Summary
The multi-kinase inhibitor rigosertib (ON 01910.Na) induces mitotic arrest and apoptosis in myeloblasts, while sparing normal cells. The purpose of this study was to determine the pharmacokinetic profile, maximum-tolerated dose (MTD), safety, and clinical activity of an oral formulation of rigosertib in patients with myelodysplastic syndromes (MDS). For pharmacokinetic studies, patients received rigosertib in single escalating weekly doses. To determine the MTD, patient cohorts received escalating doses of rigosertib twice daily for 14 days of a 21-day cycle. Overall, 37 patients were treated. Rigosertib exposure increased with escalating oral doses. Mean absolute oral bioavailability ranged from 13.9% (fed) to 34.8% (fasting) in 12 patients treated at the 560 mg dose level. Dose-limiting toxicity (grade 3 dysuria and shortness of breath) occurred at the 700 mg b.i.d. dose. Five patients experienced grade 3 non-haematological toxicity, including symptoms of urothelial inflammation, hypotension and syncope, fatigue and abdominal pain. Encouraging signs of clinical activity included 2 bone marrow complete remissions in refractory anaemia with excess blasts type 1 patients previously treated with azacitidine. In addition, 4 patients each achieved transfusion independence and haematological improvements. In conclusion, oral rigosertib is bioavailable and well tolerated, and has clinical activity in patients with MDS.
Keywords: myelodysplastic syndromes, polo-like kinase, phosphoinositide-3-kinase, rigosertib
Introduction
The myelodysplastic syndromes (MDS) are a heterogeneous group of clonal myeloid malignancies characterized by ineffective haematopoiesis with a propensity to transform to acute myeloid leukemia (AML) (Fenaux 2004, Komrokji, et al 2011). Based on the International Prognostic Scoring System (IPSS), patients can be stratified into risk categories with differing expected survival and progression to AML (Greenberg, et al 1997, Greenberg, et al 2012). For patients with higher risk MDS, the primary goal of therapy is to modify the natural history of the disease (Fenaux, et al 2009, Greenberg, et al 2011, Kantarjian, et al 2007). For patients eligible for high intensity therapy, allogeneic haematopoietic stem cell transplantation (HSCT) is the treatment of choice. However, for the majority of patients who lack a suitable donor or who are ineligible for high intensity therapy due to advanced age or significant co-morbidities, treatment with azanucleosides, 5-azacitidine or decitabine, has become the standard of care (Fenaux, et al 2009, Kantarjian, et al 2007). Although azanucleosides are beneficial for a subset of high-risk MDS patients, patients who have not responded to or have progressed after an initial response to azanucleosides have a short overall survival and limited treatment options (Jabbour, et al 2010, Lin, et al 2010, Prebet, et al 2011).
Attempts to improve the outcome of patients whose disease is resistant to azanucleosides have included investigations of novel agents. Rigosertib (ON 01910.Na) is a novel benzyl styryl sulfone that inhibits several protein kinases including polo-like kinase 1 (PLK1), phosphoinositide 3-kinase (PI3K) and Akt/Protein Kinase B (PKB). Rigosertib’s inhibitory effects on PLK1 are indirect, whereas it is a direct inhibitor of PI3K, preferentially the p110α and p110β isoforms, resulting in decreased Akt phosphorylation and cyclin D1 translation (Prasad, et al 2009). Its biological activity relates in part to sustained and/or hyperphosphorylation of RanGAP1.SUMO1, causing mitotic arrest and apoptosis (Gumireddy, et al 2005, Oussenko, et al 2011, Reddy, et al 2011). While rigosertib has potent antimitotic and antineoplastic activity demonstrable both in vitro and in xenograft models, it is relatively non-toxic for normal cells (Chapman, et al 2012, Gumireddy, et al 2005, Prasad, et al 2009).
Four phase I/II clinical trials examined various treatment schedules of the original intravenous rigosertib formulation in patients with MDS (Olnes, et al 2012, Seetharam, et al 2012, Silverman, et al 2010). Intravenous rigosertib was found to have a favourable safety profile without significant myelosuppression. Most common drug-related toxicities included nausea, diarrhoea, constipation, fatigue, dysuria and abdominal pain. Among 30 evaluable patients, 5 achieved a complete bone marrow response according to International Working Group (IWG) criteria (Cheson, et al 2000, Cheson, et al 2006) and 7 achieved a ≥50% decrease of bone marrow blasts. In addition, one patient achieved a major and another a minor cytogenetic response. Five patients had haematological improvements.
Based on the encouraging clinical efficacy and favourable toxicity profile of the intravenous formulation of rigosertib, we conducted a phase I study to determine the maximum tolerated dose (MTD), to investigate bioavailability and elimination and to preliminarily assess the clinical activity of a novel oral formulation of rigosertib in patients with MDS.
