Abstract
The objective of this study was to evaluate whether long-term shift work is associated with both the total hormonal secretion after awakening and the pattern of the cortisol levels during the first hour following awakening, among 65 randomly selected police officers who represent a high stress occupation. Dominant shift (Day, Afternoon, or Midnight) was ascertained using daily payroll records of each participant’s work activities during the 6–8 yrs prior to saliva sampling. Four salivary samples were collected at 15 min intervals upon first awakening. After accounting for potential confounders, salivary cortisol concentrations averaged across all four time points and total area under the curve differed significantly across shift with midnight shift workers showing suppressed awakening cortisol response relative to the afternoon and day shift. The percent of hours worked on midnight shift was inversely correlated with total awakening cortisol output. In contrast, the pattern of cortisol secretion during the first hour following waking appeared not to be affected as no significant interaction effect was found between time since awakening and shift work. The results show that long-term midnight shift work is associated with decreased absolute mean level and total volume of cortisol released over the waking period.
Keywords: Shift work, Waking, Cortisol, Police, Saliva, HPA-axis
Introduction
Research indicates that shift work may lead to disruption of circadian rhythms1–8) and adverse health outcomes including metabolic disorders and cardiovascular disease (CVD)9–11). In fact, long-term shift work, particularly evening or rotating shift work, has been linked to chronic stress with possible adverse consequences on the neuro-endocrine- immune systems12). Policing by its nature is a stressful occupation where officers are exposed to a high level of traumatic and non-traumatic stressors13) and shift work, a major stressor in police work as it is in many occupations, is an integral part of law enforcement. Consequently police officers are shown to have higher rates of CVD and CVD risk factors compared to the general population14, 15). They represent an important group in which to examine the relationship between long-term shift work and cortisol levels yet studies in this high risk and understudied occupational group are limited.
Following acute stressors, the activation of the hypothalamic- pituitary adrenal (HPA) axis and the subsequent increase in cortisol secretion is a known physiologic stress response16–18). However, few studies have investigated the influence of long-term (or extended) shift work on the HPA axis and the awakening cortisol. Also, the findings from previous studies on the association between shiftwork and awakening cortisol have been inconsistent19–28); both, high and low waking cortisol levels have been reported among shift workers.
In a prospective study of nurses, morning serum cortisol levels did not differ between shift working and day time nurses after adjusting for baseline values and job seniority26), while another study on a similar study population21) showed plasma morning cortisol concentrations were lower after night work than in the day of rest. In an experimental study of healthy young men24), cortisol concentrations upon awakening were significantly higher in morning than in evening-oriented subjects. A study23) reported that implementation of night work in former day workers initially lead to blunted cortisol profiles that normalized after a short period of adjustment. In a study of textile factory workers where total cortisol levels were assessed using hair samples, shift workers had significantly higher cortisol levels compared to day workers27).
Although a number of factors including occupation, type of job performed, specific work schedule, level of job-related stress, and design for cortisol sampling may play a part in explaining differences in results across studies, the studies may also share some common limitations. Previous investigations on the association between shift work and waking cortisol have relied heavily upon self-report data concerning shift work. More importantly, information on the duration or length of time (in years) that study participants have been working on a given shift prior to collection of blood, saliva or hair for determining cortisol levels was not available in these studies. Data from a random sample of active duty police officers enrolled in the Buffalo Cardio-Metabolic Occupational Police Stress (BCOPS) pilot study29) were used. The objective of the current study, therefore, was to evaluate how long-term shift work, ascertained retrospectively from payroll records of participant’s activity, influences the amount of and pattern of secretion of salivary cortisol at awakening. We present comparisons of the absolute mean waking cortisol, the integrated volume of cortisol released over the waking period, and the pattern of cortisol during the first hour after waking among long-term day, afternoon, and night shift officers.
Methods
Study participants and design
For this study, participants from the Buffalo Cardio- Metabolic Occupational Police Stress (BCOPS) Pilot Study29) were evaluated. The Buffalo, New York Police department, a midsized urban police force, was the selected sample site. A random sample stratified on gender (n = 100, 42 females, 58 males; n=65 participants with non-missing data were used for statistical analyses) was selected from all police officers in the department using a computer-generated random number table. No specific inclusion criteria were used for the study, other than that the participant would be a sworn police officer. One hundred percent of the random sample voluntarily agreed to participate in the study. The subjects were examined between 2001 and 2003 and data collection was performed at The Center for Preventive Medicine, School of Public Health and Health Professions, State University of New York at Buffalo. The State University of New York at Buffalo Internal Review Board and the National Institute for Occupational Safety and Health (NIOSH) Human Subjects Review Board (HSRB) approved the study and informed consent was obtained from all participants. The study is a retrospective cohort in design (or a historical cohort design) as past payroll records (from 1994 to date of examination) were used to assess the exposure of interest (dominant shift worked) prior to the outcome variable (awakening cortisol response). The historical exposure data was transformed into a single integrated measure and was analyzed cross-sectionally with the outcome data that was collected on the date of examination. Further details about the study have been described previously29).
Measures, Shift work
Work history data, from May of 1994 to date of each officer’s exam (2001–2003), were available in an electronic format for 96 of the 100 participants from payroll records of Buffalo police officers. The database from payroll records contained a day-by-day account of activities for each officer over the entire time period. Information included the start time of work, the type of activity (regular work, court work, overtime work), the type of leave (weekend, sickness, work-related injury, vacation), and the number of hours worked on each activity. The distribution of work start times for the regularly scheduled activity was examined and 30% of the records started at 7am, 24% at 8am, 28% at 4pm, and 13% at 9pm. The time a participant started his/her regular work was used to classify the shift for that day into one of the following three shifts: day shift if the start time was between 4 am and 11 am; after-noon shift if the start time was between 12 pm and 7 pm; and midnight shift if the start time was between 8 pm and 3 am. For the time period spanning from 1994 to date of exam, total hours worked as well as hours worked at the day, afternoon and midnight shift were computed for each participant. Taking into account the length of time a participant was working (from first date when electronic work history records were available to date of exam), the computed hours were standardized to a weekly basis (hours worked per week) and percent of total hours worked on each shift was calculated. A dominant shift for each subject was determined by comparing the percent of total hours worked at day, afternoon, and midnight shifts and was defined as the shift with the largest percentage of the total hours. For example, from 1994 to date of exam, an officer who worked 20% on the day shift, 60% on the afternoon shift, and 20% on midnight shift is classified as an afternoon shift worker (the dominant shift). Shift work variables of interest for analyses include (a) the dominant shift, a categorical variable with three levels (day, afternoon, and midnight), and (b) the percent of hours worked on midnight shift, a numeric variable that ranges from 0 to 100.
