Abstract
Aims
Growth differentiation factor 11 and/or its homologue growth differentiation factor 8 (GDF11/8) reverses age-related cardiac hypertrophy and vascular ageing in mice. We investigated whether GDF11/8 associates with cardiovascular outcomes, left ventricular hypertrophy (LVH), or age in humans.
Methods and results
We measured plasma GDF11/8 levels in 928 participants with stable ischaemic heart disease in the Heart and Soul study. We adjudicated heart failure hospitalization, stroke, myocardial infarction, death, and their composite endpoint. Left ventricular hypertrophy was evaluated by echocardiography. We used multivariable Cox proportional hazards models to compare rates of cardiovascular events and death across GDF11/8 quartiles and logistic regression models to evaluate the association between GDF11/8 and LVH. Four hundred and fifty participants (48.5%) experienced a cardiovascular event or death during 8.9 years of follow-up. The adjusted risk of the composite endpoint was lower in the highest compared with the lowest GDF11/8 quartile [hazard ratio (HR), 0.45; 95% confidence interval (CI), 0.33–0.60; P < 0.001]. We replicated this relationship of GDF11/8 to adverse events in 971 participants in the HUNT3 cohort (adjusted HR, 0.34; 95% CI, 0.23–0.51; P < 0.001). Left ventricular hypertrophy was present in 368 participants (39.7%) at baseline. Participants in the highest quartile of GDF11/8 were less likely to have LVH than those in the lowest quartile (adjusted OR, 0.55; 95% CI, 0.35–0.86; P = 0.009). GDF11/8 levels were lower in older individuals (P < 0.001).
Conclusion
In patients with stable ischaemic heart disease, higher GDF11/8 levels are associated with lower risk of cardiovascular events and death. Our findings suggest that GDF11/8 has similar cardioprotective properties in humans to those demonstrated in mice.
Keywords: Growth differentiation factor 11 and 8, Ageing, Cardiovascular outcomes, Epidemiology, Hypertrophy
See page 3435 for the editorial comment on this article (doi:10.1093/eurheartj/ehv412)
Introduction
Growth differentiation factor 11 (GDF11), a circulating TGF-β superfamily member, is a recently discovered anti-ageing factor in mice.1–3 Its age-related decline has been reported to lead to ageing of the heart, brain, and skeletal muscle in mice.1–3 Administration of recombinant GDF11 to aged mice that restores circulating GDF11 to its youthful levels reverses age-related changes in cardiovascular structure and function; it reverses age-related cardiac hypertrophy,2 and favourably affects vascular remodelling and blood flow.1 To date, GDF11 has not been investigated in humans. Specifically, in humans no evidence links circulating levels of GDF11 to the risk of cardiovascular events, all-cause death, cardiac hypertrophy, or to advancing age.
Accordingly, we aimed to characterize the association of GDF11 with (i) cardiovascular events and overall deaths, (ii) left ventricular hypertrophy (LVH), and (iii) age, using data from the San Francisco-based Heart and Soul study, a prospective cohort of participants with stable coronary heart disease (CHD).4,5 We hypothesized that lower levels of GDF11 are associated with higher rates of cardiovascular events, overall deaths, and LVH and that circulating GDF11 levels are lower in older individuals. We replicated the key findings from the Heart and Soul study in a European (Norwegian) cohort of subjects with stable CHD from the HUNT3 study.6
Myostatin (GDF8) is a close structural homologue of GDF11, with 90% amino acid sequence identity shared in their mature active forms.7 Growth differentiation factor 11 and 8 are also functionally redundant as they share the same activin type II receptors and activate the SMAD2/3, p38, and ERK pathways to a similar degree.8 Most assays, including ours and those in published studies,1–3 do not distinguish between these homologous proteins8,9 and we will thus refer to the protein as GDF11/8.
Methods
Study population
The Heart and Soul study is a prospective cohort study originally designed to investigate psychosocial factors and health outcomes in patients with stable CHD. Recruitment methods and study design have been published.4 Briefly, between September 2000 and December 2002, we recruited 1024 outpatients with stable CHD from 2 Veterans Administration Medical Centers (Palo Alto and San Francisco), 1 university medical center (University of California, San Francisco), and 9 public health clinics in the Community Health Network of San Francisco. Eligible participants met one or more of the following criteria: (i) history of myocardial infarction (MI); (ii) evidence of at least 50% stenosis in one or more coronary arteries on cardiac catheterization; (iii) evidence of exercise-induced ischaemia by treadmill electrocardiogram or nuclear perfusion stress imaging; or (iv) a history of coronary revascularization. We excluded individuals with a history of MI in the previous 6 months, inability to walk one block, or planning to move out of the local area within 3 years.
