Abstract
Significance: Mitochondria, vital cellular power plants to generate energy, are involved in immune responses. Mitochondrial damage-associated molecular patterns (DAMPs) are molecules that are released from mitochondria to extracellular space during cell death and include not only proteins but also DNA or lipids. Mitochondrial DAMPs induce inflammatory responses and are critically involved in the pathogenesis of various diseases. Recent Advances: Recent studies elucidate the molecular mechanisms by which mitochondrial DAMPs are released and initiate immune responses by use of genetically modulated cells or animals. Importantly, the levels of mitochondrial DAMPs in patients are often associated with severity and prognosis of human diseases, such as infection, asthma, ischemic heart disease, and cancer. Critical Issues: Although mitochondrial DAMPs can represent proinflammatory molecules in various experimental models, their roles in human diseases may be multifunctional and complex. It remains unclear where and how mitochondrial DAMPs are liberated into extracellular spaces and exert their biological functions particularly in vivo. In addition, while mitochondria can secrete several types of DAMPs during cell death, the interaction of each mitochondrial DAMP (e.g., synergistic effects) remains unclear. Future Directions: Regulation of mitochondrial DAMP-mediated immune responses may be important to alter the progression of human diseases. In addition, measuring mitochondrial DAMPs in patients may be clinically useful as biomarkers to predict prognosis or response to therapies. Further studies of the mechanisms by which mitochondrial DAMPs impact the initiation and progression of diseases may lead to the development of therapeutics specifically targeting this pathway. Antioxid. Redox Signal. 23, 1329–1350.
Introduction
For the last decades, mitochondria have been extensively studied as critical cellular organelles for energy generation, protein synthesis, catabolism, and cell death (135, 158). Mitochondria are thought to be evolved from an endosymbiont α-proteobacterium and uniquely have their own DNA, which is duplicated during mitochondrial division (43). Recent studies reveal that mitochondria are diversely associated with immune responses (5, 158) and diseases (135, 138, 158). When mitochondria are damaged, the dysfunctional mitochondria increase generation of mitochondrial reactive oxygen species (ROS) in cells (107, 182). These dysfunctional mitochondria are prone to enhance immune responses (107, 142). In addition, recent reports suggest that various mitochondrial molecules can be translocated to the outsides of mitochondria (e.g., cytosol, cell surface, or extracellular spaces) and promote immune responses.
Against invading pathogens, cells exert their defense system by secretion of inflammatory cytokines/chemokines, activating the adaptive immune system and promoting phagocytosis (2). These immune responses are initiated by recognition of pathogen-associated molecular patterns (PAMPs) through both the plasma membrane receptors and the intracellular receptors such as toll-like receptors (TLRs) or nucleotide-binding and oligomerization domain (NOD)-like receptors (2, 30, 125, 136). Each receptor distinctively recognizes various microbe-associated components. These receptor molecules can also activate immune function in response to nonmicrobe-associated molecules (21). When cells are injured or dying by mechanical stress, microbial infection, or other various environmental stresses, these cells release their own components to the extracellular space called damage-associated molecular patterns (DAMPs) (21, 140). DAMPs include DNA, high-mobility group box 1 (HMGB1), or heat shock proteins (21, 140). Similar to PAMPs, these host-derived molecules can be recognized by the receptors, including TLRs or NOD-like receptors (NLRs), and trigger immune responses in various immune cells (e.g., macrophages, dendritic cells [DCs], neutrophils) (21, 140). While original sources of DAMPs include nuclear, plasma membrane, and intracellular proteins (49), recent reports suggest that mitochondria are also major sources of DAMPs (179). The roles of isolated mitochondria on immune responses have been studied before the concept of mitochondrial DAMPs was proposed. For example, in 1982, Carp reported that human mitochondria disrupted by detergent or sonication display chemotaxis of polymorphonuclear leukocyte (PMN) (19). It is also reported that condition media harvested from necrotic cells contain mitochondrial components such as N-formyl peptides (NFPs) and trigger chemotaxis of platelets (28). Although the concise roles of mitochondria on inflammatory response and tissue injuries need to be further elucidated (10, 13, 178, 179), there are a number of reports showing that mitochondria-associated molecules exert various pathophysiological functions. Mitochondria can release the mitochondria-associated molecules when cells are dying in response to the cellular stress, including mechanical stress or infection (Fig. 1). These molecules are called mitochondrial DAMPs and show various immune responses on immune cells such as macrophages or neutrophils (Fig. 1 and Table 1). Although the studies of DAMPs have been focusing on the molecules released to extracellular compartments, recent data suggest that mitochondrial DAMPs (particularly mitochondrial DNA [mtDNA]) released from mitochondria initiate immune responses in the cytosol (107, 131, 142, 163, 164). In addition, the molecular mechanisms by which cells control the escape of mitochondrial DAMPs also have been suggested (e.g., autophagy-related proteins or deoxyribonuclease II [DNase II]) (107, 111). Since the impairment of these molecules increases inflammatory responses and susceptibility to the oxidative stress in vivo (107, 111), the roles of mitochondrial DAMPs in the subcellular compartment are likely to be critical in the pathogenesis of human diseases.
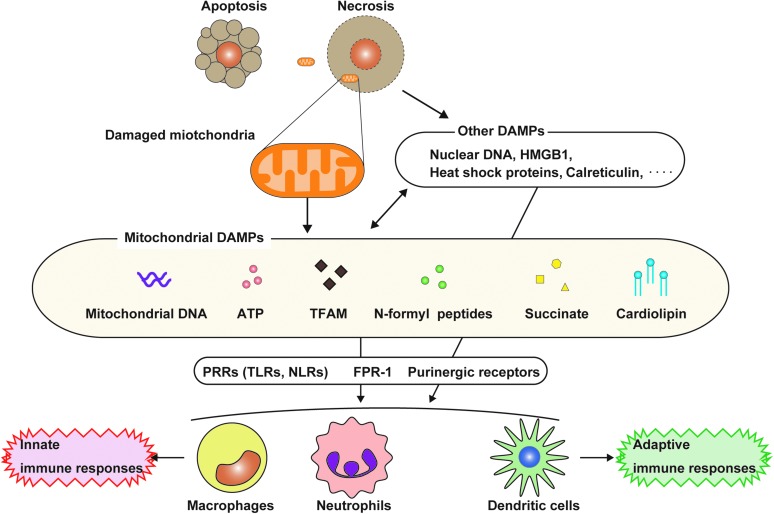
FIG. 1.
DAMPs released from mitochondria. During cell death, various cellular components are released to the extracellular space or exposed to cell surface. The molecules released from dying cells have immune functions and are involved in inflammatory response. These molecules are called DAMPs. Of note, mitochondria release various mitochondrial components, which are involved in inflammatory responses (mitochondrial DAMPs). Damaged cells caused by trauma or infection may undergo cell death, including necrosis. Under these conditions, the necrotic cells can massively leak intracellular components, including mitochondria-related molecules, whereas it is believed that the release of DAMPs from apoptotic cells is limited. Under these conditions, the cells can leak intracellular components, including mitochondria-related molecules. The released or exposed mitochondrial DAMPs can initiate innate or adaptive immune responses by activating cell surface receptors (e.g., P2X7R or FPRs) or intracellular receptors (e.g., TLR9 or NLRP3) after their internalization into the cells. DAMPs, damage-associated molecular patterns; FPR, formyl peptide receptor; TLR, toll-like receptor. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Table 1.
Mitochondria-Derived Damage-Associated Molecular Patterns
| Mitochondrial DAMPs | Models releasing mitochondrial DAMPs into cytosol, cell surface, or extracellular space (in vitro and in vivo) (Ref.) | The roles as mitochondrial DAMPs (Ref.) | The roles of mitochondrial DAMPs in animal disease models (Ref.) |
|---|---|---|---|
| mtDNA | Cytosol: ATP treatment in TLR ligand-primed macrophages (70, 106, 107, 142). Cytosol: Activation of Bak and Bax (131, 164). Cytosol: TFAM depletion (163). Media: Treatment with nitric oxide donor or PMA in neutrophils (75). Media: Treatment with LPS and IL-5 or IL-5 and C5a in eosinophils (171). Media: Treatment with IgE/anti-IgE in mast cells (176). Media: LPS or C5a treatment in GM-CSF-primed neutrophils (172). Serum or brain (cytosol): Irradiated mice (41). Plasma: Trauma/hemorrhagic shock in rats (178). Plasma: Bacillus anthracis infection in baboons (155). Serum: Acetaminophen-induced acute liver injury in mice (97). Media: Treatment with recombinant sPLA2-IIA in human platelet (10). |
AIM2 inflammasome activation (107). NLRP3 inflammasome activation (107, 142). Adhesion of neutrophils and endothelial cells (153). Activation of TLR9 and NF-κB. Secretion of proinflammatory cytokine/chemokine (128, 177, 179). Secretion of proteases (e.g., MMP-8 and -9) (178). Production of type I INF through the cGAS/STING pathway (131, 163, 164). |
IL-5 transgenic mice display intestinal eosinophil infiltration and extracellular mtDNA deposition in response to CLP-induced polymicrobial sepsis (171). Intravenous injection of disrupted mitochondria induces acute lung injury and inflammatory responses in rats (59, 179). Intravenous injection of mtDNA induces inflammation and lung injury in rats (177). Intra-articular injection of mtDNA induces arthritis in mice (25). Activated protein C treatment reduces plasma level of mtDNA and lethality in response to Bacillus anthracis-induced sepsis in baboons (155). Cardiac-specific deletion of DNase IIa increases the mortality and causes severe myocarditis with accumulation of mtDNA deposition in autolysosome in the myocardioum of mice with cardiac hypertrophy (111). Deletion of LOX-1 shows arteriosclerosis with accumulation of mtDNA and inflammation in aorta of the mice (36). Acetaminophen treatment induces marked systemic inflammation and lung injury in mice, which is prevented in TLR9-deficient mice (97). |
| ATP | Media: Fungal exposure (Alternaria alternate) in human bronchial epithelial cells (79). Media: Coculture with epithelial HaCat cells (an immortalized human keratinocyte line) and allogeneic PBMCs or buccal epithelial cells and autologous PBMCs (165). Media: Coculture of blood clots and primary retinal cells (109). Media: IgE and anti-IgE in mast cells (176). Media: NFP treatment in neutrophils (22). Media: MTX treatment in CT26 colorectal carcinoma cells (101). Media: Bleomycin (BLM) treatment in BEAS2B or MLE12 lung epithelial cells (129). BALF: Mice sensitized by i.p. injection of OVA (65). BALF: BLM-injected mice (129). Peritoneal fluid: Irradiated mice (165). |
Potassium efflux through P2XR or P2YR (71, 79). Calcium mobilization (8, 106) NLRP3 inflammasome activation and release of mtDNA to cytosol (106, 107, 142). Mitochondrial ROS generation and dysfunction (106, 107, 142). NADPH oxidase-dependent ROS generation (61, 105). Activation of MAPK pathways (116, 143). Fusion of phagosome and lysosome (promoting killing of intracellular pathogens) (42). Chemotaxis (e.g., neutrophils) (22, 63, 166). Cell death (69, 149, 181) |
Administration of apyrase (ATP-diphosphatase) or suramin inhibits eosinophilic airway inflammation, Th2 cytokine production, and bronchial hyper-reactivity in mice sensitized by the injection of OVA with alum (65). Treatment of suramin and oxidized ATP (antagonists of P2receptor) or genetic deletion of P2X7R inhibits IL-33 release and Th2 responses in fungus-induced airway inflammation in mice (79). Neutralization of ATP by apyrase and genetic deficiency of P2X7R during irradiation-induced GVHD development improve survival of the mice. Stimulation of APCs with ATP promotes IFN-γ production and donor T-cell expansion (165). Deficiency of P2Y2R suppresses recruitment of neutrophils in the peritoneal cavity of mice after intraperitoneal injection of Staphylococcus bacteria (22). Administration of suramin and apyrase inhibits BLM-induced lung inflammation in mice, while injection of ATP further enhances the lung inflammation. Treatment of Pannexin-1 mimetic inhibitory peptide or genetic deletion of P2X7R inhibits bleomycin-induced lung inflammation and fibrosis in mice (129). Subcutaneous injection of MTX-treated cancer cells induces antitumor effects in vivo, which are neutralized by addition of oxidized ATP or suramin (101). |
| TFAM | Serum: Hemorrhagic shock in rats (20). | Triggering secretion of proinflammatory cytokines (e.g., TNF and IL-6) in macrophage (20). Promoting secretion of proinflammatory cytokine by costimulation with CpG DNA or NFP (27, 68). |
Deletion of TFAM in mice displays severe mtDNA depletion and embryonic lethal (86). Administration of recombinant TFAM increases the level of IL-6 and TNF and lactate in serum and MPO activity in the lungs of rats (20). |
| NFP | Media: Hypoxia-induced necrosis in platelets (28). | Calcium influx (179). Promoting secretion of IL-8 by costimulation with mtDNA or CpG (179). Secretion of proteases (MMP-8) (179). Chemotaxis of neutrophils or platelets through FPR1 (19, 28, 179). |
Intravenous injection of disrupted mitochondria induces acute inflammatory lung injury in rats (179). FPR1−/− mice increase susceptibility with Listeria monocytogenes infection (48). Acetaminophen-treated mice exhibit marked systemic inflammation and lung injury, which is prevented by CXCR2-FPR1 blockage (97). |
| Cardiolipin | Cell surface: Fas ligand-induced cell death in U937 (148). | NLRP3 inflammasome activation (66). A potent surfactant inhibitor (127). Binding to Atp8b1 (127). Binding to CD1d and stimulating CD1d-restricted γδ T cells (35). Cell death (cytochrome c) (113). |
Mice given intratracheal injection of cardiolipin display significantly lower lung compliance and higher elastance and resistance compared with the controls (127). Atp8b1 bounds and internalizes cardiolipin from extracellular fluid to lung epithelia. Atp8b1 mutant mice display higher levels of cardiolipin in BALF and abrogate bacteria-induced acute lung injuries. Administration of a peptide, including the cardiolipin-binding motif or Atp8b1 gene transfer in mice, lessens bacteria-induced lung injury and improves survival (127). |
| Succinate | Media: Antimycin treatment in myoblasts (141). | Intracellular calcium mobilization (132). Chemotaxis and cytokine production in DC through GPR91 (132). Antigen-specific activation of helper T cells through the G protein-coupled receptor GPR91 of DCs (132). IL-1β production in macrophages by stabilizing HIF-1α (157). |
Knockout of succinate receptor GRP91 improves survival of skin allograft in mice (132). Succinate treatment further increases the expression of GRP91 and aggravates right ventricular hypertrophy in rats with pulmonary artery banding (168). |
AIM2, absent in melanoma 2; APC, antigen-presenting cells; ATP, adenosine triphosphate; BALF, bronchial alveolar lavage fluid; cGAS, cyclic GMP-AMP synthase; CLP, cecal ligation and puncture; DAMPs, damage-associated molecular patterns; DC, dendritic cell; DNase IIa, deoxyribonuclease IIa; FPR1, formyl peptide receptor 1; GM-CSF, granulocyte-macrophage colony-stimulating factor; GVHD, graft-versus-host disease; HIF-1α, hypoxia-inducible factor 1-alpha; IFN-γ, interferon-γ; IgE, immunoglobulin E; IL, interleukin; LOX-1, lectin-like, oxidized low-density lipoprotein receptor-1; LPS, lipopolysaccharide; Media, cell culture media; MAPK, mitogen-activated protein kinase; MMP, matrix metalloproteinase; MPO, myeloperoxidase; mtDNA, mitochondrial DNA; MTX, mitoxantrone; NADPH oxidase, nicotinamide adenine dinucleotide phosphate oxidase; NFP, N-formyl peptides; OVA, ovalbumin; PMA, phorbol 12-myristate 13-acetate; ROS, reactive oxygen species; STING, stimulator of interferon genes; TFAM, mitochondrial transcription factor A; Th2, T helper 2; TLRs, toll-like receptors; TNF, tumor necrosis factor.
