Abstract
Objective
While valvular endothelial cells (VECs) have unique responses compared to vascular ECs, valvular regulation of hemostasis is not well understood. Heart valves remodel throughout a person's lifetime, resulting in changes in extracellular matrix (ECM) composition and tissue mechanical properties that may affect VEC hemostatic function. This work assessed VEC regulation of hemostasis in situ and in vitro as a function of specimen age.
Approach and Results
Porcine aortic valves (PAV) were assigned into one of three age groups: YNG (6 weeks), ADT (6 months), or OLD (2 years). Histology of valves showed that secreted thrombotic/anti-thrombotic proteins localize at the valve endothelium, and tissue interior. Gene expression and immunostains for von Willebrand factor (VWF), tissue factor pathway inhibitor, and tissue plasminogen activator in YNG PAVECs was higher than in OLD, while plasminogen activator inhibitor-1 levels in OLD were higher than in YNG and ADT. Histamine stimulated YNG PAVECs released higher concentrations of VWF proteins than OLD, while the fraction of cleaved VWF-140 fragments were not different between age groups. A CAVD in vitro model using valvular interstitial cells (VICs) confirmed that VWF in culture significantly increased VIC nodule formation and calcification.
Conclusions
Hemostatic protein regulation in AV tissues and VECs changes with age. The presence of VWF and other potential hemostatic proteins increase VIC calcification in vitro. Therefore, the increased capacity of elderly valves to sequester the hemostatic proteins, together with age-associated loss of ECM organization warrants investigation into potential roles for these proteins in the formation of calcific nodules.
Keywords: aortic valve, valvular endothelial cell, hemostasis, aging, anti-thrombotic
Introduction
Semilunar valve diseases, particularly those affecting the aortic valve (AV), cause >60% of valve disease mortality, and approximately 50,000 procedures to replace or repair semilunar valves are performed each year in the US.1 Semilunar valve dysfunction affects all ages, from congenital valve defects experienced by neonates and children to the growing number of calcified valves in the elderly.1–6 These dysfunctional heart valves most often require surgical replacement using mechanical or bioprosthetic valves that may themselves fail over time due to structural or thrombosis related problems.6,7
In order to understand valvular disease and develop non-invasive therapeutic solutions, it is necessary to improve fundamental knowledge about the response of valve cells and extracellular matrix (ECM) to surrounding environments in various physiological states. The characterization of changes in valvular biology with respect to aging has become especially important, as the occurrence of aortic valve sclerosis and calcification continues to rise in elderly populations.4,5,8
While most studies of valvular biology employ AV tissues and cells from adults (either animal models or human specimens resected at surgery), it is clear that consideration of specimen age is necessary to evaluate age-specific conditions and pathologies. Previous studies from our research group and others have identified numerous age-related changes in the ECM composition and mechanical properties of the AV, as well as in the valve cell phenotypes.6,9–12 Considerable tissue remodeling and growth occurs in AV tissues prior to adulthood.4,11,13 During fetal development, AV tissues have not yet formed tri-laminate ECM structures or high degree of elastin and collagen alignment that is apparent in adult valves.6,11,13 To mediate this microstructural organization of the mature AV, the developing AV also demonstrates cellular activation of both valvular interstitial cells (VICs) and valvular endothelial cells (VECs).6,11,12
As subjects reach adulthood, valve cells become quiescent and maintain homeostasis of the highly organized valve leaflet structure and function.6 However, the AV continues to change with aging. Older valves have increased thickness and increased levels of collagen type III interspersed with proteoglycans and glycosaminoglycans throughout the leaflets.10,14–16 Corresponding to the altered ECM organization in older valves, the stiffness in the circumferential direction increases with age as well.10 Several studies have suggested that the remodeling occurring in elderly AV tissues is mediated by the activation of VICs, which can lead to osteogenic differentiation and ultimately valve calcification.2,6,10,17 However, additional factors including VEC dysfunction, changes in hemodynamics and valve mechanics, and ECM remodeling may also influence calcific formation in valve tissues. Thus, as valves remodel throughout a lifetime, the resulting changes in ECM composition and tissue mechanical properties are likely to affect cellular behavior and increase the risk of valvular diseases.
VECs have been identified to play an important role in maintaining valve homeostasis as well as affect the onset of valvular disease. However, many aspects of VEC function and behavior remain unknown. Recent work has shown that VECs have distinct phenotypes compared to vascular and cardiac endothelial cells, e.g., VECs align perpendicular to the direction of fluid flow, unlike vascular ECs, and have different mechanotransduction pathways and gene expression, likely due to the unique flow and mechanics to which VECs are subjected.18–20 VECs have been observed to have strong interactions with VICs and ECM, and appear to be highly sensitive to their surrounding environments.19,21,22 These cells can undergo endothelial to mesenchymal transdifferentiation, and thus play an important role in valvulogenesis during fetal development.23,24 Furthermore, previous studies have suggested that VEC dysfunction may play a role in initiating valvular diseases.24–27
Despite these links between VECs and valve diseases, little has been done to characterize the fundamental hemostatic behavior of VECs and the relation between this behavior and the valvular ECM in health, disease, and aging conditions. It is well-known that vascular endothelial cells perform substantial anti-thrombotic roles via production and release of tissue plasminogen activator (tPA), tissue factor pathway inhibitor (TFPI), VWF cleaving enzyme (ADAMTS-13), nitric oxide, and heparin.28–30 Endothelial cells are also responsible for the production and release of thrombotic protein mediators such as plasminogen activator inhibitor (PAI-1), tissue factor (TF), von Willebrand factor (VWF), and P-selectin.28–30 Under homeostatic conditions, vascular endothelial cells constitutively express and produce both anti-thrombotic and thrombotic mediators to balance hemostatic effects and prevent thrombus formation. However, blood coagulation and thrombus formation are quickly initiated by endothelial cells in response to mechanical damage, ECM degradation, and disease.28–30 The production and regulation of these hemostatic factors have not been well characterized in VECs.
This study will evaluate the aging-related changes in anti-thrombotic and thrombotic capacity of AV tissues and VECs. We hypothesize that aging affects the VEC regulation of hemostatic proteins and their interaction with valve ECM components, resulting in an unbalanced regulation of anti-thrombotic and thrombotic proteins within the tissues.. Thus, the evaluation of AV tissues and VEC function with respect to anti-thrombotic and thrombotic functions in aging valves will provide insight into the specific factors and conditions that affect VEC hemostatic regulation. This information is also relevant to the development of valve replacement devices, since the breakdown and thrombosis formation associated with mechanical and decellularized valve implants has generated interest in understanding how the natural anti-thrombotic behavior of VECs can be integrated into age-specific tissue engineered heart valves. Overall, there is a critical need to understand the hemostatic behavior of VECs.