All participants completed a baseline examination that included an interview, fasting blood draw, questionnaire, echocardiogram, and exercise treadmill test. Of the 1024 original study participants, 85 were excluded because of missing or inadequate blood specimens and 11 were excluded due to missing echocardiographic data. The remaining 928 participants are the analytic cohort for this study. This study complies with the Declaration of Helsinki. The institutional review board approved this protocol, and all participants provided written, informed consent.
Baseline measurements
Before the study appointment, participants fasted overnight except for taking their prescribed medications. A 21-gauge butterfly needle was inserted intravenously in the forearm after a 30-min supine rest, and blood samples were drawn into chilled EDTA tubes, divided into aliquots and stored at −70°C. We measured plasma GDF11/8 levels using a modified aptamer-based proteomic platform (SOMAscan™, SomaLogic, Inc., Boulder, CO).9 The lower limit of quantitation of this assay is 6.5 pg/mL and intra-assay and inter-assay CV is <6%.
Participants underwent transthoracic echocardiography at rest during baseline examination. Details of echocardiographic methods in the Heart and Soul cohort have been published.5,10 Left ventricular mass was derived from wall-thickness measurements using a truncated ellipsoid technique and normalized to body surface area to calculate left ventricular mass index (LVMI). We defined LVH as LVMI > 88 g/m2 in women and >102 g/m2 in men per American Society of Echocardiography/European Association of Cardiovascular Imaging guidelines.11 Left ventricular ejection fraction (LVEF) was calculated as end-diastolic volume minus end-systolic volume divided by end-diastolic volume. Patterns of LV diastolic dysfunction were based on mitral inflow E/A ratios of peak velocities at early rapid filling (E) and late filling due to atrial contraction (A) and systolic or LV diastolic dominant pulmonary venous flow using velocity time integral based on previously published criteria.12 A single experienced cardiologist (N.B.S.) who was blinded to all other clinical information interpreted the echocardiograms.5,10 Reliability of LVMI measurements is presented in Supplementary material online.
Age, sex, race, medical history, and smoking status were determined by questionnaire. We measured height and weight and calculated body mass index (kg/m2). The New York Heart Association (NYHA) functional classification was assessed as a self-reported limitation of physical activity due to cardiovascular symptoms (fatigue, shortness of breath, or chest pain).
We measured serum creatinine, cystatin C, total cholesterol, and high-density lipoprotein from fasting blood samples drawn at the baseline study appointment. Estimated glomerular filtration rate (eGFR) was calculated using the CKD Epi equation.13
Follow-up
We conducted annual follow-up interviews with participants (or their proxy) to inquire about death or interval hospitalization for ‘heart trouble’. For any reported event, two independent and blinded physician adjudicators reviewed medical records, electrocardiograms, death certificates, and coroner's reports. If the adjudicators agreed on the outcome classification, their classification was binding. In the event of a disagreement, a third blinded adjudicator was consulted.
We defined heart failure (HF) as hospitalization for a clinical syndrome based on the Framingham congestive HF criteria.14 Stroke was defined as a new neurological deficit not known to be secondary to brain trauma, tumour, infection, or other cause. Myocardial infarction was defined using standard diagnostic criteria.15 Death and cause of death were determined through review of death certificates, medical records, and coroner's reports. Cardiovascular death was defined as (i) death during the same hospitalization in which an acute MI was documented or (ii) death not explained by other causes and that occurred within 1 h of the onset of terminal symptoms.
HUNT3 cohort
We replicated the association of GDF11/8 with cardiovascular events discovered in the Heart and Soul study in 971 individuals from the European HUNT3 cohort. The HUNT cohort is a population-based cohort for health-related research in the Nord-Trøndelag County, Norway.6 A detailed description of the HUNT3 cohort can be found in the Supplementary material online.
Statistical analysis
GDF11/8 levels were normally distributed. We divided participants into quartiles by plasma GDF11/8 levels. We compared baseline characteristics by quartile of GDF11/8 level using χ2 tests for categorical characteristics and analysis of variance for continuous characteristics.
