Abstract
Objective
Pulmonary embolism (PE) can cause intracardiac hemolysis and increased plasma hemoglobin and arginase-1, which can worsen pulmonary vasoconstriction. We test the hypothesis that patients with PE that causes tricuspid regurgitation (TR), indicative of higher pulmonary arterial pressures, have decreased leukocyte expression of hmox-1 compared with patients with PE and no TR and patients without PE.
Design
Prospective, noninterventional study.
Patients
Normotensive patients with suspected PE (n=87) who underwent CT pulmonary angiography and transthoracic Doppler-echocardiography.
Measurements
Significant TR was defined as a jet velocity > 2.7m/s. Leukocyte expression of hmox-1, haptoglobin, haptoglobin related gene, the haptoglobin receptor, CD163 and cox-2 genes were assessed by quantitative rtPCR, and the hmox-1 promoter was examined for the −413 A→T SNP and GT repeat polymorphisms.
Results
Of the 44 (50%) with PE+, 22 had TR+, and their mean pulmonary vascular occlusion (39±32%) did not differ significantly from patients who were TR− (28±26%, P=0.15). Patients with PE+ and TR+ had significantly lower expression of hmox-1 and haptoglobin genes than patients without PE+ and no TR. Expression of hmox-1 varied inversely with TR velocity (r2=0.45, P<0.001) for PE+ (n=22) but not patients without PE. Hmox-1 expression did not vary significantly with genotype. Cox-2 did not differ between groups and had no correlation with TR.
Conclusions
Severity of TR varied inversely with hmox-1 expression, suggesting that hmox-1 expression affects pulmonary vascular reactivity after PE.
Keywords: heme oxygenase, fibrinolysis, pulmonary hypertension, hemolysis, haptoglobin
Graphical Abstract
Introduction
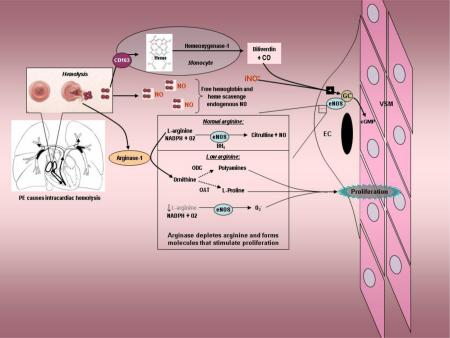
Acute pulmonary embolism (PE) causes obstruction of the pulmonary arteries, leading to aberrant flow within the pulmonary arterial tree. With more severe PE, the pulmonary arterial pressure increases, leading to turbulent blood flow within the right ventricle from regurgitation across the tricuspid and pulmonary valves, as well as increased shear forces exerted on erythrocytes within the pulmonary arteries.[1-5] The degree of pulmonary hypertension correlates poorly with the percentage pulmonary vascular obstruction produced by thrombus, suggesting variable effect of PE on the soluble determinants of the pulmonary arteriolar constrictor-dilator tone.[6-9] The potent vasodilator, nitric oxide (NO), produced tonically by vascular endothelial nitric oxide synthase (eNOS), plays a pivotal role in maintaining normal pulmonary vascular resistance under these conditions.[10] Thus, it can be hypothesized that different patients with PE causing the same degree of pulmonary vascular obstruction may manifest widely different pulmonary arterial resistances, depending in part upon the balance of their pulmonary arterial vasoconstrictor-dilator phenotype, which in turn, depends upon NO availability.
Intravascular hemolysis liberates hemoglobin and diffusible heme, which both directly bind NO, and arginase-1, which cleaves the eNOS substrate, L-arginine.[11,12] Prior work has found evidence of hemolysis with PE, shown by a severity-dependent reduction in serum haptoglobin in animals and humans with acute PE.[1,2,13] Humans with severe PE have increased arginase-1 and decreased plasma L-arginine concentrations.[14] These data suggest that the cognate ability of haptoglobin to sequester hemoglobin, and thus limit its vasoconstrictive effect, provides a protective effect—a finding already observed in humans with sepsis and subarachnoid hemorrhage.[15,16] Similarly, inducible heme oxygenase-1 (hmox-1) converts free heme from a potent vasoconstrictor, oxidant and inflammatory molecule into the vasodilator carbon monoxide and the antioxidant biliverdin, possibly explaining elevations in carboxyhemoglobin concentrations observed in nonsmoking patients with PE.[17] Experimentally increased expression of heme oxygenase-1 improves, and decreased expression worsens right heart injury in experimental models of pulmonary hypertension.[18-20]
We hypothesize an overarching cause-effect relationship, such that patients who have poor ability to induce hmox-1, have impaired ability to control the vasoconstrictive response to PE, and therefore have higher pulmonary arterial pressures. In this report, we test the specific hypotheses that patients with severe PE (1) express hmox-1 transcripts in peripheral leukocytes that is inversely proportional to the velocity of the regurgitant jet observed across the tricuspid valve on Doppler-echocardiography, and (2) exhibit lower hmox-1 expression, compared with patients who have minor PE (defined by the degree of TR) as well as patients without PE.
