Abstract
Significance: Deceased patients who have suffered severe traumatic brain injury (TBI) are the largest source of organs for lung transplantation. However, due to severely compromised pulmonary lung function, only one-third of these patients are eligible organ donors, with far fewer capable of donating lungs (∼20%). As a result of this organ scarcity, understanding and controlling the pulmonary pathophysiology of potential donors are key to improving the health and long-term success of transplanted lungs. Recent Advances: Although the exact mechanism by which TBI produces pulmonary pathophysiology remains unclear, it may be related to the release of damage-associated molecular patterns (DAMPs) from the injured tissue. These heterogeneous, endogenous host molecules can be rapidly released from damaged or dying cells and mediate sterile inflammation following trauma. In this review, we highlight the interaction of the DAMP, high-mobility group box protein 1 (HMGB1) with the receptor for advanced glycation end-products (RAGE), and toll-like receptor 4 (TLR4). Critical Issues: Recently published studies are reviewed, implicating the release of HMGB1 as producing marked changes in pulmonary inflammation and physiology following trauma, followed by an overview of the experimental evidence demonstrating the benefits of blocking the HMGB1-RAGE axis. Future Directions: Targeting the HMGB1 signaling axis may increase the number of lungs available for transplantation and improve long-term benefits for organ recipient patient outcomes. Antioxid. Redox Signal. 23, 1316–1328.
Introduction
Severe neurologic injury due to trauma often results in numerous cells that are killed in a nonspecific manner as well as displacement of physical structures of the brain, including damage to blood vessels, axonal shearing, alterations in the blood–brain barrier, and intracranial hemorrhaging. Subsequent to the initial traumatic brain injury (TBI) is a secondary injury cycle, which includes ischemia, cerebral hypoxia, hypotension, cerebral edema, and raised intracranial pressure. This array of events is also accompanied by the release of excitotoxic neurotransmitters, which damage both neural and non-neural cell types and further amplify the induction of numerous biochemical cascades and initiate neurodegeneration (61). Depending on the severity of the trauma, TBI patients may also be subjected to complications of non-neurologic organ dysfunction (NNOD).
The pathophysiology of NNOD following TBI is unclear. Apart from the direct nervous system involvement, including the hypothalamus–pituitary axis and sympathetic nervous system efferent limbs and resultant massive release of catecholamines (31), there is also systemic release of inflammatory mediators, such as pro- and anti-inflammatory cytokines, chemokines, complement factors, and reactive oxygen species, which can produce direct injury within the heart and lungs (43, 48, 54, 65). Although the concentrations of many of these factors may be highest within the brain extracellular compartment due to decreased cerebral blood flow (18, 102), release into venous drainage can occur rapidly following disturbances of the blood–brain barrier (48, 118), propagating tissue damage in susceptible organ systems, leading to further inflammation, tissue damage, and mortality (29). By some analyses, NNOD produces mortality that is proportionally similar to initiating neurological complications (91).
Pulmonary Complications Related to TBI
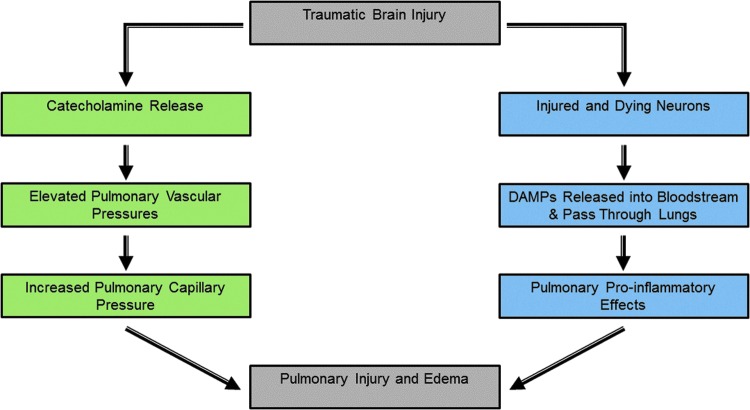
Pulmonary complications are among the most prevalent NNODs encountered in the TBI population (95). Although intensive care units (ICUs) strive to optimize oxygen delivery following severe brain injury, little is known regarding the pathophysiology of pulmonary dysfunction secondary to the neurologic insult. The most frequent NNODs present in the pulmonary system include the acute respiratory distress syndrome (ARDS) and neurogenic pulmonary edema (NPE). Clinical recognition of ARDS is classically associated with inflammatory processes, including the onset of hypoxemia, reduction in pulmonary compliance, and presence of cellular infiltrates (14). In contrast, NPE often occurs in the absence of direct lung injury and is marked by pulmonary interstitial and alveolar fluid accumulation likely due to disruptions in the integrity of the alveolar capillary membrane (9, 10). Although exploration of either condition can be justified based upon its own specific merits and mechanisms, one must also consider that the temporal elements may potentially act as a continuum; one condition may essentially contribute to the onset of the other. This common pathway may exhibit characteristics of both hemodynamic and inflammatory responses (Fig. 1). The hemodynamic attribute may serve to increase in pulmonary vascular pressure, resulting in hydrostatic edema, while the inflammatory mechanism of brain cytokine and chemokine release causes an increase in the permeability of pulmonary capillaries causing both exudative edema and leukocytic infiltration of the tissue.
FIG. 1.
Canonical and noncanonical mechanisms of traumatic brain injury induced pulmonary dysfunction. The canonical pathway (green rectangles) described for traumatic brain injury-induced effects in the lung depends on the release of catecholamines, which enter the bloodstream and cause elevated pulmonary capillary pressures and permeability. Fluid is able to cross capillary endothelial cells causing pulmonary edema. The noncanonical pathway (blue rectangles) for the effects of traumatic brain injury on pulmonary function involves the release of damage-associated molecular patterns (DAMPs) by injured or dead neurons, which then enter venous blood in the lungs via the pulmonary circulation and cause pulmonary injury and dysfunction. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Numerous inflammatory mediators are thought to contribute to the latter response and include interleukin (IL)-1 beta, IL-6, and tumor necrosis factor (TNF)-alpha (43, 48, 54, 65). In addition to these factors are damage-associated molecular patterns (DAMPs) (80). Extracellular DAMPs are released from injured or stressed cells and trigger an immune response to injury or trauma. Damaged cells of all types release DAMPs and can influence the inflammatory reactions that follow sterile traumatic injury, such as TBI.
The exact mechanism by which TBI causes pulmonary dysfunction remains unclear, but it may be related specifically to nervous system release of the DAMP, high-mobility group box protein 1 (HMGB1). This review focuses on the numerous inflammatory roles of HMGB1 and its respective receptors, receptor for advanced glycation end-products (RAGE) and toll-like receptor 4 (TLR4), and how they contribute to lung injury, and then specifically addresses the role of this pathway in acute lung injury (ALI) caused by TBI.
High-Mobility Group Box Protein 1
HMGB1, formerly known as amphoterin, was originally discovered as an important protein in neurite outgrowth. HMGB1 was rediscovered and identified as a nuclear factor to enhance DNA transcription (30, 73). HMGB1 has also been called HMG-1, p30, sulfoglucuronyl carbohydrate-binding protein-1 (SBP1), and differentiation-enhancing factor (56). The nomenclature was eventually revised to HMGB1 (21).
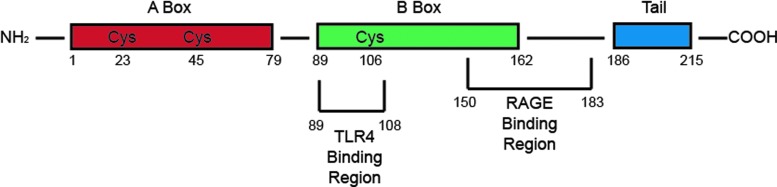
HMGB1 belongs to a family of nonhistone chromosomal proteins, including HMGB2-4 (107). The structure of HMGB1 is very important to its function as post-translational modifications of the molecule can result in not only a difference in functionality but also a change in the localization of the ligand entirely. Expressed as a single polypeptide chain of 215 amino acids, HMGB1 contains two N-terminal DNA-binding domains, HMG box A and box B, and an acidic C-terminal domain. Interestingly, HMGB1 lacks endoplasmic reticulum localization sequences, but instead has two nuclear localization sequences (17, 46, 93, 126). When not acetylated, HMGB1 remains localized in the nucleus and is not secreted or released regardless of injury or insult (34, 64, 70). However, hyperacetylation of the molecule results in cytosolic relocation, allowing for further secretion into the cellular milieu under proper signaling stimulation (34, 70). The N-terminal DNA-binding domains, box A and box B, demonstrate nonspecific interaction with DNA and transcription factors to alter chromosomal architecture. Additional molecular properties of three cysteine residues have been implicated to be involved in extracellular HMGB1 activity, including the redox state of cysteine (C) 106, and a disulfide bond between C23 and C45 (50, 96, 97, 111) (Fig. 2).
FIG. 2.
