Abstract
Microsatellite DNA and mitochondrial DNA control region sequence analyses were used to determine the population and Distinct Population Segment (DPS) origin of 173 Atlantic sturgeon Acipenser oxyrinchus oxyrinchus encountered from the Gulf of Maine to Cape Hatteras, North Carolina in NOAA's Northeast Fisheries Observer Program. It was found that the Hudson River was by far the greatest contributor to this coastal bycatch, with 42.2-46.3% of specimens originating there. Generally, specimens respected the geographic province of the river in which they were spawned, but some specimens, particularly those originating in the South Atlantic DPS, moved great distances. Genetic mixed stock analyses provides an accurate approach to determine the DPS and population origin of Atlantic sturgeon bycaught in coastal waters but most informative management requires that these results be partitioned by locale, season, target fishery, and gear type.
Keywords: Coastal bycatch, Individual Based Assignment Testing, Genetic Mixed Stock Analysis, Microsatellite DNA, Mitochondrial DNA
INTRODUCTION
The U.S. Endangered Species Act (ESA) was enacted in 1973 to protect imperilled plant and animal species from extinction. Under ESA, a species that is in danger of extinction throughout all or a significant part of its range is listed as “endangered,” whereas a species likely to become extinct in the foreseeable future is listed as “threatened.” Vertebrate species that are widely distributed can be listed under ESA as Distinct Population Segments (DPS) if these units are discrete from similar units and are “significant” in relation to the entire species. Discreteness is determined by the extent of reproductive isolation among units and evolutionary significance by the contribution that the unit makes to the ecological and genetic diversity of the species as a whole (Waples 2006).
Since early 2012, Atlantic sturgeon Acipenser oxyrinchus oxyrinchus (Mitchill 1815) have been federally listed under the U.S. ESA as five DPS (Federal Register 2012 a,b). The Gulf of Maine (GOM) DPS was designated as “threatened” while Distinct Population Segments in the New York Bight (NYB), Chesapeake Bay (CB), Carolinas (CAR), and the South Atlantic (SA) were listed as “endangered” (Federal Register 2012 a,b). In the late 1880s, adult A. oxyrinchus supported lucrative fisheries within many of their spawning rivers with the largest fishery accounting for 75% of reported landings occurring in the Delaware River (Secor & Waldman, 1999) with smaller in-river fisheries prosecuted in the tributaries of the Chesapeake Bay, rivers in North Carolina, South Carolina, and Georgia (Smith, 1985), and the Hudson River, New York (Secor, 2002). However, at the close of the 19th century, landings in most of these fisheries collapsed to less than 10% of their historic highs (Smith & Clugston, 1997). Harvest coast wide on spawning fish remained low for many decades until the late 1980s when a targeted coastal fishery for subadults developed in the coastal waters of the New York Bight (Waldman et al., 1996) and accounted for over 90% of U.S. landings at that time (Smith & Clugston, 1997). This coastal fishery reduced abundances of some populations, particularly that in the Hudson River, to even lower levels than previously. Increased harvest prompted several states to close their fisheries and eventually resulted in the 1998 imposition by the Atlantic States Marine Fisheries Commission (ASMFC) of a federal coast wide 40-year moratorium on the harvest of A. oxyrinchus. Despite implementation of this measure, populations were perceived by some to not rebound at all, or too slowly, resulting in the filing of several petitions to federally list A. oxyrinchus in the U.S. with their eventual acceptance.
At least two rivers are known to support successful natural reproduction in Canada, the St. Lawrence River, Quebec, and Saint John River, New Brunswick, and both currently support very limited fisheries. Canadian populations are not listed as separate units under the U.S. ESA but have been designated as “threatened” by the Committee on the Status of Endangered Wildlife in Canada, but are not yet listed under the Species at Risk Act (Stewart et al. In press).
The life history of A. oxyrinchus makes an evaluation of the status of individual populations and the species as a whole problematic. Acipenser oxyrinchus are anadromous and historically they were believed to spawn in up to 35 rivers coast wide extending from the St. Lawrence River to the St. Johns River, Florida (Dadswell 2006) with contemporary spawning reported to persist in 18-20 rivers coast wide (Atlantic Sturgeon Status Review Team, 2007). However, our analysis based on efforts to collect adults and juveniles from historic spawning rivers suggests that successful natural reproduction may be limited to only 13 rivers today (Table 1 Supplementary Information). Acipenser oxyrinchus are long lived (up to 60 years) (Dadswell 2006) and spawn at advanced ages and perhaps only intermittently (Smith, 1985). Females spawn at older ages than males and both genders spawn at younger ages in populations at southern compared to northern latitudes (Smith, 1985). For example, females from the St. Lawrence River do not spawn until age 27-28 and males at age 16-24 (Caron et al., 2002) compared to ages 16-20 for females and ages 12-19 for males from the Hudson River (Van Eenennaam et al. 1996; Dovel & Berggren, 1983). Furthermore, it was reported that females spawn at 3-5 years intervals with males spawning at 1-5 year intervals in South Carolina (Smith 1985). However, recent observations of captive hatchery broodstock housed at the Bears Bluff National Fish Hatchery, South Carolina, and collected from the Altamaha River, Georgia, suggest that spawning for both genders may occur annually (Kent Ware, Bears Bluff Hatchery,, pers. comm.). Similarly, results from acoustic telemetry studies indicate that some adults return to spawning grounds in consecutive years (Dewayne Fox, Delaware State University, pers. comm.). Traditionally, it was believed that spawning within all populations occurred during the spring and early summer months with earliest spawning (March-April) occurring in southern latitudes (Georgia and South Carolina) with later spawning (mid June to mid July) occurring in Canadian populations. However, recent evidence suggests that spawning occurs in late summer-early fall in two tributaries of the Chesapeake Bay (James River and York River, Virginia) (Balazik et al., 2012; Hager et al., 2014) and in the Altamaha River, Georgia (Ingram, 2014, Doug Peterson, University of Georgia, pers. comm.). Whether, spring spawning also occurs in these rivers has yet to determined. Thus, there is still much uncertainty about the basic life history of A. oxyrinchus that may impact efforts to accurately census these populations and determine levels of genetic diversity within and among populations.
Importantly for this study, juvenile A. oxyrinchus are resident within their natal estuaries for 2-6 years dependent on their natal river origin after which they emigrate as subadults to coastal waters (Dovel & Berggren 1983) or other estuaries seasonally (Waldman et al., 2013). Specific details on the duration, destination, and circuit of migrations are largely unknown as is the reason for these movements. It is known that these migrations can be extensive. For example, an adult specimen tagged on the Hudson River spawning grounds during spawning season was detected off the Nova Scotia coast (Erickson et al., 2011) and A. oxyrinchus migrants from Canada are believed to have repopulated the Baltic Sea after extirpation of the native Sea Sturgeon Acipenser sturio there (Ludwig et al., 2002). The presence of subadult and adult A. oxyrinchus outside of their natal estuaries for the vast majority of their life histories makes it difficult, if not impossible, to obtain an accurate census of population abundances. This is compounded by the difficulties in collecting young life-stages (embryos and larvae) and juveniles from many estuaries prior to their emigration. Despite these limitations, it is believed that the Saint John River, New Brunswick (Dadswell, 2006), Hudson River, New York (Peterson et al. 2000; Kahnle et al., 2007), and Altamaha River, Georgia (Peterson et al., 2008) host some of the largest populations coast wide, whereas those in the Delaware River, Delaware-Pennsylvania (Kahn & Fisher 2012), Ogeechee River (Farrae et al. 2009) and Satilla River, Georgia (D. Peterson, University Georgia pers. Comm.) are among the smallest.
