Abstract
Our aim was to optimize the cryoprotectant treatment for the preservation of immature porcine cumulus-oocyte complexes (COCs) by solid surface vitrification. In each experiment, the vitrification solution consisted of 50 mg/ml polyvinyl pyrrolidone, 0.3 M of the actual sugar and in total 35% (v/v) of the actual permeating cryoprotectant (pCPA) combination. After warming, the COCs were subjected to in vitro maturation, fertilization and embryo culture. In Experiment 1, trehalose and sucrose were equally effective during vitrification and warming in terms of facilitating oocyte survival and subsequent embryo development. In Experiment 2, when equilibration was performed at 38.5 C in a total of 4% (v/v) pCPA for 15 min, the combination of ethylene glycol and propylene glycol (EG + PG = 1:1) was superior to EG and dimethyl sulfoxide (EG + DMSO = 1:1) in terms of oocyte survival after vitrification and the quality of resultant blastocysts. In Experiment 3, equilibration in 4% (v/v) pCPA for 15 min before vitrification was superior to that in 15% (v/v) CPA for 5 min for achievement of high survival rates irrespective of the pCPA combination used. In Experiment 4, when equilibration was performed in 4% EG + PG for 5 min, 15 min or 25 min, there was no difference in oocyte survival and subsequent embryo development after vitrification and warming; however, the developmental competence of cleaved embryos was tendentiously reduced when equilibration was performed for 25 min. In conclusion, trehalose and sucrose were equally effective in facilitating vitrification, and the optimum pCPA treatment was 5–15 min equilibration in 4% (v/v) of EG + PG followed by vitrification in 35% (v/v) EG + PG.
Keywords: Cryoprotectant, Equilibration, Immature oocyte, Porcine, Vitrification
Oocyte cryopreservation combined with assisted reproductive techniques is a potent way for gene banking of female germplasm [1]. Compared with other species, oocyte cryopreservation in pigs is still considered a challenge because of the enormous sensitivity of porcine oocytes to low temperatures [2]. Application of vitrification techniques to matured porcine oocytes has resulted in limited rates of embryo development after in vitro fertilization (IVF) or intracytoplasmic sperm injection [2,3,4]. In recent years, we have been developing a protocol for the solid surface vitrification (SSV) of immature porcine oocytes, which resulted in high survival rates and the production of piglets for the first time [5]. Nevertheless, the frequency of blastocyst development from vitrified oocytes is still low, despite their normal ability to undergo in vitro maturation and fertilization [5]. Therefore, further optimization of this protocol is necessary.
Previously, we demonstrated that the permeating cryoprotectants (CPAs) used for SSV greatly determine the survival of immature porcine oocytes after warming, with the use of a CPA with high permeability (such as propylene glycol) being associated with high survival [6]. Furthermore, it has been demonstrated that combinations of different CPAs give better results than the use of a single type of CPA in pigs [6] as well as in other mammalian species [7]. To date, three main vitrification techniques have been applied to porcine oocytes with various results: the SSV [5, 6, 8,9,10,11,12], Cryotop [13,14,15,16,17,18,19,20] and Open Pulled Straw (OPS) [15, 18, 21,22,23,24,25] methods. Although the main difference among these techniques is the cryodevice (i.e., the carrier that gives the distinctive name to each method), these methods also differ in terms of the CPA treatment including the type of sugars and permeating CPA, their concentrations during equilibration and the duration of equilibration (Table 1). To complicate things further, a number of studies using the same carrier applied fundamentally different CPA treatment regimens (Table 1). In general, we can conclude that these methods use ethylene glycol (EG) alone or EG combined with either dimethyl sulfoxide (DMSO) or propylene glycol (PG) as the permeating CPA. Also, as a non-permeating CPA, either trehalose or sucrose is often applied during vitrification (Table 1). To our knowledge, the distinctive features of CPA treatment regimens applied by different reports using different vitrification methods have not been compared for the vitrification of porcine oocytes using a single carrier. Therefore, the optimum CPA treatment protocol for this purpose has remained a matter of debate. The aim of the present study was to find out the optimum CPA treatment protocol for the vitrification of immature porcine oocytes by a fixed vitrification method (SSV), which allowed quick preservation of cumulus-enclosed oocytes in a group of approximately 100 per treatment. To do so, we compared 1) two of the most frequently used sugars (trehalose vs. sucrose), 2) three combinations of permeating CPA (EG + DMSO vs. EG + PG vs. EG + DMSO + PG) and 3) two common but fundamentally different equilibration regimens (4% CPA for 15 min vs. 15% CPA for 5 min) and 4) determined the optimum length for the equilibration treatment.
Table 1. Overview of vitrification methods and cryoprotectant treatment regimens used in previous reports for porcine oocyte cryopreservation.
| Method (device) |
Equilibration |
Vitrification solution |
Highest oocyte survival reported (if available) |
References | ||||
| Steps | Permeable CPA (% v/v) | Total duration (min) |
Permeable CPA (% v/v) | Sugar (M) | Total duration (min) |
|||
| SSV | 1 | EG (4%) | 15 min | EG (35%) | Sucrose (0.4 M) | 30–60 sec | GV: 80% (approx.) | [9] |
| MII: 80% (approx.) | ||||||||
| SSV | 1 | EG (4%) | 15 min | EG (35%) | Trehalose (0.3 M) | 30–60 sec | GV: 27.7% | [10, 11] |
| MII: 61.4% | ||||||||
| SSV | 1 | EG (2%) + PG (2%) | 13–15 min | EG (17.5%) + PG (17.5%) | Trehalose (0.3 M) | 30–40 sec | GV: 42.3–87.1% | [5, 12] |
| MII: 85.4% | ||||||||
| Cryotop | 1 | EG (7.5%) + DMSO (7.5 %) | 5–15 min | EG (15%) + DMSO (15%) | Sucrose (0.5 M) | 30–60 sec | MII: 52–99.2% | [14, 15, 17] |
| Cryotop | 1 | EG (15%) | 5–9 min | EG (30%) | Sucrose (0.5 M) | 30–60 sec | MII: 80% (approx.) | [19] |
| Cryotop | 1 | EG (4%) | 15 min | EG (35%) | Trehalose (0.4 M) | 30–60 sec | GV: 4% | [18] |
| Cryotop | 2 | EG (7.5 → 15%) | 1–4 min | EG (30–35%) | Sucrose (0.5–0.6 M) | 30–60 sec | GV: 40% (approx.) | [13, 16, 20] |
| MII: 80% (approx.) | ||||||||
| OPS | 1 | EG (10%) + DMSO (10%) | 3 (5)–10 min | EG (20%) + DMSO (20%) | Sucrose (0.6 M) | 30–50 sec | MII: 68.2% | [15, 23, 24] |
| OPS | 6 | EG (5→10→15→20→25→30%) | 12.5 min | EG (40%) | None | 25 sec | No data | [21] |
| OPS | 1 | EG (10%) + DMSO (10%) | 90 sec | EG (20%) + DMSO (20%) | Sucrose (0.3 M) | 30 sec | MII: 62.1% | [22] |
| OPS | 2 | EG (0.6%) + FOR (0.5%) + DMSO (0.8%) → EG (3.6%) + FOR (2.8%) + DMSO (4.8%) | 4 min | EG (12.6%) + FOR (9.6%) + DMSO (16.7%) | None | 60 sec | MII: 98.8% | [25] |
SSV = solid surface vitrification; OPS = open pulled straw; CPA = cryoprotectant agent; EG = ethylene glycol; PG = propylene glycol; DMSO = dimethyl sulfoxide; FOR = formamide; GV = germinal vesicle (stage); MII = metaphase II (stage).
