Abstract
The International Prognostic Scoring System (IPSS) was recently revised (IPSS-R) under the auspices of the MDS Foundation as a collaborative international effort to refine its prognostic power. Our purpose was to externally validate this new risk model using a large single-institution cohort, determine its prognostic power in patients receiving active treatment, and explore its utility in guiding therapeutic decisions. Data were collected retrospectively from our myelodysplastic syndrome (MDS) database and verified by chart review. Of the data available for 1,088 patients, 152 (14%), 353 (32%), 237 (22%), 190 (18%), and 156 (14%) patients were classified as very low, low, intermediate, high, and very high risk, respectively, with median overall survival (OS) of 90 (95%CI 71–109), 54 (95%CI 50–59), 34 (95%CI 26–43), 21 (95%CI 17–25), and 13 months (95%CI 11– 15), respectively (P < 0.005). We found that the IPSS-R further refined prognostic discrimination in all IPSS risk categories, particularly in the intermediate 1 and 2 groups. Among high and very high IPSS-R patients receiving azacitidine, OS was significantly improved versus patients not receiving azacitidine, with corresponding median OS of 25 versus 18 months (P = 0.028) and 15 versus 9 months (P = 0.005), respectively. Similarly, patients with IPSS-R high- and very high-risk disease who underwent allogeneic hematopoietic stem cell transplantation had significantly improved OS versus nontransplant approaches (P < 0.005). High and very high IPSS-R patients derived a survival advantage from disease-modifying therapies. Our data validate the prognostic value of the proposed IPSS-R and show that its refined IPSS prognostic discrimination can be applied to actively treated patients.
Introduction
Myelodysplastic syndromes (MDS) comprise a heterogeneous spectrum of hematopoietic stem cell malignancies with variable clinical presentation and risk for transformation to acute myeloid leukemia (AML) [1]. In an effort to appropriately tailor therapeutic decisions to disease outcome, prognostic risk models have been utilized [2]. The International Prognostic Scoring System (IPSS) has been the most widely adopted tool in clinical practice for risk stratification of untreated MDS patients [3]. Covariates for prognostic discrimination include percentage of bone marrow blasts, cytogenetic abnormalities, and number of cytopenias at the time of initial diagnosis, which distinguish four prognostic categories with differences in overall survival (OS) and rate of AML transformation, that is, low-risk, intermediate-1, intermediate-2, and high-risk disease [3]. Contemporary treatment paradigms are adapted to IPSS risk category. In patients with “lower-risk” MDS, encompassing both low and intermediate-1 IPSS risk categories, treatment objectives are to improve hematopoiesis, cytopenias, and quality of life. Alternatively, in patients with “higher risk” MDS, including both intermediate-2 and high-risk categories, the goals of treatment are to alter disease natural history by extending OS and suppressing leukemia potential using disease-modifying treatment strategies such as azanucleosides and hematopoietic cell transplantation [4].
The IPSS was recently revised (IPSS-R) under the auspices of the MDS Foundation as a collaborative international effort [5]. The proposed IPSS-R is intended to further refine the prognostic value of IPSS by capturing additional prognostic elements and chromosomal abnormalities to generate five distinct prognostic categories. The revised tool utilizes the IPSS platform, with the addition of seven chromosomal abnormalities, to create five cytogenetic risk groups, further discrimination of the percentage of bone marrow blasts, and incorporation of the severity of each cytopenia. The IPSS-R was developed using a large cohort of patients who were either untreated or received primarily supportive therapy such as hematopoietic growth factors.
The primary objective of our study was to externally validate this new risk model using a large single-institution cohort, determine its prognostic power in patients receiving active treatment, and explore its utility in guiding therapeutic decisions.