The reason most cited for the failure of A. oxyrinchus populations to stabilize and rebuild is their vulnerability to bycatch in non-natal estuaries and coastal waters (Federal Register 2012a,b). It has been demonstrated that subadult and adult A. oxyrinchus are vulnerable to bycatch in nearshore coastal waters (< 30-40 m depths) from the Gulf of Maine to Cape Hatteras, North Carolina, and that bycatch occurred in 30 different target fisheries, with 7 target species accounting for 85% of bycatch (Stein et al., 2004). Furthermore, bycatch in sink gill nets was most damaging, incurring a 13.8% (ASMFC 2007) to 22% (Stein et al., 2004) immediate mortality rate in specimens bycaught in that gear compared to a near absence of immediate mortality (ASMFC 2007; Stein et al., 2004), or a maximum 6% longer-term mortality (Beardsall et al., 2013) in otter trawls. Because of its high rate of bycatch, and use of sink gill nets, the greatest concern has been expressed regarding the detrimental effects of the American monkfish (Lophius americanus) fishery. Bycatch is thought to be particularly devastating to highly depleted populations with low recruitment although it was projected that an anthropogenically induced mortality rate as low as 4% could lead to the decline of even relatively large populations such as that in the Hudson River (ASMFC 2007).
To protect those populations in greatest jeopardy of decline from coastal bycatch, it is essential to know the population and DPS origin of individual A. oxyrinchus and their aggregations that are bycaught in different target fisheries, gears, seasons, and coastal or estuarine locales. This information would allow resource managers to develop management strategies for target fisheries that minimize their interactions with specimens from the most vulnerable populations with the most damaging gear. The objective of this study was to utilize mitochondrial DNA (mtDNA) and microsatellite DNA markers to determine the population and DPS origin of specimens encountered in NOAA's Northeast Fisheries Observer Program (Observer Program). The ASMFC (2007) Bycatch Workshop participants identified “an evaluation of relative population contributions to regions of high bycatch,” as their highest research priority. The Observer Program is a fishery dependent survey that uses contracted observers to monitor catch and bycatch in a variety of fisheries conducted in near shore coastal (within 4.8 km of shore) and offshore waters in the federal Exclusive Economic Zone that extend from the Gulf of Maine to Cape Hatteras, North Carolina. The objectives in this study differ from our earlier efforts that determined the population and DPS origins of mixed aggregations of A. .oxyrinchus in the Bay of Fundy (Wirgin et al. 2012) and Long Island Sound (Waldman et al. 2013). Additionally, the current fishery dependent study analysed specimens that were bycaught in target fisheries rather than from fishery independent surveys as had been done previously.
MATERIALS AND METHODS
SAMPLE COLLECTIONS
All specimens were collected in the Observer Program and the distribution of collections extended to the limits of the program's coverage from the Gulf of Maine to Cape Hatteras. Observer Program coverage across this entire area is 8% of all trips with the exception that Observer coverage for the New England ground fish fisheries, extending from Maine to Rhode Island, is an additional 18% (26% coverage in total). Fin clips were taken from all specimens and were preserved in DMSO. Harvested tissues were shipped from the individual observers to the Northeast Fisheries Science Center, Woods Hole, Massachusetts, where collection data was centralized and the tissues were then shipped to the National Ocean Science Forensics Branch, Charleston, South Carolina, for archiving and distribution. All specimens (n=173) that were collected between March, 2009 and February, 2012 were analysed and they had a mean total length of 122.5 cm. When the species was federally listed 2012, sample collections were suspended until the appropriate collection permits could be obtained. These samples were collected primarily from 8 target fisheries; Atlantic cod, Atlantic croaker, dogfish (smooth and spiny), long-finned squid, monkfish, skate, striped bass, and summer flounder.
LABORATORY ANALYSIS
DNAs were analysed at mitochondrial DNA (mtDNA) control region sequences and 11 microsatellite loci as described in Wirgin et al. (2012) and Waldman et al. (2013). Briefly, total DNA was isolated from fin clips that were initially washed with PBS, incubated in CTAB buffer (Saghai-Maroof et al. 1984) and digested with proteinase K at 65° C, followed by standard phenol-chloroform extractions and alcohol precipitations. DNA concentrations and purities were evaluated by using a Nanodrop ND-1000 Spectophotometer (NanoDrop Technologies, http://www.nanodrop.com). DNA concentrations of samples were adjusted to 50 ng/μl for standardization of subsequent procedures.
Microsatellite loci that were analysed included LS19, LS39, LS54, LS68 (May et al., 1997), Aox23, AoxD45 (King et al., 2001), and Aox44, AoxD165, AoxD170, AoxD188, AoxD241 (Henderson-Arzapalo & King, 2002). PCR amplification and sequencing conditions were done exactly as detailed in Waldman et al. (2013). All mtDNA sequence and microsatellite DNA analyses were performed on a Beckman Coulter (www.beckmancoulter.com) CEQ8000™ capillary-based sequencer.
STATISTICAL ANALYSES
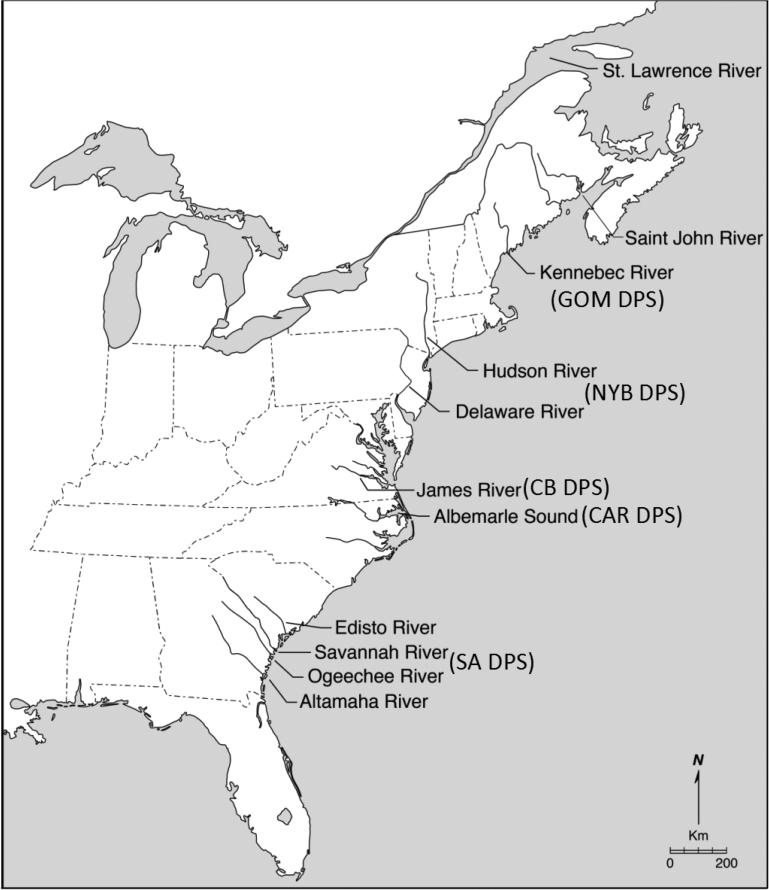
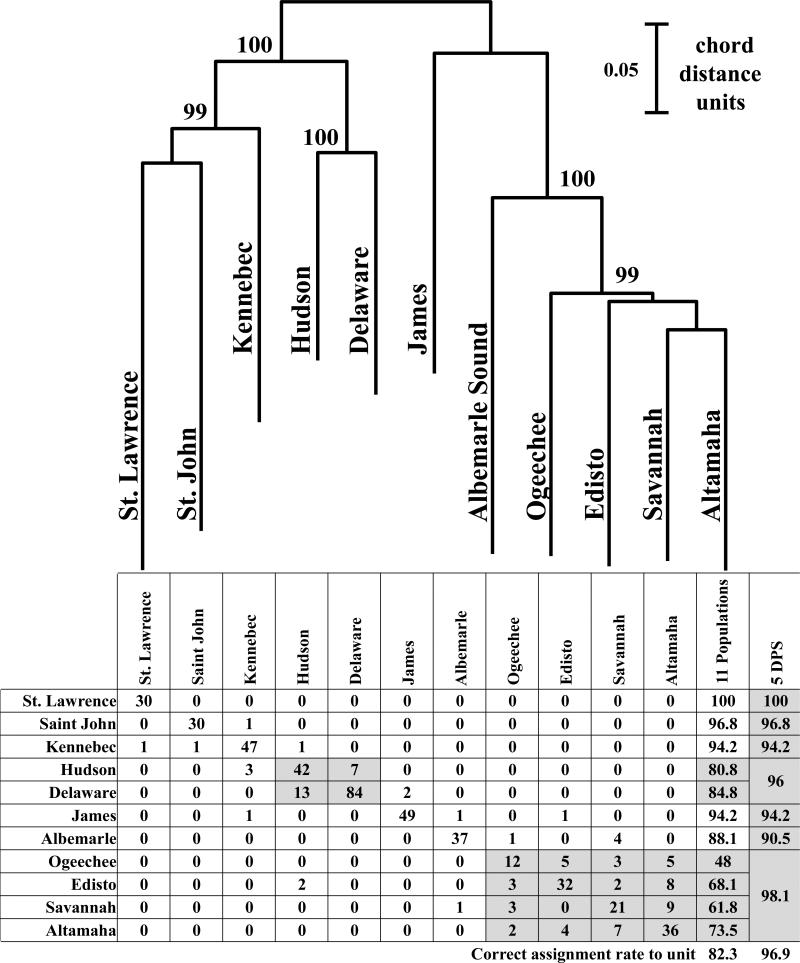
Reference collection data were compiled for eleven spawning populations including the St. Lawrence River, Quebec; Saint John River, New Brunswick; Kennebec River, Maine; Hudson River, New York; Delaware River, Delaware-Pennsylvania; James River, Virginia; Roanoke River-Albemarle Sound, North Carolina; Edisto River, South Carolina; Savannah River, South Carolina-Georgia; Ogeechee River, Georgia; and Altamaha River, Georgia (Fig. 1). All specimens in reference collections were either juveniles < 50 cm (total length) TL or spawning adults > 130 cm TL that were collected within spawning rivers and were assumed to be natal to the rivers in which they were captured based on size. Data for nine of the reference collections is exactly as reported in Fig. 2 of Waldman et al., (2013) and data for the St. Lawrence River and Edisto River is new to this study. Assignment accuracies to the five U.S. DPS and two Canadian systems was high at x=96.9% with population-level assignments less accurate at x=82.3% (Fig. 2ab). Almost all of the population level misassignments occurred within the same DPS and were most frequent among collections in the South Atlantic DPS.