Salivary Cortisol
For the assessment of the awakening cortisol, subjects were instructed to collect saliva samples immediately after awakening, and 15, 30, and 45 min thereafter. Officers were provided with Salivettes (Sarstedt, USA), a commercially available collection device consisting of a dental roll and a centrifuge tube, for the collection of saliva samples. Participants were asked to refrain from taking stimulant medication, smoking, eating and drinking, and brushing their teeth before completing salivary sampling to avoid contamination of saliva with food or blood caused my micro-injuries of the oral cavity. At the designated collection time, the officers removed the dental roll from the centrifuge tube and placed it in their mouth for approximately 2 min allowing for saturation of the roll. Once in the laboratory, the tube is centrifuged to provide a non-viscous saliva sample for assay; centrifuged samples are maintained at −20°C until assayed for cortisol by a commercially available chemiluminescence immunoassay (IBL, Hamburg, Germany) at the Technical University of Dresden. The saliva samples were analyzed within a year from the date of collection (between 2001 and 2003). In samples frozen at −20°, cortisol concentrations remain stable for 1 yr30) and are relatively insensitive to repeated thawing and refreezing. Using the four cortisol samples various summary measures were derived. Previous assessment of the current study population31) showed that derived awakening cortisol parameters can be classified into two components: those that measure total hormonal excretion (primarily, average of the waking samples (AVE), the peak morning sample (PK) and total area under the curve (AUCG)) and those that measure the profile (pattern) of cortisol over time (primarily, reactivity (RT), slope of the regression line fitted to all four waking samples (SP), and area under the curve with respect to increase (AUCI). Reactivity (RT) refers to the change in salivary cortisol during the observation period and was defined as the difference between the last awakening cortisol value and the first. These parameters were derived and used as response variables of interest in this study.
Demographic and Lifestyle Characteristics
Questionnaires were administered to collect demographic and lifestyle characteristics including years of education, sleep duration, physical activity, marital status, years of police service, and police rank. Hours of sleep and physical activity were assessed using the Seven-Day Physical Activity Recall questionnaire developed in the Stanford Five-City Project32). Smoking and alcohol intake information were gathered at a baseline study conducted two-years earlier (n=115) and 87 of the 100 officers in the present study participated at the baseline. Officers were categorized as current, former and never smokers, and alcohol intake was based on the number of drinks reported per week using categories of 0, <1, 1–6, and ≥ 6 drinks per week. Body mass index was derived using physical measurements of weight in kg divided by height in meters squared.
A number of psychosocial factors including depression, traumatic events, major stressful life events, and PTSD symptoms were assessed. Stressful life events encountered during the previous year were assessed using a 41- item life events questionnaire. Participants were asked to respond (yes or no) to each question and a total life event score was computed by summing the responses for the sessed by asking participants to respond (yes/no) to 9 traumatic events (being involved in a shooting, witnessing the shooting of another police officer, seeing abused children, seeing victims of serious traffic accident, seeing someone die, seeing dead bodies, seeing severely assaulted victims, seeing victims of homicide, seeing other disturbing events) experienced during the past year. A total score was computed by summing the responses to the 9 questions. Depressive symptoms were measured using the Center for Epidemiologic Studies-Depression (CES-D) scale. Participants were asked to complete a 20-item questionnaire designed to measure symptoms of depression during the past 7 days. The Impact of Event Scale (IES) was used to measure psychological symptoms of PTSD experienced by participants during the past 7 days and was assessed using a 15-item questionnaire on a 4 point Likert scale.
Statistical analyses
Only officers with complete data on all four waking cortisol samples and time of collection of the samples were used for statistical analyses (n=65). Descriptive statistics were used to describe the demographic and life style characteristics of the study sample by shift work. The primary relationship of interest between shift work and awakening cortisol was examined by comparing mean values of the cortisol parameters across the three shift work categories using analysis of variance (ANOVA). Analysis of covariance (ANCOVA) was used to examine the effect of adjustment for various covariates (age, gender, education, smoking, alcohol consumption, physical activity, body mass index, and awakening time) on the relationship between shift work and awakening cortisol. Due to the skewed nature of the cortisol concentration, the derived parameters (except for RT and AUCI) were log transformed prior to analyses and back-transformed means and their 95% confidence intervals were reported. The distributions of AUCI and RT were fairly normal and hence no data transformation was applied for these two variables.
Differences in cortisol response profile over time across the three shift work groups was tested using two approaches: (a) by comparing the mean values of derived cortisol parameters that are known to reflect pattern over time (RT, SP and AUCI) across the three groups and (b) by modeling cortisol response over time using a repeated measures analysis and testing the interaction between time since awakening and shift work. The MIXED procedure in SAS was used to model the repeated measures with the spatial power covariance structure as a model for correlations among measurements made on the same subject. In addition, correlation analyses between the derived measures of awakening cortisol and the continuous measure of shift work (percent of hours worked on midnight shift) were performed with adjustment for possible confounding covariates.
Residuals from the fitted models (ANOVA, ANCOVA, multiple regression models) were tested for normality and homogeneity of variance using formal and graphical procedures. Due to the small number of female participants in the midnight shift (n=1), stratified analyses by gender were not conducted. Instead adjustment for the effect of gender was made in all analyses. All statistical analyses were performed using the SAS software version 9.2 (SAS Institute, Inc., Cary, NC) and significance level was set at 5% except for interaction terms (15%).