As shown in Table 1, mitochondrial DAMPs include various types of mitochondrial molecules and are released into extracellular spaces or cytosolic spaces. In addition, the functional roles of mitochondrial DAMPs are observed in various disease models both in vitro and in vivo (Table 1 and Fig. 2). Thus, besides the well-known roles of mitochondria as energy generators, mitochondria are DAMP-enriched cellular storage spaces where various cellular stresses can release mitochondrial components with their new biological roles on immune systems.
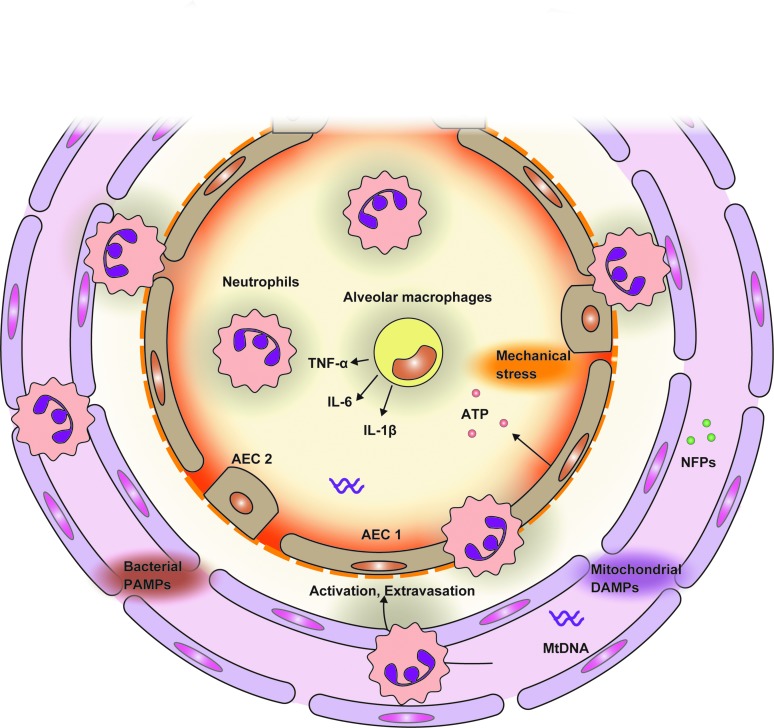
FIG. 2.
The roles of mitochondrial DAMPs on organ injuries. Circulating mitochondrial DAMPs can induce peripheral distant organ injuries. After mitochondrial DAMPs are released into circulation from damaged tissue sites, mitochondrial DAMPs promote adhesion of activated neutrophils and vascular endothelial cells and transmigration of immune cells into distant organs such as the lung. The transmigrated immune cells secrete proinflammatory cytokines and proteinases, leading to inflammatory responses in alveolar spaces. In addition, this immune response may release another mitochondrial DAMP (e.g., ATP) and further enhance inflammatory responses, resulting in exacerbating lung injuries. ATP, adenosine triphosphate. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
In this study, we review the functional roles of mitochondrial DAMPs in the various disease models. We will also discuss the association between mitochondrial DAMPs and human diseases.
Mitochondrial DAMPs
Mitochondrial DNA
MtDNA encodes 37 genes and all of them are essential for normal mitochondrial function (43). The association between mutation of mtDNA and human diseases is well characterized and studied (135, 158). However, it has been unclear if mtDNA has its own direct biological activities, including immune responses. The roles of mtDNA in immune systems and its molecular mechanisms were elucidated from the studies of bacterial DNA (2, 112). TLR9 is a member of the TLR family and is expressed in various types of cells, especially immune cells. TLR9 is localized within the intracellular endosomal compartment and recognizes unmethylated CpG sequences in DNA molecules, which are abundant in bacterial genome and virus DNA (2, 80, 112). Upon recognition, TLR9 initiates immune response, including production of proinflammatory cytokine, chemotaxis, and phagocytosis, through myeloid differentiation primary response gene 88 (MyD88)-dependent pathways (2, 80). Thus, TLR9 exerts various immune responses by binding the unmethylated CpG site of microbial DNA. Mitochondria evolve from saprophytic bacteria to endosymbionts to organelles; therefore, mtDNA contains CpG DNA repeats and is mostly unmethylated (31, 51, 54, 133, 156). Stimulation with mtDNA can increase TLR9 expression in macrophages (177), and mtDNA-mediated activation of p38 MAPK is blocked by cotreatment of inhibitory oligodeoxynucleotides (TTAGGG) that bind CpG motifs (179). These suggest that endogenous host mtDNA can be an inside self-activator of immune systems.
The roles of mtDNA in diseases have been studied by exogenous treatment of mtDNA or mice deficient in mtDNA receptors or binding molecules (Table 1). For example, the roles of mtDNA on inflammatory responses through TLR9 are studied in mice lacking DNase II, an acid DNase localized in the lysosome (36, 111). DNase II has an essential role in the degradation of the DNA of apoptotic cells after macrophages engulf them (64, 73, 74). Cardiac-specific deletion of lysosomal DNase II shows increased mortality and causes severe myocarditis and dilated cardiomyopathy after treatment with pressure overload (111). In addition, DNase II-deficient hearts display infiltration of inflammatory cells, increased gene expression of proinflammatory cytokines, and accumulation of mtDNA deposits in autolysosomes (111). Furthermore, administration of the inhibitory oligodeoxynucleotides against TLR9 or deletion of tlr9 attenuated the development of cardiomyopathy in DNase II-deficient mice (111). Similar roles of mtDNA on inflammatory responses and tissue injuries are also observed in lectin-like, oxidized low-density lipoprotein receptor-1 (LOX-1)-deficient mice, a model of atherosclerosis (36). Accumulated damaged mtDNA due to deficiency of DNase II activates TLR9, leading to the development of atherosclerosis (36). Acetaminophen treatment increases the levels of circulating mtDNA and liver dysfunction in mice, and TLR9-deficent mice display decreased neutrophil-mediated inflammatory responses and liver injury in the acetaminophen-treated mice (97). Recent studies show that extracellular mtDNA activates not only neutrophils (178, 179) but also vascular endothelial cells (153). The activation of these cells by mtDNA promotes adhesion of neutrophils to the endothelium and transmigration of the immune cells, leading to distant organ inflammatory responses (Fig. 3A). Importantly, injection of mtDNA induces acute lung injury (177) and arthritis with infiltration of mononuclear cells in mice (25), suggesting the direct roles of mtDNA on inflammation and tissue injuries in vivo. Furthermore, fragmented mtDNA containing CpG motif spontaneously induces plasmacytoid DC activation (128). These data suggest mtDNA is critically involved in the inflammatory responses and tissue injuries of animal disease models through TLR9 signaling pathways.
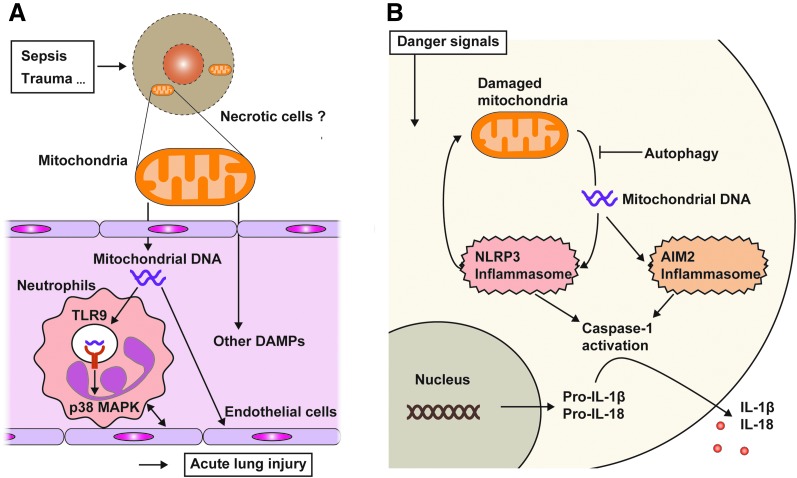
FIG. 3.
Released mtDNA activates TLR9 and inflammasome. (A) MtDNA can be released to the cytosol or extracellular space when cells receive various stresses, including mechanical stress or infection. Released mtDNA activates the TLR9-mediated signaling pathway in immune cells such as neutrophils and initiates the proinflammatory cascade to produce proinflammatory cytokines. Extracellular mtDNA also causes adherence of neutrophils and endothelial cells by activating their adherence molecules (e.g., ICAM1 or e-selectin in endothelial cells; CD18 or L-selectin in neutrophils). These events result in systemic endothelia permeability. (B) In the normal condition, aged or damaged mitochondria in cells are eliminated by the autophagy machinery, called mitophagy, to avoid excess generation of oxidative stress. However, when autophagy (mitophagy) is inhibited or the number of damaged mitochondria is beyond the capacity of the autophagy machinery, mtDNA is released into the cytosol. The cytosolic mtDNA activates NLRP3 inflammasome-mediated caspase-1 activation, leading to the secretion of IL-1β and IL-18. Cytosolic mtDNA can also activate AIM2-dependent inflammasome. AIM2, absent in melanoma 2; ICAM1, intercellular adhesion molecule 1; IL-1β, interleukin-1 beta; mtDNA, mitochondrial DNA. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Another important mechanism by which mtDNA triggers immune responses is the inflammasome. Inflammasomes are multiprotein complexes that activate caspase-1 and downstream immune responses, including the maturation and secretion of proinflammatory cytokine (30, 125, 136). The inflammasomes contain a member of the NLR family (Nod and leucine-rich repeat-containing) or PYHIN family (pyrin domain [PYD] and HIN domain-containing) (30, 125). NLR proteins are key mediators of the inflammasome as studies of gene-deficient mice and cells show that NLR inflammasomes are critically implicated in host defense, cancers, and metabolic and autoimmune disorders (30, 137). Among identified inflammasomes, the NLRP3 inflammasome is the most studied member of this family and is activated by a wide range of signals of pathogenic and nonpathogenic origins (30). Importantly, NLRP3 is also activated by host cell-derived molecules, including extracellular adenosine triphosphate (ATP), hyaluronan, amyloid-β fibrils, and uric acid crystals (30). In addition, the NLRP3 inflammasome is activated by cytosolic mtDNA released from damaged mitochondria in host cells (Fig. 3B) (107). Mitochondria of the cell treated with lipopolysaccharide (LPS) and ATP display severe swelling and destruction of the cristae structure (107). Extracellular ATP treatment rapidly induces mitochondrial dysfunction, including mitochondrial membrane potential transition and generation of mitochondrial ROS in LPS-primed macrophages by activating P2X7 receptor (106, 107). In normal conditions, autophagy, an intracellular process to maintain cellular homeostasis by facilitating the turnover of damaged organelles (104, 126), can remove the dysfunctional mitochondria (mitophagy) (6, 170). However, when the autophagy machinery is inhibited, the dysfunctional mitochondria are accumulated with increased generation of mitochondrial ROS, which leads to mtDNA release into the cytosol and further caspase-1 activation (52, 107) (Fig. 3B). Importantly, exogenous treatment of mtDNA enhances secretion of interleukin-1 beta (IL-1β) in macrophages treated with LPS and ATP, while delivery of DNase I into the cytosol inhibits cytokine secretion (107). In contrast, nuclear DNA is not translocated to the cytosol during this response (107). Similar to the results observed in DNase II-deficient mice with heart failure (111), the accumulation of mtDNA due to the failure of proper elimination of mitochondria can promote immune responses. In addition, autophagy- or DNase-dependent mtDNA digestion may be an important cellular mechanism in the prevention of mitochondrial DAMP-mediated immune responses. In fact, both mice lacking autophagy protein (e.g., LC3B) or DNase II display increased proinflammatory responses and are more susceptible to septic shock or cardiac failure (74, 107, 111).
Recent studies suggest that cytosolic mtDNA promotes cyclic GMP-AMP synthase (cGAS)/stimulator of interferon genes (STING)-mediated type I interferon (IFN) production (131, 163, 164). Moderate mtDNA stress elicited by TFAM deficiency engages cytosolic antiviral signaling to enhance the expression of a subset of IFN-stimulated genes (163). Mechanistically, aberrant mtDNA packaging promotes escape of mtDNA into the cytosol, where it engages the DNA sensor cGAS and promotes STING-IRF3-dependent signaling to potentiate type I IFN responses (163). Interestingly, the mtDNA-released mitochondria also activate the proapoptotic caspase pathway simultaneously, leading to inhibition of type I IFN production (131, 164). Thus, mitochondria play a central role to determine whether dying cells trigger mtDNA-mediated inflammatory responses or remain immunologically silent responses through apoptotic caspases (131, 163, 164).
