Abstract
Fat distribution is strongly associated with insulin resistance, a risk factor for type 2 diabetes and cardiovascular diseases. However, associations of different adipose tissue depots or/and obesity indices with insulin resistance have not been systematically evaluated. In this study we examined associations of different adipose tissue depots/obesity indices with insulin resistance, as measured by homeostatic model assessment of insulin resistance (HOMA-IR) in observational studies. A total of 40 studies with 56 populations and 29 adipose tissue depots/obesity indices were included in the meta-analysis. There were strong correlation between HOMA-IR and visceral fat mass (r = 0.570, 95% confidence interval(CI): 0.424~0.687), total fat mass (r = 0.492, 95%CI: 0.407~0.570), body mass index (r = 0.482, 95%CI: 0.445~0.518) and waist circumference (r = 0.466, 95%CI: 0.432~0.500), except lower extremity fat (r = 0.088, 95%CI: −0.116~0.285). Sample size, diabetic status, gender, mean of body mass index, and race contributed to heterogeneity of these associations. This study showed a positive correlation between insulin resistance and most adipose tissue depots/obesity indices, and the strongest association is for visceral fat mass.
Insulin resistance, a key determinant of metabolic syndrome1,2,3, is an important risk factor for type 2 diabetes1 and cardiovascular diseases4,5. Adiposity, a major determinant of insulin resistance6,7,8, and its distribution measures have been shown to be associated with insulin resistance by a number of studies7,9,10,11,12,13,14,15,16,17,18,19. However, to what extent various adipose tissue depots and obesity indices are associated with insulin resistance has not been systematically evaluated.
Among insulin resistance indices, homeostatic model assessment of insulin resistance (HOMA-IR) is the most commonly used in population studies20,21. In this meta-analysis, we systematically examined the associations of HOMA-IR with different indices of adiposity and body fat distribution, such as body mass index (BMI)12,13,14, waist circumference13,15,16, trunk fat mass17,18,19, visceral fat22,23, and total fat mass23,24 to identify which of the adipose tissue depots/obesity indices has the best association with insulin resistance.
Result
Basic characteristics of the included studies
A total of 29 adipose indices were reportedly associated with HOMA-IR. 17/29 indices were not included in the meta-analysis because they were reported only once or twice (Table 1). The remaining 12 adipose tissue depots/obesity indices that were reported more than three times were analyzed with meta-analysis.
Table 1. Correlation coefficients between HOMA-IR and the 17-adipose indices that were not included in the Meta-analysis.
| Population 1 |
Population 2 |
|||||
|---|---|---|---|---|---|---|
| n | r | P value | n | r | P value | |
| Abdominal fat | 87 | 0.585 | <0.001 | 77 | 0.477 | <0.001 |
| Intra-abdominal fat | 51 | 0.530 | <0.05 | 272 | 0.480 | <0.05 |
| Retroperitoneal adipose tissue | 51 | 0.110 | >0.05 | |||
| Subcutaneous anterior fat | 51 | 0.360 | <0.01 | |||
| Subcutaneous posterior fat | 51 | 0.390 | <0.01 | |||
| Upper extremity fat | 1579 | 0.460 | <0.0001 | |||
| Intraperitoneal fat ratio | 30 | 0.620 | 0.003 | |||
| Subcutaneous fat ratio | 30 | −0.550 | 0.011 | |||
| Liver attenuation | 5291 | −0.310 | <0.0001 | |||
| Pericardial adipose tissue | 5291 | 0.440 | <0.0001 | |||
| Sum of the skinfold thickness | 55 | 0.515 | <0.001 | 55 | 0.254 | <0.001 |
| Subscapular skinfold thickness | 55 | 0.595 | <0.001 | 55 | 0.413 | <0.01 |
| Suprailiac skinfold thickness | 55 | 0.288 | <0.001 | 55 | 0.195 | >0.05 |
| Sagittal abdominal diameter | 157 | 0.480 | <0.0001 | 138 | 0.482 | <0.001 |
| Truncal subcutaneous fat | 55 | 0.347 | 0.01 | |||
| Peripheral subcutaneous fat | 55 | 0.296 | 0.028 | |||
| Thigh fat area | 783 | 0.480 | <0.0001 | |||
The correlations between HOMA-IR and the 17 adipose indices that were excluded from the meta-analysis
Apart from retroperitoneal adipose tissue and suprailiac skinfold thickness, 15/17 adipose tissue depots/obesity indices showed significant correlations with HOMA-IR (Table 1). There were significant correlations between HOMA-IR and abdominal fat, intra-abdominal fat, subscapular skinfold thickness, intraperitoneal fat ratio, and subcutaneous fat ratio.
