Abstract
Ocular infection with Chlamydia trachomatis can lead to trachoma, a leading infectious cause of blindness. Trachoma is targeted for elimination by 2020. Clinical grading for ocular disease is currently used for evaluating trachoma elimination programs, but serological surveillance can be a sensitive measure of disease transmission and provide a more objective testing strategy than clinical grading. We calculated the basic reproduction number from serological data in settings with high, medium, and low disease transmission based on clinical disease. The data showed a striking relationship between age seroprevalence and clinical data, demonstrating the proof-of-principle that age seroprevalence predicts transmission rates and therefore could be used as an indicator of decreased transmission of ocular trachoma.
Trachoma is a neglected tropical disease caused by repeated ocular infection with the bacterium Chlamydia trachomatis (Ct). Active disease, defined as trachomatous inflammation–follicular (TF) or trachomatous inflammation–intense (TI), is self-limiting, but repeated infections can lead to pathology in the form of scarring (TS); trichiasis (TT), distinguishable by turned-in eyelashes rubbing against the globe of the eye; and irreversible blindness caused by corneal opacity (CO)1. Global efforts to eliminate trachoma as a public health problem by the year 2020 are based on the SAFE strategy: surgery for treatment of trichiasis, mass drug administration of antibiotics, and promotion of facial cleanliness and environmental improvement.
As elimination efforts proceed, defining programmatic endpoints becomes a priority. We have recently begun examining the utility of serological tests for post-endemic surveillance of trachoma elimination programs2. Antibody responses to the Ct antigens pgp3 and CT694 show high sensitivity and specificity for Ct infection2. A large percentage of children living in trachoma-endemic communities have detectable antibody responses but exhibit no clinical signs and lack bacterial nucleic acid in the conjunctiva, suggesting that these responses are indicators of historical rather than active infection2. No seroreversion was observed in seropositive individuals examined six months after drug treatment, although statistically significant decreases in antibody levels to pgp3 and CT694 were observed in younger age groups3.
Post-endemic testing has shown very low seropositivity in young children in the absence of infection4 (West et al., submitted). Program end-points are currently set at <5% TF in 1–9 year-olds, operating under the assumption that low enough levels of ocular trachoma transmission will not result in downstream blindness. Since MDA will not uniformly result in zero transmission in districts, serosurveillance will only be useful to programs if there is a clear understanding of how seroprevalence relates to currently used indicators of trachoma. To this end, we evaluated age seroprevalence in communities described as hyperendemic, mesoendemic, or hypoendemic for trachoma based on TF and rates of ocular infection, and estimated the basic reproduction number (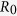 ) for each scenario.
) for each scenario.
Methods
Study Population
Studies were conducted in the Kongwa District of Tanzania as part of two separate studies in collaboration with the Kongwa Trachoma Project. The first is an ongoing study to evaluate the impact of alternative models of community-wide treatment with azithromycin and to compare nucleic acid amplification test methods5,6,7 (hyperendemic community). The second is a study to evaluate the health impact of an integrated NTD program on non-targeted diseases (mesoendemic and hypoendemic communities). Children 1–6 years of age were recruited from a single hyperendemic community and children 1–9 years of age were recruited from eight villages each to comprise the mesoendemic and hypoendemic communities. Clinical examinations for TF were performed by experienced graders, and dried blood spots (DBS) and conjunctival eye swabs for PCR were collected.
Ethics Statement
Parents or guardians provided written informed consent for children participating in the study. Children over 7 provided verbal assent. The study was approved by The Institutional Review Boards of the Tanzanian National Institute for Medical Research (Dar es Salaam, TZ), Centers for Disease Control and Prevention (Atlanta, GA) for both studies and the Johns Hopkins University School of Medicine (Baltimore, MD) for the first study (hyperendemic community) only. The study was carried out in accordance with the approved guidelines.
Grading of Ocular Trachoma
Clinical exams, using the WHO simplified grading scheme8, were performed on children aged 1 to 9 years from nine villages in Kongwa District by an experienced trachoma grader. TF was graded as negative if the ocular signs did not meet WHO criteria of positive TF with 5 or more follicles of greater than 0.5 mm8.