The translocation of mtDNA from mitochondria to the cytosol was reported in Patrushev et al. (118). The mechanism of mtDNA release includes induction of mitochondrial permeability transition pore as cyclosporine A, a potent inhibitor of pore opening, inhibits translocation of mtDNA from mitochondria to the cytosol (70, 107, 118, 119). The release of nucleic acids into the extracellular space has been thought to be related to the cell death, including apoptosis and necrosis (139). Although the concise mechanisms by which types of cells secrete mtDNA remain unclear, secretion of mtDNA to blood is increased in animal models involved with necrosis or necroptosis (e.g., cecal ligation and puncture, hemorrhagic shock, and irradiation) (38, 41, 178). In addition to the involvement of cell death, mtDNA is also released to the extracellular space in response to microbial killing stimuli, such as LPS+granulocyte-macrophage colony-stimulating factor (75), immunoglobulin E (IgE)+anti-IgE, and LPS+IL-5, respectively, from immune cells such as eosinophils or neutrophils (171, 172, 176). MtDNA is also secreted from activated platelets, leading to promote leukocyte activation (10). MtDNA administered into the peritoneal space of rats is able to be detected in the serum, suggesting that mtDNA released locally can reach the systemic circulation (176). Although concise kinetics of mtDNA in vivo remain unclear, these data also suggest the possibility that circulating mtDNA may originate from a wide range of cell types or tissues.
Finally, it should be noted that mtDNA may have unique biological roles compared with nuclear DNA. For example, while the levels of circulating mtDNA dramatically increased after trauma/hemorrhagic shock in rats, the increase of circulating nuclear DNA is modest (178). Functionally, p38-mediated secretion of matrix metalloproteinase (MMP)-8 and -9 is triggered by mtDNA treatment, but not by nuclear DNA (178). Furthermore, injection of mtDNA causes lung injuries or arthritis; however, nuclear DNA has no effect on the development of these tissue injuries in vivo (25, 177). The concise mechanisms by which mtDNA and nuclear DNA exert these differential immune responses remain unclear. Further studies will be needed to better understand these mechanisms.
Adenosine triphosphate
ATP is the primary source of energy in all living cells by donating a phosphate group during biochemical activities. ATP is synthesized mainly in mitochondria through activation of the glycolysis pathway and tricarboxylic acid (TCA) cycle. ATP is often considered as the molecular unit of currency of intracellular energy transfer. ATP was first thought to be an intracellular energy source, however, later proved to be an important extracellular signaling molecule. ATP is activated by the activation of purinergic receptor subtype, including P2X receptor (P2XR) or P2Y receptor (P2YR) (144, 154). The genetic variance of P2XR or P2YR is associated with various human diseases (47, 146). Recent studies demonstrate that the increase of extracellular ATP levels also critically contributes to pathogenesis of diseases through the activation of the P2XR or P2YR (144, 154). In normal states, secretion of ATP and its extracellular concentration are tightly regulated by ubiquitous ecto-ATP/ADPases (CD39) (91). ATP can be released from various types of cells, including epithelial, endothelial, red blood cells, and immune cells (11, 150). For example, ATP is secreted from damaged epithelial cells induced by mechanical stimuli (55, 169), hypotonic condition (60), or bacterial infection (134). The functions of extracellular ATP include muscle contraction and relaxation, vasodilatation, neurotransmission, platelet aggregation, ion transport regulation, cell growth, and immune response (16, 32, 151).
Of note, the roles of extracellular ATP in immunity and inflammation have been extensively studied in various immune cells, including macrophages, DCs, neutrophils, and lymphocytes (11, 102) (Fig. 4A, B). For example, in neutrophils, the presence of extracellular ATP triggers neutrophil chemotaxis and release of CXCL8 (also known as IL-8) and elastase. ATP also promotes adhesion of neutrophils to endothelial cells by upregulating the β2-integrin macrophage antigen (Mac)-1 (CD11b/CD18) (11). Against invading microbes, ATP promotes phagocytosis of pathogens by upregulating Mac-1 (103, 175) and promotes the degranulation of granules (99, 100) and increases generation of ROS (61, 105, 107). In addition, neutrophils release ATP during their inflammatory activation and may enhance those immune responses in an autocrine or paracrine manner. In monocytes, ATP promotes not only adhesion but also subsequently promotes transmigration through the endothelium by shedding of L-selectin from the surface of monocytes (84, 145). ATP also promotes monocyte migration through CD39 (50). One of most studied roles of ATP in macrophages and monocytes is the secretion of IL-1β and IL-18 through NLRP3 inflammasome activation (Fig. 4B). Binding of ATP to P2X7R causes opening of the P2X7 channel and triggers rapid potassium efflux, resulting in cleavage of caspase-1 (30, 125). Activation of caspase-1 catalyzes the formation of mature IL-1β and IL-18 from pro IL-1β and pro IL-18 (30, 125). Furthermore, ATP treatment also generates prostaglandin E2 (83) and promotes fusion of phagosome and lysosome to promote bacterial killing in macrophages (42) (Fig. 4B).
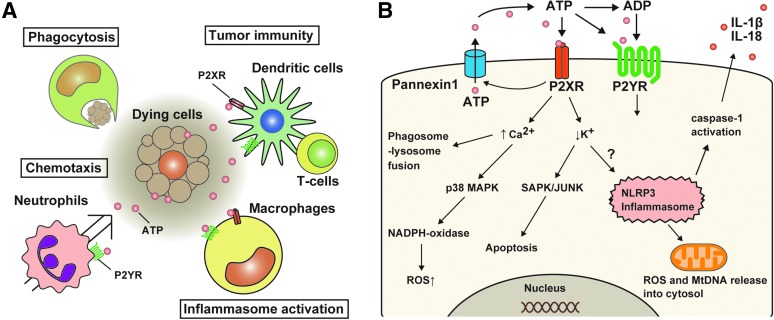
FIG. 4.
Critical roles of extracellular ATP on immune responses. (A) Extracellular ATP has diverse effects on various immune cells, such as macrophages, neutrophils, or T cells. The functional roles of ATP are mediated by P2XR or P2YR. ATP activates NLRP3 inflammasome and promotes secretion of IL-1β and IL-18 in macrophages primed with TLR ligands. ATP also promotes migration and transmigration of immune cells to inflamed sites, killing bacteria, and chemotaxis. The roles of ATP on DCs include maturation and migration of DC and cytokine production. (B) ATP-mediated P2XR or P2YR activation leads to various cellular events. Upon activation of P2XR and P2YR by ATP, potassium efflux and calcium mobilization rapidly occur, which induce activation of NLRP3 inflammasome or MAPKs, leading to initiation of proinflammatory cascade. ATP treatment also causes NADPH oxidase-dependent ROS generation and promotes fusion of phagosome and lysosome, resulting in promoting bacterial killing. DC, dendritic cell; MAPK, mitogen-activated protein kinase; NADPH, nicotinamide adenine dinucleotide phosphate; ROS, reactive oxygen species. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
In addition to the roles of ATP in innate immune, ATP is involved in adaptive immune responses. Extracellular ATP induces shedding of L-selectin from T lymphocytes by activation of P2X7 receptor (67), suggesting the role of ATP in migration of activated lymphocytes to sites of inflammation (11). ATP is also involved in proliferation of lymphocytes and negatively regulates effector function of CD4+ Th cells (11). Similar to other immune cells, T lymphocytes release ATP upon activation (93). In B cells, low concentration of ATP (10–100 μM) triggers B-cell activation, presumably through increased concentration of cytosolic-free Ca2+ (114). Thus, extracellular ATP has various immunological roles depending on its extracellular concentration and targeting of immune cells.
The roles of extracellular ATP have been studied in various disease models, such as cancer, asthma, idiopathic pulmonary fibrosis (IPF), or graft-versus-host disease (GVHD) (Table 1). ATP is secreted from dying tumor cells through the autophagic machinery and promotes the recruitment of immune cells, which improves the efficacy of antineoplastic chemotherapy (95, 101). Increased levels of extracellular ATP are observed in bronchial alveolar lavage fluid (BALF) of asthmatic humans and mice sensitized with ovalbumin and alum (65). Extracellular ATP triggers and maintains asthmatic airway inflammation by activating DCs in the mice (65). T helper 2 (Th2) cytokine production and bronchial hyper-reactivity are suppressed when ATP is neutralized by apyrase or cotreated with broad-spectrum P2-receptor antagonists (i.e., suramin) (65). Similarly, exposure of human bronchial cells to allergen extracts from the fungus Alternaria alternate induces extracellular ATP release, increased concentration of intracellular Ca2+, and secretion of IL-33 (79). The released IL-33 further triggers Th2 cell responses in vitro and in vivo after the exposure with A. alternate (79). The secretion of IL-33 is mediated by autocrine ATP-mediated activation of P2X7R, which is inhibited by antagonists for P2X7R such as oxidized ATP or the gene knockdown of P2X7R (79). Increased levels of extracellular ATP are also observed in the peritoneal fluid after total body irradiation and observed during the development of GVHD in mice and in humans (165). Stimulation of antigen-presenting cells with ATP enhances proinflammatory responses such as IFN-γ production and donor T-cell expansion (165). Neutralization of ATP by apyrase or genetic deficiency of P2X7R during irradiation-induced GVHD development improved survival of the mice (165). The levels of ATP in BALF are also elevated in mice treated with bleomycin, animal models of lung inflammation and fibrosis (129). Intranasal injection of ATP further enhances the bleomycin-induced lung inflammation, while administration of suramin and apyrase inhibits the inflammation of lungs in the mice (129). P2X7R knockout mice display attenuated lung inflammation and fibrosis after bleomycin treatment (129). Since extracellular ATP activates NLRP3 inflammasome through P2X7R, the pathogenesis of bleomycin-induced fibrosis may include the pathway of ATP-P2X7R-NLRP3 inflammasome. Thus, these reports consistently show that inhibition of ATP-mediated P2XR or P2YR signaling pathways ameliorates pathogenesis of the disease, including asthma, fibrosis, or GVHD, suggesting that extracellular ATP is a critical mitochondrial DAMP to promote pathogenesis of various diseases.
Mitochondrial transcription factor A
Mitochondrial transcription factor A (mtTFA, mtTF1, TFAM), a member of the HMG box family, is an essential protein that binds mtDNA in a sequence-independent manner to regulate both mitochondrial transcription initiation and mtDNA copy number (17, 72). The activation of mitochondrial promoters is regulated by the protein levels of TFAM (17, 72). TFAM also binds mitochondrial genome nonspecifically to ensure proper maintenance of mtDNA (17, 72). Heterozygous knockout of TFAM in mice shows reduced mtDNA copy number and respiratory chain deficiency in the heart (86). Furthermore, homozygous knockout of TFAM in mice displays severe mtDNA depletion and impairment of oxidative phosphorylation, resulting in an early embryonic lethality (86). Mice with adipose-specific deletion of TFAM exhibit the higher energy expenditure and are protected from age- and diet-induced obesity, insulin resistance, and hepatosteatosis, despite a greater food intake (162). TFAM in RKO rectal cancer cell lines carrying a TFAM-truncating mutation suppresses cell proliferation and inhibits RKO cell-induced xenograft tumor growth in nude mice (56). Thus, TFAM plays critical roles in mitochondrial functions and pathophysiological condition by regulating a copy number of mtDNA. Similar to HMGB1, an important member of DAMPs (21), TFAM is also involved in immune responses. While a recent study shows that intracellular TFAM negatively regulates antiviral immune response (163), liberated or extracellular TFAM has been shown to promote inflammatory responses. For example, costimulation with NFPs and TFAM synergistically enhances the cytokine secretion (27). Similarly, the effect of CpG DNA on tumor necrosis factor (TNF) secretion in splenocytes is augmented by additional treatment of TFAM (68). These observations suggest that extracellular TFAM in the presence of other mitochondrial DAMPs can synergistically enhance immune responses. In vivo, the serum level of TFAM is elevated in rats subjected to hemorrhagic shock, a model to induce cell death, including necrosis (20). Importantly, administration of recombinant TFAM increases the levels of IL-6 and TNF both in serum of rats and in the media of RAW264.7 macrophages (20). Furthermore, intravenous treatment of TFAM increased myeloperoxidase activity in lung and serum levels of lactate in the rats (20), a currently used biomarker for critically ill patients (1). Thus, these reports suggest that extracellular TFAM can promote inflammatory responses as DAMPs.
N-formyl peptide
Several natural formylated peptides purified form bacteria have been identified as low-molecular-weight chemoattractants to activate human phagocytes (19, 87).
Formylated peptides are recognized by formyl peptide receptors (FPRs), members of the seven transmembrane G protein-coupled receptors superfamily and highly expressing on polymorphonuclear and mononuclear phagocytes (87, 115). In particular, the NFP has been extensively studied due to its diversity of immune functions, such as phagocytosis, generation of reactive oxygen intermediate, and release of proteolytic enzymes (115). Unlike other leukocyte chemoattractants, NFP originates from either mitochondrial proteins of ruptured or dying host cells or microbes such as Escherichia coli (87). This unique character of NFPs suggests that the NFP is involved in host defense against bacterial infection and in the clearance of damaged cells. The immunomodulatory effect of NFPs was first reported as a chemoattractant for neutrophils (19) and subsequently observed in platelets (28). Treatment with purified NFPs isolated from mitochondria promotes chemotaxis of human PMN, whereas nonformylated mitochondrial proteins have no effect (19). Similarly, the synthetic peptides derived from human mitochondrial proteins or Listeria monocytogenes induce chemotaxis (124). Furthermore, knockout of formyl peptide receptor 1 (FPR1), a representative receptor for NFPs, increases susceptibility with L. monocytogenes in mice (48), suggesting that NFP is important for antibacterial immune responses. The roles of NFP on inflammation have been also reported. For example, combined treatment of antagonists for FPR1 and CXC chemokine receptor 2 improved acetaminophen-induced inflammatory responses and liver injury in mice (97). The mechanism by which NFP induces chemotaxis is mediated by increase of Ca2+ influx through FPRs (87). The functions of activated FPRs upon ligation of formylated peptides include morphological polarization, locomotion, production of ROS, and release of proteolytic enzymes (115). In humans, dysfunctional variant FPR alleles are associated with localized juvenile periodonitis (57). Neutrophils from the patients with LJP display a reduced chemotaxis in response to NFPs (29, 161), suggesting important immunological roles of NFPs in humans. The NFP also promotes chemotaxis by secreting IL-8, a potent chemoattractant (27). Costimulation with NFP and mtDNA synergistically increases IL-8 secretion (179). Importantly, the NFP is selectively secreted from necrotic cells (e.g., hypoxia-mediated necrosis), but not from apoptotic cells (e.g., staurosporine-mediated apoptosis), and triggers chemotaxis (28). These data suggest that the necrotic cells are the critical sources of extracellular NFPs.