Methods
Patient selection
Patients ≥ 18 years of age with an Eastern Cooperative Oncology Group (ECOG) Performance Status ≤ 2 and a diagnosis of MDS according to the World Health Organization (WHO) Criteria (Harris, et al 1999) with Low, Intermediate 1 or −2 or High Risk IPSS scores (Greenberg, et al 1997) and with cytopenia of least one lineage (absolute neutrophil count < 1.8 × 109/l, platelet count <100 × 109/l or haemoglobin <100 g/l) were eligible for the trial. At least 4 weeks (only 2 weeks if an erythrocyte stimulating agent [ESA] was used) had to have elapsed from prior MDS treatment. Patients had to have failed to respond to or have an inadequate response to azacitidine, decitabine, lenalidomide, or to an ESA, or had to have failed to respond to, relapsed following, or opted not to undergo a HSCT. Key exclusion criteria included anameia due to factors other than MDS, bone marrow cellularity <10%, any active malignancy within the past year, human immunodeficiency virus 1 (HIV-1) seropositivity, uncontrolled intercurrent illness, total bilirubin ≥ 26.6 not related to haemolysis or Gilbert disease, aspartate transaminase/alanine transaminase ≥2 x upper limit of normal (ULN), serum creatinine ≥1.5 x ULN, ascites, hyponatraemia, uncontrolled hypertension, new onset seizures, psychiatric illness/social situations that would limit the patient’s ability to tolerate and/or comply with study requirements, or concurrent treatment with an investigational agent or with chemotherapy, radiotherapy or immunotherapy. This trial was conducted according to the Declaration of Helsinki and was approved by the Quorum Review Institutional Review Board (IRB) (09-01; approval 28 July 2009) and by the Columbia University Medical Center IRB #4 (09-01; approval 29 Sep 2010). All patients signed an IRB-approved consent form to participate in the study.
Study Design
The objectives of this phase I trial were to explore and characterize the pharmacokinetics of rigosertib following oral capsule administration, to define the haematological and non-haematological toxicities and MTD of oral rigosertib administered twice daily for 14 of every 21 days, to determine any food effect on the absolute bioavailability of oral rigosertib, to assess the frequency and duration of clinical response (complete remission, partial remission, or haematological improvement), cytogenetic response and the bone marrow blast response (as defined by the 2006 IWG criteria (Cheson, et al 2006)) following rigosertib treatment, and to determine the time to disease progression.
For the purpose of oral administration, rigosertib was supplied as soft gelatin capsules in two strengths: A 70 mg capsule strength, which contains 1 ml of a 70 mg/ml solution of active pharmaceutical ingredient in polyethylene glycol (PEG) 400 and a 280 mg capsule strength, which contains 1 ml of a 280 mg suspension of rigosertib in PEG 400. The active pharmaceutical ingredient was identical to that used in studies of the intravenous formulation of rigosertib (Jimeno et al 2008). Capsules of oral rigosertib were supplied by Onconova Therapeutics, Inc., Newtown, PA.
In repeat-dose toxicity studies conducted in rats and dogs, the severely toxic dose to 10% of animals (STD10) was determined to be 75 mg/kg b.i.d (Investigator Brochure Rigosertib Sodium, Version 16, Onconova Therapeutics, Inc.). To determine the initial starting dose in humans, a safety factor of 10 was applied to the STD10 in rats, leading to a Human Equivalent Dose of 12.2 mg/kg or a 73 mg flat dose for a 60 kg adult. Thus, 70 mg twice daily was deemed to be a safe, non-toxic clinical starting dose level.
This phase I trial was conducted in three consecutive parts: In Part I, the bioavailability and tolerability of oral rigosertib administered as single weekly escalating doses in a fasting state were determined in 3 patients. All 3 patients received a 70 mg single oral dose and blood samples for pharmacokinetic analyses were obtained. In the absence of drug-related grade ≥2 toxicity, single dosing was escalated weekly in each patient to 140 mg (week 2), 280 mg (week 3), 560 mg (week 4) and 700 mg (week 5). At the end of Part I (week 6), all 3 patients were eligible to proceed to Part II of the trial.