Methods
Study Design
This was a secondary analysis of a four center prospective study of diagnostic accuracy conducted in patients with suspected PE (NCT00368836), which includes patients who were ultimately diagnosed with PE (PE+) or had PE excluded (PE−).[21] The criterion standard for PE was from multidetector-channel computerized tomographic pulmonary angiography (CTPA) obtained on the day of enrollment.[22] Images were obtained at each site as part of standard care, and were done on 64 slice multi-detector equipment with ≤2.5mm collimation. Intravenous contrast media was given to all patients according to local protocol using a computer-controlled mechanized timing injector in all cases. Images were obtained using energy, pitch, rotation settings as required for the patient's body habitus. All patients had reconstructions that included transverse, coronal and saggital views. Acute PE was considered present if two independent board certified radiologists interpreted a filling defect consistent with acute PE in the absence of changes associated with chronic PE (e.g., webbing). All scans read as positive for PE were further evaluated for the location of the filling defect and the percentage obstruction of the vessel(s) using an explicit data collection format to facilitate computation of the percentage of total pulmonary vascular occlusion using the method of Mastora et al.[23] Patients underwent transthoracic echocardiography as part of standard care within 24 h. Echocardiography was performed using techniques as previously described, including pulse wave Doppler interrogation of the TR jet velocity measured >2.7 m/S, corresponding to an estimated right ventricular systolic pressure of 40 mm Hg, which is elevated at all patient ages.[3]
Blood collection
All patients had blood drawn within 12 hours of CTPA scanning. Blood was drawn by a qualified phlebotomist using 18 gauge needles into two (blue-top) tubes containing sodium citrate dihydrate to yield a final concentration of citrate in plasma equal to 0.11mM (3.2%) and 6 mL of whole blood was instilled into a Paxgene® Blood RNA tube (QIAGEN USA), which was left at room temperature for 24 hours and then frozen at −20°C. Plasma samples were drawn and transported on ice and immediately centrifuged at 2500g × 15 minutes and the plasma fraction separated from red cell mass. Plasma was stored at −80°C.[24]
Measurements
RNA was isolated from peripheral leukocytes using the PAXgene blood RNA kit (Qiagen, Valencia, CA) according to the manufacturer's protocol. First-strand complementary DNA was synthesized using iScript™ cDNA systhesis kit (Bio-Rad, Hercules, CA, USA). The reverse transcription reaction was incubated at 42°C for 30 minutes and stopped by heating to 85°C for 5 minutes. 50 ng of final product was used as template for PCR. qRT-PCR was performed using TaqMan® Probe-Based Detection (Applied Biosystems, Foster City, CA, USA) per the manufacturer's instructions with an ABI Prism 7000 Sequence Detection System using Taqman® gene expression assays (Applied Biosystems) and Taqman® Gene expression master mix (Applied Biosystems). Template was amplified by 40 cycles of denaturation at 95°C for 15 seconds, annealing of primers and probe together with extension at 60°C for 1 minute in duplicate reactions. Fluorescence data were acquired during a combined anneal / extension step. RT negative reactions were run on each plate to confirm the absence of DNA contamination. Fold change values were calculated using comparative Ct analysis and normalized to those of 18s rRNA, which was an invariant.[25] We examined expression of hmox-1 (Assay ID = Hs01110251_m1), CD-163 (Assay ID = Hs01016663_m1), haptoglobin (Assay ID = Hs00978377_m1), haptoglobin related protein (Assay ID = Hs00750565_s1) because of their essential roles in the disposition of free plasma hemoglobin. We also measured cyclooxygenase-2 transcripts (Assay ID = Hs01573469_m1) in view of this enzyme's role in producing vasoactive prostaglandins. Recognizing the role of two major genotypes that affect hmox-1 expression as a potential confounder, we performed genotyping at the −413 A→T single nucleotide polymorphism and measured the length of (GT)n repeats in the hmox-1 promoter.[26,27] The hmox-1 −413 (A→T) polymorphism was assessed with a TaqMan 5’-allele discrimination assay (Custom TaqMan SNP Genotyping Assay; Applied Biosystems, Foster City, CA, USA). The primer sequences used were from Rueda et al (2007): 5’-GGG-TTG-CTA-AGT-TCC-TGA-TGT-TG-3 (forward) and 5’-CCC-AGA-AGG-TTC-CAG-AAA-GCT-3 (reverse), and the TaqMan minor groove binder probe sequences were 5’-ACC-AGG-CTT-TTG-CTC-T-3 AND 5’-ACC-AGG-CTA-TTG-CTC-T-3; the probes were labeled with the fluorescent dyes FAM and VIC, respectively. The real-time PCR reaction was performed in a total reaction volume of 20 μl with 20ng DNA, with the following amplification protocol: initial denaturation at 95°C for 10 minutes, followed by 40 cycles of denaturation at 95°C for 15 seconds and annealing and extension at 60°C for 1 minute on the ABI7500Fast (Applied Biosystems). After PCR, the genotype of each sample was attributed automatically by measuring the allele-specific fluorescence with the ABI7500 Software v.2.0.1 for allelic discrimination (Applied Biosystems). Samples were run in duplicate reactions. Positive controls, confirmed by sequencing, for all three genotypes (AA, AT, TT) were included on each plate.
Determination of variable length polymorphism (GT)n fragment lengths in the HMOX1 promoter was performed by polymerase chain reaction (PCR) and subsequent sizing using capillary electrophoresis. Specific primers used (forward: 5’- AGA GCC TGC AGC TTC TCA GA’- 3’, modified with a 5’-FAM label, and reverse: 5’- ACA AAG TCT GGC CAT AGG AC’3’), (Integrated DNA Technologies, Coralville, IA), were previously designed by Exner et al.[28] PCR amplification was performed in 15 μl reactions which consisted of 100 ng of template DNA, 0.5 μl 5 μM forward and reverse primer, 1.5 μl 250 μM dNTP, 1.5 μl 10X Buffer, 1.5 μl 25 mM MgCl2, 0.12 μl of Amplitaq Gold DNA Polymerase (Applied Biosystems, Foster City, CA), and 8.18 μl of double distilled water. All PCR reactions were performed in an Applied Biosystems 2720 Thermal Cycler, programmed for an initial denaturation at 95°C for 5 minutes, followed by 30 cycles at 95°C for 30 s, 58°C for 30 s, and 720C for 30s, with a final extension at 72°C for 7 minutes. Subsequently, 1 μl of PCR product was added to 12 μl of HiDi Formamide and 0.5 μl of Genescan 500 LIZ standard (both from Applied Biosystems). Capillary electrophoresis-based DNA sizing was performed on an ABI 310 DNA genetic analyzer (Applied Biosytems). The resultant electropherograms were analyzed using Genemapper v4.0 genotyping software (Applied Biosystems). (GT) n was determined based on the differential mobility of amplicons with different sizes. The repeat numbers were calculated using the amplicon size and the sequencing reading of the reference samples. All samples were run in duplicate. Select samples from each category were chosen to be confirmed by DNA capillary sequencing, which accurately verified the size determined by the fragment analysis. GT repeats on HO-1 alleles were classified as follows: short (<26), medium 26-30, or large (>30).
