Abstract
The relationship between the immune system and angiogenesis has been described in several contexts, both in physiological and pathological conditions, as pregnancy and cancer. In fact, different types of immune cells, such as myeloid, macrophages and denditric cells, are able to modulate tumor neovascularization. On the other hand, tumor microenvironment also includes extracellular matrix components like hyaluronan, which has a deregulated synthesis in different tumors. Hyaluronan is a glycosaminoglycan, normally present in the extracellular matrix of tissues in continuous remodeling (embryogenesis or wound healing processes) and acts as an important modulator of cell behavior by different mechanisms, including angiogenesis. In this review, we discuss hyaluronan as a modulator of tumor angiogenesis, focusing in intracellular signaling mediated by its receptors expressed on different immune cells. Recent observations suggest that the immune system is an important component in tumoural angiogenesis. Therefore, immune modulation could have an impact in anti-angiogenic therapy as a new therapeutic strategy, which in turn might improve effectiveness of treatment in cancer patients.
Angiogenesis and angiogenic factors
Angiogenesis is defined as the formation of new blood vessels from pre-existing vessels. The angiogenic process may occur by four different mechanisms: sprouting, intussusceptions, elongation/widening and potentially, incorporation of circulating endothelial precursor cells into vessel walls. This process is critical during embryonic and fetal development, but also occurs as a physiological pathway in adults during wound healing, skeletal growth, menstrual cycle and pregnancy.
Physiological angiogenesis is a sequence of cellular events comprising vascular initiation, formation, maturation, remodeling and regression, which are accurately controlled to supply tissue requirements. Nevertheless, angiogenesis has an important role in different diseases including cancer. The biochemical stimulation of angiogenesis is performed by several angiogenic factors, being the most important: vascular endothelial growth factor (VEGF) and fibroblast growth factor-2 (FGF-2). Other angiogenic factors include angiopoietins, matrix metalloproteinases (MMPs), cadherins and integrins.1
VEGF is a major player in angiogenesis: VEGF-A is the main member of a gene family that also includes VEGF-B, VEGF-C, VEGF-D and placenta growth factor. VEGF interact with multiple receptor tyrosine kinases including VEGF receptor-1 (VEGFR-1) and VEGF receptor-2 (VEGFR-2). Placenta growth factor and VEGF-B selectively bind to VEGFR-1, whereas VEGF-C and VEGF-D primarily interact with VEGFR-3.2
VEGF-A–VEGFR-2 interaction induces a tyrosine kinases signaling pathway that stimulates proliferation, migration and production of several factors in endothelial cells (ECs). In turn this interaction stimulates vessel permeability (through endothelial nitric oxide synthase and nitric oxide), proliferation/survival (FGF-2), migration (ICAMs/VCAMs/MMPs) and finally differentiation into mature blood vessels.3 The FGF family consists of 22 known proteins, being FGF-1 (acidic FGF) and FGF-2 (basic FGF) the main members. In general, FGFs stimulates cellular functions by binding to cell-surface FGF-receptors in association with heparin proteoglycans. FGF-1 stimulates the proliferation and differentiation of the cell necessary for building an arterial vessel, including ECs and smooth muscle cells. Besides, FGF-2 promotes ECs proliferation and the tube-like structure organization. Even more, FGF-1 and FGF-2 are important players in wound healing process.3
Tumor angiogenesis
Both in physiological and pathological angiogenesis, a cascade of several signals and cellular functions drive the establishment of new blood vessels, responding to an increased requirement of oxygen and nutrients.
A hypoxic microenvironment typically develops in pathological conditions such as cancer, enhancing VEGF production,4 which in turn cooperates with oncogenic events that promote angiogenesis.1, 4 The hypoxic process is driven largely by the transcriptional activity of hypoxia-inducible factors, inducing the expression of VEGF-A and other pro-angiogenic mediators.1 Tumor vessels display disorganized structure and abnormal function, as tumor-associated ECs acquire changes in morphology,5 physiology,6 cytogenetics,7 epigenetics8 and gene expression.9 The irregular perfusion impairs oxygen, nutrient and drug delivery to the tumor.10 Finally, excess of pro-angiogenic molecules stimulated by hypoxia leads to an additional disorganization driving to malignant tumor cell selection and dissemination.11
The role of tumor microenvironment in angiogenesis
Tumor cells are able to modify the environment where they grow and these changes support the formation of a special microenvironment: ‘the tumor microenvironment'.12 Tumor cells interact with tumor microenvironment, constituted by cellular and non-cellular components (the extracellular matrix (ECM)). Besides, tumor cells induce a disorder of the microenvironment homeostasis leading to a sustained proliferative signaling, evasion of growth suppressors, resistance to cell death, invasion and metastasis, reprogramming energy metabolism, evading immune responses and inducing drug resistance and angiogenesis.13
Non-cellular microenvironment: ECM
ECM contributes to the angiogenesis process by multiple mechanisms: (i) is able to regulate migration, invasion, proliferation and survival of ECs, (ii) provides a scaffold where mechanical guidance forces are established among distal ECs, providing an organized environment in the absence of cell–cell contact, (iii) serves as a control of lumen and tube formation and (iv) offers neovessel stability and maturation.14
The ECM components that mainly interact with tumor cells are fibronectin, laminin, collagen, proteoglycans and glycosaminoglycans, such as hyaluronan (HA)13 (Table 1). HA is a crucial component of the ECM. Interestingly, it has relevant implications in physiological and pathological angiogenesis, performing an importing link between ECM–angiogenesis–immune cells–cancer.
Table 1. ECM components in cancer.
| Collagens |
| The most abundant proteins in mammals ECM. Fibrillar collagens influence cellular functions through interactions with integrins, laminin and heparan-sulfate proteoglycans. Increased deposition of collagen is found during tumor formation.15 It is known that proteolytic fragments of type IV collagen can inhibit tumor angiogenesis,16 as well as a proteolytic fragment of the α1 chain of type XVIII collagen.17 |
| Glycoproteins (laminins and fibronectins) |
| Laminins are heterotrimeric glycoproteins that are composed of α, β and γ chains. They are primarily located in BMs and form networks with type IV collagen and nidogen. When laminins are cleaved by MMPs, their subunits stimulate cell migration and invasion.18 On the other hand, fibronectins are dimeric glycoproteins that are present in the ECM and in blood. They form fibrils and affect cell morphology, adhesion, migration and differentiation by binding to integrins.19 Binding domains on fibronectin were found to modulate the activity of VEGF and promote the association between integrin and VEGFR-2.20 |
| Proteoglycans and Glycosaminoglycans |
| Glycosaminoglycans (GAGs) are long, unbranched polysaccharides composed of repeating disaccharide units consisting of alternating uronic acids and amino sugars. Four classes of glycosaminoglycans have been identified: heparan sulfate, chondroitin sulfate/dermatan sulfate, keratan sulfate and HA. The main chondroitin-sulfate proteoglycan of noncartilaginous tissues is versican. Syndecans, glypicans and CD44 are cell-surface proteoglycans. All of them present relevance in cancer. Changes in expression of these molecules, as well as of enzymes involved in their biosynthesis and degradation, contribute to the different steps of tumor progression: proliferation, growth, invasion and metastasis.21 In therapeutics, targeting of GAGs and proteoglycans are highly promising. In the case of HA, it is an anionic, non-sulfated glycosaminoglycan, formed by alternating glucuronic acid and N-acetylglucosamine. One of the main components of the extracellular matrix, HA contributes significantly to cell proliferation and migration, and may also be involved in the progression of some malignant tumors.22 |
Abbreviations: ECM, extracellular matrix; HA, hyaluronan; MMP, matrix metalloproteinase; VEGF, vascular endothelial growth factor.
Cellular microenvironment
The cellular tumor microenvironment is composed of (i) non-hematopoietic cells including ECs and tumor-associated fibroblasts and (ii) stem cells, circulating endothelial precursor cells and hematopoietic cells as well as immune cells, such as myeloid-derived suppressor cells (MDSC), tumor-associated macrophages, neutrophils, natural killer cells (NK), B- and T-lymphocytes, monocytes and dendritic cells.13
Several lines of evidence indicate that different immune cells subsets contribute to blood vessel neoformation and remodeling. Even more, the immune cells functionally participate during tumor growth and progression (Table 2). These cells release pro-tumourogenic factors like cytokines and chemokines, ECM-degrading enzymes, reactive oxygen species and other bioactive molecules. Different immune cells share several angiogenic signaling pathways with other types of cells in tumor microenvironment, thus inhibition of one factor might lead to the compensatory upregulation of another angiogenic molecule release from the immune system, maintaining angiogenic activity and causing therapeutic failure in clinical treatment.