Materials and Methods
AV leaflets were dissected from fresh porcine hearts acquired from commercial abattoirs and assigned into one of three age groups: YNG (6 weeks old), ADT (6 month old), or OLD (2 years old). Immunostains for numerous thrombotic and anti-thrombotic markers (Table 1) were performed on the AV leaflets of different ages, and the proportion of the tissue stained was quantified. The same hemostatic markers were also assessed using qRT-PCR and immunocytochemistry on porcine aortic valve endothelial cell (PAVEC) cultures of the three age groups, human umbilical vein endothelial cell (HUVEC) and porcine pulmonary artery endothelial cell (PPAEC) cultures. A sandwich ELISA was performed to quantify levels of VWF protein release and cleavage from histamine stimulated PAVECs, HUVECs, and PPAECs. To assess the effects of VEC-released VWF on calcific nodule formation in vitro, conditioned culture mediums consisting of 3% (v/v) PAVEC stimulation medium supernatant (via histamine stimulation) from the three age groups (YNG, ADT, OLD) were mixed with low serum VIC culture medium, and exposed to porcine aortic valve interstitial cells PAVICs) for 10 days. For study controls, PAVICs were also cultured in VIC low serum medium only, low serum VIC medium with 3% fresh PAVEC stimulation medium and no histamine, and low serum PAVIC medium with 3% fresh PAVEC stimulation medium with 8.4 nM histamine. PAVICs were stained with Alizarin Red S to dye calcified nodules red, and the number nodules and nodule sizes were measured. A full description of the materials and methods is available in the online-only Data Supplement.
Table 1.
Antibodies used for immunohistochemistry and immunocytochemistry
| Protein | Function | Catalog No. | Dilution |
|---|---|---|---|
| Thrombotic proteins | |||
| von Willebrand factor | Binds platelets to form platelet thrombi when in ultralarge multimer form | Ab6994* | 1:200 |
| Tissue Factor | Surface protein expressed by activated endothelial cells to initiate coagulation cascade | SC18712† | 1:50 |
| Plasminogen activator inhibitor 1 | Tissue plasminogen activator inhibitor | SC6642† | 1:100 |
| Antithrombotic proteins | |||
| A disintegrin and metalloproteinase with a thrombospondin type I motif, member 13 | von Willebrand factor cleaving enzyme | BL-159‡ | 1:500 |
| Tissue factor pathway inhibitor | Major inhibitor of tissue factor, factor Xa, and thrombin | SC18712† | 1:100 |
| Tissue plasminogen activator | Regulator of fibrinolysis | Bs-1545R§ | 1:50 |
| ECM proteins | |||
| Collagen type I | Fibrillar collagen concentrated at fibrosa of AV leaflet; most abundant AV ECM component | Ab34710* | 1:50 |
| Collagen type II | Fibrillar collagen found mainly in fibrosa of AV leaflet | Ab7778* | 1:500 |
| Elastin | Highly elastic ECM component concentrated at ventricularis of AV leaflet | Ab9519* | 1:50 |
AV indicates aortic valve; and ECM, extracellular matrix
Corresponding manufacturers:
Abcam (Cambridge, MA)
Santa Cruz Biotechnology (Santa Cruz, CA)
Bethyl Laboratories (Montgomery, TX)
Bioss Laboratories (Woburn, MA)
Results
Hemostatic proteins distribute differently within aortic valve tissues
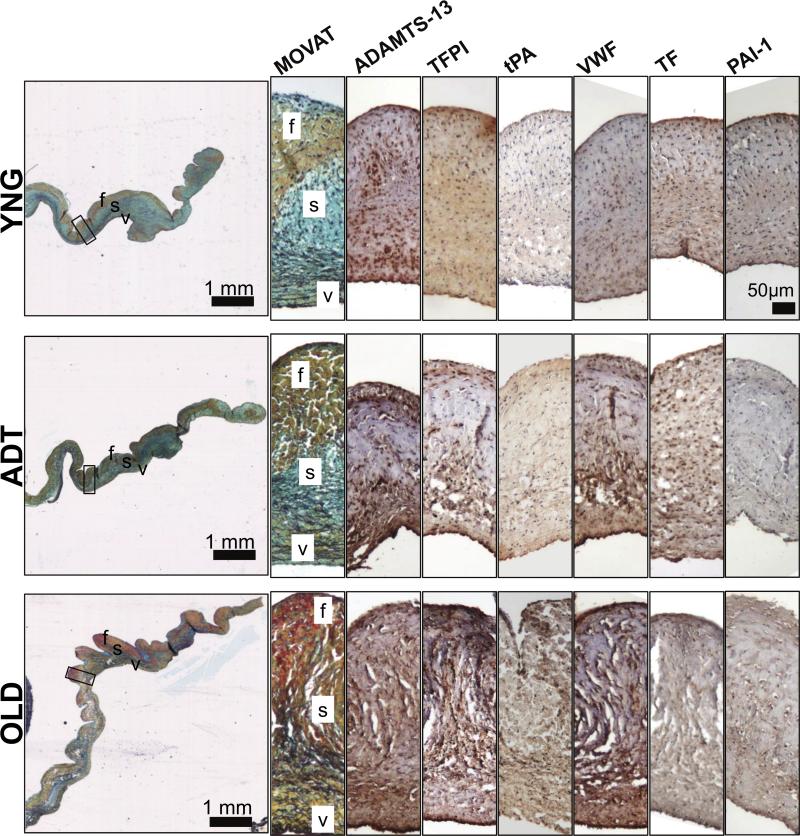
Immunostaining showed that endothelial cell-mediated anti-thrombotic and thrombotic proteins were present in each of the different aged AV leaflet groups (Fig. 1). The proteins were often localized with the endothelium at the edges of each leaflet. Many of these hemostatic mediators are actually secreted by endothelial cells, and correspondingly were also found within the tissue interior to varying degrees.30 To confirm the subendothelial localization of ADAMTS-13 and VWF, immunostains were repeated on OLD AV tissues using new antibodies specific for the proteins (Figure SIII). These additional immunostains showed the same pattern.
Figure 1.
MOVAT stains of aortic valve leaflet sections, and higher magnification views of the midleaflet regions (indicated by the black box on MOVAT) for ADAMTS13, TFPI, tPA, VWF, TF, and PAI-1. (f: fibrosa, s: spongiosa, v: ventricularis).