Multivariable Cox proportional hazards models were used to compare the rates of HF hospitalization, stroke, MI, death, and the composite of HF, stroke, MI, or death across quartiles of GDF11/8 level. Models were adjusted for age, sex, race, hypertension, diabetes mellitus, history of prior MI, LVEF, eGFR, and medication (β-blocker, statin, and ACE-inhibitor or ARB) use. We also evaluated the association between GDF11/8 as a continuous variable and cardiovascular events. Proportional hazards assumptions and the absence of co-linearity were verified for all models. No evidence of departure from linearity was present in the continuous GDF11/8 model. We evaluated for interactions by age, sex, race, the presence of LVH, LVEF < 50%, and eGFR < 60 mL/min. Supplementary material online contains details of C-statistic and net reclassification analysis.
For replication of Heart and Soul findings in the HUNT3 cohort, the same analysis strategy was used. Models were adjusted for age, sex, hypertension, diabetes, history of MI, and eGFR. Models were not adjusted for race because the HUNT3 cohort is racially homogenous. Models were not adjusted for ejection fraction or medication use because this data was not available in the HUNT3 cohort.
Logistic regression models were used to estimate the association between GDF11/8 and LVH in the Heart and Soul cohort. Models were adjusted for age, sex, race, hypertension, diabetes mellitus, history of prior MI, and LVEF. Analysis of variance was used to compare GDF11/8 levels across age strata.
Analyses were performed using Stata versions 10.0 and 12.0 (StataCorp, College Station, TX). All reported P-values are two-sided, with a P-value of <0.05 considered to indicate statistical significance.
Results
Sample characteristics
The Heart and Soul cohort was 82.2% male and 60.2% white (non-white = black: 16.5%, Hispanic: 8.5%, Asian: 11.5%, other: 3.2%), with a mean age of 66.9 years. Compared with participants in the lowest quartile, those in the highest quartile of GDF11/8 were younger, healthier, more likely male, and less likely white (Table 1). They were less likely to have a history of MI or HF. They were less likely to have impaired left ventricular relaxation or NYHA Functional Class III or IV HF symptoms. They had better renal function.
Table 1.
Baseline participant characteristics by quartile of GDF11/8a in the Heart and Soul study
| GDF11/8 quartiles |
P-Valueb | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Range in quartile (units) | 394–793c | 796–932 | 932–1106 | 1108–3333 | |
| n | 232 | 232 | 232 | 232 | |
| Demographic factors | |||||
| Age, mean (SD) | 68.9 (11.7) | 68.2 (10.7) | 65.4 (10.1) | 65.0 (10.4) | <0.001 |
| Male sex | 166 (71.6) | 193 (83.2) | 200 (86.2) | 204 (87.9) | <0.001 |
| White race | 181 (78.0) | 156 (67.2) | 126 (54.3) | 95 (41.0) | <0.001 |
| Clinical history | |||||
| Hypertension | 169 (72.8) | 166 (71.6) | 163 (70.3) | 160 (69.6) | 0.87 |
| Revascularization | 139 (59.9) | 143 (61.6) | 132 (56.9) | 134 (58.3) | 0.75 |
| Diabetes | 62 (26.8) | 57 (24.6) | 65 (28.0) | 60 (26.0) | 0.86 |
| History of MI | 137 (59.3) | 132 (57.1) | 123 (53.0) | 105 (45.9) | 0.02 |
| History of HF | 54 (23.5) | 44 (19.0) | 36 (15.5) | 27 (11.8) | 0.008 |
| Stroke | 42 (18.1) | 29 (12.5) | 34 (14.7) | 24 (10.5) | 0.11 |
| BMI, mean (SD) | 28.1 (5.5) | 28.1 (5.4) | 29.0 (5.4) | 28.3 (4.7) | 0.21 |
| Current smoking | 53 (22.8) | 44 (19.0) | 43 (18.5) | 43 (18.7) | 0.60 |
| NYHA Class III or IV | 61 (26.4) | 56 (24.1) | 48 (20.7) | 37 (16.0) | 0.04 |
| Medication use | |||||
| Aspirin | 164 (70.7) | 168 (72.7) | 183 (80.3) | 168 (74.0) | 0.11 |
| Statin | 136 (58.6) | 156 (67.5) | 155 (68.0) | 151 (66.5) | 0.12 |
| ACE-inhibitor/ARB | 130 (56.0) | 110 (47.6) | 113 (49.6) | 123 (54.2) | 0.24 |
| β-blockers | 124 (53.5) | 135 (58.4) | 144 (63.2) | 137 (60.4) | 0.19 |
| Laboratory data | |||||
| Cholesterol, mean (SD) (mg/dL) | |||||
| Total | 179 (46) | 178 (41) | 180 (42) | 174 (39) | 0.54 |
| HDL | 47 (17) | 45 (14) | 45 (14) | 45 (12) | 0.28 |
| GFR, mean (SD) (mL/min) | 64 (22) | 70 (22) | 74 (22) | 75 (21) | <0.001 |
| Cardiac function | |||||
| LVEF, mean (SD) (%) | 60 (10) | 62 (10) | 62 (9) | 62 (9) | 0.09 |
| Diastolic function | 0.001 | ||||
| Normal | 108 (52.4) | 124 (58.0) | 137 (66.5) | 144 (72.0) | – |
| Impaired relaxation | 66 (32.0) | 65 (30.4) | 51 (24.8) | 32 (16.0) | – |
| Pseudonormal | 20 (9.7) | 16 (7.5) | 6 (2.9) | 13 (6.5) | – |
| Restrictive | 12 (5.8) | 9 (4.2) | 12 (5.8) | 11 (5.5) | – |
aValues expressed as number (percentage) unless otherwise indicated.