Succinate
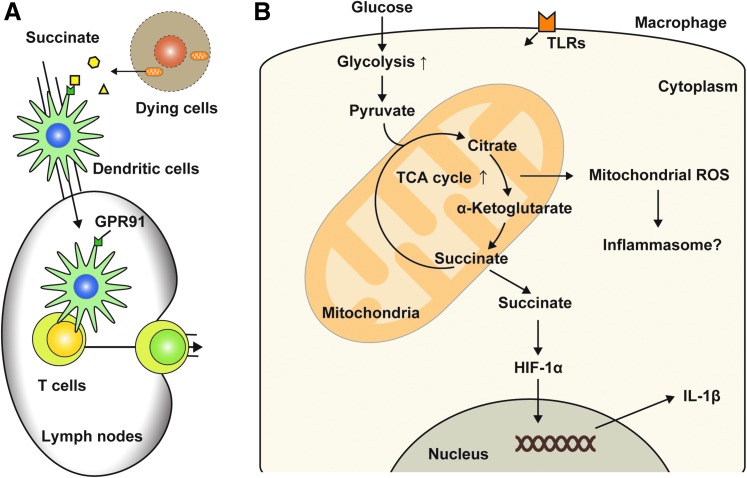
Succinate, an intermediate synthesized in the TCA cycle, donates electrons to the electron transport chain and therefore is essential for maintaining mitochondrial functions. It has been shown that succinate synthesized in mitochondria can be secreted to the extracellular space in vitro (141). The levels of succinate in cell culture media are increased by incubation with antimycin, an inhibitor for mitochondrial electron transport specifically between cytochromes, b and c1, and an inducer of cell death, including necrosis (141). Interestingly, rotenone, another potent inhibitor for the transfer of electrons from iron–sulfur centers in complex I to ubiquinone, has no effect on the secretion of succinate (141). Importantly, extracellular succinate can act as a signaling molecule in both innate and adaptive immune systems. Succinate treatment triggers an intracellular calcium mobilization and a migratory response and also synergistically enhances the production of proinflammatory cytokines (e.g., TNF and INF-γ) induced by TLR ligands in DCs (132). Succinate also enhances antigen-specific activation of human and mouse helper T cells through the G protein-coupled receptor GPR91 of DCs (132) (Fig. 5A). GPR91-deficient allografts elicit a weaker transplant rejection than did the corresponding grafts from wild-type mice (132). Succinate also promotes innate immune response, particularly increases IL-1β gene expression (157) (Fig. 5B). LPS increases the production of succinate by activating glycolysis activity, which contributes to IL-1β production in macrophages by stabilizing hypoxia-inducible factor 1-alpha (HIF-1α) (157). Exogenous treatment of succinate enhances IL-1β production, but not IL-6 and TNF in LPS-primed macrophages, while HIF-1α deficiency impairs the effect of succinate on IL-1β production (157). Succinate is also involved in pathogenesis of pulmonary artery hypertension (PAH) (46, 168). The expression of GPR91 and phosphorylated Akt (p-Akt) in the right ventricle significantly increases in a rat model of pulmonary artery banding PAH model (168). Administration of succinate further increases the p-Akt levels and aggravates right ventricular hypertrophy in vivo (168), although the roles of the succinate-mediated GPR91 signaling pathway on immune responses in the PAH model remain unclear. Thus, these reports suggest that extracellular succinate can play critical roles in pathogenesis of various disease models.
FIG. 5.
Mitochondrial metabolite in immune cells. (A) Succinate is an intermediate produced during TCA cycle in mitochondria and can secrete to the extracellular space when mitochondrial function is disturbed. Extracellular succinate enhances antigen-specific activation of helper T cells through GPR91 of DCs. (B) Glycolysis is a metabolic pathway that provides intermediates for energy generation. The induction of glycolysis by TLR ligands promotes IL-1β gene expression by succinate-mediated stabilization of HIF-1α in macrophages. In addition, high concentrations of glucose increase IL-1β secretion in an NLRP3-dependent mechanism. However, the concise mechanism by which activation of glycolysis-TCA cycle contributes in activation of inflammasome is still unclear. HIF-1α, hypoxia-inducible factor 1-alpha; TCA, tricarboxylic acid. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Cardiolipin
Cardiolipin is a lipid dimer consisting of two phosphatidyl groups bridged by a glycerol in mitochondria and is important for a diverse range of mitochondrial functions (24). Cardiolipin is required for maintaining of the optimal functions of a number of mitochondrial proteins and processes such as mitochondrial respiration and mitochondrial biogenesis (23). Therefore, it is possible that deregulation of cardiolipin metabolism contributes to various pathological conditions (23, 117). The abnormality of cardiolipin metabolism is associated with human diseases such as ischemic conditions, hypothyroidism, aging, and heart failure (23, 117). During death receptor-mediated apoptosis, cardiolipin moves from mitochondria to the cell surface and the other intracellular organelles (147, 148). Cardiolipin regulates cytochrome c release and mitochondrial outer membrane permeabilization (113), suggesting the role of cardiolipin in cell death. In addition, cardiolipin confers immunological effects. Cardiolipin binds to CD1d and stimulates CD1d-restricted γδ T cells (35). Cardiolipin is also required for NLRP3 inflammasome activation (66). Cardiolipin binds to NLRP3 directly and activates inflammasome-mediated immune responses (66). Similar to NFPs, cardiolipin is enriched in both mitochondria and bacterial membranes (78, 81, 90). Therefore, it is speculated that cardiolipin can act as PAMPs and DAMPs. In normal condition, cardiolipin comprises only ∼1–2% of alveolar surfactant and its levels are elevated in acute lung injury models (81, 90). Importantly, exogenous treatment of cardiolipin impairs the surface tension-lowering activity in mice (127). The individuals with progressive familial intrahepatic cholestasis type 1 (PFIC1 or Byler's disease) have mutations in ATP8b1 and the increased risk for pneumonia (127). Atb8b1 binds extracellular cardiolipin and imports into intracellular spaces to remove cardiolipin from the extracellular space. Gene transfer of atp8b1 or treatment of the peptide, including ATP8b1, improves acute lung injuries induced by E. coli in mice (127). In humans, the levels of cardiolipin in tracheal aspirates are elevated in patients with pneumonia (127). Although it is still unclear whether the increased cardiolipin in patients is derived from host mitochondria or bacteria, these results suggest that extracellular cardiolipin is a critical factor in the pathogenesis of acute lung injuries.
The Mitochondrial DAMPs in Human Diseases
The levels of mtDNA in human diseases
While the roles of mitochondrial DAMPs are studied in a wide range of experimental models, the association between mitochondrial DAMPs and human diseases also has been reported. Table 2 demonstrates the list of mitochondrial DAMPs in which levels are changed in the patients with various diseases.
Table 2.
Association with Human Diseases
| Mitochondrial DAMPs | Diseases | Specimen | The levels of DAMPs in diseases. Association with clinical phenotypes | References |
|---|---|---|---|---|
| mtDNA | Rheumatoid arthritis | Plasma Synovial fluid |
The levels of mtDNA are higher in the patients with RA than in the control subjects. | (58) |
| Urological malignancies | Serum | The levels of mtDNA are higher in the patients with urological malignancies (bladder cancer, renal cell carcinoma, or prostate cancer) than in the healthy subjects. | (39) | |
| Prostate cancer | Plasma | The levels of mtDNA are higher in the patients with prostate cancer than the patients with benign diseases. | (98) | |
| The levels of mtDNA are correlated with hemoglobin count and the level of PSA, a tumor marker for prostate cancer. | ||||
| The cancer patients who have higher levels of mtDNA decrease the survival compared with the cancer patients with lower levels of mtDNA. | ||||
| Ovarian cancer | Plasma | The levels of mtDNA are elevated in patients with epithelial ovarian cancer, but not with benign epithelial ovarian tumor, compared with the healthy control group. | (174) | |
| Breast cancer | Plasma | The levels of mtDNA are lower in the patients with breast cancer than control groups. (The levels of nuclear DNA are higher in the patients.) | (77) | |
| Ewing sarcoma | Serum | The levels of mtDNA are lower in the patients with Ewing sarcoma than in the healthy subjects | (173) | |
| Myocardial infarction (MI) | Plasma | The levels of mtDNA are higher and sustained in patients with MI than with stable angina pectoris. | (9) | |
| The levels of mtDNA are higher in patients with transmural MI than with nontransmural MI. | ||||
| Exposure to haloalkanes | Serum | The levels of mtDNA (both 230 and 79 bp) are higher in the group with exposed haloalkane-based pesticides than the control subjects. | (15) | |
| HIV infection | Plasma | The levels of mtDNA are higher in the HIV-positive patients than the healthy controls and the long-term nonprogressors. | (26) | |
| The levels of mtDNA are correlated with plasma viral load. | ||||
| Patients in ER | Plasma | The levels of mtDNA are higher in the patients with severe sepsis on admission to the ER than the control patients. | (82) | |
| The levels of mtDNA are higher in the nonsurvivors than in the survivors. | ||||
| Patients in ER | Plasma | The levels of mtDNA are negatively associated with organ dysfunction. | (121) | |
| Patients in medical ICU | Plasma | The levels of circulating cell-free mtDNA are associated with disease severity in critically ill patients. | (108) | |
| The medical ICU patients who have higher levels of plasma mtDNA decrease the survival compared with the patients with lower levels of mtDNA. | ||||
| Trauma | Plasma | The levels of mtDNA are higher in the patients with trauma than the control subjects. | (179) | |
| Femur fracture reamings | Plasma | The levels of mtDNA are higher in the patients with femur fracture reamings than control volunteers. | (59) | |
| ACABM | Plasma | The levels of mtDNA are higher in the patients with bacterial meningitis than with aseptic meningitis and the normal volunteers. | (94) | |
| The levels of mtDNA are negatively correlated with the modified Barthel index, a scale to measure performance in activities of daily living. Higher levels of mtDNA are associated with poor outcome in patients with ACABM | ||||
| Acute ischemic stroke | Plasma | The levels of mtDNA are elevated in patients with acute ischemic stroke compared with the control subjects. | (160) | |
| Out-of-hospital cardiac arrest | Plasma | The levels of mtDNA are higher in nonsurvivors than in survivors after out-of-hospital cardiac arrest. | (3) | |
| Acute liver failure | Serum | The levels of mtDNA are elevated in patients with acute liver failure and are associated with liver dysfunction marker ALT levels. | (97) | |
| Massive PE | Plasma | Plasma mtDNA concentrations are higher in patients with massive PE than in patients with submassive PE or controls. | (4) | |
| Maintenance hemodialysis | Plasma | The levels of mtDNA are elevated in patients with maintenance hemodialysis compared with healthy subjects. | (18) | |
| Aging | Plasma | MtDNA plasma levels increased gradually after the fifth decade of life and the levels of mtDNA are associated with the levels of proinflammatory cytokines. | (120) | |
| ATP | COPD | BALF | ATP concentration is the highest in the patients with COPD, even after smoking cessation. | (92) |
| ATP concentration is negatively associated with lung function and positively with BALF neutrophil counts. | ||||
| Asthma | BALF | ATP concentration is elevated in the patients with asthma who undergo segmental allergen provocation compared with the control patients. | (65) | |
| Cystic fibrosis | Plasma | ATP concentration is elevated in patients with CF compared with control healthy subjects. | (85) | |
| Sputum, BALF, EBC | ATP concentration is elevated in patients with CF compared with disease control children in BALF, sputum, and EBC. ATP concentration is inversely related to lung function and strongly correlated with neutrophil counts in BALF. | (40) | ||
| IPF | BALF | ATP concentration is elevated in patients with IPF compared with control patients. | (129) | |
| Type 1 diabetes | Plasma | ATP concentration is elevated in patients with type 1 diabetes compared with control healthy subjects. ATP concentration was negatively correlated with coronary flow velocity response after intervention. | (76) | |
| GVHD | Ascites | ATP concentration is elevated in patients with GVHD compared with patients without GVHD who have HCT or patients who do not have HCT. | (165) | |
| PAACG | Aqueous humor | ATP concentration is elevated in patients with PAACG associated with intraocular pressure. | (89, 180) | |
| ATP concentration is associated with intraocular pressure. | ||||
| AMD with subretinal hemorrhage | Vitreous | ATP concentration is elevated in patients with AMD compared with control subjects. | (109) | |
| Primary pulmonary hypertension | Red blood cells | ATP release is impaired in red blood cells of patients with primary pulmonary hypertension. | (152) | |
| Succinate | Metabolic acidosis | Plasma | The levels of succinate are elevated in patients with metabolic acidosis, including diabetic ketoacidosis and lactic acidosis, compared with control subjects. | (45) |
| Acute coronary syndrome | Plasma | The levels of succinate are elevated in patients with non-ST elevation acute coronary syndrome from day 0 (after the diagnosis) to 6 months compared with the healthy control group. | (159) | |
| Cowden syndrome | Plasma | The levels of succinate are elevated in individuals with PTEN, SDHB, and SDHD mutation. | (62) | |
| Exercise | Plasma | The levels of succinate are elevated after exercises, including exercise treadmill testing, bicycle ergometer, and marathon. | (88) | |
| Exercise in patients with type 1 diabetes | Serum | The levels of succinate are elevated after an intense exercise at 80% of VO2 max for 30 min. | (14) | |
| Cardiolipin | Pneumonia | Tracheal aspirates | The levels of cardiolipin are higher in patients with pneumonia than critically ill patients with nonpulmonary illnesses or patients with congestive heart failure. | (127) |
ACABM, adult community-acquired bacterial meningitis; AMD, age-related macular degeneration; ALT, alanine transaminase; CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; EBC, exhaled breath condensate; ER, emergency room; ICU, intensive care unit; IPF, idiopathic pulmonary fibrosis; HCT, hematopoietic cell transplantation; PAACG, primary acute angle closure glaucoma; PE, pulmonary embolism; PSA, prostate-specific antigen; RA, rheumatoid arthritis; VO2 max, maximal oxygen consumption.