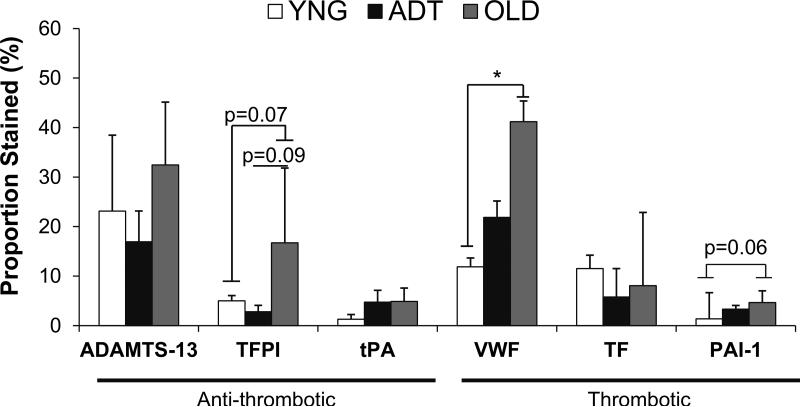
There were no significant differences in the proportions of tissues stained between these proteins within YNG and OLD samples. The proportion of VWF in ADT tissue was significantly higher than the proportions of all other proteins except for ADAMTS-13 (Fig. 2). However, the proportion of tissue stained for VWF was significantly higher in OLD tissue than in YNG tissues (p<0.05). There were trends of higher proportions of OLD valve tissues stained for PAI-1 (p=0.06), and TFPI (0.07) than in the corresponding portions of staining of YNG tissues, and a trend of higher proportion of TFPI staining in OLD tissues than in ADT tissues as well (p=0.09). The proportions of the valve tissues stained for PAI-1, TF, and tPA were low (< 10%) among all age groups in comparison to the other proteins evaluated (up to 40%) (Fig. 2), and these less abundant proteins were localized primarily in the endothelium (Fig. 1).
Figure 2.
Semi-quantitative analysis results of the proportion of assessed tissue stained for the hemostatic proteins in the different aged aortic valve leaflets. * = p-value <0.05 between noted age groups for that protein.
PAVEC hemostatic protein gene regulation changes with age
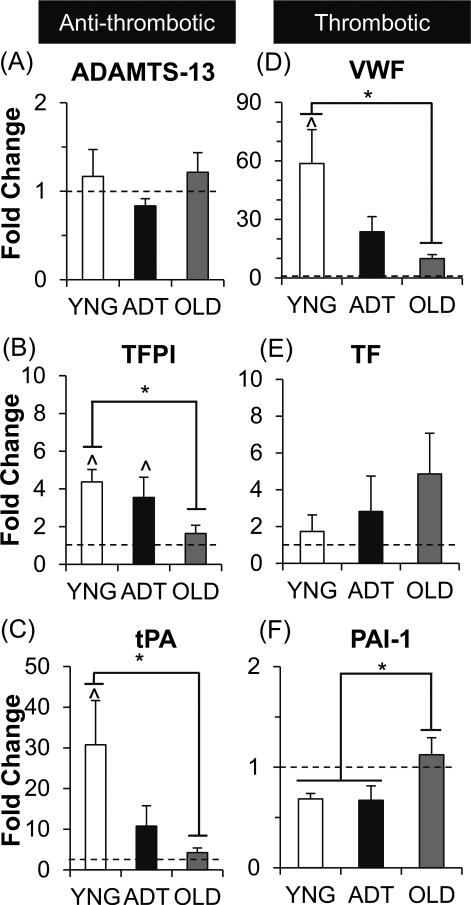
Gene expression of the anti-thrombotic and thrombotic proteins was quantified with qRTPCR (Fig. 3). PAVEC gene expression in all age groups was quantified relative to PPAECs (denoted by dotted lines in Fig. 3). Gene expression for VWF in YNG PAVECs was significantly higher than in OLD PAVECs (48×, p<0.005). Similarly, gene expression for the anti-thrombotic proteins TFPI and tPA was three-fold higher in YNG PAVECs than in OLD PAVECs (p<0.05 for each). Compared to the PPAEC control group, the YNG PAVEC gene expression for was significantly higher for VWF (58×, p<0.0005), TFPI (3×, p<0.05), and tPA (30×, p<0.05).
Figure 3.
PAVEC gene expression of anti-thrombotic (A-C) and thrombotic (D-F) proteins relative to PPAECs. ^ = p-value<0.05 between noted PAVEC age group versus the relative PPAEC gene expression (indicated by dashed line). * = p-value<0.05 between noted age groups for that protein.
There were no significant differences between ADT PAVECs and the other two age groups for all proteins. However, ADT PAVECs did have significantly higher gene expression of TFPI (2.5×, p<0.05) compared to the PPAEC controls.
The only protein that had the highest expression in the OLD PAVECs was the thrombotic protein PAI-1, which was two-fold higher than in both YNG and ADT PAVECs (p<0.05). There were no significant differences in the gene expression for ADAMTS-13 and TF between the PAVEC groups and PPAEC controls. Furthermore, all PAVEC and PPAEC groups had approximately 1000× higher gene expression levels for all hemostatic proteins than did HUVECs (data not shown).
PAVECs synthesize hemostatic mediators in vitro
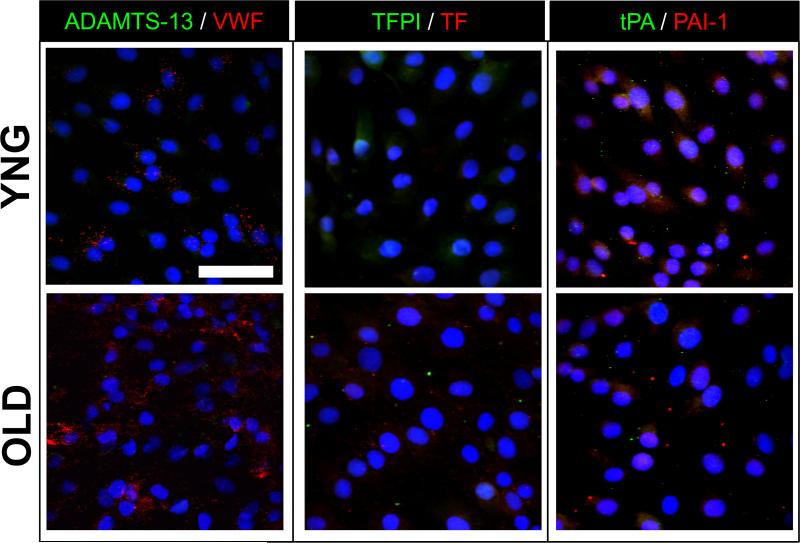
Primary PAVECs harvested from different aged AV tissues maintained their endothelial phenotype and stained positively for CD31 (data not shown). ADAMTS-13 and VWF were generally co-localized in all age groups, though there was a greater abundance of VWF staining overall (Fig. 4, first column). However, VWF levels in the OLD PAVECs appeared more profuse than in the YNG PAVEC cultures.
Figure 4.
Representative immunofluorescent stains of anti-thrombotic (ADAMTS-13, TFPI, tPA) proteins (green), and thrombotic (VWF, TF, PAI-1) proteins (red) in YNG and OLD PAVECs. Cell nuclei were stained with DAPI (blue). Scale bar = 100μm.