Table 2. Presence of immune cells in cancer.
| Macrophages |
| They adapt their phenotype to the dynamically changing microenvironment that they encounter. TAMs are derived from circulating monocytes or resident tissue macrophages, found within the stroma of many tumor types. |
| Myeloid-derived suppress cells (MDSCs) |
| This cells are a heterogeneous type of immune cell, which have a defined immunosuppressive function. Different factors derived from tumor microenvironment allow their expansion, in turn MDSC inhibit immune attack affecting T-cell response by several mechanisms.23 |
| Dendritic cells (DC) |
| Are essential to induce immunity against cancer. Immature (non-activated) DC can present self-antigens to T cells, which leads to immune tolerance either through T-cell deletion or through the differentiation of regulatory or suppressor T cells. Mature (activated) antigen-loaded DC, can start the differentiation of antigen-specific T cells into effectors T cells. DCs capture tumor antigens released from tumor cells and cross-present them to T cells, contributing to tumor rejection.24 |
| Natural killer cells (NK) |
| Provide rapid responses to transformed cancer cells. Infiltration of tumors with NK cells has been shown to represent a positive prognostic marker in colorectal,26 gastric27 and lung carcinoma.28 |
| B cells |
| High numbers of B-lymphocytes have been found in aggregates with other immune cells at the inflammatory site in tumor tissues of various human cancers.29 The intratumoral presence of B cells has been correlated with enhanced survival in patients with ovarian30 and non-small lung cancer.31 |
| T cells |
| There are several subsets of T cells, within CD4+ (helper) and CD8+ (cytotoxic), that recognize antigens expressed in most tumor cells. Enhanced intratumoral CD8+ T-cell infiltration has been observed to be a positive prognostic marker in melanoma, head and neck, breast, bladder, urothelial, ovarian, colorectal, prostatic and lung cancers.32 |
Abbreviation: TAM, tumor-associated macrophage.
As immune cells are able to bind HA through different receptors, the interaction between HA, immune system and tumor angiogenesis will be discussed in the following paragraphs of this review.
HA–immune system–tumor angiogenesis
In normal tissues, HA synthesis and degradation are strictly balanced trough HA synthases and hyaluronidases, respectively. At homeostasis, HA high-molecular weight (HMW, ranging from 0.5 × 106 to 2 × 106 Da) is predominant, having hydrodynamic properties. Although the low-molecular weight (LMW, ranging from 104 to 0.5 × 106 Da) form is present mainly during inflammation. It is well known that in malignant tumors the concentration of HA is usually altered in relation to normal tissues and in some tumors the HA level could be considered a predictor of malignancy.33 In fact, during cancer, HA is fragmented into LMW forms due to an imbalance between HA synthases/hyaluronidases activity and by reactive oxygen species present in tumor tissues. Even more, HA LMW and fragments (<104 Da) promote spreading by stimulating angiogenesis and creating a microvascular network in several tumors. Moreover, it is well documented that HA oligomers are angiogenic and enhance tumor invasiveness.34, 35 On the other hand and despite of their angiogenic properties, exogenous administration of HA oligomers might inhibit tumor growth.36, 37 Besides, HA HMW could promote a protective effect during tumor progress, delaying tumor growth in colon carcinoma38 and migration in fibrosarcoma cells.39 Moreover, several works have demonstrated that HA HMW is able to mediate cancer resistance in naked mole rats. This species of rodent present a high HA accumulation in skin, and the bases of its cancer resistance is because their fibroblast cells synthesize large concentrations of HA with a molecular mass higher (~1 × 107 Da) than other species.40 Thus, this controversial effects require further investigations in the HA science study.
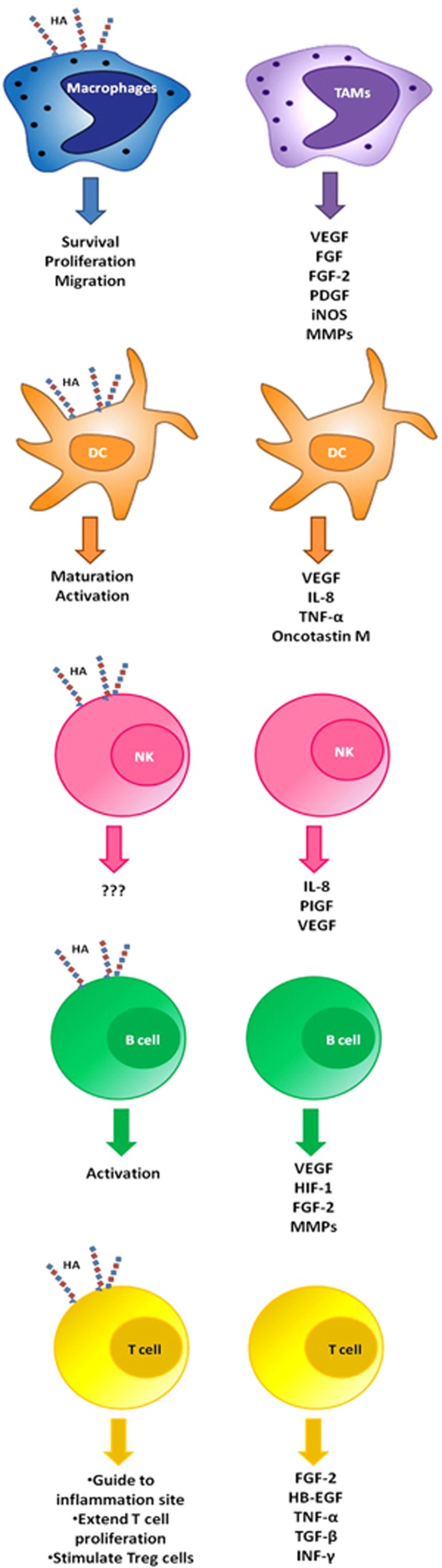
During inflammation immune cells are able to bind HA, thus their response depend on the cell type, the HA molecular weight, HA-binding proteins and structures41 (Figure 1). For instance, an inflammatory microenvironment induces macrophages to bind HA possible to promote their migration, survival and proliferation41 and it was shown that LMW HA polarize macrophages to M1 phenotype in vitro.42 On the other hand, HA present in tumor microenvironment serves as a signal for recruiting tumor-associated macrophages, which are key immune cells involved in tumor neovascularization related to HA.43 Tumor-associated macrophages suffer a metabolic adaptation in hypoxic areas and release pro-angiogenic factors: VEGF, FGF-2, PIGF, platelet-derived growth factor (PDGF).44, 45, 46, 47 Also, these cells are able to downregulate the expression of angiogenesis inhibitors, such as vasohibin-248 and stimulate the expression of angiogenesis-modulating enzymes, such as COX-2, iNOS and MMPs.47, 49, 50
Figure 1.
Immune cells: HA binding and angiogenic signaling. During inflammation immune cells are able to bind HA. Even more, it is well known that these cells are able to modulate angiogenesis by releasing cytokines and angiogenic factors.
MDSCs have an important role in tumor angiogenesis, as they are able to incorporate into endothelial vessel, displaying an endothelial like morphology and expressing both VEGFR-2 and VE-cadherin, which are EC markers. Besides, MDSCs secrete angiogenic factors, such as MMPs, VEGF, basic FGF.23 There is no evidence about their capability to bind HA, although it has been demonstrated in liver that human hepatic stellate cells induce the transformation of peripheral blood monocytes into MDSCs in a CD44-dependent form. Then it will be very interesting to investigate the linking mechanisms of the CD44–HA–MDSCs interaction.51
HA fragments and oligosaccharides, but not HA HMW, induce maturation and activation of dendritic cell (DC) in vitro and in tumor context.24, 52, 53, 54, 55 Even though DCs prime responses to tumor antigens, functions of these cells are altered by the tumor microenvironment. Immature DC can increase the expression of VEGF and interleukin-8 (IL-8) on hypoxic conditions,56 promoting a pro-angiogenic function in cancer.57, 58 DCs in tumor environment are able to release pro-angiogenic cytokines: tumor necrosis factor-α, IL-8, osteopontin57, 59 VEGF, oncotastin M,60, 61 controlling the process of DCs transdifferentiation to EC-like phenotype.61 Besides, tumor-derived factors such as hepatocyte growth factor,62 transforming growth factor-β63;64 prostaglandin E2,54 lactate65 and osteopontin66 modulate DCs maturation and thus their pro-angiogenic properties.
