Abstract
The purposes of this study were to examine the relationship between male artificial insemination (AI) fertility and sperm acrosomal conditions assessed by new and conventional staining techniques and to identify possible reproductive dysfunctions causing low conception rates in AI using frozen-thawed spermatozoa with poor acrosomal conditions in Japanese Black bulls. We investigated individual differences among bulls in the results concerning (1) acrosomal conditions of frozen-thawed spermatozoa as assessed by not merely peanut agglutinin-lectin staining (a conventional staining technique) but also immunostaining of acrosomal tyrosine-phosphorylated proteins (a new staining technique), (2) routine AI using frozen-thawed spermatozoa as assessed by pregnancy diagnosis, (3) in vivo fertilization of frozen-thawed spermatozoa and early development of fertilized eggs as assessed by superovulation/AI-embryo collection tests and (4) in vitro fertilization of frozen-thawed spermatozoa with oocytes. The percentages of frozen-thawed spermatozoa with normal acrosomal conditions assessed by the abovementioned staining techniques were significantly correlated with the conception rates of routine AI, rates of transferable embryos in superovulation/AI-embryo collection tests and in vitro fertilization rates. These results are consistent with new suggestions that the distribution of acrosomal tyrosine-phosphorylated proteins as well as the acrosomal morphology of frozen-thawed spermatozoa are AI fertility-associated markers that are valid for the prediction of AI results and that low conception rates in AI using frozen-thawed spermatozoa with poor acrosomal conditions result from reproductive dysfunctions in the processes between sperm insemination into females and early embryo development, probably failed fertilization of frozen-thawed spermatozoa with oocytes.
Keywords: Acrosome, Artificial insemination, Cattle, Sperm, Subfertility
Artificial insemination (AI) is indispensable for wide use of spermatozoa collected from high-performance sires, and it has enabled us to produce a large number of high-performance offspring of these cattle. Moreover, it has also allowed us to limit the number of sires and to reduce the high costs of feeding and transportation of sires. Currently, bovine reproduction is almost always via AI techniques using frozen-thawed spermatozoa in Japan. To our annoyance, however, conception rates in AI programs for cattle are gradually decreasing in Japan and other countries [1, 2] (see http://liaj.or.jp/giken/gijutsubu/seieki/jyutai.htm). It is apparent that reduced AI fertility (AI subfertility) in cattle is due at least partially to female reproductive dysfunctions. The reproductive dysfunctions of females result in extension of the calving interval and increase of product costs, which have bad influences on business management for farms [2,3,4]. On the other hand, male reproductive dysfunctions may be the cause of this problem, because bulls with severe AI subfertility are occasionally found in AI centers. Although there have been several investigations regarding male fertility-associated factors in cattle [5,6,7,8,9,10,11], further efforts to determine male reproductive dysfunctions causing AI subfertility are absolutely necessary to recover the high conception rates in the AI program for cattle.
In our AI program, the reproductive performance of the bull is conventionally predicted by routine examination of sperm motility and morphological shape. However, a severe problem has recently been noted regarding the validity of this examination. Namely, AI using frozen-thawed spermatozoa with high motility and a normal shape from a certain bull resulted in considerably lower conception rates. We are deeply concerned about the increase of bulls which are classified as males with severe AI subfertility [9]. In our urgent experiments using only a limited number of Japanese Black bulls to address this severe problem, we found that frozen-thawed spermatozoa from one bull with severe AI subfertility frequently had severely damaged acrosomes and showed an abnormal distribution of tyrosine-phosphorylated proteins in the principal segment of the acrosome [12]. This observation suggested the possibility that the distribution of acrosomal tyrosine-phosphorylated proteins as well as the acrosomal morphology of frozen-thawed spermatozoa might be AI fertility-associated markers, though further experiments were required to evaluate this hypothesis.
In the first part of this study, we made investigations to examine the statistical significance of the relationship between AI fertility of Japanese Black bulls and acrosomal conditions of their frozen-thawed spermatozoa (assessed by immunodetection of tyrosine-phosphorylated proteins and by observation of acrosomal morphology). In the second part of this study, the purpose of which was to identify possible male reproductive dysfunctions causing the low conception rates in AI using frozen-thawed spermatozoa with poor acrosomal conditions, we conducted AI tests using frozen-thawed spermatozoa with different acrosomal conditions and superovulated females and then examined developmental ability of in vivo fertilized eggs by nonsurgical recovery of embryos from uteri. In the final part of this study, we carried out in vitro fertilization (IVF) tests using frozen-thawed spermatozoa with different acrosomal conditions.