PAVECs positively stained for low levels of TFPI and TF (Fig. 4, second column). TFPI staining in YNG and ADT PAVECs was primarily localized intracellularly with some punctate staining outside of the cells; TFPI staining in OLD PAVECs was low, with punctate staining outside of the cells. For the YNG PAVEC cultures, there appeared to be higher levels of TFPI than of TF. Conversely, TF was more abundant than TFPI in OLD cultures.
PAI-1 and tPA were also detected in PAVEC cultures (Fig 4, third column). There was intracellular staining for PAI-1 in all PAVEC age groups, although punctate extracellular staining of PAI-1 was present in the OLD PAVEC cultures. Staining for tPA appeared to be more abundant in YNG and ADT PAVEC cultures than in OLD PAVEC cultures. Although the stains for PAI-1 and tPA were generally less abundant in the OLD PAVEC cultures, the complimentary proteins appeared to be co-localized with slightly higher levels of PAI-1.
PAVECs can mediate functional VWF release and cleavage
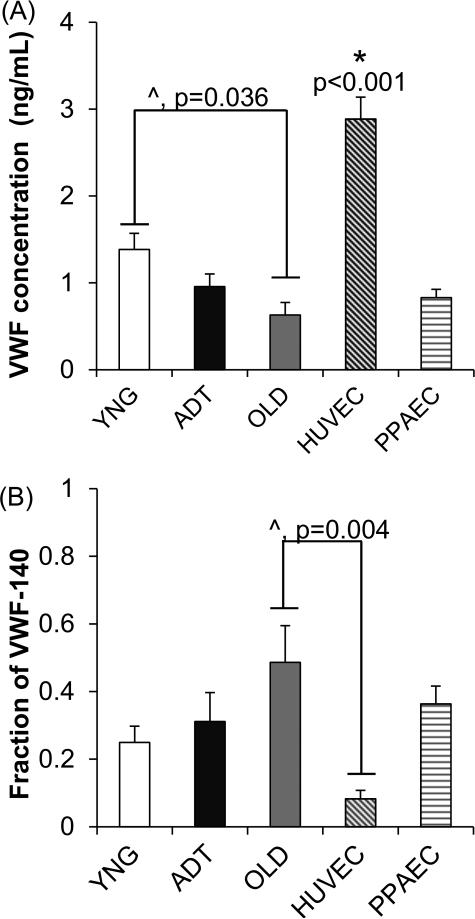
Histamine stimulation of PAVECs in vitro initiated the rapid release of thrombogenic VWF multimer proteins into the culture medium. For the comparison of PAVEC age groups, VWF protein release paralleled the VWF gene expression trend in that YNG PAVECs released a significantly higher concentration of VWF protein than did OLD PAVECs (p<0.05) (Fig. 5A). VWF release by the PPAECs and ADT PAVECs was not significantly different from that released by the YNG and OLD PAVECs. HUVECs released significantly higher amounts of VWF protein than all porcine derived PAVEC and PPAEC groups (p<0.0001).
Figure 5.
Quantification of rapidly released VWF protein from PAVEC, HUVEC, and PPAEC groups after 10 min incubation with histamine stimulation solution (A), and the fraction of cleaved VWF-140 fragments in solution (B). ^ = p-value<0.05 between noted age groups. * = p-value<0.001 between noted age group versus all groups.
The fraction of cleaved VWF-140 fragments was measured using ELISA assays with a specific monoclonal antibody for cleaved VWF proteins. There was no statistically significant difference in the fraction of VWF-140 fragments between porcine derived PAVEC and PPAECs (Fig. 5B). For the HUVECs, only approximately 10% of the VWF protein was cleaved into VWF-140 fragments, which was a significantly lower cleavage product fraction compared to the OLD PAVECs (p<0.05).
PAVEC released VWF increases PAVIC calcific nodule formation in vitro
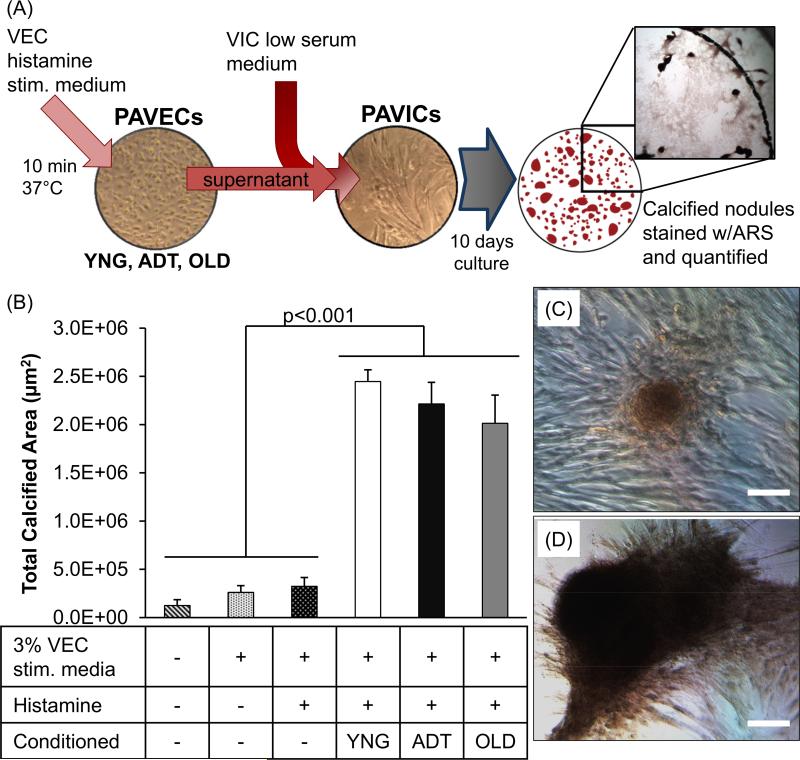
PAVICs were cultured with various culture medium conditions to assess how the addition of VEC released VWF affect calcific nodule formation in vitro. Measured levels of VWF protein present in the low serum PAVIC medium mixed with 3% (v/v) conditioned culture mediums collected from YNG, ADT, and OLD PAVEC groups were 257 pg/mL, 130 pg/mL, and 90 pg/mL, respectively. No levels of VWF protein were detected in any of the three control conditions.