Nucleic Acid Amplification Testing
Eye swabs were collected for PCR analyses of C. trachomatis from all children, with careful attention to avoid field contamination. Swabs from the hyperendemic community were sent to the International Chlamydia Research Laboratory at Johns Hopkins University and tested for the presence of chlamydial DNA using Amplicor CT/NG (Roche, Basel, Switzerland) as described3,6. According to the manufacturer’s directions, the Amplicor test was positive if the optical density read at 450 nM was >0.8, negative if the signal was <0.2, and equivocal if in-between. All equivocal tests were re-tested in duplicate, and only graded positive if at least one test was positive. PCR testing in hypo- and meso-endemic settings was done at CDC on non-pooled ocular swabs using a nested PCR protocol (Expand High Fidelity PCR System, Roche Diagnostics Corporation, Indianapolis, IN) to amplify the ompA gene as previously described9.
Serology for Assessment of chlamydial-specific Antibodies
Serum was eluted from dried blood spots and then incubated with chemically-modified microspheres (Luminex Corp., Austin, TX) conjugated to the Ct antigens pgp3 and CT6942. After washing out unbound serum antibodies, bound antibody was detected with biotinylated mouse anti-human IgG (clone H2; Southern Biotech, Birmingham, AL) and biotinylated mouse anti-human IgG4 (clone HP6025; Invitrogen, South San Francisco, CA), followed by R-phycoerythrin-labeled streptavidin (SAPE, Invitrogen, South San Francisco, CA). Beads were suspended in 125 μl PBS, shaken, and immediately read on a BioPlex 200 instrument (Bio-Rad, Hercules, CA) equipped with Bio-Plex Manager 6.0 software (Bio-Rad).
Modeling
Because it is not possible to directly measure the transmission contact pattern over age groups, the basic reproduction rate (R0) was calculated to estimate the transmission potential using a simple method that assumes a constant force of infection (cFOI) over age10. While this is a crude approximation of the true contact pattern, which is likely to differ over age, the resulting estimate of R0 serves as a simple summary parameter, allowing quick comparison of each community. In addition, by using this very simple approach, we can compare the data in this study with serological data from other studies. A close correspondence between estimates obtained by the cFOI method and those from the more complex ‘Next Generation Matrix’ (NGM) method11 for age-dependent models fitted to serological data have been observed in a recent model of cytomegalovirus infection, which has, if anything, a more complex transmission pattern and natural history than trachoma10. Furthermore, because the serological data analyzed here pertain primarily to young children, the assumptions of the cFOI model might be expected to be roughly true; they should, however, be restricted to young children and not the whole population. The proportion of the population at age a that is seronegative 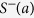 (with force of infection at age x, denoted by λ(x)) is given by:
(with force of infection at age x, denoted by λ(x)) is given by:
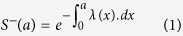 |
Assuming that the force of infection is constant with age we see that:
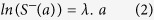 |
Lanzieri et al.1010109 state that, in this case, R0 can be approximated as:
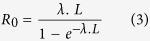 |
where L is the average lifespan of the population, which we set to 75 years here. This value of the lifespan may not correspond exactly with the true average life expectancy across the regions from which data were collected. However, the value of L is used here to set the scale of the calculated R0; since this R0 does not pertain to the whole population it should simply be set to a fixed value to allow comparison of its value across different countries. The values of  obtained were approximately 15% smaller if a value of L equal to 63 years (Tanzanian value http://data.worldbank.org/indicator/SP.DYN.LE00.FE.IN) was used. Confidence interval (CI) 95% bounds were reported by inserting the 95% upper and lower bound values for the force of infection
obtained were approximately 15% smaller if a value of L equal to 63 years (Tanzanian value http://data.worldbank.org/indicator/SP.DYN.LE00.FE.IN) was used. Confidence interval (CI) 95% bounds were reported by inserting the 95% upper and lower bound values for the force of infection  into the formula for
into the formula for 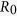 above. The values of
above. The values of 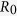 obtained by the cFOI method were checked against those calculated for hyper-, meso- and hypoendemic communities using the NGM method from a previous mathematical modeling study12. The NGM method used here assumes an initially naive susceptible population, which has experienced minimal prior infections; as such, the NGM method can also be said to apply to young children and hence correspond to the cFOI method used here for the same age group. All calculations were performed using Matlab version R2013b (Mathworks, MA).