Materials and Methods
Animals and experiment outline
The procedures of this study were approved in a research project plan titled “Improvement of fertility assay for Japanese Black bull spermatozoa” by the General Technological Center of Hyogo Prefecture for Agriculture, Forest and Fishery (General Technological Center).
This study was composed of (1) assessment of acrosomal conditions in frozen-thawed spermatozoa, (2) investigation of the AI fertility (as assessed by pregnancy diagnosis) of bulls by routine AI using frozen-thawed spermatozoa, (3) investigation of in vivo fertilization of frozen-thawed spermatozoa and early development of fertilized eggs by superovulation (SOV)/AI-embryo collection (EC) tests and (4) evaluation of in vitro fertilizing ability of frozen-thawed spermatozoa by IVF tests. All of the cattle used for the investigations in this study were Japanese Black cattle. Bulls (37 males, > 12 months old) were fed as candidate sires at the General Technological Center and used for semen collection using an artificial vagina to produce frozen spermatozoa. Frozen-thawed spermatozoa were used for the assessment of acrosomal conditions by fluorescein isothiocyanate (FITC)-conjugated peanut agglutinin (PNA)/propidium iodide (PI) staining (for samples from 37 bulls, A1–A9, B1–B9, C1–C9, D1–D9 and E1; No. of tests, 3–6 tests/bull; and No. of kinds of straw lot/bull, 1–4 lots/bull) and immunodetection of acrosomal tyrosine-phosphorylated proteins (for samples from 34 bulls, A1–A9, B1–B9, C1–C9 and D1–D7; No. of tests, 3–4 tests/bull; and No. of kinds of straw lot/bull, 1–3 lots/bull) [12]. The routine AIs (total number of AIs, 1413 tests; number of AIs per bull, 30–129 AIs) for frozen-thawed spermatozoa from 19 bulls (A2, A4–A9, B1–B9, D8, D9 and E1) were conducted at the General Technological Center and farms near the Center.
The SOV/AI-EC tests were carried out using delivered females (57 females, > 34 months old) of the General Technological Center and frozen-thawed spermatozoa from 16 bulls (A1, A3–A9, B1–B5 and C1–C3). In SOV/AI-EC tests, embryos (total number of examined embryos, 358 embryos; number of examined embryos per bull, 13–39 embryos) were collected and evaluated.
Moreover, the IVF tests were done using frozen-thawed spermatozoa from 5 bulls (A1–A5) and ovaries obtained from a local meat center.
Assessment of acrosomal conditions in frozen-thawed spermatozoa
Ejaculated spermatozoa were routinely frozen and then stored in liquid nitrogen as described previously [12]. Frozen-thawed spermatozoa were rapidly (for 10–15 sec) thawed in a warm water (at 37–39 C) immediately before use. Aliquots of the samples were used for subjective assessment of sperm motility. Briefly, they were put on a glass chamber for sperm motility assessment (Fujihira Industries, Tokyo, Japan) on a heated stage at 37 or 38.5 C and observed under a bright-field microscope in a conventional manner (subjective estimation by a technician). The samples were washed in phosphate-buffered saline (PBS) containing 0.1% (wt/vol) polyvinyl alcohol (PVA, Sigma-Aldrich, St. Louis, MO, USA) (PBS-PVA) by centrifugation (700 g, three times for 5 min). Washed spermatozoa were used for FITC-PNA/PI staining and immunodetection of acrosomal tyrosine-phosphorylated proteins by indirect immunofluorescence [12]. On each preparation, approximately 100 spermatozoa were observed under a differential interference microscope equipped with epifluorescence (mirror unit U-MWIB2: excitation filter BP 460-490, dichroic mirror DM 505, emission filter BA510IF, Olympus Optical, Tokyo, Japan). In this study, the spermatozoa with acrosomes that were classified into FITC-PNA staining patterns I and II [12] were considered those with normal acrosomes. Moreover, the distribution of acrosomal tyrosine-phosphorylated proteins was assessed according to the criteria shown in Supplementary Fig. 1 (online only).
Routine AI
Females were subjected to AI at natural estrus or estrus induced with an intramuscular injection of 150 μg of prostaglandin F2alpha (PGF2α) analog (d-cloprostenol, Dalmazin, Kyoritsu Seiyaku, Tokyo, Japan) at random stages of the estrous cycle. In this study, females that did not exhibit any estrus signs for more than 40 days after AI were examined for pregnancy by ultrasound examination and/or rectal palpation. Data were obtained in routine AI during the progeny tests at the General Technological Center and farms near the Center.
