Abstract
Purpose
Stereotactic body radiotherapy (SBRT) has emerged as an effective treatment for localized prostate cancer. However, prostate-specific antigen (PSA) kinetics after SBRT has not been well characterized. The purpose of the current study is to assess the kinetics of PSA for low- and intermediate-risk prostate cancer patients treated with SBRT using Cyberknife as both monotherapy and boost after whole pelvic radiotherapy (WPRT) in the absence of androgen deprivation therapy.
Methods
A total of 61 patients with low- and intermediated-risk prostate cancer treated with SBRT as monotherapy (36.25 Gy in 5 fractions in 32 patients) and SBRT (21 Gy in 3 fractions in 29 patients) boost combined with WPRT (45 Gy in 25 fractions). Patients were excluded if they failed therapy by the Phoenix definition or had androgen deprivation therapy. PSA nadir and rate of change in PSA over time (slope) were calculated and compared.
Results
With a median follow-up of 52.4 months (range, 14–74 months), for SBRT monotherapy, the median PSA nadir was 0.31 ng/mL (range, 0.04–1.15 ng/mL) and slopes were –0.41 ng/mL/mo, –0.17 ng/mL/mo, –0.12 ng/mL/mo, and –0.09 ng/mL/mo, respectively, for durations of 1 year, 2 years, 3 years, and 4 years postradiotherapy. Similarly, for SBRT boost after WPRT, the median PSA nadir was 0.34 ng/mL (range, 0.04–1.44 ng/mL) and slopes were –0.53 ng/mL/mo, –0.25 ng/mL/mo, –0.14 ng/mL/mo, and –0.09 ng/mL/mo, respectively. The median nadir and slopes of SBRT monotherapy did not differ significantly from those of SBRT boost after WPRT. Benign PSA bounces were common in 30.4% of all cohorts, and the median time to PSA bounce was 12 months (range, 6–25 months).
Conclusions
In this report of low- and intermediate-risk prostate cancer patients, an initial period of rapid PSA decline was followed by a slow decline, which resulted in a lower PSA nadir. The PSA kinetics of SBRT monotherapy appears to be comparable to those achieved with SBRT boost with WPRT.
Keywords: Cyberknife, Prostate cancer, Prostate-specific antigen kinetics, Prostate-specific antigen nadir, Stereotactic body radiotherapy
1. Introduction
Prostate cancer is the most common cancer and the second leading cause of death among men in the United States,1 and its incidence rates in Korea, although relatively lower than those in western nations, continue to increase.2 At present, intensity-modulated radiotherapy (IMRT) is the standard of care in radical radiotherapy for prostate cancer.3 In IMRT delivery, accuracy is critically important as steep dose gradients may increase the risk of geographical miss.4 Cyberknife (Accuray Inc., Sunnyvale, CA, USA) is one of the tools used for hypofractionated stereotactic body radiotherapy (SBRT) and real-time image guidance to account for intrafraction prostatic motion. Advanced techniques of Cyberknife allow high doses of radiation to be delivered precisely to the target while sparing the surrounding healthy tissue.5
Accumulating recent clinical evidence has demonstrated that the α/β ratios of prostate cancer may be around 2 Gy, which is lower than that of the surrounding normal tissue.6, 7 The hypofractionated radiotherapy schema may improve the biochemical control of prostate cancer without increasing toxicities associated with late-responding tissue.6 Analogously, hypofractionation with Cyberknife has demonstrated excellent efficacy and toxicity profiles as both monotherapy and boost after whole pelvic radiotherapy (WPRT) for localized prostate cancer.8, 9, 10, 11, 12, 13, 14
Prostate-specific antigen (PSA) is a well-established biomarker for prostate cancer and available for monitoring response to treatment. In patients without androgen deprivation therapy (ADT), analysis of PSA kinetics after treatment may reveal the biologic effect of radiation on prostate cancer. The changes of PSA after radical prostatectomy, external beam radiotherapy, and brachytherapy have been researched extensively.15 A lower PSA nadir and a rapid decline in PSA after treatment have been related to improved clinical outcome.16, 17, 18, 19 Unfortunately, kinetics of PSA decline following SBRT using Cyberknife remains poorly understood, and there are only a few reports from western countries.20, 21 It is necessary to elucidate the kinetics of SBRT in Asian population. The purpose of the current study is to assess the kinetics of PSA for low- and intermediate-risk prostate cancer patients treated with SBRT using Cyberknife as both monotherapy and boost after WPRT in the absence of ADT.