In Part II, we determined the MTD of oral rigosertib administered in a fasting state twice a day for 14 consecutive days of each 21-day cycle using an adaptive design. Toxicities were graded according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 (Trotti, et al 2003). Dose-limiting toxicity (DLT) and toxicity assessments were conducted throughout the study, along with the assessment of overall adverse events. A DLT for dose escalation purposes was defined as any grade ≥3 serious adverse event (SAE) at least possibly related to rigosertib occurring within 28 days of the last dose. The starting dose of rigosertib was 70 mg. Initially, patients were enrolled in 2-patient cohorts. In the absence of drug-related grade ≥2 toxicity in 2 patients treated for a complete 21-day first cycle (14 days of treatment followed by 7 days of rest), the next 2 patients received a dose escalated by 100% of the prior dose (i.e. from 70 mg to 140 mg; the next dose levels were 280, 560 and 700 mg). If drug-related grade ≥2 toxicity was observed in at least 1 of the 2 patients treated for a full 21-day cycle, the cohort was expanded to 3 evaluable patients treated for an entire 21-day first cycle. If no DLT was observed in the first 3 patients treated for an entire 21-day first cycle, then the next 3 patients were enrolled at the next dose level. If one DLT was observed in the first 3 patients treated for an entire first 21-day cycle, then the next 3 patients were enrolled at the same dose level. If no more than one DLT was observed in 6 patients treated for an entire 21-day cycle, then the next 6 patients were treated at the next dose level. If 2 or more patients in any cohort experienced a DLT, then the MTD was deemed to have been exceeded and no further dose escalation occurred. The MTD was then defined as the immediate prior dosing level.
In Part 3, an expansion cohort, comprising an additional 22 patients, was enrolled at the MTD dose level. Twelve of these patients underwent the following procedures to assess the effect of food on absolute bioavailability of the oral formulation: On Day 1, patients were dosed with rigosertib 800 mg/m2 IV over 24 h and pharmacokinetic analysis was performed. After a washout period of 1 day (i.e., on the morning of Day 3) and after an overnight fast of at least 10 h, patients were given 560 mg oral rigosertib with 240 ml (8 fluid ounces) of water and pharmacokinetic analysis was performed. On the morning of the next day (i.e. on Day 4), after an overnight fast of at least 10 h, patients were fed a high-fat meal and then given 560 mg oral rigosertib, again followed by pharmacokinetic analysis. Daily oral dosing at the MTD level (560 mg b.i.d.) then resumed on Day 5 of the first 21-day cycle and further cycles were administered until disease progression, absence of bone marrow response or haematological improvement after 16 weeks, or unacceptable toxicity.
Pharmacokinetics (PK)
Rigosertib concentrations in plasma were determined using a Micromass Quattro LC triple-quadrupole mass spectrometric detector (Beverly, MA, USA) with electrospray positive ionization (Li, et al 2007). Levels of rigosertib were determined at specified time points in the PK profile. PK parameters were calculated from rigosertib concentration-time data using standard non-compartmental methods as implemented in WinNonlin software (Pharsight Corp., Mountain View, CA). The maximum plasma concentration (Cmax) and time to reach Cmax (Tmax) were the observed values. The area under the plasma concentration-time curve (AUC) value was calculated to the last quantifiable sample (AUClast) by using the linear trapezoidal rule. The AUC values were further extrapolated to infinity (AUC0–∞) by dividing the last quantifiable concentration by the terminal disposition rate constant (λz), which was determined from the slope of the terminal phase of the concentration-time profile. The terminal half-life (t1/2) was calculated as 0.693 divided by λz.
Response assessment
Clinical assessments (vital signs, ECOG Performance Status, complete physical examinations and clinical laboratory assessments) were performed at screening, on day 1, and every 3 weeks during treatment as well as at study completion (at least 30 days after study initiation). Bone marrow aspirates and biopsies as well as cytogenetics were obtained every 9 weeks. The response rates and the bone marrow blast- and haematopoietic responses (haematological improvement-erythroid, HI-E; haematological improvement-neutrophils, HI-N; haematological improvement-platelets, HI-P) rates were determined according to IWG 2006 criteria (Cheson, et al 2006). In patients with low/intermediate-1 risk disease, haematological improvement (HI) criteria were applied and in patients with intermediate-2/higher risk, IPSS disease altering criteria were used (Greenberg, et al 1997, Greenberg, et al 2012). The duration of stable disease (SD) was measured from the start of the treatment until the criteria for either disease progression or partial/complete remission were met. Duration of overall response was measured from when the time measurement criteria were met for complete or partial remission (whichever was first recorded) until the first date that recurrent or progressive disease was objectively documented.
Data analysis
Demographic data and summary statistics were used to describe the study population. Safety data including laboratory parameters and adverse events were tabulated for all patients by organ system, severity, and relation to treatment. Overall survival was estimated using the Kaplan-Meier method (Kaplan and Meier 1958). Analysis of the primary safety endpoint was performed on all enrolled patients who received at least one dose of rigosertib. Only patients who had received at least 8 weeks of rigosertib treatment were included in the efficacy analysis.