Results
Demographic and lifestyle characteristics
The demographic and lifestyle characteristics of the participants by the dominant shift are shown in Table 1. The study population consists of 65% males and the majority was white (71%) and had a rank of patrol officer or sergeant (57%). The mean age was 43.2 yrs (range: 29–63). Participants on the day shift were significantly older, had longer years of service, had a higher percentage of women (47%), and African Americans (35%) compared to the after-noon and midnight shift groups. The average time of awakening did not differ across shift (day: 7:29AM, afternoon: 7:55AM, and midnight: 8:12AM, p-value=0.4841). Participants on the afternoon and midnight shift had higher traumatic police incident scores than those on the day shift.
Table 1.
Demographic and lifestyle characteristics of study participants by shift work
| Day shift (n=34) | Afternoon shift (n=23) | Midnight shift (n=8) | P-value1 | |
|---|---|---|---|---|
| Age, years | 46.8 ± 7.5 | 38.7 ± 6.1 | 40.5 ± 7.1 | 0.001 |
| BMI, kg/m2 | 27.3 ± 4.5 | 28.7 ± 3.6 | 28.6 ± 4.8 | 0.439 |
| Physical activity, hours/wk* | 7.5 ± 9.6 | 7.4 ± 13.5 | 3.5 ± 2.3 | 0.609 |
| Length of work history (yrs) | 8.0 ± 0.8 | 7.5 ± 1.3 | 6.6 ± 1.9 | 0.008 |
| Hours of sleep/day* | 5.9 ± 2.0 | 6.6 ± 0.93 | 6.3 ± 1.6 | 0.399 |
| CES_D score+ | 7.9 ± 8.1 | 7.0 ± 5.1 | 6.1 ± 2.8 | 0.747 |
| IES score+ | 22.0 ± 14.7 | 18.8 ± 16.3 | 13.4 ± 12.0 | 0.321 |
| Life events stress score | 3.1 ± 3.1 | 2.3 ± 1.8 | 2.8 ± 1.7 | 0.535 |
| TPI score+ | 3.0 ± 2.4 | 5.0 ± 2.0 | 4.9 ± 2.0 | 0.004 |
| Waking time | 7:29AM ± 1.7 hrs. | 7:55AM ± 1.7 hrs. | 8:12AM ± 2.0 hrs. | 0.484 |
| Gender | ||||
| Male | 18 (52.9) | 17 (73.9) | 7 (87.5) | 0.094 |
| Female | 16 (47.1) | 6 (26.1) | 1 (12.5) | |
| Race | ||||
| White | 19 (55.9) | 21 (91.3) | 6 (75.0) | 0.043 |
| Black | 12 (35.3) | 2 (8.70) | 2 (25.0) | |
| Hispanic | 3 (8.8) | 0 (00.0) | 0 (00.0) | |
| Education | ||||
| ≤ High school | 5 (14.7) | 3 (13.0) | 2 (25.0) | 0.437 |
| College < 4 years | 9 (26.5) | 6 (26.1) | 4 (50.0) | |
| College + 4 years | 20 (58.8) | 14 (60.9) | 2 (25.0) | |
| Marital status | ||||
| Single | 6 (17.7) | 3 (13.0) | 3 (37.5) | 0.451 |
| Married | 21 (61.8) | 17 (73.9) | 5 (62.5) | |
| Divorced | 7 (20.6) | 3 (13.0) | 0 (00.0) | |
| Rank | ||||
| Police officer/Sergeant | 14 (41.2) | 15 (65.2) | 8 (100.0) | 0.044 |
| Lieutenant/Captain | 11 (32.4) | 4 (17.4) | 0 (00.0) | |
| Detective/Other | 9 (26.5) | 4 (17.4) | 0 (00.0) | |
| Smoking* | ||||
| Current | 4 (13.8) | 3 (15.0) | 2 (25.0) | 0.945 |
| Former | 11 (37.9) | 8 (40.0) | 3 (37.5) | |
| Never | 14 (48.3) | 9 (45.0) | 3 (37.5) | |
| Alcohol drinks/week* | ||||
| 0 | 6 (20.7) | 1 (5.0) | 2 (25.0) | 0.589 |
| < 1 | 6 (20.7) | 5 (25.0) | 1 (12.5) | |
| 1–5 | 14 (48.3) | 10 (50.0) | 5 (62.5) | |
| ≥ 6 | 3 (10.3) | 4 (20.0) | 0 (00.0) | |
Results are means ± SD for the continuous variables, and n (%) for categorical variables.
P-values are from χ2 tests of independence or Fisher’s exact test for categorical variables and from ANOVA testing differences in means across dominant shift for continuous variables.
Number of participants with complete data on smoking and alcohol consumption were: 29, 20, and 8 for day, afternoon, and midnight shift respectively; BMI: 31, 16 and 8; Hours of sleep and physical activity: 31, 17 and 8.
The Center for Epidemiologic Studies Depression Scale (CES-D); The Impact of Event Score (IES); Traumatic Police Incidents (TPI) score.
The dominant shift and distribution of percent of hours on each shift
Of the current sample used for analyses (n=65), the percentage of participants with dominant day, afternoon, and midnight shift were 52.3% (n=34), 35.4% (n=23), and 12.3% (n=8) respectively (Table 1). These estimates are consistent with the distribution of dominant shift for the larger sample (n=96); 53.1%, 34.4%, and 12.5% for day, afternoon, and midnight shifts respectively (not restricted to those having complete cortisol measurements). Within each dominant shift, the distribution of percent of hours worked on that specific shift was examined: 31 of the 34 with dominant day shift spent over 70% of their work hours on the day shift; 16 of the 23 with dominant afternoon shift spent over 70% of their work hours on the afternoon shift; 7 of the 8 subjects with dominant midnight shift spent over 70% of their time on midnight shift. The data suggests that dominant shift as a categorical variable captures the shift during which a participant spent the majority of his/her work hours.