There is no direct evidence that links HA with NK cells, but it was shown that LMW HA in combination with IL-2, IL-12 or IL-18 is able to enhance interferon-γ production.67 Separately, it is well documented that NK cells contribute to physiological vascular remodeling during the secretory phase of menstrual cycle and pregnancy, through secretion of cytokines and angiogenic growth factors.68 The role of NK in tumor angiogenesis has not yet been fully investigated. Bruno et al. have shown that in patients with lung cancer, tumor infiltrating NK cells exhibited an angiogenic phenotype associated with a production of VEGF, PIGF and IL-8.69 NK cells have a high cytolytic capacity against transformed cancer cells, playing an important role in tumor rejection, even though NK cells function is provably affected in tumor microenvironment.
Naive B cells bind HA in a lower degree in comparison to activated B cells. However, it was shown that HA could induce their activation.70 Angiogenic signals between tumor cells, ECs and B cells are mediated by STAT3 through the overexpression of multiple pro-angiogenic molecules, such as VEGF, hypoxia-inducible factor-1, FGF-2, MMP-9, MMP-2.47 Furthermore, activated STAT3 B cells are found in the surroundings of tumor vasculature, contributing to tumor development and angiogenesis. Therefore, STAT3 in B cells might work as potential therapeutic target for anti-angiogenesis therapy.71 In parallel, it was demonstrated that STAT3 activation through fibroblast growth factor receptor (FGFR) induces HA synthesis, contributing to tumor growth.72
T cells activation induces HA binding probably to guide them to the inflammation site.41 Even more, it was demonstrated that HA stimulates CD4+ CD25+ T cells to increase IL-2 production. An IL-2 sustain production is able to (i) extend T-cell proliferation and/or (ii) stimulate Treg cells to suppress the immune response.41 In parallel, a hypoxic tumor microenvironment recruits Treg cells which promote tumor angiogenesis through VEGF-A production and diminish T-cell function.73 Alternatively, T cells are able to recognize class I and II major histocompatibility complex-peptide and other co-stimulatory surface molecules express on ECs.74 Furthermore, T cells are able to interact with ECs through cytokines and cell–cell contact, modulating blood vessel formation and remodeling, blood flow, fluidity and ‘permselectivity'.75 T cells can synthesize pro-angiogenic factors, such as FGF-2 and heparin binding epidermal-like growth factor.76 Alternatively, T-cell cytokines, tumor necrosis factor-α, transforming growth factor-β and INF-γ were reported to have anti-angiogenesis properties in tumors such as brain and ovarian carcinoma.77
The exact mechanisms by which HA can contribute with tumor angiogenesis has not yet been well elucidated. It is possible to suggest one mechanism linked to the immune system. As it was mentioned, HA oligomers and LMW are able to induce tumor angiogenesis, as well as inhibiting tumor growth and inducing active immune responses. To understand these apparent contradictions, further investigations will be necessary regarding the signals triggered by HA and its cellular receptors in tumor context.
Modulation of intracellular signals by CD44
CD44, the major receptor of HA, includes a family of plasma membrane glycoproteins, which are involved in several cellular functions, such as cell–cell and cell–matrix adhesions, cell migration, proliferation and lymphocyte homing. CD44 is expressed in a variety of cells including tumor cells. Elevated CD44 expression was correlated with poor prognosis in many cancers, such as lung,78 ovarian,79 breast,80 colorectal,81 gastrointestinal neuroendocrine tumor.82 Even more, CD44 is emerging as a metastatic tumor marker.
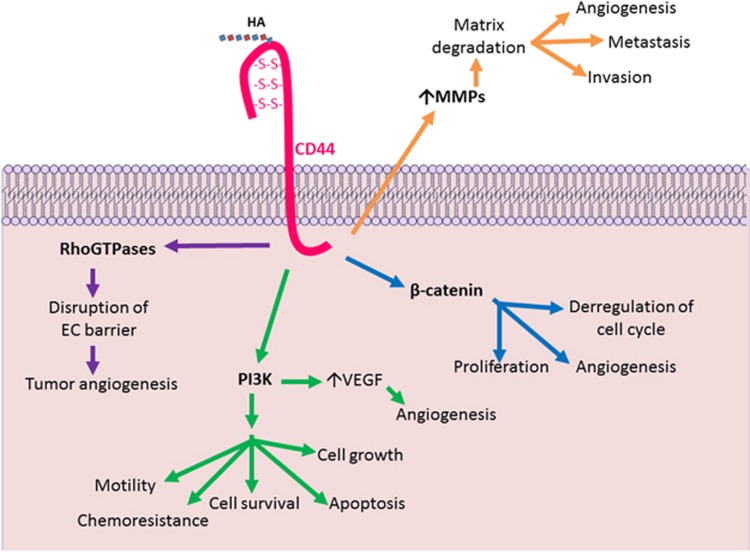
As it has been stated HA–CD44 interactions have an important role in: (i) development, (ii) inflammation, (iii) T-cell recruitment and activation and (iv) tumor growth and metastasis. CD44 has a cytoplasmatic domain with phosphorylation sites (Ser291, Ser325 and Ser316) that are regulated when ligands bind to transduce signaling and select its downstream effectors. These interactions and modulations generate multiple cellular functions such as cytoskeleton activation, tumor cell adhesion, growth migration and invasion, leading to tumor progression and angiogenesis (Figure 2).
Figure 2.
HA–CD44 interaction. CD44 has a cytoplasmic domain that can be phosphorylated when HA binds to transduce signaling and so it selects its downstream effectors, it can be cytoskeletal proteins like Ankyrin, different oncogenic signals such as RhoGTPases, PI3K, β-catenin and the release of ECM-degrading enzymes (MMPs). This interactions and modulations generate multiple cellular functions: cytoskeleton activation, tumor cell adhesion, tumor growth and invasion, tumor progression, angiogenesis and metastasis.
HA–CD44 and RhoGTPases
HA–CD44 induces activation of RhoGTPases signaling, which produce specific structural changes: as actin assembly, cytoskeleton reorganization, transcriptional activation, tumor cell growth, survival, migration and invasion.83 CD44 is linked to small GTP-binding proteins such as RhoA and Rac1. It has been shown that the interaction HMW-HA–CD44 leads to the activation of Rac1 initiating cortical actin formation and strengthening the connection between ECs.84 On the other hand, LMW HA binds to CD44 and triggers a series of events that promote the formation of RoA-GTP complex, which stimulates the serine/threonine kinase, leading to actin stress fiber formation and disruption of the EC barrier.84 These events triggered by LMW HA cause the disruption of the endothelium guiding to an important event in tumor angiogenesis and cancer metastasis.84 In immune cells, GTPases (RhoA, Rac1 and Rac2) have important roles in migration, adherence, chemotaxis and phagocytosis/endocytosis.85 However, it is not yet elucidated how the HA–CD44 interaction modulates RhoGTPases signaling in immune cells and its relation with the synthesis and function angiogenic factors.
HA–CD44 and PI3K
HA induces the formation of a complex that contains CD44, ErbB2, phosphatidylinositol 3-kinase (PI3K), ezrin and the chaperone protein HASP90.35 It was found that this complex is assembled in lipid rafts and it enables signaling through ErbB286 promoting cell survival through PI3K activation. This pathway has a central role in oncogenesis by the regulation of intracellular processes: cell survival, apoptosis, cell growth, angiogenesis, motility and chemoresistance.87 HA is able to modulate the PI3K/AKT pathway in different cancer types, such as lymphoma, colon and lung carcinoma.36, 88 Activation of the PI3K/AKT pathway in tumor cells can increase VEGF secretion by hypoxia-inducible factor-189 and modulate the expression of other angiogenic factors: nitric oxide and angiopoietins.89 There is strong evidence that suggests that the PI3K/AKT pathway modulates angiogenesis though it is not well elucidated yet how HA is involved in this angiogenic pathway.
In inflammatory context PI3K/AKT controls several functions, for example hematopoietic stem cells survival, and T- and B-cell development.90 There is no evidence that connects this pathway in immune cells with CD44 and HA, but it is possible to hypothesize that HA would modulate this signaling by CD44 present in immune cells.