Results
Patients
Thirty-seven patients (23 males, 14 females) with a median age of 74 years (range 53 to 89 years) were enrolled on the study between August 2010 and March 2011 (Table I). Subjects included 16 patients with refractory anameia with excess blasts (RAEB-1, n=7 or RAEB-2, n=9) and 21 patients with refractory cytopenia with multilineage dysplasia (RCMD). IPSS categories included low (n=7), intermediate-1 (n=16), intermediate-2 (n=10) or high (n=4) risk. When we retrospectively applied the recently revised IPSS scoring system (R-IPSS), 5 cases of intermediate-1 risk were reclassified as high (n=4) or very high (n=1) risk (Greenberg, et al 2012). Twenty-seven patients (73%) had received prior treatment with azanucleosides (azacitidine and/or decitabine).
Table I.
Patient and disease characteristics
| Rigosertib b.i.d. Dosing (mg) for 2 out of 3 weeks | Total | |||||
|---|---|---|---|---|---|---|
| 70 | 140 | 280 | 560 | 700 | ||
| Patients (n) | 3 | 2 | 2 | 24 | 6 | 37 |
| Sex | 3F | 1M/1F | 2M | 15M/9F | 5M/1F | 23M/14F |
| Age range (years) | 64–82 | 74–84 | 73–76 | 56–89 | 53–82 | 53–89 |
| Duration of treatment (weeks) | 4–18 | 9–34 | 11–31 | 1–53+ | 5–17 | 1–53+ |
| Prior azacitidine/decitabine treatment | 3 | 2 | 2 | 15 | 5 | 27 |
The study population included 23 male and 14 female patients with myelodysplastic syndrome (MDS), with a median age of 74 years (range 53 to 89 years). The study included 16 (43%) patients with refractory anaemia with excess blasts (RAEB)-1 or -2, and 21 (57%) patients with refractory cytopenia with multilineage dysplasia, as defined by the World Health Organization classification of MDS (Vardiman et al 2009). In terms of IPSS scores, 23 (62%) patients were in the low or intermediate-1 risk categories, and 14 (38%) patients were in the intermediate-2 or high-risk categories. The majority (73%) of patients had been previously treated with azanucleosides (azacitidine and/or decitabine). F, female; M, male.
The first 3 patients were treated on Part 1 (Pharmacokinetics) of the study and received single weekly escalating doses of oral rigosertib. These patients then proceeded to Part 2 of the study (Dose-escalation), which included an additional 12 patients. Once the MTD was determined, 22 additional patients were entered onto Part 3 of the trial and treated at the recommended phase II dose of oral rigosertib (Expansion cohort). In addition, 12 of the patients in the expansion cohort underwent further testing to determine the effects of food intake on PK and bioavailability of oral rigosertib.
Pharmacokinetic analysis
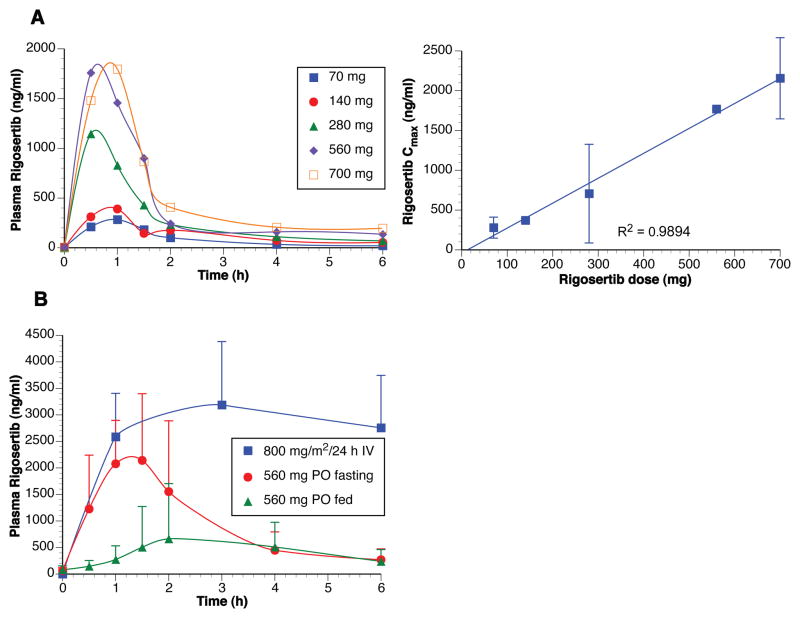
Following a single fasting dose, oral rigosertib was rapidly absorbed (Tmax ~ 1 h). Systemic exposure increased in a linear and dose-proportional manner over the dose range of 70–700 mg (Figure 1A). The elimination half-life from plasma (2.79 ± 1.23 h) was comparable to estimates obtained after intravenous dosing (3.25 ± 0.97 h). The absolute bioavailability of rigosertib (560 mg dose) was 35%. Food significantly reduced the rate and extent of rigosertib absorption. Oral administration of rigosertib after a meal decreased Cmax and AUC by 77% and 61%, respectively, compared to fasting conditions, with an estimated bioavailability of 14% (Figure 1B).