Biomarkers of hemolysis
Arginase-1 and hemoglobin were measured using commercially available enzyme-linked immunoabsorbant assays (Human Arginase-1 I ELISA kit, Cat No: HK322. Hycult Biotech, Plymouth Meeting, PA; Human Hemoglobin ELISA kit, Cat No: E88-135, Manufacturer: Bethyl Laboratories, Montgomery, TX). Plasma D-dimer and fibrinogen concentrations were measured in batches of single-freeze-thawed aliquots of plasma on FDA-cleared devices (VIDAS ELISA, bioMerieux, Durham NC, Dade® Thrombin Reagent, Dade Behring, Inc. Newark, DE).
Statistical analysis
Continuous data were examined with the Shapiro-Wilk test and P<0.1 used to reject the hypothesis of a normal distribution; non-normal data are presented as medians and interquartile ranges (IQR). Parametric and categorical baseline data were compared with an unpaired t-test or a Chi-square statistic, respectively with 2 sided p values reported. Correlation was examined with first-order regression. Between-group and paired medians were compared for non-normal data using the the Kruskal-Wallis test with pairwise comparisons. P<0.05 was considered significant. All statistical testing was performed on StatsDirect (Cheshire, England, v 2.6.2).
Results
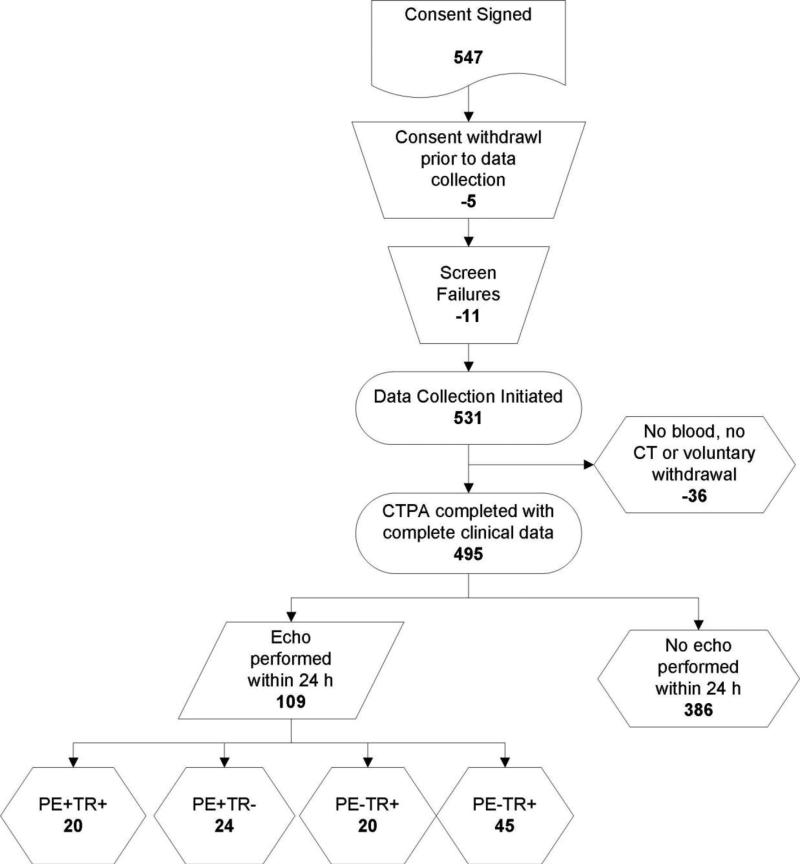
The study population included 109 patients with PE, including 44 with PE causing a mean pulmonary vascular obstruction of 33±28% and 65 without PE, (PE−). Doppler assessment found an abnormally high TR jet velocity (>2.7 m/S) in 20/44 patients (45%) with PE (PE+TR+) versus 29/65 (44%) of PE− patients.The mean pulmonary vascular occlusion of TR+ patients (39±32%) did not differ significantly from patients who were TR− (28±26%, P=0.15). The mean estimated right ventricular pressure in the PE+TR+ group was 49±14 mm Hg, which was not significantly different from the mean right ventricular systolic pressure of the 29 PE− patients who had TR jet velocity>2.7 m/S (50±15 mm Hg, P=0.55, unpaired t-test). Patients with PE+TR+ did not have a significantly different mean pulmonary vascular obstruction than patients who had PE and no TR (PE+TR−). Table 1 shows the clinical features of patients divided by PE status, and demonstrates that patients with PE were similar in terms of age and comorbidities compared with patients who did not have PE.
Table 1.