HA–CD44 and β-catenin
Another interesting outcome in the HA–CD44 signaling is the involvement of β-catenin, the main protein of the canonical Wnt pathway.91, 92 The Wnt signaling pathway controls cell polarity, self renewal and proliferation.93 In absence of Wnt ligands, β-catenin is phosphorylated at N-terminal-specific residues by a destruction complex conformed by GSK3 kinase and the adenomatous polyposis coli protein among other complexes and it is sent to ubiquitination and degradation. Otherwise, when Wnt ligands bind to Frizzled receptors and low-density receptor-related protein (LRP) co-receptors, β-catenin remains unphosphorylated at GSK3 phosphorylation sites, which prevents its destruction, then it translocates to the nucleus and activates the transcription of target genes through the interaction with ‘lymphocyte enhancer factor'/‘T-cell factor' nuclear transcription factors.93 β-Catenin-T-cell factor/lymphocyte enhancer factor complexes are involved in the transcription of cyclin D1 and c-myc target genes among others, which are important regulators of cell cycle and proliferation. In addition, β-catenin takes part in adherent junctions through binding to E-cadherin. Phosphorylation at specific C-terminal residues decreases β-catenin affinity for E-cadherin and β-catenin facilitates signaling transduction.93
Preliminary data of our laboratory shows Wnt/β-catenin pathway activation and β-catenin relocalization in murine models of prolactinomas (Demarchi et al. WNT/β-catenin pathway activation in murine models of prolactinomas. Third South American Symposium in Signal Transduction and Molecular Medicine. Argentina. 2015).
Interestingly, β-catenin signaling is a critical event in ErbB2-mediated mammary tumor94 and other tumor progression. Moreover, ErbB2 tyrosine kinase, modulated by HA–CD44 interaction, promotes β-catenin phosphorylation, which destabilizes E-cadherin-catenin complexes leading to a decrease of cell adhesion and transcriptional upregulation of T-cell factor/lymphocyte enhancer factor transcription, deregulating the cell cycle progression of ovarian tumor cells.91, 92
Furthermore, Wnt signaling regulates CD44 expression and thus its function, and also CD44 induces β-catenin instability reducing its nuclear accumulation. These reciprocal regulations between CD44 and Wnt/β-catenin result interesting in handling potential therapeutic targets, to inhibit metastasis, drug resistence and recurrent processes.95, 96
Several authors have investigated the involvement of Wnt/β-catenin signaling in vasculogenesis and angiogenesis. Franco et al.95 reviewed the emerging concepts of the role of Wnts, their receptors and signaling pathways in regulating differential behavior and/or cell function during vascular development.95 Indeed, Wnt pathway regulates the expression of several angiogenic factors like VEGF-A97 and IL-898 in different biological contexts, raising the possibility that it could promote both angiogenesis and sprouting of the pre-existing vessels.
It has been stated that treatment with WIF1, an inhibitor of the canonical Wnt signaling, as well as the knocking down of the Wnt pathway component BCL9 in different cancer models, decrease VEGF and CD44 protein and mRNA levels, suggesting a role of these factors regulated by Wnt in carcinogenesis.99, 100, 101 However, the relationship between the CD44-β-catenin crosstalk and angiogenesis is yet poorly understood.
Regard to the roles of the Wnt/β-catenin signaling in immune cells, several functions have been described for this pathway, as the modulation of CD8+ T-cell differentiation and memory formation and regulation of CD4+ T-cell polarization and survival.102 In the case of DCs, β-catenin in a Wnt-dependent and -independent manner modulates immunity and tolerance.103 Nevertheless, it is yet to be determined the mechanisms triggered by HA–CD44-β-catenin in immune cells.
Modulation of ECM-degrading enzymes
It has been reported that the interaction between HA and CD44 can induce, in tumor cells, the expression and activation of several ECM-degrading enzymes, such as MMP-2, MMP-7, MMP-9, EMMPRIN, cathepsin and MT1-MMP. This regulation has an important role in tumor cell behavior allowing matrix degradation, invasion and metastasis and also promoting angiogenesis during tumor progression.104, 105, 106, 107, 108 MMPs are important contributors to angiogenesis allowing new capillary formation and VEGF release from the ECM stores.109 The immune cells are able to produce different type of MMPs, in turn it is possible to suggest that HA may modulate MMPs in these cells. Once MMP-9 is stimulated, it activates the latent form of transforming growth factor-β modulating ECs behavior and inflammatory responses.110
Modulation of intracellular signals by TLR4
Toll-like receptors (TLRs) are transmembrane proteins that recognize not only highly conserved molecules present on microbes, known as ‘pathogen associated molecules patterns', but also responds to self-molecules produced after cell damage or death, identified as ‘damage-associated molecular patterns'. damage-associated molecular patterns include a variety of different molecules present in the host. For example, LMW HA functions as a pro-inflammatory damage-associated molecular pattern as it can modulate DC, stimulate the release of pro-inflammatory cytokines and promote proliferation. Several of these pro-inflammatory effects of LMW HA are due to the interaction with TLR4.
It has been reported that the interaction of LMW HA with TLR4 induce specific intracellular signaling pathways in immune cells and ECs.54 TLR4 activation by LMW HA has a critical role during DC maturation, inducing the signal transduction pathway involving p38/p42-44 MAPKs and NF-kB.54 Besides, macrophages and tumor cells treated with LMW HA resulted in the activation of NF-kB, a major component of TLR4 signaling pathway, and lead to the transcription of MMPs (MMP-2) and cytokines (IL-8, IL-12 and tumor necrosis factor).54, 111, 112, 113 This promotes motility and invasiveness in a TLR4 dependent manner.113 Even more, it was reported that LMW-HA fragments were found to interact with TLR4 in human dermal microvascular ECs inducing the release of IL-8.114 In parallel, the activation of NF-kB was found to stimulate angiogenesis by inducing expression of IL-8 and VEGF.115
Despite there is not enough evidence that HA-TLR4 has a role in modulating angiogenesis, this mechanism cannot be ruled out. Our preliminary results show that HA acts as a modulator of VFGF expression in spleen cells (Spinelli et al. Hyaluronan as a modulator of VEGF expression in immune cells. 10th International Conference on Hyaluronan, Italy, 2015). In parallel, we are investigating the role of HA and its receptors, CD44 and TLR4, in the regulation of tumor angiogenesis by immune cells.
Modulation of intracellular signals by RHAMM
The receptor for HA-mediated motility (RHAMM, CD168) is a cytoplasmatic protein that is unconventionally exported to the cell surface.116 Intracellular RHAMM is associated with the centrosome and mitotic spindle.117 Extracellular RHAMM is a HA-binding protein that links and activates CD44 resulting in stimulation of the Ras/Erk1,2 pathway,118 a cascade that functions in cellular proliferation and is often deregulated in cancer.
It was shown that in normal lymphocytes, RHAMM is mostly intracellular and surface RHAMM is almost always absent.119 On the other hand, RHAMM is expressed both as an intracellular and as a cell-surface protein in B lineage cancers, with important functional roles119: motility and malignant spread,120 and also modulation of apoptotic and cell cycle progression pathways.116
There are several studies that show that RHAMM is expressed in EC and it participates in modulating angiogenesis in EC. For example, it was shown that the RHAMM receptor is overexpressed in neovessels and it might be responsible for enhancing EC activation via HA oligosaccharides.121 RHAMM is necessary for EC migration122 and downregulation of RHAMM exerts a negative effect on HA-induced EC tube formation.123 Even more, it was shown that RHAMM stimulated by HA activates Rac1,124 a protein involved in the hypoxia-induced production of VEGF and VEGFR-2.125, 126
In an inflammatory context, RHAMM–HA interaction was found to regulate macrophage chemotaxis and motility in lung injury.126
Angiogenesis, immune system and HA as therapeutic targets in cancer
Inhibition of angiogenesis is an important strategy for cancer treatment. Extensive research has positioned VEGF, FGF, PDGFB/PDGFR-β and angiopoietins as therapeutic targets. As VEGF has a central role in promoting an angiogenic phenotype in most tumors, development of VEGF antagonists has become one of the central focuses for anti-angiogenic therapies. The first evidence that anti-VEGF might be useful in cancer therapy came from a mouse tumor model using a neutralizing antibody, that could prevent tumor growth in neuroblastoma in mice.127 After that, antagonists of VEGF and its receptors have been developed and evaluated for their ability to suppress tumor growth. The strategies involved are (i) anti-hypoxia-inducible factor reagents, (ii) VEGF antisense oligonucleotides, (iii) VEGF ribozymes, (iv) soluble VEGF receptors, (v) expression of a dominant negative mutant of VEGF receptors in target ECs, (vi) anti-VEGF receptor antibodies, (vii) VEGF receptor-mediated downstream signaling pathways and (viii) VEGF DNA vaccines.128, 129, 130 Several anti-angiogenic agents have been approved for clinical use: bevacizumab (monoclonal antibody that targets VEGF), sunitinib, sorafenib and axitinib (small molecules tyrosine kinase inhibitors including VEGF-R and FGF-R). However, these drugs have not demonstrated the expected effectiveness in cancer treatment. Expression patterns of pro-angiogenic factors can be altered along tumor progression and single angiogenic factor antagonists may encounter drug-resistance problems or activation of compensatory pathways.