obtained by the cFOI method were checked against those calculated for hyper-, meso- and hypoendemic communities using the NGM method from a previous mathematical modeling study12. The NGM method used here assumes an initially naive susceptible population, which has experienced minimal prior infections; as such, the NGM method can also be said to apply to young children and hence correspond to the cFOI method used here for the same age group. All calculations were performed using Matlab version R2013b (Mathworks, MA).
Results
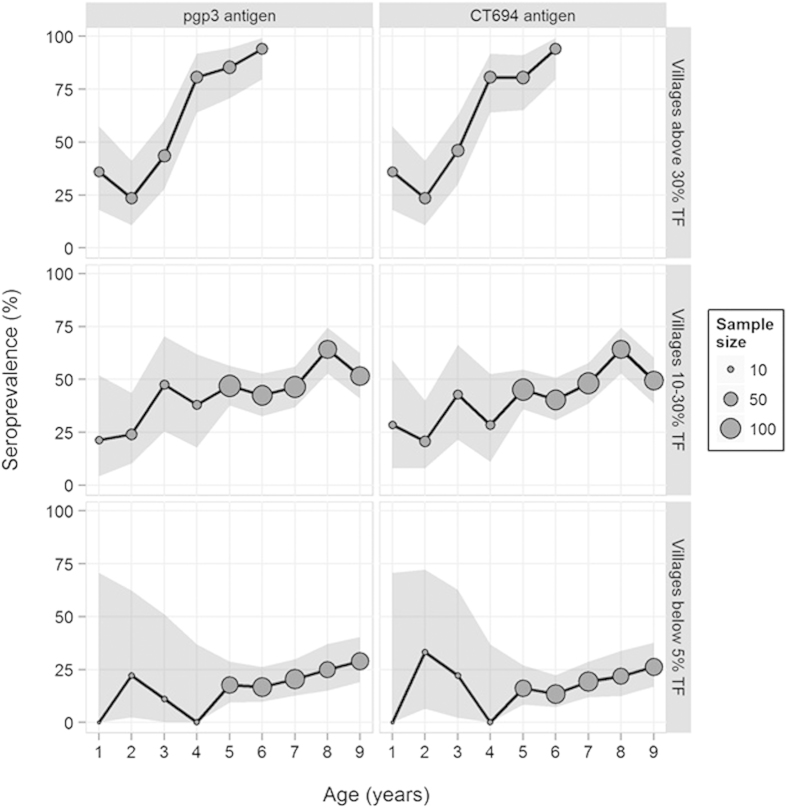
In a hyperendemic setting, 208 1–6 year-old children from a single village in Kongwa District, TZ, had a TF prevalence of 47% and ocular infection rates of 24% (previously published in3,6, Table 1). In the mesoendemic villages, TF prevalence in 987 1-9 year olds was 14·5% and ocular infection rate was 8·3% (Table 1). For the hypoendemic setting, TF was present in 2·9% and ocular infection in 1·8% of 680 1–9 year-olds evaluated (Table 1). Antibody levels against the Ct antigens pgp3 and CT694 were determined for 208 children in the hyperendemic setting, 581 children in the mesoendemic communities, and 419 children in the hypoendemic communites. The data from the four mesoendemic villages and four hypoendemic villages were aggregated into the two sample sets for analysis. TF, NAAT, and serology data are shown in Table 1. Figure 1 shows age-seroprevalence curves, with 95% CIs in the shaded area.