Fig. 1.
Map depicting rivers where 11 reference spawning populations and 5 U.S. DPS of A. oxyrinchus were sampled for this study. GOM-Gulf of Maine, NYB-New York Bight, CB-Chesapeake Bay, CAR-Carolinas, and SA-South Atlantic.
Fig. 2.
(a) Neighbor-Joining Tree depicting evolutionary relationships (as Cavalli-Sforza and Edwards chord distances) among the eleven A. oxyrinchus reference spawning populations that were surveyed at 11 microsatellite loci in this study. Numbers represent bootstrap support at each branch with 5,000 replicates of resampling across loci.
(b) Results of assignment testing of the baseline individuals from the eleven A oxyrinchus reference spawning populations using 11 microsatellite loci and mtDNA control region haplotypes (scored as a homozygous locus) to determine the likelihood of each individual's multi-locus genotype/haplotype being found in the reference collection and DPS from which it was collected (without replacement) using the program GeneClass2. The numbers in the shaded cells represent the total number of individual specimens from the populations in that DPS correctly assigned to DPS.
Two approaches were used to determine the contributions of individual populations or DPS to the Observer Program collection including Individual-Based Assignment (IBA) testing and Mixed-Stock Analysis (MSA). Both approaches require that populations potentially contributing to the mixed aggregations be characterized at the same genetic loci (mtDNA and 11 microsatellites) that were used to characterize the coastal samples of unknown origin. IBA tests were implemented in GeneClass2 (Piry et al., 2004) using Rannala & Mountain's (1997) and Cornuet et al.'s (1999) Bayesian and frequency based model. IBA tests were used to determine the likelihood of each individual's genotype in the unknown Observer collection being found in each of the eleven reference collections. The analysis determines the likelihood ratio assuming that the gene pool is in Hardy-Weinberg equilibrium with frequencies as specified in the collections.
Mixed stock analysis implemented in ONCOR (Kalinowski 2008) was used to estimate the proportion of each reference population in the mixed Observer sample of unknown origin and to determine the probability of each individual belonging to each of the reference DPS and populations. In contrast to IBA, MSA applies mixture modelling, taking into account the genotypes of individual specimens across multiple loci, the multi-locus genotype distributions of the reference samples and, in addition, the multi-locus genotype distribution in the mixture Observer sample. Mixture proportions were estimated with 95% confidence limits based on 10,000 bootstraps. Results were reported for each population in the reference collection as well as for each U.S. DPS and Canadian management unit. Unlike earlier studies (Wirgin et al. 2012; Waldman et al. 2013), the Saint John River collection was not included in the U.S. Gulf of Maine DPS and was treated as a separate management unit.
The distance travelled was determined for each individual specimen from each of the 11 reference collections that were bycaught in the Observer Program. Distance travelled was calculated from the mouth of the specimen's source estuary to its position (latitude-longitude) at the end of the trawl in which it was caught. The mean distance travelled by specimens from that population was determined using the haversine formula implemented in Movable Type Scripts (http://www.movable-type.co.uk/scripts/latlong.html).
RESULTS
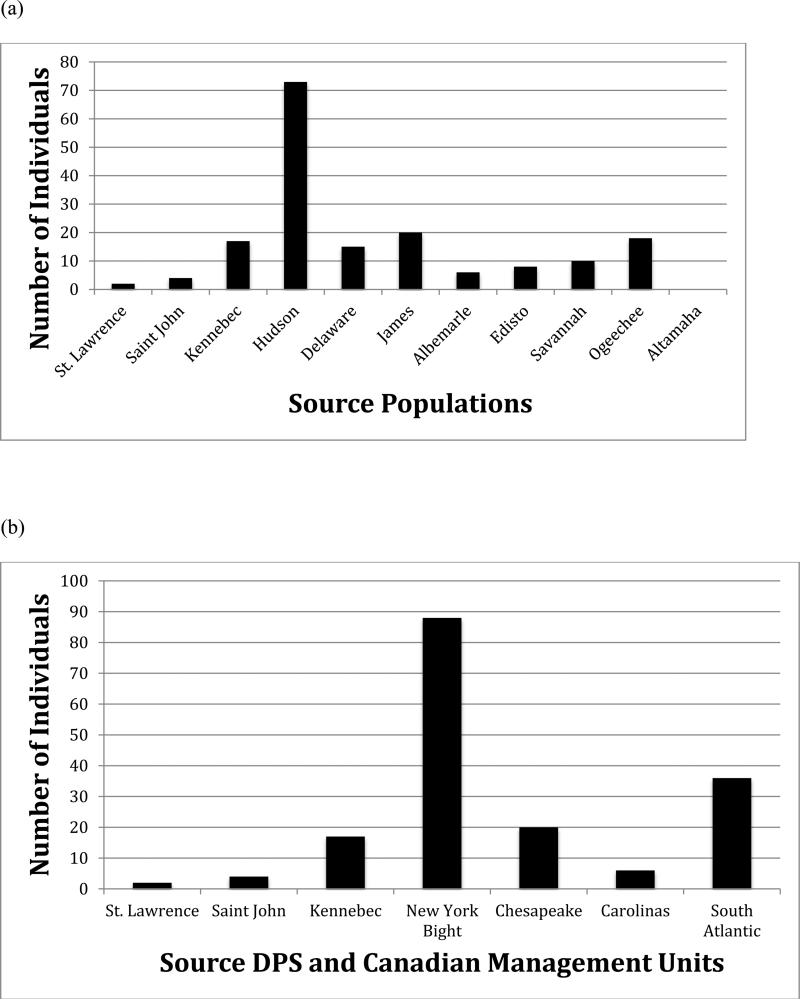
Population and DPS origin of specimens from the Observer Program were determined as a single coast wide sample (Fig. 3) and also by gear used (sink gill nets and bottom otter trawl), known immediate mortalities, and different target fisheries (monkfish and summer flounder). Without exception, IBA and MSA provided similar quantitative results in assigning ancestry to aggregations of A. oxyrinchus that interacted with coastal target fisheries. For example, coast wide it was found that the Hudson River population was the predominant source of specimens collected in the Observer Program. Using IBA testing, we found that 42.2% of all Observer Program bycatch assigned to the Hudson River population (Fig. 4a). Using a second approach, MSA, we observed a slightly higher Hudson River contribution of 46.3% (95% CI= 34.1 to 53.3%) (Table 1). Similarly, the NYB DPS (Hudson River and Delaware River populations) was the largest contributor to Observer Program bycatch, with 48.3% of specimens assigned to the NYB DPS with IBA testing (Fig 4b) and 51.7% (95% CI= 42.9 to 59.4%) using MSA (Table 1). Without exception, IBA and MSA provided similar quantitative results in assigning ancestry to aggregations of A. oxyrinchus that interacted with coastal target fisheries.