The correlations between HOMA-IR and the 12 adipose indices revealed by meta-analysis
11/12 adipose tissue depots/obesity indices except leg or lower extremity fat mass showed significant correlation with HOMA-IR (Table 2). The strongest correlation was for visceral fat(r = 0.570, 95%CI: 0.424~0.687), followed by total fat mass (r = 0.492, 95%CI: 0.407~0.570) and body mass index (r = 0.482, 95% CI: 0.445~0.518).
Table 2. Pooled correlation coefficients between HOMA-IR and adipose indices and 95% confidence interval estimated with random model by Meta-analysis.
| Variables | Number of studies | z valuea | r valueb |
|---|---|---|---|
| Visceral fat | |||
| Mass | 3 | 0.648 (0.453, 0.843) | 0.570 (0.424, 0.687) |
| Area | 9 | 0.438 (0.390, 0.487) | 0.412 (0.371, 0.452) |
| Subcutaneous fat | |||
| Mass | 3 | 0.344 (0.149, 0.539) | 0.331 (0.148, 0.492) |
| Area | 8 | 0.412 (0.265, 0.558) | 0.390 (0.259, 0.506) |
| Total fat | |||
| Mass | 7 | 0.539 (0.432, 0.647) | 0.492 (0.407, 0.570) |
| Area | 2 | 0.338 (0.188, 0.489) | 0.326 (0.186, 0.453) |
| Fat mass percentage | 6 | 0.436 (0.343, 0.529) | 0.410 (0.330, 0.485) |
| Leg/lower extremity fat mass | 7 | 0.088 (−0.117, 0.293) | 0.088 (−0.116, 0.285) |
| Body mass index | 30 | 0.526 (0.479, 0.574) | 0.482 (0.445, 0.518) |
| Waist circumference | 40 | 0.505 (0.462, 0.549) | 0.466 (0.432, 0.500) |
| Hip circumference | 10 | 0.436 (0.391, 0.481) | 0.410 (0.372, 0.447) |
| Waist/Hip circumference | 14 | 0.351 (0.290, 0.413) | 0.337 (0.282, 0.391) |
| Waist circumference to height ratio | 6 | 0.460 (0.402, 0.519) | 0.430 (0.382, 0.477) |
| Leg to trunk ratio | 4 | −0.376 (−0.672, −0.081) | −0.359 (−0.586, −0.081) |
| Trunk fat mass | 7 | 0.371 (0.186, 0.555) | 0.355 (0.183, 0.504) |
aFisher transformation from correlation coefficient, 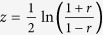 .
.
bcorrelation coefficient from z value, 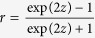 .
.
Sensitivity analyses
No study appears to drive the pooled estimation as dropping any of the studies did not materially change the pooled estimation.
Meta-regression analysis on correlation coefficients’ related factors
The Meta-regression analysis identified a number of factors that were associated with the correlation between adipose tissue depots/obesity indices and HOMA-IR, including sample size of population, gender, race, diabetic status and mean of BMI (Table 3). In detail, sample size of population was found to be associated with correlation between visceral fat and HOMA-IR while gender was associated with correlation between subcutaneous fat or waist to hip circumference ratio and HOMA-IR. In addition, race was associated with correlation between body mass index and HOMA-IR and correlation between waist circumference and HOMA-IR while diabetic status, mean of BMI and race is associated with correlation between hip circumference and HOMA-IR.