Shift work and awakening cortisol response
Salivary cortisol concentrations (nmol/liter) averaged across all four time points (AVE) differed significantly among the day, afternoon and midnight shift workers (P = 0.038, Table 2, Model I), with midnight shift workers (6.5, 95% CI: 4.0–10.6) showing diminished awakening cortisol secretion compared to the afternoon (13.3, 10.0–17.8) or the day (12.7, 10.0–16.1) shift. Total area under the curve (AUCG, nmol/liter × minutes) also differed significantly across the three shift work categories (P = 0.041) with those in the midnight shift (314.6, 191.5–517.1) showing lower mean values than those in the afternoon (647.5, 483.1–867.9) or the day shift (606.7, 476.8–772.0). Peak morning cortisol (PK) concentration (nmol/liter), although not significantly different among the shift groups (P = 0.070), was smaller for midnight shift workers (9.4, 5.7–15.6) than the afternoon (18.3, 13.6–24.6) or day (17.5, 13.7–22.3) shift workers. Adjustment for potential con-founders (age, gender, education, smoking, alcohol consumption, physical activity, body mass index and awakening time) did not alter the overall significant association between the measures of total hormonal secretion and shift work (Table 2, Model II: adjusted p-values were 0.030, 0.035, and 0.076 for AVE, AUCG and PK respectively). However, adjustment for the confounders slightly attenuated the significant pair-wise comparison between the midnight and day shifts (Table 2, Model II). Interactions between shift work and potential effect modifiers (CES-D score, PTSD score, life events score, and traumatic police incidents) were tested for each derived awakening cortisol parameter and none of the interactions were statistically significant at 15% level. Further adjustment for the psychosocial stress measures did not attenuate the association (adjusted p-values were 0.022, 0.023, and 0.048 for AVE, AUCG and PK respectively).
Table 2.
Mean values (and 95% CI) of derived awakening cortisol parameters by shift work
| Day shift (D) | Afternoon shift (A) | Midnight shift (M) | P-value1 | P-value2 for multiple comparisons and contrasts
|
|||
|---|---|---|---|---|---|---|---|
| M vs. A | M vs. D | M vs. D&A | |||||
| Model I | (n=34) | (n=23) | (n=8) | ||||
| a AVE | 12.7 (10.0–16.1) | 13.3 (10.0–17.8) | 6.5 (4.0–10.6) | 0.038 | 0.015 | 0.018 | 0.011 |
| b AUCG | 606.7 (476.8–772.0) | 647.5 (483.1–867.9) | 314.6 (191.5–517.1) | 0.041 | 0.015 | 0.021 | 0.012 |
| c AUCI | 77.1 (–20.1–174.2) | 85.2 (–32.9–203.3) | 46.6 (–153.7–246.9) | 0.946 | 0.741 | 0.785 | 0.751 |
| d PK | 17.5 (13.7–22.3) | 18.3 (13.6–24.6) | 9.4 (5.7–15.6) | 0.070 | 0.028 | 0.032 | 0.021 |
| e SP | 0.30 (0.2–0.4) | 0.24 (0.17–0.34) | 0.21 (0.12–0.37) | 0.734 | 0.671 | 0.273 | 0.385 |
| f RT | −0.32 (−4.4–3.8) | 1.41 (−3.5–6.4) | −0.16 (−8.57–8.2) | 0.860 | 0.749 | 0.974 | 0.904 |
|
| |||||||
| Model II | (n=26) | (n=14) | (n=8) | ||||
| a AVE | 10.7 (7.8–14.7) | 15.7 (10.3–23.8) | 6.2 (3.5–11.0) | 0.030 | 0.009 | 0.121 | 0.021 |
| b AUCG | 515.3 (375.8–706.7) | 757.3 (498.1–1151.4) | 306.3 (172.9–542.5) | 0.035 | 0.011 | 0.142 | 0.026 |
| c AUCI | 47.9 (−48.8–144.7) | 153.7 (25.4–282.0) | 101.0 (−74.2–276.1) | 0.437 | 0.611 | 0.620 | 0.961 |
| d PK | 14.8 (10.6–20.7) | 21.1 (13.5–32.9) | 9.1 (5.0–16.8) | 0.076 | 0.025 | 0.198 | 0.049 |
| e SP | 0.28 (0.19–0.40) | 0.27 (0.16–0.43) | 0.22 (0.11–0.42) | 0.820 | 0.612 | 0.542 | 0.538 |
| f RT | −1.2 (−5.5–3.2) | 2.7 (−3.1–8.5) | 2.5 (−5.4–10.4) | 0.572 | 0.973 | 0.444 | 0.713 |
All cortisol parameters except AUCI and RT were log-transformed prior to statistical analyses. The means and 95% CIs obtained in the log-scale were then back transformed. Model I: unadjusted means (95% CI) of cortisol parameters across shift work. Model II: adjusted means (95% CI) of cortisol parameters across shift work. Adjustment was made for age, gender, education, smoking, alcohol consumption, physical activity, body mass index and awakening time.
Overall p-value comparing differences across the three shift categories;
p-value for pairwise differences and contrasts of interest.
AVE: Average of morning samples, nmol/L;
AUCG: Total area under the curve (expressed as nmol/liter × minutes);
AUCI: Area under the curve with respect to increase (expressed as nmol/liter × minutes);
PK: Peak morning sample, nmol/L;
SP: Slope of fitted regression line, nmol/L;
RT: Reactivity, nmol/L.
The parameters that quantify awakening cortisol dynamics (pattern) over time, i.e., reactivity (RT), slope of the regression line fitted to the waking samples (SP), and the area under the curve with respect to increase (AUCI) did not differ significantly among the day, afternoon and midnight shift workers (Table 2, Model II). This result is consistent with the pattern displayed in Fig. 1 and also with the lack of interaction between time since awakening and shift work (p=0.392) from the repeated measures data analysis. We also compared the cortisol awakening response (defined as the difference between the awakening and peak cortisol samples) among the three groups. After accounting for potential confounders, there were no significant differences across shift (Day: 1.63 nmol/l, 95% CI: 0.77–3.45; Afternoon: 1.04 nmol/l, 95% CI: 0.39–2.82; Midnight: 0.75 nmol/l, 95% CI: 0.19–2.91; p=0.618).
Fig. 1.