SOV/AI-EC tests
Female Japanese Black cattle received a controlled internal drug release (CIDR) containing progesterone (1.9 g, Zoetis Japan, Tokyo, Japan) in combination with intramuscular injection of gonadotropin-releasing hormone analog (Conceral injection containing 100 µg fertirelin acetate, Intervet, Tokyo, Japan) at random stages of the estrous cycle (Day 0) at the General Technological Center. Superovulation treatments were performed during the period between Day 7 and Day 9 with six intramuscular injections of gradually decreasing doses of porcine follicle-stimulating hormone (FSH, total dose of 20 AU, Antrin R10, Kyoritsu Seiyaku) at 12-h intervals. On the third day after the first FSH injection, the CIDR was removed, and 225 µg of PGF2α analog was intramuscularly administered. Two AIs were conducted using frozen-thawed spermatozoa, one at 12 h and one at 24 h after the onset of standing estrous. On Day 7.5 after the onset of standing estrus, embryos were collected nonsurgically, as described previously [13]. Recovered embryos were morphologically evaluated to determine rates of transferable embryos [embryos developed up to the stages between late morulae and expanded blastocysts with code 1 (excellent or good) according to the IETS manual] [14].
IVF tests
Collection and culture of oocytes for in vitro maturation were performed according to the procedures described previously with minor modifications [15]. Briefly, cumulus-oocyte complexes were aspirated from small follicles (3 to 5 mm in diameter) in the ovaries with a 5 ml disposable syringe and 18-gauge needle (Terumo, Tokyo, Japan). They were cultured for 21–22 h in a droplet (100 µl) of a medium [Medium 199 Earle's liquid (buffered with 25 mM Hepes, Life Technologies, Carlsbad, CA, USA), supplemented with 5% (vol/vol) fetal bovine serum (FBS, ICN Biomedicals, Aurora, OH, USA), 100 IU/ml penicillin G potassium (Meiji Seika Pharma, Tokyo, Japan) and 100 μg/ml streptomycin sulfate (Meiji Seika)] at 38.5 C in 5% (vol/vol) CO2 in air.
The frozen-thawed spermatozoa were washed in isotonic Percoll [2 ml 90% (vol/vol) and 2 ml 45% Percoll, GE Healthcare UK, Buckinghamshire, UK] to remove egg yolk-based extender and then washed twice in the IVF100 medium (Research Institute for the Functional Peptides, Yamagata, Japan). The final concentration of the spermatozoa was adjusted to 5 × 106/ml with the IVF100 medium. Droplets (100 µl) of the sperm suspension were put on plastic dishes. Finally, approximately 20 cumulus-oocyte complexes after culture were transferred to each droplet for sperm insemination, followed by 19 h of culture at 38.5 C in 5% CO2 in air. After insemination, the fertilized (or unfertilized) eggs were separated from the cumulus by gentle pipetting with a small-bore pipette and put on glass slides. The eggs on the glass slides were fixed with a fixative consisting of ethanol and acetic acid (3 parts:1 part) and stained with 1% (wt/vol) aceto-orcein solution. Successful fertilization was determined by the presence of two pronuclei.
In the IVF tests, additional lots of frozen-thawed spermatozoa (one lot for each bull) were used for assessments of acrosomal conditions and fertilization.
Statistical analyses
Spearman rank-order correlation coefficients of the values between two parameters were computed with Ekuseru-Toukei 2010 (Social Survey Research Information, Tokyo, Japan), which is an add-in software for the Japanese version of Microsoft Excel 2010 (Microsoft Japan, Tokyo, Japan). Differences in the values between two groups were analyzed using the two-tailed unpaired t-test after arcsine transformation with the abovementioned computer software.
Results
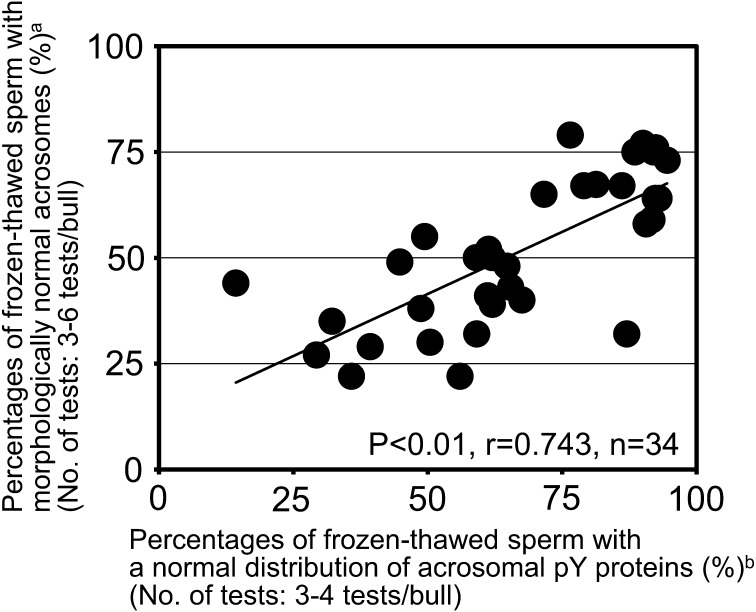
In the first part of the present study, we examined the statistical significance of the relationship between acrosomal conditions of frozen-thawed spermatozoa and results of routine AI using them. First, we observed individual differences in the morphologic and molecular acrosomal conditions of frozen-thawed spermatozoa (Fig. 1). The percentages of frozen-thawed spermatozoa with a normal distribution of acrosomal tyrosine-phosphorylated proteins largely varied among different bulls between 14% and 95% (mean ± standard error; 67 ± 4%, n = 34). Similar individual differences were observed in the percentages of frozen-thawed spermatozoa with morphologically normal acrosomes (values ranging between 22% and 79%, mean ± standard error; 51 ± 3%, n = 34). Moreover, there was a significantly positive correlation between these parameters (P < 0.01, r = 0.743, n = 34).
