Abstract
Objective
To examine trajectories of recovery and change in patterns of personal care and instrumental functional activity performance to determine whether different assessment interval designs within a 12-month period yield different estimates of improvement and decline following acute hospitalization and inpatient rehabilitation.
Design
Secondary analysis of a 12-month prospective cohort study.
Setting
Transition to the community.
Participants
Adults (N=419) admitted to acute care and receiving inpatient rehabilitation for a neurological, lower-extremity musculoskeletal, or medically complex condition.
Interventions
Not applicable
Main Outcome Measures
Improvement, no change, and decline as measured by the personal care and instrumental scale of the Activity Measure for Post-Acute Care.
Results
Assessment at the end of a single 12-month follow-up assessment interval showed that over 60% of the participants improved. In contrast, analysis of 2 fixed-length 6-month assessment intervals revealed an almost 40% decrease in the proportion who improved from 6 to 12 months. Fewer participants continued to improve in the time periods further from the acute hospitalization and the proportion of subjects who declined increased from 21.4% to 31.2% to 38.0% over the 3 consecutive assessment intervals (baseline to 1mo, 1–6mo, 6–12mo). Only 58 (19.7%) participants continued on the same path of recovery from baseline to 12 months (9.9% improved over all 3 consecutive time periods, 3.1% made no change, 6.8% declined).
Conclusions
Examination of change over shorter compared with longer assessment intervals revealed considerable variability in the trajectories of recovery. Research is needed to determine the appropriate frequency and timing for measuring and monitoring function and recovery following an acute hospitalization.
Keywords: Activities of daily living, Outcome assessment (health care), Recovery of function, Rehabilitation
The goal of rehabilitation services is to minimize the impact of impairments and illness on patients’ ability to live and function independently and in the community. The challenge for clinicians is the dynamic nature of the disability process as each patient exhibits a unique trajectory of recovery that may include periodic setbacks in the period following an acute hospitalization.1,2 Greater insight into the variability of recovery patterns would be helpful to guide planning for more effective intervention and health service strategies. However, to date there has been little empirical work on change in recovery patterns over time and the trajectories that diverse populations of rehabilitation patients follow after discharge from acute care. This study was designed to address this need by examining patterns of change in functional activity performance across 12 months in a population of adults who were hospitalized acutely and subsequently received inpatient rehabilitation.
Currently, the most common approach to evaluating functional outcomes uses two assessment points, that is, an initial measure of function is used to predict a later end-point, or a single measure of change is calculated. Analyses using 2 time points assume that patients continue in essentially the same linear trajectory over the time period studied and do not allow examination of intraindividual variations or interindividual similarities in trajectories of recovery over time.3 Furthermore, this approach may inadvertently obscure clinically meaningful patterns of change that occur between the 2 measurements. Thus, it is reasonable to question whether previous longitudinal studies of rehabilitation outcomes have provided an accurate description of improvement and decline in function PAC.
Evidence from the aging literature also suggest that rehabilitation outcomes may have been misestimated when the assessment interval has been long, that is more than 6 months, because this design overlooks the possibility of intermediate transitions between patterns of recovery.4 Studies of aging and disability (defined here as the inability to perform ADLs) have reported that it is common for community-dwelling elders to transition in and out of disability over the course of only 2 years. For example, a study of 439 community-dwelling older adults found that more than half of the episodes of decline were temporary and no longer present at 1 year.5 This pattern would go undetected with traditional analyses using only 2 assessment points or long assessment intervals.1,6 In fact, longitudinal studies using these designs (2 assessments or long assessment intervals) have been shown to substantially underestimate the occurrence of disability and recovery in the aging population.2,7,8 It is possible that the same issue may apply in rehabilitation outcomes research.
Repeated measurements of rehabilitation outcomes using shorter (≤6mo) assessment intervals may help reveal differential patterns of improvement, no change, and decline, and where and how often in the path of recovery these changes occur for different people.2,6 However, the appropriate frequency and timing for measuring functional status following an acute hospitalization has not been clinically or empirically established. One approach is the use of fixed-length follow-up intervals. A fixed-frequency for repeated measurements provides the opportunity to determine the natural course of recovery or disease by establishing rates of change and patterns of association over periods of time that are held constant. Another approach, as suggested with chronic diseases exhibiting indeterminate beginning and ending points, is to define the illness into phases such as acute, maintenance, chronic complications, and so on.9,10 Phases that define measurement points for patients who have had an acute event requiring hospitalization and inpatient rehabilitation might be divided similarly: a short initial interval as is traditionally done when assessing outcomes associated with acute and inpatient care,11 a 6-month assessment as is typically done in studies predicting recovery from a disabling event,12 and follow-up assessments over 3- to 6-month intervals to monitor change as suggested for primary and secondary prevention measures such as blood pressure and cholesterol levels associated with broader outcomes.13,14 It is unclear whether measuring function related to phases of care or using fixed-length intervals is more appropriate for studying trajectories of recovery. To our knowledge, a comparison of results obtained by using different designs has not yet been conducted in research on functional activity outcomes following an acute hospitalization and inpatient rehabilitation. Such a comparison requires availability of outcome measures that are suitable for use across the broad range of settings and that capture a broad range of patient function.
The measures of function most commonly used in rehabilitation research have limited ability to document patterns of change in function beyond the setting for which they were designed.15 Instruments designed to measure a more basic level of function16,17 are limited in the extent of change that can be captured due to ceiling effects.15,18–21 Similarly, measures developed for PAC are not useful measures for people with more severe impairments due to floor effects.22–25 Thus analysis of functional outcomes across service settings and beyond rehabilitation services using these existing measures may not accurately detect a treatment effect or the actual magnitude of change from one period of measurement to the next. This limitation is especially problematic when repeated measurements can no longer detect change.26,27 Thus, our assessment of functional recovery in this study used an instrument developed specifically to enable tracking as patients move along the continuum of health service settings.