Patients and Methods
Data were collected retrospectively from the Moffitt Cancer Center (MCC) MDS database and verified by chart review after institutional Internal Review Board approval. All patients diagnosed according to World Health Organization (WHO) 2000 criteria were included. A total of 1,158 patients with these criteria were identified as having MDS in the MCC database. The IPSS-R was calculated as published using the IPSS-R calculator found on the MDS Foundation website (http://www.mds-foundation.org/ipss-r-calculator/). The score totals were generated from blast percentage, cytogenetic category, hemoglobin value, platelet count, and absolute neutrophil count (ANC). Two authors (AM, MCY) independently calculated the score for the entire cohort; discrepancies in score were resolved by consensus, which included recalculation of the score by two other investigators (NHA, RSK). We had insufficient data for 70 patients to adequately calculate the IPSS-R score; therefore, those patients were excluded from the analysis.
Patients were divided into five prognostic categories based on the aggregate scores: very low (≤1.5 points), low (>1.5–3 points), intermediate (>3–4.5 points), high (>4.5–6 points), and very high-risk groups (>6 points) [5]. IPSS scores [3] and MD Anderson scores [6] were calculated as previously reported. Red blood cell transfusion dependence was defined as requiring 2 or more units of packed red blood cells every 8 weeks. All analyses were conducted using SPSS version 16.0 (SPSS Inc., Chicago, IL). The Kaplan–Meier method was used to estimate median OS. Log-rank test was used to compare Kaplan– Meier survival estimates between the groups. Cox regression was used for multivariable analysis.
Results
Between January 2001 and May 2012, the MCC MDS database captured 1,158 patients. Necessary data to calculate the IPSS-R score were available for 1,088 of those patients. Table 1 summarizes the baseline characteristics. The median duration of follow up was 70 months and the median age at diagnosis was 68 years, with greater than 77% of patients older than 60 years of age. Greater than two-thirds of the patients were categorized as low/intermediate-1 risk according to IPSS and 33% were intermediate-2 or high risk. The most common WHO subtype was refractory cytopenia with multilineage dysplasia (30%). Among all patients analyzed, 833 (77%) were red blood cell transfusion dependent, and 281 (26%) had a serum ferritin >1,000 ng/L. Of the 1,086 patients, 649 (60%) received a hypomethylating agent (HMA), with 580 patients receiving azacitidine (53%).
Table 1. Baseline Characteristics.
| Characteristic | N = 1088 |
|---|---|
| Age (Yr) | |
| ≤60 | 240 (22.1%) |
| >60 | 848 (77.9%) |
| Gender | |
| Male | 717 (65.9%) |
| Female | 371 (34.1%) |
| Race | |
| White | 1009 (92.7%) |
| WHO | |
| RA | 125 (11.5%) |
| RARS | 95 (8.7%) |
| RCMD | 328 (30.1%) |
| Del5q | 32 (2.9%) |
| RAEB-1 | 226 (20.8%) |
| RAEB-2 | 181 (16.6%) |
| MDS-U | 27 (2.5%) |
| CMML | 24 (2.2%) |
| MDS/MPN-U | 46 (4.2%) |
| AML | 4 (<1%) |
| IPSS | |
| Low | 181 (16.6%) |
| Int-1 | 553 (50.8%) |
| Int-2 | 265 (24.4) |
| High | 88 (8.1) |
| Missing | 1 (<1%) |
| MD Anderson score | |
| Low | 194 (17.8%) |
| Int-1 | 435 (39.9%) |
| Int-2 | 236 (21.7%) |
| High | 219 (20.1%) |
| Missing | 4 (<1%) |
| RBC transfusion | |
| No | 255 (23.4%) |
| Yes | 833 (76.6%) |
| Ferritin ≥1000 | |
| No | 658 (60.5%) |
| Yes | 281 (25.8%) |
| Missing | 149 (13.7%) |
| Treated with HMA | |
| No | 437 (40.2%) |
| Yes | 649 (59.7%) |
| Missing | 2 (<1%) |
| Treated with AZA | |
| No | 506 (46.5%) |
| Yes | 580 (53.3%) |
| Missing | 2 (<1%) |
Based on the IPSS risk score, the median OS was 90 [95% confidence interval (CI) 76–105], 44 (95% CI: 39–48), 19 (95% CI: 15–22), and 14 months (95% CI: 11–17), for low, intermediate-1, intermediate-2, and high-risk categories, respectively (P < 0.005). According to the MD Anderson Scoring System, the median OS was 109 (95% CI: 94–123), 55 (95% CI: 50–60), 25 (95% CI: 22–28), and 14 months (95% CI: 12–16), for low, intermediate-1, intermediate-2, and high-risk disease categories, respectively (P < 0.005; Fig. 1A,B).