2. Materials and methods
2.1. Patient characteristics
From 2008 to 2014, 61 patients newly diagnosed with low- and intermediate-risk (National Comprehensive Cancer Network definition) localized prostate cancer treated with SBRT using the Cyberknife robotic radiosurgery system (Accuray Inc.) were enrolled in this retrospective analysis. The treatment modality was chosen by the patient after extensive discussion of various treatment options, including surgery, SBRT, and IMRT. Of 61 patients, 32 were treated with SBRT as monotherapy and 29 received SBRT boost after WPRT. All patients had histologically confirmed primary adenocarcinoma of the prostate. None of these patients had received any other local or systemic primary treatment of prostate cancer. Prior transurethral resection of the prostate for urinary symptom relief was allowed. Patients were stratified according to 2.2014 National Comprehensive Cancer Network risk stratification guidelines.22 This study was approved by the Ethical Committee for Clinical Trials of our institution, and the retrospective data were prospectively collected from our institutional database.
In order to assess PSA kinetics in response to radiotherapy alone, patients were excluded if they failed therapy by the Phoenix definition.23 All included patients had at least 1 year of follow-up. PSA bounce was defined as an absolute increase in PSA level of 0.2 ng/mL from the previous level, followed by a subsequent decrease.24 Toxicity was documented at follow-up visits using the Radiation Therapy Oncology Group scale.
2.2. SBRT treatment planning and delivery
Four or more gold fiducial markers were implanted transperineally into the prostate. After 7 days, patients underwent magnetic resonance imaging and thin-cut computed tomography scan. Fused computed tomography and magnetic resonance images were used for treatment planning. The prostate, seminal vesicles, rectum, bladder, penile bulb, and bowel were contoured. The clinical target volume (CTV) included the prostate and proximal seminal vesicles. The planning target volume (PTV) equaled the CTV expanded by 3 mm posteriorly and 5 mm in all other dimensions. A prescription dose of 36.25 Gy, delivered in five fractions, was prescribed to the PTV. The prescription dose covered at least 95% of the PTV, normalized to the 75–85% isodose line [median homogeneity index of 1.27 (range, 1.24–1.41)]. The rectal dose–volume goals were as follows: <50% of the rectal volume receiving 50% of the prescribed dose, <20% receiving 80% of the dose, <10% receiving 90% dose, and <5% receiving 100% of the dose. Treatments were given over 5 consecutive days. All SBRT treatment plans were generated on MultiPlan (version 2.2.0; Accuray Inc.).
2.3. WPRT and SBRT boost treatment planning and delivery
The prostate gland, seminal vesicles, and area of radiographic extracapsular extension were defined as CTV1. CTV2 included the external iliac nodes, internal iliac nodes, presacral nodes, and obturator nodes following the Radiation Therapy Oncology Group consensus.15 PTV1 was extended 7 mm beyond the CTV1 in all directions, except in the posterior direction, wherein it was extended 5 mm. PTV2 was extended 7 mm in all directions. The prescription dose of WPRT was 45 Gy, which was administered in 25 fractions. A minimum of 95% of the prescription dose was assured to cover 100% of the PTV. All WPRT treatment plans were generated on a Varian Eclipse treatment planning system (version 8.8.6; Varian Medical Systems, Palo Alto, CA, USA).
In SBRT boost planning, the prostate gland, seminal vesicles, and area of radiographic extracapsular extension were defined as the CTV, which was the same as that of the WPRT treatment plans. The PTV extended 5 mm beyond the CTV in all directions, except in the posterior direction, wherein it was extended 3 mm. A prescription boost dose of 21 Gy, delivered in three fractions, was prescribed to the PTV. The prescription dose covered at least 95% of the PTV, normalized to the 75–85% isodose line ;median homogeneity index of 1.29 (range, 1.21–1.43)]. The rectal dose–volume goals were as follows: <50% of the rectal volume receiving 50% of the prescribed dose, <20% receiving 80% of the dose, <10% receiving 90% dose, and <5% receiving 100% of the dose. Treatments were given over 3 consecutive days.