Figure 1. Pharmacokinetics of oral rigosertib.
A. Three patients were treated with single escalating weekly doses of oral rigosertib to determine pharmacokinetics. Data are shown for one patient as the profiles were similar for all 3 patients. Following a single oral dose (fasting) the rigosertib pharmacokinetics were linear and dose-proportional over the dose range of 70–700 mg. B. Bioavailability of oral rigosertib (560 mg), under fasting and fed conditions, was compared to that of intravenously administered rigosertib (800 mg/m2/24 h) in 12 patients in the expansion cohort.
Safety
All 37 patients entered onto the study were evaluable for toxicity. The incidence of grade 2 or 3 non-haematological toxicity attributable to rigosertib was dose-dependent. Grade 2–3 toxicity was observed in 17 of 37 (46%) patients overall, and in 11 of 24 (46%) of the patients who received the recommended phase II dose of 560 mg b.i.d. (Table II). As shown in Table III, toxicity included predominantly urinary symptoms (dysuria, haematuria, urinary frequency, nocturia and cystitis; 12 patients), abdominal pain and/or diarrhoea (4 patients), fatigue (2 patients), hypotension and syncope (1 patient), and anorexia (1 patient). Five patients experienced grade 3 non-haematological toxicity, including urinary symptoms (3 patients), hypotension and syncope (1 patient), fatigue (1 patient) and abdominal pain (1 patient). Six patients experienced more than one type of grade 2–3 toxicity. Three patients experienced a drug-related serious adverse event (SAE). One patient experienced two DLTs at the 700 mg dose level during the first 3-week cycle (dysuria and shortness of breath). Another patient at this dose level had grade 3 dysuria during cycle 2. Thus, the recommended phase II dose was identified as 560 mg b.i.d.
Table II.
Grade 2 or 3 toxicity attributable to rigosertib by dose level
| Initial dose level (mg) | Patients treated | Patients with grade 2 or 3 drug-related toxicity (%) |
|---|---|---|
| 70 | 3 | 1 (33) |
| 140 | 2 | 0 |
| 280 | 2 | 0 |
| 560 | 24 | 11 (46) |
| 700 | 6 | 5 (83) |
| All | 37 | 17 (46) |
Table III.
Grade 2 or 3 non-haematological toxic events attributed to rigosertib
| Rigosertib b.i.d. dosing | 560 mg (n=24) | 700 mg (n=6) | ||
|---|---|---|---|---|
| Grade 2 | Grade 3 | Grade 2 | Grade 3 | |
| Dysuria | 4 | 1 | 3 | 1 (DLT) |
| Haematuria | 4 (1 SAE) | 1 | 1 | 1 |
| Urinary Frequency | 0 | 1 | 0 | 0 |
| Nocturia | 0 | 0 | 1 | 0 |
| Cystitis | 0 | 0 | 1 | 0 |
| Diarrhoea | 1 | 1 | 0 | 0 |
| Abdominal pain | 0 | 1 (SAE) | 0 | 0 |
| Hypotension, syncope | 0 | 0 | 0 | 1 (SAE) |
| Shortness of breath | 0 | 0 | 0 | 1 (DLT) |
| Fatigue | 1 | 1 | 0 | 0 |
| Anorexia | 0 | 0 | 1 | 0 |
DLT, dose-limiting toxicity; SAE, severe adverse event.
Overall, grade ≥2 urinary toxicity occurred in 7 of 24 (29%) patients treated at the 560 mg b.i.d. dose level and in 5 of 6 (83%) patients treated at the 700 mg b.i.d. dose level, suggesting a dose-dependent effect. The onset of urinary symptoms occurred at a median of 3–5 weeks and necessitated holding treatment and/or reducing the rigosertib dose in 6 patients or discontinuing treatment altogether in 7 patients. Two patients underwent cystoscopy to evaluate haematuria. One case revealed diffuse erythaema of the bladder mucosa consistent with cystitis, while the second one revealed normal bladder mucosa.
Efficacy
The median duration of rigosertib treatment was 15 weeks (range 1–53+ weeks). Responses were assessed according to the modified IWG 2006 criteria (Cheson, et al 2000, Cheson, et al 2006). The median time to progression was 16 weeks for the intermediate-2/high risk group and had not been reached for the low/intermediate-1 risk group at the time of analysis. When patients were reclassified according to the revised IPSS (Greenberg, et al 2012), the median time to progression was 18 weeks for the high/very high-risk group, and had not been reached for the low/intermediate risk group. Eleven patients discontinued treatment because of disease progression (2 prior to re-evaluation at 8 weeks), 7 patients because of lack of response, 8 patients because of toxicity, and 1 patient because of a new diagnosis of bladder cancer. In addition, 2 patients died on study and 2 patients withdrew. At the time of analysis, 5 patients still remained on study for 32+ to 55+ weeks.