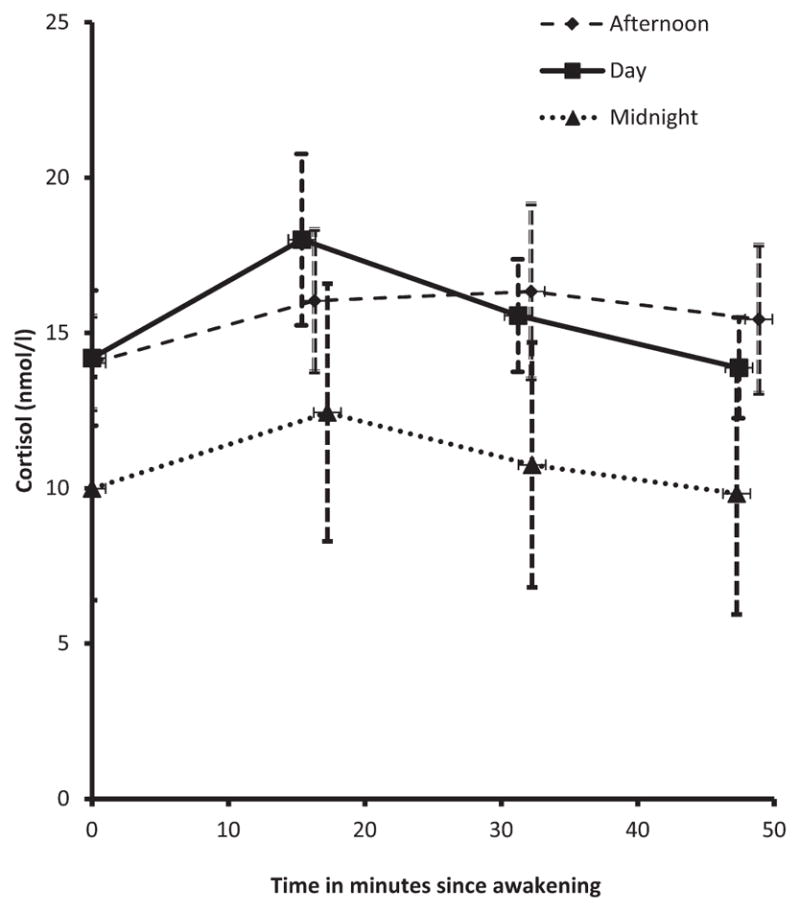
Profile of awakening salivary cortisol among day, after-noon, and midnight shift workers (mean ± SE).
The simple and partial (adjusted) correlation coefficients between the percent of hours per week worked on the midnight shift and the six derived measures of awakening cortisol response are presented in Table 3. The average cortisol over all four time points (AVE: r=−0.42, p=0.010), the peak cortisol (PK: r=−0.37, p=0.024) and the total area under the curve (AUCG: r=−0.41, p=0.012) showed negative and significant correlation with percent of hours worked on the midnight shift, whereas the parameters measuring morning cortisol pattern over time (RT, SP, and AUCI) were not significantly correlated.
Table 3.
Pearson correlations (r) between percent of hours worked on midnight shift and derived awakening cortisol parameters
| Simple correlations
|
Partial correlations
|
|||
|---|---|---|---|---|
| Derived cortisol Parameters | r | P-value1 | r | P-value2 |
| a AVE | −0.32 | 0.010 | −0.42 | 0.010 |
| b AUCG | −0.32 | 0.009 | −0.41 | 0.012 |
| c AUCI | −0.04 | 0.749 | −0.09 | 0.584 |
| d PK | −0.3 | 0.014 | −0.37 | 0.024 |
| e SP | −0.13 | 0.319 | −0.18 | 0.306 |
| f RT | 0 | 0.991 | 0.04 | 0.829 |
All cortisol parameters except AUCI and RT were log-transformed prior to statistical analyses.
unadjusted p-value (n=65).
p-value adjusted for age, gender, education, smoking, alcohol consumption, physical activity, body mass index and awakening time (n=48).
AVE: Average of morning samples, nmol/L;
AUCG: Total area under the curve (expressed as nmol/ liter × minutes);
AUCI: Area under the curve with respect to increase (expressed as nmol/liter × minutes);
PK: Peak morning sample, nmol/L;
SP: Slope of fitted regression line, nmol/L;
RT: Reactivity, nmol/L.
Discussion
Previous research linking shift work and HPA axis function as measured by awakening cortisol response was contradictory with some studies reporting increased activation21, 23, 24) and others reporting the opposite26, 27). The central aim of this study was to examine differences in waking salivary cortisol secretion (hormonal output) and profile (pattern over time) across three work shift schedules (day, afternoon, and midnight) among police officers who represent a high stress occupation. The participants were officers who worked on their respective shift for 6–8 yrs (i.e., long term shift workers). Within this context, we found, after controlling for age, gender, education, smoking status, alcohol consumption, physical activity, BMI, and awakening time that officers working in the midnight shift had lower levels of cortisol after awakening compared to those in the afternoon or day shifts. On the other hand, the general pattern (profile) of the repeated measures of salivary cortisol during the first hour after awakening were similar across all three shifts; characterized by a peak cortisol 20–30 min after waking and a gradual decline thereafter. The results from the current study suggest that working dominantly on the midnight shift (for extended period of time) may influence aspects of HPA axis regulation leading to a suppressed release of cortisol following awakening.
Repeated or chronic challenges of the HPA axis by environmental stressors may cause alterations in HPA axis activity leading to small diurnal variations and flattening of the diurnal cortisol curve as a consequence of significant reductions in awakening cortisol accompanied by a rise in evening cortisol33). A meta-analysis34) presented a similar argument that following a stress the HPA axis exhibits initial activation in the form of elevated cortisol release. However, following prolonged exposure to the stressors, the body’s biological systems could mount a counter-regulatory response (dysregulation) such that the cortisol output rebounds to less than normal. The results from the current study are consistent with this hypothesis and in agreement with other studies that investigated cortisol rhythms in shift workers and found that the cortisol awakening response is decreased and evening cortisol levels are increased during shift work19, 25, 35). In a study of police officers from the current sample, the association between short-term shift work (3, 5, 7 and 14 days prior to saliva collection) and waking cortisol was examined and waking salivary cortisol levels were lower among night or afternoon shift officers compared to those on day shift28). In an experimental study of 8 morning-oriented vs. 8 evening-oriented healthy young men, cortisol concentrations upon awakening were higher in the morning oriented than in the evening oriented subjects24). In a study of workers at an electronic manufacturing facility, cortisol profiles in permanent night workers appear to be blunted during night work and days off and implementation of night work in former day workers seems to lead to initially blunted cortisol profiles that normalize after a short adjustment period23). Among nurses, a study showed significant reduction in morning salivary cortisol (at 6AM) after 7 days of consecutive night shifts compared to those working days20).