2.4. Statistical analysis
To eliminate the effect of differing follow-up durations between SBRT monotherapy and SBRT boost after WPRT, we calculated the rate of change in PSA over time intervals from the completion of radiotherapy to 1 year, 2 years, 3 years, and 4 years post-treatment. The slope of PSA decline (ng/mL/mo) was calculated as the regression coefficient in a linear regression model for each individual.25 The t test was performed to compare slopes of PSA and other mean values and analysis of variance in continuous variables.
3. Results
All patients completed the treatment. Sixty-one patients with a median 52.4-month (range, 14–74 months) follow-up were analyzed. The pretreatment median PSA level was 7.68 ng/mL (range, 3.45–19.50 ng/mL). Patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics (n = 61).
| variables | SBRT monotherapy (n = 32) | SBRT boost + WPRT (n = 29) |
|---|---|---|
| Median age (range) | 66.5 (56–77) | 68.7 (60–78) |
| ECOG | ||
| 0 | 22 (68.8%) | 18 (62.1%) |
| 1 | 10 (31.2%) | 11 (37.9%) |
| T stage | ||
| T1–T2a | 10 (31.3%) | 6 (20.7%) |
| T2b–T2c | 22 (68.7%) | 23 (79.3%) |
| Gleason score | ||
| ≤6 | 16 (50%) | 5 (17.2%) |
| 7 | 16 (50%) | 24 (82.8%) |
| Pretreatment PSA (ng/mL) | ||
| Mean (range) | 7.68 (3.45–14.90) | 9.04 (6.76–19.50) |
| <10 | 25 (78.1%) | 21 (72.4%) |
| 10–20 | 7 (21.9%) | 8 (27.6%) |
| NCCN risk group | ||
| Low | 9 (28.1%) | 0 (0%) |
| Intermediate | 23 (71.9%) | 29 (100%) |
ECOG, Eastern Cooperative Oncology Group; NCCN, National Comprehensive Cancer Network; PSA, prostate–specific antigen; SBRT, stereotactic body radiotherapy; WPRT, whole pelvis radiotherapy.
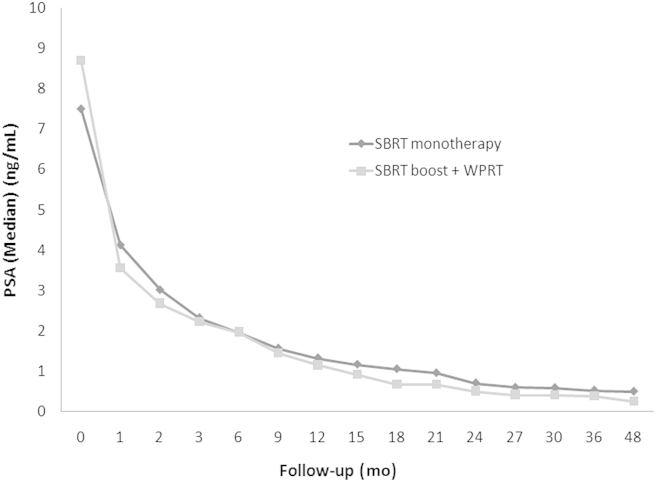
Fig. 1 shows PSA changes over time, with a different rate of PSA decline for each time interval since the end of radiotherapy. To investigate the PSA kinetics after radiotherapy, the rate of PSA decline (slope) was calculated for four intervals following radiotherapy (0–1 year, 0–2 years, 0–3 years, and 0–4 years). The slope for all cohorts was maximal in the 1st year, but tapered off quickly in the following years, with median values of –0.43 ng/mL/mo, –0.22 ng/mL/mo, –0.13 ng/mL/mo, and –0.09 ng/mL/mo for durations of 1 year, 2 years, 3 years, and 4 years after radiotherapy, respectively. Distribution of the slope for SBRT monotherapy did not differ from that of the SBRT boost and WPRT through the years (Table 2). Owing to relatively homogenous inclusion criteria, there were no significant differences in the comparison of the rate of PSA decline by the Gleason score (≤6 vs. 7) and pretreatment PSA (≤10 vs. >10).
Fig. 1.
PSA changes after SBRT as monotherapy and SBRT boost after WPRT. PSA, prostate-specific antigen; SBRT, stereotactic body radiotherapy; WPRT, whole pelvic radiotherapy.
Table 2.