bSignificance tests for comparisons by quartile of GDF11/8 based on analysis of variance for continuous participant characteristics and Pearson's χ2 test for categorical participant characteristics.
cUnits = relative fluorescence units.
Cardiovascular outcomes and death
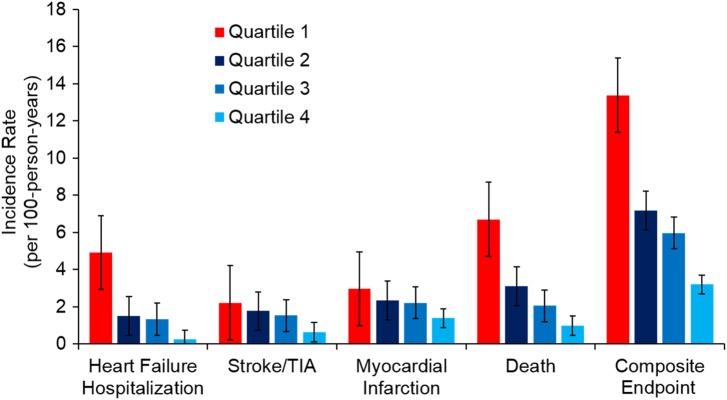
After a median follow-up of 8.9 years, 367 participants (39.6%) died, 166 participants (17.9%) were hospitalized for HF, 45 participants (4.9%) experienced a stroke, 128 participants (13.8%) suffered an MI, and 450 participants (48.5%) experienced any cardiovascular event or death (Figure 1 and Supplementary material online, Table S1).
Figure 1.
Incidence of heart failure hospitalization, stroke, myocardial infarction, death, and composite endpoint in Heart and Soul cohort, unadjusted, stratified by quartile of GDF11/8. P-Values for trend are <0.001 for heart failure, death, and composite endpoint, 0.18 for stroke, and 0.004 for myocardial infarction.
When participants in the highest quartile of GDF11/8 were compared with those in the lowest quartile in unadjusted analysis, hazard ratios for individual cardiovascular outcomes or death ranged from 0.35 to 0.47 and were all significant at P < 0.05 level (Figure 1 and Table 2). These relationships persisted after adjustment for demographic variables, clinical risk factors, and medication use. Notably, participants in the highest quartile of GDF11/8 had a lower risk of all-cause death than those in the lowest quartile [adjusted hazard ratio (HR), 0.37; 95% confidence interval (CI), 0.26–0.52; P < 0.001]. GDF11/8 was likewise associated with cardiovascular-specific mortality (adjusted HR for Q4 vs. Q1, 0.26; 95% CI, 0.14–0.46; P < 0.001). After multivariable adjustment, participants in the highest quartile of GDF11/8 had lower risk of HF hospitalization (HR, 0.57; 95% CI, 0.36–0.92; P = 0.02), stroke (HR, 0.32; 95% CI, 0.12–0.83; P = 0.02), and MI (HR, 0.52; 95% CI, 0.29–0.92; P = 0.03) than did participants in the lowest quartile of GDF11/8 (Table 2). Participants in the highest quartile of GDF11/8 had lower risk of the composite endpoint (HF, stroke, MI, or death) than those in the lowest quartile in multivariable adjusted analysis (HR, 0.45; 95% CI, 0.33–0.60; P < 0.001) (Table 2 and Supplementary material online, Figure S1A). Estimates of the association between GDF11/8 and cardiovascular events were not meaningfully changed (<5% change in estimate) with the addition of LVH, LVMI, or diastolic function to the model.
Table 2.