Materials and Methods
Collection and vitrification of cumulus-oocyte complexes (COCs)
Ovaries from prepubertal cross-bred gilts (Landrace × Large White) were collected at a local slaughterhouse and brought to the laboratory within 1 h in Dulbecco’s phosphate-buffered saline (PBS) (Nissui Pharmaceutical, Tokyo, Japan) at 35−37 C. COCs were collected by scraping follicles 3 to 6 mm in diameter into a collection medium consisting of medium 199 (with Hank’s salts; Sigma-Aldrich, St. Louis, MO, USA) supplemented with 5% fetal bovine serum (Gibco; Invitrogen, Carlsbad, CA, USA), 20 mM HEPES (Dojindo Laboratories, Kumamoto, Japan) and antibiotics [100 units/ml penicillin G potassium (Sigma-Aldrich) and 0.1 mg/ml streptomycin sulfate (Sigma-Aldrich)]. The COCs were cryopreserved as reported previously [26], with modifications. In brief, COCs were treated for 30 min in a basic medium (BM) consisting of modified NCSU-37 [27] without glucose but supplemented with 20 mM HEPES, 50 µM β-mercaptoethanol, 0.17 mM sodium pyruvate and 2.73 mM sodium lactate. The BM medium was further supplemented with 4 mg/ml bovine serum albumin (BSA; Fraction V, Sigma-Aldrich) and 7.5 µg/ml cytochalasin B (C-6762, Sigma-Aldrich) for the first 30 min treatment before equilibration. Further treatments varied in terms of the components of the equilibration and vitrification media and the treatment intervals among experiments (see Experimental design). In general, for equilibration, a group of approximately 100 COCs were treated all at once in an equilibration medium comprised of BM supplemented with 4 mg/ml BSA, 7.5 µg/ml cytochalasin B and either 4% (v/v) or 15% (v/v) of a permeating CPA combination for 15 min or 5 min, respectively, at 38.5 C according to the experimental design. The vitrification solution was BM supplemented with 50 mg/ml polyvinylpyrrolidone (P-0930, Sigma-Aldrich), 0.3 M trehalose (T-0167, Sigma-Aldrich) or 0.3 M sucrose (196-00015, Wako Pure Chemical Industries, Osaka, Japan,) and, in total, 35% (v/v) of a permeating CPA combination, depending on the experimental design. After equilibration, approximately 50 COCs were picked up, washed three times in 20-µl drops of vitrification solution at 38.5 C, pipetted into a glass capillary tube and then finally dropped in 2–3 microdrops (each about 2–3 µl,. containing 10–25 COCs) onto aluminum foil floating on the surface of liquid nitrogen (LN). Washing in vitrification medium and placement of microdrops with COCs onto the cold aluminum surface were performed over the course of about 30–40 sec. The vitrified droplets were transferred to 2-ml cryotubes (Iwaki 2732-002; AGC Techno Glass, Tokyo, Japan) partly immersed in LN and then stored in LN until use. Before the start of the warming procedure, the warming medium (kept airtight in 15 ml centrifuge tubes) was pre-warmed to 42 C in a dry block tube heater fitted on a hot plate (SP-45D, Hirasawa, Tokyo, Japan). Vitrified droplets were warmed by transfer into 2.5 ml of a warming solution, which was either 0.4 M trehalose or 0.4 M sucrose depending on the experimental design in BM, supplemented with 4 mg/ml BSA in a 35-mm plastic dish (Falcon 351008, Thomas Scientific, Swedesboro, NJ, USA) kept on a hot plate at 42 C according to our previous report [5]. One to two minutes later, oocytes were consecutively transferred for periods of 1 min (each) to 500-µl droplets of BM supplemented with 0.2, 0.1 or 0.05 M trehalose or sucrose (see Experimental design) at 38.0 C. They were then washed in BM without trehalose/sucrose at 38.0 C and subjected to in vitro maturation (IVM).
IVM
The maturation culture medium was a modified NCSU-37 solution [27] containing 10% (v/v) porcine follicular fluid, 0.6 mM cysteine, 50 μM β-mercaptoethanol, 1 mM dibutyryl cAMP (dbcAMP; Sigma), 10 IU/ml eCG (Serotropin; ASKA Pharmaceutical, Tokyo, Japan) and 10 IU/ml hCG (500 units; Puberogen, Novartis Animal Health, Tokyo, Japan). Maturation was performed in 4-well dishes (Nunc MultiDishes, Thomas Scientific) in 500-µl droplets of IVM medium without oil coverage for 22 h in an atmosphere of 5% CO2, 5% O2 and 90% N2 at 39 C. The COCs were subsequently cultured in the maturation medium without dbcAMP and hormones for an additional 22 h under the same atmosphere. Forty to 50 COCs were cultured in each droplet.
IVF, evaluation of oocyte survival and in vitro embryo culture
The IVF and in vitro embryo culture (IVC) procedures were performed by the method of Kikuchi et al. [28]. The medium used for IVF was a modified Pig-FM [29] medium containing 10 mM HEPES, 2 mM caffeine and 5 mg/ml BSA. After being washed 3 times in IVF medium, all cumulus-enclosed oocytes were transferred into 90-µl IVF droplets (approximately 20 oocytes in each droplet) covered by paraffin oil (Paraffin Liquid; Nacalai Tesque, Kyoto, Japan). Frozen-thawed epididymal spermatozoa from a Landrace boar were preincubated at 37 C in medium 199 (with Earle’s salts, Gibco, pH adjusted to 7.8) for 15 min [30]. To obtain the final sperm concentration (1 × 105 cells/ml), 10 µl of the sperm suspension was introduced into the IVF medium containing oocytes and co-incubated for 3 h at 39 C under 5% CO2, 5% O2 and 90% N2. The day of IVF was defined as day 0. At the end of IVF, spermatozoa and cumulus cells were removed from the surface of the zona pellucida by gentle pipetting with a fine glass pipette. At this time, oocyte viability was assessed under a stereomicroscope. Oocytes with clear signs of membrane damage (brownish, faded cytoplasm) were removed, and only oocytes with a normal spherical shape, smooth surface and dark and evenly granulated cytoplasm were considered live. Only live oocytes were used for IVC, which was performed in 500-µl drops of IVC-PyrLac on days 0 to 2 and 500-µl drops of IVC-Glu on days 2 to 6 [28] in 4-well dishes in an atmosphere of 5% CO2, 5% O2 and 90% N2 at 39 C. IVC-PyrLac was BM medium without HEPES supplementation. IVC-Glu was the original NCSU-37 medium containing 5.55 mM glucose and supplemented with 4 mg/ml BSA and 50 µM β-mercaptoethanol.
Evaluation of maturation and fertilization
The maturation and fertilization statuses of oocytes were assessed 10 h after IVF. In each experimental replication, 25 oocytes were mounted on glass slides in each group and fixed with acetic alcohol (acetic acid 1:ethanol 3) for at least 5 days, stained with 1% (w/v) orcein (Sigma) in acetic acid, rinsed in glycerol:acetic acid:water (1:1:3) and then examined under a phase-contrast microscope with × 40 or × 100 objectives. The status of the oocyte chromatin, the presence and numbers of female and male pronuclei and/or the sperm head(s) and existence of the first and second polar bodies (1PB and 2PB, respectively) were investigated in the oocytes. When a nuclear envelope was not detected around the female chromatin, the oocyte was considered one that had undergone germinal vesicle breakdown (GVBD). Oocytes were considered matured when they had a metaphase plate and 1PB (unfertilized metaphase-II stage oocytes) or if they were penetrated and had both a 1PB and 2PB (fertilized oocytes progressing beyond the metaphase-II stage). Oocytes were considered penetrated when a sperm head(s), male pronucleus with the corresponding sperm tail or both were detected in the cytoplasm. Oocytes with one penetrating sperm in the cytoplasm were defined as monospermic. Normal fertilization was defined by the presence of 1 female pronucleus and 1 male pronucleus and extrusion of both the 1 PB and 2PB. Frequencies of total penetration and normal fertilization were calculated as their percentage from GVBD oocytes, since at the end of IVM, porcine oocytes arrested at any stage beyond the germinal vesicle (GV) stage show similar fertilization and monospermy rates [31].