Comparison of the rate of PSA decline of SBRT monotherapy and SBRT boost + WPRT cohort.
| Through year | SBRT monotherapy | SBRT boost + WPRT | P |
|---|---|---|---|
| 1 | –0.41 (–0.19, 0.04) | –0.53 (–0.21, 0.06) | 0.35 |
| 2 | –0.17 (–0.11, 0.03) | –0.25 (–0.14, 0.04) | 0.48 |
| 3 | –0.12 (–0.03, 0.09) | –0.14 (–0.05, 0.03) | 0.73 |
| 4 | –0.09 (–0.04, 0.01) | –0.09 (–0.04, 0.01) | 0.96 |
PSA, prostate–specific antigen; SBRT, stereotactic body radiotherapy; WPRT, whole pelvis radiotherapy.
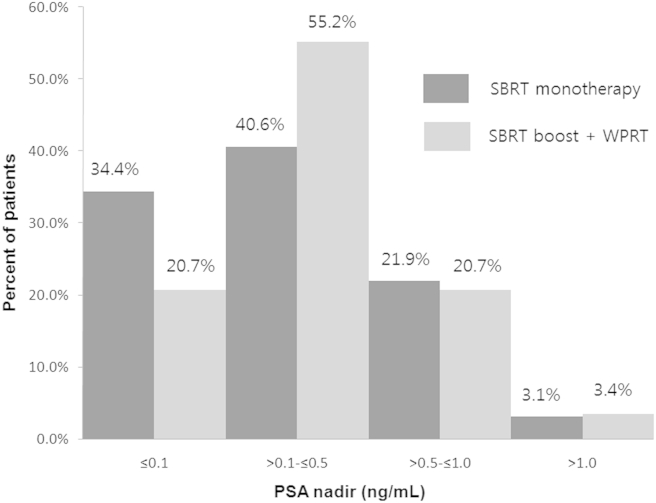
The PSA response defined by the PSA nadir was excellent. The entire cohort achieved a median PSA nadir of 0.32 ng/mL (range, 0.04–1.44 ng/mL). The SBRT monotherapy cohort achieved a median PSA nadir of 0.31 ng/mL (range, 0.04–1.15 ng/mL) with a median follow-up of 32.8 months, and the SBRT boost after WPRT cohort achieved a median PSA nadir of 0.34 ng/mL (range, 0.04–1.44 ng/mL) with a median follow-up of 33 months (Fig. 2 and Table 3). Benign PSA bounces were common in 30.4% of all cohorts. The median time to PSA bounce was 12 months (range, 6–25 months). The median height of PSA bounce was 0.3 ng/mL (range, 0.21–1.39 ng/mL). Patients with PSA bounces had lower pretreatment PSA levels (9.26 ng/mL vs. 6.43 ng/mL, P = 0.005) and were associate with the low-risk group (P = 0.07).
Fig. 2.
PSA nadir by SBRT as monotherapy and SBRT boost after WPRT. PSA, prostate-specific antigen; SBRT, stereotactic body radiotherapy; WPRT, whole pelvic radiotherapy.
Table 3.
PSA kinetics for SBRT monotherapy and SBRT boost and WPRT.
| SBRT monotherapy | SBRT boost + WPRT | |
|---|---|---|
| Median PSA nadir (ng/mL) | 0.31 (0.04–1.15) | 0.34 (0.04–1.44) |
| PSA nadir ≤ 0.5 ng/mL (%) | 75.0 | 75.9 |
| Median time to nadir (mo) | 32.8 (9–52) | 33 (12–51) |
| PSA bounce (%) | 37.50 | 27.60 |
| Median PSA bounce (ng/mL) | 0.34 (0.21–1.39) | 0.26 (0.21–0.58) |
| Median time to PSA bounce (mo) | 13.5 (6–18) | 11.5 (6–25) |
PSA, prostate–specific antigen; SBRT, stereotactic body radiotherapy; WPRT, whole pelvis radiotherapy.
The prevalent acute complaints after radiotherapy were urinary frequency and rectal pain; acute Grade II genitourinary (GU) and gastrointestinal (GI) toxicity were seen in 19.7% and 14.8% of patients, respectively. Late Grade II GU and GI toxicities were observed in 11.5% and 8.2% of patients, respectively. No Grade III GI or GU toxicities occurred.