Association of GDF11/8 with cardiovascular events and death in the Heart and Soul study
| Outcome | Model | Q4 vs. Q1 |
Per 1SD increase |
||
|---|---|---|---|---|---|
| HR (95% CI) | P-Value | HR (95% CI) | P-Value | ||
| Heart failure hospitalization | Unadjusted | 0.47 (0.31–0.73) | 0.001 | 0.67 (0.55–0.82) | <0.001 |
| Model 1a | 0.49 (0.31–0.77) | 0.002 | 0.69 (0.56–0.85) | <0.001 | |
| Model 2b | 0.57 (0.36–0.92) | 0.02 | 0.79 (0.64–0.97) | 0.02 | |
| Stroke | Unadjusted | 0.41 (0.17–0.97) | 0.04 | 0.72 (0.50– 1.03) | 0.07 |
| Model 1 | 0.33 (0.13–0.81) | 0.02 | 0.65 (0.44–0.97) | 0.04 | |
| Model 2 | 0.32 (0.12–0.83) | 0.02 | 0.69 (0.45–1.02) | 0.06 | |
| Myocardial infarction | Unadjusted | 0.45 (0.27–0.76) | 0.003 | 0.68 (0.55–0.85) | 0.001 |
| Model 1 | 0.42 (0.24–0.74) | 0.002 | 0.67 (0.53– 0.85) | 0.001 | |
| Model 2 | 0.52 (0.29–0.92) | 0.03 | 0.75 (0.59–0.95) | 0.02 | |
| Death | Unadjusted | 0.35 (0.26–0.47) | <0.001 | 0.66 (0.58–0.76) | <0.001 |
| Model 1 | 0.33 (0.24–0.46) | <0.001 | 0.67 (0.58–0.77) | <0.001 | |
| Model 2 | 0.37 (0.26–0.52) | <0.001 | 0.74 (0.64–0.85) | <0.001 | |
| Composite HF/stroke/MI/death | Unadjusted | 0.43 (0.32–0.56) | <0.001 | 0.71 (0.63–0.80) | <0.001 |
| Model 1 | 0.40 (0.30–0.53) | <0.001 | 0.70 (0.62–0.79) | <0.001 | |
| Model 2 | 0.45 (0.33– 0.60) | <0.001 | 0.76 (0.67–0.86) | <0.001 | |
aCox proportional hazards Model 1, adjusted for age, sex, and race.
bCox proportional hazards Model 2, adjusted for age, sex, race, hypertension, diabetes, history of myocardial infarction, eGFR, ejection fraction, and β-blocker, angiotensin converting enzyme-inhibitor or angiotensin receptor blocker, and statin use.
A significant interaction was present between race and GDF11/8 as a continuous variable for the composite outcome (P = 0.02 for interaction). Compared with whites with low GDF11/8, whites with high levels of GDF11/8 had a lower risk of the composite outcome (adjusted HR, 0.67; 95% CI, 0.56–0.80 per SD increase in GDF11/8 level), while GDF11/8 level was not significantly associated with increased risk among non-whites (adjusted HR, 0.89; 95% CI, 0.74–1.07 per SD increase in GDF11/8 level). The association between GDF11/8 and the composite outcome did not vary by age, sex, LVEF, LVH, or eGFR (P-value for interaction >0.10 for all).
In the Norwegian HUNT3 cohort, after a median follow-up of 4.5 years, 133 participants died, 107 participants were hospitalized for HF, 62 participants experienced a stroke or transient ischaemic attack (TIA), 90 participants suffered an MI, and 273 participants experienced any cardiovascular event or death. The relationship between GDF11/8 and key baseline characteristics observed in the Heart and Soul cohort was reinforced in HUNT3, with participants in the highest quartile being younger and more predominantly male (Supplementary material online, Table S2). The relationship between plasma GDF11/8 levels and individual cardiovascular endpoints (HF, stroke/TIA, MI, and death) or their composite endpoint observed in our Heart and Soul cohort was replicated in the Norwegian HUNT3 cohort (Figure 2, Supplementary material online, Table S3 and Figure S1B). Participants in the highest quartile of GDF11/8 had markedly lower risk of composite cardiovascular endpoint (HF, stroke/TIA, MI, or death) than those in the lowest quartile, both unadjusted (HR, 0.24; 95% CI, 0.16–0.35; P < 0.001) and in multivariable adjusted analysis (HR, 0.34; 95% CI, 0.23–0.51; P < 0.001).
Figure 2.