Figure 1.
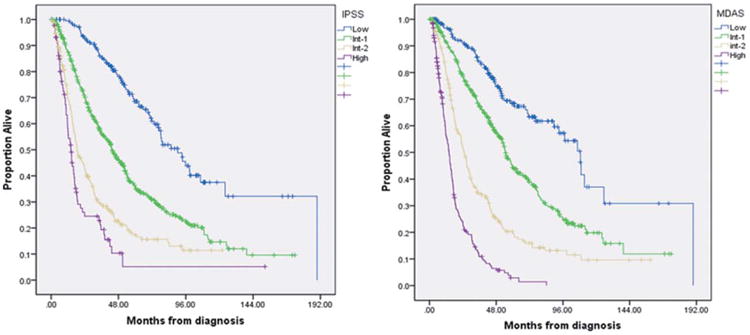
Median OS in patients with MDS based on International Prognostic Scoring System (A) and MD Anderson Scoring System (B). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
According to IPSS-R, 152 (14%), 353 (32%), 237 (22%), 190 (18%), and 156 (14%) patients were classified as very low, low, intermediate, high, and very high risk, respectively. Low-risk IPSS patients were redistributed using IPSS-R as very low risk in 36%, low risk in 59%, and intermediate risk in 5%. Among patients categorized as intermediate-1 by IPSS, 16% were categorized as very low risk, 42% as low risk, 32% as intermediate, and 10% as high risk by IPSS-R. IPSS intermediate-2 patients were redistributed as low risk in 5%, intermediate risk in 20%, high risk in 43%, and very high risk in 32% by IPSS-R. Among high-risk IPSS patients, only 3% were assigned to the intermediate risk category, 21% to high, and 76% to very high risk in IPSS-R. Combining all IPSS “lower risk” (low and intermediate-1) patients, 67% were reclassified as very low and low risk, 25% as intermediate risk, and 8% as high risk by IPSS-R classification. For IPSS “higher risk” (intermediate-2 and high) patients, 81% were assigned to the high- and very high-risk categories, 15% to intermediate risk, and 4% to low by IPSS-R (Table II). The IPSS-R further refined the prognostic power of the IPSS in all risk categories, which was statistically significant, particularly in intermediate-1 and intermediate-2 IPSS (P < 0.005).
Table II. Classification of Each IPSS Risk Group by IPSS-R.
| IPSS | IPSS-R | Median overall survival (months) | P-value | |
|---|---|---|---|---|
| Very low | 65 (36%) | 96 | ||
| Low (n = 181) | Low | 106 (59%) | 93 | 0.03 |
| Int | 10 (5%) | 58 | ||
| Very low | 86 (16%) | 76 | ||
| Int-1 (n = 547) | Low | 232 (42%) | 51 | <0.005 |
| Int | 172 (32%) | 30 | ||
| High | 57 (10%) | 22 | ||
| Low | 13 (5%) | 46 | ||
| Int-2 (n = 265) | Int | 52 (20%) | 41 | <0.005 |
| High | 114 (43%) | 19 | ||
| Very High | 86 (32%) | 13 | ||
| Int | 3 (3%) | 42 | ||
| High (n = 87) | High | 18 (21%) | 24 | 0.02 |
| Very High | 66 (76%) | 12 | ||
The median OS was significantly different for each IPSS-R prognostic category, including 90 months in very low risk (95% CI: 71–109), 54 months in low risk (95% CI: 50–59), 34 months (95% CI: 26–43) in intermediate risk, 21 months (95% CI: 17–25) in high risk, and 13 months in very high risk (95% CI: 11–15) patients, respectively (P < 0.005; Fig. 2). Patients with therapy-related MDS (t-MDS) (n = 187, 17%) were also included in the analysis with 16, 36, 48, 46, and 41 patients assigned to the very low, low, intermediate, high, and very high-risk IPSS-R groups, respectively. The median OS was significantly different in each of the IPSS-R risk groups, ranging from 58 months in the very low-risk group, to 35, 30, 18, and 11 months in the remaining respective groups (P < 0.005). In multivariate analysis, IPSS-R [hazard ratio (HR)51.6 (95% CI: 1.5–1.8); P < 0.005], age >60 years [HR=1.3 (95% CI: 1.1–1.7); P = 0.008], serum albumin level [HR=0.7 (95% CI: 0.6– 0.8); P < 0.005], serum ferritin >350 ng/mL [HR=1.3 (95% CI: 1.0–1.5); P = 0.015], and presence of bone marrow fibrosis [grades 2 and 3; HR 51.1 (95% CI: 1.0–1.2); P = 0.045] were independent prognostic factors for OS. The rate of transformation to AML among IPSS-R risk groups was 16%, 19%, 31%, 46%, and 53% for very low, low, intermediate, high, and very high groups, respectively (P < 0.005).