Evaluation of embryo development and blastocyst cell numbers
Cleavage rates on day 2, blastocyst rates on day 6 and total cell numbers in blastocysts on day 6 were recorded. On day 2, embryos with 2−4 blastomeres were considered cleaved [32]. On day 6, expanded embryos without a visible perivitelline space containing more than 10 blastomeres and a blastocoel were considered blastocysts. To assess total cell numbers in embryos, blastocysts were placed in 25 µg/ml Hoechst 33342 (H33342, Calbiochem, San Diego, CA, USA) dissolved in 99.5% ethanol and kept at 4 C for overnight. After washing in 99.5% ethanol, they were mounted on glass slides in glycerol droplets, flattened by cover slips and examined under UV light with an excitation wavelength of 330–385 nm by using an epifluorescence microscope (IX-71, Olympus, Tokyo, Japan). A digital image of each embryo was taken, and the total numbers of nuclei labeled by H33342 were counted using the NIH ImageJ (v. 1.40) software [33].
Experimental design
Experiment 1: This experiment was performed to compare the feasibilities of trehalose and sucrose for oocyte vitrification and warming. Immature COCs were equilibrated for 15 min in a medium containing 2% (v/v) EG (E-9129, Sigma-Aldrich) + 2% (v/v) PG (29218-35, Nacalai Tesque). In all groups, oocytes were treated with 7.5 µg/ml cytochalasin B for 45 min (30 min pretreatment + 15 min equilibration) before vitrification as described above. Vitrification was performed in a medium containing 50 mg/ml PVP and 17.5% (v/v) EG + 17.5% (v/v) PG supplemented either with 0.3 M trehalose (vitrified trehalose group) or 0.3 M sucrose (vitrified sucrose group). Warming of vitrified COCs was performed in media supplemented either with 0.4 M trehalose (vitrified trehalose group) or 0.4 M sucrose (vitrified sucrose group) as detailed above. After warming, all vitrified COCs were subjected to IVM and IVF. Viability status of oocytes was evaluated after IVF. Live oocytes were subsequently used either for the assessment of nuclear and fertilization statuses or for embryo culture.
Survival, maturation, fertilization rates after IVM/IVF, cleavage and blastocyst developmental rates and blastocyst cell numbers during culture in the two vitrified groups were compared with those of non-vitrified COCs (control). Four replications were performed.
Experiment 2: This experiment was performed to compare the feasibilities of 3 combinations of permeating CPA for the vitrification of immature COCs. Immature COCs were equilibrated for 15 min in a medium containing a total of 4% (v/v) permeating CPA, which was 2% (v/v) EG + 2% (v/v) PG (vitrified EG+PG group), 2% (v/v) EG + 2% (v/v) DMSO (043-07216, Wako) (vitrified EG+DMSO group) or 1.33% (v/v) EG + 1.33% (v/v) PG + 1.33% (v/v) DMSO (vitrified EG+PG+DMSO group). In all groups, oocytes were treated with 7.5 µg/ml cytochalasin B for 45 min (30 min pretreatment + 15 min equilibration) before vitrification as described above. Then COCs were vitrified in media supplemented with 50 mg/ml PVP and 0.3 M sucrose (based on the results of Experiment 1) containing a total of 35% (v/v) permeating CPA, which was 17.5% (v/v) EG + 17.5% (v/v) PG (vitrified EG+PG group), 17.5% (v/v) EG + 17.5% (v/v) DMSO (vitrified EG+DMSO group) or 11.66% (v/v) EG + 11.66% (v/v) PG + 11.66% (v/v) DMSO (vitrified EG + PG + DMSO group). Warming of vitrified COCs was performed in media supplemented with sucrose as detailed above. After warming, all vitrified COCs were subjected to IVM and IVF. Viability status of oocytes was evaluated after IVF. Live oocytes were subsequently used either for assessment of their nuclear and fertilization statuses or for embryo culture. Survival, maturation of the oocytes, fertilization rates after IVM/IVF, cleavage and blastocyst developmental rates and also blastocyst cell numbers during culture in the three vitrified groups were compared to those of non-vitrified COCs (control). Four replications were performed.
Experiment 3: This experiment was performed to compare the feasibilities of two frequently used equilibration regimens for the vitrification of immature COCs. Immature COCs were equilibrated in a medium containing 2% (v/v) EG + 2% (v/v) PG for 15 min (Regimen A/EG + PG), 7.5% (v/v) EG + 7.5% (v/v) PG for 5 min (Regimen B/EG + PG) or 7.5% (v/v) EG + 7.5% (v/v) DMSO for 5 min (Regimen B/EG + DMSO). (Note: In this experiment, Regimen A/ EG + DMSO was not examined because it was compared with Regimen A/EG + PG in Experiment 1). In all groups, oocytes were treated with 7.5 µg/ml cytochalasin B for 45 min (30 min pretreatment + 15 min equilibration for Regimen A or 40 min pretreatment + 5 min equilibration for Regimen B) before vitrification as described above. Vitrification was performed in a medium containing 50 mg/ml PVP, 0.3 M sucrose and either 17.5% (v/v) EG + 17.5% (v/v) PG (for the Regimen A/EG + PG and Regimen B/EG + PG groups) or 17.5% (v/v) EG + 17.5% (v/v) DMSO (for the Regimen B/EG + DMSO group). Warming of vitrified COCs was performed in media supplemented with sucrose as detailed above. After warming, all vitrified COCs were subjected to IVM and IVF. Viability status of oocytes was evaluated after IVF. All live oocytes were subjected to embryo culture. Oocyte survival, cleavage and blastocyst developmental rates during culture in the three vitrified groups were compared with those of non-vitrified COCs (control). Three replications were performed.
Experiment 4: This experiment was performed to determine the optimum interval for equilibration treatment in a total of 4% permeating CPA. Immature COCs were equilibrated in a medium containing 2% (v/v) EG + 2% (v/v) PG for 5 min (5 min group), 15 min (15 min group) or 25 min (25 min group). (Note: The equilibration medium contained 7.5 µg/ml cytochalasin B as mentioned above. In order to provide a total of 45 min of treatment with cytochalasin B in each group, 5 min, 15 min and 25 min groups were pretreated cytochalasin B in rinsing medium for 40 min, 30 min and 20 min, respectively). Then COCs were vitrified in a medium containing 50 mg/ml PVP, 0.3 M sucrose and 17.5% (v/v) EG + 17.5% (v/v) PG. Warming of vitrified COCs was performed in media supplemented with sucrose as detailed above. After warming, all vitrified COCs were subjected to IVM and IVF. Viability status of oocytes was evaluated after IVF. All live oocytes were subjected to embryo culture. Oocyte survival, cleavage and blastocyst developmental rates during IVC were compared among the three groups. Four replications were performed.
Statistical analysis
All data were expressed as mean ± SEM values. Percentage data were arcsine transformed before analysis. Data on survival, maturation, fertilization status, in vitro development and embryo cell numbers were analyzed by one-way ANOVA followed by Tukey’s multiple comparison test. All data were analyzed using the KyPlot package (Ver. 2.0, KyensLab, Tokyo, Japan). P < 0.05 was defined as the significance level.