Incidence of heart failure, stroke or transient ischaemic attack (TIA), myocardial infarction, death, and composite endpoint in HUNT3 cohort, unadjusted, stratified by quartile of GDF11/8. P-Values for trend are <0.001 for heart failure, death, and composite endpoint, 0.004 for stroke, and 0.02 for myocardial infarction.
While the focus of this study is on the cardiovascular pathobiology of GDF11/8 in humans and not on GDF11/8 as a potential biomarker of cardiovascular risk, for interested readers we have included discrimination and reclassification performance metrics for GDF11/8 when added to an existing clinical secondary cardiovascular risk prediction model (Supplementary material online, Tables S4 and S5).
Left ventricular hypertrophy
Of the 928 participants in the Heart and Soul cohort, 368 (39.7%) had LVH by echocardiogram. The percentage of participants with LVH increased with decreasing quartile of GDF11/8 (Figure 3). Thirty-one per cent of participants in the highest quartile had LVH compared with 46% in the lowest quartile (unadjusted OR, 0.55; 95% CI, 0.37–0.80; P = 0.002, Table 3). This relationship remained after adjustment for demographics and clinical risk factors (OR, 0.55; 95% CI, 0.35–0.86; P = 0.009). Similarly, when we modelled GDF11/8 as a continuous variable, higher levels of GDF11/8 were associated with lower odds of LVH (adjusted OR, 0.80; 95% CI, 0.67–0.95; P = 0.01 per SD increase in GDF11/8 level).
Figure 3.

Prevalence of left ventricular hypertrophy by quartile of GDF11/8. Unadjusted. Left ventricular hypertrophy defined as left ventricular mass index >88 g/m2 in females, >102 g/m2 in males. P-Value is 0.02.
Table 3.
Cross-sectional association of GDF11/8 with left ventricular hypertrophy in the Heart and Soul study
| Q4 vs. Q1 |
Per 1SD increase |
|||
|---|---|---|---|---|
| OR (95% CI) | P-Value | OR (95% CI) | P-Value | |
| Unadjusted | 0.55 (0.37–0.80) | 0.002 | 0.79 (0.69–0.92) | 0.002 |
| Model 1a | 0.47 (0.31–0.70) | <0.001 | 0.75 (0.64–0.88) | <0.001 |
| Model 2a | 0.55 (0.35–0.86) | 0.009 | 0.80 (0.67–0.95) | 0.01 |
aLogistic regression model Model 1, adjusted for age, sex, and race.
bLogistic regression Model 2, adjusted for age, sex, race, hypertension, diabetes, history of myocardial infarction, and ejection fraction.
GDF11/8 and age
In both the Heart and Soul and the HUNT3 cohorts, GDF11/8 levels were lower in older participants. Figure 4 demonstrates the relationship of plasma GDF11/8 levels to age in the Heart and Soul (A) and HUNT3 (B) cohorts.
Figure 4.
GDF11/8 levels by age, unadjusted. Inner line = median, box 25th–75th percentile, outer whiskers denote adjacent value 1.5 times height of box. Units = relative fluorescence units. (A) Heart and Soul cohort and (B) HUNT3 cohort. P-Value is <0.001 for both Heart and Soul and HUNT3 cohorts.
Discussion
To the best of our knowledge, this is the first study to investigate the association of GDF11/8, factor(s) that putatively regulate the ageing process in mice,1–3 with cardiovascular outcomes and all-cause mortality in humans. In two independent cohorts across two continents totalling 1899 subjects with stable CHD, we found markedly reduced risk of incident HF hospitalization, stroke, MI, and all-cause death in those with higher circulating GDF11/8 levels. These relationships remained significant after adjustment for demographic and clinical covariates. Additionally, we found that higher levels of GDF11/8 are associated with lower prevalence of LVH. We also found that GDF11/8 levels are lower in older individuals. These data suggest that GDF11/8 characteristics in humans are similar to those reported in mice.2 Taken in the context of mechanistic studies in mice,1,2 our findings support the hypothesis that GDF11/8 is a circulating factor that protects against adverse cardiovascular events, cardiac hypertrophy, and death.
Despite the relatively narrow age range of our human subjects compared with the extremes of age reported in mice, we detected an age-related decline in GDF11/8 in both cohorts (Figure 1A and B). In context of the studies in mice relating biological ageing to a decline in GDF11/8 levels,1–3 our findings support the hypothesis that age-related decline in GDF11/8 might also contribute to cardiovascular ageing in humans.