Figure 2.
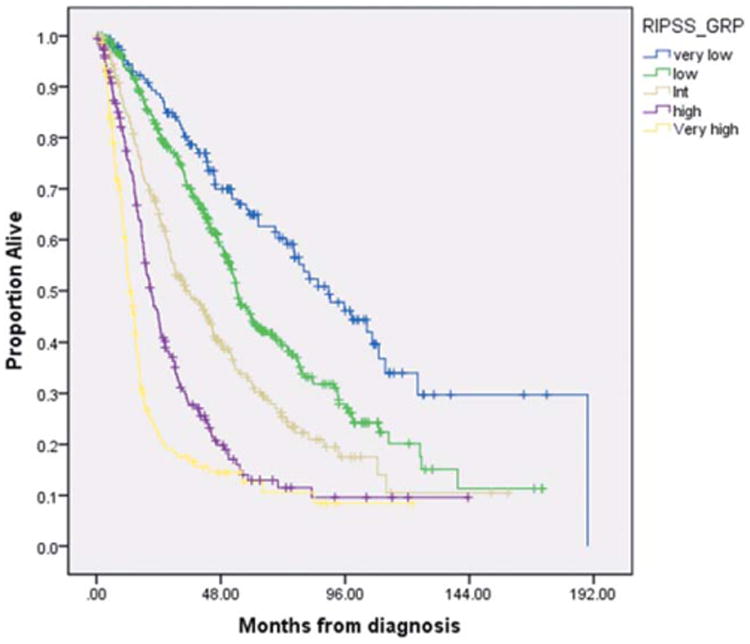
Median OS for patients with MDS based on revised IPSS (IPSS-R). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
To address the therapeutic utility of the IPSS-R, we assessed outcome among each IPSS-R group based on disease-modifying therapy with azacitidine or allogeneic hematopoietic stem cell transplantation (alloHSCT). For patients who were treated with azacitidine (n = 580), the median time from diagnosis to azacitidine initiation was 156 days. The median OS results for azacitidine-treated groups were 78, 55, 43, 25, and 15 months for very low, low, intermediate, high, and very high-risk patients, respectively (P < 0.005). There was a statistically significant survival benefit for those patients treated with azacitidine in the high- and very high-risk IPSS-R groups compared to those who did not receive azacitidine. Median OS was 25 months (95% CI: 20–30) versus 18 months (95% CI: 15–21) (P = 0.028) and 15 months (95% CI: 13–17) versus 9 months (95% CI: 6–12; P = 0.005) in the high- and very high-risk IPSS-R groups, respectively (Table III). Similarly, patients with high- and very high-risk IPSS-R scores who underwent alloHSCT had a better OS than those who were managed solely with nontransplant approaches. The median time from diagnosis to alloHSCT for this group was 255 days. Patients with low or very low risk IPSS-R scores had no discernable survival benefit from alloHSCT, although the total number of patients proceeding to transplant was small (n = 28). There was a trend toward improved OS among patients with intermediate risk disease who received alloHSCT, although statistical significance was not reached (Table IV).