Results
Feasibility of trehalose and sucrose for oocyte vitrification and warming
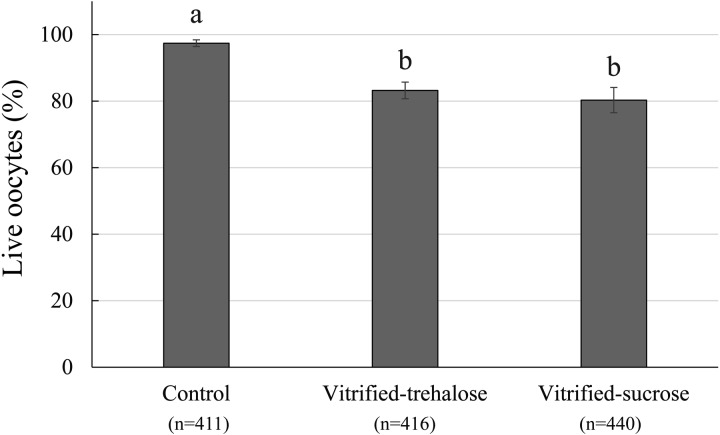
The proportions of surviving oocytes after vitrification and warming were similar when trehalose or sucrose was used (83.2% and 80.3%); however, these values were significantly lower than that of the control group (97.4%) (Fig. 1). The percentages of matured oocytes, fertilization, monospermy, male pronuclear formation and normal fertilization were not statistically different among the groups (Table 2). During embryo culture, the cleavage rates were similar between the vitrified-trehalose and vitrified-sucrose groups (39.7% and 42.4%, respectively) but significantly lower than that in the control group (62.5%) (Table 3). Also, the percentages of cultured oocytes developed to the blastocyst stage on day 6 were not different in the vitrified-trehalose and vitrified-sucrose groups (3.5% and 5.9%), but both of these values were significantly lower than that of the control group (26.9%) (Table 3). Likewise, blastocyst formation rates calculated from the numbers of cleaved embryos were similar in the vitrified-trehalose and vitrified-sucrose groups (Table 3). There was no significant difference in the mean total number of cells in day 6 blastocysts among the control, vitrified-trehalose and vitrified-sucrose groups (43.4, 44.8 and 39.2, respectively) (Table 3).
Fig. 1.
Survival of immature cumulus-enclosed porcine oocytes after vitrification in the presence of either trehalose or sucrose. Four replications were performed. Total numbers of oocytes subjected to each treatment are given in parentheses (n). Data are presented as mean ± SEM values. a, b Columns with different letters are significantly different (P < 0.05). After warming, all vitrified COCs were subjected to IVM and IVF. Viability status of oocytes was evaluated after IVF. Live oocytes were subsequently used either for assessment of nuclear and fertilization status or for embryo culture (results are given in Tables 2 and 3, respectively).
Table 2. Maturation and fertilization status following IVM and IVF of oocytes vitrified in the presence of either trehalose or sucrose.
| Treatment groups | No. of oocytes |
||||||
| Total* | GVBD | Matured$ | Penetrated | Normal | MPN | Monospermy | |
| (% total) | (% total) | (% GVBD) | (% GVBD) | (% penetrated) | (% penetrated) | ||
| Control | 100 | 95 (95.0 ± 3.0) | 73 (70.0 ± 6.8) | 43 (44.6 ± 9.3) | 12 (12.5 ± 2.7) | 43 (100) | 17 (43.7 ± 6.3) |
| Vitrified-trehalose | 100 | 94 (94.0 ± 2.0) | 57 (57. 0 ± 1.9) | 48 (61.4 ± 4.4) | 18 (19.3 ± 3.0) | 55 (94.6 ± 1.8) | 31 (54.1 ± 3.4) |
| Vitrified-sucrose | 100 | 88 (88.0 ± 4.3) | 55 (55.0 ± 1.0) | 41 (45.9 ± 10.1) | 19 (21.0 ± 7.0) | 37 (90.7 ± 3.6) | 27 (65.9 ± 7.5) |
Before vitrification, oocytes were equilibrated in a total of 4% (v/v) CPA (ethylene glycol + propylene glycol (1:1)) for 15 min followed by a brief (approx. 40 sec) washing and vitrification in a total of 35% (v/v) CPA supplemented with either trehalose or sucrose (0.3 M). Four replicates were performed. Percentage data are presented as mean ± SEM values. No significant differences were detected among treatment groups (P < 0.05). MPN = male pronucleus; GVBD = germinal vesicle breakdown. * After vitrification IVM and IVF, only live oocytes used to assess nuclear and fertilization status. $At or beyond the MII stage at 10 h after IVF.
Table 3. In vitro embryo development after IVF of oocytes vitrified in the presence of either trehalose or sucrose.
| Treatment groups | Total cultured* | Cleaved embryos | Blastocysts (day 6) |
Total cells in blastocysts | |
| (% cultured) | (% cultured) | (% cleaved) | |||
| Control | 300 | 189 | 82 | 43.4 ± 2.3 | |
| (62.5 ± 4.3)a | (26.9 ± 2.8)a | (42.7 ± 2.1)a | |||
| Vitrified-trehalose | 247 | 98 | 9 | 44.8 ± 3.7 | |
| (39.7 ± 2.7)b | (3.5 ± 0.6)b | (9.4 ± 2.0)b | |||
| Vitrified-sucrose | 255 | 110 | 15 | 39.2 ± 3.7 | |
| (42.4 ± 6.0)b | (5.9 ± 1.7)b | (13.5 ± 2.5)b | |||
Four replications were performed. Data are presented as mean ± SEM values. * After vitrification, IVM and IVF, only surviving oocytes were subjected to subsequent culture. a, b Percentages with different letters in the same column differ significantly (P < 0.05).
Comparison of permeating CPA combinations for oocyte vitrification
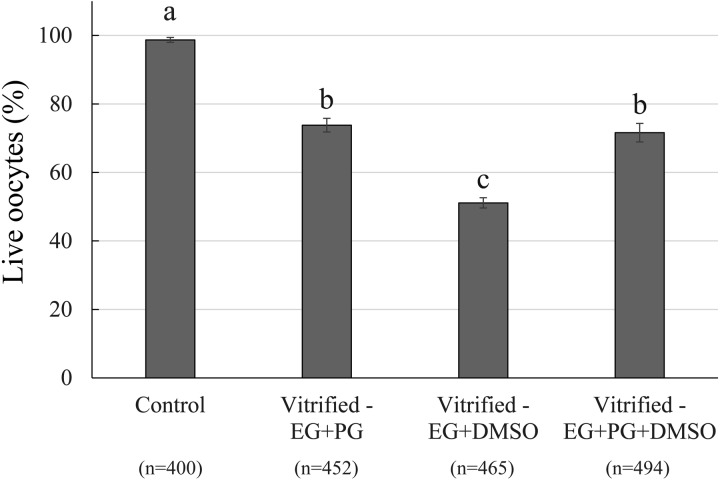
The proportions of surviving oocytes after vitrification and warming were similar when EG + PG and EG + PG + DMSO combinations were used as a CPA (73.8% and 71.6%, respectively) (Fig. 2). However, these values were significantly higher than that in the group vitrified in the EG + DMSO combination (51.1%) but lower than that in the control group (98.7%). The percentages of matured oocytes were not significantly different among the control group and the vitrified groups applying EG + PG or EG+DMSO as cryoprotectants (77.0%, 69.0% and 81.0%, respectively) (Table 4). However, the maturation rate of oocytes in the vitrified group applying EG + PG + DMSO (58.0%) was significantly lower (P < 0.05) compared with those of the control and vitrified EG + DMSO group but did not differ statistically from that of the vitrified EG + PG group (Table 4). Fertilization, monospermy, male pronuclear formation and normal fertilization were not statistically different among the groups (Table 4). During embryo culture, the cleavage rates were similar among all vitrified groups (ranging between 30.5%, and 44.5%), but significantly lower than that in the control group (64.7%) (Table 5). Similarly, the percentages of cultured oocytes developing to the blastocyst stage by day 6 were not significantly different among any of the vitrified groups (ranging between 4.1%, and 6.3%), but all of the values were significantly lower than that of the control group (24.4%) (Table 5). Likewise, blastocyst formation rates calculated from the numbers of cleaved embryos were similar among vitrified groups but lower than that in the control (Table 5). There was no significant difference in the mean total number of cells in day 6 blastocysts among the control and vitrified groups (ranging between 31.2 and 48.1) (Table 5). Nevertheless, cell numbers in blastocysts obtained from oocytes vitrified in EG + DMSO were tendentiously lower compared with those in the control (P = 0.06), EG + PG (P = 0.06) and EG + PG + DMSO groups (P = 0.08).