The purpose of the present study was to compare different designs for analyzing change in functional activity performance over 12 months to determine how the choice of design affects the estimate of recovery patterns. In order to achieve this aim we examined patterns of improvement, no change, and decline in functional activity performance comparing results from a single assessment interval of 12 months with those from analyses of shorter intervals within the same year. Several comparisons were made to elucidate whether there are common trajectories of recovery in a diverse population of rehabilitation patients, and whether use of traditional longitudinal analyses under or overestimates improvement and decline.
METHODS
For this study, we conducted secondary analyses of data collected in a 12-month prospective longitudinal study. The first section below provides an overview of the ROS during which the original patient data were collected. We then describe the sample, measures, and analyses for the present study of recovery patterns. The university institutional review board approved both the original and the present studies.
Patient Recruitment and Data Collection
The ROS, conducted from 1999 to 2003, was designed to examine the performance of a comprehensive set of measures to monitor functional recovery in patients who had an acute hospitalization and subsequently received rehabilitation in the hospital or in an independent rehabilitation facility. Patients who were receiving rehabilitation services in either setting were recruited. To be eligible, subjects had to be 18 years of age or older, English speaking, and receiving inpatient rehabilitation services at the time of recruitment. Patients who were not expected to live 12 months after the acute hospitalization (according to the physician report) were excluded from the study. The treating clinician assessed the patient’s ability to participate based on whether the subject could understand the interview questions, sustain attention for an hour, and reliably respond to the questions.28–30
The sample was stratified with the intent to include an approximately equal number of subjects in 3 major diagnostic groups: (1) neurologic disorders including stroke, multiple sclerosis, Parkinson’s disease, brain injury, spinal cord injury, and neuropathy, (2) lower-extremity musculoskeletal disorders including fractures, joint replacements, orthopedic surgery, and joint or muscular pain, and (3) medically complex disorders including debility resulting from illness, cardiopulmonary conditions, or postsurgical recovery. Of the 516 participants recruited, 23.3% were admitted for a neurologic disorder, 32.4% for a lower-extremity musculoskeletal disorder, and 44.4% for a complex medical condition. Using scores on the adapted Modified Rankin Scale, the sample was also stratified to assure good representation of levels of disability severity.31 There was a similar proportion of subjects representing mild, moderate, and severe levels.
The majority of participants (63.4%) were recruited from an independent rehabilitation facility as opposed to an acute hospital, and the average number of days between admission to the acute hospital and date of the baseline interview ± standard deviation was 19.6±18.2. A trained data collector conducted personal interviews with each participant lasting approximately 45 minutes to an hour at baseline, 1-, 6-, and 12-month follow-up. Interviews at baseline were conducted in the location of recruitment (either the acute hospital or independent rehabilitation facility), and interviews following baseline were conducted at the subject’s living location or a mutually convenient location. All data were self-report with the exception of diagnosis, disease severity, and acute hospital LOS that were obtained from the medical record. Receipt of nursing or rehabilitation services following baseline were also self-report and were not confirmed with health service records.
Sample
Timing of the baseline assessment is an important factor for predicting the potential for recovery.32 The influence of intervening factors such as spontaneous recovery or receipt of rehabilitation intervention is more likely for patients with a baseline assessment of the primary outcome further from the acute hospital admission date (an administrative proxy variable for the onset of the acute event). To better align the timing of the follow-up assessments in relation to the acute hospital admission date and reduce the variability in the recovery period analyzed for each patient, this study of recovery patterns and trajectories was restricted to the 419 patients who had their baseline assessment completed within 28 days of the acute hospital admission (81.2% of the original study’s sample). Compared to the full ROS sample population of 516 patients, this study’s sample population of 419 patients was not significantly different in age, sex, race, diagnosis, medical or sensory comorbidity, perception of health, or function at baseline but did have a larger proportion living at home prior to the hospitalization (P=.02) and had a shorter acute hospital LOS (P<.001).
Measures
Patient characteristics measured at baseline included age, sex, race, level of educational attainment, living location and situation prior to hospitalization, and the participants’ perceived health status prior to hospitalization as measured by the first question of the Medical Outcomes Study 8-item Short-Form.33 This item was self-rated on a 6-point Likert scale from very poor to excellent, and has been used previously as a self-report measure of general health status.34,35 Clinical characteristics included admitting diagnosis, presence of medical and sensory comorbidities, baseline personal care and instrumental functional activity performance score, acute hospital LOS, and number of days between hospital admission and the baseline assessment.
Outcome measure
The AM-PAC was developed specifically to enable tracking of functional recovery as patients move along the continuum of health service settings. The instrument’s content, guided by the ICF,36 covers 3 domains of functional recovery: physical and movement activities, applied cognitive activities, and personal care and instrumental activities.28–30,37 The PCI scale was selected as the outcome measure of the present study as it includes the majority of functional activities most commonly used in the United States to assess the impact of chronic disease and impairments on the ability of adults to live independently.35,38 The activities examined by the PCI scale are frequently referred to as basic and instrumental ADLs, and most closely parallel the self-care and domestic ICF domains.
The PCI scale consists of 2 linked forms: an inpatient form and a community form. The inpatient form, designed for use only with inpatients, contains activities typically performed in this environment and was used with all participants at baseline and any participant who was in an inpatient setting for any of the 3 follow-up assessments. On the other hand, the community form was used with participants beyond baseline who were no longer in inpatient settings as it includes activities more typically performed at home. Each form of the instrument consists of 10 items that ask about the difficulty of performing an activity (a 5-point rating) or the use of assistance to perform an activity (6-point rating). The inpatient instrument asks about difficulty performing and use of assistance with basic self-care activities, while the community instrument asks about difficulty and assistance needed with more complex activities. Together the linked PCI instruments assess a range of relevant activities from personal hygiene to putting the dishes away. All items were selected from a common item pool based on their collective ability to provide useful measurement information across a broad spectrum of functional activity performance. In contrast to other measures of function or ADLs, the PCI scale does not include mobility or communication items as they are examined by the physical and movement and applied cognitive scales of the AM-PAC. The range of activities included reduces the likelihood of floor and ceiling effects, as demonstrated in previous analyses.39 Furthermore, this design allows clinicians and researchers to measure functional activity performance outcomes across a wide variety of patient groups and health delivery settings, capturing the greatest range of abilities from the acute setting to at least 1 year posthospitalization.30,39
Raw scores on the PCI scale are transformed onto a 0- to 100-interval level scale using tables derived from Rasch analyses, which also provide a unique SE of measurement estimate for each possible score. Higher scores reflect less difficulty and less use of assistance.30 Test-retest reliability estimates for the longer scale from which these short 10-item forms were derived ranged from .91 to .97.40 Additional psychometric information about the PCI scale and the AM-PAC has been reported previously.28,30
Statistical Analysis
Benchmark
The benchmark for determining the minimal detectable change, the amount of change necessary to conclude that the difference is not attributable to measurement error,41 was specific to the time period in question (interval of time defined by 2 assessments) and to the sample with data available for both assessments defining the interval. A unique benchmark was calculated for each assessment interval: the traditional longitudinal interval starting from baseline to a specific end-point of 12 months, and the 5 comparison intervals of baseline to 1 month, baseline to 6 months, 1 to 6 months, and 6 to 12 months. As is common in the literature,42–44 the benchmark for change in function used for this study was ±1 SE of measurement, calculated as the mean of the median SE scores (table 1) from the 2 time points.