We also found that GDF11/8 levels vary by sex, being lower in females compared with males (Table 1 and Supplementary material online, Table S2), a finding not previously described in mice. Furthermore, we found that whites compared with non-whites had both lower levels of GDF11/8 (Table 1) and a stronger link between circulating GDF11/8 levels and adverse cardiovascular outcomes. This finding may explain why the association between GDF11/8 and cardiovascular events was numerically stronger in HUNT3, a homogeneous white cohort, compared with the racially mixed Heart and Soul cohort.
The overarching purpose of our study was to establish whether the protective cardiovascular and anti-ageing characteristics of GDF11/8 described in mice may apply to humans, a goal met affirmatively. The potential biological mechanisms behind these observations in humans have to be viewed in context of parallel experimental investigations. GDF11/8 in mice has both myocardial (anti-hypertrophic) and vascular effects (favourable vascular remodelling and increased blood flow).1,2 In our study, LVH and diastolic dysfunction were not found to meaningfully weaken the association between GDF11/8 and outcomes when added to the models as covariates. The reasons for this finding are not entirely clear, as we have demonstrated the association of GDF11/8 with both LVH and outcomes, and it is well documented that LVH is associated with outcomes of HF, stroke, MI, and death.5,10,16–18 Perhaps the accuracy of measurement was not sufficient to characterize this relationship. However, it is also likely that the favourable impact of GDF11/8 on cardiovascular outcomes and cardiovascular deaths is mediated largely by its known benefits on arterial remodelling and blood flow.1 The remarkably strong association between GDF11/8 and overall deaths observed in both of our cohorts is also consistent with benefits of GDF11/8 that extend beyond the cardiovascular system to other organs. This notion is supported by the broad tissue distribution of GDF11 and GDF8.8
Clinical implications
Given reports of GDF11/8 in mice as cardioprotective and anti-ageing,1–3 now reinforced with our human outcomes data, it is plausible to consider targeting this pathway to reduce cardiovascular risk and other adverse outcomes associated with ageing.
Limitations
We have studied two cohorts of subjects with stable CHD across two continents. Future studies will need to generalize our findings to other populations, including populations free of cardiovascular disease and populations with higher proportions of women (18% in Heart and Soul and 28% in HUNT3). In this study, as in any observational study, residual confounding may influence results. Because of their high structural homology, our assay does not distinguish circulating GDF11 and GDF8.8,9 This does not diminish the significance of our findings as the functions of the two circulating proteins appear identical, both proteins binding to the same activin II receptors and activating the same effector pathways.8 The relative contributions of GDF11 and GDF8 will require further study as more selective SOMAmer reagents become available.9
In summary, in two independent cohorts with baseline CHD, we found that lower levels of GDF11/8 are associated with higher rates of incident cardiovascular events and overall deaths as well as higher prevalence of LVH. GDF11/8 levels are lower with higher age, a finding relevant to understanding the biology of human ageing. Based on findings in mice1–3 translated to humans in our study, GDF11/8 may represent a valuable therapeutic target for treating cardiovascular diseases and possibly even modulating the ageing process.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This study was supported by grants from the Department of Veterans Affairs (Epidemiology Merit Review Program); the National Heart, Lung and Blood Institute (grant number: R01 HL079235); the Robert Wood Johnson Foundation (Generalist Physician Faculty Scholars Program); the American Federation for Aging Research (Paul Beeson Faculty Scholars in Aging Research Program); and the Ischemia Research and Education Foundation. None of these funding sources had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The contents of this research are solely the responsibility of the investigators and do not necessarily represent the official views of the NIH. A.L.B. is supported by the National Center for Advancing Translational Sciences (grant number: KL2TR000143). This study was also supported in part by a research grant to M.A.W. from SomaLogic.
Conflict of interest: E.N.B., R.E.M., B.S.S., D.G.S., and S.A.W. are employees of SomaLogic. T.F. and R.M.L. are former Somalogic employees. The SomaLogic co-authors used aptamer-based technology to measure GDF11 levels while blinded to clinical outcome data. The UCSF authors, specifically K.A.O., A.L.B., P.G., and M.C.R., had the sole responsibility for the study design, for data analyses, and for preparation of the manuscript. K.A.O., A.L.B., M.C.R., M.A.W., S.K., D.G.S., and P.G.: performed statistical analysis. P.G. and S.A.W.: handled funding and supervision. K.A.O., A.L.B., B.H., T.F., K.H., H.D., D.G.S., S.A.W., M.A.W., N.B.S., and P.G.: acquired the data. K.A.O., A.L.B., B.H., E.N.B., T.F., M.C.R., S.K., K.H., H.D., R.E.M., B.S.S., R.M.L., D.G.S., S.A.W., M.A.W., N.B.S., and P.G.: conceived and designed the research. K.A.O., A.L.B., B.H., R.M.L., D.G.S., S.A.W., M.A.W., N.B.S., and P.G.: drafted the manuscript. K.A.O., A.L.B., B.H., E.N.B., T.F., M.C.R., S.K., K.H., H.D., R.E.M., B.S.S., R.M.L., D.G.S., S.A.W., M.A.W., N.B.S., and P.G.: made critical revision of the manuscript for key intellectual content.