Table 3. Summary of Meta-regression analysis of z valuea.
| Visceral fat |
Subcutaneous fat |
Body mass index |
Waist circumference |
Hip circumference |
Waist/Hip |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b | P value | b | P value | b | P value | b | P value | b | P value | b | P value | |
| Sample size | ||||||||||||
| <100 | reference | reference | reference | reference | reference | reference | ||||||
| 100 ~ 1000 | −0.136 | 0.035 | −0.052 | 0.669 | −0.131 | 0.148 | −0.134 | 0.102 | −0.378 | 0.165 | 0.400 | 0.149 |
| >1000 | 0.023 | − | 0.0381 | 0.836 | −0.099 | 0.282 | −0.077 | 0.395 | −0.368 | 0.173 | 0.351 | 0.204 |
| Diabetic status | ||||||||||||
| No diabetes | reference | reference | reference | reference | reference | reference | ||||||
| With diabetes | −0.013 | 0.928 | −0.122 | 0.554 | −0.175 | 0.089 | −0.153 | 0.127 | −0.097 | 0.097 | −0.031 | 0.742 |
| Mixed | 0.029 | 0.733 | −0.176 | 0.147 | −0.070 | 0.284 | −0.118 | 0.077 | −0.092 | 0.027 | 0.016 | 0.833 |
| Gender | ||||||||||||
| Male | reference | reference | reference | reference | reference | reference | ||||||
| Female | 0.063 | 0.581 | 0.058 | 0.660 | 0.095 | 0.259 | 0.010 | 0.906 | −0.005 | 0.926 | −0.179 | 0.020 |
| mixed | −0.008 | 0.926 | −0.402 | 0.003 | 0.014 | 0.845 | −0.028 | 0.720 | −0.040 | 0.496 | −0.101 | 0.142 |
| Method of correlation | ||||||||||||
| Pearson | reference | reference | reference | reference | reference | reference | ||||||
| Pearson with Logb | −0.116 | 0.149 | −0.040 | 0.766 | −0.013 | 0.869 | 0.034 | 0.645 | −0.025 | 0.719 | 0.054 | 0.519 |
| Spearman | −0.143 | 0.398 | 0.070 | 0.686 | 0.541 | 0.773 | 0.062 | 0.476 | −0.070 | 0.120 | −0.008 | 0.917 |
| Mean age | ||||||||||||
| <60 years | reference | reference | reference | reference | reference | reference | ||||||
| ≥60 years | 0.004 | 0.968 | 0.219 | 0.292 | 0.021 | 0.847 | −0.065 | 0.393 | −0.045 | 0.445 | −0.041 | 0.623 |
| Mean BMI | ||||||||||||
| <28 kg/m2 | reference | reference | reference | reference | reference | reference | ||||||
| ≥28 kg/m2 | 0.167 | 0.802 | −0.006 | 0.979 | 0.043 | 0.525 | 0.083 | 0.232 | −0.084 | 0.027 | −0.023 | 0.728 |
| Race | ||||||||||||
| Caucasian | reference | reference | reference | reference | reference | reference | ||||||
| Asian | −0.021 | 0.770 | −0.110 | 0.292 | −0.051 | 0.372 | −0.041 | 0.418 | 0.099 | 0.016 | 0.021 | 0.760 |
| Other | − | − | − | − | 0.604 | 0.002 | 0.983 | <0.001 | 0.046 | 0.449 | 0.139 | 0.154 |
aFisher transformation of correlation coefficient, 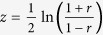 .
.
bPearson correlation with logarithm transformation, – Sample size in the subgroup is not enough for regression analysis.
Statistical tests of publication bias
No publication bias was found for the 12 indices included in the meta-analyses by Begg’s test (P > 0.05, Table 4). Using Egger’s test, we found that 2/12 P values for leg (or lower extremity fat) and trunk fat respectively, fell lower than 0.05 (Table 4).