HMGB1 structure. HMGB1 structure with the A and B box-binding regions as well as the acidic tail. The TLR4 and RAGE4-binding regions are also highlighted. The three cysteine residues are apparent at the 23, 45, and 106 positions. HMGB1, high-mobility group box protein 1; RAGE, receptor for advanced glycation end-products; TLR4, toll-like receptor 4. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
HMGB1 is known to interact with transcription factors and chromatin, although this interaction is generally transient or unstable (116). For example, HMGB1 interacts with numerous transcriptional regulators, such as p53, HOX proteins, Rel, NFAT2, and PU.1, to facilitate expression or repression of targeted genes (2, 68, 71, 76, 140). There is also substantial evidence demonstrating that HMGB1 and histone H1, a chromatin-binding protein, compete for binding sites on chromatin, further regulating transcriptional activation (25, 59, 77). Collectively, HMGB1 can interact directly with DNA, chromatin, and transcription factors to regulate transcription and genetic recombination. HMGB1 is also able to activate cells via several surface receptors, including TLR2, TLR4, and RAGE (51, 57, 84).
Extracellular Forms of HMGB1
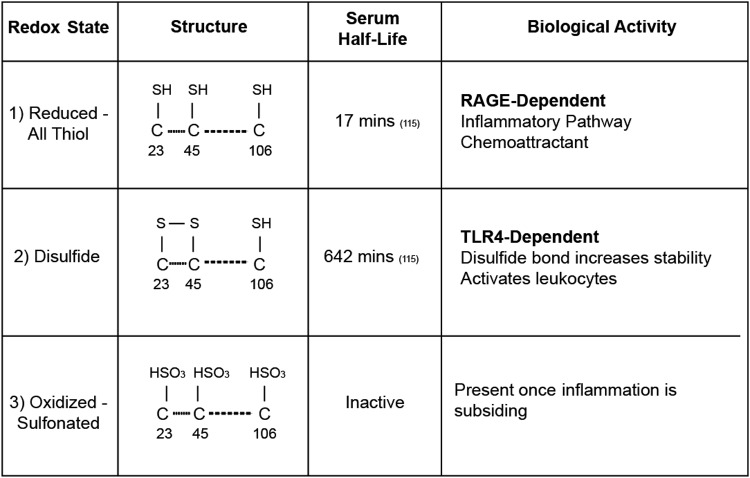
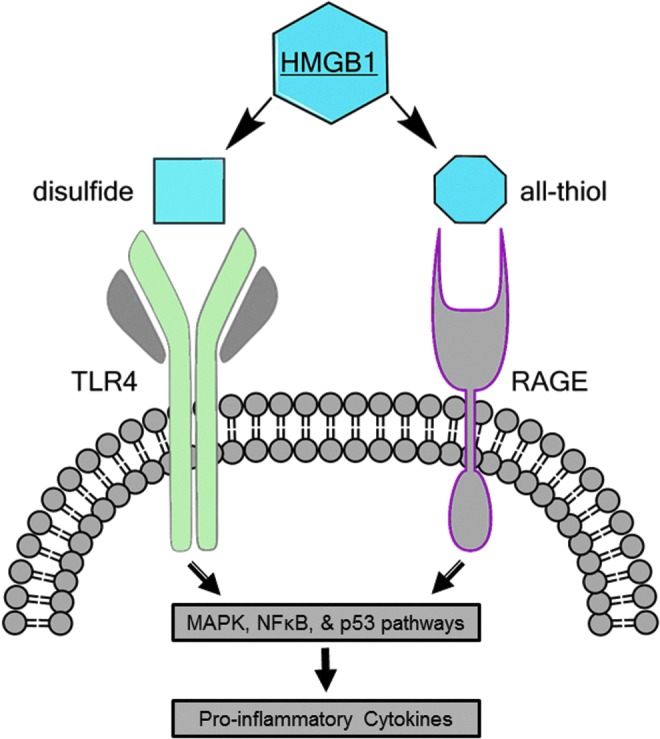
Although HMGB1 is typically associated with chromatin in normal cells, it can be quickly released into the cytoplasm following stress, injury, or disease. The extracellular form of HMGB1 is known to differ dramatically based on the oxidative environment, be it saliva, serum, or tissue parenchyma, and subsequently produces different cellular action (4). For example, the initial form of HMGB1 present upon release into the extracellular space is the all-thiol state. All-thiol HMGB1 is thought to largely act on a member of the Ig superfamily, RAGE, and produces chemoattractant actions on leukocytes (120). There are also reports that all-thiol HMGB1 can form a complex with the chemokine, CXCL12, and act through its cognate receptor, CXCR4 (101, 120). When present in the oxidative environment, cysteines 23 and 46 present in the HMGB1 A box form a sulfide bond, effectively producing the disulfide form of HMGB1 (133). Disulfide HMGB1 can only act on the receptor, TLR4 (110), and influences the production of inflammatory cytokines (133–135). Important work using an NMR-based approach to distinguish the oxidation states and half-lives of HMGB1 in serum, saliva, and cell culture media revealed that the half-life of all-thiol HMGB1 is as short as 17–18 min in human serum and saliva, and the subsequent clearance of the disulfide HMGB1 varies between 65 and 642 min depending on the extracellular fluid (139) (Fig. 3). Disulfide HMGB1 is then further reduced following oxidation of Cys-106 in the B-domain to an inert form (139).
FIG. 3.
HMGB1 redox states and associated biological activity. The three redox states of HMGB1 differ by structure, half-life, and activity. The reduced form contains a thiol group at all three cysteine residues with a serum half-life of 17 min. It is this form that is able to bind to RAGE and activate inflammatory pathways. The disulfide form contains a disulfide bond between the cysteine residues at positions 23 and 45. This bond increases it stability and half-life to 642 min. Additionally, this is the form that is TLR4 dependent. The final inactivated form is known as the oxidized form and has sulfonated cysteines at all three positions. This is the predominant form once inflammation has begun to subside.
HMGB1 Release
Numerous cells types throughout the body can undergo passive release of loosely bound nuclear HMGB1 following tissue injury, including cells of the nervous system (41, 62, 63, 100, 114). However, 25 years after HMGB1 was discovered, Tracey and colleagues determined that HMGB1 can also be actively released from primary monocytes and it functions as a critical cytokine to mediate the immune response to infection and injury (123). Other cell types known to actively release HMGB1 include astrocytes, microglia, and neurons (37, 38, 40, 62, 86, 123). In both ways, HMGB1 orchestrates different cellular functions in consequence to environmental and homeostatic cues to act as a signal for tissue damage, injury, and/or infection.
As noted previously, extracellular HMGB1 may form a complex with other molecules to enhance proinflammatory responses, including lipopolysaccharide (LPS), IL-1, bacterial DNA, CXCL12, CD24, and viral RNA (24, 52, 103). Interestingly, in some cases, application of recombinant HMGB1 alone was shown to lack cytokine function (113). It is likely that this protein took the all-thiol form of HMGB1 as it had chemotactic qualities to recruit enterocytes and smooth muscle, endothelial, and stem cells (32, 51, 74, 81, 99, 121, 131). During active inflammation, the predominant form of HMGB1 is thought to be the disulfide bond between C23 and C45 (disulfide HMGB1); however, when inflammation begins to subside, HMGB1 is terminally oxidized at the cysteine residues, rendering it biological inactive (oxidized HMGB1) (6, 121, 135). Subsequently, an oxidizing environment following inflammation or injury may promote HMGB1 cytokine activity instead of cellular repair by monocyte recruitment (121).
Less is known about HMGB1-mediated degradation. Extracellular HMGB1 undergoes limited proteolysis by serine proteinases that are secreted by stimulated cells. Although the degradation of HMGB1 can produce a 10-amino acid fragment that retains some functional activity in leukocytic cells (105), the kinetics of HMGB1 oxidation and the half-lives of all-thiol and disulfide HMGB1 species in serum, saliva, and cell culture medium are relatively restricted (139).
HMGB1 Receptors: RAGE and TLR4
Extracellular HMGB1 transduces cellular signals through plasma membrane receptors, including TLR2, TLR4, and RAGE (51, 57, 84). Notable binding domains present on the all-thiol HMGB1 form are amino acids 150–183 on the RAGE-binding portion (55), whereas the disulfide HMGB1-binding domains to TLR4 are amino acids 89–108 (133, 135) (Fig. 3). The disulfide bond between C23 and C45 further increases the stability of the folded full-length HMGB1 molecule (97).
Downstream signaling of HMGB1 is facilitated by a number of adaptor proteins, which converge through pathways involving mitogen-activated protein kinase (MAPK) and nuclear factor kappa B (NFκB) and transcriptional regulator, p53 (82, 83, 89, 98) (Fig. 4). Activation of these cascades is known to result in the production and release of proinflammatory cytokines, TNFα, IL-1, IL-6, and IL-8, and several chemokines (5, 85, 87, 94, 128).
FIG. 4.