4. Discussion
In this report, we described the changes in PSA levels in patients with low- and intermediate-risk prostate cancer treated with SBRT as monotherapy and SBRT boost after WPRT. The majority of PSA decline occurred in the 1st year but tapered off quickly in the following years. Katz et al8 demonstrated that PSA level declines steadily after treatment, and a very low mean level of 0.25 ng/mL is achieved within 4–5 years. Anwar et al20 compared the PSA kinetics between hypofractionated SBRT and conventionally fractionated external beam radiotherapy for localized prostate cancer, and reported that median slopes for SBRT were –0.09 ng/mL/mo, –0.06 ng/mL/mo, and –0.05 ng/mL/mo, respectively, for durations of 1 year, 2 years, and 3 years postradiotherapy. In our study, rates of PSA decline after SBRT as monotherapy and SBRT boost with WPRT were –0.43 ng/mL/mo, –0.22 ng/mL/mo, –0.13 ng/mL/mo, and –0.09 ng/mL/mo for durations of 1 year, 2 years, 3years, and 4 years, respectively. Although, a direct comparison with other studies is not proper, the rate of PSA decline in our study tended to be more rapid than that in Anwar et al’s20 study; however, the median pretreatment PSA level of 7.68 ng/mL in our study was slightly higher than that of 6.2 ng/mL in the report of Anwar et al. Shi et al25 described that a lower PSA at diagnosis had a lower PSA velocity following radiotherapy. However, the difference in the rate of PSA decline after radiotherapy may be due to underlying biologic differences between Asian and Western men, but any racial differences in PSA kinetics after hypofractionated radiotherapy need further studies.
Recent clinical evidence has demonstrated that the α/β ratios of prostate cancer may be around 2 Gy.6, 7 SBRT as monotherapy delivered in five fractions of 7.25 Gy has a biologic equivalent dose (BED) of 167.7 Gy, assuming an α/β ratio of 2 (e.g., BED2), and is comparable to a BED2 of 180 Gy with SBRT boost (3 fractions of 7 Gy) and WPRT (35 fractions of 1.8 Gy). Consistent with dose escalation trials that have showed a lower PSA nadir with an increase in total dose,26 we expect the SBRT monotherapy and SBRT boost regimen to produce a similar effect of a lower PSA nadir and rapid rate decline of PSA. In our study, the rate of PSA decline was not significantly different between SBRT as monotherapy and SBRT. Lamb et al27 showed that the postradiation nadir PSA is the strongest indicator. Zelefsky et al28 demonstrated that nadir PSA values of ≤1.5 ng/mL at 2 years after radiation therapy for prostate cancer could predict long-term distant metastases and cause-specific mortality. We consider the low nadir of 0.32 ng/mL in our report as an indication of a favorable outcome despite the limited follow-up.
In this study, PSA bounce was seen in 30.4% of patients after SBRT monotherapy and SBRT boost after WPRT with a median time of 12 months. McBride et al11 found that the mean age of those who experienced a bounce was significantly lower than those who did not. Vu et al29 reported that younger age was the only factor that predicted PSA bounce following SBRT for prostate cancer. Park et al30 showed that only the pretreatment PSA level was associated with an increased risk of PSA bounce. However, the pretreatment PSA level, Gleason score, and low-risk group were associated with PSA bounce in our study.
Zelefsky et al28 reported on late toxicity using a 81 Gy dose with IMRT in conventional fractionation. The 8-year actuarial likelihood of Grade II GI toxicity was 1.6%, and 0.1% of patients experienced Grade III rectal toxicity. The 8-year likelihood of late Grade II and III GU toxicities was 9% and 3%, respectively. Our current study shows a similar proportion of toxicity.
Our study is limited by the retrospective nature of analysis and a small number of patients. There were no strict protocols for the clinical decision-making process. Future studies should employ more comprehensive instruments to assess the effect of prostate SBRT.
In this report of low- and intermediate-risk prostate cancer patients, an initial period of rapid PSA decline was followed by a slow decline, which resulted in a lower PSA nadir. The PSA kinetics of SBRT monotherapy appears to be comparable to those achieved with SBRT boost with WPRT. Hypofractionated SBRT as monotherapy and SBRT boost combined with WPRT is a promising treatment option for men with low- and intermediate-risk prostate cancer. Continued accrual and follow-up would be necessary to confirm the biochemical control rate and toxicity profiles.
Conflicts of interest
The authors have no conflicts of interest or financial ties to disclose.
Acknowledgments
This work was supported by an INHA University research grant.