Fig. 1.
Relationship between acrosomal integrity and distributive normality of acrosomal tyrosine-phosphorylated (pY) proteins in frozen-thawed spermatozoa from Japanese Black bulls. Filled circles (●) indicate the results obtained for each of 34 bulls (A1–A9, B1–B9, C1–C9 and D1–D7). a Acrosomal integrity was examined by the fluorescein isothiocyanate (FITC)-conjugated peanut agglutinin (PNA)/propidium iodide (PI) staining. bDistributive normality of acrosomal pY proteins was examined by indirect immunofluorescence.
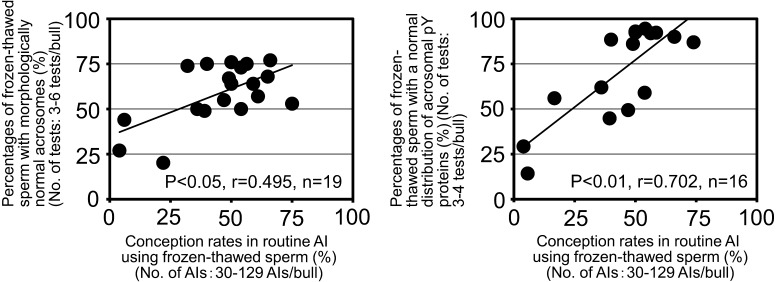
For bulls with different conception rates of routine AI [Fig. 2 left panel, n = 19 including three bulls with severe AI subfertility (conception rates, < 25%), rates between 4% and 75% and mean ± standard error of 46 ± 4% (cf. n = 16 excluding three bulls with severe AI subfertility, mean ± standard error of 52 ± 3%); right panel, rates between 4% and 74%, mean ± standard error of 44 ± 5%, n = 16 including three bulls with severe AI subfertility], we assessed acrosomal conditions of frozen-thawed spermatozoa. As expected, both the percentages of frozen-thawed spermatozoa with morphologically normal acrosomes (Fig. 2 left panel, P < 0.05, r = 0.495, n = 19) and the percentages of frozen-thawed spermatozoa with a normal distribution of acrosomal tyrosine-phosphorylated proteins (Fig. 2 right panel, P < 0.01, r = 0.702, n = 16) showed significant positive correlations with the conception rates of routine AI.
Fig. 2.
Relationship between acrosomal conditions of frozen-thawed spermatozoa and results of artificial insemination (AI) using frozen-thawed spermatozoa in Japanese Black bulls. Filled circles (●) indicate the results obtained for each of the bulls (left panel, 19 bulls, A2, A4–A9, B1–B9, D8, D9 and E1; right panel, 16 bulls A2, A4–A9 and B1–B9).