Table 4. Statistical tests of publication bias.
| Begg’s test |
Egger’s test |
|||
|---|---|---|---|---|
| z value | P value | bias | P value | |
| Visceral fat | 1.83 | 0.067 | −0.5812 | 0.392 |
| Subcutaneous fat | −0.96 | 0.337 | −0.9006 | 0.362 |
| Total fat | 0.42 | 0.677 | −1.1902 | 0.398 |
| Fat mass percentage | 0.19 | 0.851 | −0.6610 | 0.617 |
| Leg/lower extremity fat | −1.05 | 0.293 | −6.1400 | 0.015 |
| Body mass index | −0.39 | 0.695 | −0.9814 | 0.296 |
| Waist circumference | −0.08 | 0.935 | −1.0613 | 0.186 |
| Hip circumference | −0.27 | 0.788 | −0.3973 | 0.763 |
| Waist/Hip circumference | 0.05 | 0.956 | −0.0496 | 0.972 |
| Waist circumference to height ratio | −0.19 | 0.851 | −0.1624 | 0.911 |
| Leg to trunk ratio | −0.68 | 0.497 | −0.4371 | 0.956 |
| Trunk fat | 0.45 | 0.652 | −3.6389 | 0.026 |
Discussion
This meta-analysis study is the first to assess correlation between different adipose tissue depots/obesity indices and insulin resistance. We found significant correlations between most adipose tissue depots/obesity indices and insulin resistance. Among these indices, visceral fat mass showed the strongest correlation with HOMA-IR, followed by total fat mass, BMI and waist circumference. Notably, the leg fat (or lower extremity fat) had no significant correlation with HOMA-IR. In addition, diabetic status, gender, mean BMI, and race were associated with correlation estimates in meta-regression analysis. These findings may have important clinical and public health implications for prevention and treatment of diabetes.
In this study visceral fat mass showed the strongest correlation with HOMA-IR, followed by total fat mass, BMI and waist circumference. Other studies, which were not included in this meta-analysis, also reported significant correlation between HOMA-IR and intraperitoneal fat ratio25, intra-abdominal fat23, abdominal fat26 and sagittal abdominal diameter14,27 with correlation coefficients around 0.5. Visceral adipose tissue appeared to be the best predictor of insulin resistance28,29,30, measured by the clamp technique. Kelley et al.30 reported that insulin-stimulated glucose utilization was significantly correlated with both visceral adipose tissue and deep subcutaneous adipose tissue (r = −0.61 and −0.64, respectively; both P < 0.001). Nevertheless, visceral fat mass and total fat mass are measured with DEXA or magnetic resonance imaging, whereas BMI and waist circumference measurements are quick and easy using simple measuring instruments. Therefore, BMI and waist circumference are probably better predictors to be used for insulin resistance for economic reasons.
In this study, factors such as diabetic status, gender, obesity status and race were found to be associated with pooled correlation estimates. Gender difference has been widely reported regarding obesity, especially central obesity. Machann et al.31 reported that females were characterized by lower visceral adipose tissue and higher subcutaneous adipose tissue. Bouchard et al.32 also described a more pronounced increase in visceral adipose tissue in men compared to women, in normal weight, overweight, and obese individuals. Differences in HOMA-IR levels in men and women (2.06 vs. 1.93, respectively; P = 0.047) may also be a contributing factor. Insulin resistance deteriorates with age in women 50 years or older, but not so in men33. Many women are going through menopause at 50; therefore, menopause may also contribute to insulin resistance and obesity in women of 50 years or older.
There are some limits in our study. First, we only used HOMA-IR as an index to measure insulin resistance without testing any other method; nevertheless, indexes other than HOMA-IR are not widely used. Secondly, race/ethnicity was not well defined in some of the studies included in this work. Lastly, there is always considerable heterogeneity presented in the meta-analyses. This work is no exception and we identified a few contributing factors.
In conclusion, we found significant positive correlation between most adipose tissue depots/obesity indices and insulin resistance, as measured by HOMA-IR. Visceral fat showed the strongest correlation whereas lower extremity fat had no correlation with insulin resistance. Diabetic status, gender, race/ethnicity, and mean BMI contributed to the heterogeneity of the overall estimates.
Methods
Literature collection
We systematically searched PubMed, Web of Science, and Dissertation Theses to identify all relevant reports that met our inclusion criteria (see below) until September 2014. “Body mass index”, “ waist circumference”, “waist to hip ratio”, “waist to height ratio”, “abdominal height”, “fat mass”, “skinfold”, “adiposity”, “adipose tissue”, “fatness”, “body fat distribution” and “insulin resistance” in Title or Abstract, as well as MeSH terms “Body Fat Distribution”, “Body Mass Index”, “ Waist Circumference”, “Adipose Tissue”, “Skinfold Thickness” and “Insulin Resistance” were used as search terms. We also performed a manual search of references cited in published original and review articles.