References
- 1.Siegel R., Naishadham D., Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Jung K.W., Won Y.J., Kong H.J., Oh C.M., Cho H.S., Lee H. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat. 2015;47:127–141. doi: 10.4143/crt.2015.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheets N.C., Goldin G.H., Meyer A.M., Wu Y., Chang Y., Sturmer T. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA. 2012;307:1,611–1,620. doi: 10.1001/jama.2012.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kupelian P.A., Langen K.M., Zeidan O.A., Meeks S.L., Willoughby T.R., Wagner T.H. Daily variations in delivered doses in patients treated with radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2006;66:876–882. doi: 10.1016/j.ijrobp.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Seisen T., Drouin S.J., Phe V., Parra J., Mozer P., Bitker M.O. Current role of image-guided robotic radiosurgery (Cyberknife) for prostate cancer treatment. BJU Int. 2013;111:761–766. doi: 10.1111/bju.12000. [DOI] [PubMed] [Google Scholar]
- 6.Fowler J.F., Ritter M.A., Chappell R.J., Brenner D.J. What hypofractionated protocols should be tested for prostate cancer? Int J Radiat Oncol Biol Phys. 2003;56:1093–1104. doi: 10.1016/s0360-3016(03)00132-9. [DOI] [PubMed] [Google Scholar]
- 7.Brenner D.J., Hall E.J. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys. 1999;43:1,095–1,101. doi: 10.1016/s0360-3016(98)00438-6. [DOI] [PubMed] [Google Scholar]
- 8.Katz A.J., Santoro M., Ashley R., Diblasio F. Stereotactic body radiotherapy for localized prostate cancer: disease control and quality of life at 6 years. Radiat Oncol. 2013;8 doi: 10.1186/1748-717X-8-118. 118–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L.N., Suy S., Uhm S., Oermann E.K., Ju A.W., Chen V. Stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer: the Georgetown University experience. Radiat Oncol. 2013;8:58–68. doi: 10.1186/1748-717X-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King C.R., Brooks J.D., Gill H., Presti J.R. Long-term outcomes from a prospective trial of stereotactic body radiotherapy for low-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82:877–882. doi: 10.1016/j.ijrobp.2010.11.054. [DOI] [PubMed] [Google Scholar]
- 11.McBride S.M., Wong D.S., Dombrowski J.J., Harkins B. Hypofractionated stereotactic body radiotherapy in low-risk prostate adenocarcinoma. Cancer. 2012;118:3,681–3,690. doi: 10.1002/cncr.26699. [DOI] [PubMed] [Google Scholar]
- 12.Jabbari S., Weinberg V.K., Kaprealian T., Hsu I.C., Ma L., Chuang C. Stereotactic body radiotherapy as monotherapy or post-external beam radiotherapy boost for prostate cancer: technique, early toxicity, and PSA response. Int J Radiat Oncol Biol Phys. 2012;82:228–234. doi: 10.1016/j.ijrobp.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Katz A.J., Santoro M., Ashley R., Diblasio F., Witten M. Stereotactic body radio-therapy as boost for organ-confined prostate cancer. Technol Cancer Res Treat. 2010;9:575–582. doi: 10.1177/153303461000900605. [DOI] [PubMed] [Google Scholar]
- 14.Oermann E.K., Slack R.S., Hanscom H.N., Lei S., Suy S., Park H.U. A pilot study of intensity modulated radiation therapy with hypofractionated stereo-tactic body radiation therapy (SBRT) boost in the treatment of intermediate- to high-risk prostate cancer. Technol Cancer Res Treat. 2010;9:453–462. doi: 10.1177/153303461000900503. [DOI] [PubMed] [Google Scholar]
- 15.Ciezki J.P., Reddy C.A., Garcia J., Angermeier K., Ulchaker J., Mahadevan A. PSA kinetics after prostate brachytherapy: PSA bounce phenomenon and its implications for PSA doubling time. Int J Radiat Oncol Biol Phys. 2006;64:512–527. doi: 10.1016/j.ijrobp.2005.07.960. [DOI] [PubMed] [Google Scholar]
- 16.Lee W.R., Hanlon A.L., Hanks G.E. Prostate specific antigen nadir following external beam radiation therapy for clinically localized prostate cancer: the relationship between nadir level and disease-free survival. J Urol. 1996;156:450–453. doi: 10.1097/00005392-199608000-00033. [DOI] [PubMed] [Google Scholar]