Recent studies suggest that anti-angiogenic therapies are efficient to reverse some levels of immunosuppression in patients with cancer; therefore the link between anti-angiogenic strategies and immune system might lead to the appearance of new therapies, for example metastatic renal cancer patients treated with bevacizumab and interferon-α presented a superior progression-free survival than those treated with interferon-α monotherapy.131
Furthermore, different anti-cancer therapies that are being consider are those that target other aspects related to HA. HA production, for example, through chemical inhibitors of HA synthesis like 4-Methylumbelliferone, an orally dietary supplement. It would be interesting and valuable to evaluate HA-related strategies in the context of anti-angiogenic therapy and immunotherapy.
Conclusion and future directions
In summary, immune cells could modulate angiogenesis in different tumor contexts, having a crucial participation in neovascularization, beyond the known mechanism that involves EC. Thus, the factors and mechanisms that allow immune cells to acquire pro-angiogenic functions will be an important tool to improve and develop anti-angiogenic therapies in cancer. It is possible to hypothesize that abnormal ECM, is in part responsible for this unusual response of the immune system. We also considered that HA, as a component of ECM, is able to modulate angiogenesis and immune responses, working as a linker in tumor microenvironment.
Acknowledgments
This work was supported by CONICET, UNNOBA, Fundación Florencio Fiorini and Agencia Nacional de Promoción Científica y Tecnológica, Ministerio de Ciencia y Tecnología, Argentina.
The authors declare no conflict of interest.
References
- 1Chung AS, Ferrara N. Developmental and pathological angiogenesis. Annu Rev Cell Dev Biol 2011; 27: 563–584. [DOI] [PubMed] [Google Scholar]
- 2Ferrara N. Vascular endothelial growth factor as a target for anticancer therapy. Oncologist 2004; 9 (Suppl 1): 2–10. [DOI] [PubMed] [Google Scholar]
- 3Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol 2001; 2: REVIEWS3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Cristina C, Luque GM, Demarchi G, Lopez Vicchi F, Zubeldia-Brenner L, Perez Millan MI et al. Angiogenesis in pituitary adenomas: human studies and new mutant mouse models. Int J Endocrinol 2014; 2014: 608497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5McDonald DM, Choyke PL. Imaging of angiogenesis: from microscope to clinic. Nat Med 2003; 9: 713–725. [DOI] [PubMed] [Google Scholar]
- 6Hagendoorn J, Tong R, Fukumura D, Lin Q, Lobo J, Padera TP et al. Onset of abnormal blood and lymphatic vessel function and interstitial hypertension in early stages of carcinogenesis. Cancer Res 2006; 66: 3360–3364. [DOI] [PubMed] [Google Scholar]
- 7Hida K, Hida Y, Amin DN, Flint AF, Panigrahy D, Morton CC et al. Tumour-associated endothelial cells with cytogenetic abnormalities. Cancer Res 2004; 64: 8249–8255. [DOI] [PubMed] [Google Scholar]
- 8Grover AC, Tangrea MA, Woodson KG, Wallis BS, Hanson JC, Chuaqui RF et al. Tumour-associated endothelial cells display GSTP1 and RARbeta2 promoter methylation in human prostate cancer. J Transl Med 2006; 4: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E et al. Genes expressed in human tumour endothelium. Science 2000; 289: 1197–1202. [DOI] [PubMed] [Google Scholar]
- 10Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev 2011; 91: 1071–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell 2011; 146: 873–887. [DOI] [PubMed] [Google Scholar]
- 12Bissell MJ, Hines WC. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med 2011; 17: 320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Fan F, Schimming A, Jaeger D, Podar K. Targeting the tumour microenvironment: focus on angiogenesis. J Oncol 2012; 2012: 281261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res 2005; 97: 1093–1107. [DOI] [PubMed] [Google Scholar]
- 15Huijbers IJ, Iravani M, Popov S, Robertson D, Al-Sarraj S, Jones C et al. A role for fibrillar collagen deposition and the collagen internalization receptor endo180 in glioma invasion. PLoS ONE 2010; 5: e9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Maeshima Y, Sudhakar A, Lively JC, Ueki K, Kharbanda S, Kahn CR et al. Tumstatin, an endothelial cell-specific inhibitor of protein synthesis. Science 2002; 295: 140–143. [DOI] [PubMed] [Google Scholar]
- 17O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS et al. Endostatin: an endogenous inhibitor of angiogenesis and tumour growth. Cell 1997; 88: 277–285. [DOI] [PubMed] [Google Scholar]
- 18Giannelli G, Antonaci S. Biological and clinical relevance of laminin-5 in cancer. Clin Exp Metastasis 2000; 18: 439–443. [DOI] [PubMed] [Google Scholar]
- 19George EL, Baldwin HS, Hynes RO. Fibronectins are essential for heart and blood vessel morphogenesis but are dispensable for initial specification of precursor cells. Blood 1997; 90: 3073–3081. [PubMed] [Google Scholar]
- 20Wijelath ES, Murray J, Rahman S, Patel Y, Ishida A, Strand K et al. Novel vascular endothelial growth factor binding domains of fibronectin enhance vascular endothelial growth factor biological activity. Circ Res 2002; 91: 25–31. [DOI] [PubMed] [Google Scholar]
- 21Yip GW, Smollich M, Gotte M. Therapeutic value of glycosaminoglycans in cancer. Mol Cancer Ther 2006; 5: 2139–2148. [DOI] [PubMed] [Google Scholar]
- 22Stern R. Hyaluronan in cancer biology. Semin Cancer Biol 2008; 18: 237. [DOI] [PubMed] [Google Scholar]
- 23Ye XZ, Yu SC, Bian XW. Contribution of myeloid-derived suppressor cells to tumour-induced immune suppression, angiogenesis, invasion and metastasis. J Genet Genomics 2010; 37: 423–430. [DOI] [PubMed] [Google Scholar]
- 24Rizzo M, Bayo J, Piccioni F, Malvicini M, Fiore E, Peixoto E et al. Low molecular weight hyaluronan-pulsed human dendritic cells showed increased migration capacity and induced resistance to tumour chemoattraction. PLoS ONE 2014; 9: e107944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer 2012; 12: 265–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26Coca S, Perez-Piqueras J, Martinez D, Colmenarejo A, Saez MA, Vallejo C et al. The prognostic significance of intratumoural natural killer cells in patients with colorectal carcinoma. Cancer 1997; 79: 2320–2328. [DOI] [PubMed] [Google Scholar]
- 27Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Che X, Iwashige H et al. Prognostic value of intratumoural natural killer cells in gastric carcinoma. Cancer 2000; 88: 577–583. [PubMed] [Google Scholar]
- 28Villegas FR, Coca S, Villarrubia VG, Jimenez R, Chillon MJ, Jareno J et al. Prognostic significance of tumour infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer 2002; 35: 23–28. [DOI] [PubMed] [Google Scholar]
- 29Nelson BH. CD20+ B cells: the other tumour-infiltrating lymphocytes. J Immunol 2010; 185: 4977–4982. [DOI] [PubMed] [Google Scholar]
- 30Milne K, Kobel M, Kalloger SE, Barnes RO, Gao D, Gilks CB et al. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS ONE 2009; 4: e6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund LT. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res 2008; 14: 5220–5227. [DOI] [PubMed] [Google Scholar]
- 32Feldmeyer L, Gaide O, Speiser DE. Clinical Implications of CD8+ T-cell infiltration in frequent and rare cancers. J Invest Dermatol 2013; 133: 1929–1932. [DOI] [PubMed] [Google Scholar]