The inclusion criteria were as follows: (1) the study was observational, either cross-sectional or of a case-control design; (2) conducted in humans; and (3) correlation coefficients between HOMA-IR and fat indices and their variance were reported. Studies were excluded if (1) the sample was under 19-year old; (2) the sample had chronic conditions such as cancer, heart failure, chronic kidney disease, and infectious disease. Studies of type 2 diabetes with no severe complication were included in this work.
Data retrieval
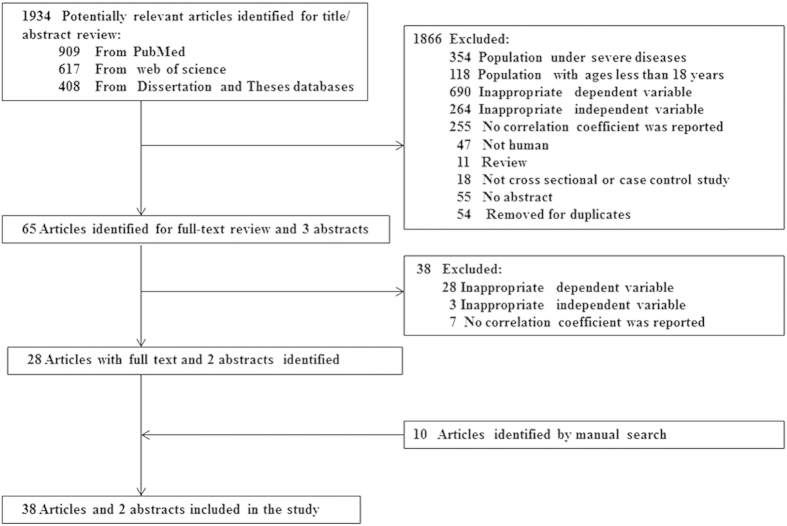
All data were independently retrieved by two investigators (Zhang, M and Zhou, L) according to a standardized protocol and data-collection form. Disagreements were resolved by discussion with the third investigator (Zhang, S). First author’s name and year of publication, study design (case-control or cross-sectional), characteristics of the study subjects including sample size, mean age, mean BMI, sex, race, diabetic status, indices of adiposity, HOMA-IR transformation, and measures of associations (correlation coefficient and P value) were recorded. The schematic view for data retrieval is presented in Fig. 1. A total of 40 studies including 29 adipose depots or adipose indices were identified. Twelve adipose indices with at least 3 individual results were analyzed with meta-analysis.
Figure 1. The flow chart of literature search.
Data analysis
Z value from Fisher’s z-transformation of correlation coefficient by equation (1) 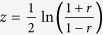 was served as effect size, and standard error of z value was calculated with equation (2)
was served as effect size, and standard error of z value was calculated with equation (2) 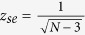 . The pooled z value and 95% confidence interval was transformed into correlation coefficient and 95% CI with equation (3)
. The pooled z value and 95% confidence interval was transformed into correlation coefficient and 95% CI with equation (3) 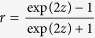 . Fixed and random effect models were used to combine z values for those with more than 3 populations. Heterogeneity of z values was assessed by I2. Meta regression was performed to investigate the association between z values and sample characteristics while Begg’s and Egger’s tests were used to assess publication bias.
. Fixed and random effect models were used to combine z values for those with more than 3 populations. Heterogeneity of z values was assessed by I2. Meta regression was performed to investigate the association between z values and sample characteristics while Begg’s and Egger’s tests were used to assess publication bias.
Additional Information
How to cite this article: Zhang, M. et al. Associations of Different Adipose Tissue Depots with Insulin Resistance: A Systematic Review and Meta-analysis of Observational Studies. Sci. Rep. 5, 18495; doi: 10.1038/srep18495 (2015).
Acknowledgments
This study is supported by Youth Found of the National Natural Science Foundation of China (grant No. 81202271).