Fig. 2.
Survival of immature cumulus-enclosed porcine oocytes after vitrification in different combinations of penetrating CPA. Four replications were performed. Total numbers of oocytes subjected to each treatment are given in parentheses (n). Data are presented as mean ± SEM values. a, b, c Percentages with different letters in the same column differ significantly (P < 0.05). After warming, all vitrified COCs were subjected to IVM and IVF. Viability status of oocytes was evaluated after IVF. Live oocytes were subsequently used either for assessment of nuclear and fertilization status or for embryo culture (results are given in Tables 4 and 5, respectively).
Table 4. Maturation and fertilization status following IVM and IVF of oocytes vitrified in different combinations of penetrating CPA.
| Treatment groups | No. of oocytes |
||||||
| Total* | GVBD | Matured$ | Penetrated | Normal | MPN | Monospermy | |
| (% total) | (% total) | (% GVBD) | (% GVBD) | (% penetrated) | (% penetrated) | ||
| Control | 100 | 98 (98.0 ± 1.1) | 77 (77.0 ± 3.0)a | 64 (65.1 ± 12.0) | 14 (14.2 ± 2.6) | 64 (100) | 19 (32.7 ± 9.2) |
| Vitrified-EG + PG | 100 | 98 (98.0 ± 1.1) | 69 (69.0 ± 6.6)ab | 57 (58.3 ± 6.2) | 20 (20.4 ± 6.0) | 54 (94.6 ± 3.1) | 29 (50.4 ± 3.5) |
| Vitrified-EG + DMSO | 100 | 99 (99.0 ± 1.0) | 81 (81.0 ± 3.7)a | 52 (52.5 ± 9.0) | 19 (19.1 ± 2.9) | 52 (100) | 23 (45.7 ± 3.1) |
| Vitrified-EG + PG + DMSO | 100 | 99 (99.0 ± 1.0) | 58 (58.0 ± 2.5)b | 47 (47.2 ± 12.7) | 8 (8.0 ± 1.5) | 45 (97.6 ± 2.3) | 16 (41.0 ± 8.9) |
Before vitrification, oocytes were equilibrated in a total of 4% (v/v) CPA of the actual CPA combination (treatment groups) for 15 min followed by a brief (approx. 40 sec) washing and vitrification in a total of 35% (v/v) CPA supplemented with sucrose (0.3 M). EG + PG = ethylene glycol + propylene glycol (1:1). EG + DMSO = ethylene glycol + dimethyl sulfoxide (1:1). EG + PG + DMSO = ethylene glycol + propylene glycol + dimethyl sulfoxide (1:1:1). Four replicates were performed. Percentage data are presented as mean ± SEM values. a, b Percentages with different letters in the same column differ significantly (P < 0.05). MPN = male pronucleus; GVBD = germinal vesicle breakdown. * After vitrification, IVM and IVF, only live oocytes used to assess nuclear and fertilization status. $At or beyond the MII stage at 10 h after IVF.
Table 5. In vitro embryo development after IVF of oocytes vitrified in different combinations of penetrating CPA.
| Treatment groups | Cultured* | Cleaved embryos (% cultured) | Blastocysts (day 6) |
Total cells in blastocysts | |
| (% cultured) | (% cleaved) | ||||
| Control | 295 | 191 | 72 | 46.6 ± 2.3 | |
| (64.7 ± 2.4)a | (24.4 ± 3.0)a | (37.4 ± 3.2)a | |||
| Vitrified-EG + PG | 234 | 73 | 10 | 48.1 ± 5.3 | |
| (30.5 ± 6.5)b | (4.1 ± 2.3)b | (11.7 ± 4.2)b | |||
| Vitrified-EG + DMSO | 138 | 62 | 9 | 31.2 ± 6.3$ | |
| (44.5 ± 7.9)b | (6.3 ± 2.0)b | (13.2 ± 2.1)b | |||
| Vitrified-EG + PG + DMSO | 252 | 88 | 12 | 45.2 ± 5.8 | |
| (34.5 ± 4.1)b | (4.8 ± 0.7)b | (14.6 ± 2.7)b | |||
Four replications were performed. Data are presented as mean ± SEM values. * After vitrification, IVM and IVF, only surviving oocytes were subjected to subsequent culture. a, b, c Percentages with different letters in the same column differ significantly (P < 0.05). $ Tendentious difference compared with the other groups (P = 0.06–0.08).
Comparison of equilibration regimens for oocyte vitrification
The percentage of surviving oocytes after vitrification and warming was significantly higher (P < 0.05) when oocytes were equilibrated with Regimen A using EG + PG compared with equilibration with Regimen B either with EG + PG or EG + DMSO, which in turn were similar to one another (82.5%, 22.4% and 26.6%, respectively) (Table 6). Nevertheless, the rates of live oocytes in all vitrified groups were lower (P < 0.05) than that in the control (99.0%). After IVF, the cleavage rates were not statistically different when equilibration was performed with Regimen A using EG + PG and Regimen B using EG + DMSO (24.0% and 31.5%, respectively) and both of these values were significantly higher (P < 0.05) than that obtained by Regimen B with EG + PG (7.6%) but lower than that in the control (63.4%). Similarly, blastocyst development calculated as a percentage either from live oocytes or from cleaved oocytes was similar in vitrified groups after equilibration with Regimen A using EG + PG and Regimen B using EG + DMSO; however, these values were lower than those obtained in the control, whereas blastocysts were not obtained when oocytes were vitrified after equilibration with Regimen B using EG + PG (Table 6). Among the vitrified groups, the highest blastocyst development yield calculated as the percentage of blastocysts from the original number of oocytes subjected to vitrification was significantly higher (P < 0.05) after equilibration with Regimen A in EG + PG compared with the other vitrified groups (Table 6).