Clinical characteristics of the first study population
| Table 1 | PE+ N=44 | PE− N= 65 | P* | ||
|---|---|---|---|---|---|
| Vital signs and demographics | Mean or N | Standard deviation or % | Mean or N | Standard deviation or % | |
| Age, years | 50 | 16 | 58 | 18 | 0.07 |
| Body Mass Index | 31.9 | 8.2 | 28.9 | 7.6 | 0.16 |
| SaO2, % | 97 | 2 | 97 | 2 | 0.90 |
| Systolic blood pressure, mm Hg | 116 | 13 | 135 | 18 | <0.001 |
| Heart Rate, beats/min | 94 | 21 | 89 | 18 | 0.46 |
| Caucasian race | 20 | 45% | 44 | 68% | 0.02 |
| Black race | 16 | 36% | 18 | 28% | 0.34 |
| Female gender | 26 | 59% | 34 | 52% | 0.48 |
| Symptoms | |||||
| Syncope | 2 | 5% | 2 | 3% | 0.69 |
| Pleuritic chest pain | 20 | 48% | 17 | 26% | 0.04 |
| Dyspnea | 25 | 56% | 35 | 54% | 0.76 |
| Hemoptysis | 0 | 0% | 2 | 3% | 0.24 |
| Cough | 11 | 25% | 27 | 42% | 0.08 |
| Limb swelling | 10 | 23% | 8 | 12% | 0.15 |
| Risk Factors** | |||||
| Prior stroke | 0 | 0% | 2 | 3% | 0.24 |
| Recent surgery | 6 | 14% | 10 | 15% | 0.80 |
| Heart failure | 4 | 9% | 8 | 12% | 0.60 |
| Bed rest >72h | 10 | 23% | 15 | 23% | 0.97 |
| Prior DVT without PE | 8 | 18% | 7 | 10% | 0.27 |
| Active connective tissue disease | 1 | 2% | 6 | 9% | 0.15 |
| Known thrombophilia | 1 | 2% | 0 | 0% | 0.22 |
| Active malignancy | 5 | 11% | 6 | 9% | 0.72 |
| Sickle cell disease | 0 | 0% | 1 | 2% | 0.41 |
| Anemia | 3 | 7% | 5 | 8% | 0.86 |
| Diabetes | 4 | 9% | 13 | 20% | 0.12 |
| Focal Infection | 0 | 0% | 5 | 8% | 0.06 |
| Current estrogen use | 6 | 13% | 3 | 5% | 0.09 |
| History of COPD | 1 | 2% | 6 | 9% | 0.15 |
| History of Asthma | 5 | 12% | 13 | 20% | 0.23 |
| Never smoked | 30 | 68% | 47 | 72% | 0.64 |
| Aspirin use in past 7 days | 19 | 43% | 27 | 42% | 0.86 |
P values from unpaired t-test or Chi-Square
Prior history requiring treatment; reported by patient or from documented past medical history at time of diagnosis
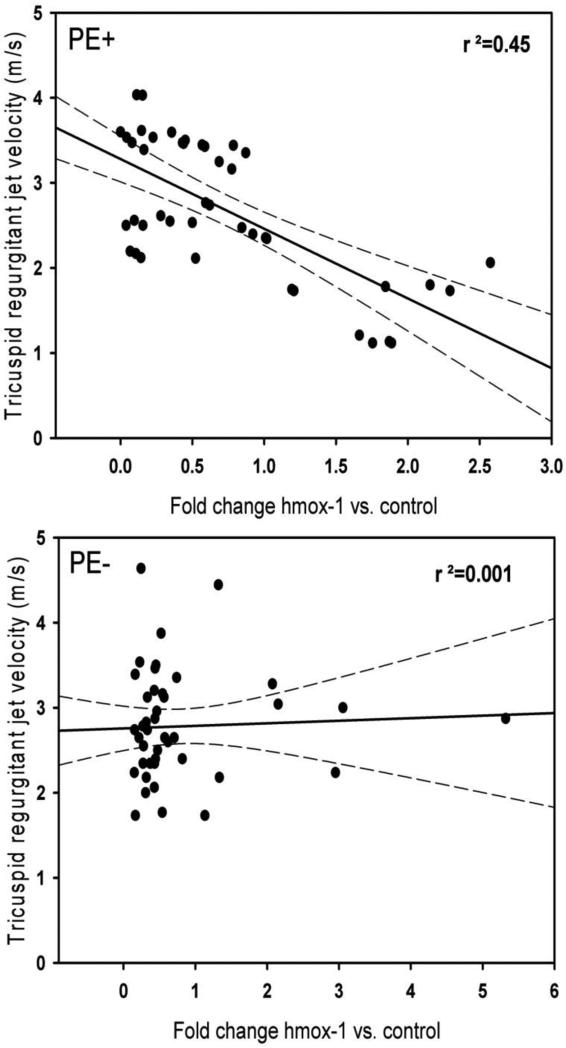
Table 2 shows the fold change in gene expression based upon presence or absence of PE and significant TR relative to the housekeeper 18S RNA for hmox-1, haptoglobin receptor, CD163, haptoglobin, haptoglobin related protein and cyclooxygenase-2. Medians are shown because the data were not normally distributed. The median values and associated interquartile ranges for hmox-1 and haptoglobin show that patients with PE+TR+ had lower expression of these genes compared with patients who were PE+TR− or PE−. To explore this potential relationship further, Figures 2A and 2B plot the first order regression line of the fold change in hmox-1 versus the TR jet velocity. The results demonstrate a significant inverse relationship for patients with PE (r2,=0.45, P<0.001) but this inverse relationship was absent in patients without acute PE (r2,=0.001, P=0.52). The cyclooxygenase-2 gene was not significantly different between groups and had no correlation with the TR jet velocity in patients with PE (R2=0.03). These data suggest an hmox-1 specific transcriptional difference in the way that patients with PE adapt to increased right heart pressure compared with patients who have TR from non thrombotic causes.
Table 2.