Footnotes
Author Contributions Z.M. conducted the literature search, designed the study, decided the data exclusion and inclusion criteria, retrieved data from published studies, performed data analysis and drafted the manuscript; H.T. extracted data from retrieved studies; Z.S. decided the data exclusion and inclusion criteria; Z.L. conducted the literature search. All authors reviewed the paper and approved the final manuscript.
References
- Ruige J. B. et al. Insulin and risk of cardiovascular disease: a meta-analysis. Circulation 97, 996–1001 (1998). [DOI] [PubMed] [Google Scholar]
- Hanley A. J. et al. Prediction of type 2 diabetes using simple measures of insulin resistance: combined results from the San Antonio Heart Study, the Mexico City Diabetes Study, and the Insulin Resistance Atherosclerosis Study. Diabetes 52, 463–469 (2003). [DOI] [PubMed] [Google Scholar]
- Meigs J. B. et al. Risk variable clustering in the insulin resistance syndrome. The Framingham Offspring Study. Diabetes 46, 1594–1600 (1997). [DOI] [PubMed] [Google Scholar]
- Lai T. S., Curhan G. C. & Forman J. P. Insulin resistance and risk of incident hypertension among men. J Clin Hypertens (Greenwich) 11, 483–490 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh S. et al. Insulin resistance and the development of cardiovascular disease in a Japanese community: the hisayama study. J Atheroscler Thromb 19, 977–985 (2012). [DOI] [PubMed] [Google Scholar]
- Brotons C. et al. [Prediction of glucose and insulin resistance disorders by using anthropometric parameters of central adiposity: PRED-IR study]. Med Clin (Barc) 131, 366–370 (2008). [PubMed] [Google Scholar]
- Bari M. R., Ostgren C. J., Rastam L. & Lindblad U. Abdominal obesity and insulin resistance in patients with type 2 diabetes in a Swedish community. Skaraborg hypertension and diabetes project. Scand J Prim Health Care 24, 211–217 (2006). [DOI] [PubMed] [Google Scholar]
- Mueller N. T. et al. Adiposity indices in the prediction of insulin resistance in prepubertal Colombian children. Public Health Nutr 16, 248–255 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abaci A., Ozdemir O., Hizli S., Razi C. H. & Kabakus N. Subepicardial adipose tissue thickness and its relation with anthropometric and clinical parameters in pubertal obese children. J Endocrinol Invest 33, 715–719 (2010). [DOI] [PubMed] [Google Scholar]
- Dwimartutie N., Setiati S. & Oemardi M. The correlation between body fat distribution and insulin resistance in elderly. Acta Med Indones 42, 66–73 (2010). [PubMed] [Google Scholar]
- Orsatti F. L. et al. Association between anthropometric indicators of body fat and metabolic risk markers in post-menopausal women. Gynecol Endocrinol 26, 16–22 (2010). [DOI] [PubMed] [Google Scholar]
- Can A. S. et al. Anthropometric indices and their relationship with cardiometabolic risk factors in a sample of Turkish adults. Public Health Nutr 12, 538–546 (2009). [DOI] [PubMed] [Google Scholar]
- Lovegrove J. A. et al. Lack of association between central adiposity and lipaemia in UK Sikh men. Int J Obes Relat Metab Disord 27, 1373–1382 (2003). [DOI] [PubMed] [Google Scholar]
- Petersson H., Daryani A. & Riserus U. Sagittal abdominal diameter as a marker of inflammation and insulin resistance among immigrant women from the Middle East and native Swedish women: a cross-sectional study. Cardiovasc Diabetol 6, 10 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H., Salim A., Lee J., Tai E. S. & Van Dam R. M. Can body fat distribution, adiponectin levels and inflammation explain differences in insulin resistance between ethnic Chinese, Malays and Asian Indians? Int J Obes (Lond) 36, 1086–1093 (2012). [DOI] [PubMed] [Google Scholar]
- Tai E. S., Lau T. N., Ho S. C., Fok A. C. & Tan C. E. Body fat distribution and cardiovascular risk in normal weight women. Associations with insulin resistance, lipids and plasma leptin. Int J Obes Relat Metab Disord 24, 751–757 (2000). [DOI] [PubMed] [Google Scholar]