The levels of circulating mtDNA are increased in patients with diseases, such as sepsis (82, 167), strokes (160), acute liver failure (97), massive pulmonary embolism (4), or cancers (39, 98, 174), and are also associated with severities or prognosis of the diseases (Table 2). While the levels of circulating mtDNA are elevated in the patients with urological cancers (39, 98) and ovarian cancer (174), the levels of mtDNA are not increased in patients with Ewing sarcoma (173) or breast cancer (77). It is likely that the changes of circulating mtDNA may be dependent on types of cancers or sarcomas. ATP is also elevated in plasma, serum, or BALF from patients with various diseases (Table 2). Importantly, there are many cases where the association between the levels of mitochondrial DAMPs and the severities of human diseases is consistent or similar to that observed in vivo studies. For example, while the elevation of ATP in BALF is observed in patients with IPF (129) or asthma (65), the elevated levels of ATP are also observed in BALF from the experimental mice models of fibrosis (129) or bronchial hypersensitivity (65). Similarly, the levels of circulating cell-free mtDNA are elevated in patients with trauma (167, 179) or sepsis (82, 167) and also observed in animal models of trauma/hemorrhagic shock (178) or bacterial sepsis (155). In addition, the exogenous administration of mitochondrial DAMPs is able to develop the similar pathological conditions with human diseases in animals. Intratracheal injection of ATP abrogates the pathogenesis of bleomycin-induced fibrosis in mice, whereas the levels of ATP in BALF are further elevated in patients with exacerbated IPF compared with stable IPF (129). The levels of mtDNA are elevated in both plasma and synovial fluid of patients with rheumatoid arthritis (58), and the administration of mtDNA develops arthritis in mice (25). These data suggest that mitochondrial DAMPs elevated in the patients may be involved in the pathogenesis of human diseases.
MtDNA: a biomarker in medical intensive care unit
Circulating mtDNA levels are higher in patients admitted in medical intensive care units (ICUs), who died within 28 days of the medical ICU admission, and are also higher in patients with diagnoses commonly associated with mortality in the ICU such as sepsis and acute respiratory distress syndrome (ARDS) (108). Medical ICU patients with elevated mtDNA levels have an increase in their odds of dying within 28 days of ICU admission, although there is no evidence that the association between the mtDNA level and medical ICU mortality is attenuated in models adjusted for clinical covariates, including acute physiology and chronic health evaluation (APACHE) II score, sepsis, or ARDS (108). Furthermore, mtDNA can improve risk prediction even after accounting for the levels of lactate or procalcitonin and APACHE II score among the medical ICU patients (108). Similar findings are observed in the patients presenting at the emergency room. The levels of mtDNA in plasma are elevated in patients with sepsis and only the plasma mtDNA level is independently predictive of fatality among the variables used in the logistic regression (e.g., mechanical ventilation, mean sequential organ failure assessment score, serum lactate, and plasma mtDNA) (82). Importantly, in contrast to the strong association between mtDNA and the mortality in the patients admitted in medical ICUs, no evidence of association is observed in patients in nonmedical ICU patients such as surgical ICU, although there is no evidence that the mtDNA level is higher in patients from medical ICUs when compared with surgical ICUs (108). MtDNA could be released from cells or tissue as the result of surgery, and in this setting, an elevated mtDNA level may be unlikely to be associated with an increased mortality.
Since mtDNA acts as a potent activator of inflammasome (107), it is possible that the inflammasome is also associated with pathogenesis of critical care illness. In fact, the levels of circulating IL-18, a representative inflammasome-mediated proinflammatory cytokine, are elevated among the patients with sepsis and ARDS and are associated with the disease severity and mortality (37, 53, 110). Importantly, the plasma levels of mtDNA and IL-18 are positively associated among the medical ICU patients (108).
The levels of mtDNA are decreased in mononuclear cells from septic patients compared with healthy volunteers, and the cellular mtDNA copy numbers are negatively associated with APACHE II score (122), suggesting the possible sources of circulating cell-free mtDNA in critically ill patients. In addition, a recent study suggests that platelets can secrete mitochondria upon platelet activation (10), which can also explain the increased levels of extracellular mtDNA reported in blood in various pathological conditions. However, at this point, it remains unclear which type of cells are responsible for secretion of mtDNA into blood in those critically ill patients. Further studies will be needed to clarify this point. In summary, circulating cell-free mtDNA could be a valuable addition to assessment of patients in the ICU or emergency room and point the way to the possibility of new diagnostic and/or therapeutic approaches for patients with critical illness.
ATP: a biomarker in lung diseases
While the roles of extracellular ATP and P2X7R have been reported in various experimental disease models, clinical relevance of ATP and P2XR or P2YR is also studied in patients. Of note, recent studies demonstrate the association between the levels of extracellular ATP and the various lung diseases, such as chronic obstructive pulmonary disease (COPD) (92), asthma (65), IPF (129), and cystic fibrosis (40) (Table 2). The levels of ATP in BALF are elevated in patients with COPD compared with smokers or ex-smokers (92). ATP levels in BALF are correlated negatively with lung function and positively with BALF neutrophil counts (92). In addition, blood neutrophils harvested from the patients with COPD show a stronger chemotaxis and an elastase release in response to ATP treatment compared with the control subjects (92). In patients with asthma, in addition to increased levels of ATP in BALF, the functional activity or Single Nucleotide Polymorphism of P2X7R is also associated with the exacerbation or severity of asthma (33, 96). While the pathogenesis of these lung diseases differs from each other, inflammatory responses are commonly involved in the development and acute exacerbation of these diseases. When lung epithelial cells are damaged or injured by oxidative stress or invading microbes, these cells may secrete ATP into the alveolar space. Subsequently, the released ATP activates P2X7R of immune cells (e.g., alveolar macrophages) and may further enhance inflammatory responses in the lungs. In addition, the activation of P2X7R by ATP often leads to cell death in immune cells (7). Therefore, this cell death may release other DAMPs (e.g., ATP, mtDNA, or NFP) to cytosol or extracellular spaces and further promotes inflammatory response (e.g., inflammasome) in the lung through autocrine or paracrine systems. Finally, these series of inflammatory events may result in the disruption of alveolar structures and functions or abrogation of fibrosis. Thus, it is likely that extracellular ATP is critically involved in inflammatory responses in human lung diseases. Since other mitochondrial DAMPs may also be involved in the lung diseases (138), it would be worth measuring the extracellular levels of other mitochondrial DAMPs in these patients. Measurement of mitochondrial DMAPs, including ATP, may be useful to predict disease severity or outcome.
Concluding Remarks
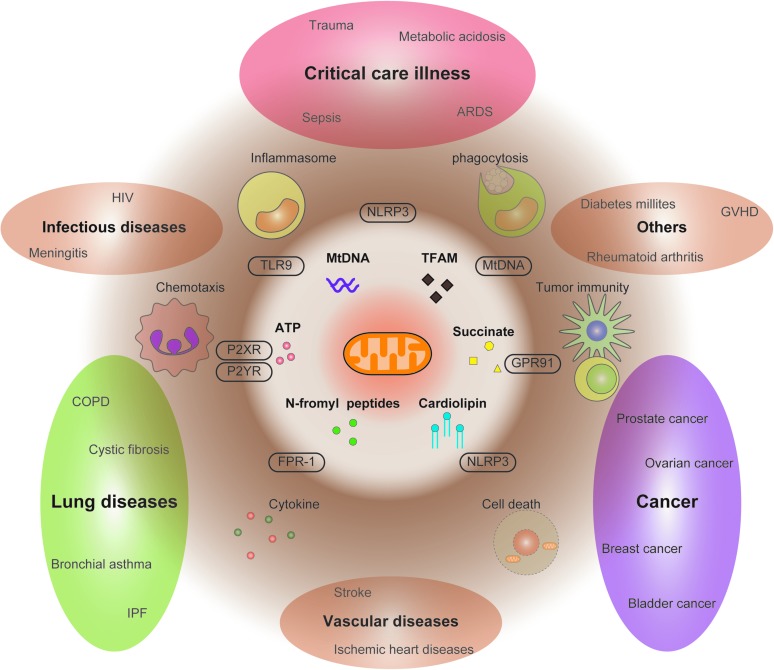
We have reviewed the roles of mitochondrial DAMPs in experimental disease models and also the association between mitochondrial DAMPs and human diseases (Fig. 6 and Table 2). Although there are ample data to support the critical roles of mitochondrial DAMPs in diseases, there are also many questions to be resolved. First, it is unclear how individual mitochondrial DAMPs cross talk with each other and affect immune responses in vivo or in patients. For example, in vitro, treatment of multiple mitochondrial DAMPs (e.g., TFAM and NFP) synergistically enhances immune function. It is possible that mitochondrial DAMPs may exert potent biological roles when they interact with other mitochondrial DAMPs or other extracellular molecules. Second, it also remains unclear what types of cell death are responsible for release of mitochondrial DAMPs in vivo or in patients. Some DAMP molecules such as ATP can be secreted from necrotic cells or apoptotic cells (123, 130). During the development of diseases, the host may have different types of cell death (e.g., apoptosis, necrosis, necroptosis, autophagic cell death, or pyroptosis in various cells or tissues) (7, 34, 44). Third, it is also challenging to elucidate which cell types are responsible for the release of mitochondrial DAMPs and contribute to the immune responses in vivo. While a number of reports suggest immune function of extracellular ATP in human lung diseases, it is also important to note that circulating ATP secreted from red blood cells may play a protective role in the pathogenesis of pulmonary hypertension (12, 151, 152). Mitochondrial DAMPs secreted from different types of cells may have different biological roles in vivo. Thus, further studies will be needed to elucidate the concise mechanism by which mitochondrial DAMPs contribute to the pathogenesis of human diseases.
FIG. 6.
Diverse effects of mitochondrial DAMPs on disease progression. The functional modes of mitochondrial DAMPs at cellular levels include secretion of proinflammatory cytokines (e.g., inflammasome activation), promoting phagocytosis, triggering chemotaxis, and regulating tumor immunity. Mitochondrial DAMPs are involved in pathogenesis of various diseases. The roles of mitochondrial DAMPs in diseases are shown. Various types of mitochondrial DAMPs may additionally or synergistically contribute to the pathogenesis of the diseases. For example, in sepsis, ATP and NFP promote chemotaxis of neutrophils or monocytes. ATP also activates inflammasome and triggers release of mtDNA into the cytosol, which further enhance caspase-1 activation. In trauma, the secreted mitochondrial DAMPs from damaged tissues, including NFP and mtDNA, activate chemotaxis, secretion of proinflammatory cytokines, and adhesion of neutrophil to the endothelium. NFP, N-formyl peptides. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Given the critical roles of mitochondrial DAMPs in the pathogenesis of diseases and correlation between the levels of mitochondrial DAMPs and severities of human diseases, it is also worth studying the therapeutic aspect of regulating mitochondrial DAMPs. Regulating the secretion or the signaling pathways of mitochondrial DAMPs may lead to dampening inflammatory response and tissue injuries and further ameliorating the pathogenesis of diseases.