- 17.Pollack A., Zagars G.K., Antolak J.A., Kuban D.A., Rosen Prostate biopsy status and PSA nadir level as early surrogates for treatment failure: analysis of a prostate cancer randomized radiation dose escalation trial. Int J Radiat Oncol Biol Phys. 2002;54:677–685. doi: 10.1016/s0360-3016(02)02977-2. [DOI] [PubMed] [Google Scholar]
- 18.Shipley W.U., Thames H.D., Sandler H.M., Hanks G.E., Zietman A.L., Perez C.A. Radiation therapy for clinically localized prostate cancer: a multi-institutional pooled analysis. JAMA. 1999;281:1598–1604. doi: 10.1001/jama.281.17.1598. [DOI] [PubMed] [Google Scholar]
- 19.Zietman A.L., Tibbs M.K., Dallow K.C., Smith C.T., Althausen A.F., Zlotecki R.A. Use of PSA nadir to predict subsequent biochemical outcome following external beam radiation therapy for T1-2 adenocarcinoma of the prostate. Radiother Oncol. 1996;40:159–162. doi: 10.1016/0167-8140(96)01770-7. [DOI] [PubMed] [Google Scholar]
- 20.Anwar M., Weinberg V., Chang A.J., Hsu I.C., Roach M., 3rd, Gottschalk A. Hypofractionated SBRT versus conventionally fractionated EBRT for prostate cancer: comparison of PSA slope and nadir. Radiat Oncol. 2014;9:42–51. doi: 10.1186/1748-717X-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kole T.P., Chen L.N., Obayomi-Davies O., Kim J.S., Lei S., Suy S. Prostate specific antigen kinetics following robotic stereotactic body radiotherapy for localized prostate cancer. Acta Oncol. 2015;54:832–838. doi: 10.3109/0284186X.2014.983656. [DOI] [PubMed] [Google Scholar]
- 22.Network N.C.C. NCCN clinical practice guidelines in oncology (NCCN Guideline®): prostate cancer. Version 1. 2014. [Google Scholar]
- 23.Roach M., III, Hanks G., Thames H., Jr., Schellhammer P., Shipley W.U., Sokol G.H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO phoenix consensus conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 24.Patel C., Elshaikh M.A., Angermeier K., Ulchaker J., Klein E.A., Chehade N. PSA bounce predicts early success in patients with permanent iodine-125 prostate implant. Urology. 2004;63:110–113. doi: 10.1016/j.urology.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 25.Shi Z., Pinnock C.B., Kinsey-Trotman S., Borg M., Moretti K.L., Walsh S. Prostate-specific antigen (PSA) rate of decline post external beam radiotherapy predicts prostate cancer death. Radiother Oncol. 2013;107:129–133. doi: 10.1016/j.radonc.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 26.Martinez A.A., Gustafson G., Gonzalez J., Armour E., Mitchell C., Edmundson G. Dose escalation using conformal high-dose-rate brachytherapy improves outcome in unfavorable prostate cancer. Int J Radiat Oncol Biol Phys. 2002;53:316–327. doi: 10.1016/s0360-3016(02)02733-5. [DOI] [PubMed] [Google Scholar]
- 27.Lamb D.S., Denham J.W., Joseph D., Matthews J., Atkinson C., Spry N.A. A comparison of the prognostic value of early PSA test-based variables following external beam radiotherapy, with or without preceding androgen deprivation: analysis of data from the TROG 96.01 randomized trial. Int J Radiat Oncol Biol Phys. 2011;79:385–391. doi: 10.1016/j.ijrobp.2009.10.071. [DOI] [PubMed] [Google Scholar]
- 28.Zelefsky M.J., Chan H., Hunt M., Yamada Y., Shippy A.M., Amols H. Long-term outcome of high dose intensity modulated radiation therapy for patients with clinically localized prostate cancer. J Urol. 2006;176:1415–1419. doi: 10.1016/j.juro.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Vu C.C., Haas J.A., Katz A.E., Witten M.R. Prostate-specific antigen bounce following stereotactic body radiation therapy for prostate cancer. Front Oncol. 2014;4:8–16. doi: 10.3389/fonc.2014.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park Y.H., Choi I.Y., Yoon S.H., Jang H.S., Moon H.W., Hong S.H. Prostate-specific antigen kinetics after primary stereotactic body radiation therapy using Cyberknife for localized prostate cancer. Prostate Int. 2015;3:6–9. doi: 10.1016/j.prnil.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]