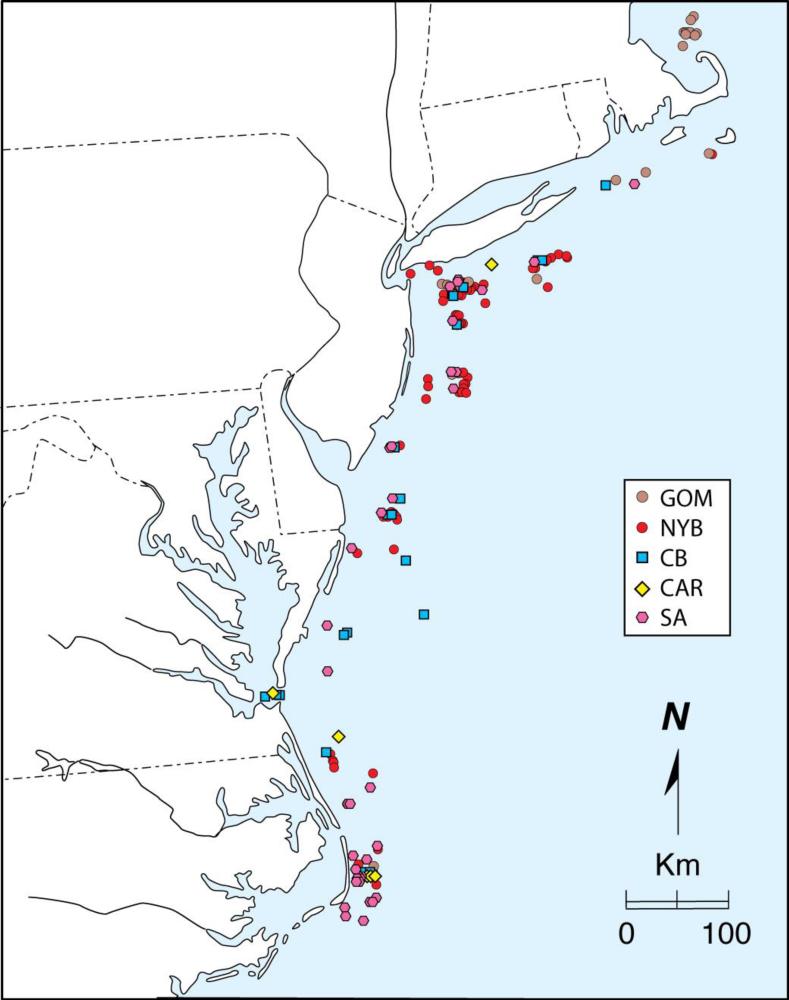
Fig. 3.
Encounter locations of individual A. oxyrinchus specimens (n=173) encountered in the Northeast Fisheries Observer Program from the Gulf of Maine to Cape Hatteras, North Carolina, and assigned to 5 source DPS using IBA testing implemented in GeneClass2 and based on mtDNA control region sequence and microsatellite DNA analyses at 11 loci. Gulf of Maine (GOM), New York Bight (NYB), Chesapeake Bay (CB), Carolinas (CAR), and South Atlantic (SA).
Fig. 4.
Contributions of 11 reference A. oxyrinchus source populations (a) and 5 DPS and 2 Canadian management units (b) to the Northeast Fisheries Observer Program interactions with Atlantic sturgeon (n=173) using IBA testing implemented in GeneClass2 based on mtDNA control region sequence and 11 microsatellite DNA loci.
Table I.
Population and DPS (including Canadian management units) origin determined by Mixed Stock Analysis implemented in ONCOR of 173 adult and subadult A. oxyrinchus encountered in the Northeast Fisheries Observer Program based on mtDNA control region sequence and microsatellite DNA data at 11 loci.
| Population or DPS Origin | Estimate | 95% CI |
|---|---|---|
| Population | ||
| St. Lawrence River | 0.010 | (0.000, 0.028) |
| Saint John River | 0.012 | (0.000, 0.040) |
| Kennebec River | 0.101 | (0.051, 0.164) |
| Hudson River | 0.463 | (0.341, 0.533) |
| Delaware River | 0.055 | (0.022, 0.145) |
| James River | 0.118 | (0.069, 0.180) |
| Albemarle Sound | 0.024 | (0.000, 0.068) |
| Edisto River | 0.039 | (0.000, 0.089) |
| Savannah River | 0.053 | (0.006, 0.114) |
| Ogeechee River | 0.126 | (0.056, 0.182) |
| Altamaha River | 0.000 | (0.000, 0.018) |
| U.S. DPS and Canadian Management Units | ||
| St. Lawrence River | 0.010 | (0.000, 0.029) |
| Saint John River | 0.012 | (0.000, 0.040) |
| Gulf of Maine (GOM) | 0.101 | (0.051, 0.164) |
| New York Bight (NYB) | 0.517 | (0.430, 0.594) |
| Chesapeake Bay (CB) | 0.118 | (0.070, 0.180) |
| Carolinas (CAR) | 0.024 | (0.000, 0.068) |
| South Atlantic (SA) | 0.219 | (0.142, 0.279) |
Populations at one time thought to be extirpated, or nearly so (Grogan and Boreman 1998), also made substantial contributions to the Observer Program samples. For example, the James River population contributed 11.6% (IBA) to 11.8% (MSA) (95% CI= 6.9 to 18.0%) of coastal bycatch. Similarly, the Delaware River with an estimated population size of < 300 adults, contributed 8.7% (IBA) to 5.5% (MSA) (95% CI 2.2 to 14.5%) of coastal bycatch.
Surprisingly, the third largest contributing population coast wide was the Ogeechee River which the use of IBA determined contributed 10.4% and MSA 12.6% (95% CI= 5.6 to 18.2%) of coast wide bycaught specimens. The Altamaha River, Georgia, believed to host the second largest U.S. population and the largest in the SA DPS did not contribute at all to the Observer Program collection. In total, the SA DPS's contribution was the second largest coast wide and contributed 20.8% of Observer Program bycatch using IBA testing and 21.9% (95% CI= 14.2 to 27.9%) using MSA.