Table 1.
Benchmarks for Change in PCI Functional Activity Performance
| Time Period | Change in Function Benchmark (mean of the median SE for 2 assessments in a time period) |
|---|---|
| Longitudinal Interval | |
| Baseline to 12mo | 3.755 |
| Comparison Intervals | |
| Baseline to 1mo | 3.055 |
| Baseline to 6mo | 3.465 |
| One to 6mo | 3.330 |
| Six to 12mo | 4.000 |
Assessment of change
Change in PCI was assessed by computing raw change scores (eg, PCI at 12mo minus PCI at 6mo) for each assessment interval for each person and then comparing the magnitude of change for each assessment interval to the calculated benchmark. The results were categorized into 3 patterns for each assessment interval: improvement, no change, and decline. A difference smaller than the benchmark for any specific interval was categorized as no change as compared to improvement (positive difference) and decline (negative difference). Subjects who died or could not participate due to illness were classified as having declined. Participants with undocumented status for a follow-up interview or missing a PCI score were excluded from analysis of recovery patterns (table 2).
Table 2.
Participation in this Study and Sample Size by Follow-Up Interview With the Percentage of the Total Indicating Missing Data
| Study Participation | Baseline | 1 Month | 6 Months | 12 Months |
|---|---|---|---|---|
| Participated | 419 | 338 (80.7) | 316 (75.4) | 284 (67.8) |
| Deceased (total) | NA | 12 (2.9) | 24 (5.7) | 34 (8.1) |
| Too ill | NA | 19 (4.5) | 8 (1.9) | 13 (3.1) |
| Missing | NA | 50 (11.9) | 71 (17.0) | 88 (21.0) |
| Outcome measure missing | 9 (2.1) | 3 (0.7) | 14 (3.3) | 12 (2.9) |
| Total sample by follow-up interview* | 410 (97.9) | 366 (87.4) | 334 (79.7) | 319 (76.1) |
NOTE. Values are n (%).
Abbreviation: NA, not applicable.
The sample included in the analysis of each assessment interval was dependent on the availability of a complete outcome score for the relevant time points or documentation of illness or having died in order to be categorized into the decline group.
(Sample total calculated for each follow-up assessment as the number who participated + the deceased + participants who were too ill – the number missing a measured outcome.)
Demographics, comparison of patterns, and illustration of trajectories
The central purpose of this study was to compare different designs for analyzing change in function over 12 months, and therefore, patient characteristics were only used to describe the sample and not examined for their association with the different patterns of recovery. Descriptive statistics were calculated for the full sample of 419 patients and for the group who had complete data for analyzing trajectories of recovery (N=295). Chi-square tests for specified proportions (goodness of fit test) and 1-sample t tests were used to determine if the trajectory subsample was significantly different than the full sample for this study, and also to compare baseline characteristics for those who completed the study, including those who were documented as too ill or dead, with those patients who were missing (undocumented status) for the 12-month assessment.
The proportion of the sample exhibiting each pattern for each assessment interval was calculated and presented in three alternative designs: (1) a single 12-month assessment interval capturing change from baseline to 12 months; (2) two 6-month fixed-length assessment intervals illustrating change from baseline to 6 months and 6 to 12 months; and (3) 3 assessment intervals representing a model for phases of rehabilitation and recovery including intervals for baseline to 1 month, 1 to 6 months, and 6 to 12 months. The patterns assessed prospectively were mapped to illustrate the most common 12-month trajectories of recovery for the participants in this sample with data at all time points (N=295).
RESULTS
The sample of patients with a baseline study assessment within 28 days of their acute hospital admission (N=419) had a wide range in age from 19 to 100 years. The largest diagnostic group included those patients admitted with complex medical conditions (42.0%). More than half of the sample was female (54.2%), and a large majority were white (90.7%) and living at home prior to the acute hospitalization (95.3%). Table 3 presents the baseline characteristics for the total sample of 419 patients and compares these characteristics with those in the group with data at all time points and with those whose status was not documented at 12 months.
Table 3.