Within 5 days of culture, nodule formation was visible in PAVIC cultures treated with each of the conditioned mediums from the different aged PAVECs. At the end of the 10 day culture period, there were no significant differences in average number of nodules per well, average nodule size, or total calcified area between the control groups of low serum PAVIC medium, 3% PAVEC stimulation medium without histamine, and 3% PAVEC stimulation medium with histamine (Figures 6, SVI,). The total calcified area in PAVICs treated with the conditioned mediums from YNG, ADT, and OLD PAVEC supernatants (2.0-2.4 × 106 μm2) were all significantly greater than all three control groups (1.2-3.2 × 104 μm2, p<0.001) (Figure 6). PAVICs in both YNG (40 nodules) and ADT (45 nodules) conditioned medium groups had significantly more nodules per well than the three control groups (9-13 nodules, p<0.001, supplemental figure SVI-A). Furthermore, the average nodule size in PAVICs treated with one of the three conditioned mediums (52-78 ×103 μm2) were significantly larger that each of the three control conditions (13-23 ×103 μm2, p<0.001) (supplemental figure SVI-B).
Figure 6.
Schematic of in vitro calcific nodule experiment to test the effects of VEC-released VWF on PAVIC calcification (A). Conditioned culture mediums consisted of VIC low serum medium with VWF-enriched media supernatant from histamine stimulated PAVECs from each age group. PAVICs were cultured in conditioned culture medium and control mediums for 10 days. Calcified nodules were stained red with Alizarin Red S (ARS) and the average total calcified area per culture condition was quantified (B). Representative images of PAVIC calcific nodules formed in low serum VIC medium (C) and conditioned OLD medium (D) after 10 days in culture. Scale bar = 100μm.
Discussion
The endothelial cell-mediated process of hemostasis is essential for the function of all living heart valve tissues. As these tissues undergo remodeling with age and disease, VEC management of hemostatic protein regulation also changes. This study is the first to examine the production and expression of numerous hemostatic proteins in AV tissues and in vitro PAVEC cultures from three distinct age groups.
There are several age-related differences in the abundance and localization of thrombotic and anti-thrombotic proteins within the aortic valve. As expected, all examined hemostatic proteins were strongly present at the leaflet edges in each age group. However, many of these soluble components, namely ADAMTS-13, TFPI, VWF, TF, and PAI-1, were found throughout the interior of the valves as well. These proteins were primarily localized in the ventricularis layer of the AV in YNG and ADT tissues. Conversely, they were distributed more evenly across the valve layers in OLD AV samples. Due to their interior location, it is possible that these hemostatic components interact with sub-endothelial extracellular matrix components such as elastin and collagen type I. The ventricularis layer contains densely packed elastin, which may sequester the soluble hemostatic proteins that have permeated into the subendothelium. As elastin becomes more disperse throughout the OLD AV tissues10, the hemostatic proteins may be able to permeate throughout the entire interior of the AV.
These proteins may also interact with collagen type I, as it is most abundant valve ECM protein and is present throughout the leaflet. Previous work investigating fiber alignment in articular cartilage tissues suggest that highly organized collagen alignment limits diffusion of proteins through anisotropic tissues.31,32 Similarly, the lower amount of hemostatic proteins within the fibrosa layer of the YNG and ADT AV tissues may be due to the highly organized and aligned collagen fibrils serving as a barrier to limit the diffusion of hemostatic proteins into the aortic side of the AV leaflet. Given the age-associated collagen remodeling and turnover observed in older tissues14, this barrier function of the fibrosa may be attenuated with age and result in the increased permeation of VEC-secreted hemostatic proteins throughout the valve layers.
In addition, qRT-PCR analyses showed highly significant changes in gene expression of many hemostatic proteins with age in PAVEC cultures. PAI-1, the protein inhibitor for tPA in the fibrinolysis pathways, was significantly elevated with age as shown by mRNA analysis, immunocytochemistry, and a trend in the proportion of tissue stained. The increased accumulation of PAI-1 in older valves suggests that this smaller molecular weight protein can easily permeate through the remodeled ECM architectures in OLD valves. Previous studies have shown that inflammatory or cytokine stimuli can increase endothelial secretion of PAI-1 while not affecting tPA secretion.30,33 It seems likely that elderly patients with higher levels of inflammation, cholesterol, and tissue remodeling, will also experience more pro-coagulant states, in which PAI-1 levels in the blood and ECM overcome the basally secreted levels of tPA.33–35 Interestingly, there were no differences in tPA accumulation in AV tissues despite the significant drop in tPA gene expression in OLD PAVECs relative to YNG PAVECs. This may suggest that the expressed tPA is released more into the bloodstream as opposed to accumulating within valve tissues. The reduction in PAVEC tPA expression also reflects the hemostatic imbalance between tPA and PAI-1 associated with age.
PAVEC expression and tissue accumulation of TFPI and VWF were affected by age-related changes as well. In both cases, YNG PAVECs had significantly higher levels of TFPI and VWF gene expression than did OLD PAVECs. Consistent with qRT-PCR results, stains for TFPI in YNG PAVECs appeared to be more abundant than in OLD PAVEC cultures. Conversely, OLD PAVEC cultures had slightly higher levels of VWF staining than in YNG PAVEC cultures. However, the polyclonal anti-VWF antibody used in the stain binds to both cleaved and uncleaved VWF proteins. Therefore, the increased staining observed in the OLD PAVEC cultures is likely from the higher proportion of cleaved VWF proteins in the condition, as observed in the ELISA experiments. Furthermore, YNG valves showed trends of lower proportions of tissue stained for both TFPI and VWF than in OLD tissues. This discrepancy could be a result of the retention of these basally secreted proteins within the disorganized OLD tissue ECM through interactions with heparin and collagen type I.30,36
PAVEC expression of ADAMTS-13 and TF was not affected by age for gene expression or the proportion of tissue stained. Thus, these specific hemostatic proteins seem less likely to be direct players in causing age-related valve disease. These results, however, suggest that the valvular hemostasis may become unbalanced over a lifetime, as many of the associated counteracting hemostatic proteins (VWF for ADAMTS-13, TFPI for TF) were shown to be affected by age. This imbalance was also observed in the immunostaining of PAVECs, as co-localization of the associated hemostatic component pairs were less present in the OLD PAVEC cultures.
Functional testing of the fragmentation of histamine-stimulated VWF confirmed that PAVECs secrete ADAMTS-13 to cleave VWF multimers. The total amount of VWF protein released by OLD PAVECs was significantly lower than by YNG PAVECs, which was consistent with the gene expression results. The fractions of cleaved VWF (140 kDa fragments) were not significantly different between all porcine derived endothelial cell groups, which supports the previous finding that ADAMTS-13 expression and function is not affected by age.37 On the contrary, since VWF expression and release is affected by age, with the elderly age group having a drop in VWF expression and production, there will be lower proportions of complete VWF proteins present in patient plasma that can mediate coagulation. This phenomenon is consistent with clinical reports that elderly patients require lower doses of anti-coagulants after heart valve surgeries than do adult patients, due to lower clearance kinetics of the drugs and decreased clotting ability in elderly patients.38
The changes in hemostatic protein regulation by VECs from our results matches with the age-related hemostatic protein plasma levels previously reported.33–35,38 We believe that these age-related imbalances in hemostatic protein regulation and production by VECs are not likely to cause spontaneous development of thrombus. However, when injury or insult occur at the valve endothelium, the aging-induced imbalance in the expression of anti-thrombotic and thrombotic proteins by VECs will lower the VEC's capability to maintain hemostasis; thus causes AV tissues to be more susceptible to thrombotic complications. Also, injury to the endothelium exposes the underlying tissue and therefore the accumulation of these proteins in valve tissues along with the structural changes can induce further thrombotic events.