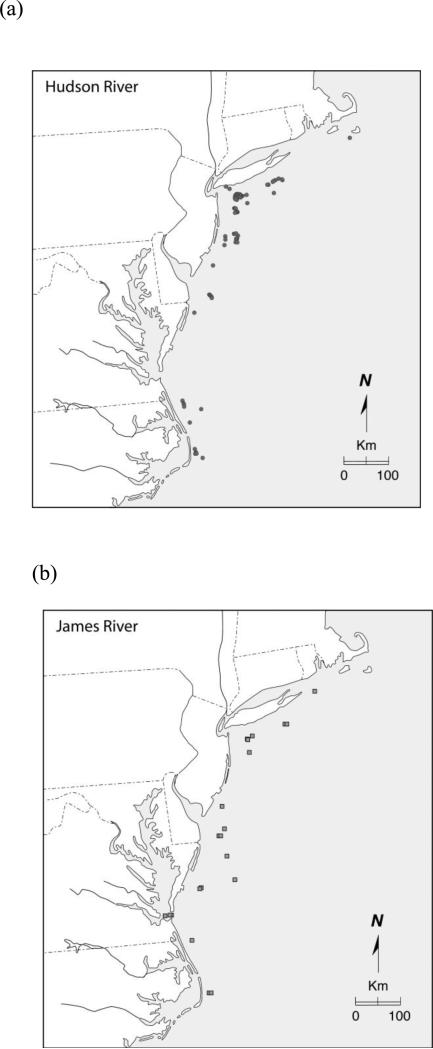
Specimens encountered in the Observer Program were distributed across the Program's entire coverage extending from the Gulf of Maine to Cape Hatteras (Fig. 3). While some specimens from some populations tended to be encountered in close proximity to their natal estuaries others did not. For example, most Hudson River specimens were encountered in the New York Bight, however a moderate number were encountered off Cape Hatteras and one off Nantucket, Massachusetts (Fig. 5a). Similarly, while some James River specimens were encountered off the mouth of Chesapeake Bay, many more were detected to the northeast in the New York Bight extending as far as Montauk Point, New York (Fig. 5b). Most interestingly, all specimens from the Savannah River and Ogeechee River populations in the SA DPS were only encountered distant from their natal estuaries. Of course, part of that observation results from the absence of Observer Program coverage south of Cape Hatteras, North Carolina.
Fig. 5.
Map of capture locations of individual A. oxyrinchus bycaught in Northeast Fisheries Observer Program and assigned ancestry by IBA testing to the (a) Hudson River and (b) James River populations
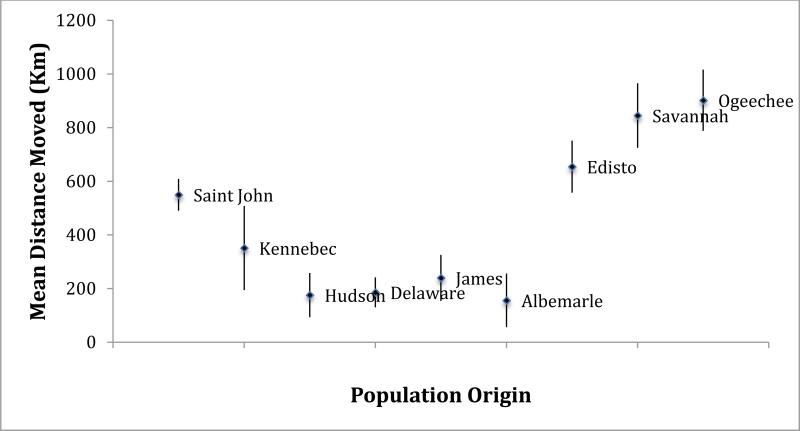
We were interested in determining the minimum distance travelled by each individual specimen from each of the 11 reference collections that were bycaught in the Observer Program. Using ANOVA, statistically significant differences in distance travelled were found among the 11 reference populations (Fig. 6) (P < 0.001), with specimens from two populations in the SA DPS moving significantly further than specimens from the two populations in the NYB DPS. For example, specimens from the Savannah and Ogeechee rivers moved a mean of 845 km (range: 639-1147 km) and 902 km (range: 633-1344 km), respectively, before being bycaught in coastal fisheries. In comparison, specimens from the Hudson and Delaware rivers moved a mean of 176 km (range 23.3-600 km) and 186 km (range 57 to 380 km), respectively, before their coastal bycatch.
Fig. 6.
Comparison of mean minimum distance (km) travelled by A. oxyrinchus specimens from the mouth of their natal estuary to the coastal location of their encounter with the Northeast Fisheries Observer Program. The St. Lawrence River is omitted because only 2 specimens were assigned to that population. Population origin for individual specimens was determined using IBA testing implemented in GeneClass2 and based on mtDNA control region sequence and microsatellite DNA data at 11 loci.
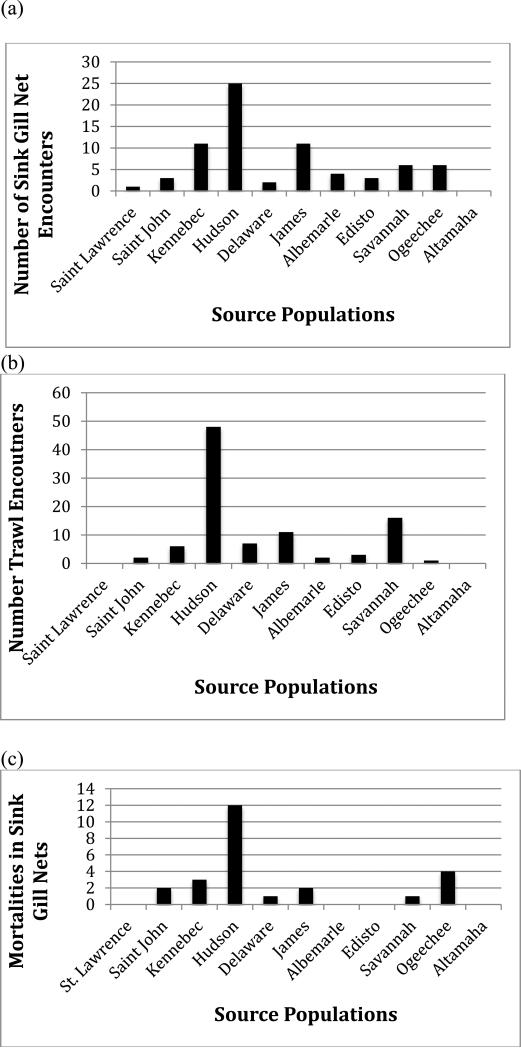
We next examined reference population contributions to coastal bycatch on the basis of two gear types; sink gill net and bottom otter trawl. For both gear types, specimens of Hudson River origin predominated. In the coast wide sink gill net fishery, the Hudson River was the source for approximately 35% of specimens (Fig. 7a), while in the bottom trawl fishery the Hudson River contribution was even greater at 50% (Fig. 7b). Because almost all mortality of A. oxyrinchus bycatch in coastal fisheries occurs in the sink gill net fishery, we determined the stock origin of all mortalities from that gear type. As expected, we found that of 25 total mortalities, 48% of specimens were of Hudson River origin but we also found that 1 and 2 specimens, respectively, were from populations at one time thought to be near extirpation in the Delaware River and James River, respectively (Fig. 7c).
Fig. 7.
Contributions of 11 reference populations of A. oxyrinchus to Northeast Fisheries Observer Program (a) sink gill net encounters (n=72), (b) encounters in bottom trawls (n=96), and (c) mortalities in sink gill nets (n=25) determined using IBA testing implemented in GeneClass2 based on mtDNA control region sequence and microsatellite DNA analysis at 11 loci.