Abbreviations Used
- ACABM
adult community-acquired bacterial meningitis
- AIM2
absent in melanoma 2
- ALT
alanine transaminase
- APC
antigen-presenting cells
- APACHE II
acute physiology and chronic health evaluation II
- ARDS
acute respiratory distress syndrome
- ATP
adenosine triphosphate
- BALF
bronchial alveolar lavage fluid
- CD4+ Th cells
cluster of differentiation 4 (+) helper T cells
- CF
cystic fibrosis
- cGAS
cyclic GMP-AMP synthase
- CLP
cecal ligation and puncture
- COPD
chronic obstructive pulmonary disease
- CXCL8
chemokine (C-X-C motif) ligand 8
- DAMPs
damage-associated molecular patterns
- DC
dendritic cell
- DNase II
deoxyribonuclease II
- EBC
exhaled breath condensate
- ER
emergency room
- FPRs
formyl peptide receptors
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- GPR91
G protein-coupled receptor
- GVHD
graft-versus-host disease
- HCT
hematopoietic cell transplantation
- HIF-1α
hypoxia-inducible factor 1-alpha
- HMG
high-mobility group
- ICU
intensive care unit
- IFN-γ
interferon-γ
- IgE
immunoglobulin E
- IL-1β
interleukin-1 beta
- IPF
idiopathic pulmonary fibrosis
- LOX-1
lectin-like, oxidized low-density lipoprotein receptor-1
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MMP
matrix metalloproteinase
- MPO
myeloperoxidase
- mtDNA
mitochondrial DNA
- MTX
mitoxantrone
- NADPH
nicotinamide adenine dinucleotide phosphate
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NFP
N-formyl peptides
- NLR
NOD-like receptors
- NLRP3
NLR family, pyrin domain-containing 3
- NOD
nucleotide-binding and oligomerization domain
- OVA
ovalbumin
- P2X7R
P2X purinoceptor 7
- PAACG
primary acute angle closure glaucoma
- PAH
pulmonary artery hypertension
- PAMPs
pathogen-associated molecular patterns
- PE
pulmonary embolism
- PMA
phorbol 12-myristate 13-acetate
- PMN
polymorphonuclear leukocyte
- PSA
prostate-specific antigen
- RA
rheumatoid arthritis
- ROS
reactive oxygen species
- STING
stimulator of interferon genes
- TCA
tricarboxylic acid
- TFAM
mitochondrial transcription factor A
- Th2
T helper 2
- TLRs
toll-like receptors
- TNF
tumor necrosis factor
References
- 1.Aduen J, Bernstein WK, Khastgir T, Miller J, Kerzner R, Bhatiani A, Lustgarten J, Bassin AS, Davison L, and Chernow B. The use and clinical importance of a substrate-specific electrode for rapid determination of blood lactate concentrations. JAMA 272: 1678–1685, 1994 [PubMed] [Google Scholar]
- 2.Akira S. and Takeda K. Toll-like receptor signalling. Nat Rev Immunol 4: 499–511, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Arnalich F, Codoceo R, Lopez-Collazo E, and Montiel C. Circulating cell-free mitochondrial DNA: a better early prognostic marker in patients with out-of-hospital cardiac arrest. Resuscitation 83: e162–e163, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Arnalich F, Maldifassi MC, Ciria E, Codoceo R, Renart J, Fernandez-Capitan C, Herruzo R, Garcia-Rio F, Lopez-Collazo E, and Montiel C. Plasma levels of mitochondrial and nuclear DNA in patients with massive pulmonary embolism in the emergency department: a prospective cohort study. Crit Care 17: R90, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnoult D, Soares F, Tattoli I, and Girardin SE. Mitochondria in innate immunity. EMBO Rep 12: 901–910, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashrafi G. and Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ 20: 31–42, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergsbaken T, Fink SL, and Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 7: 99–109, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biswas D, Qureshi OS, Lee WY, Croudace JE, Mura M, and Lammas DA. ATP-induced autophagy is associated with rapid killing of intracellular mycobacteria within human monocytes/macrophages. BMC Immunol 9: 35, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bliksoen M, Mariero LH, Ohm IK, Haugen F, Yndestad A, Solheim S, Seljeflot I, Ranheim T, Andersen GO, Aukrust P, Valen G, and Vinge LE. Increased circulating mitochondrial DNA after myocardial infarction. Int J Cardiol 158: 132–134, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Boudreau LH, Duchez AC, Cloutier N, Soulet D, Martin N, Bollinger J, Pare A, Rousseau M, Naika GS, Levesque T, Laflamme C, Marcoux G, Lambeau G, Farndale RW, Pouliot M, Hamzeh-Cognasse H, Cognasse F, Garraud O, Nigrovic PA, Guderley H, Lacroix S, Thibault L, Semple JW, Gelb MH, and Boilard E. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood 124: 2173–2183, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, and Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther 112: 358–404, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Bowles EA, Moody GN, Yeragunta Y, Stephenson AH, Ellsworth ML, and Sprague RS. Phosphodiesterase 5 inhibitors augment UT-15C-stimulated ATP release from erythrocytes of humans with pulmonary arterial hypertension. Exp Biol Med (Maywood), 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brinkmann CR, Jensen L, Dagnaes-Hansen F, Holm IE, Endo Y, Fujita T, Thiel S, Jensenius JC, and Degn SE. Mitochondria and the lectin pathway of complement. J Biol Chem 288: 8016–8027, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brugnara L, Vinaixa M, Murillo S, Samino S, Rodriguez MA, Beltran A, Lerin C, Davison G, Correig X, and Novials A. Metabolomics approach for analyzing the effects of exercise in subjects with type 1 diabetes mellitus. PLoS One 7: e40600, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Budnik LT, Kloth S, Baur X, Preisser AM, and Schwarzenbach H. Circulating mitochondrial DNA as biomarker linking environmental chemical exposure to early preclinical lesions elevation of mtDNA in human serum after exposure to carcinogenic halo-alkane-based pesticides. PLoS One 8: e64413, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev 58: 58–86, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Campbell CT, Kolesar JE, and Kaufman BA. Mitochondrial transcription factor A regulates mitochondrial transcription initiation, DNA packaging, and genome copy number. Biochim Biophys Acta 1819: 921–929, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Cao H, Ye H, Sun Z, Shen X, Song Z, Wu X, He W, Dai C, and Yang J. Circulatory mitochondrial DNA is a pro-inflammatory agent in maintenance hemodialysis patients. PLoS One 9: e113179, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carp H. Mitochondrial N-formylmethionyl proteins as chemoattractants for neutrophils. J Exp Med 155: 264–275, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaung WW, Wu R, Ji Y, Dong W, and Wang P. Mitochondrial transcription factor A is a proinflammatory mediator in hemorrhagic shock. Int J Mol Med 30: 199–203, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen GY. and Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 10: 826–837, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, and Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 314: 1792–1795, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Chicco AJ. and Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol 292: C33–C44, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Claypool SM. and Koehler CM. The complexity of cardiolipin in health and disease. Trends Biochem Sci 37: 32–41, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins LV, Hajizadeh S, Holme E, Jonsson IM, and Tarkowski A. Endogenously oxidized mitochondrial DNA induces in vivo and in vitro inflammatory responses. J Leukoc Biol 75: 995–1000, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Cossarizza A, Pinti M, Nasi M, Gibellini L, Manzini S, Roat E, De Biasi S, Bertoncelli L, Montagna JP, Bisi L, Manzini L, Trenti T, Borghi V, and Mussini C. Increased plasma levels of extracellular mitochondrial DNA during HIV infection: a new role for mitochondrial damage-associated molecular patterns during inflammation. Mitochondrion 11: 750–755, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Crouser ED, Shao G, Julian MW, Macre JE, Shadel GS, Tridandapani S, Huang Q, and Wewers MD. Monocyte activation by necrotic cells is promoted by mitochondrial proteins and formyl peptide receptors. Crit Care Med 37: 2000–2009, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Czapiga M, Gao JL, Kirk A, and Lekstrom-Himes J. Human platelets exhibit chemotaxis using functional N-formyl peptide receptors. Exp Hematol 33: 73–84, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Daniel MA, McDonald G, Offenbacher S, and Van Dyke TE. Defective chemotaxis and calcium response in localized juvenile periodontitis neutrophils. J Periodontol 64: 617–621, 1993 [DOI] [PubMed] [Google Scholar]
- 30.Davis BK, Wen H, and Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol 29: 707–735, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dawid IB. 5-methylcytidylic acid: absence from mitochondrial DNA of frogs and HeLa cells. Science 184: 80–81, 1974 [DOI] [PubMed] [Google Scholar]
- 32.Deli T. and Csernoch L. Extracellular ATP and cancer: an overview with special reference to P2 purinergic receptors. Pathol Oncol Res 14: 219–231, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Denlinger LC, Manthei DM, Seibold MA, Ahn K, Bleecker E, Boushey HA, Calhoun WJ, Castro M, Chinchili VM, Fahy JV, Hawkins GA, Icitovic N, Israel E, Jarjour NN, King T, Kraft M, Lazarus SC, Lehman E, Martin RJ, Meyers DA, Peters SP, Sheerar D, Shi L, Sutherland ER, Szefler SJ, Wechsler ME, Sorkness CA, Lemanske RF, Jr.; NHLBI Asthma Clinical Research Network Investigators. P2X7-regulated protection from exacerbations and loss of control is independent of asthma maintenance therapy. Am J Respir Crit Care Med 187: 28–33, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Denton D, Nicolson S, and Kumar S. Cell death by autophagy: facts and apparent artefacts. Cell Death Differ 19: 87–95, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dieude M, Striegl H, Tyznik AJ, Wang J, Behar SM, Piccirillo CA, Levine JS, Zajonc DM, and Rauch J. Cardiolipin binds to CD1d and stimulates CD1d-restricted gammadelta T cells in the normal murine repertoire. J Immunol 186: 4771–4781, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding Z, Liu S, Wang X, Khaidakov M, Dai Y, and Mehta JL. Oxidant stress in mitochondrial DNA damage, autophagy and inflammation in atherosclerosis. Sci Rep 3: 1077, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dolinay T, Kim YS, Howrylak J, Hunninghake GM, An CH, Fredenburgh L, Massaro AF, Rogers A, Gazourian L, Nakahira K, Haspel JA, Landazury R, Eppanapally S, Christie JD, Meyer NJ, Ware LB, Christiani DC, Ryter SW, Baron RM, and Choi AM. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med 185: 1225–1234, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duprez L, Takahashi N, Van Hauwermeiren F, Vandendriessche B, Goossens V, Vanden Berghe T, Declercq W, Libert C, Cauwels A, and Vandenabeele P. RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity 35: 908–918, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Ellinger J, Muller DC, Muller SC, Hauser S, Heukamp LC, von Ruecker A, Bastian PJ, and Walgenbach-Brunagel G. Circulating mitochondrial DNA in serum: a universal diagnostic biomarker for patients with urological malignancies. Urol Oncol 30: 509–515, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Esther CR, Jr., Alexis NE, Clas ML, Lazarowski ER, Donaldson SH, Ribeiro CM, Moore CG, Davis SD, and Boucher RC. Extracellular purines are biomarkers of neutrophilic airway inflammation. Eur Respir J 31: 949–956, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Evdokimovsky EV, Ushakova TE, Kudriavtcev AA, and Gaziev AI. Alteration of mtDNA copy number, mitochondrial gene expression and extracellular DNA content in mice after irradiation at lethal dose. Radiat Environ Biophys 50: 181–188, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Fairbairn IP, Stober CB, Kumararatne DS, and Lammas DA. ATP-mediated killing of intracellular mycobacteria by macrophages is a P2X(7)-dependent process inducing bacterial death by phagosome-lysosome fusion. J Immunol 167: 3300–3307, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Falkenberg M, Larsson NG, and Gustafsson CM. DNA replication and transcription in mammalian mitochondria. Annu Rev Biochem 76: 679–699, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Fink SL. and Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun 73: 1907–1916, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Forni LG, McKinnon W, Lord GA, Treacher DF, Peron JM, and Hilton PJ. Circulating anions usually associated with the Krebs cycle in patients with metabolic acidosis. Crit Care 9: R591–R595, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freund-Michel V, Khoyrattee N, Savineau JP, Muller B, and Guibert C. Mitochondria: roles in pulmonary hypertension. Int J Biochem Cell Biol 55: 93–97, 2014 [DOI] [PubMed] [Google Scholar]
- 47.Fuller SJ, Stokes L, Skarratt KK, Gu BJ, and Wiley JS. Genetics of the P2X7 receptor and human disease. Purinergic Signal 5: 257–262, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao JL, Lee EJ, and Murphy PM. Impaired antibacterial host defense in mice lacking the N-formylpeptide receptor. J Exp Med 189: 657–662, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, and Agostinis P. Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochim Biophys Acta 1805: 53–71, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Goepfert C, Sundberg C, Sevigny J, Enjyoji K, Hoshi T, Csizmadia E, and Robson S. Disordered cellular migration and angiogenesis in cd39-null mice. Circulation 104: 3109–3115, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Gray MW, Burger G, and Lang BF. Mitochondrial evolution. Science 283: 1476–1481, 1999 [DOI] [PubMed] [Google Scholar]
- 52.Green DR, Galluzzi L, and Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science 333: 1109–1112, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grobmyer SR, Lin E, Lowry SF, Rivadeneira DE, Potter S, Barie PS, and Nathan CF. Elevation of IL-18 in human sepsis. J Clin Immunol 20: 212–215, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Groot GS. and Kroon AM. Mitochondrial DNA from various organisms does not contain internally methylated cytosine in -CCGG- sequences. Biochim Biophys Acta 564: 355–357, 1979 [DOI] [PubMed] [Google Scholar]
- 55.Grygorczyk R. and Hanrahan JW. CFTR-independent ATP release from epithelial cells triggered by mechanical stimuli. Am J Physiol 272: C1058–C1066, 1997 [DOI] [PubMed] [Google Scholar]
- 56.Guo J, Zheng L, Liu W, Wang X, Wang Z, Wang Z, French AJ, Kang D, Chen L, Thibodeau SN, and Liu W. Frequent truncating mutation of TFAM induces mitochondrial DNA depletion and apoptotic resistance in microsatellite-unstable colorectal cancer. Cancer Res 71: 2978–2987, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gwinn MR, Sharma A, and De Nardin E. Single nucleotide polymorphisms of the N-formyl peptide receptor in localized juvenile periodontitis. J Periodontol 70: 1194–1201, 1999 [DOI] [PubMed] [Google Scholar]