The interaction between HMGB1 and RAGE/TLR4. Downstream signaling of HMGB1 via RAGE and TLR4 receptor activation is facilitated by a number of adaptor proteins, which converge through pathways involving MAPK, NFκB, and the transcriptional regulator, p53. Activation of these cascades is known to result in the production and release of proinflammatory cytokines, TNFα, IL-1, IL-6, and IL-8, and several chemokines. IL, interleukin; MAPK, mitogen-activated protein kinase; NFκB, nuclear factor kappa B. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
TLR4: Form and Function
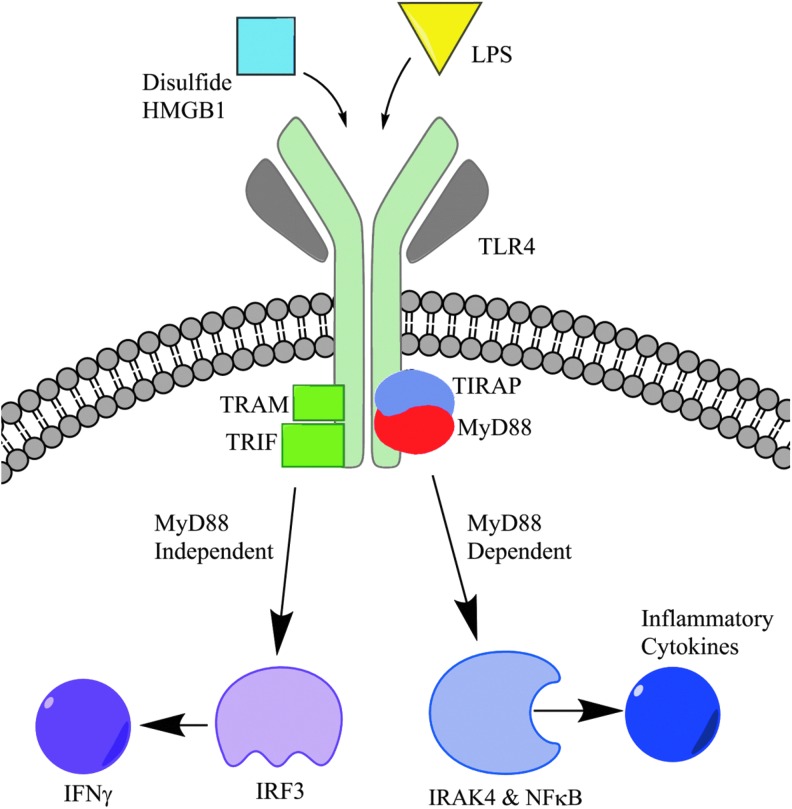
Toll-like receptors, or TLRs, are membrane receptors that play a vital role in host defense by recognizing pathogen-associated molecular patterns or DAMPs with subsequent activation of immune responses. In the 1990s, Janeway and colleagues discovered TLR4, the first identified TLR, by ligating antibodies to induced immune responses (72). Since that discovery, a total of 13 TLRs have been identified, which play a role in multiple pathways, including pathogen recognition and cellular repair and regeneration (39). Ligands for TLRs are numerous and those known to interact with TLR4 include exogenous molecules such as LPS as well as endogenous ligands (DAMPs), such as heat shock proteins, fibrinogen, peptidoglycan, and HMGB1, and others (15, 80, 138). In particular, the HMGB1-TLR4 axis has been implicated in several disease processes such as cerebral ischemia (135, 136) as well as ischemia reperfusion (IR) injury in the heart, liver, and lungs (35, 132). In particular, a translational study using clinical samples and a mouse model demonstrated that TLR4 activation by HMGB1 contributes to the development of pulmonary hypertension and that HMGB1 levels correct with pulmonary arterial pressures (13). After binding with HMGB1, TLR4 downstream inflammatory pathways are both myeloid differentiation primary response gene 88 (MyD88) independent and dependent (Fig. 5).
FIG. 5.
HMGB1-TLR4 binding and downstream activation. TLR4 has two distinct pathway choices, varying by the involvement of the MyD88 adaptor protein. Both pathway products are key to the inflammatory process. LPS, lipopolysaccharide; MyD88, myeloid differentiation primary response gene 88. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
RAGE: Form and Function
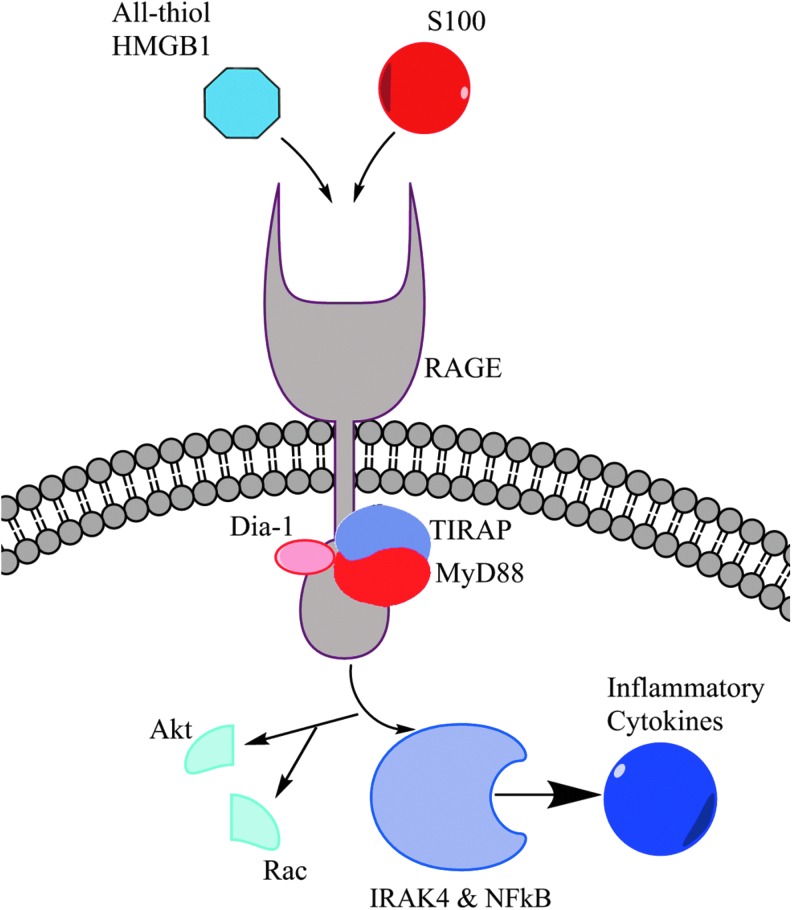
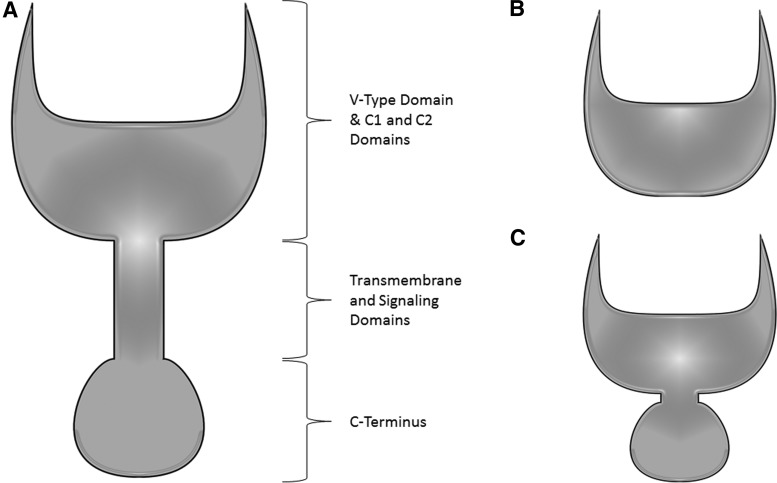
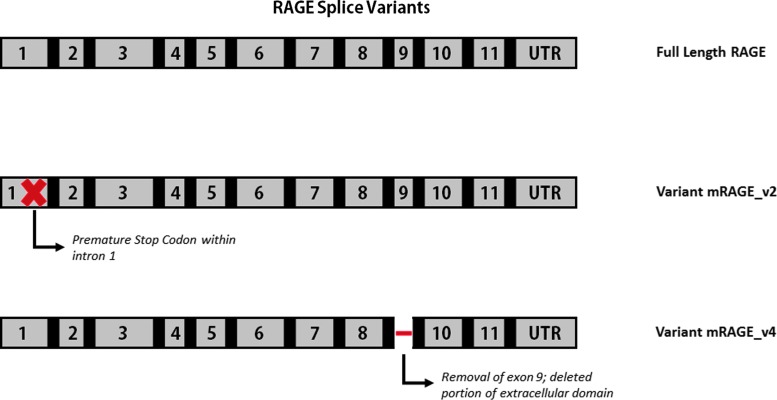
In addition to TLR-mediated pathways of inflammation, HMGB1 is known to bind to the transmembrane receptor, RAGE. RAGE is highly conserved across the mouse, rat, and human and is constitutively expressed at high levels both on pulmonary endothelial and alveolar cells (104). It binds to several proinflammatory molecules, including S100, amyloid fibrils, and HMGB1. Upon binding, the downstream signaling pathway is MyD88 dependent, which is common to one of the TLR4 pathways (Fig. 6). RAGE exists primarily in two forms: a full-length transmembrane form as well as a soluble isoform generated either by splicing or proteolytic cleavage (20, 45). An inert form of RAGE is also known to exist (Fig. 7). This soluble form is able to bind to RAGE ligands in the extracellular space before interaction with the transmembrane form, suggesting its role as a decoy receptor (20). Additionally, several studies have documented that RAGE has multiple splice variants, which have various ligands and functions (36, 53, 84, 137). Recent work has demonstrated that the different variants are distributed in different tissues with the full-length form being found most frequently in all tissues (60). However, over 15 variants were discovered, demonstrating a unique variant distribution in the body (Fig. 8).