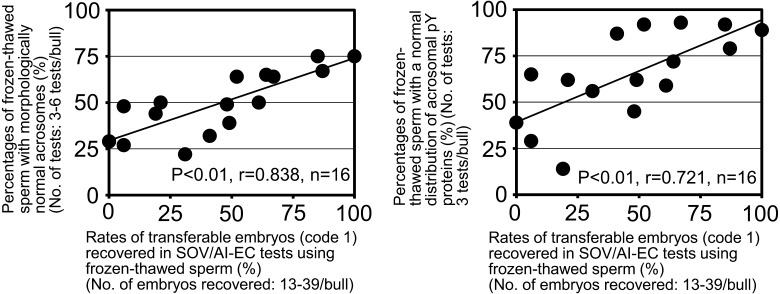
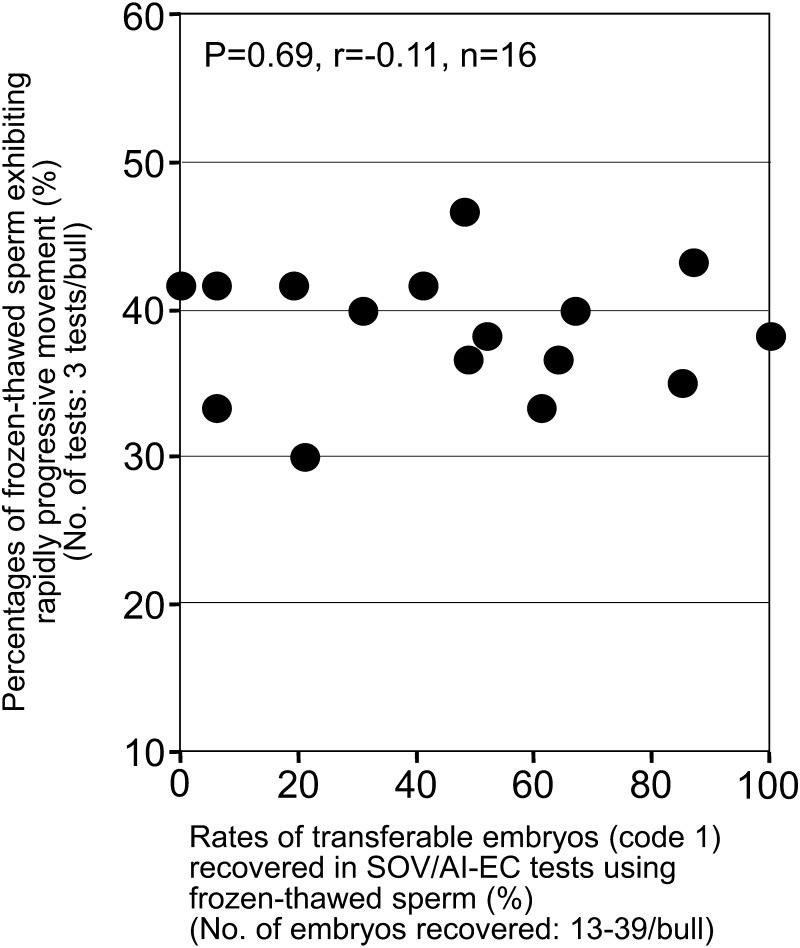
In the second part of this study, we examined the relationship between sperm acrosomal conditions and results of SOV/AI-EC tests. For bulls with different rates of transferable embryos in the SOV/AI-EC tests (Fig. 3, n = 16, rates between 0% and 100%, mean ± standard error of 46 ± 8%, No. of embryos recovered, 13–39 embryos/bull), we assessed acrosomal conditions of frozen-thawed spermatozoa. Both the percentages of frozen-thawed spermatozoa with morphologically normal acrosomes (Fig. 3 left panel, P < 0.01, r = 0.838, n = 16) and the percentages of frozen-thawed spermatozoa with a normal distribution of acrosomal tyrosine-phosphorylated proteins (Fig. 3 right panel, P < 0.01, r = 0.721, n = 16) showed significant positive correlations with the rates of transferable embryos in the SOV/AI-EC tests. In addition, the percentages of frozen-thawed spermatozoa exhibiting rapidly progressive movement showed only small variations among bulls examined in the SOV/AI-EC tests and did not show any significant correlations with the rates of transferable embryos in the SOV/AI-EC tests (Fig. 4, P = 0.69, r = –0.11, n = 16).
Fig. 3.
Relationship between acrosomal conditions of frozen-thawed spermatozoa and results of superovulation/artificial insemination-embryo collection (SOV/AI-EC) tests using frozen-thawed spermatozoa in Japanese Black bulls. Filled circles (●) indicate the results obtained for each of 16 bulls (A1, A3–A9, B1-B5 and C1–C3).
Fig. 4.
Relationship between rapidly progressive movement of frozen-thawed spermatozoa and results of SOV/AI-EC tests using frozen-thawed spermatozoa in Japanese Black bulls. Filled circles (●) indicate the results obtained for each of 16 bulls (A1, A3–A9, B1–B5 and C1–C3).