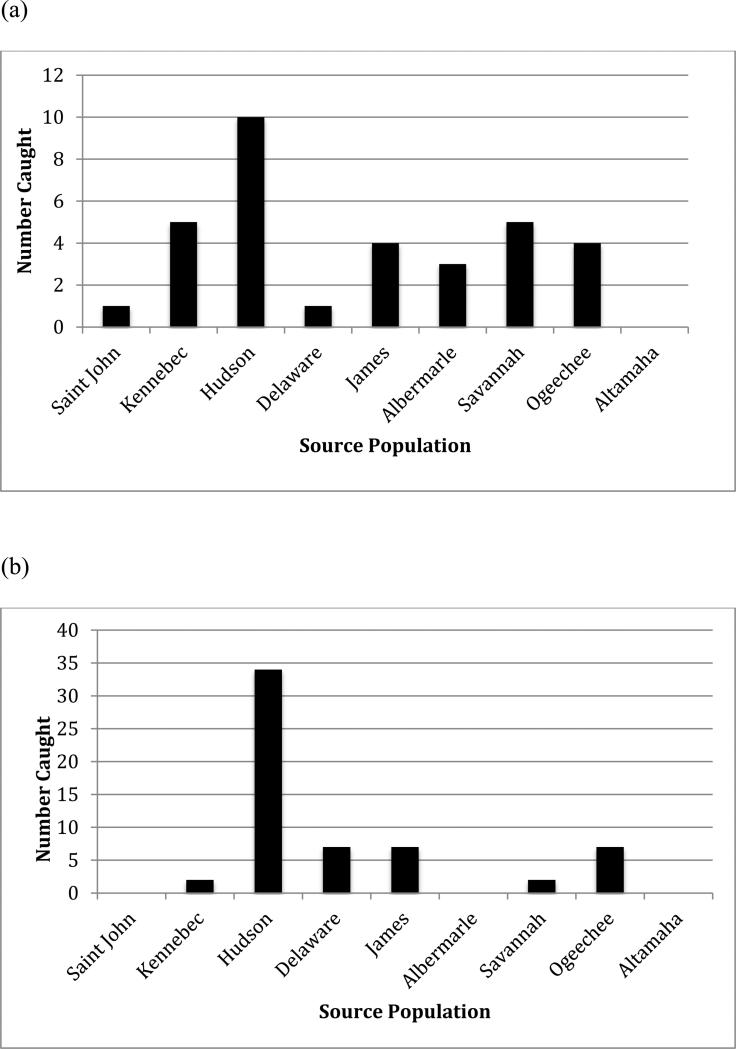
The stock origin of bycaught A. oxyrinchus was also determined by target fishery. Eleven different targeted fisheries bycaught the 173 A. oxyrinchus specimens that were analysed. The largest numbers of bycaught A. oxyrinchus were in the summer flounder (34.6%), monkfish (19%), squid (9.2%) and skate (8.1%) fisheries. Because summer flounder are fished with otter trawls which incur little mortality of bycaught A. oxyrinchus and monkfish are caught with sink gill nets with high associated mortality, the stock origin of A. oxyrinchus in the monkfish fishery was also determined. It should be noted that the monkfish fishery extends the entire limits of the Gulf of Maine to Cape Hatteras Observer Program coverage although we are not aware of the relative efforts of the monkfish fishery within this overall area. We found that 30% of A. oxyrinchus bycaught in the monkfish fishery were of Hudson River origin (Fig. 8).
Fig. 8.
Source populations of A. oxyrinchus encountered in the Northeast Fisheries Observer Program and captured in the (a) monkfish (n=33) and (b) summer flounder (n=60) fisheries determined using IBA testing implemented in GeneClass2 with data from mtDNA control region sequence and microsatellite DNA analysis at 11 loci.
DISCUSSION
We found that A. oxyrinchus from all eleven reference populations interacted with target fisheries along the U.S. Atlantic Coast from the Gulf of Maine to Cape Hatteras and probably beyond. The spatial limits to A. oxyrinchus interactions in our study should not be considered the geographic boundaries to coastal movement of A. oxyrinchus from these populations. For example, in previous studies adult A. oxyrinchus acoustically tagged off the Delaware coast during the spring months were detected off the Georgia and mid-Florida coasts and in the Cabot Strait entering the Gulf of St. Lawrence (D. Fox, Delaware State University, pers Comm.). Clearly, A. oxyrinchus migrations and interactions with target fisheries extend beyond the limited geographic boundaries of our study.
Not unexpectedly, the Hudson River in the NYB DPS was the largest contributor of bycatch to these fisheries with its contribution exceeding 40% in both IBA and MSA analyses. Similarly, in both analyses the NYB DPS contributed almost half of specimens to Observer Program bycatch. This high percentage is consistent with the relatively robust size of the Hudson River spawning population (Kahnle et al., 2007) and its position near the centre of the geographic distribution of the Observer Program sample collections. In previous studies, we found that the Hudson River made only a minor contribution (<2%) to bycatch in weirs and bottom trawls in the Minas Basin of the inner Bay of Fundy, Canada (Wirgin et al., 2012), a predominant contribution (65-70%) to fishery independent collections of subadults in Long Island Sound, Connecticut, and its tributary Connecticut River (Waldman et al., 2013), and a major contribution (39% to 44%) to the aggregation of adults and subadults off the Delaware Coast during the spring months (Wirgin et al., In press). Importantly for management, we also found that the Hudson River population interacted with target fisheries outside of the boundaries of the NYB DPS off Martha's Vineyard, Massachusetts, and very frequently with fisheries off the Outer Banks of North Carolina (Fig. 5a). Thus, our finding of the Hudson River population being the largest contributor to the Observer Program bycatch is consistent with its relative size, geographic position near the centre of the Observer Program's coverage, and the large impact that coastal bycatch could potentially have on its abundance. Whether, the mortality from coastal bycatch approaches the 4% level thought to impede the rebuilding of populations (ASMFC 2007) such as that in the Hudson River is open to question. Perhaps more importantly, the impact of coastal bycatch on the effective population sizes (Ne) of individual populations has yet to be assessed.
Surprisingly, the James River, Virginia population, at one time thought to be extirpated, or nearly so (Grogan & Boreman 1998), still contributed moderate numbers of specimens to coastal bycatch. We found that 11.6% (IBA) to 11.8% (MSA) of coast wide Observer Program samples assigned to the James River population. Prior to the institution of a reward program in 1996-1998, and the subsequent capture of numerous young-of-the-year specimens there, there was little evidence of successful spawning of A. oxyrinchus in the James River or elsewhere in the Chesapeake Bay for many decades. However, recent efforts have detected the presence of fall spawning aggregations of A. oxyrinchus in the James River (Balazik et al., 2012) and the adjacent York River system (Hager et al., submitted), a finding consistent with their frequent occurrence in Observer Program bycatch. Interestingly, the majority of this bycatch of James River specimens occurred in the NYB DPS with smaller numbers off the Outer Banks of North Carolina in the CAR DPS.
Based on census size, the Delaware River population is probably one of the smallest along the U.S. coast with recent estimates suggesting that its size is less than 300 adults (Breece et al., 2013). Despite this, based on IBA testing, 15 (8.7%) specimens assigned to the Delaware River collection. Given the estimated size of the Delaware River population and the high immediate mortality rate in sink gill nets, this bycatch of Delaware River specimens may be sufficiently high to contribute to the impaired rebuilding of its population. Our accuracy in assigning reference specimens to the Delaware River collection was only moderate at 84.8%, with 13 of 15 inaccuracies (of 99 specimens) assigning to the Hudson River population (Fig. 1) and thus it might be argued that our Delaware assigned specimens were actually misassigned Hudson River specimens. However, we are certain that at least 3 of the specimens that were assigned to the Delaware River population were accurate because they exhibited mtDNA haplotypes that are common in Delaware River juvenile collections (32%) but are absent from our Hudson River reference collection of more than 200 adult specimens (Grunwald et al., 2008; Wirgin et al., unpublished data).
The monkfish fishery is considered to pose the greatest potential threat to migrating A. oxyrinchus because of its use of sink gill nets with high associated immediate mortality of A. oxyrinchus (ASMFC, 2007) and the overlap of the fishery's distribution with that of A. oxyrinchus in nearshore mid-Atlantic U.S. coastal waters (Stein et al., 2004; ASMFC, 2007). As expected, specimens from the Hudson River population interacted most frequently with the monkfish fishery, with about 30% of bycaught Observer Program specimens assigning to the Hudson River population. Previously, it was projected that a 4% anthropogenically induced mortality rate on the Hudson River population could jeopardize its ability to rebuild (ASMFC, 2007). Because of the absence of empirical data on the census size nor effective population size of the Hudson River population in marine waters, it is impossible to determine if the current rate of A. oxyrinchus bycatch in the monkfish fishery is sufficiently high to impede its rebuilding.