Table III. Impact of AZA Among Each IPSS-R Group.
| IPSS-R | Azacitidine (n) | Median OS in months (95% CI) | P-value |
|---|---|---|---|
| Very Low | Yes (53) | 78 (57–100) | |
| No (99) | 109 (62–156) | 0.16 | |
| Low | Yes (182) | 55 (49–60) | |
| No (170) | 54 (44–64) | 0.685 | |
| Intermediate | Yes (125) | 43 (35–52) | |
| No (112) | 29 (23–35) | 0.153 | |
| High | Yes (119) | 25 (20–30) | |
| No (68) | 18 (15–21) | 0.028 | |
| Very High | Yes (99) | 15 (13–17) | |
| No (57) | 9 (6–12) | 0.005 |
Table IV. Impact of Allo HSCT Among Each IPSS-R Group.
| IPSS-R | alloHSCT (n) | Median OS in months (95% CI) | P-value |
|---|---|---|---|
| Very low | Yes (6) | Not reached (00) | |
| No (146) | 90 (71–108) | 0.550 | |
| Low | Yes (22) | 42 (17–67) | |
| No (330) | 54 (50–59) | 0.307 | |
| Intermediate | Yes (35) | 54 (36–71) | |
| No (202) | 34 (26–42) | 0.079 | |
| High | Yes (42) | 40 (22–57) | |
| No (147) | 19 (16–21) | <0.005 | |
| Very high | Yes (34) | 31 (8–54) | |
| No (122) | 12 (10–14) | <0.005 |
Discussion
Currently, the IPSS is the most widely adopted tool for risk stratification and prognostic assessment in newly diagnosed MDS patients [3]. Application of the IPSS has been useful in tailoring management decisions based on prognosis of disease. Current guidelines recommend alloHSCT for patients with intermediate-2/high-risk IPSS who are candidates for the procedure [4] and treatment with azacitidine, in which there is recognized survival advantage for patients with higher risk MDS [7]. Despite its utility, modifications of the existing parameters and incorporation of new prognostic variables have been recommended to overcome deficiencies of the initial IPSS model [6,8,9]. A limitation of the original IPSS was its development based on a pooled cohort of predominantly newly diagnosed MDS, the majority of whom were treated either with supportive care measures or hematopoietic growth factor support. Moreover, it was not intended to serve as a dynamic model [10], and patients with secondary MDS and proliferative chronic myelomonocytic leukemia (CMML) were excluded [11,12]. In addition, the IPSS did not account for severity of cytopenias or relevant surrogates of anemia severity duration such as red blood cell transfusion dependence and serum ferritin level, respectively.
The collaborative effort by the International Working Group for the Prognosis of MDS used coalesced data from 11 different countries to create a large database from which to develop a refined prognostic tool, known as the IPSS-R [5]. Provisions within this new model, when compared to IPSS, include further stratification of the percentage of bone marrow blasts, five cytogenetic categories versus three in the original model, inclusion of measures of the severity of cytopenias, and inclusion of additional risk categories (5 vs. 4). Patients with secondary or t-MDS were excluded from the analysis because of their perceived distinct disease behavior compared to patients with primary de novo MDS. Patients with proliferative MDS were also excluded from the analysis.
Our study aimed to validate the IPSS-R utilizing a large external institutional cohort of MDS patients who, contrary to the original cohort, had received disease-modifying treatment. Our data demonstrate that the new model refines the prognostic value of the IPSS in all risk categories, with particular improvement in prognostic discrimination for those patients in the intermediate-1 and intermediate-2 IPSS categories. In comparison to the original IPSS-R cohort of patients, our study of treated MDS patients had a higher percentage of high- and very high-risk patients. The most common risk group reported between both studies was low-risk disease [5] (38% and 32%). Interestingly, despite the differences in treatment, OS among each of the risk groups was similar in our study to that which was originally reported by Greenberg et al. [5]. Our inclusion of t-MDS patients (17%) may have obscured any benefit of disease modifying in the overall patient cohort.