Aside from intravascular complications, accumulation of these proteins in valve tissues along with the structural changes can cause tissue level progression of age related diseases such as CAVD. Utilizing an in vitro CAVD model, PAVIC nodule formation experiments showed that the presence of VEC-released VWF significantly increased the total number of nodules, nodule area, and total calcified area in PAVICs relative to control groups. Having no differences in nodule formation between the three control culture conditions confirmed that the significant increase in calcific nodule formation by PAVICs was not due to the addition of PAVEC stimulation medium or residual levels of histamine in solution, but due to the presence of VEC-released proteins including VWF, after histamine stimulation. Therefore, the secretion and accumulation of VWF and other hemostatic proteins within the valve tissues affect the development and progression of CAVD and other acquired valvular diseases.
Though there were no significant differences in PAVIC nodule formation between the different aged PAVEC-based conditions, the significant increase in VWF present within OLD valve tissues in conjunction with age-associated ECM disorganization seen in the histology slides suggest that calcification could result from increased pro-osteogenic VWF-VIC interactions, which correlated with the classical clinical presentation of CAVD in the elderly. Although VWF is the main component of histamine stimulated PAVEC supernatant, other factors in the supernatant (e.g. prostacyclin, platelet activating factor, angiopoietin-2, and interleukin-8) may also intensity PAVIC nodule formation, and warrants further investigation into how other hemostatic proteins within valve tissues can affect AV calcification.39–41 Nonetheless, our studies have shown that PAVECs will constitutively secrete VWF and other hemostatic factors, which will result in their accumulation of these proteins within the valve subendothelium. As ECM organization changes with age and the valve hemostatic protein regulation becomes more imbalanced, there is potential that VWF and some of these proteins may play a substantial role in the progression of age-related valve disease.
This study was also the first to perform an extensive characterization of PAVEC hemostatic protein expression and production relative to vascular endothelial cell types PPAECs and HUVECs. PPAECs have been documented to produce significant levels of VWF and were chosen as a porcine vascular endothelial cell control group.42 Previous findings have found that porcine endothelial cultures isolated from the thoracic aorta expressed low levels of VWF mRNA and did not contain Weibel Palade bodies, indicating that porcine endothelial cells have different regional capacities for hemostatic regulation.42,43 The gene expression of TFPI in YNG and ADT PAVECs, and of tPA and VWF in YNG PAVECs were significantly higher than in the baseline PPAECs, suggesting that PAVECs have distinct hemostatic regulation activity. Gene expression levels differed greatly between PAVECs and HUVECs, with expression of the investigated proteins being approximately three orders of magnitude higher in the PAVECs. HUVECs have been well documented to produce and release VWF in vitro,30,44 and thus are frequently used in endothelial cell studies and hemostatic experiments. However, the concentration of VWF protein released from histamine stimulated HUVECs was significantly higher than all porcine derived endothelial cell groups, suggesting that HUVECs are capable of storing more VWF in their Weibel Palade bodies than are porcine endothelial cells. Therefore, future studies regarding PAVEC expression and mediated protein functions should consider using porcine derived vascular endothelial cells as baseline controls.
Limitations of this study include the inherent variability in the semi-quantitative analysis of immunohistochemistry, which reduces the ability to distinguish between age groups. A larger sample size may help in confirming the promising trends. Still, this study examined several key endothelial cell-mediated hemostatic proteins. Additional factors such as ectonucleotidases, prostacyclin, and thrombomodulin have yet to be studied with respect to PAVEC hemostatic regulation. Future studies using a gene array for a wider panel of thrombotic and anti-thrombotic proteins in conjunction with ECM components and inflammatory markers may be informative in elucidating the relationship between the VECs regulation of their environment and how the protein accumulation influences the biology and pathology of the AV. Furthermore, while the interaction and balance of thrombotic and anti-thrombotic proteins is well understood at the apical side of the endothelium, further studies to investigate the interactions of each protein with sub-endothelial ECM and cells may be important in understanding the mechanisms that can promote the onset of valvular dysfunction. Lastly, further investigation into the molecular mechanisms behind the age-related imbalances in PAVEC hemostatic protein regulation will greatly enhance our knowledge of these changes and may provide potential targets for the prevention and treatment of acquired valve abnormalities.
In conclusion, it is well understood that valve tissues experience different environments with respect to age as a result of changes in valve mechanics, hemodynamics, and matrix composition. This study has identified age-related differences in PAVEC hemostatic protein regulation and the ability of basally secreted proteins to aggregate within different aged AV tissues. In addition, we have verified that PAVECs express many of the same thrombotic and anti-thrombotic proteins as vascular endothelial cells. Though there was not one pattern for all hemostatic protein expression and aggregation with respect to age, we demonstrated age-related differences in the overall expression and localization of tPA, PAI-1, TFPI, and VWF in AV tissues and cells. Though thrombosis does not commonly occur as an age-associated dysfunction in valve tissues, we observed that thickened elderly valve tissues with ubiquitous distributions of elastin and collagen sequester high levels of the hemostatic proteins in the subendothelium, which warrants further investigation into potential roles for these aggregated proteins in the formation of calcific nodules that are so prevalent in older AVs.5,6 Our in vitro PAVIC studies suggest that the presence of these VWF promotes PAVIC calcification, and that VWF and other hemostatic proteins within the tissue interior may have a potential role in the development of CAVD and other acquired abnormalities as age-related valve tissue ECM remodeling occurs. Thus, future studies regarding valve biology, pathology, and tissue engineering require consideration of environments that reflect the specific age group in question, including the necessary hemostatic framework.
Significance.
Valvular endothelial cells (VECs) play an important role in maintaining valve homeostasis and affect the onset of valve disease. However, little has been done to characterize the fundamental hemostatic behavior of VECs and its relation to the extracellular matrix (ECM) in health, disease, and aging. This study was the first to perform an extensive age-dependent characterization for hemostatic proteins in valves, and VEC hemostatic regulation relative to vascular endothelial cell types. Results identified age-related differences in VEC hemostatic protein regulation, and the increased capacity of specific proteins to aggregate within regions of elderly valves shown to have age-associated loss of ECM organization. In vitro CAVD model studies show that the presence of VWF significantly increases valvular interstitial cell formation of calcific nodules relative to baseline controls. Therefore, the hemostasis imbalance with aging and the accumulations of hemostatic proteins may contribute to the formation of calcific nodules, and warrants further investigation to determine the connection between VEC hemostatic mechanisms and the progression of valve disease. Furthermore, this works proposes the importance of selecting appropriate age and species based controls in future VEC and hemostatic work.