References
- 1.Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 2014;344:630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, Sinha M, Dall'Osso C, Khong D, Shadrach JL, Miller CM, Singer BS, Stewart A, Psychogios N, Gerszten RE, Hartigan AJ, Kim MJ, Serwold T, Wagers AJ, Lee RT. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell 2013;153:828–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinha M, Jang YC, Oh J, Khong D, Wu EY, Manohar R, Miller C, Regalado SG, Loffredo FS, Pancoast JR, Hirshman MF, Lebowitz J, Shadrach JL, Cerletti M, Kim MJ, Serwold T, Goodyear LJ, Rosner B, Lee RT, Wagers AJ. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science 2014;344:649–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA 2003;290:215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stevens SM, Farzaneh-Far R, Na B, Whooley MA, Schiller NB. Development of an echocardiographic risk-stratification index to predict heart failure in patients with stable coronary artery disease: The Heart and Soul Study. JACC Cardiovasc Imaging 2009;2:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krokstad S, Langhammer A, Hveem K, Holmen TL, Midthjell K, Stene TR, Bratberg G, Heggland J, Holmen J. Cohort Profile: the HUNT Study, Norway. Int J Epidemiol 2013;42:968–977. [DOI] [PubMed] [Google Scholar]
- 7.Nakashima M, Toyono T, Akamine A, Joyner A. Expression of growth/differentiation factor 11, a new member of the BMP/TGFbeta superfamily during mouse embryogenesis. Mech Dev 1999;80:185–189. [DOI] [PubMed] [Google Scholar]
- 8.Egerman MA, Cadena SM, Gilbert JA, Meyer A, Nelson HN, Swalley SE, Mallozzi C, Jacobi C, Jennings LL, Clay I, Laurent G, Ma S, Brachat S, Lach-Trifilieff E, Shavlakadze T, Trendelenburg AU, Brack AS, Glass DJ. GDF11 increases with age and inhibits skeletal muscle regeneration. Cell Metab 2015;22:164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hathout Y, Brody E, Clemens PR, Cripe L, DeLisle RK, Furlong P, Gordish-Dressman H, Hache L, Henricson E, Hoffman EP, Kobayashi YM, Lorts A, Mah JK, McDonald C, Mehler B, Nelson S, Nikrad M, Singer B, Steele F, Sterling D, Sweeney HL, Williams S, Gold L. Large-scale serum protein biomarker discovery in Duchenne muscular dystrophy. Proc Natl Acad Sci USA 2015;112:7153–7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turakhia MP, Schiller NB, Whooley MA. Prognostic significance of increased left ventricular mass index to mortality and sudden death in patients with stable coronary heart disease (from The Heart and Soul Study). Am J Cardiol 2008;102:1131–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–271. [DOI] [PubMed] [Google Scholar]
- 12.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003;289:194–202. [DOI] [PubMed] [Google Scholar]
- 13.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012;367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: The Framingham Study. N Engl J Med 1971;285:1441–1446. [DOI] [PubMed] [Google Scholar]
- 15.Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, Goldberg RJ, Hand MM, Jaffe AS, Julian DG, Levy D, Manolio T, Mendis S, Mensah G, Pajak A, Prineas RJ, Reddy KS, Roger VL, Rosamond WD, Shahar E, Sharrett AR, Sorlie P, Tunstall-Pedoe H. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation 2003;108:2543–2549. [DOI] [PubMed] [Google Scholar]
- 16.Di Tullio MR, Zwas DR, Sacco RL, Sciacca RR, Homma S. Left ventricular mass and geometry and the risk of ischemic stroke. Stroke 2003;34:2380–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Left ventricular mass and incidence of coronary heart disease in an elderly cohort. The Framingham Heart Study. Ann Intern Med 1989;110:101–107. [DOI] [PubMed] [Google Scholar]
- 18.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990;322:1561–1566. [DOI] [PubMed] [Google Scholar]