Table 1. Overall prevalence of clinical signs, ocular infection, antibody-positivity, and calculated
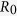 in hyperendemic, mesoendemic, and hypoendemic settings.
in hyperendemic, mesoendemic, and hypoendemic settings.
| Age range | %TF+ [95% CI) | %NAAT + [95% CI] | % pgp3Ab + (95% CI) | %CT694 Ab+ [95% CI] |
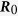 pgp3 [95% CI] pgp3 [95% CI] |
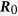 CT694 [95% CI] CT694 [95% CI] |
|
|---|---|---|---|---|---|---|---|
| High | 1–6 | 47.0 [44.7–49.3] | 24·0 [17.2–30.8] | 62·0 [55.4–68.6] | 61·5 [54.9–68.1] | 29·4 [21·1–37·7] | 28·3 [19·9–36·8] |
| Medium | 1–9 | 14·5 [12.3–16.7] | 8·1 [6.4–9.8] | 3·0 [30.0–36.0] | 34·0 [31.0–37.0] | 8·1 [6·3–10·0] | 7·8 [6·0–9·6] |
| Low | 1–9 | 2·8 [1.4–4.2] | 2·0 [0.82–3.2] | 21·2 [17.7–24.6] | 18·4 [15.1–21.7] | 2·8 [2·1–3·6] | 2·8 [1·6–4·0] |
Figure 1. Seroprevalence percentages by age broken down by antigen (pgp3 and CT694) and village prevalence (below 5% TF, 10-30% TF, and above 30% TF).
Seroprevalences are represented with circles scaled by the sample size and shaded regions denote 95% confidence intervals. For antigen-specific seroprevalences by age, confidence intervals use the incomplete beta function19 to account for village-level clustering (except when the seroprevalence was zero and Clopper-Pearson (1934) limit19 were used).
Using the cFOI method, we estimated the value of R0 in young children for each setting using age seroprevalence against each antigen (pgp3 and CT694; Table 1). 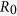 (pgp3) was 29·4 for the hyperendemic community, 8·1 for the mesoendemic setting, and 2·8 for the hypoendemic setting.
(pgp3) was 29·4 for the hyperendemic community, 8·1 for the mesoendemic setting, and 2·8 for the hypoendemic setting. 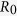 (CT694) was 28·3 for the hyperendemic community, 7·8 for the mesoendemic setting, and 2·8 for the hypoendemic setting (95% confidence intervals for these values are provided in Table 1). These values of the basic reproduction number correspond well with equivalent values in young children calculated for hyper-, meso- and hypoendemic communities using the NGM method for a more complex epidemiological model fitted to baseline infection prevalence data over all age groups in the community12: hyperendemic 34·9 [27.5, 44.2] (95% CIs, based upon the transmission rate uncertainty), mesoendemic 3·0 [2.5, 3.6], hypoendemic 2·3 [2.0, 2.7]. It should be noted that the meso- and hypoendemic study sites whose data were used in the NGM calculation differed from the Kongwa District, Tanzania, study site used for the serological calculations.
(CT694) was 28·3 for the hyperendemic community, 7·8 for the mesoendemic setting, and 2·8 for the hypoendemic setting (95% confidence intervals for these values are provided in Table 1). These values of the basic reproduction number correspond well with equivalent values in young children calculated for hyper-, meso- and hypoendemic communities using the NGM method for a more complex epidemiological model fitted to baseline infection prevalence data over all age groups in the community12: hyperendemic 34·9 [27.5, 44.2] (95% CIs, based upon the transmission rate uncertainty), mesoendemic 3·0 [2.5, 3.6], hypoendemic 2·3 [2.0, 2.7]. It should be noted that the meso- and hypoendemic study sites whose data were used in the NGM calculation differed from the Kongwa District, Tanzania, study site used for the serological calculations.
Discussion
Our data demonstrate that age seroprevalence provides a proxy for clinical disease in determining transmission rates for trachoma. A conspicuous increase in the slope of the age seroprevalence curve was seen with increasing TF prevalence in the setting, which was reflected in the numerical value of 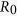 for each setting. Modelling showed clear differences in the seroprevalence-determined
for each setting. Modelling showed clear differences in the seroprevalence-determined 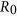 among young children in the hyperendemic, mesoendemic, and hypoendemic settings, regardless of the antigen used.
among young children in the hyperendemic, mesoendemic, and hypoendemic settings, regardless of the antigen used.