Acknowledgements
We are very grateful to Dr. Joel L. Moake for extensive project support including discussion of the data and manuscript. We thankfully acknowledge Nancy Turner and Leticia Nolasco for providing harvested primary HUVECs and guidance in conducting VWF release and cleavage assays. We also thank Adam Lin for his thoughtful discussion of the study results.
Sources of Funding
This research was funded by a pre-doctoral fellowship from the American Heart Association (to L.R.B), NIH HL110063, the Mary R. Gibson Foundation, and the Mabel and Everett Hinkson Memorial Fund.
Abbreviations
- AV
aortic valve
- ECM
extracellular matrix
- VIC
valvular interstitial cell
- VEC
valvular endothelial cell
- tPA
tissue plasminogen activator
- TFPI
tissue factor pathway inhibitor
- ADAMTS-13
A disintegrin and metalloproteinase with a thrombospondin type I motif, member 13
- PAI-1
plasminogen activator inhibitor 1
- TF
tissue factor
- VWF
von Willebrand factor
- PAVEC
porcine aortic valve endothelial cell
- YNG
6 week old porcine aortic valve sample
- ADT
6 month old porcine aortic valve sample
- OLD
2 years old porcine aortic valve sample
- PPAEC
porcine pulmonary artery endothelial cell
- HUVEC
human umbilical vein endothelial cell
- qRT-PCR
quantitative real time polymerase chain reaction
- PAVIC
porcine aortic valve interstitial cell
- CAVD
calcific aortic valve disease
Footnotes
Disclosure
None
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hakuno D, Kimura N, Yoshioka M, Fukuda K. Molecular mechanisms underlying the onset of degenerative aortic valve disease. Journal of Molecular Medicine. 2009;87:17–24. doi: 10.1007/s00109-008-0400-9. [DOI] [PubMed] [Google Scholar]
- 3.Leask RL, Jain N, Butany J. Endothelium and valvular diseases of the heart. Microscopy Research and Technique. 2003;60:129–37. doi: 10.1002/jemt.10251. [DOI] [PubMed] [Google Scholar]
- 4.Hinton RB, Yutzey KE. Heart valve structure and function in development and disease. Annual review of physiology. 2011;73:29–46. doi: 10.1146/annurev-physiol-012110-142145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–11. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 6.Schoen FJ. Evolving concepts of cardiac valve dynamics: the continuum of development, functional structure, pathobiology, and tissue engineering. Circulation. 2008;118:1864–80. doi: 10.1161/CIRCULATIONAHA.108.805911. [DOI] [PubMed] [Google Scholar]
- 7.Breuer CK, Mettler BA, Anthony T, Sales VL, Schoen FJ, Mayer JE. Application of tissue-engineering principles toward the development of a semilunar heart valve substitute. Tissue Engineering. 2004;10:1725–36. doi: 10.1089/ten.2004.10.1725. [DOI] [PubMed] [Google Scholar]
- 8.Roosens B, Bala G, Droogmans S, Hostens J, Somja J, Delvenne E, Schiettecatte J, Delvenne P, Lahoutte T, Van Camp G, Cosyns B. Occurrence of cardiovascular calcifications in normal, aging rats. Experimental Gerontology. 2012;47:614–9. doi: 10.1016/j.exger.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Stephens EH, Saltarrelli JG, Baggett LS, Nandi I, Kuo JJ, Davis AR, Olmsted-Davis E a, Reardon MJ, Morrisett JD, Grande-Allen KJ. Differential proteoglycan and hyaluronan distribution in calcified aortic valves. Cardiovascular pathology : the official journal of the Society for Cardiovascular Pathology. 2011;20:334–42. doi: 10.1016/j.carpath.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stephens EH, de Jonge N, McNeill MP, Durst CA, Grande-Allen KJ. Age-related changes in material behavior of porcine mitral and aortic valves and correlation to matrix composition. Tissue engineering Part A. 2010;16:867–78. doi: 10.1089/ten.tea.2009.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aikawa E, Whittaker P, Farber M, Mendelson K, Padera RF, Aikawa M, Schoen FJ, Rabkin-Aikawa E. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation. 2006;113:1344–52. doi: 10.1161/CIRCULATIONAHA.105.591768. [DOI] [PubMed] [Google Scholar]
- 12.Rabkin-Aikawa E, Farber M, Aikawa M, Schoen FJ. Dynamic and reversible changes of interstitial cell phenotype during remodeling of cardiac valves. The Journal of Heart Valve Disease. 2004;13:841–7. [PubMed] [Google Scholar]
- 13.Stephens EH, Post AD, Laucirica DR, Grande-Allen KJ. Perinatal changes in mitral and aortic valve structure and composition. Pediatric and Developmental Pathology : the Official Journal of the Society for Pediatric Pathology and the Paediatric Pathology Society. 2010;13:447–58. doi: 10.2350/09-11-0749-OA.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stephens EH, Grande-Allen KJ. Age-related changes in collagen synthesis and turnover in porcine heart valves. The Journal of Heart Valve Disease. 2007;16:672–82. [PubMed] [Google Scholar]
- 15.Stephens EH, Chu CK, Grande-Allen KJ. Valve proteoglycan content and glycosaminoglycan fine structure are unique to microstructure, mechanical load and age: relevance to an age-specific tissue-engineered heart valve. Acta Biomaterialia. 2008;4:1148–1160. doi: 10.1016/j.actbio.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin S-L, Liu C-P, Young S-T, Lin M, Chiou C-W. Age-related changes in aortic valve with emphasis on the relation between pressure loading and thickened leaflets of the aortic valves. International Journal of Cardiology. 2005;103:272–9. doi: 10.1016/j.ijcard.2004.08.079. [DOI] [PubMed] [Google Scholar]
- 17.Mohler ER. Mechanisms of aortic valve calcification. The American Journal of Cardiology. 2004;94:1396–402. A6. doi: 10.1016/j.amjcard.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Butcher JT, Penrod AM, García AJ, Nerem RM. Unique morphology and focal adhesion development of valvular endothelial cells in static and fluid flow environments. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24:1429–34. doi: 10.1161/01.ATV.0000130462.50769.5a. [DOI] [PubMed] [Google Scholar]
- 19.Butcher JT, Nerem RM. Valvular endothelial cells regulate the phenotype of interstitial cells in co-culture: effects of steady shear stress. Tissue engineering. 2006;12:905–15. doi: 10.1089/ten.2006.12.905. [DOI] [PubMed] [Google Scholar]