Baseline characteristics of All Participants in This Study, for Participants With Data at All Time Points (complete data), and for Those Missing (undocumented status) at 12 Months
| Variable | Full Sample (n=419) |
Complete Data (n=295) |
Missing (n=88) |
|---|---|---|---|
| Categorical | |||
| Diagnostic group | |||
| Neurologic | 99 (23.6) | 70 (23.7) | 22 (25.0) |
| Lower-extremity musculoskeletal | 144 (34.4) | 103 (34.9) | 27 (30.7) |
| Complex medical | 176 (42.0) | 122 (41.4) | 39 (44.3) |
| Sex: females | 227 (54.2) | 156 (52.9) | 49 (55.7) |
| Race: white | 380 (90.7) | 268 (90.9) | 78 (88.6) |
| Education: high school diploma or less† | 181 (45.3) | 123 (42.9) | 42 (52.5) |
| Medical comorbidity (reported at least 1 of 6) | 336 (81.0) | 242 (82.9) | 64 (72.7)* |
| Sensory comorbidity: vision and/or hearing | 149 (35.9) | 105 (35.8) | 33 (37.9) |
| Good-excellent perceived health status prior | 243 (59.3) | 175 (59.7) | 50 (59.5) |
| Living alone prior‡ | 149 (39.3) | 103 (38.0) | 36 (47.4) |
| Living location prior: home | 385 (95.3) | 275 (96.2) | 77 (92.8) |
| Continuous | |||
| Age | 69.1±15.0 | 69.5±13.6 | 66.0±19.5* |
| Baseline personal care & instrumental function | 57.8±12.4 | 57.0±12.7 | 60.0±11.9 |
| Acute hospital LOS§ | 7.6±4.9 | 7.5±5.1 | 7.4±3.9 |
| Days from hospital admission to baseline | 13.4±7.1 | 13.6±7.1 | 13.3±7.1 |
NOTE. Values are n (%) or mean ± standard deviation.
P range, .01–.05.
5% not reported;
9.6% not reported;
19% not documented.
Of the 419 participants in the sample, 88 (21.0%) were missing at 12 months. These patients were significantly younger in age than the total sample (mean, 66.0y vs 69.1y, P=.03) and fewer reported a medical comorbidity (72.7% vs 81.0%, P=.03). Admitting diagnosis, sex, race, education, presence of a sensory comorbidity, baseline PCI, acute hospital LOS, and living situation (alone), location (home), and perception of health prior to acute hospitalization were not associated with being lost to follow-up with undocumented status in this study. Furthermore, there were no significant differences between the full sample and those who completed each assessment in this study (N=295).
Recovery Patterns
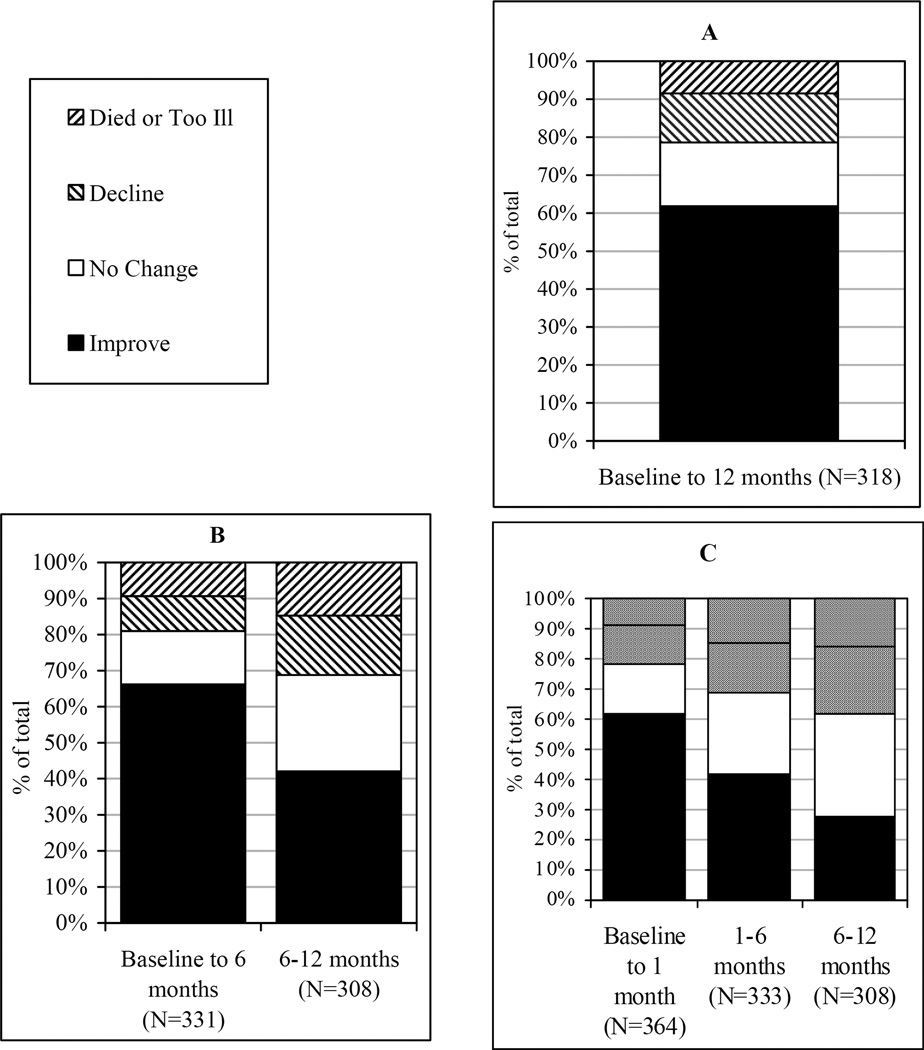
Figures 1A–1C display the change in PCI for 3 assessment interval designs over 12 months: a single assessment interval of 12 months; 2 fixed-length assessment intervals of 6 months each; and assessment intervals in phases over 12 months (baseline to 1mo, 1–6mo, 6–12mo). Each figure illustrates the proportion of those who improved, made no change, or declined, died, or were too ill in the intervals indicated.
Figure 1A illustrates results obtained from a traditional outcomes analysis model of 2 time points where baseline data are compared to functional activity performance 12 months post hospitalization. Comparing figure 1A to data in figures 1B and 1C suggests that the proportion of people who declined or did not change in 12 months is underrepresented when change in PCI is assessed over a 12-month interval from baseline. Variability in recovery and decline is not captured by this long 12-month assessment interval.
Fig 1.
Proportion of the sample exhibiting each pattern for the specified interval. (A) Single assessment interval. (B) Two fixed-length intervals. (C) Intervals by phase of recovery.
Figure 1B depicts 2 fixed-length assessment intervals of 6 months. In this analysis, PCI performance seems to plateau for the majority after 6 months (34.4%), more than twice the proportion that made no change in PCI from baseline to 6 months (14.8%). This finding contrasts with results from the 12-month interval in figure 1A where there appeared to be fewer people who made no change (10.1%) over the duration of the study. Analysis of 2 fixed-length intervals of 6 months also revealed the proportion who improved dropped almost 40% after 6 months.