FIG. 6.
HMGB1-RAGE binding and downstream activation. The signaling pathway of RAGE involves the key adaptor proteins, MyD88 and TIRAP, which it shares in common with TLR4 signaling. All-thiol HMGB1 also serves to signal an increase in chemotactic activity. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
FIG. 7.
Forms of RAGE. The RAGE receptor can be found in several forms throughout the organism due to splicing variations. (A) Full-length RAGE is the most prominent isoform of the receptor. (B) A soluble form of the receptor exists without the transmembrane domain, signaling domain, or C-Terminus. (C) Inert RAGE receptors can be found with modifications to the signaling domain, preventing signal transduction down the pathway.
FIG. 8.
Splice variants of RAGE. Although full-length RAGE is the most common form isolated in various tissues, multiple variants exist. mRAGE_v1 and mRAGE_v3 have an inclusion of intron 9 causing a shift in the reading frame hypothesis to be a source of soluble RAGE. mRAGE_v2 has a premature stop codon and is most prevalent in the brain. Exon 9 is deleted in mRAGE_v4, which does not affect the reading frame or remove either the transmembrane or cytoplasmic domains. This form is most prominent in the lung (60). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
HMGB1 and TBI
Severe TBI is a major public health problem that accounts for one-third of early mortality due to trauma in the United States. Late mortality is caused by injuries secondary to head trauma, which impact distal organ function and increase susceptibility to infections. In particular, severe brain injury often produces a pronounced impact on lung function (16, 124). The mechanisms by which TBI contributes to pulmonary dysfunction are poorly understood, but are thought to involve a catecholamine surge-associated pulmonary vascular permeability change leading to NPE (7). This injury-induced excess of catecholamine also lends itself to hypertension, abnormal heart variability, and neurological deficits (44, 90, 112). More recent clinical studies implicate the production and release of inflammatory mediators, including HMGB1 (8, 49, 75, 122). Although organ failure scoring systems, present clinical markers, and single cytokine estimates have failed to predict the onset of organ dysfunction in the clinical setting, patterns of early circulating trauma markers such as HMGB1 may serve to guide and streamline damage control following TBI.
There is currently no absolute definition of pathophysiology associated with HMGB1 release following traumatic injury with or without TBI. Some clinical studies of mechanical trauma in the absence of head injury fail to find a correlation between HMGB1 levels in plasma and measures of morbidity (88). However, other studies, which included patients with trauma to the head, suggest that the release of HMGB1 is predictive of both mortality and neurological dysfunction in adult and pediatric patients (8, 28, 42). The presence of the increased HMGB1 levels in both plasma and cerebrospinal fluid may ultimately reflect release from damaged and dying cells in the brain.
No single animal model can adequately mimic all aspects of the human TBI. However, recent investigations using the controlled cortical impact model or fluid percussion in rodents reveal similar rapid changes in HMGB1 in blood and brain tissue (78, 125). The different isoforms of the protein may be integral to pulmonary functional changes and cellular responses by immune cells. For example, the initial all-thiol form of HMGB1 released from the brain may contribute to RAGE-dependent functional chemotaxis of innate immune system cells (119, 130). However, as the half-life of this isoform is relatively short, the disulfide form of HMGB1 may also contribute to organ dysfunction and changes in blood–brain permeability in a TLR4-dependent manner (78, 125, 139). Subsequently, HMGB1 isoforms present in injury conditions may suitably act as priming signals, which are perceived in both the nervous and immune systems (3, 38, 109, 120).
Alternative interpretations include the suggestion that macrophages exposed to catecholamine induce HMGB1 release (68). Additional evidence to support this observation can be obtained by blocking adrenergic receptors or using an agonist of α2-adrenergic receptors, dexmedetomidine, to reduce levels of HMGB1 in blood post-TBI (26, 58).
RAGE and Lung Disease
RAGE has been implicated in several disease processes, such as cancer, diabetes, and Alzheimer's disease. Considering its high expression in the lung, it is not surprising that RAGE has also been associated with several pulmonary diseases, including lung cancer, pulmonary fibrosis, and ALI (12, 33). Multiple studies have demonstrated that several RAGE ligands, including HMGB1, are upregulated in lung cancer and are associated with metastasis and poor outcomes. The HMGB1-RAGE interaction appears to contribute to tumor invasion and metastasis and is believed to be an important target and opportunity for antitumor therapy (69). Additionally, recent work has demonstrated that blocking of the HMGB1-RAGE pathway decreases tumor cell proliferation, invasion, and MMP activity (108).
In addition to lung cancer, RAGE has also been implicated in idiopathic pulmonary fibrosis (IPF). IPF is a progressive debilitating disease with an unclear pathogenesis and no reliable treatment. In a mouse model of pulmonary fibrosis induced by bleomycin, HMGB1 production was elevated from inflammatory cells in the airway and RAGE−/− mice did not respond to HMGB1, suggesting that RAGE may contribute to bleomycin-induced pulmonary fibrosis (47). Conversely, other studies have reported that the loss of RAGE may contribute to pulmonary fibrosis. In clinical studies, patients with IPF demonstrated RAGE expression that was downregulated in lung homogenates and alveolar cells (92). Additionally, soluble RAGE (sRAGE) levels were lower in the BAL fluid of patients with IPF (11). These conflicting reports highlight that RAGE may be implicated in the pathogenesis of fibrosis, but the underlying mechanisms remain uncertain.
RAGE, IR Injury, and Lung Transplantation
Work has also been done to explore the connection between RAGE and IR injury in the setting of lung transplantation. In a murine model of pulmonary reperfusion injury, pharmacologic blockade of RAGE diminished pulmonary function measured by arterial oxygenation, capillary leakage, and histologic injury. Additionally, genetic deletion of RAGE attenuated evidence of ischemic reperfusion injury (106). Similar findings were also obtained in the setting of IR injury of the liver where RAGE blockade protected against hepatocellular death and necrosis in a murine model (141). More recently, lung injury and pulmonary dysfunction were attenuated in RAGE−/− mice. Additionally, the deletion of RAGE was also able to prevent IR injury in a hyperglycemia-enhanced IR animal model (67).
The connection between RAGE and short-term outcomes after lung transplantation has also been studied. Among 20 patients who underwent lung transplantation, plasma RAGE levels were obtained 4 h after the transplant. Among these patients, higher levels of RAGE predicted long durations of mechanical ventilation and ICU stays (22). Among 317 patients undergoing lung transplantation, plasma levels of sRAGE were measured 6 and 24 h post-transplant. Patients who developed primary graft dysfunction had higher levels of sRAGE. Higher levels of sRAGE were also associated with blood transfusions and cardiopulmonary bypass (27). Both these studies demonstrate that elevated levels of sRAGE are associated with worse short-term outcomes after transplantation.
The HMGB1-RAGE Axis and ALI
Several recent studies have demonstrated a connection between HMGB1, RAGE, and ALI and ARDS. HMGB1 has been identified as a marker of ALI in both human and animal studies (1, 117). Aside from the brain injury, elevated HMGB1 levels have been associated with diseases such as sepsis, hemorrhagic shock, and rheumatologic disorders (66, 79). Additionally, both experimental and clinical studies have implicated HMGB1 in ALI (129). Analysis of plasma and lung epithelial lining fluid of patients with ALI secondary to sepsis demonstrated increased levels of HMGB1. HMGB1 was also elevated in plasma and lung fluid from LPS-induced ALI in a mouse model (117). In this scenario, it is believed that HMGB1 is released from normal airways and leaks into the bloodstream after destruction of the alveolar capillary barrier (127).
Additionally, clinical studies in patients with ALI have demonstrated a correlation with plasma sRAGE levels and worse outcomes as defined by severity of lung injury, ventilator-free days, and mortality (23). One study demonstrated sRAGE to be a marker of alveolar cell injury in a rat model. In both experimental and human studies, higher sRAGE concentrations were found in the alveolar space compared with controls (115). Furthermore, RAGE levels in the alveolar fluid were measured after human lungs declined for transplant were perfused, ventilated, and rewarmed. RAGE was found to be inversely correlated with alveolar fluid clearance, suggesting that RAGE can be used as a marker for alveolar cell injury in donor lungs and possibly ALI (19).