- 33Tammi RH, Kultti A, Kosma VM, Pirinen R, Auvinen P, Tammi MI. Hyaluronan in human tumours: pathobiological and prognostic messages from cell-associated and stromal hyaluronan. Semin Cancer Biol 2008; 18: 288–295. [DOI] [PubMed] [Google Scholar]
- 34Koyama H, Hibi T, Isogai Z, Yoneda M, Fujimori M, Amano J et al. Hyperproduction of hyaluronan in neu-induced mammary tumour accelerates angiogenesis through stromal cell recruitment: possible involvement of versican/PG-M. Am J Pathol 2007; 170: 1086–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer 2004; 4: 528–539. [DOI] [PubMed] [Google Scholar]
- 36Ghatak S, Misra S, Toole BP. Hyaluronan oligosaccharides inhibit anchorage-independent growth of tumour cells by suppressing the phosphoinositide 3-kinase/Akt cell survival pathway. J Biol Chem 2002; 277: 38013–38020. [DOI] [PubMed] [Google Scholar]
- 37Zeng C, Toole BP, Kinney SD, Kuo JW, Stamenkovic I. Inhibition of tumour growth in vivo by hyaluronan oligomers. Int J Cancer 1998; 77: 396–401. [DOI] [PubMed] [Google Scholar]
- 38Mueller BM, Schraufstatter IU, Goncharova V, Povaliy T, DiScipio R, Khaldoyanidi SK. Hyaluronan inhibits postchemotherapy tumour regrowth in a colon carcinoma xenograft model. Mol Cancer Ther 2010; 9: 3024–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39Berdiaki A, Nikitovic D, Tsatsakis A, Katonis P, Karamanos NK, Tzanakakis GN. bFGF induces changes in hyaluronan synthase and hyaluronidase isoform expression and modulates the migration capacity of fibrosarcoma cells. Biochim Biophys Acta 2009; 1790: 1258–1265. [DOI] [PubMed] [Google Scholar]
- 40Tian X, Azpurua J, Hine C, Vaidya A, Myakishev-Rempel M, Ablaeva J et al. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature 2013; 499: 346–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41Lee-Sayer SS, Dong Y, Arif AA, Olsson M, Brown KL, Johnson P. The where, when, how, and why of hyaluronan binding by immune cells. Front Immunol 2015; 6: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42Sokolowska M, Chen LY, Eberlein M, Martinez-Anton A, Liu Y, Alsaaty S et al. Low molecular weight hyaluronan activates cytosolic phospholipase A2alpha and eicosanoid production in monocytes and macrophages. J Biol Chem 2014; 289: 4470–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43Kobayashi N, Miyoshi S, Mikami T, Koyama H, Kitazawa M, Takeoka M et al. Hyaluronan deficiency in tumour stroma impairs macrophage trafficking and tumour neovascularization. Cancer Res 2010; 70: 7073–7083. [DOI] [PubMed] [Google Scholar]
- 44Burke B, Tang N, Corke KP, Tazzyman D, Ameri K, Wells M et al. Expression of HIF-1alpha by human macrophages: implications for the use of macrophages in hypoxia-regulated cancer gene therapy. J Pathol 2002; 196: 204–212. [DOI] [PubMed] [Google Scholar]
- 45Murdoch C, Lewis CE. Macrophage migration and gene expression in response to tumour hypoxia. Int J Cancer 2005; 117: 701–708. [DOI] [PubMed] [Google Scholar]
- 46Dirkx AE, Oude Egbrink MG, Wagstaff J, Griffioen AW. Monocyte/macrophage infiltration in tumours: modulators of angiogenesis. J Leukoc Biol 2006; 80: 1183–1196. [DOI] [PubMed] [Google Scholar]
- 47Stockmann C, Schadendorf D, Klose R, Helfrich I. The impact of the immune system on tumour: angiogenesis and vascular remodeling. Front Oncol 2014; 4: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48Shen Z, Kauttu T, Seppanen H, Vainionpaa S, Ye Y, Wang S et al. Vasohibin-1 and vasohibin-2 expression in gastric cancer cells and TAMs. Med Oncol 2012; 29: 2718–2726. [DOI] [PubMed] [Google Scholar]
- 49Klimp AH, Hollema H, Kempinga C, van der Zee AG. de Vries EG, Daemen T. Expression of cyclooxygenase-2 and inducible nitric oxide synthase in human ovarian tumours and tumour-associated macrophages. Cancer Res 2001; 61: 7305–7309. [PubMed] [Google Scholar]
- 50Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest 2004; 114: 623–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51Hochst B, Schildberg FA, Sauerborn P, Gabel YA, Gevensleben H, Goltz D et al. Activated human hepatic stellate cells induce myeloid derived suppressor cells from peripheral blood monocytes in a CD44-dependent fashion. J Hepatol 2013; 59: 528–535. [DOI] [PubMed] [Google Scholar]
- 52Alaniz L, Rizzo M, Garcia MG, Piccioni F, Aquino JB, Malvicini M et al. Low molecular weight hyaluronan preconditioning of tumour-pulsed dendritic cells increases their migratory ability and induces immunity against murine colorectal carcinoma. Cancer Immunol Immunother 2011; 60: 1383–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53Alaniz L, Rizzo M, Malvicini M, Jaunarena J, Avella D, Atorrasagasti C et al. Low molecular weight hyaluronan inhibits colorectal carcinoma growth by decreasing tumour cell proliferation and stimulating immune response. Cancer Lett 2009; 278: 9–16. [DOI] [PubMed] [Google Scholar]
- 54Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T et al. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med 2002; 195: 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55Termeer CC, Hennies J, Voith U, Ahrens T, Weiss JM, Prehm P et al. Oligosaccharides of hyaluronan are potent activators of dendritic cells. J Immunol 2000; 165: 1863–1870. [DOI] [PubMed] [Google Scholar]
- 56Ricciardi A, Elia AR, Cappello P, Puppo M, Vanni C, Fardin P et al. Transcriptome of hypoxic immature dendritic cells: modulation of chemokine/receptor expression. Mol Cancer Res 2008; 6: 175–185. [DOI] [PubMed] [Google Scholar]
- 57Feijoo E, Alfaro C, Mazzolini G, Serra P, Penuelas I, Arina A et al. Dendritic cells delivered inside human carcinomas are sequestered by interleukin-8. Int J Cancer 2005; 116: 275–281. [DOI] [PubMed] [Google Scholar]
- 58Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 1998; 92: 4150–4166. [PubMed] [Google Scholar]
- 59Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004; 10: 942–949. [DOI] [PubMed] [Google Scholar]
- 60Conejo-Garcia JR, Benencia F, Courreges MC, Kang E, Mohamed-Hadley A, Buckanovich RJ et al. Tumour-infiltrating dendritic cell precursors recruited by a beta-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat Med 2004; 10: 950–958. [DOI] [PubMed] [Google Scholar]
- 61Gottfried E, Kreutz M, Haffner S, Holler E, Iacobelli M, Andreesen R et al. Differentiation of human tumour-associated dendritic cells into endothelial-like cells: an alternative pathway of tumour angiogenesis. Scand J Immunol 2007; 65: 329–335. [DOI] [PubMed] [Google Scholar]
- 62Okunishi K, Dohi M, Nakagome K, Tanaka R, Mizuno S, Matsumoto K et al. A novel role of hepatocyte growth factor as an immune regulator through suppressing dendritic cell function. J Immunol 2005; 175: 4745–4753. [DOI] [PubMed] [Google Scholar]
- 63Alard P, Clark SL, Kosiewicz MM. Mechanisms of tolerance induced by TGF beta-treated APC: CD4 regulatory T cells prevent the induction of the immune response possibly through a mechanism involving TGF beta. Eur J Immunol 2004; 34: 1021–1030. [DOI] [PubMed] [Google Scholar]
- 64Pockaj BA, Basu GD, Pathangey LB, Gray RJ, Hernandez JL, Gendler SJ et al. Reduced T-cell and dendritic cell function is related to cyclooxygenase-2 overexpression and prostaglandin E2 secretion in patients with breast cancer. Ann Surg Oncol 2004; 11: 328–339. [DOI] [PubMed] [Google Scholar]