Fold expression of genes in peripheral leukocytes relative to 18S RNA in the first population
| Gene | PE+ TR+ | PE+ TR− | PE− | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Median fold change | 1st-3rd quartiles | Median fold change | 1st-3rd quartiles | Median fold change | 1st-3rd quartiles | ||||
| hmox-1 | 0.59* | 0.34 | 0.77 | 1.11 | 0.46 | 1.41 | 0.54 | 0.36 | 1.18 |
| CD163 | 0.81 | 0.44 | 1.47 | 0.80 | 0.57 | 0.92 | 0.65 | 0.37 | 1.21 |
| Haptoglobin | 0.23** | 0.04 | 0.39 | 0.49 | 0.23 | 1.68 | 0.70 | 0.17 | 1.70 |
| Haptoglobin related protein | 2.07 | 0.16 | 4.05 | 0.50 | 0.22 | 2.00 | 1.76 | 0.28 | 8.36 |
| Cyclooxygenase-2 | 0.52 | 0.42 | 0.87 | 0.76 | 0.33 | 1.14 | 0.71 | 0.44 | 1.14 |
Abbreviations: PE, pulmonary embolism; TR, tricuspid regurgitation > 2.7 m/S
P<0.05 vs. PE+TR−
P<0.05 vs. PE+TR− and PE−
Figure 2.
First order regression plots of the TR jet velocity, estimated by Doppler echocardiography versus fold expression of hmox-1 relative to 18S RNA. A. Patients with acute PE, B. Patients with PE excluded.
Table 3 shows a significant increase in the median value for the plasma biomarker of hemolysis, arginase-1 in patients with PE+TR+ by pairwise comparison using the Kruskal-Wallis test. This finding is consistent with previous work has found that arginase-1 elevated in acute PE.[14,29] Cell-free hemoglobin, D-dimer and fibrinogen median concentrations were not different between groups.
Table 3.
Biomarkers of hemolysis and coagulation
| Gene | PE+ TR+ | PE+ TR− | PE− | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Median value | 1st-3rd quartiles | Median value | 1st-3rd quartiles | Median value | 1st-3rd quartiles | ||||
| Arginase-1 (ug/L) | 41.6** | 22.7 | 49.5 | 14.8 | 8.8 | 28.4 | 18.6 | 9.5 | 50.1 |
| Cell free hemoglobin (ug/L) | 35.8 | 24.7 | 42.6 | 29.0 | 21.8 | 47.4 | 22.1 | 15.6 | 42.2 |
| D-dimer (ug/L) | 2845 | 1660 | 4426 | 3104 | 2219 | 3611 | 1167* | 537 | 2176 |
| Fibrinogen (mg/dL) | 359.0 | 299.8 | 473.8 | 385.0 | 324.0 | 530.0 | 408.0 | 323.0 | 483.5 |
P<0.05 vs. PE+TR−
P<0.05 vs. PE+TR− and PE−
Table 4 is a matrix that shows distribution of hmox-1 genotypes for the −413 A→T sequence variation and for the GT repeats in the promoter region and the median fold change in expression associated with the genotype. The range of the median fold change in hmox-1 expression was 0.34 to 1.89 and neither genotype was more frequent among patients with PE and significant TR. These data indicate at most a mild effect of genotype on hmox-1 expression in this patient sample. The results also suggest that other still unknown inherited or acquired factors may influence expression of hmox-1.
Table 4.
Counts of patients by two hmox-1 genotypes and PE status (median fold change in hmox-1 expression versus 18SRNA shown in parenthesis).
| –413 A→T status | PE+, GT repeat length per allele | Subtotal PE+ | –413 A→T status | PE−, GT repeat length per allele | Subtotal PE− | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LL | ML | SL | MM | SM | SS | LL | ML | SL | MM | SM | SS | ||||
| AA (wild type) | 3 (0.34) | 4 (1.34) | 1 | 5 (0.74) | 2 | 1 | 16 (0.78) | AA (wild type) | 0 | 1 (0.92) | 0 | 17 (1.14) | 0 | 0 | 18 (1.05) |
| AT (heterozygous) | 1 | 4 (0.65) | 4 (0.57) | 3 (0.41) | 2 (1.06) | 2 | 15 (0.77) | AT (heterozygous) | 1 (0.81) | 5 (1.10) | 1 (1.50) | 5 (0.32) | 15 (0.92) | 0 | 27 (0.85) |
| TT (homozygous) | 0 | 4 (0.73) | 8 (0.55) | 1 (0.16) | (1.89) | 0 | 13 (0.71) | TT (homozygous) | 2 (0.51) | 1 (0.32) | 6 (1.08) | 2 (1.30) | 1 (0.45) | 8 (0.50) | 20 (0.73) |
| Subtotal PE+ | 4 (0.34) | 12 (0.79) | 13 (0.56) | 9 (0.59) | 6 (1.34) | 0 | 44 (0.75) | Subtotal PE− | 3 (0.66) | 7 (0.98) | 7 (1.14) | 24 (0.98) | 16 (0.89) | 8 (0.50) | 65 (0.90) |
Abbreviations: A, adenosine; T, thymidine; PE, pulmonary embolism; LL, long-long; ML, medium-long; SL, short-long; MM, medium-medium; SM, short-medium; SS, short-short
DISCUSSION
This study documents an inverse relationship between leukocyte hmox-1 expression in patients with PE and the severity of pulmonary hypertension, as assessed by degree of TR, and lower hmox-1 and haptoglobin expression in patients with more severe PE compared with patients with PE and no TR, and patients without PE. The patients without PE included 44% with significant TR, suggesting evidence of pulmonary hypertension from non-thrombotic causes. Patients with PE were well matched in terms of age and comorbidities to patients without PE. The inverse correlation between hmox-1 expression in circulating leukocytes and the TR jet velocity in patients with acute PE was significant (r2=0.45, p <0.001), but this significance was absent in patients without PE (r2=0.01, P=0.52). The magnitude of hmox-1 expression varied minimally with variations in the hmox-1 promotor (−413 (A→T) polymorphism or GT repeats), providing evidence that the reduced hmox-1 expression with severe PE was not a result of either of these genotypes. Taken together, these data support the hypothesis that the heme oxygenase-1 enzyme has a role in determining the natural history of pulmonary hypertensive response to acute PE. We believe that heme oxygenase helps to mitigate a secondary pulmonary vasoconstriction observed with acute thrombotic PE that results from intracardiac hemolysis, possibly related to shear stresses on the erythrocyte.[1,2,13,14] We found no significant difference in expression of the inducible cyclooxygenase-2 enzyme, which synthesizes both vasodilatory and vasoconstrictive prostaglandins in the setting of PE.[9] This finding suggests a more specific adaptive role of increasing leukocyte hemeoxygenase-1 transcripts. A large body of literature has implicated hemolysis as a cause of chronic pulmonary hypertension in hemoglobinopathies, primarily sickle cell disease, but this is the first report of evidence linking hemolysis with acute pulmonary hypertension from thrombotic PE in humans.[30]