Conclusions
This review has sought to describe evidence for the role of HMGB1-RAGE and the HMGB1-TLR4 axis associated with pulmonary dysfunction and central nervous system injury. Better understanding of the manner in which the redox isoforms of HMGB1 influence organ systems following trauma or disease may directly lead to the development of new therapeutic strategies, applying to not only patients with TBI but also the patients who demonstrate subsequent pulmonary complications as a result, as well as the recipients of transplanted lungs. Moreover, the pathogenesis manifested by release of HMGB1 throughout a number of organ systems suggests that therapeutic blockade of the protein will lead to novel directions in transplantation research. Further investigation of the mechanisms behind HMGB1 release, as well as its subsequent signaling pathways, is necessary to advancing understanding of the inflammatory process given that it is no longer restricted or solely related to the immune system. The study of HMGB1 and its receptors provides an exciting direction for the improvement of clinical outcomes of a significant portion of the patient population in a novel manner, all the while allowing for a direct translational connection from the bench to the bedside.
Abbreviations Used
- ALI
acute lung injury
- ARDS
acute respiratory distress syndrome
- DAMP
damage-associated molecular pattern
- HMGB1
high-mobility group box protein 1
- ICU
intensive care unit
- IL
interleukin
- IPF
idiopathic pulmonary fibrosis
- IR
ischemia reperfusion
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MyD88
myeloid differentiation primary response gene 88
- NFκB
nuclear factor kappa B
- NNOD
non-neurologic organ dysfunction
- NPE
neurogenic pulmonary edema
- RAGE
receptor for advanced glycation end-products
- SBP1
sulfoglucuronyl carbohydrate-binding protein-1
- sRAGE
soluble RAGE
- TBI
traumatic brain injury
- TLR4
toll-like receptor 4
- TNF
tumor necrosis factor
References
- 1.Abraham E, Arcaroli J, Carmody A, Wang H, and Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol 165: 2950–2954, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Agresti A, Lupo R, Bianchi ME, and Muller S. HMGB1 interacts differentially with members of the Rel family of transcription factors. Biochem Biophys Res Commun 302: 421–426, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Allette YM, Due MR, Wilson SM, Feldman P, Ripsch MS, Khanna R, and White FA. Identification of a functional interaction of HMGB1 with receptor for advanced glycation end-products in a model of neuropathic pain. Brain Behav Immun 42: 169–177, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson U. and Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol 29: 139–162, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, and Tracey KJ. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med 192: 565–570, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antoine DJ, Williams DP, Kipar A, Laverty H, and Park BK. Diet restriction inhibits apoptosis and HMGB1 oxidation and promotes inflammatory cell recruitment during acetaminophen hepatotoxicity. Mol Med 16: 479–490, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Atkinson JL. The neglected prehospital phase of head injury: apnea and catecholamine surge. Mayo Clin Proc 75: 37–47, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Au AK, Aneja RK, Bell MJ, Bayir H, Feldman K, Adelson PD, Fink EL, Kochanek PM, and Clark RS. Cerebrospinal fluid levels of high-mobility group box 1 and cytochrome C predict outcome after pediatric traumatic brain injury. J Neurotrauma 29: 2013–2021, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avlonitis VS, Wigfield CH, Golledge HD, Kirby JA, and Dark JH. Early hemodynamic injury during donor brain death determines the severity of primary graft dysfunction after lung transplantation. Am J Transplant 7: 83–90, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Avlonitis VS, Wigfield CH, Kirby JA, and Dark JH. The hemodynamic mechanisms of lung injury and systemic inflammatory response following brain death in the transplant donor. Am J Transplant 5: 684–693, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Bargagli E, Penza F, Bianchi N, Olivieri C, Bennett D, Prasse A, and Rottoli P. Controversial role of RAGE in the pathogenesis of idiopathic pulmonary fibrosis. Respir Physiol Neurobiol 165: 119–120; author reply 121–122, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Barrett T, Troup DB, Wilhite SE, Ledoux P, Rudnev D, Evangelista C, Kim IF, Soboleva A, Tomashevsky M, and Edgar R. NCBI GEO: mining tens of millions of expression profiles—database and tools update. Nucleic Acids Res 35: D760–D765, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bauer EM, Shapiro R, Zheng H, Ahmad F, Ishizawar D, Comhair SA, Erzurum SC, Billiar TR, and Bauer PM. High mobility group box 1 contributes to the pathogenesis of experimental pulmonary hypertension via activation of Toll-like receptor 4. Mol Med 18: 1509–1518, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, and Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149: 818–824, 1994 [DOI] [PubMed] [Google Scholar]
- 15.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81: 1–5, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Bittner HB, Kendall SW, Chen EP, Craig D, and Van Trigt P. The effects of brain death on cardiopulmonary hemodynamics and pulmonary blood flow characteristics. Chest 108: 1358–1363, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, and Bianchi ME. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J 22: 5551–5560, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouma GJ, Muizelaar JP, Choi SC, Newlon PG, and Young HF. Cerebral circulation and metabolism after severe traumatic brain injury: the elusive role of ischemia. J Neurosurg 75: 685–693, 1991 [DOI] [PubMed] [Google Scholar]
- 19.Briot R, Frank JA, Uchida T, Lee JW, Calfee CS, and Matthay MA. Elevated levels of the receptor for advanced glycation end products, a marker of alveolar epithelial type I cell injury, predict impaired alveolar fluid clearance in isolated perfused human lungs. Chest 135: 269–275, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buckley ST. and Ehrhardt C. The receptor for advanced glycation end products (RAGE) and the lung. J Biomed Biotechnol 2010: 917108, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bustin M. Revised nomenclature for high mobility group (HMG) chromosomal proteins. Trends Biochem Sci 26: 152–153, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Calfee CS, Budev MM, Matthay MA, Church G, Brady S, Uchida T, Ishizaka A, Lara A, Ranes JL, deCamp MM, and Arroliga AC. Plasma receptor for advanced glycation end-products predicts duration of ICU stay and mechanical ventilation in patients after lung transplantation. J Heart Lung Transplant 26: 675–680, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calfee CS, Ware LB, Eisner MD, Parsons PE, Thompson BT, Wickersham N, Matthay MA, and Network NA. Plasma receptor for advanced glycation end products and clinical outcomes in acute lung injury. Thorax 63: 1083–1089, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campana L, Bosurgi L, Bianchi ME, Manfredi AA, and Rovere-Querini P. Requirement of HMGB1 for stromal cell-derived factor-1/CXCL12-dependent migration of macrophages and dendritic cells. J Leukoc Biol 86: 609–615, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Catez F, Yang H, Tracey KJ, Reeves R, Misteli T, and Bustin M. Network of dynamic interactions between histone H1 and high-mobility-group proteins in chromatin. Mol Cell Biol 24: 4321–4328, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang Y, Huang X, Liu Z, Han G, Huang L, Xiong YC, and Wang Z. Dexmedetomidine inhibits the secretion of high mobility group box 1 from lipopolysaccharide-activated macrophages in vitro. J Surg Res 181: 308–314, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Christie JD, Shah CV, Kawut SM, Mangalmurti N, Lederer DJ, Sonett JR, Ahya VN, Palmer SM, Wille K, Lama V, Shah PD, Shah A, Weinacker A, Deutschman CS, Kohl BA, Demissie E, Bellamy S, Ware LB; Lung Transplant Outcomes Group. Plasma levels of receptor for advanced glycation end products, blood transfusion, and risk of primary graft dysfunction. Am J Respir Crit Care Med 180: 1010–1015, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen MJ, Brohi K, Calfee CS, Rahn P, Chesebro BB, Christiaans SC, Carles M, Howard M, and Pittet JF. Early release of high mobility group box nuclear protein 1 after severe trauma in humans: role of injury severity and tissue hypoperfusion. Crit Care 13: R174, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corral L, Javierre CF, Ventura JL, Marcos P, Herrero JI, and Manez R. Impact of non-neurological complications in severe traumatic brain injury outcome. Crit Care 16: R44, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daston MM. and Ratner N. Expression of P30, a protein with adhesive properties, in Schwann cells and neurons of the developing and regenerating peripheral nerve. J Cell Biol 112: 1229–1239, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davison DL, Chawla LS, Selassie L, Tevar R, Junker C, and Seneff MG. Neurogenic pulmonary edema: successful treatment with IV phentolamine. Chest 141: 793–795, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Degryse B, Bonaldi T, Scaffidi P, Muller S, Resnati M, Sanvito F, Arrigoni G, and Bianchi ME. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J Cell Biol 152: 1197–1206, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demling N, Ehrhardt C, Kasper M, Laue M, Knels L, and Rieber EP. Promotion of cell adherence and spreading: a novel function of RAGE, the highly selective differentiation marker of human alveolar epithelial type I cells. Cell Tissue Res 323: 475–488, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Diener KR, Al-Dasooqi N, Lousberg EL, and Hayball JD. The multifunctional alarmin HMGB1 with roles in the pathophysiology of sepsis and cancer. Immunol Cell Biol 91: 443–450, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Ding HS, Yang J, Chen P, Bo SQ, Ding JW, and Yu QQ. The HMGB1-TLR4 axis contributes to myocardial ischemia/reperfusion injury via regulation of cardiomyocyte apoptosis. Gene 527: 389–393, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Ding Q. and Keller JN. Splice variants of the receptor for advanced glycosylation end products (RAGE) in human brain. Neurosci Lett 373: 67–72, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Faraco G, Fossati S, Bianchi ME, Patrone M, Pedrazzi M, Sparatore B, Moroni F, and Chiarugi A. High mobility group box 1 protein is released by neural cells upon different stresses and worsens ischemic neurodegeneration in vitro and in vivo. J Neurochem 103: 590–603, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Feldman P, Due MR, Ripsch MS, Khanna R, and White FA. The persistent release of HMGB1 contributes to tactile hyperalgesia in a rodent model of neuropathic pain. J Neuroinflammation 9: 180, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gangloff M, Weber AN, Gibbard RJ, and Gay NJ. Evolutionary relationships, but functional differences, between the Drosophila and human Toll-like receptor families. Biochem Soc Trans 31: 659–663, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Gao HM, Zhou H, Zhang F, Wilson BC, Kam W, and Hong JS. HMGB1 acts on microglia Mac1 to mediate chronic neuroinflammation that drives progressive neurodegeneration. J Neurosci 31: 1081–1092, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giancotti V, Bandiera A, Sindici C, Perissin L, and Crane-Robinson C. Calcium-dependent ADP-ribosylation of high-mobility-group I (HMGI) proteins. Biochem J 317 (Pt 3): 865–870, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gibot S, Massin F, Cravoisy A, Barraud D, Nace L, Levy B, and Bollaert PE. High-mobility group box 1 protein plasma concentrations during septic shock. Intensive Care Med 33: 1347–1353, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Goodman JC, Van M, Gopinath SP, and Robertson CS. Pro-inflammatory and pro-apoptotic elements of the neuroinflammatory response are activated in traumatic brain injury. Acta Neurochir Suppl 102: 437–439, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Hamill RW, Woolf PD, McDonald JV, Lee LA, and Kelly M. Catecholamines predict outcome in traumatic brain injury. Ann Neurol 21: 438–443, 1987 [DOI] [PubMed] [Google Scholar]
- 45.Hanford LE, Enghild JJ, Valnickova Z, Petersen SV, Schaefer LM, Schaefer TM, Reinhart TA, and Oury TD. Purification and characterization of mouse soluble receptor for advanced glycation end products (sRAGE). J Biol Chem 279: 50019–50024, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hardman CH, Broadhurst RW, Raine AR, Grasser KD, Thomas JO, and Laue ED. Structure of the A-domain of HMG1 and its interaction with DNA as studied by heteronuclear three- and four-dimensional NMR spectroscopy. Biochemistry 34: 16596–16607, 1995 [DOI] [PubMed] [Google Scholar]
- 47.He M, Kubo H, Ishizawa K, Hegab AE, Yamamoto Y, Yamamoto H, and Yamaya M. The role of the receptor for advanced glycation end-products in lung fibrosis. Am J Physiol Lung Cell Mol Physiol 293: L1427–L1436, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Helmy A, Carpenter KL, Menon DK, Pickard JD, and Hutchinson PJ. The cytokine response to human traumatic brain injury: temporal profiles and evidence for cerebral parenchymal production. J Cereb Blood Flow Metab 31: 658–670, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hinson HE, Rowell S, and Schreiber M. Clinical evidence of inflammation driving secondary brain injury: a systematic review. J Trauma Acute Care Surg 78: 184–191, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoppe G, Talcott KE, Bhattacharya SK, Crabb JW, and Sears JE. Molecular basis for the redox control of nuclear transport of the structural chromatin protein Hmgb1. Exp Cell Res 312: 3526–3538, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, Nagashima M, Lundh ER, Vijay S, Nitecki D, et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem 270: 25752–25761, 1995 [DOI] [PubMed] [Google Scholar]
- 52.Hreggvidsdottir HS, Lundberg AM, Aveberger AC, Klevenvall L, Andersson U, and Harris HE. High mobility group box protein 1 (HMGB1)-partner molecule complexes enhance cytokine production by signaling through the partner molecule receptor. Mol Med 18: 224–230, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hudson BI, Carter AM, Harja E, Kalea AZ, Arriero M, Yang H, Grant PJ, and Schmidt AM. Identification, classification, and expression of RAGE gene splice variants. FASEB J 22: 1572–1580, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Hutchinson PJ, O'Connell MT, Rothwell NJ, Hopkins SJ, Nortje J, Carpenter KL, Timofeev I, Al-Rawi PG, Menon DK, and Pickard JD. Inflammation in human brain injury: intracerebral concentrations of IL-1alpha, IL-1beta, and their endogenous inhibitor IL-1ra. J Neurotrauma 24: 1545–1557, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Huttunen HJ, Fages C, Kuja-Panula J, Ridley AJ, and Rauvala H. Receptor for advanced glycation end products-binding COOH-terminal motif of amphoterin inhibits invasive migration and metastasis. Cancer Res 62: 4805–4811, 2002 [PubMed] [Google Scholar]
- 56.Huttunen HJ. and Rauvala H. Amphoterin as an extracellular regulator of cell motility: from discovery to disease. J Intern Med 255: 351–366, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Ivanov S, Dragoi AM, Wang X, Dallacosta C, Louten J, Musco G, Sitia G, Yap GS, Wan Y, Biron CA, Bianchi ME, Wang H, and Chu WM. A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood 110: 1970–1981, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ji MH, Zhu XL, Liu FF, Li GM, Tian M, Wu J, Fan YX, Li N, and Yang JJ. Alpha 2A-adrenoreceptor blockade improves sepsis-induced acute lung injury accompanied with depressed high mobility group box-1 levels in rats. Cytokine 60: 639–645, 2012 [DOI] [PubMed] [Google Scholar]
- 59.Ju BG, Lunyak VV, Perissi V, Garcia-Bassets I, Rose DW, Glass CK, and Rosenfeld MG. A topoisomerase IIbeta-mediated dsDNA break required for regulated transcription. Science 312: 1798–1802, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Kalea AZ, Reiniger N, Yang H, Arriero M, Schmidt AM, and Hudson BI. Alternative splicing of the murine receptor for advanced glycation end-products (RAGE) gene. FASEB J 23: 1766–1774, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Katayama Y, Maeda T, Koshinaga M, Kawamata T, and Tsubokawa T. Role of excitatory amino acid-mediated ionic fluxes in traumatic brain injury. Brain Pathol 5: 427–435, 1995 [DOI] [PubMed] [Google Scholar]
- 62.Kim JB, Lim CM, Yu YM, and Lee JK. Induction and subcellular localization of high-mobility group box-1 (HMGB1) in the postischemic rat brain. J Neurosci Res 86: 1125–1131, 2008 [DOI] [PubMed] [Google Scholar]
- 63.Kim JB, Sig Choi J, Yu YM, Nam K, Piao CS, Kim SW, Lee MH, Han PL, Park JS, and Lee JK. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J Neurosci 26: 6413–6421, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klune JR, Dhupar R, Cardinal J, Billiar TR, and Tsung A. HMGB1: endogenous danger signaling. Mol Med 14: 476–484, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kobori N, Clifton GL, and Dash P. Altered expression of novel genes in the cerebral cortex following experimental brain injury. Brain Res Mol Brain Res 104: 148–158, 2002 [DOI] [PubMed] [Google Scholar]