- 65Puig-Kroger A, Pello OM, Selgas R, Criado G, Bajo MA, Sanchez-Tomero JA et al. Peritoneal dialysis solutions inhibit the differentiation and maturation of human monocyte-derived dendritic cells: effect of lactate and glucose-degradation products. J Leukoc Biol 2003; 73: 482–492. [DOI] [PubMed] [Google Scholar]
- 66Konno S, Eckman JA, Plunkett B, Li X, Berman JS, Schroeder J et al. Interleukin-10 and Th2 cytokines differentially regulate osteopontin expression in human monocytes and dendritic cells. J Interferon Cytokine Res 2006; 26: 562–567. [DOI] [PubMed] [Google Scholar]
- 67Sague SL, Tato C, Pure E, Hunter CA. The regulation and activation of CD44 by natural killer (NK) cells and its role in the production of IFN-gamma. J Interferon Cytokine Res 2004; 24: 301–309. [DOI] [PubMed] [Google Scholar]
- 68Zhang J, Chen Z, Smith GN, Croy BA. Natural killer cell-triggered vascular transformation: maternal care before birth? Cell Mol Immunol 2011; 8: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69Bruno A, Focaccetti C, Pagani A, Imperatori AS, Spagnoletti M, Rotolo N et al. The proangiogenic phenotype of natural killer cells in patients with non-small cell lung cancer. Neoplasia 2013; 15: 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70Rafi A, Nagarkatti M, Nagarkatti PS. Hyaluronate-CD44 interactions can induce murine B-cell activation. Blood 1997; 89: 2901–2908. [PubMed] [Google Scholar]
- 71Yang C, Lee H, Pal S, Jove V, Deng J, Zhang W et al. B cells promote tumour progression via STAT3 regulated-angiogenesis. PLoS ONE 2013; 8: e64159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72Bohrer LR, Chuntova P, Bade LK, Beadnell TC, Leon RP, Brady NJ et al. Activation of the FGFR-STAT3 pathway in breast cancer cells induces a hyaluronan-rich microenvironment that licenses tumour formation. Cancer Res 2014; 74: 374–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature 2011; 475: 226–230. [DOI] [PubMed] [Google Scholar]
- 74Pober JS, Tellides G. Participation of blood vessel cells in human adaptive immune responses. Trends Immunol 2012; 33: 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75Choi J, Enis DR, Koh KP, Shiao SL, Pober JS. T lymphocyte–endothelial cell interactions. Annu Rev Immunol 2004; 22: 683–709. [DOI] [PubMed] [Google Scholar]
- 76Blotnick S, Peoples GE, Freeman MR, Eberlein TJ, Klagsbrun M. T lymphocytes synthesize and export heparin-binding epidermal growth factor-like growth factor and basic fibroblast growth factor, mitogens for vascular cells and fibroblasts: differential production and release by CD4+ and CD8+ T cells. Proc Natl Acad Sci USA 1994; 91: 2890–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77Fathallah-Shaykh HM, Zhao LJ, Kafrouni AI, Smith GM, Forman J. Gene transfer of IFN-gamma into established brain tumours represses growth by antiangiogenesis. J Immunol 2000; 164: 217–222. [DOI] [PubMed] [Google Scholar]
- 78Le QT, Chen E, Salim A, Cao H, Kong CS, Whyte R et al. An evaluation of tumour oxygenation and gene expression in patients with early stage non-small cell lung cancers. Clin Cancer Res 2006; 12: 1507–1514. [DOI] [PubMed] [Google Scholar]
- 79Cho EY, Choi Y, Chae SW, Sohn JH, Ahn GH. Immunohistochemical study of the expression of adhesion molecules in ovarian serous neoplasms. Pathol Int 2006; 56: 62–70. [DOI] [PubMed] [Google Scholar]
- 80Watanabe O, Kinoshita J, Shimizu T, Imamura H, Hirano A, Okabe T et al. Expression of a CD44 variant and VEGF-C and the implications for lymphatic metastasis and long-term prognosis of human breast cancer. J Exp Clin Cancer Res 2005; 24: 75–82. [PubMed] [Google Scholar]
- 81Zavrides HN, Zizi-Sermpetzoglou A, Panousopoulos D, Athanasas G, Elemenoglou I, Peros G. Prognostic evaluation of CD44 expression in correlation with bcl-2 and p53 in colorectal cancer. Folia Histochem Cytobiol 2005; 43: 31–36. [PubMed] [Google Scholar]
- 82Lai CH, Shan YS, Sy ED, Hsieh YH, Tsai HW, Lee JC et al. The significance of CD44 expression in gastrointestinal neuroendocrine tumours. Hepatogastroenterology 2005; 52: 1071–1076. [PubMed] [Google Scholar]
- 83Bourguignon LY. Hyaluronan-mediated CD44 activation of RhoGTPase signaling and cytoskeleton function promotes tumour progression. Semin Cancer Biol 2008; 18: 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84Singleton PA, Dudek SM, Ma SF, Garcia JG. Transactivation of sphingosine 1-phosphate receptors is essential for vascular barrier regulation. Novel role for hyaluronan and CD44 receptor family. J Biol Chem 2006; 281: 34381–34393. [DOI] [PubMed] [Google Scholar]
- 85Bokoch GM. Regulation of innate immunity by Rho GTPases. Trends Cell Biol 2005; 15: 163–171. [DOI] [PubMed] [Google Scholar]
- 86Ghatak S, Misra S, Toole BP. Hyaluronan constitutively regulates ErbB2 phosphorylation and signaling complex formation in carcinoma cells. J Biol Chem 2005; 280: 8875–8883. [DOI] [PubMed] [Google Scholar]
- 87Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res 2005; 94: 29–86. [DOI] [PubMed] [Google Scholar]
- 88Alaniz L, Garcia MG, Gallo-Rodriguez C, Agusti R, Sterin-Speziale N, Hajos SE et al. Hyaluronan oligosaccharides induce cell death through PI3-K/Akt pathway independently of NF-kappaB transcription factor. Glycobiology 2006; 16: 359–367. [DOI] [PubMed] [Google Scholar]
- 89Karar J, Maity A. PI3K/AKT/mTOR Pathway in Angiogenesis. Front Mol Neurosci 2011; 4: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90Okkenhaug K. Signaling by the phosphoinositide 3-kinase family in immune cells. Annu Rev Immunol 2013; 31: 675–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91Bourguignon LY, Gilad E, Peyrollier K. Heregulin-mediated ErbB2-ERK signaling activates hyaluronan synthases leading to CD44-dependent ovarian tumour cell growth and migration. J Biol Chem 2007; 282: 19426–19441. [DOI] [PubMed] [Google Scholar]
- 92Bourguignon LY, Peyrollier K, Gilad E, Brightman A. Hyaluronan-CD44 interaction with neural Wiskott-Aldrich syndrome protein (N-WASP) promotes actin polymerization and ErbB2 activation leading to beta-catenin nuclear translocation, transcriptional up-regulation, and cell migration in ovarian tumour cells. J Biol Chem 2007; 282: 1265–1280. [DOI] [PubMed] [Google Scholar]
- 93Daugherty RL, Gottardi CJ. Phospho-regulation of Beta-catenin adhesion and signaling functions. Physiology (Bethesda) 2007; 22: 303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94Schade B, Lesurf R, Sanguin-Gendreau V, Bui T, Deblois G, O'Toole SA et al. beta-Catenin signaling is a critical event in ErbB2-mediated mammary tumour progression. Cancer Res 2013; 73: 4474–4487. [DOI] [PubMed] [Google Scholar]
- 95Franco CA, Liebner S, Gerhardt H. Vascular morphogenesis: a Wnt for every vessel? Curr Opin Genet Dev 2009; 19: 476–483. [DOI] [PubMed] [Google Scholar]
- 96Chang G, Zhang H, Wang J, Zhang Y, Xu H, Wang C et al. CD44 targets Wnt/beta-catenin pathway to mediate the proliferation of K562 cells. Cancer Cell Int 2013; 13: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97Zhang X, Gaspard JP, Chung DC. Regulation of vascular endothelial growth factor by the Wnt and K-ras pathways in colonic neoplasia. Cancer Res 2001; 61: 6050–6054. [PubMed] [Google Scholar]
- 98Masckauchan TN, Shawber CJ, Funahashi Y, Li CM, Kitajewski J. Wnt/beta-catenin signaling induces proliferation, survival and interleukin-8 in human endothelial cells. Angiogenesis 2005; 8: 43–51. [DOI] [PubMed] [Google Scholar]