The assessment intervals in figure 1C represent phases associated with rehabilitation and recovery: the baseline to 1-month interval, postacute recovery from 1 to 6 months, and a 6-month follow-up interval. The characteristics of this rehabilitation population support this phase model of assessing outcomes. The majority of patients had transitioned from the acute hospital or inpatient rehabilitation to the community by the 1-month assessment and had been discharged either to home or to an assisted living environment (83.4%). By 6 months, rehabilitation services for most patients had been discontinued (78.0%) and the living location was stable (94.7% at home).
The intervals presented in figure 1C provide detail on recovery patterns over the course of 12 months that cannot be estimated from the 12-month interval in figure 1A. Examining change across the most acute 1-month period separately from the following 5-month interval in figure 1C provides additional information about recovery patterns in the period closer to acute hospitalization that is not available in the baseline to 6-month assessment in figure 1B. There were 85 people who no longer improved from 1 to 6 months. Furthermore, the intermediate assessment at 1 month identifies a group who declined during the postacute rehabilitation and recovery interval who would have been missed otherwise.
In summary, the recovery patterns in figure 1C show that fewer people continued to improve in time periods further from the acute hospitalization, while the proportion of individuals who declined increased from 21.4% to 31.2% to 38.0% over the 3 consecutive intervals. Although a large proportion (34.4% of the sample with data at 12mo) experienced a plateau in PCI and made no further change after 6 months, 27.6% improved in this 6-month follow-up interval.
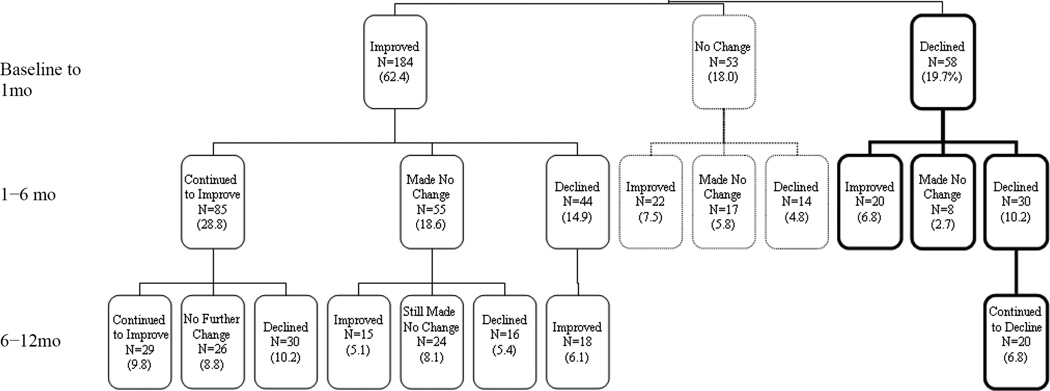
Trajectories
Figure 2 presents a tree diagram displaying each of the trajectories of recovery over 12 months that were experienced by at least 5% of the participants who had PCI data for all 4 interview points. The figure collectively describes 60.3% of the sample population. Mapping the 3 possible patterns of improvement, no change, or decline resulted in a total of 27 possible patient trajectories over 3 intervals of time across 12 months. In this rehabilitation sample, all 27 trajectories were present. Only 58 of the 295 participants continued in the same pattern over time: 9.9% improved over all three consecutive periods, 3.1% made no change, and 6.8% continued to decline over 12 months.
Fig 2.
Twelve-month trajectories of recovery experienced by at least 5% of those participants with PCI functional activity performance measured at all 4 follow-up interviews over the course of 12 months (N=295). NOTE. Each box indicates the number of participants exhibiting the indicated pattern of improvement, no change, or decline and the percentage of the sample with complete PCI data at all 4 time points.
Of the patients who declined from baseline to 1 month, more than 50% continued to decline from 1 to 6 months and then two thirds continued to decline from 6 to 12 months. The number of people who improved in PCI in the first month was more than 3 times greater than the number who made no change or declined. Despite this, only 46.2% continued to improve from 1 to 6 months after which there was an almost equal prognosis of improvement, no change, or decline from 6 to 12 months (χ22=11.7, P<.009).
DISCUSSION
The purpose of the present study was to examine the possibility that different approaches to analyzing change over time may yield a different picture of recovery patterns in a population of adults who were hospitalized and subsequently received inpatient rehabilitation. Our results suggest that traditional methods of analysis for longitudinal studies may overshadow the extent of improvement and decline revealed by assessments conducted over shorter intervals. The finding of 27 different trajectories of recovery in the sample of 295 patients who were assessed four times in a year further illustrates the interindividual heterogeneity possible in the recovery process. These findings suggest that the number of assessments, length of each assessment interval, and sensitivity of the measurement instrument are significant factors affecting estimates of improvement and decline following an acute hospitalization with inpatient rehabilitation. Results from our baseline to 12-month design for examining change illustrate 2 primary limitations of outcome studies with a lengthy interval between the 2 assessment points. This design assumes a linear trajectory of recovery, which we found was experienced by only 20% of our sample. In addition, this design greatly misrepresented the proportion that improved, declined, or made no change during this time period. For example, results from the single 12-month assessment interval suggest that over 60% of the sample improved over 12 months when in fact, analysis by the smallest intervals possible in this study revealed that 60% improved in the first month and only 27% improved from 6 to 12 months. Findings demonstrate the value of examining recovery in the year after hospitalization using shorter assessment intervals.
Measuring functional activity performance with shorter and more frequent assessment intervals would also provide the opportunity to examine intraindividual change and interindividual similarities in trajectories of recovery and factors related to these variations. Several of the patterns revealed by trajectory mapping are worth noting. The importance of making gains in function early in the episode of illness is suggested by the finding that a greater proportion of these patients improved further from the acute hospitalization or transitioned toward better performance in the latest phase evaluated. However, even among those who showed improvement in their scores from baseline to 12 months, many experienced phases of no change or decline while recovering. These findings are clinically important as they suggest that improvement from baseline to 1 month is important for continued gains in PCI.