Table 6. Survival after warming and IVM and subsequent in vitro embryo development after IVF of immature cumulus-enclosed oocytes vitrified by different equilibration regimens.
| Treatment | CPA treatment regimen |
CPA | Total | Live* (% total) | Cleaved embryos (% live) | Blastocysts (day 6) |
||
| (% live) | (% cleaved) | (% total) | ||||||
| Control | - | - | 310 | 307 | 194 | 62 | ||
| (99.0 ± 0.5)a | (63.4 ± 4.6)a | (20.3 ± 2.0)a | (32.0 ± 2.2)a | (20.1 ± 1.9)a | ||||
| Vitrified | Regimen A | EG + PG | 368 | 304 | 73 | 10 | ||
| (82.5 ± 4.6)b | (24.0 ± 4.5)b | (3.2 ± 0.1)b | (13.6 ± 0.9)b | (2.7 ± 0.2)b | ||||
| Vitrified | Regimen B | EG + PG | 363 | 80 | 7 | 0 | ||
| (22.4 ± 4.3)c | (7.6 ± 3.8)c | (0 ± 0)c | (0 ± 0)c | (0 ± 0)c | ||||
| Vitrified | Regimen B | EG + DMSO | 350 | 92 | 29 | 3 | ||
| (26.6 ± 4.5)c | (31.5 ± 1.6)b | (2.8 ± 1.6)b | (9.4 ± 5.8)b | (0.9 ± 0.5)c | ||||
Three replications were performed. Data are presented as mean ± SEM values. * After IVF, all live oocytes were subsequently cultured for 6 days (day 0 = IVF). a, b, c Percentages with different letters in the same column differ significantly (P < 0.05). Regimen A = equilibration in a total of 4% (v/v) CPA for 15 min followed by a brief (approx. 40 sec) washing and vitrification in 35% (v/v) CPA. Regimen B = equilibration in a total of 15% (v/v) CPA for 5 min followed by a brief (approx. 40 sec) washing and vitrification in 35% (v/v) CPA. EG + PG = ethylene glycol + propylene glycol (1:1). EG + DMSO = ethylene glycol + dimethyl sulfoxide (1:1).
Effect of equilibration interval in 4% CPA on oocyte survival and development after vitrification and warming
When oocytes were equilibrated for different intervals in a total of 4% EG + PG, there was no significant difference in the percentage of surviving oocytes after vitrification and warming (Table 7). Also, embryo development in terms of cleavage and blastocyst formation rates was not statistically different among the groups. Nevertheless, the percentage of blastocyst development calculated from the cleaved embryos appeared to be tendentiously lower (P = 0.08) after 25 min equilibration compared with the 5 min group (13.2% and 33.1%, respectively).
Table 7. Survival after warming and IVM and subsequent in vitro embryo development after IVF of immature cumulus-enclosed oocytes vitrified in a total of 35% (v/v) CPA (EG + PG = 1:1) after different durations of equilibration in a total of 4% (v/v) CPA.
| Treatment | Equilibration duration |
Total | Live (% total) | Cleaved embryos (% live*) | Blastocysts (day 6) |
||
| (% cultured) | (% cleaved) | (% total) | |||||
| Vitrified | 5 min | 418 | 260 | 65 | 14 | ||
| (62.5 ± 4.6) | (25.3 ± 4.6) | (5.6 ± 2.0) | (33.1 ± 4.7) | (3.4 ± 1.2) | |||
| Vitrified | 15 min | 432 | 302 | 68 | 10 | ||
| (70.1 ± 3.2) | (22.4 ± 3.9) | (3.3 ± 0.9) | (19.1 ± 2.8) | (2.3 ± 0.6) | |||
| Vitrified | 25 min | 440 | 315 | 69 | 7 | ||
| (71.8 ± 3.1) | (21.9 ± 1.0) | (2.3 ± 1.0) | (13.2 ± 5.9)$ | (1.6 ± 0.6) | |||
Four replications were performed. Data are presented as mean ± SEM values. * After IVF, all live oocytes were subsequently cultured for 6 days (day 0 = IVF). $ Tendentious difference compared with vitrified oocytes equilibrated for 5 min (P = 0.08).
Discussion
The aim of the present study was to determine the optimum CPA treatment for the vitrification of porcine oocytes in terms of 1) sugars, 2) permeating CPA combinations, 3) CPA treatment regimens and 4) equilibration intervals.
Sugars are considered non-permeating cryoprotectants and are widely used in vitrification and warming protocols for the control of osmolarity [34]. Also, sugars are known to stabilize and thus protect cell membranes under dehydrated conditions [35]. The most available and therefore most commonly used sugars for this purpose are trehalose and sucrose (Table 1), which are rather similar in molecular weight (378.3 and 342.3, respectively). Our first experiment demonstrated nearly identical survival rates and subsequent embryo development when oocytes were vitrified and warmed in the presence of either trehalose or sucrose applied at the same concentrations. Therefore we concluded that trehalose and sucrose are equally effective in supporting the process of vitrification and warming. Based on this outcome and the lower cost of this method, we decided to use sucrose in the following experiments.
Previous studies on the vitrification of mouse, rabbit and bovine embryos and porcine oocytes have demonstrated that combining more than one permeating CPA is advantageous, as it promotes the permeation of the CPAs [7] and reduces the toxic effects of specific CPAs (such as that of PG) that occur when they are used individually at high concentration [6]. We determined previously that the 1:1 combination of EG and PG applied as a total of 4% (v/v) CPA for 13–15 min for equilibration and a total of 35% (v/v) CPA for 40 sec for vitrification (referred to as Regimen A in this study) significantly improved the survival rates of vitrified/warmed oocytes compared with use of EG alone, without any obvious toxic effects on developmental competence [6]. On the other hand, other authors have used the combination of EG with DMSO (references summarized in Table 1). The second experiment in this study demonstrated that use of a 1:1 combination of EG and PG in our original CPA treatment regimen (Regimen A) resulted in higher oocyte survival after vitrification and warming compared with EG and DMSO. Furthermore, cell numbers in blastocysts appeared to be tendentiously reduced with the EG + DMSO combination, whereas cell numbers of blastocysts with the EG + PG combination were almost identical to those of blastocysts obtained from non-vitrified oocytes. The reduction of survival rate by replacement of PG with DMSO is likely attributable to the lower permeability of DMSO compared with PG [36], whereas reduced cell numbers in blastocysts might reflect the toxic effect of DMSO on porcine oocytes [9]. These results suggest the superiority of the EG + PG combination compared with EG + DMSO when immature porcine oocytes are vitrified by using the Regimen A CPA treatment protocol. Furthermore, Experiment 2 also demonstrated that combining all three permeating CPAs (EG + PG + DMSO) for vitrification using the Regimen A CPA treatment protocol had no advantages, since oocyte survival and further embryo development with the EG + PG + DMSO combination was similar to that with EG + PG. Therefore, it is evident that even when combined with other CPAs, application of DMSO is not advantageous by any means for immature oocyte vitrification using our protocol. Nevertheless, it is important to point out that previous reports applied the EG + DMSO combination using a regimen that was different from our original CPA treatment protocol (Regimen A); equilibration was performed with a higher concentration of total CPA (15–20%) for a shorter interval (5–10 min), whereas the CPA concentration (30–40%) and treatment interval (30–60 sec) in vitrification medium was similar to that of our protocol (Table 1).