- 99Jones M, Tussey L, Athanasou N, Jackson DG. Heparan sulfate proteoglycan isoforms of the CD44 hyaluronan receptor induced in human inflammatory macrophages can function as paracrine regulators of fibroblast growth factor action. J Biol Chem 2000; 275: 7964–7974. [DOI] [PubMed] [Google Scholar]
- 100Ramachandran I, Thavathiru E, Ramalingam S, Natarajan G, Mills WK, Benbrook DM et al. Wnt inhibitory factor 1 induces apoptosis and inhibits cervical cancer growth, invasion and angiogenesis in vivo. Oncogene 2012; 31: 2725–2737. [DOI] [PubMed] [Google Scholar]
- 101Mani M, Carrasco DE, Zhang Y, Takada K, Gatt ME, Dutta-Simmons J et al. BCL9 promotes tumour progression by conferring enhanced proliferative, metastatic, and angiogenic properties to cancer cells. Cancer Res 2009; 69: 7577–7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102Gattinoni L, Ji Y, Restifo NP. Wnt/beta-catenin signaling in T-cell immunity and cancer immunotherapy. Clin Cancer Res 2010; 16: 4695–4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103Swafford D, Manicassamy S. Wnt signaling in dendritic cells: its role in regulation of immunity and tolerance. Discov Med 2015; 19: 303–310. [PMC free article] [PubMed] [Google Scholar]
- 104Abecassis I, Olofsson B, Schmid M, Zalcman G, Karniguian A. RhoA induces MMP-9 expression at CD44 lamellipodial focal complexes and promotes HMEC-1 cell invasion. Exp Cell Res 2003; 291: 363–376. [DOI] [PubMed] [Google Scholar]
- 105Bourguignon LY, Singleton PA, Diedrich F, Stern R, Gilad E. CD44 interaction with Na+-H+ exchanger (NHE1) creates acidic microenvironments leading to hyaluronidase-2 and cathepsin B activation and breast tumour cell invasion. J Biol Chem 2004; 279: 26991–27007. [DOI] [PubMed] [Google Scholar]
- 106Lokeshwar VB, Iida N, Bourguignon LY. The cell adhesion molecule, GP116, is a new CD44 variant (ex14/v10) involved in hyaluronic acid binding and endothelial cell proliferation. J Biol Chem 1996; 271: 23853–23864. [DOI] [PubMed] [Google Scholar]
- 107Marrero-Diaz R, Bravo-Cordero JJ, Megias D, Garcia MA, Bartolome RA, Teixido J et al. Polarized MT1-MMP-CD44 interaction and CD44 cleavage during cell retraction reveal an essential role for MT1-MMP in CD44-mediated invasion. Cell Motil Cytoskeleton 2009; 66: 48–61. [DOI] [PubMed] [Google Scholar]
- 108Lokeshwar VB, Mirza S, Jordan A. Targeting hyaluronic acid family for cancer chemoprevention and therapy. Adv Cancer Res 2014; 123: 35–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumours. J Cell Biol 2005; 169: 681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumour invasion and angiogenesis. Genes Dev 2000; 14: 163–176. [PMC free article] [PubMed] [Google Scholar]
- 111Fieber C, Baumann P, Vallon R, Termeer C, Simon JC, Hofmann M et al. Hyaluronan-oligosaccharide-induced transcription of metalloproteases. J Cell Sci 2004; 117: 359–367. [DOI] [PubMed] [Google Scholar]
- 112Noble PW, McKee CM, Cowman M, Shin HS. Hyaluronan fragments activate an NF-kappa B/I-kappa B alpha autoregulatory loop in murine macrophages. J Exp Med 1996; 183: 2373–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113Voelcker V, Gebhardt C, Averbeck M, Saalbach A, Wolf V, Weih F et al. Hyaluronan fragments induce cytokine and metalloprotease upregulation in human melanoma cells in part by signalling via TLR4. Exp Dermatol 2008; 17: 100–107. [DOI] [PubMed] [Google Scholar]
- 114Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem 2004; 279: 17079–17084. [DOI] [PubMed] [Google Scholar]
- 115Huang S, Robinson JB, Deguzman A, Bucana CD, Fidler IJ. Blockade of nuclear factor-kappaB signaling inhibits angiogenesis and tumourigenicity of human ovarian cancer cells by suppressing expression of vascular endothelial growth factor and interleukin 8. Cancer Res 2000; 60: 5334–5339. [PubMed] [Google Scholar]
- 116Maxwell CA, McCarthy J, Turley E. Cell-surface and mitotic-spindle RHAMM: moonlighting or dual oncogenic functions? J Cell Sci 2008; 121: 925–932. [DOI] [PubMed] [Google Scholar]
- 117Eck M, Schmausser B, Scheller K, Brandlein S, Muller-Hermelink HK. Pleiotropic effects of CXC chemokines in gastric carcinoma: differences in CXCL8 and CXCL1 expression between diffuse and intestinal types of gastric carcinoma. Clin Exp Immunol 2003; 134: 508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118Hamilton SR, Fard SF, Paiwand FF, Tolg C, Veiseh M, Wang C et al. The hyaluronan receptors CD44 and Rhamm (CD168) form complexes with ERK1,2 that sustain high basal motility in breast cancer cells. J Biol Chem 2007; 282: 16667–16680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119Adamia S, Kriangkum J, Belch AR, Pilarski LM. Aberrant posttranscriptional processing of hyaluronan synthase 1 in malignant transformation and tumour progression. Adv Cancer Res 2014; 123: 67–94. [DOI] [PubMed] [Google Scholar]
- 120Masellis-Smith A, Belch AR, Mant MJ, Turley EA, Pilarski LM. Hyaluronan-dependent motility of B cells and leukemic plasma cells in blood, but not of bone marrow plasma cells, in multiple myeloma: alternate use of receptor for hyaluronan-mediated motility (RHAMM) and CD44. Blood 1996; 87: 1891–1899. [PubMed] [Google Scholar]
- 121Slevin M, Krupinski J, Gaffney J, Matou S, West D, Delisser H et al. Hyaluronan-mediated angiogenesis in vascular disease: uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol 2007; 26: 58–68. [DOI] [PubMed] [Google Scholar]
- 122Savani RC, Cao G, Pooler PM, Zaman A, Zhou Z, DeLisser HM. Differential involvement of the hyaluronan (HA) receptors CD44 and receptor for HA-mediated motility in endothelial cell function and angiogenesis. J Biol Chem 2001; 276: 36770–36778. [DOI] [PubMed] [Google Scholar]
- 123Matou-Nasri S, Gaffney J, Kumar S, Slevin M. Oligosaccharides of hyaluronan induce angiogenesis through distinct CD44 and RHAMM-mediated signalling pathways involving Cdc2 and gamma-adducin. Int J Oncol 2009; 35: 761–773. [DOI] [PubMed] [Google Scholar]
- 124Hirota K, Semenza GL. Rac1 activity is required for the activation of hypoxia-inducible factor 1. J Biol Chem 2001; 276: 21166–21172. [DOI] [PubMed] [Google Scholar]
- 125Park D, Kim Y, Kim H, Kim K, Lee YS, Choe J et al. Hyaluronic acid promotes angiogenesis by inducing RHAMM-TGFbeta receptor interaction via CD44-PKCdelta. Mol Cells 2012; 33: 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126Zaman A, Cui Z, Foley JP, Zhao H, Grimm PC, Delisser HM et al. Expression and role of the hyaluronan receptor RHAMM in inflammation after bleomycin injury. Am J Respir Cell Mol Biol 2005; 33: 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127Kim WY, Lee HY. Brain angiogenesis in developmental and pathological processes: mechanism and therapeutic intervention in brain tumours. FEBS J 2009; 276: 4653–4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128Garber K. Angiogenesis inhibitors suffer new setback. Nat Biotechnol 2002; 20: 1067–1068. [DOI] [PubMed] [Google Scholar]
- 129Niethammer AG, Xiang R, Becker JC, Wodrich H, Pertl U, Karsten G et al. A DNA vaccine against VEGF receptor 2 prevents effective angiogenesis and inhibits tumour growth. Nat Med 2002; 8: 1369–1375. [DOI] [PubMed] [Google Scholar]
- 130Makino Y, Cao R, Svensson K, Bertilsson G, Asman M, Tanaka H et al. Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature 2001; 414: 550–554. [DOI] [PubMed] [Google Scholar]
- 131Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Ou SS et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol 2008; 26: 5422–5428. [DOI] [PMC free article] [PubMed] [Google Scholar]