The improved risk stratification seen in the IPSS intermediate groups (majority of MDS patients) with the IPSS-R may significantly modify therapeutic decisions. Ten percent (n = 57) of patients who were initially stratified as intermediate-1 by IPSS were instead upstaged to advanced disease by IPSS-R. Conversely, fewer patients with intermediate-2 IPSS had evidence of low-risk disease by IPSS-R (5%, n = 13). Patients previously assigned into intermediate-1 and intermediate-2 categories collectively spanned all five of the IPSS-R categories. Similar findings were also reported by Greenberg et al. [5].
To our knowledge, this is the first study to examine the potential benefit of disease-modifying treatment modalities such as HMA and alloHSCT according to IPSS-R risk modeling. Given that the IPSS-R was expanded to include five risk groups, it is unclear how management should be tailored for these prognostically distinct subgroups. Further investigation is necessary to clarify therapeutic decisions, particularly for management of the intermediate risk group and whether treatment should be tailored toward “lower risk or supportive” strategies versus “higher risk” disease-modifying approaches. A major limitation in assessing therapeutic outcomes using the new model remains a more limited sample size of patients who received disease-altering therapy in each subgroup (e.g., only 35 patients reclassified as intermediate risk group underwent alloHSCT). The IPSS-R was found to be a valid prognostic model for patients treated with HMA, a finding that was also recently reported by the French MDS group [13]. Interestingly, the survival benefit observed in azacitidine-treated patients was statistically significant only in patients with high- and very high-risk IPSS-R categories. Despite the apparent benefit of azacitidine treatment, these analyses are retrospective and cannot account for decision variables of the treating physicians.
We also assessed the benefit of alloHSCT within the IPSS-R groups. Similar to azacitidine-based therapy, the benefit of alloHSCT was observed only in higher-risk patients. Only high (P < 0.005) and very high (P < 0.005) IPSS-R groups showed a statistically significant improvement in the OS. These findings support current recommendations for intensification of therapy with alloHSCT in intermediate-2 and high-risk patients by IPSS early in the disease course [4,14]. Our data also suggest a trend for OS improvement with alloHSCT in patients in the intermediate-risk IPSS-R group. The number of patients who underwent alloHSCT in this group was relatively small (n = 35). Accordingly, treatment decisions should be individualized in this group by considering other risk features such as age, presence of bone marrow fibrosis, serum lactate dehydrogenase level, serum ferritin, or incorporation of molecular data. Further, randomized studies are needed to validate treatment benefits using IPSS-R in this setting.
Despite the apparent refinements, the IPSS-R does have limitations. First, the score is relatively complicated, and its general adoption in the community setting may pose challenges. Second, the distinction between 2% or more bone marrow myeloblasts is not uniformly reported and may lack reliability depending on pathologist expertise and total number of cells enumerated. Furthermore, the IPSS-R did not include patients with secondary MDS or MDS/MPN. Appropriate risk stratification for these patients remains an unmet need. Our data demonstrate a statistically significant difference in median OS in patients with t-MDS, suggesting that the IPSS-R can be applied in this population; however, its utility in MDS/MPN subtypes such as CMML is unclear. Additionally, although further cytogenetic categories have been added to adequately segregate the risk groups and perhaps improve its utility in t-MDS, several new questions and concerns have emerged. How should cytogenetic abnormalities noted in two different clones be assessed? Does the presence of more than one clone confer worse outcome? Additionally, the presence of marker chromosomes and its associated phenotypic implications and malignant potential in MDS are currently unclear [15].
A limitation in evaluating the utility of this model in treated patients includes calculation of the IPSS-R at the time of diagnosis rather than at the time of treatment with disease-modifying therapy. Additionally, in this retrospective analysis, criteria for utilizing one treatment modality versus another were not evaluated. Risk stratification at time of disease modifying therapy may be altered compared to IPSS-R group at the time of diagnosis. However, in our cohort, the median time to treatment for both azacitidine (median=156 days) and alloHSCT (median=255 days) was under 1 year.