- 66.Kokkola R, Sundberg E, Ulfgren AK, Palmblad K, Li J, Wang H, Ulloa L, Yang H, Yan XJ, Furie R, Chiorazzi N, Tracey KJ, Andersson U, and Harris HE. High mobility group box chromosomal protein 1: a novel proinflammatory mediator in synovitis. Arthritis Rheum 46: 2598–2603, 2002 [DOI] [PubMed] [Google Scholar]
- 67.Lapar DJ, Hajzus VA, Zhao Y, Lau CL, French BA, Kron IL, Sharma AK, and Laubach VE. Acute hyperglycemic exacerbation of lung ischemia-reperfusion injury is mediated by receptor for advanced glycation end-products signaling. Am J Respir Cell Mol Biol 46: 299–305, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu H, Yao YM, Ding LH, Zhang H, Yuan B, Song Q, Ye QN, Huang CF, and Sheng ZY. High mobility group box-1 protein acts as a coactivator of nuclear factor of activated T cells-2 in promoting interleukin-2 transcription. Int J Biochem Cell Biol 41: 641–648, 2009 [DOI] [PubMed] [Google Scholar]
- 69.Logsdon CD, Fuentes MK, Huang EH, and Arumugam T. RAGE and RAGE ligands in cancer. Curr Mol Med 7: 777–789, 2007 [DOI] [PubMed] [Google Scholar]
- 70.Lotze MT. and Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol 5: 331–342, 2005 [DOI] [PubMed] [Google Scholar]
- 71.McKinney K. and Prives C. Efficient specific DNA binding by p53 requires both its central and C-terminal domains as revealed by studies with high-mobility group 1 protein. Mol Cell Biol 22: 6797–6808, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Medzhitov R, Preston-Hurlburt P, and Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388: 394–397, 1997 [DOI] [PubMed] [Google Scholar]
- 73.Merenmies J, Pihlaskari R, Laitinen J, Wartiovaara J, and Rauvala H. 30-kDa heparin-binding protein of brain (amphoterin) involved in neurite outgrowth. Amino acid sequence and localization in the filopodia of the advancing plasma membrane. J Biol Chem 266: 16722–16729, 1991 [PubMed] [Google Scholar]
- 74.Mitola S, Belleri M, Urbinati C, Coltrini D, Sparatore B, Pedrazzi M, Melloni E, and Presta M. Cutting edge: extracellular high mobility group box-1 protein is a proangiogenic cytokine. J Immunol 176: 12–15, 2006 [DOI] [PubMed] [Google Scholar]
- 75.Morganti-Kossmann MC, Satgunaseelan L, Bye N, and Kossmann T. Modulation of immune response by head injury. Injury 38: 1392–1400, 2007 [DOI] [PubMed] [Google Scholar]
- 76.Mouri F, Tsukada J, Mizobe T, Higashi T, Yoshida Y, Minami Y, Izumi H, Kominato Y, Kohno K, and Tanaka Y. Intracellular HMGB1 transactivates the human IL1B gene promoter through association with an Ets transcription factor PU.1. Eur J Haematol 80: 10–19, 2008 [DOI] [PubMed] [Google Scholar]
- 77.Ogawa Y, Aizawa S, Shirakawa H, and Yoshida M. Stimulation of transcription accompanying relaxation of chromatin structure in cells overexpressing high mobility group 1 protein. J Biol Chem 270: 9272–9280, 1995 [DOI] [PubMed] [Google Scholar]
- 78.Okuma Y, Liu K, Wake H, Zhang J, Maruo T, Date I, Yoshino T, Ohtsuka A, Otani N, Tomura S, Shima K, Yamamoto Y, Yamamoto H, Takahashi HK, Mori S, and Nishibori M. Anti-high mobility group box-1 antibody therapy for traumatic brain injury. Ann Neurol 72: 373–384, 2012 [DOI] [PubMed] [Google Scholar]
- 79.Ombrellino M, Wang H, Ajemian MS, Talhouk A, Scher LA, Friedman SG, and Tracey KJ. Increased serum concentrations of high-mobility-group protein 1 in haemorrhagic shock. Lancet 354: 1446–1447, 1999 [DOI] [PubMed] [Google Scholar]
- 80.Oppenheim JJ. and Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol 17: 359–365, 2005 [DOI] [PubMed] [Google Scholar]
- 81.Palumbo R. and Bianchi ME. High mobility group box 1 protein, a cue for stem cell recruitment. Biochem Pharmacol 68: 1165–1170, 2004 [DOI] [PubMed] [Google Scholar]
- 82.Palumbo R, De Marchis F, Pusterla T, Conti A, Alessio M, and Bianchi ME. Src family kinases are necessary for cell migration induced by extracellular HMGB1. J Leukoc Biol 86: 617–623, 2009 [DOI] [PubMed] [Google Scholar]
- 83.Palumbo R, Galvez BG, Pusterla T, De Marchis F, Cossu G, Marcu KB, and Bianchi ME. Cells migrating to sites of tissue damage in response to the danger signal HMGB1 require NF-kappaB activation. J Cell Biol 179: 33–40, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park IH, Yeon SI, Youn JH, Choi JE, Sasaki N, Choi IH, and Shin JS. Expression of a novel secreted splice variant of the receptor for advanced glycation end products (RAGE) in human brain astrocytes and peripheral blood mononuclear cells. Mol Immunol 40: 1203–1211, 2004 [DOI] [PubMed] [Google Scholar]
- 85.Park JS, Arcaroli J, Yum HK, Yang H, Wang H, Yang KY, Choe KH, Strassheim D, Pitts TM, Tracey KJ, and Abraham E. Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am J Physiol Cell Physiol 284: C870–C879, 2003 [DOI] [PubMed] [Google Scholar]
- 86.Passalacqua M, Patrone M, Picotti GB, Del Rio M, Sparatore B, Melloni E, and Pontremoli S. Stimulated astrocytes release high-mobility group 1 protein, an inducer of LAN-5 neuroblastoma cell differentiation. Neuroscience 82: 1021–1028, 1998 [DOI] [PubMed] [Google Scholar]
- 87.Pedrazzi M, Patrone M, Passalacqua M, Ranzato E, Colamassaro D, Sparatore B, Pontremoli S, and Melloni E. Selective proinflammatory activation of astrocytes by high-mobility group box 1 protein signaling. J Immunol 179: 8525–8532, 2007 [DOI] [PubMed] [Google Scholar]
- 88.Peltz ED, Moore EE, Eckels PC, Damle SS, Tsuruta Y, Johnson JL, Sauaia A, Silliman CC, Banerjee A, and Abraham E. HMGB1 is markedly elevated within 6 hours of mechanical trauma in humans. Shock 32: 17–22, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Penzo M, Molteni R, Suda T, Samaniego S, Raucci A, Habiel DM, Miller F, Jiang HP, Li J, Pardi R, Palumbo R, Olivotto E, Kew RR, Bianchi ME, and Marcu KB. Inhibitor of NF-kappa B kinases alpha and beta are both essential for high mobility group box 1-mediated chemotaxis [corrected]. J Immunol 184: 4497–4509, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Perkes I, Baguley IJ, Nott MT, and Menon DK. A review of paroxysmal sympathetic hyperactivity after acquired brain injury. Ann Neurol 68: 126–135, 2010 [DOI] [PubMed] [Google Scholar]
- 91.Piazza O, Venditto A, and Tufano R. Neurogenic pulmonary edema in subarachnoid hemorrage. Panminerva Med 53: 203–210, 2011 [PubMed] [Google Scholar]
- 92.Queisser MA, Kouri FM, Konigshoff M, Wygrecka M, Schubert U, Eickelberg O, and Preissner KT. Loss of RAGE in pulmonary fibrosis: molecular relations to functional changes in pulmonary cell types. Am J Respir Cell Mol Biol 39: 337–345, 2008 [DOI] [PubMed] [Google Scholar]
- 93.Read CM, Cary PD, Crane-Robinson C, Driscoll PC, and Norman DG. Solution structure of a DNA-binding domain from HMG1. Nucleic Acids Res 21: 3427–3436, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ren PC, Zhang Y, Zhang XD, An LJ, Lv HG, He J, Gao CJ, and Sun XD. High-mobility group box 1 contributes to mechanical allodynia and spinal astrocytic activation in a mouse model of type 2 diabetes. Brain Res Bull 88: 332–337, 2012 [DOI] [PubMed] [Google Scholar]
- 95.Rincon F, Ghosh S, Dey S, Maltenfort M, Vibbert M, Urtecho J, McBride W, Moussouttas M, Bell R, Ratliff JK, and Jallo J. Impact of acute lung injury and acute respiratory distress syndrome after traumatic brain injury in the United States. Neurosurgery 71: 795–803, 2012 [DOI] [PubMed] [Google Scholar]
- 96.Rubartelli A. and Lotze MT. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol 28: 429–436, 2007 [DOI] [PubMed] [Google Scholar]
- 97.Sahu D, Debnath P, Takayama Y, and Iwahara J. Redox properties of the A-domain of the HMGB1 protein. FEBS Lett 582: 3973–3978, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sakaguchi M, Murata H, Yamamoto K, Ono T, Sakaguchi Y, Motoyama A, Hibino T, Kataoka K, and Huh NH. TIRAP, an adaptor protein for TLR2/4, transduces a signal from RAGE phosphorylated upon ligand binding. PLoS One 6: e23132, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sappington PL, Yang R, Yang H, Tracey KJ, Delude RL, and Fink MP. HMGB1 B box increases the permeability of Caco-2 enterocytic monolayers and impairs intestinal barrier function in mice. Gastroenterology 123: 790–802, 2002 [DOI] [PubMed] [Google Scholar]
- 100.Scaffidi P. and Misteli T, and Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418: 191–195, 2002 [DOI] [PubMed] [Google Scholar]
- 101.Schiraldi M, Raucci A, Munoz LM, Livoti E, Celona B, Venereau E, Apuzzo T, De Marchis F, Pedotti M, Bachi A, Thelen M, Varani L, Mellado M, Proudfoot A, Bianchi ME, and Uguccioni M. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med 209: 551–563, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schroder ML, Muizelaar JP, Kuta AJ, and Choi SC. Thresholds for cerebral ischemia after severe head injury: relationship with late CT findings and outcome. J Neurotrauma 13: 17–23, 1996 [DOI] [PubMed] [Google Scholar]
- 103.Sha Y, Zmijewski J, Xu Z, and Abraham E. HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J Immunol 180: 2531–2537, 2008 [DOI] [PubMed] [Google Scholar]