To determine which is the better approach for CPA treatment, we compared Regimen A using the EG + PG combination with another treatment protocol employing a total of 15% (v/v) permeating CPA for 5 min for equilibration and a total of 35% (v/v) permeating CPA for 40 sec for vitrification (referred to as Regimen B in this study). Regimen B was tested both by using EG + PG (1:1) and EG + DMSO (1:1) combinations. The results demonstrated the superiority of Regimen A compared with Regimen B in terms of oocyte survival. Irrespective of the CPA used, the survival rate of vitrified/warmed oocytes remained below 30% when Regimen B was used, whereas Regimen A resulted in 82.5% oocyte survival. Since the two regimens applied the same treatment with vitrification solution but a fundamentally different equilibration process, it is evident that the great difference in survival rates was attributed to the different methods of equilibration. A plausible explanation for different survival rates between Regimens A and B may be the different osmotic behavior of the oocytes when placed in the vitrification solution after equilibration. At 30–40 sec after their placement in the vitrification solution (right before cooling), we observed a higher degree of oocyte shrinking with Regimen A compared with Regimen B. This could be explained by a higher content of CPA within the oocyte after equilibration at a higher (15%) CPA concentration with Regimen B even after a shorter equilibration interval (5 min). (Note: During equilibration in both regimens, the oocytes re-expanded to their original size in approximately 5 min, which suggests that by that time the oocytes had finished CPA uptake in both groups.). Subsequently, when oocytes were placed in vitrification medium with the same permeating CPA concentration (35%), those with a lower initial CPA content (Regimen A) showed a higher grade of shrinking. It is possible that the actual volume of the oocyte at the time of vitrification (affected by the degree of shrinking) determines the survival of oocytes during vitrification and warming. It is known that there is a negative correlation between the volume of the cell and the speed of heat transfer during cooling and warming, with smaller cells cooled/warmed quicker than larger cells showing higher survival abilities during cryopreservation [37]. Taken together, our results demonstrate that the method of CPA equilibration greatly determines the survival of porcine oocytes after vitrification and warming. In agreement with the present results, a recent study demonstrated that during the first equilibration treatment in low concentrations of CPA, cattle oocytes can uptake the amount of permeating CPA sufficient to prevent cellular damage later when the oocytes are vitrified in a medium with a high concentration of CPA [38]. In such a system, the most important function of the relatively short treatment with the vitrification solution seems to be the osmotic withdrawal of water from cells, which concentrates intracellular CPAs to a level that allows the process of vitrification during placement in liquid nitrogen [38]. Nevertheless, it must be noted that, at least in porcine oocytes, insufficient permeation of CPA during the treatment with vitrification solution may also result in reduced survival after vitrification and warming when a CPA with relatively low permeability is used [6]. The abovementioned facts suggest that an optimum balance of intracellular CPA content and cell volume may exist that is necessary for oocyte survival during vitrification/warming and that this balance can be achieved by the control of CPA permeation during equilibration and right before vitrification. Furthermore, Experiment 3 revealed that the developmental competence of surviving oocytes in terms of cleavage and blastocyst development was severely compromised after vitrification in EG + PG with Regimen B compared with Regimen A using the same CPA combination. On the other hand, when Regimen B was used, the developmental competence of surviving oocytes after vitrification in EG + DMSO was significantly higher than that of those vitrified in EG + PG and did not differ significantly from that obtained after vitrification with Regimen A using EG + PG. Even so, among the vitrified groups, the application of EG + PG with Regimen A resulted in the highest blastocyst development efficiency based on the total number of vitrified oocytes because of the remarkably higher survival rate compared with the other groups. Nevertheless, when permeating CPA combinations were applied at a higher concentration (15%) for equilibration (Regimen B), EG + DMSO was superior to EG + PG. This difference may be attributed to the toxicity of PG at high doses, which compromises the competence of immature porcine oocytes to develop to embryos after fertilization [6].
In Experiment 4, we investigated the impact of equilibration duration in 4% (v/v) EG + PG before vitrification. Our results demonstrated that a duration of equilibration at 38.5 C of between 5 min and 25 min does not affect statistically the survival rate of oocytes and embryo production. Nevertheless, extending the equilibration duration to 25 min before oocyte vitrification tendentiously reduced the ability of resultant cleaved embryos to develop to the blastocyst stage, probably due to their toxic effects. These results suggest that, under the given conditions, equilibration for 5 min is sufficient to facilitate vitrification and that the equilibration duration should not exceed 15 min.
In conclusion, the present study clarified that trehalose and sucrose are equally effective during vitrification and warming in facilitating oocyte survival and subsequent embryo development. Under 38.5 C, equilibration in 4% permeating CPA for 15 min before vitrification is superior to that in 15% CPA for 5 min in terms of achievement of high survival rates. When equilibration is performed in 4% permeating CPA for 15 min, the combination of EG + PG is superior to EG + DMSO in terms of oocyte survival after vitrification and the quality of resultant blastocysts. Under these conditions, the optimum duration for equilibration appears to be between 5 min and 15 min.
Acknowledgments
The authors are grateful to Ms M Osaki, Ms M Nagai and Mr S Takakusa for technical assistance. This work was supported by a JSPS KAKENHI (Grant Number: 26870839) and also by the Science and Technology Research Partnership for Sustainable Development (SATREPS) of the Japan Science and Technology Agency (JST)/Japan International Cooperation Agency (JICA).
References
- 1.Dinnyes A, Liu J, Nedambale TL. Novel gamete storage. Reprod Fertil Dev 2007; 19: 719–731. [DOI] [PubMed] [Google Scholar]
- 2.Zhou GB, Li N. Cryopreservation of porcine oocytes: recent advances. Mol Hum Reprod 2009; 15: 279–285. [DOI] [PubMed] [Google Scholar]
- 3.Somfai T, Kikuchi K, Nagai T. Factors affecting cryopreservation of porcine oocytes. J Reprod Dev 2012; 58: 17–24. [DOI] [PubMed] [Google Scholar]
- 4.Mullen SF, Fahy GM. A chronologic review of mature oocyte vitrification research in cattle, pigs, and sheep. Theriogenology 2012; 78: 1709–1719. [DOI] [PubMed] [Google Scholar]
- 5.Somfai T, Yoshioka K, Tanihara F, Kaneko H, Noguchi J, Kashiwazaki N, Nagai T, Kikuchi K. Generation of live piglets from cryopreserved oocytes for the first time using a defined system for in vitro embryo production. PLoS ONE 2014; 9: e97731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Somfai T, Nakai M, Tanihara F, Noguchi J, Kaneko H, Kashiwazaki N, Egerszegi I, Nagai T, Kikuchi K. Comparison of ethylene glycol and propylene glycol for the vitrification of immature porcine oocytes. J Reprod Dev 2013; 59: 378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenology 2007; 67: 73–80. [DOI] [PubMed] [Google Scholar]