Results from other studies differed from ours26, 27, 36, 37). In a longitudinal study, morning cortisol was measured in 68 shift-working and 28 daytime nurses at baseline and at 12 months. Cortisol levels at 12 months did not differ between the two groups after adjustment for baseline values and job seniority26). A study of total cortisol levels from hair samples27) compared 33 shift workers and 89 day workers where shift workers were shown to have higher hair cortisol levels than day workers but the hair cortisol in this study measured total amount of cortisol released over the last 3 months while our measure concerns how much is released in response to the awakening signal. In a study of chronic stress associated with unemployment, unemployed subjects had higher morning and lower evening salivary cortisol levels compared to employed subjects35). In a sample of 100 university students, those who perceived a higher level of chronic stress due to workload had higher early morning cortisol increase after awakening than those who did not36).
The mechanism linking shift work and awakening cortisol response can be complex and some studies34, 38) elude that some of the variability in results across studies is attributable to differences in stressors (e.g. the specific work schedule and flexibility, job type, perceived stress, years worked on the specific shift, etc.) and personal characteristics (e.g. individual differences in tolerance to shift work). A number of previous studies that examined associations of perceived stress, job strain, and fatigue/burnout with awakening cortisol response indirectly support the current results34, 39, 40, 41–43). A meta-analyses of studies on the relationship between psychosocial factors and waking cortisol found that waking cortisol is positively associated with job and general life stress but was negatively associated with fatigue, burnout and exhaustion42). In a study of school teachers where saliva samples were collected at 15, 30, and 60 min following awakening, the study found that those scoring high on burnout had lower overall cortisol secretion44). Studies of middle-aged women42) and hospital nurses40) found perceived stress level was negatively correlated with waking salivary cortisol concentration where those in the high stress group secreted lower levels of waking cortisol compared to those in the low stress group. A longitudinal study of retail industry workers39) found that salivary cortisol was inversely associated with job strain; higher concentrations were observed among subjects with low job strain compared to those experiencing high job strain. In the current study the negative correlation between percent of hours worked in midnight shift and the total awakening cortisol secretion suggests that those who spend the majority of their weekly work hours on the midnight shift tend to have lower morning cortisol output.
Cortisol is secreted according to a well-defined circadian rhythm45), and generally morning cortisol level increases rapidly following awakening with the peak level occurring 15 to 30 min after waking. In the current study the nonsignificant interaction between shift category and time of saliva sampling from repeated measures data analysis and the non-significant association between cortisol parameters that measure overall pattern (RT, SP, and AUCI) and shift category (% of hours worked on midnight shift) suggest that the pattern of morning cortisol levels during the first 45–60 min after awakening is similar regardless of shift.
The HPA-axis response to shift work is probably highly variable and may be associated with several confounding factors including job stress, individual characteristics, the type of work, duration of work hours, and intervals between shifts26). For example, several studies reported that shift work is overall well-tolerated by nurses and does not impair their health or sleep quality46, 47). Hence future comprehensive studies should consider including the above factors, particularly the type of occupation and level of job-related stress in order to gain a better understanding of the association between shift work and HPA-axis activity in general not just the waking response. Night shift work as a stressor may influence future health risk indirectly through changing health behaviors (e.g. unhealthy eating, less exercise, poor sleep, etc.) and therefore understanding the extent to which these changes in health behaviors influence awakening cortisol secretion17) and pattern are important in estimating the independent effect of shift work. In future studies it would be worthwhile to compare and contrast the influence of recent night shift work experience and long-term night shift work on awakening cortisol response.
The current study has limitations and strengths. Shortcomings include the retrospective cohort design of the study which precludes causal inference, recruitment limited to police officers only, small sample size for those on the midnight shift, and single day of saliva sampling48). The sub-sample used for statistical analyses (n=65) were compared to those excluded (n=35) with respect to dominant shift (Day: 52% vs. 55%, Afternoon: 35% vs. 32%, Midnight: 12% vs. 13%, p-value=0.9555), demographic, lifestyle, and psychosocial variables. The results showed that the two samples did not differ significantly in all variables except CES-D score (7.4 ± 6.6 vs. 11.2 ± 8.7, p-value=0.015). The sample used for analysis (n=65) is therefore representative of the whole group (n=100). Generalizability of the findings may be limited to police departments with similar characteristics. To our knowledge, this study is among the first to examine long-term shift work and waking salivary cortisol among police officers, an understudied and high risk population. More importantly, the study provided a unique opportunity to examine the association based on multiple years (8–10 yrs) of objective, day-to-day work records of officers while previous studies relied primarily upon self-reported data concerning shift work. We were also able to account for awakening time and a number of physical (e.g. BMI), lifestyle (smoking, alcohol consumption, physical activity), and psychosocial factors (e.g. depression, traumatic events, life events) that could confound the association between shift work and waking salivary cortisol.