The transition from improvement to decline and no change (transition toward worse performance) was more common than the transition from decline or no change to improvement (transition toward better performance). Examination of those who declined from baseline to 1 month revealed that they were most likely to decline in the following periods. However, decline for some was a temporary state. These trends and highly variable trajectories were not unlike those found in studies of functional change in community-dwelling elders.5,45
The transition between patterns, as shown in this study when assessing change over consecutive shorter assessment intervals, would not have been captured in studies designed with a long single assessment interval or measurement instruments insensitive to change for both high and low functioning persons. Using one instrument to measure function that is both reliable and valid across the continuum of PAC is key to assessing change in function and across providers and health services. Instruments commonly used for measuring function in clinical rehabilitation practice and research do not meet this requirement. The AM-PAC was found to have larger standardized response means than the FIM across patient and severity groups and to be more sensitive to small changes in activity performance.39 In addition, the instrument’s ability to capture a large range in performance helped reveal patterns of improvement and decline even across late periods. Only 5% of the subjects in this study reached ceiling on the PCI scale and no subjects were at floor prior to the final measurement at 12 months. Further advances in understanding functional outcomes over an extended period will depend on the use of measures that are applicable across the continuum of PAC settings and that capture the performance of both high and low functioning people.
Studies extending longer than 1 year with frequent measures of function, across short, fixed-length assessment intervals are needed to more clearly delineate the epidemiology of recovery. Although this research design can be financially and administratively resource intensive and may be subject to high drop-out rates due to participant burnout, the approach has the potential to identify an optimal schedule for measurement to capture clinically meaningful change based on the patients’ condition. Ongoing monitoring, as has been implemented with several chronic and infectious disease measures, is needed to minimize the frequency and severity of subsequent periods of decline,45 to detect a change in health status, and to make clinicians aware of the need for intervention.46 An incomplete understanding of the epidemiology of recovery, particularly in periods further from the acute hospitalization, may be an impediment to the provision of rehabilitation services in later phases of recovery.
From a health policy perspective, functional decline is one of the most distressing health outcomes for society and the health system as it has been shown to lead to deteriorating health status, dependence, institutionalization, high health care costs, and death.47–50 This study clearly illustrates the possibility that the extent of decline following an acute hospitalization and inpatient rehabilitation may have been seriously underrepresented in previous studies that did not assess change over short consecutive intervals of time with an instrument both valid and reliable across health delivery settings for high to low functioning people.
Study Limitations
The primary purpose of the present study was to examine the effect of using different designs to analyze change. However, it is important to note that patterns of recovery and trajectories over time may be influenced by patient demographic and clinical characteristics, continued health service use, living location, and the role of other environmental factors. These factors were not accounted for in this study and are the focus of future work designed to identify the predictors of recovery. Because the study involved secondary analysis of existing data analyses were limited to the time periods of the original assessments, which were determined by the purposes of the original study. Future prospective longitudinal studies are needed that a more representative sample, including patients from rural and urban health facilities, more from minority groups, and more patients admitted from institutional care.
CONCLUSIONS
These findings illustrate the importance of short assessment intervals in providing more detailed estimates of change in functional activity performance over 12 months. Previous studies with traditional analysis of 2 time points and long assessment intervals have potentially misrepresented actual change over time. Although the phase approach to analyzing outcomes data provided the most specific representation of recovery in this study, even shorter fixed-length assessment intervals should be evaluated over a period longer than 1 year for their utility in examining function. Research to determine the appropriate frequency and timing for measuring and monitoring function following an acute hospitalization and the risk factors for change will more clearly delineate the epidemiology of recovery and may help determine the optimal timing of the delivery of health care services to prevent decline and promote improvement.
Acknowledgments
Source of support for this study: This work was supported in part by a Post-Doctoral Fellowship for Dr. Prvu Bettger (T32-HD-007425) awarded to the University of Pennsylvania from the National Institute of Child Health and Human Development (NICHD) National Center for Medical Rehabilitation Research (NCMRR). The original study was supported by the National Institute of Disability and Rehabilitation Research (NIDRR), United States Department of Education (grant no. H133B990005). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the funding agencies.
Financial disclosure(s) and conflict(s) of interest: We certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated AND, we certify that all financial and material support for this research (eg, NIH or NHS grants) and work are clearly identified in the title page of the manuscript.
List of Abbreviations
- ADLs
activities of daily living
- AM-PAC
Activity Measure for Post-Acute Care
- ICF
International Classification of Functioning, Disability and Health
- LOS
length of stay
- PAC
postacute care
- PCI
personal care and instrumental
- ROS
Rehabilitation Outcomes Study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimers: none
Poster presentation of part of this material: American Congress of Rehabilitation Medicine and American Society of Neurorehabilitation Annual Education Conference held in Boston, MA September 28 – October 1, 2006.