In conclusion, our study validates the prognostic value of the IPSS-R using a large external cohort of actively treated MDS patients, including patients who received disease-modifying therapy. The IPSS-R had improved prognostic precision, particularly in patients with intermediate-1 and intermediate-2 risk disease, versus the original IPSS. The utility of IPSS-R as a tool for specific therapeutic assignment requires further investigation; however, our findings suggest that patients who benefit from disease-modifying therapy may be better identified using this tool. Patients classified as high and very high risk drove the OS benefit from alloHSCT and HMA, whereas in patients classified as intermediate risk, treatment must be individualized. Molecular models [16] for evaluating MDS prognosis are promising and in the future may complement IPSS-R.
Acknowledgments
We thank Rasa Hamilton (Moffitt Cancer Center) for editorial assistance.
Footnotes
Conflict of interest: Nothing to report.
Presented in abstract form at the American Society of Hematology 54th Annual Meeting, December 2012, Atlanta, Georgia (Abstract #633).
References
- 1.Bennett JM, Komrokji R, Kouides P. The myelodysplastic syndromes. In: Abel-off MD, Armitage JO, Niederhuber JE, editors. Clinical Oncology. New York: Churchill Livingstone; 2004. pp. 2849–2881. [Google Scholar]
- 2.Komrokji RS, Zhang L, Bennett JM. Myelodysplastic syndromes classification and risk stratification. Hematol Oncol Clin North Am. 2010;24:443–457. doi: 10.1016/j.hoc.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 4.NCCN Clinical Practice Guidelines in Oncology. Myelodysplastic Syndromes (Version 2, 2013) [Accessed December 16, 2012]; // www.nccn.org/professionals/physician_gls/f_guidelines.asp#mds.
- 5.Greenberg P, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–2465. doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kantarjian H, O'Brien S, Ravandi F, et al. Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original International Prognostic Scoring System. Cancer. 2008;113:1351–1361. doi: 10.1002/cncr.23697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malcovati L, Della Porta M, Pascutto C, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: A basis for clinical decision- making. J Clin Oncol. 2005;23:7594–7603. doi: 10.1200/JCO.2005.01.7038. [DOI] [PubMed] [Google Scholar]
- 9.Schanz J, Steidl C, Fonatsch C, et al. Coalesced multicentric analysis of 2,351 patients with myelodysplastic syndromes indicates an underestimation of poor-risk cytogenetics of myelodysplastic syndromes in the international prognostic scoring system. J Clin Oncol. 2011;29:1963–1970. doi: 10.1200/JCO.2010.28.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malcovati L, Germing U, Kuendgen A, et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol. 2007;25:3503–3510. doi: 10.1200/JCO.2006.08.5696. [DOI] [PubMed] [Google Scholar]
- 11.Larson RA. Therapy-related myeloid neoplasms. Haematologica. 2009;94:454–459. doi: 10.3324/haematol.2008.005157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Germing U, Kundgen A, Gattermann N. Risk assessment in chronic myelomonocytic leukemia (CMML) Leuk Lymphoma. 2004;45:1311–1318. doi: 10.1080/1042819042000207271. [DOI] [PubMed] [Google Scholar]
- 13.Ades L, Lamarque M, Raynaud S, et al. Revised-IPSS (IPSS-R) is a powerful tool to evaluate the outcome of MDS patient treated with azacitidine (AZA): The Groupe Francophone Des Myelodysplasies (GFM) Experience. Blood. 2012;120:422. doi: 10.1182/blood-2012-09-453555. abstr. [DOI] [PubMed] [Google Scholar]
- 14.Cutler CS, Lee SJ, Greenberg P, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: Delayed for low-risk myelodysplasia is associated with improved outcome. Blood. 2004;104:579–585. doi: 10.1182/blood-2004-01-0338. [DOI] [PubMed] [Google Scholar]
- 15.Fan YS, editor. Molecular Cytogenetics: Protocols and Applications, Vol 2. Totowa, NJ: Humana Press; 2002. Methods in Molecular Biology. [Google Scholar]
- 16.Bejar R, Stevenson K, Abdel-Wahab O, et al. Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med. 2011;364:2496–2506. doi: 10.1056/NEJMoa1013343. [DOI] [PMC free article] [PubMed] [Google Scholar]