Interestingly, the mean minimum movement of specimens from two populations in the SA DPS, the Savannah (845 km) and Ogeechee (902 km) rivers, before their interactions with the Observer Program, was significantly greater than those from the Hudson (176 km) and Delaware (186 km) rivers populations in the NYB DPS. This variation could be due to actual behavioural differences between populations in the central and southern portions of the species’ range or differences in the intensity of Observer Program sampling between areas. Clearly, Observer coverage only extends to Cape Hatteras and there is an absence of coverage off the mouths of rivers in the SA DPS compared to heavy coverage off the Hudson and Delaware rivers in the New York Bight. This difference would inflate distances moved by specimens originating in the SA DPS. We feel that there is a behavioural difference in migration patterns between populations, but that our sampling program accentuated the difference. Clearly, specimens from the SA DPS frequently interact with target fisheries that are 2-3 DPS removed from their natal DPS.
Several reservations to our conclusions should be considered because of deficiencies in our reference and Observer Program collections. First, our mixed stock results are only based on eleven reference populations contributing to the Observer Program collection, whereas 18-20 populations coast wide have been hypothesized to currently support successful natural reproduction of A. oxyrinchus (ASSRT 2007). As a result, it is possible that our population specific, but probably not DPS, assignments are inflated because of our omission of other possible contributing reference populations. However, the omitted populations are usually those in which attempts at the collection of juvenile or adult specimens has unproductive reflecting their small sizes and likely minor contributions to coastal bycatch. Furthermore, the perusal of records for A. oxyrinchus from which tissues were deposited over the past 15 years in the NOS Marine Forensics Branch Archive repository in Charleston, South Carolina, indicates that there are only two populations from which juvenile or adult A. oxyrinchus were successfully sampled and which are not represented in our reference collection (Table 1 Supplementary Information). For example, recent evidence suggests the persistence of successful natural reproduction in the York River, Virginia (a tributary of the Chesapeake Bay), based on the collection of spawning adults in late summer of 2013 and 2014 (Hager et al. 2014; J. Kahn pers. comm.) and the discovery of moderate numbers of juvenile A. oxyrinchus in the Satilla River, Georgia in 2010 after 2 years of failure to catch any juveniles there in 2008 and 2009 (D. Peterson, University of Georgia). Second, our reference collections have not been systematically tested for the inter-annual temporal stability of diagnostic genotype/haplotype frequencies. The likelihood of temporal instability may be particularly problematic for juvenile collections from small A. oxyrinchus populations where successful natural reproductions may be supported by a limited number of parents in any single year. These conditions can potentially lead to significant temporal instability of allelic and haplotype frequencies among year classes. However, our preliminary studies do support the temporal stability of microsatellite allelic frequencies between two years classes of juveniles from the large Hudson River and Altamaha River populations. Whether results from these two relatively large populations are representative of similar results from smaller populations remains to be empirically determined. Finally, the most effective use of genetic mixed stock analysis in a management context demands that the effects of coastal bycatch on individual populations or DPS be partitioned spatially, temporally, by target fisheries, and by gear type. Given the unequal impacts of bycatch by gear type and fishery and the varying migratory dynamics of different populations, it is essential that future studies analyse the larger collections needed to address these disparities.
For the first time, mixed stock analysis of Observer Program bycatch provides a comprehensive picture of the distribution of A. oxyrinchus from different populations in U.S. coastal waters. We found that the Hudson River population is by far the largest contributor to overall bycatch of A. oxyrinchus in U.S. marine waters from the Gulf of Maine to Cape Hatteras and the greatest number of interactions with damaging sink gill net fisheries such as those for monkfish. Surprisingly, we found that the number of interactions of presumably small populations, such as those in the Delaware and James rivers, with targeted fisheries are not infrequent and support their possible impediment to population rebuilding. Unlike fisheries independent surveys such as reported in Waldman et al. (2013) and Wirgin et al. (In press), analysis of Observer Program collections provides a direct evaluation of the effects of bycatch in targeted fisheries on individual A. oxyrinchus populations and DPS.
Genetic mixed stock analysis provides the only empirical approach to quantitatively evaluate the effects of specific coastal fisheries or other anthropogenic disturbances outside natal estuaries on individual A. oxyrinchus populations or DPS that differ in abundance or listing status. Use of this methodology can provide the data needed to better manage target coastal fisheries to minimize interactions between the most damaging of fisheries and gears with the most vulnerable of A. oxyrinchus populations. Furthermore, we feel that genetic mixed stock analysis in coastal waters may provide the most viable means to estimate the size of individual populations because of the absence of A. oxyrinchus from natal rivers except for their brief and intermittent spawning windows. Precise estimates of total coastal population size such as attempted in Kocik et al. (2013) combined with accurate genetic mixed stock analysis and compared to effective population sizes (Ne) may provide the most robust estimates of the size of individual populations and DPS.
Supplementary Material
Acknowledgments
All the Observers who provided tissue samples for our analyses, Carl Lemire and K.B. McArdle of the Northeast Fisheries Science Center of NOAA who coordinated their efforts, and Julie Carter of the NOS Marine Forensics Branch who archived and shipped samples are thanked. Support of New York Sea Grant (Grant R/SG-20), the Protected Resources Division of NOAA, and use of the Molecular Facility Core of the NYU NIEHS Center ES000260 are acknowledged. J.R. Waldman is thanked for commenting on an earlier draft of this manuscript. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. government.
References
- Atlantic States Marine Fisheries Commission (ASMFC) Special Report of Workshop. Estimate of Atlantic sturgeon bycatch in coastal Atlantic commercial fisheries of New England and the mid-Atlantic. 2007:95. [Google Scholar]
- Atlantic Sturgeon Status Review Team (ASSRT) Status review of Atlantic sturgeon (Acipenser oxyrinchus oxyrinchus). Report to National Marine Fisheries Service, Northeast Regional Office. February. 2007;23:2007. [Google Scholar]
- Balazik MT, Garman GC, Van Eenennaam JP, Mohler J, Woods LC., III Empirical evidence of fall spawning by Atlantic sturgeon in the James River, Virginia. Transactions of the American Fisheries Society. 2012;141:1465–1471. [Google Scholar]
- Beardsall JW, McLean MF, Cooke SJ, Wilson BC, Dadswell MJ, Redden AM, Stokesbury MJW. Consequences of incidental otter trawl capture on survival and physiological condition of threatened Atlantic sturgeon. Transactions of the American Fisheries Society. 2013;142:1202–1214. [Google Scholar]
- Breece MW, Oliver MJ, Cimino MA, Fox DA. Shifting distributions of adult Atlantic sturgeon amidst post-industrialization and future impacts in the Delaware River: a maximum entropy approach. PLOS ONE. 2013 doi: 10.1371/journal.pone.0081321. DOI: 10.1371/journal.pone.0081321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron F, Hatin D, Fortin R. Biological characteristics of adult Atlantic sturgeon (Acipenser oxyrinchus) in the St. Lawrence River estuary and the effectiveness of management rules. Journal of Applied Ichthyology. 2002;18:580–585. [Google Scholar]