Repeated bone marrow blast counts were available for 8 patients with RAEB-1 or -2 MDS. Bone marrow blast reductions were seen in 5 of these patients. The 2 patients with RAEB-1 and intermediate-1 risk disease achieved a bone marrow complete response (mCR). The first patient eventually developed progressive disease to AML at 37 weeks, while the second patient (high-risk by R-IPSS) was alive and well at 55+ weeks follow-up. Two out of 8 (25%) evaluable patients with intermediate-2 MDS achieved a major and a minor cytogenetic response, respectively. Both patients were classified as high-risk by R-IPSS. Of the 16 evaluable patients with RCMD, only 2 (12.5%) had marrow blast increases while on rigosertib treatment (to 9% and 22% respectively).
Twelve evaluable patients who were transfusion dependent at study entry were treated with oral rigosertib at 560 mg b.i.d. (n=10) or 700 mg b.i.d. (n=2). The transfusion requirement decreased by >25% in 8 (75%) of these patients. Moreover, 4 of these patients achieved true red blood cell transfusion independence during rigosertib treatment that lasted a median of 12.5 weeks (range: 8–24 weeks). One additional patient achieved an erythroid response lasting 18 weeks (Patient 29). Finally, two of 15 neutropenic patients achieved a neutrophil response lasting 27 and 22 weeks, respectively, and two of 26 thrombocytopenic patients achieved a platelet response lasting 79+ and 27 weeks, respectively. Prior treatment with an ESA was not predictive of transfusion independence or haematological improvement in response to rigosertib (data not shown).
Discussion
The pharmacokinetic profile of oral rigosertib highlights the potential for oral delivery of rigosertib, which might be preferred by patients over continuous intravenous infusion. Moreover, the lower bioavailability of the oral compared to the intravenous formulation of rigosertib did not appear to affect toxicity or clinical activity of the drug, suggesting that prolonged exposure achieved with repeated oral dosing rather than peak plasma levels may be a key determinant of its pharmacodynamic and therapeutic effects.
Oral rigosertib was moderately well tolerated. The predominant clinically significant (grade ≥2) toxicity encountered was cystitis, associated with symptoms of dysuria, urinary frequency and haematuria, which accounted for one of the two DLTs. The effects of rigosertib on the urothelium were clearly dose-dependent, as urinary toxicity was not reported at the lowest three dose levels, and was more common at the 700 mg b.i.d. than at the 560 mg b.i.d. dose levels. Urothelial toxicity is most probably due to a direct effect of rigosertib, which is not metabolized in vivo and is excreted largely unchanged in the urine (Li, et al 2007). This probably results in prolonged exposure of the urothelium to active drug. Cystoscopy performed in two patients revealed generalized mucosal inflammation in one and normal mucosa in the other. Precisely how rigosertib causes inflammation of the bladder mucosa is not clear at this time and continues to be investigated.
Intravenous and oral rigosertib share a remarkable lack of myelosuppression in these heavily pretreated patients despite suppression of bone marrow blast percentages, suggesting that the drug has limited suppressive effects on the normal haematopoietic reserve and a favourable therapeutic index in this patient population (Olnes, et al 2012, Seetharam, et al 2012). Moreover, we found no relationship between prior treatment with azanucleosides, lenalidomide, cytotoxic or ESAs and subsequent rigosertib-induced toxicity (data not shown).
Finally, our study suggests that oral rigosertib, like the intravenous formulation, has activity in patients with MDS who have failed to respond to azanucleoside therapy. Bone marrow complete responses, cytogenetic responses, transfusion-independence and haematological improvements were observed. Within the context of this phase I study, we cannot be certain whether the reduction in transfusion requirement in response to rigosertib was dependent on concurrent ESA treatment or not. A phase II trial to further clarify this interaction is currently underway.
When we applied the revised IPSS (Greenberg, et al 2012), 5 cases of intermediate-1 risk were reclassified as high (n=4) or very high (n=1) risk. When we then reanalysed the response data using the R-IPSS, one of the two patients who achieved a mCR was reclassified as being in the high-risk category. Moreover, both patients who achieved a cytogenetic response were in the high-risk category. On the other hand, all 7 patients who achieved transfusion independence and/or haematological improvement were reclassified as having low or intermediate risk disease. Thus, patients with both high and low risk MDS appear to have benefitted from oral rigosertib treatment.