- 58.Hajizadeh S, DeGroot J, TeKoppele JM, Tarkowski A, and Collins LV. Extracellular mitochondrial DNA and oxidatively damaged DNA in synovial fluid of patients with rheumatoid arthritis. Arthritis Res Ther 5: R234–R240, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hauser CJ, Sursal T, Rodriguez EK, Appleton PT, Zhang Q, and Itagaki K. Mitochondrial damage associated molecular patterns from femoral reamings activate neutrophils through formyl peptide receptors and P44/42 MAP kinase. J Orthop Trauma 24: 534–538, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hazama A, Shimizu T, Ando-Akatsuka Y, Hayashi S, Tanaka S, Maeno E, and Okada Y. Swelling-induced, CFTR-independent ATP release from a human epithelial cell line: lack of correlation with volume-sensitive cl(-) channels. J Gen Physiol 114: 525–533, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hewinson J, Moore SF, Glover C, Watts AG, and MacKenzie AB. A key role for redox signaling in rapid P2X7 receptor-induced IL-1 beta processing in human monocytes. J Immunol 180: 8410–8420, 2008 [DOI] [PubMed] [Google Scholar]
- 62.Hobert JA, Mester JL, Moline J, and Eng C. Elevated plasma succinate in PTEN, SDHB, and SDHD mutation-positive individuals. Genet Med 14: 616–619, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Honda S, Sasaki Y, Ohsawa K, Imai Y, Nakamura Y, Inoue K, and Kohsaka S. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J Neurosci 21: 1975–1982, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hornung V. and Latz E. Intracellular DNA recognition. Nat Rev Immunol 10: 123–130, 2010 [DOI] [PubMed] [Google Scholar]
- 65.Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MA, Muskens F, Hoogsteden HC, Luttmann W, Ferrari D, Di Virgilio F, Virchow JC, Jr., and Lambrecht BN. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med 13: 913–919, 2007 [DOI] [PubMed] [Google Scholar]
- 66.Iyer SS, He Q, Janczy JR, Elliott EI, Zhong Z, Olivier AK, Sadler JJ, Knepper-Adrian V, Han R, Qiao L, Eisenbarth SC, Nauseef WM, Cassel SL, and Sutterwala FS. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity 39: 311–323, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jamieson GP, Snook MB, Thurlow PJ, and Wiley JS. Extracellular ATP causes of loss of L-selectin from human lymphocytes via occupancy of P2Z purinocepters. J Cell Physiol 166: 637–642, 1996 [DOI] [PubMed] [Google Scholar]
- 68.Julian MW, Shao G, Vangundy ZC, Papenfuss TL, and Crouser ED. Mitochondrial transcription factor A, an endogenous danger signal, promotes TNFalpha release via RAGE- and TLR9-responsive plasmacytoid dendritic cells. PLoS One 8: e72354, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jun DJ, Kim J, Jung SY, Song R, Noh JH, Park YS, Ryu SH, Kim JH, Kong YY, Chung JM, and Kim KT. Extracellular ATP mediates necrotic cell swelling in SN4741 dopaminergic neurons through P2X7 receptors. J Biol Chem 282: 37350–37358, 2007 [DOI] [PubMed] [Google Scholar]
- 70.Jung SS, Moon JS, Xu JF, Ifedigbo E, Ryter SW, Choi AM, and Nakahira K. Carbon monoxide negatively regulates NLRP3 inflammasome activation in macrophages. Am J Physiol Lung Cell Mol Physiol 308: L1058–L1067, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol 11: 201–212, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kang D, Kim SH, and Hamasaki N. Mitochondrial transcription factor A (TFAM): roles in maintenance of mtDNA and cellular functions. Mitochondrion 7: 39–44, 2007 [DOI] [PubMed] [Google Scholar]
- 73.Kawane K, Fukuyama H, Kondoh G, Takeda J, Ohsawa Y, Uchiyama Y, and Nagata S. Requirement of DNase II for definitive erythropoiesis in the mouse fetal liver. Science 292: 1546–1549, 2001 [DOI] [PubMed] [Google Scholar]
- 74.Kawane K, Ohtani M, Miwa K, Kizawa T, Kanbara Y, Yoshioka Y, Yoshikawa H, and Nagata S. Chronic polyarthritis caused by mammalian DNA that escapes from degradation in macrophages. Nature 443: 998–1002, 2006 [DOI] [PubMed] [Google Scholar]
- 75.Keshari RS, Jyoti A, Kumar S, Dubey M, Verma A, Srinag BS, Krishnamurthy H, Barthwal MK, and Dikshit M. Neutrophil extracellular traps contain mitochondrial as well as nuclear DNA and exhibit inflammatory potential. Cytometry A 81: 238–247, 2012 [DOI] [PubMed] [Google Scholar]
- 76.Kiviniemi TO, Yegutkin GG, Toikka JO, Paul S, Aittokallio T, Janatuinen T, Knuuti J, Ronnemaa T, Koskenvuo JW, Hartiala JJ, Jalkanen S, and Raitakari OT. Pravastatin-induced improvement in coronary reactivity and circulating ATP and ADP levels in young adults with type 1 diabetes. Front Physiol 3: 338, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kohler C, Radpour R, Barekati Z, Asadollahi R, Bitzer J, Wight E, Burki N, Diesch C, Holzgreve W, and Zhong XY. Levels of plasma circulating cell free nuclear and mitochondrial DNA as potential biomarkers for breast tumors. Mol Cancer 8: 105, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koppelman CM, Den Blaauwen T, Duursma MC, Heeren RM, and Nanninga N. Escherichia coli minicell membranes are enriched in cardiolipin. J Bacteriol 183: 6144–6147, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kouzaki H, Iijima K, Kobayashi T, O'Grady SM, and Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol 186: 4375–4387, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov 5: 471–484, 2006 [DOI] [PubMed] [Google Scholar]
- 81.Ksenzenko SM, Davidson SB, Saba AA, Franko AP, Raafat AM, Diebel LN, and Dulchavsky SA. Effect of triiodothyronine augmentation on rat lung surfactant phospholipids during sepsis. J Appl Physiol (1985) 82: 2020–2027, 1997 [DOI] [PubMed] [Google Scholar]
- 82.Kung CT, Hsiao SY, Tsai TC, Su CM, Chang WN, Huang CR, Wang HC, Lin WC, Chang HW, Lin YJ, Cheng BC, Su BY, Tsai NW, and Lu CH. Plasma nuclear and mitochondrial DNA levels as predictors of outcome in severe sepsis patients in the emergency room. J Transl Med 10: 130, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kuroda E, Ishii KJ, Uematsu S, Ohata K, Coban C, Akira S, Aritake K, Urade Y, and Morimoto Y. Silica crystals and aluminum salts regulate the production of prostaglandin in macrophages via NALP3 inflammasome-independent mechanisms. Immunity 34: 514–526, 2011 [DOI] [PubMed] [Google Scholar]
- 84.Labasi JM, Petrushova N, Donovan C, McCurdy S, Lira P, Payette MM, Brissette W, Wicks JR, Audoly L, and Gabel CA. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol 168: 6436–6445, 2002 [DOI] [PubMed] [Google Scholar]
- 85.Lader AS, Prat AG, Jackson GR, Jr., Chervinsky KL, Lapey A, Kinane TB, and Cantiello HF. Increased circulating levels of plasma ATP in cystic fibrosis patients. Clin Physiol 20: 348–353, 2000 [DOI] [PubMed] [Google Scholar]
- 86.Larsson NG, Wang J, Wilhelmsson H, Oldfors A, Rustin P, Lewandoski M, Barsh GS, and Clayton DA. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet 18: 231–236, 1998 [DOI] [PubMed] [Google Scholar]
- 87.Le Y, Murphy PM, and Wang JM. Formyl-peptide receptors revisited. Trends Immunol 23: 541–548, 2002 [DOI] [PubMed] [Google Scholar]
- 88.Lewis GD, Farrell L, Wood MJ, Martinovic M, Arany Z, Rowe GC, Souza A, Cheng S, McCabe EL, Yang E, Shi X, Deo R, Roth FP, Asnani A, Rhee EP, Systrom DM, Semigran MJ, Vasan RS, Carr SA, Wang TJ, Sabatine MS, Clish CB, and Gerszten RE. Metabolic signatures of exercise in human plasma. Sci Transl Med 2: 33ra37, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li A, Zhang X, Zheng D, Ge J, Laties AM, and Mitchell CH. Sustained elevation of extracellular ATP in aqueous humor from humans with primary chronic angle-closure glaucoma. Exp Eye Res 93: 528–533, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liau DF, Barrett CR, Bell AL, Cernansky G, and Ryan SF. Diphosphatidylglycerol in experimental acute alveolar injury in the dog. J Lipid Res 25: 678–683, 1984 [PubMed] [Google Scholar]
- 91.Lohman AW, Billaud M, and Isakson BE. Mechanisms of ATP release and signalling in the blood vessel wall. Cardiovasc Res 95: 269–280, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lommatzsch M, Cicko S, Muller T, Lucattelli M, Bratke K, Stoll P, Grimm M, Durk T, Zissel G, Ferrari D, Di Virgilio F, Sorichter S, Lungarella G, Virchow JC, and Idzko M. Extracellular adenosine triphosphate and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 181: 928–934, 2010 [DOI] [PubMed] [Google Scholar]
- 93.Loomis WH, Namiki S, Ostrom RS, Insel PA, and Junger WG. Hypertonic stress increases T cell interleukin-2 expression through a mechanism that involves ATP release, P2 receptor, and p38 MAPK activation. J Biol Chem 278: 4590–4596, 2003 [DOI] [PubMed] [Google Scholar]
- 94.Lu CH, Chang WN, Tsai NW, Chuang YC, Huang CR, and Wang HC. The value of serial plasma nuclear and mitochondrial DNA levels in adult community-acquired bacterial meningitis. QJM 103: 169–175, 2010 [DOI] [PubMed] [Google Scholar]
- 95.Ma Y, Adjemian S, Yang H, Catani JP, Hannani D, Martins I, Michaud M, Kepp O, Sukkurwala AQ, Vacchelli E, Galluzzi L, Zitvogel L, and Kroemer G. ATP-dependent recruitment, survival and differentiation of dendritic cell precursors in the tumor bed after anticancer chemotherapy. Oncoimmunology 2: e24568, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Manthei DM, Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Gern JE, Lemanske RF, Jr., and Denlinger LC. Protection from asthma in a high-risk birth cohort by attenuated P2X(7) function. J Allergy Clin Immunol 130: 496–502, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Marques PE, Amaral SS, Pires DA, Nogueira LL, Soriani FM, Lima BH, Lopes GA, Russo RC, Avila TV, Melgaco JG, Oliveira AG, Pinto MA, Lima CX, De Paula AM, Cara DC, Leite MF, Teixeira MM, and Menezes GB. Chemokines and mitochondrial products activate neutrophils to amplify organ injury during mouse acute liver failure. Hepatology 56: 1971–1982, 2012 [DOI] [PubMed] [Google Scholar]
- 98.Mehra N, Penning M, Maas J, van Daal N, Giles RH, and Voest EE. Circulating mitochondrial nucleic acids have prognostic value for survival in patients with advanced prostate cancer. Clin Cancer Res 13: 421–426, 2007 [DOI] [PubMed] [Google Scholar]
- 99.Melloni E, Pontremoli S, Salamino F, Sparatore B, Michetti M, Sacco O, and Horecker BL. ATP induces the release of a neutral serine proteinase and enhances the production of superoxide anion in membranes from phorbol ester-activated neutrophils. J Biol Chem 261: 11437–11439, 1986 [PubMed] [Google Scholar]
- 100.Meshki J, Tuluc F, Bredetean O, Ding Z, and Kunapuli SP. Molecular mechanism of nucleotide-induced primary granule release in human neutrophils: role for the P2Y2 receptor. Am J Physiol Cell Physiol 286: C264–C271, 2004 [DOI] [PubMed] [Google Scholar]
- 101.Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G, Rello-Varona S, Tailler M, Menger L, Vacchelli E, Galluzzi L, Ghiringhelli F, di Virgilio F, Zitvogel L, and Kroemer G. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 334: 1573–1577, 2011 [DOI] [PubMed] [Google Scholar]
- 102.Miller CM, Boulter NR, Fuller SJ, Zakrzewski AM, Lees MP, Saunders BM, Wiley JS, and Smith NC. The role of the P2X(7) receptor in infectious diseases. PLoS Pathog 7: e1002212, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Miyabe K, Sakamoto N, Wu YH, Mori N, and Sakamoto H. Effects of platelet release products on neutrophilic phagocytosis and complement receptors. Thromb Res 114: 29–36, 2004 [DOI] [PubMed] [Google Scholar]
- 104.Mizushima N. and Komatsu M. Autophagy: renovation of cells and tissues. Cell 147: 728–741, 2011 [DOI] [PubMed] [Google Scholar]
- 105.Moore SF. and MacKenzie AB. NADPH oxidase NOX2 mediates rapid cellular oxidation following ATP stimulation of endotoxin-primed macrophages. J Immunol 183: 3302–3308, 2009 [DOI] [PubMed] [Google Scholar]
- 106.Murakami T, Ockinger J, Yu J, Byles V, McColl A, Hofer AM, and Horng T. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci U S A 109: 11282–11287, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, Fitzgerald KA, Ryter SW, and Choi AM. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol 12: 222–230, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nakahira K, Kyung SY, Rogers AJ, Gazourian L, Youn S, Massaro AF, Quintana C, Osorio JC, Wang Z, Zhao Y, Lawler LA, Christie JD, Meyer NJ, Mc Causland FR, Waikar SS, Waxman AB, Chung RT, Bueno R, Rosas IO, Fredenburgh LE, Baron RM, Christiani DC, Hunninghake GM, and Choi AM. Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: derivation and validation. PLoS Med 10: e1001577; discussion e1001577, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Notomi S, Hisatomi T, Murakami Y, Terasaki H, Sonoda S, Asato R, Takeda A, Ikeda Y, Enaida H, Sakamoto T, and Ishibashi T. Dynamic increase in extracellular ATP accelerates photoreceptor cell apoptosis via ligation of P2RX7 in subretinal hemorrhage. PLoS One 8: e53338, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Oberholzer A, Steckholzer U, Kurimoto M, Trentz O, and Ertel W. Interleukin-18 plasma levels are increased in patients with sepsis compared to severely injured patients. Shock 16: 411–414, 2001 [DOI] [PubMed] [Google Scholar]
- 111.Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, Oyabu J, Murakawa T, Nakayama H, Nishida K, Akira S, Yamamoto A, Komuro I, and Otsu K. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature 485: 251–255, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.O'Neill LA, Golenbock D, and Bowie AG. The history of Toll-like receptors—redefining innate immunity. Nat Rev Immunol 13: 453–460, 2013 [DOI] [PubMed] [Google Scholar]
- 113.Ott M, Zhivotovsky B, and Orrenius S. Role of cardiolipin in cytochrome c release from mitochondria. Cell Death Differ 14: 1243–1247, 2007 [DOI] [PubMed] [Google Scholar]
- 114.Padeh S, Cohen A, and Roifman CM. ATP-induced activation of human B lymphocytes via P2-purinoceptors. J Immunol 146: 1626–1632, 1991 [PubMed] [Google Scholar]
- 115.Panaro MA, Acquafredda A, Sisto M, Lisi S, Maffione AB, and Mitolo V. Biological role of the N-formyl peptide receptors. Immunopharmacol Immunotoxicol 28: 103–127, 2006 [DOI] [PubMed] [Google Scholar]
- 116.Papp L, Vizi ES, and Sperlagh B. P2X7 receptor mediated phosphorylation of p38MAP kinase in the hippocampus. Biochem Biophys Res Commun 355: 568–574, 2007 [DOI] [PubMed] [Google Scholar]
- 117.Paradies G, Paradies V, Ruggiero FM, and Petrosillo G. Cardiolipin and mitochondrial function in health and disease. Antioxid Redox Signal 20: 1925–1953, 2014 [DOI] [PubMed] [Google Scholar]
- 118.Patrushev M, Kasymov V, Patrusheva V, Ushakova T, Gogvadze V, and Gaziev A. Mitochondrial permeability transition triggers the release of mtDNA fragments. Cell Mol Life Sci 61: 3100–3103, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Patrushev M, Kasymov V, Patrusheva V, Ushakova T, Gogvadze V, and Gaziev AI. Release of mitochondrial DNA fragments from brain mitochondria of irradiated mice. Mitochondrion 6: 43–47, 2006 [DOI] [PubMed] [Google Scholar]