- 8.Somfai T, Dinnyés A, Sage D, Marosán M, Carnwath JW, Ozawa M, Kikuchi K, Niemann H. Development to the blastocyst stage of parthenogenetically activated in vitro matured porcine oocytes after solid surface vitrification (SSV). Theriogenology 2006; 66: 415–422. [DOI] [PubMed] [Google Scholar]
- 9.Gupta MK, Uhm SJ, Lee HT. Cryopreservation of immature and in vitro matured porcine oocytes by solid surface vitrification. Theriogenology 2007; 67: 238–248. [DOI] [PubMed] [Google Scholar]
- 10.Somfai T, Ozawa M, Noguchi J, Kaneko H, Kuriani Karja NW, Farhudin M, Dinnyés A, Nagai T, Kikuchi K. Developmental competence of in vitro-fertilized porcine oocytes after in vitro maturation and solid surface vitrification: effect of cryopreservation on oocyte antioxidative system and cell cycle stage. Cryobiology 2007; 55: 115–126. [DOI] [PubMed] [Google Scholar]
- 11.Somfai T, Noguchi J, Kaneko H, Nakai M, Ozawa M, Kashiwazaki N, Egerszegi I, Rátky J, Nagai T, Kikuchi K. Production of good-quality porcine blastocysts by in vitro fertilization of follicular oocytes vitrified at the germinal vesicle stage. Theriogenology 2010; 73: 147–156. [DOI] [PubMed] [Google Scholar]
- 12.Egerszegi I, Somfai T, Nakai M, Tanihara F, Noguchi J, Kaneko H, Nagai T, Rátky J, Kikuchi K. Comparison of cytoskeletal integrity, fertilization and developmental competence of oocytes vitrified before or after in vitro maturation in a porcine model. Cryobiology 2013; 67: 287–292. [DOI] [PubMed] [Google Scholar]
- 13.Fujihira T, Nagai H, Fukui Y. Relationship between equilibration times and the presence of cumulus cells, and effect of taxol treatment for vitrification of in vitro matured porcine oocytes. Cryobiology 2005; 51: 339–343. [DOI] [PubMed] [Google Scholar]
- 14.Pribenszky C, Du Y, Molnár M, Harnos A, Vajta G. Increased stress tolerance of matured pig oocytes after high hydrostatic pressure treatment. Anim Reprod Sci 2008; 106: 200–207. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Du Y, Lin L, Li J, Kragh PM, Kuwayama M, Bolund L, Yang H, Vajta G. Comparison of efficiency of open pulled straw (OPS) and Cryotop vitrification for cryopreservation of in vitro matured pig oocytes. Cryo Lett 2008; 29: 315–320. [PubMed] [Google Scholar]
- 16.Ogawa B, Ueno S, Nakayama N, Matsunari H, Nakano K, Fujiwara T, Ikezawa Y, Nagashima H. Developmental ability of porcine in vitro matured oocytes at the meiosis II stage after vitrification. J Reprod Dev 2010; 56: 356–361. [DOI] [PubMed] [Google Scholar]
- 17.Galeati G, Spinaci M, Vallorani C, Bucci D, Porcu E, Tamanini C. Pig oocyte vitrification by cryotop method: effects on viability, spindle and chromosome configuration and in vitro fertilization. Anim Reprod Sci 2011; 127: 43–49. [DOI] [PubMed] [Google Scholar]
- 18.Fernández-Reyez F, Ducolomb Y, Romo S, Casas E, Salazar Z, Betancourt M. Viability, maturation and embryo development in vitro of immature porcine and ovine oocytes vitrified in different devices. Cryobiology 2012; 64: 261–266. [DOI] [PubMed] [Google Scholar]
- 19.Hirose M, Kamoshita M, Fujiwara K, Kato T, Nakamura A, Wojcikiewicz RJ, Parys JB, Ito J, Kashiwazaki N. Vitrification procedure decreases inositol 1,4,5-trisphophate receptor expression, resulting in low fertility of pig oocytes. Anim Sci J 2013; 84: 693–701. [DOI] [PubMed] [Google Scholar]
- 20.Wu G, Jia B, Mo X, Liu C, Fu X, Zhu S, Hou Y. Nuclear maturation and embryo development of porcine oocytes vitrified by cryotop: effect of different stages of in vitro maturation. Cryobiology 2013; 67: 95–101. [DOI] [PubMed] [Google Scholar]
- 21.Rojas C, Palomo MJ, Albarracín JL, Mogas T. Vitrification of immature and in vitro matured pig oocytes: study of distribution of chromosomes, microtubules, and actin microfilaments. Cryobiology 2004; 49: 211–220. [DOI] [PubMed] [Google Scholar]
- 22.Shi WQ, Zhu SE, Zhang D, Wang WH, Tang GL, Hou YP, Tian SJ. Improved development by Taxol pretreatment after vitrification of in vitro matured porcine oocytes. Reproduction 2006; 131: 795–804. [DOI] [PubMed] [Google Scholar]
- 23.Varga E, Gardón JC, Papp AB. Effect of open pulled straw (OPS) vitrification on the fertilisation rate and developmental competence of porcine oocytes. Acta Vet Hung 2006; 54: 107–116. [DOI] [PubMed] [Google Scholar]
- 24.Macedo MC, Jr, Lucia T, Jr, Rambo G, Ferreira Filho EB, Rosa AP, Fabiane C, Cabral M, Deschamps JC. In vitro penetration of swine oocytes by homologous spermatozoa: Distinct systems for gamete’s co-incubation and oocyte’s cryopreservation. Anim Reprod Sci 2010; 117: 295–301. [DOI] [PubMed] [Google Scholar]
- 25.Marco-Jiménez F, Casares-Crespo L, Vicente JS. Effect of cytochalasin B pre-treatment of in vitro matured porcine oocytes before vitrification. Cryo Lett 2012; 33: 24–30. [PubMed] [Google Scholar]
- 26.Dinnyés A, Dai Y, Jiang S, Yang X. High developmental rates of vitrified bovine oocytes following parthenogenetic activation, in vitro fertilization, and somatic cell nuclear transfer. Biol Reprod 2000; 63: 513–518. [DOI] [PubMed] [Google Scholar]
- 27.Petters RM, Wells KD. Culture of pig embryos. J Reprod Fertil Suppl 1993; 48: 61–73. [PubMed] [Google Scholar]
- 28.Kikuchi K, Onishi A, Kashiwazaki N, Iwamoto M, Noguchi J, Kaneko H, Akita T, Nagai T. Successful piglet production after transfer of blastocysts produced by a modified in vitro system. Biol Reprod 2002; 66: 1033–1041. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki K, Asano A, Eriksson B, Niwa K, Nagai T, Rodriguez-Martinez H. Capacitation status and in vitro fertility of boar spermatozoa: effects of seminal plasma, cumulus-oocyte-complexes-conditioned medium and hyaluronan. Int J Androl 2002; 25: 84–93. [DOI] [PubMed] [Google Scholar]
- 30.Kikuchi K, Nagai T, Kashiwazaki N, Ikeda H, Noguchi J, Shimada A, Soloy E, Kaneko H. Cryopreservation and ensuing in vitro fertilization ability of boar spermatozoa from epididymides stored at 4°C. Theriogenology 1998; 50: 615–623. [DOI] [PubMed] [Google Scholar]
- 31.Somfai T, Kikuchi K, Medvedev S, Onishi A, Iwamoto M, Fuchimoto D, Ozawa M, Noguchi J, Kaneko H, Ohnuma K, Sato E, Nagai T. Development to the blastocyst stage of immature pig oocytes arrested before the metaphase-II stage and fertilized in vitro. Anim Reprod Sci 2005; 90: 307–328. [DOI] [PubMed] [Google Scholar]
- 32.Dang-Nguyen TQ, Kikuchi K, Somfai T, Ozawa M, Nakai M, Maedomari N, Viet-Linh N, Kanai Y, Nguyen BX, Nagai T. Evaluation of developmental competence of in vitro-produced porcine embryos based on the timing, pattern and evenness of the first cleavage and onset of the second cleavage. J Reprod Dev 2010; 56: 593–600. [DOI] [PubMed] [Google Scholar]
- 33.Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophoton Int 2004; 11: 36–42. [Google Scholar]
- 34.Arav A, Shehu D, Mattioli M. Osmotic and cytotoxic study of vitrification of immature bovine oocytes. J Reprod Fertil 1993; 99: 353–358. [DOI] [PubMed] [Google Scholar]
- 35.Crowe LM, Mouradian R, Crowe JH, Jackson SA, Womersley C. Effects of carbohydrates on membrane stability at low water activities. Biochim Biophys Acta 1984; 769: 141–150. [DOI] [PubMed] [Google Scholar]
- 36.Pedro PB, Yokoyama E, Zhu SE, Yoshida N, Valdez DM, Jr, Tanaka M, Edashige K, Kasai M. Permeability of mouse oocytes and embryos at various developmental stages to five cryoprotectants. J Reprod Dev 2005; 51: 235–246. [DOI] [PubMed] [Google Scholar]
- 37.Dumont F, Marechal PA, Gervais P. Cell size and water permeability as determining factors for cell viability after freezing at different cooling rates. Appl Environ Microbiol 2004; 70: 268–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Y, Fu X, Zhou G, Jia B, Fang Y, Hou Y, Zhu S. An efficient method for the sanitary vitrification of bovine oocytes in straws. J Anim Sci Biotechnol 2014; 5: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]