- 20.Butcher JT, Tressel S, Johnson T, Turner D, Sorescu G, Jo H, Nerem RM. Transcriptional profiles of valvular and vascular endothelial cells reveal phenotypic differences: influence of shear stress. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26:69–77. doi: 10.1161/01.ATV.0000196624.70507.0d. [DOI] [PubMed] [Google Scholar]
- 21.Simmons CA, Grant GR, Manduchi E, Davies PF. Spatial heterogeneity of endothelial phenotypes correlates with side-specific vulnerability to calcification in normal porcine aortic valves. Circulation Research. 2005;96:792–9. doi: 10.1161/01.RES.0000161998.92009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young EWK, Wheeler AR, Simmons CA. Matrix-dependent adhesion of vascular and valvular endothelial cells in microfluidic channels. Lab on a Chip. 2007;7:1759–66. doi: 10.1039/b712486d. [DOI] [PubMed] [Google Scholar]
- 23.Bischoff J, Aikawa E. Progenitor cells confer plasticity to cardiac valve endothelium. Journal of Cardiovascular Translational Research. 2011;4:710–9. doi: 10.1007/s12265-011-9312-0. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong EJ, Bischoff J. Heart valve development: endothelial cell signaling and differentiation. Circulation Research. 2004;95:459–70. doi: 10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Hamamsy I, Balachandran K, Yacoub MH, Stevens LM, Sarathchandra P, Taylor PM, Yoganathan AP, Chester AH. Endothelium-dependent regulation of the mechanical properties of aortic valve cusps. Journal of the American College of Cardiology. 2009;53:1448–55. doi: 10.1016/j.jacc.2008.11.056. [DOI] [PubMed] [Google Scholar]
- 26.Merryman WD. Mechano-potential etiologies of aortic valve disease. Journal of Biomechanics. 2010;43:87–92. doi: 10.1016/j.jbiomech.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simmons CA. Aortic valve mechanics: an emerging role for the endothelium. Journal of the American College of Cardiology. 2009;53:1456–8. doi: 10.1016/j.jacc.2008.12.052. [DOI] [PubMed] [Google Scholar]
- 28.Widmaier EP, Raff H, Keving ST. Vander's Human Physiology: The Mechanisms of Body Function. 11th ed. McGraw-Hill Higher Education; 2006. p. 770. [Google Scholar]
- 29.Li S, Henry JJD. Nonthrombogenic approaches to cardiovascular bioengineering. Annual Review of Biomedical Engineering. 2011;13:451–75. doi: 10.1146/annurev-bioeng-071910-124733. [DOI] [PubMed] [Google Scholar]
- 30.Pearson JD. Endothelial cell function and thrombosis. Baillière's Best Practice & Research Clinical Haematology. 1999;12:329–41. doi: 10.1053/beha.1999.0028. [DOI] [PubMed] [Google Scholar]
- 31.Federico S, Grillo A, La Rosa G, Giaquinta G, Herzog W. A transversely isotropic, transversely homogeneous microstructural-statistical model of articular cartilage. Journal of Biomechanics. 2005;38:2008–18. doi: 10.1016/j.jbiomech.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 32.Federico S, Herzog W. On the anisotropy and inhomogeneity of permeability in articular cartilage. Biomechanics and Modeling in Mechanobiology. 2008;7:367–78. doi: 10.1007/s10237-007-0091-0. [DOI] [PubMed] [Google Scholar]
- 33.Mari D, Coppola R, Provenzano R. Hemostasis factors and aging. Experimental Gerontology. 2008;43:66–73. doi: 10.1016/j.exger.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 34.Burg P Van den, van den Burg PJ, Hospers JE, Mosterd WL, Bouma BN, Huisveld IA. Aging, physical conditioning, and exercise-induced changes in hemostatic factors and reaction products. Journal of Applied Physiology. 2000;88:1558–64. doi: 10.1152/jappl.2000.88.5.1558. [DOI] [PubMed] [Google Scholar]
- 35.Gharacholou SM, Becker RC. Hemostasis and thrombosis in older adults. Journal of Thrombosis and Thrombolysis. 2009;27:249–51. doi: 10.1007/s11239-009-0308-4. [DOI] [PubMed] [Google Scholar]
- 36.Yuan H. Interaction of the von Willebrand Factor (vWF) with Collagen. Journal of Biological Chemistry. 1995;270:10822–10827. doi: 10.1074/jbc.270.18.10822. [DOI] [PubMed] [Google Scholar]
- 37.Turner NA, Nolasco L, Ruggeri ZM, Moake JL. Endothelial cell ADAMTS-13 and VWF: production, release, and VWF string cleavage. Blood. 2009;114:5102–11. doi: 10.1182/blood-2009-07-231597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russmann S, Gohlke-Bärwolf C, Jähnchen E, Trenk D, Roskamm H. Age-dependent differences in the anticoagulant effect of phenprocoumon in patients after heart valve surgery. European Journal of Clinical Pharmacology. 1997;52:31–5. doi: 10.1007/s002280050245. [DOI] [PubMed] [Google Scholar]
- 39.McIntyre TM, Zimmerman GA, Satoh K, Prescott SM. Cultured endothelial cells synthesize both platelet-activating factor and prostacyclin in response to histamine, bradykinin, and adenosine triphosphate. The Journal of Clinical Investigation. 1985;76:271–80. doi: 10.1172/JCI111957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, Kriz W, Thurston G, Augustin HG. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood. 2004;103:4150–6. doi: 10.1182/blood-2003-10-3685. [DOI] [PubMed] [Google Scholar]
- 41.Jeannin P, Delneste Y, Gosset P, Molet S, Lassalle P, Hamid Q, Tsicopoulos A, Tonnel AB. Histamine induces interleukin-8 secretion by endothelial cells. Blood. 1994;84:2229–33. [PubMed] [Google Scholar]
- 42.Rand JH, Badimon L, Gordon RE, Uson RR, Fuster V. Distribution of von Willebrand factor in porcine intima varies with blood vessel type and location. Arteriosclerosis & Thrombosis, and Vascular Biology. 1987;7:287–91. doi: 10.1161/01.atv.7.3.287. [DOI] [PubMed] [Google Scholar]
- 43.Royo T, Martínez-González J, Vilahur G, Badimon L. Differential intracellular trafficking of von Willebrand factor (vWF) and vWF propeptide in porcine endothelial cells lacking Weibel-Palade bodies and in human endothelial cells. Atherosclerosis. 2003;167:55–63. doi: 10.1016/s0021-9150(02)00393-3. [DOI] [PubMed] [Google Scholar]
- 44.Turner N, Nolasco L, Moake J. Generation and breakdown of soluble ultralarge von Willebrand factor multimers. Seminars in Thrombosis and Hemostasis. 2012;38:38–46. doi: 10.1055/s-0031-1300950. [DOI] [PubMed] [Google Scholar]