- Cornuet JM, Piry S, Luikart G, Estroup A, Solignac M. New methods employing multilocus genotypes to select or exclude populations as origins of individuals. Genetics. 1999;153:1989–2000. doi: 10.1093/genetics/153.4.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadswell M. A review of the status of Atlantic sturgeon in Canada, with comparisons to populations in the United States and Europe. Fisheries. 2006;31:218–229. [Google Scholar]
- Dovel WL, Berggren TJ. Atlantic sturgeon of the Hudson River estuary, New York. New York Fish and Game Journal. 1983;30:140–172. [Google Scholar]
- Erickson DL, Kahnle A, Millard MJ, Mora EA, Bryja M, Higgs A, Mohler J, DuFour M, Kenney G, Sweka J, Pikitch EK. Use of pop-up satellite archival tags to identify oceanic-migratory patterns for adult Atlantic sturgeon, Acipenser oxyrinchus oxyrinchus Mitchell, 1815. Journal of Applied Ichthyology. 2011;27:356–365. [Google Scholar]
- Farrae DJ, Schueller PM, Peterson DL. Abundance of juvenile Atlantic sturgeon in the Ogeechee River, Georgia. Proceedings Annual Conference Southeastern Association of Fish and Wildlife Agencies. 2009;63:172–176. [Google Scholar]
- Federal Register Endangered and threatened wildlife and plants; threatened and endangered status for distinct population segments of Atlantic sturgeon in the Northeast region. 2012a Feb 6;77:5880–5912. 2012. [Google Scholar]
- Federal Register Endangered and threatened wildlife and plants; threatened and endangered status for two distinct population segments of Atlantic sturgeon Acipenser oxyrinchus oxyrinchus in the southeast. 2012b Feb 6;77:5914–5984. 2012. [Google Scholar]
- Grogan CS, Boreman J. Estimating the probability the historical populations of fish species are extirpated. North American Journal of Fisheries Management. 1998;18:522–529. [Google Scholar]
- Grunwald C, Maceda L, Waldman J, Stabile J, Wirgin I. Conservation of Atlantic sturgeon Acipenser oxyrinchus oxyrinchus: delineation of stock structure and distinct population segments. Conservation Genetics. 2008;9:1111–1124. [Google Scholar]
- Hager C, Kahn J, Watterson C, Russo J, Hartman K. Evidence of Atlantic sturgeon spawning in the York River system. Transactions of the American Fisheries Society. 2014;5:1217–129. [Google Scholar]
- Henderson-Arzapalo A, King TL. Novel microsatellite markers for Atlantic sturgeon population delineation and broodstock management. Molecular Ecology Notes. 2002;2:437–439. [Google Scholar]
- Ingram E. Master thesis submitted to the University of Georgia. 2014. Temporal and spatial patterns of shortnose sturgeon (Acipenser brevirostrum) and Atlantic sturgeon (Acipenser oxyrinchus oxyrinchus) spawning migrations in the Altamaha River system. p. 87. [Google Scholar]
- Kahn DM, Fisher M. Delaware Division of Fish and Wildlife. Dover, Delaware: 2012. Endangered Atlantic sturgeon in the Delaware River require higher standards for dissolved oxygen. p. 6. [Google Scholar]
- Kahnle AW, Hattala KA, McKown KA. Status of Atlantic sturgeon of the Hudson River estuary, New York, USA. In: Munro J, Hatin D, Hightower JE, McKown KA, Sulak KJ, Kahnle A, Caron F, editors. Proceedings of the Symposium on Anadromous Sturgeon: Status and Trends, Anthropogenic Impact, and Essential Habitat. American Fisheries Society Symposium 56; Bethesda, Maryland: 2007. pp. 347–363. [Google Scholar]
- Kalinowski ST. ONCOR: Software for genetic stock identification. 2008 Available at: http://www.montana.edu/kalinowski/Software.htm.
- King TL, Lubinski BA, Spidle AP. Microsatellite DNA variation in Atlantic sturgeon (Acipenser oxyrinchus oxyrinchus) and cross-species amplification in the Acipenseridae. Conservation Genetics. 2001;2:103–119. [Google Scholar]
- Kocik J, Lipsky C, Miller T, Rago P, Shepherd . An Atlantic Sturgeon Population Index for ESA Management Analysis. US Dept Commer, Northeast Fish Sci Cent; 2013. p. 36. Ref Doc. 13-06 Available from: National Marine Fisheries Service, 166 Water Street, Woods Hole, MA 02543-1026, or online at http://www.nefsc.noaa.gov/nefsc/ publications/ [Google Scholar]
- Ludwig A, Debus L, Lieckfeldt D, Wirgin I, Benecke N, Jenneckens I, Williot P, Waldman JR, Pitra C. When the American sea sturgeon swam east: a colder Baltic Sea greeted this fish from across the Atlantic Ocean in the Middle Ages. Nature. 2002;419:447–448. doi: 10.1038/419447a. [DOI] [PubMed] [Google Scholar]
- May B, Krueger CC, Kincaid HL. Genetic variation at microsatellite loci in sturgeon: primer sequence homology in Acipenser and Scaphirhynchus. Canadian Journal of Fisheries and Aquatic Sciences. 1997;54:1542–1547. [Google Scholar]
- Peterson DL, Bain M, Haley N. Evidence of declining recruitment of Atlantic sturgeon in the Hudson River. North American Journal of Fisheries Management. 2000;20:231–238. [Google Scholar]
- Peterson DL, Schueller P, DeVries R, Fleming J, Grunwald C, Wirgin I. Annual run size and genetic characteristics of Atlantic sturgeon in the Altamaha River, Georgia. Transactions of the American Fisheries Society. 2008;137:393–401. [Google Scholar]
- Piry S, Alapetite A, Cornuet JM, Paetkau D, Baudouin L, Estoup A. GeneClass2: A software for genetic assignment and first-generation migrant detection. Journal of Heredity. 2004;95:536–539. doi: 10.1093/jhered/esh074. [DOI] [PubMed] [Google Scholar]
- Rannala B, Mountain JL. Detecting immigration by using multilocus genotypes. Proceedings National Academy of Sciences USA. 1997;94:9197–9221. doi: 10.1073/pnas.94.17.9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghai-Maroof MA, Soliman KM, Jorgensen RA, Allard RW. Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proceedings National Academy of Sciences USA. 1984;81:8014–8018. doi: 10.1073/pnas.81.24.8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secor DH. Atlantic sturgeon fisheries and stock abundances during the late nineteenth century. American Fisheries Society Symposium. 2002;28:89–98. [Google Scholar]
- Secor DH, Waldman JR. Historical abundance of Delaware Bay Atlantic sturgeon and potential rate of recovery. American Fisheries Society Symposium. 1999;23:203–216. [Google Scholar]
- Smith TIJ. The fishery, biology, and management of Atlantic sturgeon, Acipenser oxyrhynchus, in North America. Environmental Biology of Fishes. 1985;14:61–72. [Google Scholar]
- Smith TIJ, Clugston JP. Status and management of Atlantic sturgeon, Acipenser oxyrinchus, in North America. Environmental Biology of Fishes. 1997;48:335–346. [Google Scholar]
- Stein AB, Friedland KD, Sutherland M. Atlantic sturgeon marine distribution and habitat use along the Northeastern Coast of the United States. Transactions of the American Fisheries Society. 2004;133:527–537. [Google Scholar]
- Stewart ND, Dadswell MJ, Leblanc P, Bradford RG, Ceapa C, Stokesbury MJW. North American Journal Fisheries Management. In press. [Google Scholar]
- Van Eenennaam JP, Doroshov SI, Moberg GP, Watson JG, Moore DS, Linares J. Reproductive conditions of the Atlantic sturgeon (Acipenser oxyrinchus) in the Hudson River. Estuaries. 1996;19:769–777. [Google Scholar]
- Waldman JR, Hart JT, Wirgin II. Stock composition of the New York Bight Atlantic sturgeon fishery based on analysis of mitochondrial DNA. Transactions of the American Fisheries Society. 1996;125:364–371. [Google Scholar]
- Waldman JR, King T, Savoy T, Maceda L, Grunwald C, Wirgin I. Stock origins of subadult and adult Atlantic sturgeon, Acipenser oxyrinchus, in a non-natal estuary, Long Island Sound. Estuaries and Coasts. 2013;36:257–267. [Google Scholar]
- Waples RS. Distinct Population Segments. Publications, Agencies and Staff of the U.S. Department of Commerce; 2006. p. 489. [Google Scholar]
- Wirgin I, Breece MW, Fox DA, Maceda L, Wark KW, King TL. Origin of Atlantic sturgeon oxyrinchus oxyrinchus collected off the Delaware Coast during spring months. North American Journal of Fisheries Management. In Press. [Google Scholar]
- Wirgin I, Maceda L, Waldman JR, Wehrell S, Dadswell M, King T. Stock origin of migratory Atlantic sturgeon in the Minas Basin, Inner Bay of Fundy, Canada, determined by microsatellite and mitochondrial DNA analyses. Transactions of the American Fisheries Society. 2012;141:1389–1398. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.