- 120.Pinti M, Cevenini E, Nasi M, De Biasi S, Salvioli S, Monti D, Benatti S, Gibellini L, Cotichini R, Stazi MA, Trenti T, Franceschi C, and Cossarizza A. Circulating mitochondrial DNA increases with age and is a familiar trait: Implications for “inflamm-aging”. Eur J Immunol 44: 1552–1562, 2014 [DOI] [PubMed] [Google Scholar]
- 121.Puskarich MA, Shapiro NI, Trzeciak S, Kline JA, and Jones AE. Plasma levels of mitochondrial DNA in patients presenting to the emergency department with sepsis. Shock 38: 337–340, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pyle A, Burn DJ, Gordon C, Swan C, Chinnery PF, and Baudouin SV. Fall in circulating mononuclear cell mitochondrial DNA content in human sepsis. Intensive Care Med 36: 956–962, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Qu Y, Misaghi S, Newton K, Gilmour LL, Louie S, Cupp JE, Dubyak GR, Hackos D, and Dixit VM. Pannexin-1 is required for ATP release during apoptosis but not for inflammasome activation. J Immunol 186: 6553–6561, 2011 [DOI] [PubMed] [Google Scholar]
- 124.Rabiet MJ, Huet E, and Boulay F. Human mitochondria-derived N-formylated peptides are novel agonists equally active on FPR and FPRL1, while Listeria monocytogenes-derived peptides preferentially activate FPR. Eur J Immunol 35: 2486–2495, 2005 [DOI] [PubMed] [Google Scholar]
- 125.Rathinam VA, Vanaja SK, and Fitzgerald KA. Regulation of inflammasome signaling. Nat Immunol 13: 333–342, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, Massey DC, Menzies FM, Moreau K, Narayanan U, Renna M, Siddiqi FH, Underwood BR, Winslow AR, and Rubinsztein DC. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev 90: 1383–1435, 2010 [DOI] [PubMed] [Google Scholar]
- 127.Ray NB, Durairaj L, Chen BB, McVerry BJ, Ryan AJ, Donahoe M, Waltenbaugh AK, O'Donnell CP, Henderson FC, Etscheidt CA, McCoy DM, Agassandian M, Hayes-Rowan EC, Coon TA, Butler PL, Gakhar L, Mathur SN, Sieren JC, Tyurina YY, Kagan VE, McLennan G, and Mallampalli RK. Dynamic regulation of cardiolipin by the lipid pump Atp8b1 determines the severity of lung injury in experimental pneumonia. Nat Med 16: 1120–1127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ries M, Schuster P, Thomann S, Donhauser N, Vollmer J, and Schmidt B. Identification of novel oligonucleotides from mitochondrial DNA that spontaneously induce plasmacytoid dendritic cell activation. J Leukoc Biol 94: 123–135, 2013 [DOI] [PubMed] [Google Scholar]
- 129.Riteau N, Gasse P, Fauconnier L, Gombault A, Couegnat M, Fick L, Kanellopoulos J, Quesniaux VF, Marchand-Adam S, Crestani B, Ryffel B, and Couillin I. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am J Respir Crit Care Med 182: 774–783, 2010 [DOI] [PubMed] [Google Scholar]
- 130.Rock KL. and Kono H. The inflammatory response to cell death. Annu Rev Pathol 3: 99–126, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rongvaux A, Jackson R, Harman CC, Li T, West AP, de Zoete MR, Wu Y, Yordy B, Lakhani SA, Kuan CY, Taniguchi T, Shadel GS, Chen ZJ, Iwasaki A, and Flavell RA. Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell 159: 1563–1577, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rubic T, Lametschwandtner G, Jost S, Hinteregger S, Kund J, Carballido-Perrig N, Schwarzler C, Junt T, Voshol H, Meingassner JG, Mao X, Werner G, Rot A, and Carballido JM. Triggering the succinate receptor GPR91 on dendritic cells enhances immunity. Nat Immunol 9: 1261–1269, 2008 [DOI] [PubMed] [Google Scholar]
- 133.Sagan L. On the origin of mitosing cells. J Theor Biol 14: 255–274, 1967 [DOI] [PubMed] [Google Scholar]
- 134.Save S. and Persson K. Extracellular ATP and P2Y receptor activation induce a proinflammatory host response in the human urinary tract. Infect Immun 78: 3609–3615, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schapira AH. Mitochondrial disease. Lancet 368: 70–82, 2006 [DOI] [PubMed] [Google Scholar]
- 136.Schroder K. and Tschopp J. The inflammasomes. Cell 140: 821–832, 2010 [DOI] [PubMed] [Google Scholar]
- 137.Schroder K, Zhou R, and Tschopp J. The NLRP3 inflammasome: a sensor for metabolic danger? Science 327: 296–300, 2010 [DOI] [PubMed] [Google Scholar]
- 138.Schumacker PT, Gillespie MN, Nakahira K, Choi AM, Crouser ED, Piantadosi CA, and Bhattacharya J. Mitochondria in lung biology and pathology: more than just a powerhouse. Am J Physiol Lung Cell Mol Physiol 306: L962–L974, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schwarzenbach H, Hoon DS, and Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer 11: 426–437, 2011 [DOI] [PubMed] [Google Scholar]
- 140.Seong SY. and Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol 4: 469–478, 2004 [DOI] [PubMed] [Google Scholar]
- 141.Shaham O, Slate NG, Goldberger O, Xu Q, Ramanathan A, Souza AL, Clish CB, Sims KB, and Mootha VK. A plasma signature of human mitochondrial disease revealed through metabolic profiling of spent media from cultured muscle cells. Proc Natl Acad Sci U S A 107: 1571–1575, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, Rentsendorj A, Vargas M, Guerrero C, Wang Y, Fitzgerald KA, Underhill DM, Town T, and Arditi M. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36: 401–414, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shiratori M, Tozaki-Saitoh H, Yoshitake M, Tsuda M, and Inoue K. P2X7 receptor activation induces CXCL2 production in microglia through NFAT and PKC/MAPK pathways. J Neurochem 114: 810–819, 2010 [DOI] [PubMed] [Google Scholar]
- 144.Skaper SD, Debetto P, and Giusti P. The P2X7 purinergic receptor: from physiology to neurological disorders. FASEB J 24: 337–345, 2010 [DOI] [PubMed] [Google Scholar]
- 145.Sluyter R, Shemon AN, and Wiley JS. Glu496 to Ala polymorphism in the P2X7 receptor impairs ATP-induced IL-1 beta release from human monocytes. J Immunol 172: 3399–3405, 2004 [DOI] [PubMed] [Google Scholar]
- 146.Sluyter R. and Stokes L. Significance of P2X7 receptor variants to human health and disease. Recent Pat DNA Gene Seq 5: 41–54, 2011 [DOI] [PubMed] [Google Scholar]
- 147.Sorice M, Circella A, Cristea IM, Garofalo T, Di Renzo L, Alessandri C, Valesini G, and Esposti MD. Cardiolipin and its metabolites move from mitochondria to other cellular membranes during death receptor-mediated apoptosis. Cell Death Differ 11: 1133–1145, 2004 [DOI] [PubMed] [Google Scholar]
- 148.Sorice M, Circella A, Misasi R, Pittoni V, Garofalo T, Cirelli A, Pavan A, Pontieri GM, and Valesini G. Cardiolipin on the surface of apoptotic cells as a possible trigger for antiphospholipids antibodies. Clin Exp Immunol 122: 277–284, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Souza CO, Santoro GF, Figliuolo VR, Nanini HF, de Souza HS, Castelo-Branco MT, Abalo AA, Paiva MM, Coutinho CM, and Coutinho-Silva R. Extracellular ATP induces cell death in human intestinal epithelial cells. Biochim Biophys Acta 1820: 1867–1878, 2012 [DOI] [PubMed] [Google Scholar]
- 150.Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, and Lonigro AJ. Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol 275: H1726–H1732, 1998 [DOI] [PubMed] [Google Scholar]
- 151.Sprague RS, Olearczyk JJ, Spence DM, Stephenson AH, Sprung RW, and Lonigro AJ. Extracellular ATP signaling in the rabbit lung: erythrocytes as determinants of vascular resistance. Am J Physiol Heart Circ Physiol 285: H693–H700, 2003 [DOI] [PubMed] [Google Scholar]
- 152.Sprague RS, Stephenson AH, Ellsworth ML, Keller C, and Lonigro AJ. Impaired release of ATP from red blood cells of humans with primary pulmonary hypertension. Exp Biol Med (Maywood) 226: 434–439, 2001 [DOI] [PubMed] [Google Scholar]
- 153.Sun S, Sursal T, Adibnia Y, Zhao C, Zheng Y, Li H, Otterbein LE, Hauser CJ, and Itagaki K. Mitochondrial DAMPs increase endothelial permeability through neutrophil dependent and independent pathways. PLoS One 8: e59989, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Surprenant A. and North RA. Signaling at purinergic P2X receptors. Annu Rev Physiol 71: 333–359, 2009 [DOI] [PubMed] [Google Scholar]
- 155.Sursal T, Stearns-Kurosawa DJ, Itagaki K, Oh SY, Sun S, Kurosawa S, and Hauser CJ. Plasma bacterial and mitochondrial DNA distinguish bacterial sepsis from sterile systemic inflammatory response syndrome and quantify inflammatory tissue injury in nonhuman primates. Shock 39: 55–62, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Taanman JW. The mitochondrial genome: structure, transcription, translation and replication. Biochim Biophys Acta 1410: 103–123, 1999 [DOI] [PubMed] [Google Scholar]
- 157.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, Zheng L, Gardet A, Tong Z, Jany SS, Corr SC, Haneklaus M, Caffrey BE, Pierce K, Walmsley S, Beasley FC, Cummins E, Nizet V, Whyte M, Taylor CT, Lin H, Masters SL, Gottlieb E, Kelly VP, Clish C, Auron PE, Xavier RJ, and O'Neill LA. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature 496: 238–242, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Taylor RW. and Turnbull DM. Mitochondrial DNA mutations in human disease. Nat Rev Genet 6: 389–402, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Teul J, Garcia A, Tunon J, Martin-Ventura JL, Tarin N, Bescos LL, Egido J, Barbas C, and Ruperez FJ. Targeted and non-targeted metabolic time trajectory in plasma of patients after acute coronary syndrome. J Pharm Biomed Anal 56: 343–351, 2011 [DOI] [PubMed] [Google Scholar]
- 160.Tsai NW, Lin TK, Chen SD, Chang WN, Wang HC, Yang TM, Lin YJ, Jan CR, Huang CR, Liou CW, and Lu CH. The value of serial plasma nuclear and mitochondrial DNA levels in patients with acute ischemic stroke. Clin Chim Acta 412: 476–479, 2011 [DOI] [PubMed] [Google Scholar]
- 161.Van Dyke TE, Levine MJ, Tabak LA, and Genco RJ. Reduced chemotactic peptide binding in juvenile periodontitis: a model for neutrophil function. Biochem Biophys Res Commun 100: 1278–1284, 1981 [DOI] [PubMed] [Google Scholar]
- 162.Vernochet C, Mourier A, Bezy O, Macotela Y, Boucher J, Rardin MJ, An D, Lee KY, Ilkayeva OR, Zingaretti CM, Emanuelli B, Smyth G, Cinti S, Newgard CB, Gibson BW, Larsson NG, and Kahn CR. Adipose-specific deletion of TFAM increases mitochondrial oxidation and protects mice against obesity and insulin resistance. Cell Metab 16: 765–776, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.West AP, Khoury-Hanold W, Staron M, Tal MC, Pineda CM, Lang SM, Bestwick M, Duguay BA, Raimundo N, MacDuff DA, Kaech SM, Smiley JR, Means RE, Iwasaki A, and Shadel GS. Mitochondrial DNA stress primes the antiviral innate immune response. Nature 520: 553–557, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.White MJ, McArthur K, Metcalf D, Lane RM, Cambier JC, Herold MJ, van Delft MF, Bedoui S, Lessene G, Ritchie ME, Huang DC, and Kile BT. Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell 159: 1549–1562, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wilhelm K, Ganesan J, Muller T, Durr C, Grimm M, Beilhack A, Krempl CD, Sorichter S, Gerlach UV, Juttner E, Zerweck A, Gartner F, Pellegatti P, Di Virgilio F, Ferrari D, Kambham N, Fisch P, Finke J, Idzko M, and Zeiser R. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat Med 16: 1434–1438, 2010 [DOI] [PubMed] [Google Scholar]
- 166.Wu LJ, Vadakkan KI, and Zhuo M. ATP-induced chemotaxis of microglial processes requires P2Y receptor-activated initiation of outward potassium currents. Glia 55: 810–821, 2007 [DOI] [PubMed] [Google Scholar]
- 167.Yamanouchi S, Kudo D, Yamada M, Miyagawa N, Furukawa H, and Kushimoto S. Plasma mitochondrial DNA levels in patients with trauma and severe sepsis: time course and the association with clinical status. J Crit Care 28: 1027–1031, 2013 [DOI] [PubMed] [Google Scholar]
- 168.Yang L, Yu D, Fan HH, Feng Y, Hu L, Zhang WY, Zhou K, and Mo XM. Triggering the succinate receptor GPR91 enhances pressure overload-induced right ventricular hypertrophy. Int J Clin Exp Pathol 7: 5415–5428, 2014 [PMC free article] [PubMed] [Google Scholar]
- 169.Yin J, Xu K, Zhang J, Kumar A, and Yu FS. Wound-induced ATP release and EGF receptor activation in epithelial cells. J Cell Sci 120: 815–825, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Youle RJ. and Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol 12: 9–14, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, Schmid I, Straumann A, Reichenbach J, Gleich GJ, and Simon HU. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med 14: 949–953, 2008 [DOI] [PubMed] [Google Scholar]
- 172.Yousefi S, Mihalache C, Kozlowski E, Schmid I, and Simon HU. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ 16: 1438–1444, 2009 [DOI] [PubMed] [Google Scholar]
- 173.Yu M, Wan YF, and Zou QH. Cell-free circulating mitochondrial DNA in the serum: a potential non-invasive biomarker for Ewing's sarcoma. Arch Med Res 43: 389–394, 2012 [DOI] [PubMed] [Google Scholar]
- 174.Zachariah RR, Schmid S, Buerki N, Radpour R, Holzgreve W, and Zhong X. Levels of circulating cell-free nuclear and mitochondrial DNA in benign and malignant ovarian tumors. Obstet Gynecol 112: 843–850, 2008 [DOI] [PubMed] [Google Scholar]
- 175.Zalavary S, Grenegard M, Stendahl O, and Bengtsson T. Platelets enhance Fc(gamma) receptor-mediated phagocytosis and respiratory burst in neutrophils: the role of purinergic modulation and actin polymerization. J Leukoc Biol 60: 58–68, 1996 [DOI] [PubMed] [Google Scholar]
- 176.Zhang B, Asadi S, Weng Z, Sismanopoulos N, and Theoharides TC. Stimulated human mast cells secrete mitochondrial components that have autocrine and paracrine inflammatory actions. PLoS One 7: e49767, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhang JZ, Liu Z, Liu J, Ren JX, and Sun TS. Mitochondrial DNA induces inflammation and increases TLR9/NF-kappaB expression in lung tissue. Int J Mol Med 33: 817–824, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 178.Zhang Q, Itagaki K, and Hauser CJ. Mitochondrial DNA is released by shock and activates neutrophils via p38 map kinase. Shock 34: 55–59, 2010 [DOI] [PubMed] [Google Scholar]
- 179.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, and Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464: 104–107, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhang X, Li A, Ge J, Reigada D, Laties AM, and Mitchell CH. Acute increase of intraocular pressure releases ATP into the anterior chamber. Exp Eye Res 85: 637–643, 2007 [DOI] [PubMed] [Google Scholar]
- 181.Zheng LM, Zychlinsky A, Liu CC, Ojcius DM, and Young JD. Extracellular ATP as a trigger for apoptosis or programmed cell death. J Cell Biol 112: 279–288, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhou R, Yazdi AS, Menu P, and Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469: 221–225, 2011 [DOI] [PubMed] [Google Scholar]