura H, Okajima F. Quantitative measurement of sphingosine 1-phosphate by radioreceptor-binding assay. Anal Biochem. 2000;282(1):115–120. doi: 10.1006/abio.2000.4580. [DOI] [PubMed] [Google Scholar]
- 129.Yatomi Y, Igarashi Y, Yang L, et al. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J Biochem. 1997;121(5):969–973. doi: 10.1093/oxfordjournals.jbchem.a021681. [DOI] [PubMed] [Google Scholar]
- 130.Murata N, Sato K, Kon J, et al. Interaction of sphingosine 1-phosphate with plasma components, including lipoproteins, regulates the lipid receptor-mediated actions. Biochem J. 2000;352(pt 3):809–815. [PMC free article] [PubMed] [Google Scholar]
- 131.Kawahara A, Nishi T, Hisano Y, Fukui H, Yamaguchi A, Mochizuki N. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science. 2009;323(5913):524–527. doi: 10.1126/science.1167449. [DOI] [PubMed] [Google Scholar]
- 132.Hisano Y, Kobayashi N, Kawahara A, Yamaguchi A, Nishi T. The sphingosine 1-phosphate transporter, SPNS2, functions as a transporter of the phosphorylated form of the immunomodulating agent FTY720. J Biol Chem. 2011;286(3):1758–1766. doi: 10.1074/jbc.M110.171116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Adada MM, Canals D, Jeong N, et al. Intracellular sphingosine kinase 2-derived sphingosine-1-phosphate mediates epidermal growth factor-induced ezrinradixin-moesin phosphorylation and cancer cell invasion. FASEB J. 2015;29(11):4654–4669. doi: 10.1096/fj.15-274340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Laurenzana A, Cencetti F, Serratì S, et al. Endothelial sphingosine kinase/SPNS2 axis is critical for vessel-like formation by human mesoangioblasts. J Mol Med (Berl) 2015;93(10):1145–1157. doi: 10.1007/s00109-015-1292-0. [DOI] [PubMed] [Google Scholar]
- 135.Degagné E, Pandurangan A, Bandhuvula P, et al. Sphingosine-1-phosphate lyase downregulation promotes colon carcinogenesis through STAT3-activated microRNAs. J Clin Invest. 2014;124(12):5368–5384. doi: 10.1172/JCI74188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bradley E, Dasgupta S, Jiang X, et al. Critical role of Spns2, a sphingosine-1-phosphate transporter, in lung cancer cell survival and migration. PLoS One. 2014;9(10):e110119. doi: 10.1371/journal.pone.0110119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen J, Ingham N, Kelly J, et al. Spinster homolog 2 (spns2) deficiency causes early onset progressive hearing loss. PLoS Genet. 2014;10(10):e1004688. doi: 10.1371/journal.pgen.1004688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mendoza A, Bréart B, Ramos-Perez WD, et al. The transporter Spns2 is required for secretion of lymph but not plasma sphingosine-1-phosphate. Cell Rep. 2012;2(5):1104–1110. doi: 10.1016/j.celrep.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Huang Y, Lemieux MJ, Song J, Auer M, Wang DN. Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science. 2003;301(5633):616–620. doi: 10.1126/science.1087619. [DOI] [PubMed] [Google Scholar]
- 140.Hanson MA, Roth CB, Jo E, et al. Crystal structure of a lipid G protein-coupled receptor. Science. 2012;335(6070):851–855. doi: 10.1126/science.1215904. [DOI] [PMC free article] [PubMed] [Google Scholar]