- Aasen G., Fagertun H. & Halse J. Regional fat mass by DXA: high leg fat mass attenuates the relative risk of insulin resistance and dyslipidaemia in obese but not in overweight postmenopausal women. Scand J Clin Lab Invest 68, 204–211 (2008). [DOI] [PubMed] [Google Scholar]
- Aasen G., Fagertun H., Tonstad S. & Halse J. Leg fat mass as measured by dual X-ray absorptiometry (DXA) impacts insulin resistance differently in obese women versus men. Scand J Clin Lab Invest 69, 181–189 (2009). [DOI] [PubMed] [Google Scholar]
- Boutcher S. H., Dunn S. L., Gail Trapp E. & Freund J. Regional adiposity distribution and insulin resistance in young Chinese and European Australian women. Scand J Clin Lab Invest 71, 653–657 (2011). [DOI] [PubMed] [Google Scholar]
- Hirose H., Saito I., Kawabe H. & Saruta T. Insulin resistance and hypertension: seven-year follow-up study in middle-aged Japanese men (the KEIO study). Hypertens Res 26, 795–800 (2003). [DOI] [PubMed] [Google Scholar]
- Kim K., Valentine R. J., Shin Y. & Gong K. Associations of visceral adiposity and exercise participation with C-reactive protein, insulin resistance, and endothelial dysfunction in Korean healthy adults. Metabolism 57, 1181–1189 (2008). [DOI] [PubMed] [Google Scholar]
- Bu J. et al. Visceral fat mass is always, but adipokines (adiponectin and resistin) are diversely associated with insulin resistance in Chinese type 2 diabetic and normoglycemic subjects. Diabetes Res Clin Pract 96, 163–169 (2012). [DOI] [PubMed] [Google Scholar]
- Chan D. C. et al. Adipose tissue compartments and insulin resistance in overweight-obese Caucasian men. Diabetes Res Clin Pract 63, 77–85 (2004). [DOI] [PubMed] [Google Scholar]
- Frederiksen L. et al. Subcutaneous rather than visceral adipose tissue is associated with adiponectin levels and insulin resistance in young men. J Clin Endocrinol Metab 94, 4010–4015 (2009). [DOI] [PubMed] [Google Scholar]
- Basat O. et al. Visceral adipose tissue as an indicator of insulin resistance in nonobese patients with new onset type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes 114, 58–62 (2006). [DOI] [PubMed] [Google Scholar]
- Usui C. et al. Visceral fat is a strong predictor of insulin resistance regardless of cardiorespiratory fitness in non-diabetic people. J Nutr Sci Vitaminol (Tokyo) 56, 109–116 (2010). [DOI] [PubMed] [Google Scholar]
- Vasques A. C. et al. [Predictive ability of anthropometric and body composition indicators in the identification of insulin resistance]. Arq Bras Endocrinol Metabol 53, 72–79 (2009). [DOI] [PubMed] [Google Scholar]
- Bonora E. et al. Total body fat content and fat topography are associated differently with in vivo glucose metabolism in nonobese and obese nondiabetic women. Diabetes 41, 1151–1159 (1992). [PubMed] [Google Scholar]
- Goodpaster B. H., Thaete F. L., Simoneau J. A. & Kelley D. E. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes 46, 1579–1585 (1997). [DOI] [PubMed] [Google Scholar]
- Kelley D. E., Thaete F. L., Troost F., Huwe T. & Goodpaster B. H. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab 278, E941–948 (2000). [DOI] [PubMed] [Google Scholar]
- Machann J. et al. Age and gender related effects on adipose tissue compartments of subjects with increased risk for type 2 diabetes: a whole body MRI/MRS study. MAGMA 18, 128–137 (2005). [DOI] [PubMed] [Google Scholar]
- Bouchard C., Despres J. P. & Mauriege P. Genetic and nongenetic determinants of regional fat distribution. Endocr Rev 14, 72–93 (1993). [DOI] [PubMed] [Google Scholar]
- Gayoso-Diz P. et al. Insulin resistance index (HOMA-IR) levels in a general adult population: curves percentile by gender and age. The EPIRCE study. Diabetes Res Clin Pract 94, 146–155 (2011). [DOI] [PubMed] [Google Scholar]