References
- 1.Gill TM, Kurland B. The burden and patterns of disability in activities of daily living among community-living older persons. J Gerontol A Biol Sci Med Sci. 2003;58:70–75. doi: 10.1093/gerona/58.1.m70. [DOI] [PubMed] [Google Scholar]
- 2.Verbrugge LM, Reoma JM, Gruber-Baldini AL. Short-term dynamics of disability and well-being. J Health Soc Behav. 1994;35:97–117. [PubMed] [Google Scholar]
- 3.Beckett LA, Brock DB, Lemke JH, et al. Analysis of change in self-reported physical function among older persons in four population studies. Am J Epidemiol. 1996;143:766–778. doi: 10.1093/oxfordjournals.aje.a008814. [DOI] [PubMed] [Google Scholar]
- 4.Hardy SE, Dubin JA, Holford TR, Gill TM. Transitions between states of disability and independence among older persons. Am J Epidemiol. 2005;161:575–584. doi: 10.1093/aje/kwi083. [DOI] [PubMed] [Google Scholar]
- 5.Perera S, Studenski S, Chandler JM, Guralnik JM. Magnitude and patterns of decline in health and function in 1 year affect subsequent 5-year survival. J Gerontol A Biol Sci Med Sci. 2005;60:894–900. doi: 10.1093/gerona/60.7.894. [DOI] [PubMed] [Google Scholar]
- 6.Hardy SE, Gill TM. Recovery from disability among community-dwelling older persons. JAMA. 2004;291:1596–1602. doi: 10.1001/jama.291.13.1596. [DOI] [PubMed] [Google Scholar]
- 7.Gill TM, Allore H, Holford TR, Guo Z. The development of insidious disability in activities of daily living among community-living older persons. Am J Med. 2004;117:484–491. doi: 10.1016/j.amjmed.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 8.Gill TM, Hardy SE, Williams CS. Underestimation of disability in community-living older persons. J Am Geriatr Soc. 2002;50:1492–1497. doi: 10.1046/j.1532-5415.2002.50403.x. [DOI] [PubMed] [Google Scholar]
- 9.Hornbrook MC, Hurtado AV, Johnson RE. Health care episodes: definition, measurement and use. Med Care Rev. 1985;42:163–218. doi: 10.1177/107755878504200202. [DOI] [PubMed] [Google Scholar]
- 10.Rosen AK, Mayer-Oakes A. Episodes of care: theoretical frameworks versus current operational realities. Jt Comm J Qual Improv. 1999;25:111–128. doi: 10.1016/s1070-3241(16)30431-x. [DOI] [PubMed] [Google Scholar]
- 11.Rosen AK. Windows of observation. In: Iezzoni L, editor. Risk adjustment for measuring health care outcomes. 3rd ed. Chicago: Health Administration Pr; 2003. pp. 71–82. [Google Scholar]
- 12.Gill TM, Allore HG, Hardy SE, Guo Z. The dynamic nature of mobility disability in older persons. J Am Geriatr Soc. 2006;54:248–254. doi: 10.1111/j.1532-5415.2005.00586.x. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein LB, Adams R, Alberts MJ, et al. American Heart Association/American Stroke Association Stroke Council; Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; Quality of Care and Outcomes Research Interdisciplinary Working Group; American Academy of Neurology. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:1583–1633. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- 14.Sacco RL, Adams R, Albers G, et al. American Heart Association/American Stroke Association Council on Stroke; Council on Cardiovascular Radiology and Intervention; American Academy of Neurology. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Circulation. 2006;113:e409–e449. [PubMed] [Google Scholar]
- 15.Tilling K, Sterne JA, Rudd AG, Glass TA, Wityk RJ, Wolfe CD. A new method for predicting recovery after stroke. Stroke. 2001;32:2867–2873. doi: 10.1161/hs1201.099413. [DOI] [PubMed] [Google Scholar]
- 16.Version 3.1. Buffalo: Research Foundation, State Univ New York; 1990. Guide for the uniform data set for medical rehabilitation (adult FIM) [Google Scholar]
- 17.Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 18.Dromerick AW, Edwards DF, Diringer MN. Sensitivity to changes in disability after stroke: a comparison of four scales useful in clinical trials. J Rehabil Res Dev. 2003;40:1–8. doi: 10.1682/jrrd.2003.01.0001. [DOI] [PubMed] [Google Scholar]
- 19.Kwon S, Hartzema AG, Duncan PW, Min-Lai S. Disability measures in stroke: relationship among the Barthel Index, the Functional Independence Measure, and the Modified Rankin Scale. Stroke. 2004;35:918–923. doi: 10.1161/01.STR.0000119385.56094.32. [DOI] [PubMed] [Google Scholar]
- 20.van der Putten JJ, Hobart JC, Freeman JA, Thompson AJ. Measuring change in disability after inpatient rehabilitation: comparison of the responsiveness of the Barthel index and the Functional Independence Measure. J Neurol Neurosurg Psychiatry. 1999;66:480–484. doi: 10.1136/jnnp.66.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen ME, Marino RJ. The tools of disability outcomes research functional status measures. Arch Phys Med Rehabil. 2000;81(12) Suppl 2:S21–S29. doi: 10.1053/apmr.2000.20620. [DOI] [PubMed] [Google Scholar]
- 22.Kersten P, Mullee MA, Smith JA, McLellan L, George S. Generic health status measures are unsuitable for measuring health status in severely disabled people. Clin Rehabil. 1999;13:219–228. doi: 10.1177/026921559901300306. [DOI] [PubMed] [Google Scholar]
- 23.Hobart JC, Williams LS, Moran K, Thompson AJ. Quality of life measurement after stroke: uses and abuses of the SF-36. Stroke. 2002;33:1348–1356. doi: 10.1161/01.str.0000015030.59594.b3. [DOI] [PubMed] [Google Scholar]
- 24.Lai SM, Perera S, Duncan PW, Bode R. Physical and social functioning after stroke: comparison of the Stroke Impact Scale and Short Form-36. Stroke. 2003;34:488–493. doi: 10.1161/01.str.0000054162.94998.c0. [DOI] [PubMed] [Google Scholar]
- 25.Andresen EM, Meyers AR. Health-related quality of life outcomes measures. Arch Phys Med Rehabil. 2000;81(12) Suppl 2:S30–S45. doi: 10.1053/apmr.2000.20621. [DOI] [PubMed] [Google Scholar]