In summary, despite the small sample limitation, our study has shown that independent of confounding factors long-term night work is associated with attenuation of the awakening cortisol response, yet the cortisol pattern through the first hour after waking was not affected by shift work. The findings are consistent with accumulating evidence indicating that working night shift for an extended period of time may lead to chronic stress and reduced HPA axis activity. This study adds to the body of knowledge concerning the association between long-term shift work and awakening salivary cortisol among high stress occupations and may have implications for future health risk of officers working night shifts for an extended period of time. Due to the small sample size of night shift officers these results are preliminary and should, in the future, be replicated with larger samples and other populations. In the context of our finding of a suppressed awakening cortisol in police officers who worked a greater percentage of time on the midnight shift, it is worth noting that a low awakening cortisol level may signify a dysfunction of the HPA axis. Importantly, this type of dysfunction may serve as a pathophysiological link to CVD through a number of mechanisms such as heightened inflammatory responses or by an impact on other established risk factors49). Given the susceptibility of this population to CVD, the possible pathophysiological links between dysfunction of the HPA axis and preclinical or clinical indicators of CVD should be the focus of future investigations in the full BCOPS Study cohort
References
- 1.Åkerstedt T. Work hours, sleepiness and the underlying mechanisms. J Sleep Res. 1995;4:15–22. doi: 10.1111/j.1365-2869.1995.tb00221.x. [DOI] [PubMed] [Google Scholar]
- 2.Rosa RR. Extended work shifts and excessive fatigue. J Sleep Res. 1995;4:51–6. doi: 10.1111/j.1365-2869.1995.tb00227.x. [DOI] [PubMed] [Google Scholar]
- 3.Costa G. The problem: shiftwork. Chronobiol Int. 1997;14:89–98. doi: 10.3109/07420529709001147. [DOI] [PubMed] [Google Scholar]
- 4.Weibel L, Follénius M, Brandenberger G. Biologic rhythms: their changes in night-shift workers. Presse Med. 1999;28:252–8. [PubMed] [Google Scholar]
- 5.Kuhn G. Circadian rhythm, shift work, and emergency medicine. Ann Emerg Med. 2001;37:88–98. doi: 10.1067/mem.2001.111571. [DOI] [PubMed] [Google Scholar]
- 6.Folkard S. Do permanent night workers show circadian adjustment? A review based on the endogenous melatonin rhythm. Chronobiol Int. 2008;25:215–24. doi: 10.1080/07420520802106835. [DOI] [PubMed] [Google Scholar]
- 7.Reinberg A, Ashkenazi I. Internal desynchronization of circadian rhythms and tolerance to shift work. Chronobiol Int. 2008;25:625–43. doi: 10.1080/07420520802256101. [DOI] [PubMed] [Google Scholar]
- 8.Machi MS, Staum M, Callaway CW, Moore C, Jeong K, Suyama J, Patterson PD, Hostler D. The relationship between shift work, sleep, and cognition in career emergency physicians. Acad Emerg Med. 2012;19:85–91. doi: 10.1111/j.1553-2712.2011.01254.x. [DOI] [PubMed] [Google Scholar]
- 9.Knutsson A, Boggild H. Shiftwork and cardiovascular disease: Review of disease mechanisms. Rev Environ Health. 2000;15:359–72. doi: 10.1515/reveh.2000.15.4.359. [DOI] [PubMed] [Google Scholar]
- 10.De Bacquer D, Van Risseghem M, Clays E, Kittel F, De Backer G, Braeckman L. Rotating shift work and the metabolic syndrome: a prospective study. Int J Epidemiol. 2009;38:848–54. doi: 10.1093/ije/dyn360. [DOI] [PubMed] [Google Scholar]
- 11.Wang XS, Armstrong ME, Cairns BJ, Key TJ, Travis RC. Shift work and chronic disease: the epidemiological evidence. Occup Med (Lond) 2011;61:78–89. doi: 10.1093/occmed/kqr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shields M. Shift work and health. Health Rep. 2002;13:11–33. [PubMed] [Google Scholar]
- 13.Liberman A, Best S, Metzler T, Fagan J, Weiss D, Marmar C. Routine occupational stress and psychological distress in police. Polic Int J Police Strateg Manage. 2002;25:421–39. [Google Scholar]
- 14.Franke WD, Collins SA, Hinz PN. Cardiovascular disease morbidity in an Iowa law enforcement cohort, compared with the general Iowa population. J Occup Environ Med. 1998;40:441–4. doi: 10.1097/00043764-199805000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Franke WD, Ramey SL, Shelley MC., II Relationship between cardiovascular disease morbidity, risk factors and stress in a law enforcement cohort. J Occup Environ Med. 2002;44:1182–9. doi: 10.1097/00043764-200212000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Schedlowski M, Wiechert D, Wagner TO, Tewes U. Acute psychological stress increases plasma levels of cortisol, prolactin and TSH. Life Sci. 1992;50:1201–5. doi: 10.1016/0024-3205(92)90319-k. [DOI] [PubMed] [Google Scholar]
- 17.Fukuda S, Morimoto K. Lifestyle, stress and cortisol response: Review I: Mental stress. Environ Health Prev Med. 2001;6:9–14. doi: 10.1007/BF02897303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kudielka BM, Hellhammer DH, Wust S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology. 2009;34:2–18. doi: 10.1016/j.psyneuen.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Touitou Y, Motohashi Y, Reinberg A, Touitou C, Bourdeleau P, Bogdan A, Auzéby A. Effect of shift work on the night-time secretory patterns of melatonin, prolactin, cortisol and testosterone. Eur J Appl Physiol Occup Physiol. 1990;60:288–92. doi: 10.1007/BF00379398. [DOI] [PubMed] [Google Scholar]
- 20.Hennig J, Kieferdorf P, Moritz C, Huwe S, Netter P. Changes in cortisol secretion during shiftwork: implications for tolerance to shiftwork? Ergonomics. 1998;41:610–21. doi: 10.1080/001401398186784. [DOI] [PubMed] [Google Scholar]
- 21.Munakata M, Ichi S, Nunokawa T, Saito Y, Ito N, Fukudo S, Yoshinaga K. Influence of night shift work on psychologic state and cardiovascular and neuroendocrine responses in healthy nurses. Hypertens Res. 2001;24:25–31. doi: 10.1291/hypres.24.25. [DOI] [PubMed] [Google Scholar]
- 22.Lac G, Chamoux A. Biological and psychological responses to two rapid shiftwork schedules. Ergonomics. 2004;47:1339–49. doi: 10.1080/00140130410001724237. [DOI] [PubMed] [Google Scholar]