In summary, oral rigosertib has adequate and predictable bioavailability in a fasting state, can be administered safely to patients with MDS, and displays a favourable toxicity profile that overlaps, at least in part, with that of the intravenous formulation (Olnes, et al 2012, Seetharam, et al 2012). Moreover, preliminary evidence of clinical activity with bone marrow blast suppression and haematological and cytogenetic improvements is encouraging, and appears to be comparable to the experience with the intravenous formulation (Olnes, et al 2012, Seetharam, et al 2012). Given the practical advantages of an oral agent in terms of patient acceptance and the potential to be administered over extended periods of time, oral rigosertib deserves further investigation for the treatment of MDS.
Acknowledgments
We acknowledge the contributions of Stephen Cosenza, Edward O’Rourke and Daniel Fox who assembled the rigosertib IND, Isabelle Darnis-Wilhelm who analysed overall rigosertib safety data and of Michael Reiss, who provided medical writing services on behalf of Onconova, Inc.
Funding: This study was funded by Onconova Therapeutics, Inc.
Footnotes
Author contributions:
RK, AL, FW, and MM designed the research study. RK, AR, and JL carried out the study. CR contributed essential reagents. RK, FW, MM and DT performed data analysis. RK, AL, FW, and MM wrote the manuscript.
Competing interest disclosures: CR, MM and FW are paid employees of Onconova Therapeutics, Inc. The other authors have no conflict of interest to declare.
Personal financial interests: FW owns Onconova Therapeutics, Inc. stock options.
Employment: CR, MM and FW are paid employees of Onconova Therapeutics, Inc. The other authors have no conflict of interest to declare.
Patent rights: None
References
- Chapman CM, Sun X, Roschewski M, Aue G, Farooqui M, Stennett L, Gibellini F, Arthur D, Perez-Galan P, Wiestner A. ON 01910.Na is selectively cytotoxic for chronic lymphocytic leukemia cells through a dual mechanism of action involving PI3K/AKT inhibition and induction of oxidative stress. Clinical Cancer Research. 2012;18:1979–1991. doi: 10.1158/1078-0432.CCR-11-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheson BD, Bennett JM, Kantarjian H, Pinto A, Schiffer CA, Nimer SD, Lowenberg B, Beran M, de Witte TM, Stone RM, Mittelman M, Sanz GF, Wijermans PW, Gore S, Greenberg PL. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood. 2000;96:3671–3674. [PubMed] [Google Scholar]
- Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, Pinto A, Beran M, de Witte TM, Stone RM, Mittelman M, Sanz GF, Gore SD, Schiffer CA, Kantarjian H. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–425. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- Fenaux P. Myelodysplastic syndromes: From pathogenesis and prognosis to treatment. Seminars in Hematology. 2004;41:6–12. doi: 10.1053/j.seminhematol.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, Schoch R, Gattermann N, Sanz G, List A, Gore SD, Seymour JF, Bennett JM, Byrd J, Backstrom J, Zimmerman L, McKenzie D, Beach C, Silverman LR. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncology. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, Sanz M, Vallespi T, Hamblin T, Oscier D, Ohyashiki K, Toyama K, Aul C, Mufti G, Bennett J. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- Greenberg PL, Attar E, Bennett JM, Bloomfield CD, De Castro CM, Deeg HJ, Foran JM, Gaensler K, Garcia-Manero G, Gore SD, Head D, Komrokji R, Maness LJ, Millenson M, Nimer SD, O’Donnell MR, Schroeder MA, Shami PJ, Stone RM, Thompson JE, Westervelt P. NCCN Clinical Practice Guidelines in Oncology: myelodysplastic syndromes. Journal of the National Comprehensive Cancer Network: JNCCN. 2011;9:30–56. doi: 10.6004/jnccn.2011.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Sole F, Bennett JM, Bowen D, Fenaux P, Dreyfus F, Kantarjian H, Kuendgen A, Levis A, Malcovati L, Cazzola M, Cermak J, Fonatsch C, Le Beau MM, Slovak ML, Krieger O, Luebbert M, Maciejewski J, Magalhaes SM, Miyazaki Y, Pfeilstocker M, Sekeres M, Sperr WR, Stauder R, Tauro S, Valent P, Vallespi T, van de Loosdrecht AA, Germing U, Haase D. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–2465. doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumireddy K, Reddy MV, Cosenza SC, Boominathan R, Baker SJ, Papathi N, Jiang J, Holland J, Reddy EP. ON01910, a non-ATP-competitive small molecule inhibitor of Plk1, is a potent anticancer agent. Cancer Cell. 2005;7:275–286. doi: 10.1016/j.ccr.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, Lister TA, Bloomfield CD. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. Journal of Clinical Oncology. 1999;17:3835–3849. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- Jabbour E, Garcia-Manero G, Batty N, Shan J, O’Brien S, Cortes J, Ravandi F, Issa JP, Kantarjian H. Outcome of patients with myelodysplastic syndrome after failure of decitabine therapy. Cancer. 2010;116:3830–3834. doi: 10.1002/cncr.25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimeno A, Li J, Messersmith WA, Laheru D, Rudek MA, Maniar M, Hidalgo M, Baker SD, Donehower RC. Phase I study of ON 01910.Na, a novel modulator of the Polo-like kinase 1 pathway, in adult patients with solid tumors. J Clin Oncol. 2008;26:5504–10. doi: 10.1200/JCO.2008.17.9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarjian HM, O’Brien S, Shan J, Aribi A, Garcia-Manero G, Jabbour E, Ravandi F, Cortes J, Davisson J, Issa JP. Update of the decitabine experience in higher risk myelodysplastic syndrome and analysis of prognostic factors associated with outcome. Cancer. 2007;109:265–273. doi: 10.1002/cncr.22376. [DOI] [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- Komrokji RS, Sekeres MA, List AF. Management of lower-risk myelodysplastic syndromes: the art and evidence. Current Hematologic Malignancy Reports. 2011;6:145–153. doi: 10.1007/s11899-011-0086-x. [DOI] [PubMed] [Google Scholar]
- Li J, Zhao M, Jimeno A, He P, Ramana Reddy MV, Hidalgo M, Donehower RC, Rudek MA. Validation and implementation of a liquid chromatography/tandem mass spectrometry assay to quantitate ON 01910.Na, a mitotic progression modulator, in human plasma. Journal of Chromatography B. 2007;856:198–204. doi: 10.1016/j.jchromb.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K, Reljic T, Kumar A, Lancet JE, List AF, Komrokji RS. Poor outcome of patients with myelodysplastic syndrome (MDS) after azacitidine treatment failure. Blood (ASH Annual Meeting Abstracts) 2010;116:2913. doi: 10.1016/j.clml.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Olnes MJ, Shenoy A, Weinstein B, Pfannes L, Loeliger K, Tucker Z, Tian X, Kwak M, Wilhelm F, Yong AS, Maric I, Maniar M, Scheinberg P, Groopman J, Young NS, Sloand EM. Directed therapy for patients with myelodysplastic syndromes (MDS) by suppression of cyclin D1 with ON 01910.Na. Leukemia Research. 2012;36:982–989. doi: 10.1016/j.leukres.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oussenko IA, Holland JF, Reddy EP, Ohnuma T. Effect of ON 01910.Na, an anticancer mitotic inhibitor, on cell-cycle progression correlates with RanGAP1 hyperphosphorylation. Cancer Research. 2011;71:4968–4976. doi: 10.1158/0008-5472.CAN-10-1603. [DOI] [PubMed] [Google Scholar]
- Prasad A, Park IW, Allen H, Zhang X, Reddy MV, Boominathan R, Reddy EP, Groopman JE. Styryl sulfonyl compounds inhibit translation of cyclin D1 in mantle cell lymphoma cells. Oncogene. 2009;28:1518–1528. doi: 10.1038/onc.2008.502. [DOI] [PubMed] [Google Scholar]
- Prebet T, Gore SD, Esterni B, Gardin C, Itzykson R, Thepot S, Dreyfus F, Rauzy OB, Recher C, Ades L, Quesnel B, Beach CL, Fenaux P, Vey N. Outcome of high-risk myelodysplastic syndrome after azacitidine treatment failure. Journal of Clinical Oncology. 2011;29:3322–3327. doi: 10.1200/JCO.2011.35.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy MV, Venkatapuram P, Mallireddigari MR, Pallela VR, Cosenza SC, Robell KA, Akula B, Hoffman BS, Reddy EP. Discovery of a clinical stage multi-kinase inhibitor sodium (E)-2-{2-methoxy-5-[(2′,4′,6′-trimethoxystyrylsulfonyl)methyl]phenylamino}acetate (ON 01910.Na): synthesis, structure-activity relationship, and biological activity. Journal of Medicinal Chemistry. 2011;54:6254–6276. doi: 10.1021/jm200570p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seetharam M, Fan AC, Tran M, Xu L, Renschler JP, Felsher DW, Sridhar K, Wilhelm F, Greenberg PL. Treatment of higher risk myelodysplastic syndrome patients unresponsive to hypomethylating agents with ON 01910.Na. Leukemia Research. 2012;36:98–103. doi: 10.1016/j.leukres.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman LR, Raza A, Sloand EM, Greenberg PL, Wilhelm FE. Overall survival In myelodysplastic syndrome or acute myeloid leukemia patients treated with ON 01910.Na correlates with bone marrow blast response. Blood (ASH Annual Meeting Abstracts) 2010;116:3998. [Google Scholar]
- Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN, Rubin P. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Seminars in Radiation Oncology. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellstrom-Lindberg E, Tefferi A, Bloomfield CD. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]