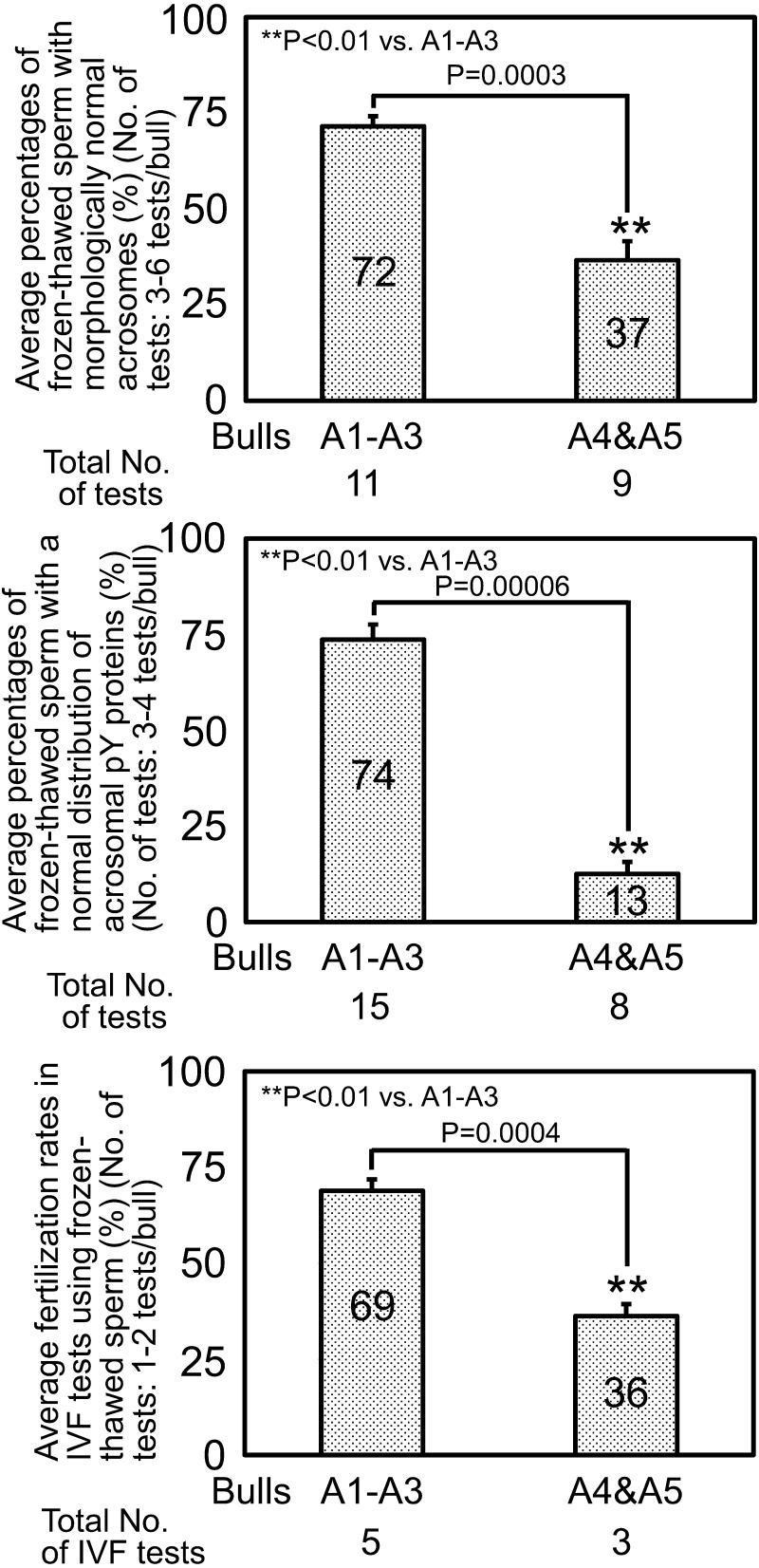
The IVF test is a good laboratory assay for evaluation of sperm fertilizing ability in vitro [16]. In the final part of this study, we assessed fertilization rates in the IVF tests among bulls with different acrosomal conditions in their frozen-thawed spermatozoa (Fig. 5). The average fertilization rate of bulls A4–A5 (relatively poor acrosomal conditions) (mean ± standard error of 36 ± 3%) was approximately half the value of that of bulls A1–A3 (relatively good acrosomal conditions) (mean ± standard error of 69 ± 3%).
Fig. 5.
Results of in vitro fertilization (IVF) tests using frozen-thawed spermatozoa with different acrosomal conditions in Japanese Black bulls. Values are means ± SEM.
Discussion
Among 34 Japanese Black bulls examined in this study (Fig. 1), the percentages of frozen-thawed spermatozoa with a normal distribution of acrosomal tyrosine-phosphorylated proteins (ranging between 14% and 95%) showed individual differences similar to those with morphologically normal acrosomes (ranging between 22% and 79%). Moreover, there was a significantly positive correlation between these parameters (P < 0.01, r = 0.743), indicating possible linkages between acrosomal tyrosine-phosphorylated proteins and morphological normality of the acrosomes. In our preliminary investigations of freshly ejaculated spermatozoa from 15 Japanese Black bulls fed at the same AI center [17], there were large individual differences in the percentages of frozen-thawed spermatozoa with a normal distribution of acrosomal tyrosine-phosphorylated proteins (22–99%). However, most of the freshly ejaculated spermatozoa (> 80%) had normal acrosomes in the ejaculates from these bulls. These findings suggest that the abnormal distribution of acrosomal tyrosine-phosphorylated proteins observed in frozen-thawed spermatozoa was probably detectable in freshly ejaculated spermatozoa and that morphological damage of the acrosomes occurred during the process for freezing-thawing of the spermatozoa.