- 104.Sims GP, Rowe DC, Rietdijk ST, Herbst R, and Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol 28: 367–388, 2010 [DOI] [PubMed] [Google Scholar]
- 105.Sparatore B, Patrone M, Passalacqua M, Pedrazzi M, Gaggero D, Pontremoli S, and Melloni E. Extracellular processing of amphoterin generates a peptide active on erythroleukaemia cell differentiation. Biochem J 357: 569–574, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sternberg DI, Gowda R, Mehra D, Qu W, Weinberg A, Twaddell W, Sarkar J, Wallace A, Hudson B, D'Ovidio F, Arcasoy S, Ramasamy R, D'Armiento J, Schmidt AM, and Sonett JR. Blockade of receptor for advanced glycation end product attenuates pulmonary reperfusion injury in mice. J Thorac Cardiovasc Surg 136: 1576–1585, 2008 [DOI] [PubMed] [Google Scholar]
- 107.Stros M, Launholt D, and Grasser KD. The HMG-box: a versatile protein domain occurring in a wide variety of DNA-binding proteins. Cell Mol Life Sci 64: 2590–2606, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C, Hofmann MA, Kislinger T, Ingram M, Lu A, Tanaka H, Hori O, Ogawa S, Stern DM, and Schmidt AM. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature 405: 354–360, 2000 [DOI] [PubMed] [Google Scholar]
- 109.Tang D, Billiar TR, and Lotze MT. A Janus tale of two active high mobility group box 1 (HMGB1) redox states. Mol Med 18: 1360–1362, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tang D, Kang R, Cheh CW, Livesey KM, Liang X, Schapiro NE, Benschop R, Sparvero LJ, Amoscato AA, Tracey KJ, Zeh HJ, and Lotze MT. HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene 29: 5299–5310, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tang D, Kang R, Zeh HJ, 3rd, and Lotze MT. High-mobility group box 1, oxidative stress, and disease. Antioxid Redox Signal 14: 1315–1335, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tran TY, Dunne IE, and German JW. Beta blockers exposure and traumatic brain injury: a literature review. Neurosurg Focus 25: E8, 2008 [DOI] [PubMed] [Google Scholar]
- 113.Tsan MF. Heat shock proteins and high mobility group box 1 protein lack cytokine function. J Leukoc Biol 89: 847–853, 2011 [DOI] [PubMed] [Google Scholar]
- 114.Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, Peng X, Stolz DB, Geller DA, Rosengart MR, and Billiar TR. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med 204: 2913–2923, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Uchida T, Shirasawa M, Ware LB, Kojima K, Hata Y, Makita K, Mednick G, Matthay ZA, and Matthay MA. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med 173: 1008–1015, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ueda T. and Yoshida M. HMGB proteins and transcriptional regulation. Biochim Biophys Acta 1799: 114–118, 2010 [DOI] [PubMed] [Google Scholar]
- 117.Ueno H, Matsuda T, Hashimoto S, Amaya F, Kitamura Y, Tanaka M, Kobayashi A, Maruyama I, Yamada S, Hasegawa N, Soejima J, Koh H, and Ishizaka A. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am J Respir Crit Care Med 170: 1310–1316, 2004 [DOI] [PubMed] [Google Scholar]
- 118.Unterberg AW, Stover J, Kress B, and Kiening KL. Edema and brain trauma. Neuroscience 129: 1021–1029, 2004 [DOI] [PubMed] [Google Scholar]
- 119.van Zoelen MA, Achouiti A, and van der Poll T. RAGE during infectious diseases. Front Biosci (Schol Ed) 3: 1119–1132, 2011 [DOI] [PubMed] [Google Scholar]
- 120.Venereau E, Schiraldi M, Uguccioni M, and Bianchi ME. HMGB1 and leukocyte migration during trauma and sterile inflammation. Mol Immunol 55: 76–82, 2013 [DOI] [PubMed] [Google Scholar]
- 121.Vezzoli M, Castellani P, Corna G, Castiglioni A, Bosurgi L, Monno A, Brunelli S, Manfredi AA, Rubartelli A, and Rovere-Querini P. High-mobility group box 1 release and redox regulation accompany regeneration and remodeling of skeletal muscle. Antioxid Redox Signal 15: 2161–2174, 2011 [DOI] [PubMed] [Google Scholar]
- 122.Walko TD, 3rd, Bola RA, Hong JD, Au AK, Bell MJ, Kochanek PM, Clark RS, and Aneja RK. Cerebrospinal fluid mitochondrial DNA: a novel DAMP in pediatric traumatic brain injury. Shock 41: 499–503, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, and Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285: 248–251, 1999 [DOI] [PubMed] [Google Scholar]
- 124.Wauters S, Somers J, De Vleeschauwer S, Verbeken E, Verleden GM, van Loon J, and Van Raemdonck DE. Evaluating lung injury at increasing time intervals in a murine brain death model. J Surg Res 183: 419–426, 2013 [DOI] [PubMed] [Google Scholar]
- 125.Weber DJ, Gracon AS, Ripsch MS, Fisher AJ, Cheon BM, Pandya PH, Vittal R, Capitano ML, Kim Y, Allette YM, Riley AA, McCarthy BP, Territo PR, Hutchins GD, Broxmeyer HE, Sandusky GE, White FA, and Wilkes DS. The HMGB1-RAGE axis mediates traumatic brain injury-induced pulmonary dysfunction in lung transplantation. Sci Transl Med 6: 252ra124, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 126.Weir HM, Kraulis PJ, Hill CS, Raine AR, Laue ED, and Thomas JO. Structure of the HMG box motif in the B-domain of HMG1. EMBO J 12: 1311–1319, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wolfson RK, Chiang ET, and Garcia JGN. HMGB1 induces human lung endothelial cell cytoskeletal rearrangement and barrier disruption. Microvasc Res 81: 189–197, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wu H, Ma J, Wang P, Corpuz TM, Panchapakesan U, Wyburn KR, and Chadban SJ. HMGB1 contributes to kidney ischemia reperfusion injury. J Am Soc Nephrol 21: 1878–1890, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wu X, Mi Y, Yang H, Hu A, Zhang Q, and Shang C. The activation of HMGB1 as a progression factor on inflammation response in normal human bronchial epithelial cells through RAGE/JNK/NF-kappaB pathway. Mol Cell Biochem 380: 249–257, 2013 [DOI] [PubMed] [Google Scholar]
- 130.Yan SF, Ramasamy R, and Schmidt AM. Mechanisms of disease: advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat Clin Pract Endocrinol Metab 4: 285–293, 2008 [DOI] [PubMed] [Google Scholar]
- 131.Yang D, Chen Q, Yang H, Tracey KJ, Bustin M, and Oppenheim JJ. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J Leukoc Biol 81: 59–66, 2007 [DOI] [PubMed] [Google Scholar]
- 132.Yang H, Antoine DJ, Andersson U, and Tracey KJ. The many faces of HMGB1: molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J Leukoc Biol 93: 865–873, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, Lu B, Chavan S, Rosas-Ballina M, Al-Abed Y, Akira S, Bierhaus A, Erlandsson-Harris H, Andersson U, and Tracey KJ. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci U S A 107: 11942–11947, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang H, Lundback P, Ottosson L, Erlandsson-Harris H, Venereau E, Bianchi ME, Al-Abed Y, Andersson U, Tracey KJ, and Antoine DJ. Redox modification of cysteine residues regulates the cytokine activity of high mobility group box-1 (HMGB1). Mol Med 18: 250–259, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 135.Yang QW, Lu FL, Zhou Y, Wang L, Zhong Q, Lin S, Xiang J, Li JC, Fang CQ, and Wang JZ. HMBG1 mediates ischemia-reperfusion injury by TRIF-adaptor independent Toll-like receptor 4 signaling. J Cereb Blood Flow Metab 31: 593–605, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang QW, Wang JZ, Li JC, Zhou Y, Zhong Q, Lu FL, and Xiang J. High-mobility group protein box-1 and its relevance to cerebral ischemia. J Cereb Blood Flow Metab 30: 243–254, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yonekura H, Yamamoto Y, Sakurai S, Petrova RG, Abedin MJ, Li H, Yasui K, Takeuchi M, Makita Z, Takasawa S, Okamoto H, Watanabe T, and Yamamoto H. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem J 370: 1097–1109, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yu DH, Nho DH, Song RH, Kim SH, Lee MJ, Nemzek JA, and Park J. High-mobility group box 1 as a surrogate prognostic marker in dogs with systemic inflammatory response syndrome. J Vet Emerg Crit Care (San Antonio) 20: 298–302, 2010 [DOI] [PubMed] [Google Scholar]
- 139.Zandarashvili L, Sahu D, Lee K, Lee YS, Singh P, Rajarathnam K, and Iwahara J. Real-time kinetics of high-mobility group box 1 (HMGB1) oxidation in extracellular fluids studied by in situ protein NMR spectroscopy. J Biol Chem 288: 11621–11627, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zappavigna V, Falciola L, Helmer-Citterich M, Mavilio F, and Bianchi ME. HMG1 interacts with HOX proteins and enhances their DNA binding and transcriptional activation. EMBO J 15: 4981–4991, 1996 [PMC free article] [PubMed] [Google Scholar]
- 141.Zeng S, Feirt N, Goldstein M, Guarrera J, Ippagunta N, Ekong U, Dun H, Lu Y, Qu W, Schmidt AM, and Emond JC. Blockade of receptor for advanced glycation end product (RAGE) attenuates ischemia and reperfusion injury to the liver in mice. Hepatology 39: 422–432, 2004 [DOI] [PubMed] [Google Scholar]