The 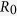 values obtained here are higher than those previously described using model-based estimates13,14, although those studies estimated
values obtained here are higher than those previously described using model-based estimates13,14, although those studies estimated 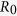 over the whole population, with all age groups combined, using a stochastic transmission model used to fit cross-sectional ocular infection data. This would be expected to result in a substantially different
over the whole population, with all age groups combined, using a stochastic transmission model used to fit cross-sectional ocular infection data. This would be expected to result in a substantially different 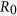 estimate because the rate of recovery from infection is thought to increase and the bacterial load per infection to decrease with each subsequent infection, and therefore with age15,16,17,18. Contact rates are also likely to drop in older age groups, and these effects all lead to lower forces of infection and contributions to the overall reproduction number. We emphasize that we are calculating an
estimate because the rate of recovery from infection is thought to increase and the bacterial load per infection to decrease with each subsequent infection, and therefore with age15,16,17,18. Contact rates are also likely to drop in older age groups, and these effects all lead to lower forces of infection and contributions to the overall reproduction number. We emphasize that we are calculating an 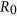 here that applies to the age groups surveyed i.e. young children.
here that applies to the age groups surveyed i.e. young children.
The major limitations of this study are the relatively small sample size, particularly the small number of 1–4 year olds in the mesoendemic and hypoendemic settings, and the lack of 7–9 year olds in the high-prevalence village. This is especially apparent in the wide confidence intervals for antibody responses in 1–4 year olds in the hypo and mesoendemic communities as shown in Fig. 1. We would predict that since 92% of 6-year-olds in the high prevalence village were seropositive that this trend would continue with the older ages but still need to generate the data to support that. Having the 7–9 year-olds in that village may also have changed the 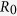 calculated by the model, although if the seroprevalence trend continued at >90% in 7–9 year-olds in that village, the R0 would almost certainly be greater than the current estimate. Changes in antibody levels after treatment have only been examined in a single high prevalence community3, and changes over time in the absence of treatment have not been evaluated at all. Therefore assumptions about negligible seroreversion in all settings may be premature. Currently, studies are underway examining
calculated by the model, although if the seroprevalence trend continued at >90% in 7–9 year-olds in that village, the R0 would almost certainly be greater than the current estimate. Changes in antibody levels after treatment have only been examined in a single high prevalence community3, and changes over time in the absence of treatment have not been evaluated at all. Therefore assumptions about negligible seroreversion in all settings may be premature. Currently, studies are underway examining 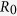 and force of infection in hyperendemic, mesoendemic, and hypoendemic-prevalence settings at the district level, the implementation and evaluation units for trachoma programs. We will also evaluate changes in antibody levels in a variety of transmission settings, as the high attack rate in hyperendemic communities may result in earlier formation of long-lived antibody-producing plasma cells than in areas with lower attack rates.
and force of infection in hyperendemic, mesoendemic, and hypoendemic-prevalence settings at the district level, the implementation and evaluation units for trachoma programs. We will also evaluate changes in antibody levels in a variety of transmission settings, as the high attack rate in hyperendemic communities may result in earlier formation of long-lived antibody-producing plasma cells than in areas with lower attack rates.
Despite the limitations, the data provide a powerful proof-of-concept that age seroprevalence predicts transmission rates of trachoma and therefore could be used as an indicator of decreased exposure. Post-endemic surveillance is a difficult sell to donors and to programs, so creative approaches to surveillance are urgently needed. This is especially true for trachoma programs, for which decision-making is guided by personnel and training-intensive ocular examinations. A serosurveillance platform has the potential for integration with other surveillance activities, making the most of the increasingly limited resources available to elimination programs.
Additional Information
How to cite this article: Martin, D. L. et al. Serological Measures of Trachoma Transmission Intensity. Sci. Rep. 5, 18532; doi: 10.1038/srep18532 (2015).
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. This work was supported by funding through the United States Agency for International Development (USAID) and the Bill and Melinda Gates Foundation.