Next, we analyzed the relationship between sperm acrosomal conditions and routine AI results (Fig. 2). The conception rates of routine AIs were significantly positive correlated with the percentages of frozen-thawed spermatozoa with a normal distribution of acrosomal tyrosine-phosphorylated proteins as well as those of frozen-thawed spermatozoa with morphologically normal acrosomes. These results support the suggestion we previously made with only a limited number of bulls [12] that poor acrosomal conditions of frozen-thawed spermatozoa are related to the occurrence of AI subfertility in Japanese Black bulls. Moreover, the results indicate that the distribution of acrosomal tyrosine-phosphorylated proteins in frozen-thawed spermatozoa is an AI fertility-associated marker that is valid for the prediction of AI results.
Research using genetically modified mice has demonstrated that successful IVF of spermatozoa with oocytes requires active serine proteases (acrosin and testisin) of the acrosomal contents [18] but that acrosome-reacted spermatozoa without intact acrosomes can penetrate the zona pellucida and then ooplasm in vitro [19, 20]. In the first part of this study, however, we showed that AI using frozen-thawed spermatozoa with poor acrosomal conditions (namely without intact acrosomes) resulted in low conception rates (Fig. 2). This indicates that the normal acrosomal conditions of frozen-thawed spermatozoa are important for the maintenance of high conception rates in an AI program. Thus, we attempted to determine possible reproductive dysfunctions causing low conception rates in AI using frozen-thawed spermatozoa with poor acrosomal conditions by analyzing the relationships between sperm acrosomal conditions and results of SOV/AI-EC tests in the second part of this study (Figs. 3 and 4). The obtained data indicate that AI of frozen-thawed spermatozoa with poor acrosomal conditions is often accompanied by reproductive dysfunctions in the processes before embryo development up to the blastocyst stage, possibly by failed fertilization of frozen-thawed spermatozoa in vivo and/or in vivo development of early embryos. Moreover, our IVF tests in the final part of this study showed that in vitro fertilization rates were significantly lower in the frozen-thawed spermatozoa with poor acrosomal conditions (Fig. 5). This result suggests that poor acrosomal conditions reduce the penetrability of bull frozen-thawed spermatozoa into zona pellucida and ooplasm in vitro.
In conclusion, to our knowledge, this is the first report indicating that low conception rates in AI using frozen-thawed spermatozoa with poor acrosomal conditions in Japanese Black cattle result from reproductive dysfunctions in the processes between sperm insemination into females and early embryo development, probably from failed fertilization of frozen-thawed spermatozoa with oocytes. Our investigations of the reproductive dysfunctions causing male AI subfertility should contribute to the recovery of high conception rates in AI programs for Japanese Black cattle.
Supplementary
Acknowledgments
This work was supported in part by Grant-in-Aid 25450394 from the Japan Society for the Promotion of Science to HH.