We hypothesize acute moderate to severe PE causes turbulent flow across the tricuspid and pulmonic valves and in the pulmonary tree, causing rupture of a small percentage of red cells in or immediately proximal to or within the pulmonary vascular tree. This timing and location of this shear effect requires release of a only a small amount of free hemoglobin to have a profound effect on pulmonary vascular tone. Upon their rupture, erythrocytes release tetrameric (α2β2) hemoglobin, which can avidly bind NO, but must first dissociate into αβ dimers before haptoglobin can bind them with high avidity and inactivate this NO scavenging effect.[31] In contrast to the millimolar concentrations required to constrict peripheral vasculature, free hemoglobin in the low micromolar concentration will significantly increase pulmonary vascular resistance. [32,33] Thus, any free hemoglobin produced in the right ventricle occurs at a highly vulnerable time and place for a patient with large clots that are mechanically obstructing the pulmonary arteries. The pulmonary vasoconstrictive effect of hemolysate has been well documented in isolated lung preparations.[1,34] Release of hemoglobin (and ultimately heme), and arginase-1 into the plasma results in binding of nitric oxide and also reduction in L-arginine, respectively, causing a relative reduction in vasodilatory effect.[14,29] At the same time, acute PE can increase concentrations of vasoconstrictive molecules in the pulmonary vascular tree, resulting in a shift toward vasoconstriction. [7-9] In this scenario, the efficiency with which cells in the blood and lung can remove the constrictor effect of heme via haptoglobin and heme oxygenase-1, and maintain tonic de novo nitric oxide synthesis via eNOS should have a pivotal role in this balance.
Strengths of the study include the relatively large number of patients examined for both hmox-1 mRNA and DNA sequencing for the two most common genotypes known to decrease its expression. We believe our data provide useful evidence that neither the −413 A→T single nucleotide polymorphism nor the length of (GT)n repeats in the hmox-1 promoter play a clinically significant role in hmox-1 expression in patients with PE. Also blood was drawn within 12 hours of PE diagnosis or exclusion, and therefore captured hmox-1 transcripts in the acute setting. This is important because the patient's clinical condition can change with time and treatments, which may also affect the steady state of mRNA content in peripheral leukocytes.
Limitations of present work include that the blood samples drawn from an antecubital vein probably do not contain the same concentration of plasma free hemoglobin and heme that is released in the right heart and pulmonary vascular tree with severe PE. We examined circulating leukocytes because of their convenience, but also because these cells are likely to be the first cells to contact molecules released from ruptured erythrocytes, and their genotype, assessed by mRNA content, and should provide insight into the magnitude of effect of hemolysis. However, this approach precludes ability to determine which leukocyte is responsible for the change in expression. We also did not examine haptoglobin phenotype, which may affect ability of haptoglobin to scavenge hemoglobin.[31] Additionally, the clot burden tended to be higher in TR+ patients (P=0.15), suggesting that clot severity as a possible source of bias. However, preclinical and clinical data support the hypothesis that impared hmox-1 activity may increase the initial embolic load and reduce endogenous rate of resolution.[35,36]
In conclusion, this report presents the first published data from humans to show an inverse correlation between the steady-state level of leukocyte hmox-1 transcripts and the estimated degree of TR in patients. This is consistent with our previous publications showing an increase in circulating arginase-1 and a simultaneous, but transient reduction in plasma L-arginine in patients and animals with PE.[14,29] The data suggest that haptoglobin and heme oxygenase-1 expression have important modulatory roles in the development of acute pulmonary hypertension in patients with PE.
Highlights.
Severe acute pulmonary embolism (PE) causes intravascular hemolysis
Hemolysis releases free hemoglobin and heme, which scavenge nitric oxide
Induction of hmox-1 may be beneficial as it detoxifies heme
hmox-1 transcripts were inversely proportional to right ventricular pressure
Leukocyte expression of hmox-1 may be an adaptive response to PE
Figure 1.
Flow diagram of patients with complete data including echocardiography.
Acknowledgments
JAK received funding from NIH/NHLBI R42 HL086316 to support this work.
Abbreviations
- CT
computerized tomography
- CTPA
CT pulmonary angiography
- CTEPH
Chronic thromboembolic pulmonary hypertension
- eNOS
endothelial cell derived nitric oxide synthase
- NO
nitric oxide
- PE
pulmonary embolism
- RV
right ventricle
- SBP
systolic blood pressure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest statement. JAW, NMS, MBF, DMC and HLB have no conflicts to disclose.