- 26.Johnston KC, Connors AF, Jr, Wagner DP, Haley EC Predicting outcome in ischemic stroke: external validation of predictive risk models. Stroke. 2003;34:200–202. doi: 10.1161/01.str.0000047102.61863.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnston KC, Connors AF, Jr, Wagner DP, Knaus WA, Wang X, Haley EC., Jr A predictive risk model for outcomes of ischemic stroke. Stroke. 2000;31:448–455. doi: 10.1161/01.str.31.2.448. [DOI] [PubMed] [Google Scholar]
- 28.Coster WJ, Haley SM, Andres PL, Ludlow LH, Bond TL, Ni PS. Refining the conceptual basis for rehabilitation outcome measurement: personal care and instrumental activities domain. Med Care. 2004;42(1 Suppl):I62–I72. doi: 10.1097/01.mlr.0000103521.84103.21. [DOI] [PubMed] [Google Scholar]
- 29.Haley SM, Coster WJ, Andres PL, et al. Activity outcome measurement for postacute care. Med Care. 2004;42(1 Suppl):I49–I61. doi: 10.1097/01.mlr.0000103520.43902.6c. [DOI] [PubMed] [Google Scholar]
- 30.Haley SM, Andres PL, Coster WJ, Kosinski M, Ni P, Jette AM. Short-form activity measure for post-acute care. Am J Phys Med Rehabil. 2004;85:649–660. doi: 10.1016/j.apmr.2003.08.098. [DOI] [PubMed] [Google Scholar]
- 31.van Swieten J, Koudstaal P, Visser M, Schouten H, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 32.Hankey GJ, Spiesser J, Hakimi Z, Bego G, Carita P, Gabriel S. Rate, degree, and predictors of recovery from disability following ischemic stroke. Neurology. 2007;68:1583–1587. doi: 10.1212/01.wnl.0000260967.77422.97. [DOI] [PubMed] [Google Scholar]
- 33.Ware JE, Kosinski M, Dewey JE, Gandek B. How to score and interpret single-item health status measures: s manual for users of the SF-8 Health Survey. Lincoln: QualityMetric; 1999. [Google Scholar]
- 34.Stuck AE, Walthert JM, Nikolaus T, Bula CJ, Hohmann C, Beck JC. Risk factors for functional status decline in community-living elderly people: a systematic literature review. Soc Sci Med. 1999;48:445–469. doi: 10.1016/s0277-9536(98)00370-0. [DOI] [PubMed] [Google Scholar]
- 35.Campbell VA, Crews JE, Moriarty DG, Zack MM, Blackman DK. Surveillance for sensory impairment, activity limitation, and health-related quality of life among older adults: United States 1993–1997. MMWR Surveill Summ. 1999;48:131–156. [PubMed] [Google Scholar]
- 36.World Health Organization. Geneva: WHO; 2001. International classification of functioning, disability and health: IDF. [Google Scholar]
- 37.Coster WJ, Haley SM, Ludlow LH, Andres PL, Ni PS. Development of an applied cognition scale to measure rehabilitation outcomes. Am J Phys Med Rehabil. 2004;85:2030–2035. doi: 10.1016/j.apmr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Manton KG, Gu X, Lamb VL. Change in chronic disability from 1982 to 2004/2005 as measured by long-term changes in function and health in the U.S. elderly population. Proc Natl Acad Sci U S A. 2006;103:18374–18379. doi: 10.1073/pnas.0608483103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coster WJ, Haley SM, Jette AM. Measuring patient reported outcomes after rehabilitation using the Short-Form Activity Measure for Post-Acute Care (AM-PAC) J Rehabil Med. 2006;38:237–242. doi: 10.1080/16501970600609774. [DOI] [PubMed] [Google Scholar]
- 40.Andres PL, Haley SM, Ni PS. Is patient-reported function reliable for monitoring postacute outcomes? Am J Phys Med Rehabil. 2003;82:614–621. doi: 10.1097/01.PHM.0000073818.34847.F0. [DOI] [PubMed] [Google Scholar]
- 41.Fritz JM, Irrgang JJ. A comparison of a modified Oswestry Low Back Pain Disability Questionnaire and the Quebec Back Pain Disability Scale. Phys Ther. 2001;81:776–788. doi: 10.1093/ptj/81.2.776. [DOI] [PubMed] [Google Scholar]
- 42.Wyrwich KW, Nienaber NA, Tierney WM, Wolinsky FD. Linking clinical relevance and statistical significance in evaluating intra-individual changes in health-related quality of life. Med Care. 1999;37:469–478. doi: 10.1097/00005650-199905000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Wyrwich KW, Tierney WM, Wolinsky FD. Further evidence supporting an SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life. J Clin Epidemiol. 1999;52:861–873. doi: 10.1016/s0895-4356(99)00071-2. [DOI] [PubMed] [Google Scholar]
- 44.Hebert R, Spiegelhalter DJ, Brayne C. Setting the minimal metrically detectable change on disability rating scales. Arch Phys Med Rehabil. 1997;78:1305–1308. doi: 10.1016/s0003-9993(97)90301-4. [DOI] [PubMed] [Google Scholar]
- 45.Hardy SE, Allore HG, Guo Z, Dubin JA, Gill TM. The effect of prior disability history on subsequent functional transitions. J Gerontol A Biol Sci Med Sci. 2006;61:272–277. doi: 10.1093/gerona/61.3.272. [DOI] [PubMed] [Google Scholar]
- 46.Chobanian AV, Bakris GL, Black HR, et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The JNC 7 Report. JAMA. 2003;289:2560–2571. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 47.Tinetti ME, Inouye SK, Gill TM, Doucette JT. Shared risk factors for falls, incontinence, and functional dependence. Unifying the approach to geriatric syndromes. JAMA. 1995;273:1348–1353. [PubMed] [Google Scholar]
- 48.Mahoney JE, Sager MA, Jalaluddin M. Use of an ambulation assistive device predicts functional decline associated with hospitalization. J Gerontol A Biol Sci Med Sci. 1999;54:M83–M88. doi: 10.1093/gerona/54.2.m83. [DOI] [PubMed] [Google Scholar]
- 49.Ramos LR, Simoes EJ, Albert MS. Dependence in activities of daily living and cognitive impairment strongly predicted mortality in older urban residents in Brazil: a 2-year follow-up. J Am Geriatr Soc. 2001;49:1168–1175. doi: 10.1046/j.1532-5415.2001.49233.x. [DOI] [PubMed] [Google Scholar]
- 50.Dunlop DD, Semanik P, Song J, Manheim LM, Shih V, Chang RW. Risk factors for functional decline in older adults with arthritis. Arthritis Rheum. 2005;52:1274–1282. doi: 10.1002/art.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gill TM, Kurland BF. Prognostic effect of prior disability episodes among nondisabled community-living older persons. Am J Epidemiol. 2003;158:1090–1096. doi: 10.1093/aje/kwg237. [DOI] [PubMed] [Google Scholar]