References
- 1.Barbat A, Le Mézec P, Ducrocq V, Mattalia S, Fritz S, Boichard D, Ponsart C, Humblot P. Female fertility in French dairy breeds: current situation and strategies for improvement. J Reprod Dev 2010; 56(Suppl): S15–S21. [DOI] [PubMed] [Google Scholar]
- 2.Dochi O, Kabeya S, Koyama H. Factors affecting reproductive performance in high milk-producing Holstein cows. J Reprod Dev 2010; 56(Suppl): S61–S65. [DOI] [PubMed] [Google Scholar]
- 3.Nabenishi H, Ohta H, Nishimoto T, Morita T, Ashizawa K, Tsuzuki Y. Effect of the temperature-humidity index on body temperature and conception rate of lactating dairy cows in southwestern Japan. J Reprod Dev 2011; 57: 450–456. [DOI] [PubMed] [Google Scholar]
- 4.Kadokawa H, Sakatani M, Hansen PJ. Perspectives on improvement of reproduction in cattle during heat stress in a future Japan. Anim Sci J 2012; 83: 439–445. [DOI] [PubMed] [Google Scholar]
- 5.Cancel AM, Chapman DA, Killian GJ. Osteopontin is the 55-kilodalton fertility-associated protein in Holstein bull seminal plasma. Biol Reprod 1997; 57: 1293–1301. [DOI] [PubMed] [Google Scholar]
- 6.Gerena RL, Irikura D, Urade Y, Eguchi N, Chapman DA, Killian GJ. Identification of a fertility-associated protein in bull seminal plasma as lipocalin-type prostaglandin D synthase. Biol Reprod 1998; 58: 826–833. [DOI] [PubMed] [Google Scholar]
- 7.Murase T, Mukaijima K, Sakaguchi S, Ohtani T, Tubota T, Kita I. Characterization of frozen-thawed Japanese Black bull spermatozoa by standard semen analysis, mucus penetration test and the ability to undergo the acrosome reaction in response to calcium and the calcium ionophore A23187. J Reprod Dev 2001; 47: 237–243. [Google Scholar]
- 8.Kohsaka T, Hamano K, Sasada H, Watanabe S, Ogine T, Suzuki E, Nishida S, Takahara H, Sato E. Seminal immunoreactive relaxin in domestic animals and its relationship to sperm motility as a possible index for predicting the fertilizing ability of sires. Int J Androl 2003; 26: 115–120. [DOI] [PubMed] [Google Scholar]
- 9.Kuroda K, Fukushima M, Harayama H. Premature capacitation of frozen-thawed spermatozoa from subfertile Japanese black cattle. J Reprod Dev 2007; 53: 1079–1086. [DOI] [PubMed] [Google Scholar]
- 10.Karoui S, Díaz C, González-Marín C, Amenabar ME, Serrano M, Ugarte E, Gosálvez J, Roy R, López-Fernández C, Carabaño MJ. Is sperm DNA fragmentation a good marker for field AI bull fertility? J Anim Sci 2012; 90: 2437–2449. [DOI] [PubMed] [Google Scholar]
- 11.Dogan S, Vargovic P, Oliveira R, Belser LE, Kaya A, Moura A, Sutovsky P, Parrish J, Topper E, Memili E. Sperm protamine-status correlates to the fertility of breeding bulls. Biol Reprod 2015; 92: 92. [DOI] [PubMed] [Google Scholar]
- 12.Harayama H, Nishijima K, Murase T, Sakase M, Fukushima M. Relationship of protein tyrosine phosphorylation state with tolerance to frozen storage and the potential to undergo cyclic AMP-dependent hyperactivation in the spermatozoa of Japanese Black bulls. Mol Reprod Dev 2010; 77: 910–921. [DOI] [PubMed] [Google Scholar]
- 13.Kawate N, Sakase M, Watanabe K, Fukushima M, Noda M, Takeda K, Ueno S, Inaba T, Kida K, Tamada H, Sawada T. Ovsynch plus CIDR protocol for timed embryo transfer in suckled postpartum Japanese Black beef cows. J Reprod Dev 2007; 53: 811–817. [DOI] [PubMed] [Google Scholar]
- 14.Robertson I, Nelson RE. Certification and identification of the embryo. In: Stringfellow, DA, Seidel SM (eds.), Manual of the International Embryo Transfer Society, 3rd edition. Savoy, IL: International Embryo Transfer Society; 1998: 103–134.
- 15.Imai K, Matoba S, Dochi O, Shimohira I. Different factors affect developmental competence and cryotolerance in in vitro produced bovine embryo. J Vet Med Sci 2002; 64: 887–891. [DOI] [PubMed] [Google Scholar]
- 16.Graham JK, Mocé E. Fertility evaluation of frozen/thawed semen. Theriogenology 2005; 64: 492–504. [DOI] [PubMed] [Google Scholar]
- 17.Arai M, Minami K, Ogura Y, Sakase M, Fukushima M, Harayama H.Effects of distribution of tyrosine-phosphorylated proteins on the acrosomal stability in Japanese Black bull spermatozoa. J Reprod Dev 2014; 60 (Suppl): j111.(abstract P-24, in Japanese). [Google Scholar]
- 18.Kawano N, Kang W, Yamashita M, Koga Y, Yamazaki T, Hata T, Miyado K, Baba T. Mice lacking two sperm serine proteases, ACR and PRSS21, are subfertile, but the mutant sperm are infertile in vitro. Biol Reprod 2010; 83: 359–369. [DOI] [PubMed] [Google Scholar]
- 19.Jin M, Fujiwara E, Kakiuchi Y, Okabe M, Satouh Y, Baba SA, Chiba K, Hirohashi N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc Natl Acad Sci USA 2011; 108: 4892–4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inoue N, Satouh Y, Ikawa M, Okabe M, Yanagimachi R. Acrosome-reacted mouse spermatozoa recovered from the perivitelline space can fertilize other eggs. Proc Natl Acad Sci USA 2011; 108: 20008–20011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.