Reference List
- 1.Zagorski J, Marchick MR, Kline JA. Rapid clearance of circulating haptoglobin from plasma during acute pulmonary embolism in rats results in HMOX1 up-regulation in peripheral blood leukocytes. Journal of Thrombosis & Haemostasis. 2010;8:289–96. doi: 10.1111/j.1538-7836.2009.03704.x. [DOI] [PubMed] [Google Scholar]
- 2.Kline JA, Marchick MR, Hogg MM. Reduction in plasma haptoglobin in humans with acute pulmonary embolism causing tricuspid regurgitation. J Thromb Haemost. 2009;7:1597–9. doi: 10.1111/j.1538-7836.2009.03535.x. [DOI] [PubMed] [Google Scholar]
- 3.Kline JA, Steuerwald MT, Marchick MR, Hernandez-Nino J, Rose GA. Prospective Evaluation of Right Ventricular Function and Functional Status Six Months After Acute Submassive Pulmonary Embolism: Frequency of Persistent or Subsequent Elevation in Estimated Pulmonary Artery Pressure. Chest. 2009;136:1202–10. doi: 10.1378/chest.08-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levi M. Disseminated intravascular coagulation or extended intravascular coagulation in massive pulmonary embolism. J Thromb Haemost. 2010;8:1475–6. doi: 10.1111/j.1538-7836.2010.03891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leitner JM, Jilma B, Spiel AO, Sterz F, Laggner AN, Janata KM. Massive pulmonary embolism leading to cardiac arrest is associated with consumptive coagulopathy presenting as disseminated intravascular coagulation. J Thromb Haemost. 2010;8:1477–82. doi: 10.1111/j.1538-7836.2010.03862.x. [DOI] [PubMed] [Google Scholar]
- 6.Kline JA, Zeitouni R, Hernandez-Nino J, Rose GA. Size isn't everything. J Thromb Haemost. 2008;6:397–9. doi: 10.1111/j.1538-7836.2008.02834.x. [DOI] [PubMed] [Google Scholar]
- 7.Smulders Y. Pathophysiology and treatment of haemodynamic instability in acute pulmonary embolism: the pivotal role of pulmonary vasoconstriction. Cardiovascular Research. 2000;48:23–33. doi: 10.1016/s0008-6363(00)00168-1. [DOI] [PubMed] [Google Scholar]
- 8.Nakos G, Kitsiouli EI, Lekka ME. Bronchoalveolar lavage alterations in pulmonary embolism. American Journal of Respiratory & Critical Care Medicine. 1998;158:1504–10. doi: 10.1164/ajrccm.158.5.9802036. [DOI] [PubMed] [Google Scholar]
- 9.Jones AE, Watts JA, Debelak JP, Thornton LR, Younger JG, Kline JA. Inhibition of prostaglandin synthesis during polystyrene microsphere-induced pulmonary embolism in the rat. Am J Physiol Lung Cell Mol Physiol. 2003;284:L1072–L1081. doi: 10.1152/ajplung.00283.2002. [DOI] [PubMed] [Google Scholar]
- 10.Gladwin MT, Crawford JH, Patel RP. The biochemistry of nitric oxide, nitrite, and hemoglobin: role in blood flow regulation. Free Radic Biol Med. 2004;36:707–17. doi: 10.1016/j.freeradbiomed.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 11.Minneci PC, Deans KJ, Zhi H, Yuen PS, Star RA, Banks SM, Schechter AN, Natanson C, Gladwin MT, Solomon SB. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest. 2005;115:3409–17. doi: 10.1172/JCI25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293:1653–62. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 13.Insenser M, Montes-Nieto R, Martinez-Garcia MA, Duran EF, Santiuste C, Gomez V, Kline JA, Escobar-Morreale HF, Jimenez D. Identification of reduced circulating haptoglobin concentration as a biomarker of the severity of pulmonary embolism: a nontargeted proteomic study. PLoS One. 2014;9:e100902. doi: 10.1371/journal.pone.0100902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kline JA, Watts J, Courtney D, Lee Y, Hwang S. Severe pulmonary embolism decreases plasma L-arginine. Eur Respir J. 2013 doi: 10.1183/09031936.00171913. [DOI] [PubMed] [Google Scholar]
- 15.Janz DR, Bastarache JA, Sills G, Wickersham N, May AK, Bernard GR, Ware LB. Association between haptoglobin, hemopexin and mortality in adults with sepsis. Crit Care. 2013;17:R272. doi: 10.1186/cc13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leclerc JL, Blackburn S, Neal D, Mendez NV, Wharton JA, Waters MF, Dore S. Haptoglobin phenotype predicts the development of focal and global cerebral vasospasm and may influence outcomes after aneurysmal subarachnoid hemorrhage. Proc Natl Acad Sci U S A. 2015;112:1155–60. doi: 10.1073/pnas.1412833112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kakavas S, Papanikolaou A, Ballis E, Tatsis N, Goga C, Tatsis G. Carboxyhemoglobin and methemoglobin levels as prognostic markers in acute pulmonary embolism. Am J Emerg Med. 2015 doi: 10.1016/j.ajem.2015.01.046. [DOI] [PubMed] [Google Scholar]
- 18.Yet SF, Perrella MA, Layne MD, Hsieh CM, Maemura K, Kobzik L, Wiesel P, Christou H, Kourembanas S, Lee ME. Hypoxia induces severe right ventricular dilatation and infarction in heme oxygenase-1 null mice. J Clin Invest. 1999;103:R23–R29. doi: 10.1172/JCI6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimzu K, Takahashi T, Iwasaki T, Shimizu H, Inoue K, Morimatsu H, Omori E, Matsumi M, Akagi R, Morita K. Hemin treatment abrogates monocrotaline-induced pulmonary hypertension. Med Chem. 2008;4:572–6. doi: 10.2174/157340608786241972. [DOI] [PubMed] [Google Scholar]