- 23.Kudielka BM, Buchtal J, Uhde A, Wüst S. Circadian cortisol profiles and psychological self-reports in shift workers with and without recent change in the shift rotation system. Biol Psychol. 2007;74:92–103. doi: 10.1016/j.biopsycho.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Griefahn B, Robens S. The cortisol awakening response: a pilot study on the effects of shift work, morningness and sleep duration. Psychoneuroendocrinology. 2008;33:981–8. doi: 10.1016/j.psyneuen.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Vangelova K. The effect of shift rotation on variations of cortisol, fatigue and sleep in sound engineers. Ind Health. 2008;46:490–3. doi: 10.2486/indhealth.46.490. [DOI] [PubMed] [Google Scholar]
- 26.Copertaro A, Bracci M, Gesuita R, Carle F, Amati M, Baldassari M, Mocchegiani E, Santarelli L. Influence of shift-work on selected immune variables in nurses. Ind Health. 2011;49:597–604. doi: 10.2486/indhealth.ms1210. [DOI] [PubMed] [Google Scholar]
- 27.Manenschijn L, van Kruysbergen RG, de Jong FH, Koper JW, van Rossum EF. Shift work at young age is associated with elevated long-term cortisol levels and body mass index. J Clin Endocrinol Metab. 2011;96:E1862–5. doi: 10.1210/jc.2011-1551. [DOI] [PubMed] [Google Scholar]
- 28.Wirth M, Burch J, Violanti J, Burchfiel C, Fekedulegn D, Andrew M, Zhang H, Miller DB, Hébert JR, Vena JE. Shiftwork duration and the awakening cortisol response among police officers. Chronobiol Int. 2011;28:446–57. doi: 10.3109/07420528.2011.573112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Violanti JM, Burchfiel CM, Miller DB, Andrew ME, Dorn J, Wactawski-Wende J, Beighley CM, Pierino K, Joseph PN, Vena JE, Sharp DS, Trevisan M. The Buffalo Cardio- Metabolic Occupational Police Stress (BCOPS) pilot study: methods and participant characteristics. Ann Epidemiol. 2006;16:148–56. doi: 10.1016/j.annepidem.2005.07.054. [DOI] [PubMed] [Google Scholar]
- 30.Garde AH, Hansen AM. Long-term stability of salivary cortisol. Scand J Clin Lab Invest. 2005;65:433–6. doi: 10.1080/00365510510025773. [DOI] [PubMed] [Google Scholar]
- 31.Fekedulegn DB, Andrew ME, Burchfiel CM, Violanti JM, Hartley TA, Charles LE, Miller DB. Area under the curve and other summary indicators of repeated waking cortisol measurements. Psychosom Med. 2007;69:651–9. doi: 10.1097/PSY.0b013e31814c405c. [DOI] [PubMed] [Google Scholar]
- 32.Sallis JF, Haskell WL, Wood PD, Fortmann SP, Rogers T, Blair SN, Paffenbarger RS. Physical activity assessment methodology in the 5-City Project. Am J Epidemiol. 1985;121:91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- 33.Dallman MF. Stress update Adaptation of the hypothalamic-pituitary-adrenal axis to chronic stress. Trends Endocrinol Metab. 1993;4:62–9. doi: 10.1016/s1043-2760(05)80017-7. [DOI] [PubMed] [Google Scholar]
- 34.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 35.Harris A, Waage S, Ursin H, Hansen AM, Bjorvatn B, Eriksen HR. Cortisol, reaction time test and health among offshore shift workers. Psychoneuroendocrinology. 2010;35:1339–47. doi: 10.1016/j.psyneuen.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 36.Ockenfels MC, Porter L, Smyth J, Kirschbaum C, Hellhammer DH, Stone AA. Effect of chronic stress associated with unemployment on salivary cortisol: overall cortisol levels, diurnal rhythm, and acute stress reactivity. Psychosom Med. 1995;57:460–7. doi: 10.1097/00006842-199509000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Schulz P, Kirschbaum C, Pruessner J, Hellhammer D. Increased free cortisol secretion after awakening in chronically stressed individuals due to work overload. Stress Med. 1998;14:91–7. [Google Scholar]
- 38.Härmä M. Individual differences in tolerance to shiftwork: a review. Ergonomics. 1993;36:101–9. doi: 10.1080/00140139308967860. [DOI] [PubMed] [Google Scholar]
- 39.Steptoe A, Wardle J, Lipsey Z, Mills R, Oliver G, Jarvis M, Kirschbaum C. A longitudinal study of work load and variations in psychological well-being, cortisol, smoking, and alcohol consumption. Ann Behav Med. 1998;20:84–91. doi: 10.1007/BF02884453. [DOI] [PubMed] [Google Scholar]
- 40.Yang Y, Koh D, Ng V, Lee FC, Chan G, Dong F, Chia SE. Salivary cortisol levels and work-related stress among emergency department nurses. J Occup Environ Med. 2001;43:1011–8. doi: 10.1097/00043764-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Nater UM, Youngblood LS, Jones JF, Unger ER, Miller AH, Reeves WC, Heim C. Alterations in diurnal salivary cortisol rhythm in a population-based sample of cases with chronic fatigue syndrome. Psychosom Med. 2008;70:298–305. doi: 10.1097/PSY.0b013e3181651025. [DOI] [PubMed] [Google Scholar]
- 42.O’Connor DB, Hendrickx H, Dadd T, Elliman TD, Willis TA, Talbot D, Mayes AE, Thethi K, Powell J, Dye L. Cortisol awakening rise in middle-aged women in relation to psychological stress. Psychoneuroendocrinology. 2009;34:1486–94. doi: 10.1016/j.psyneuen.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 43.Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol Psychol. 2009;80:265–78. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Pruessner JC, Hellhammer DH, Kirschbaum C. Burnout, perceived stress, and cortisol responses to awakening. Psychosom Med. 1999;61:197–204. doi: 10.1097/00006842-199903000-00012. [DOI] [PubMed] [Google Scholar]
- 45.Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, Kaspers F, Kirschbaum C. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997;61:2539–49. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- 46.Sveinsdóttir H. Self-assessed quality of sleep, occupational health, working environment, illness experience and job satisfaction of female nurses working different combination of shifts. Scand J Caring Sci. 2006;20:229–37. doi: 10.1111/j.1471-6712.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- 47.Admi H, Tzischinsky O, Epstein R, Herer P, Lavie P. Shift work in nursing: is it really a risk factor for nurses’ health and patients’ safety? Nurs Econ. 2008;26:250–7. [PubMed] [Google Scholar]
- 48.Hellhammer J, Fries E, Schweisthal OW, Schlotz W, Stone AA, Hagemann D. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: state- and trait components. Psychoneuroendocrinology. 2007;32:80–6. doi: 10.1016/j.psyneuen.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 49.Rosmond R, Bjorntorp P. The hypothalamic –pituitary-adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes and stroke. J Intern Med. 2000;247:188–97. doi: 10.1046/j.1365-2796.2000.00603.x. [DOI] [PubMed] [Google Scholar]