Footnotes
Author Contributions D.M. and M.G. designed the study and wrote the paper, R.W. contributed Figure 1, M.G. performed the 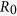 analysis, D.M., B.G., P.L., S.W., H.M. and M.K. organized field data collection, B.G. performed serological analysis, C.B., S.W., C.G. and L.D. performed PCR analysis. All authors reviewed and approved the manuscript.
analysis, D.M., B.G., P.L., S.W., H.M. and M.K. organized field data collection, B.G. performed serological analysis, C.B., S.W., C.G. and L.D. performed PCR analysis. All authors reviewed and approved the manuscript.
References
- Solomon A. W. Z. M., K. H., Buchan J. C., Mabey D. C. W. & Foster A. Trachoma Control: A Guide for Program Managers. World Health Organization: Geneva, (2006). [Google Scholar]
- Goodhew E. B. et al. CT694 and pgp3 as serological tools for monitoring trachoma programs. PLoS Negl Trop Dis 6, e1873 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodhew E. B. et al. Longitudinal analysis of antibody responses to trachoma antigens before and after mass drug administration. BMC Infect Dis 14, 216 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. L. et al. Serology for trachoma surveillance after cessation of mass drug administration. PLoS Negl Trop Dis 9, e0003555 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dize L., Gaydos C. A., Quinn T. C. & West S. K. Stability of Chlamydia trachomatis on storage of dry swabs for accurate detection by nucleic acid amplification tests. J Clin Microbiol doi: 10.1128/JCM.03218-14 (2014). [DOI] [PMC free article] [PubMed]
- Dize L. et al. Comparison of the Abbott m2000 RealTime CT assay and the Cepheid GeneXpert CT/NG assay to the Roche Amplicor CT assay for detection of Chlamydia trachomatis in ocular samples from Tanzania. J Clin Microbiol 51, 1611–3 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dize L., Gaydos C. A., Quinn T. C. & West S. K. Stability of Chlamydia trachomatis on storage of dry swabs for accurate detection by nucleic acid amplification tests. J Clin Microbiol 53, 1046–7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thylefors B., Dawson C. R., Jones B. R., West S. K. & Taylor H. R. A simple system for the assessment of trachoma and its complications. Bull World Health Organ 65, 477–83 (1987). [PMC free article] [PubMed] [Google Scholar]
- Bandea C. et al. Typing of Chlamydia trachomatis strains from urine samples by amplificaiton and sequencing the major outer membrance protein gene (omp1). Sexually Transmitted Infections 77, 419–22 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzieri T. M., Bialek S. R., Ortega-Sanchez I. R. & Gambhir M. Modeling the potential impact of vaccination on the epidemiology of congenital cytomegalovirus infection. Vaccine 32, 3780–6 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekmann O., Heesterbeek J. A. & Roberts M. G. The construction of next-generation matrices for compartmental epidemic models. J R Soc Interface 7, 873–85 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambhir M. et al. The development of an age-structured model for trachoma transmission dynamics, pathogenesis and control. PLoS Negl Trop Dis 3, e462 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F. et al. Assessment of transmission in trachoma programs over time suggests no short-term loss of immunity. PLoS Negl Trop Dis 7, e2303 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray K. J. et al. When can antibiotic treatments for trachoma be discontinued? Graduating communities in three African countries. PLoS Negl Trop Dis 3, e458 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey R., Duong T., Carpenter R., Whittle H. & Mabey D. The duration of human ocular Chlamydia trachomatis infection is age dependent. Epidemiol Infect 123, 479–86 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassly N. C., Ward M. E., Ferris S., Mabey D. C. & Bailey R. L. The natural history of trachoma infection and disease in a Gambian cohort with frequent follow-up. PLoS Negl Trop Dis 2, e341 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton M. J. et al. Re-emergence of Chlamydia trachomatis infection after mass antibiotic treatment of a trachoma-endemic Gambian community: a longitudinal study. Lancet 365, 1321–8 (2005). [DOI] [PubMed] [Google Scholar]
- Solomon A. W. et al. Strategies for control of trachoma: observational study with quantitative PCR. Lancet 362, 198–204 (2003). [DOI] [PubMed] [Google Scholar]
- Graubard B. & Korn E. Confidence Intervals for Proportions with Small Expected Number of Positive Counts Estimated from Survey Data. Survey Methodology 24, 193–201 (1998). [Google Scholar]