- 20.Minamino T, Christou H, Hsieh CM, Liu Y, Dhawan V, Abraham NG, Perrella MA, Mitsialis SA, Kourembanas S. Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular responses to hypoxia. Proc Natl Acad Sci U S A. 2001;98:8798–803. doi: 10.1073/pnas.161272598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kline JA, Hogg MM, Courtney DM, Miller CD, Jones AE, Smithline HA. D-Dimer Threshold Increase with Pretest Probability Unlikely for Pulmonary Embolism to Decrease Unnecessary Computerized Tomographic Pulmonary Angiography. J Thromb Haemost. 2012;10:572–81. doi: 10.1111/j.1538-7836.2012.04647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Courtney DM, Miller CD, Smithline HA, Klekowski N, Hogg MM, Kline JA. Prospective multi-center assessment of interobserver agreement for radiologist interpretation of multidetector CT angiography for pulmonary embolism. Journal of Thrombosis & Haemostasis. 2010;8:533–40. doi: 10.1111/j.1538-7836.2009.03724.x. [DOI] [PubMed] [Google Scholar]
- 23.Mastora I, Remy-Jardin M, Masson P, Galland E, Delannoy V, Bauchart JJ, Remy J. Severity of acute pulmonary embolism: evaluation of a new spiral CT angiographic score in correlation with echocardiographic data. European Radiology. 2003;13:29–35. doi: 10.1007/s00330-002-1515-y. [DOI] [PubMed] [Google Scholar]
- 24.Woodhams B, Girardot O, Blanco MJ, Colesse G, Gourmelin Y. Stability of coagulation proteins in frozen plasma. Blood Coagulation & Fibrinolysis. 2001;12:229–36. doi: 10.1097/00001721-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Exner M, Minar E, Wagner O, Schillinger M. The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Radic Biol Med. 2004;37:1097–104. doi: 10.1016/j.freeradbiomed.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Mustafa S, Weltermann A, Fritsche R, Marsik C, Wagner O, Kyrle PA, Eichinger S. Genetic variation in heme oxygenase 1 (HMOX1) and the risk of recurrent venous thromboembolism. J Vasc Surg. 2008;47:566–70. doi: 10.1016/j.jvs.2007.09.060. [DOI] [PubMed] [Google Scholar]
- 28.Exner M, Bohmig GA, Schillinger M, Regele H, Watschinger B, Horl WH, Raith M, Mannhalter C, Wagner OF. Donor heme oxygenase-1 genotype is associated with renal allograft function. Transplantation. 2004;77:538–42. doi: 10.1097/01.tp.0000113467.36269.f8. [DOI] [PubMed] [Google Scholar]
- 29.Watts JA, Gellar MA, Fulkerson MB, Das SK, Kline JA. Arginase depletes plasma l-arginine and decreases pulmonary vascular reserve during experimental pulmonary embolism. Pulm Pharmacol Ther. 2011 doi: 10.1016/j.pupt.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Klings ES, Machado RF, Barst RJ, Morris CR, Mubarak KK, Gordeuk VR, Kato GJ, Ataga KI, Gibbs JS, Castro O, Rosenzweig EB, Sood N, Hsu L, Wilson KC, Telen MJ, Decastro LM, Krishnamurti L, Steinberg MH, Badesch DB, Gladwin MT. An official American Thoracic Society clinical practice guideline: diagnosis, risk stratification, and management of pulmonary hypertension of sickle cell disease. Am J Respir Crit Care Med. 2014;189:727–40. doi: 10.1164/rccm.201401-0065ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nielsen MJ, Moestrup SK. Receptor targeting of hemoglobin mediated by the haptoglobins: roles beyond heme scavenging. Blood. 2009;114:764–71. doi: 10.1182/blood-2009-01-198309. [DOI] [PubMed] [Google Scholar]
- 32.Jeffers A, Gladwin MT, Kim-Shapiro DB. Computation of plasma hemoglobin nitric oxide scavenging in hemolytic anemias. Free Radic Biol Med. 2006;41:1557–65. doi: 10.1016/j.freeradbiomed.2006.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blood AB, Schroeder HJ, Terry MH, Merrill-Henry J, Bragg SL, Vrancken K, Liu T, Herring JL, Sowers LC, Wilson SM, Power GG. Inhaled nitrite reverses hemolysis-induced pulmonary vasoconstriction in newborn lambs without blood participation. Circulation. 2011;123:605–12. doi: 10.1161/CIRCULATIONAHA.110.001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voelkel NF, Lobel K, Westcott JY, Burke TJ. Nitric oxide-related vasoconstriction in lungs perfused with red cell lysate. FASEB J. 1995;9:379–86. doi: 10.1096/fasebj.9.5.7896007. [DOI] [PubMed] [Google Scholar]
- 35.Bean CJ, Boulet SL, Ellingsen D, Trau H, Ghaji N, Hooper WC, Austin H. Increased risk of venous thromboembolism is associated with genetic variation in heme oxygenase-1 in Blacks. Thromb Res. 2012;130:942–7. doi: 10.1016/j.thromres.2012.08.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gabre J, Chabasse C, Cao C, Mukhopadhyay S, Siefert S, Bi Y, Netzel-Arnett S, Sarkar R, Zhang L. Activated protein C accelerates venous thrombus resolution through heme oxygenase-1 induction. J Thromb Haemost. 2014;12:93–102. doi: 10.1111/jth.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]