Abstract
Sortilin, as a member of Vps10p-domain sorting receptor family, is overexpressed in a number of malignancies, including ovarian carcinoma. Antibodies against sortilin may contribute to further clarification of sortilin functional activities in signal transduction, intracellular sorting of proteins, and endocytosis. The aim of this study was to produce a monoclonal antibody against a synthetic peptide derived from extracellular N-terminal region of sortilin to be used as a tool for investigating sortilin characteristics in ovarian carcinoma. A synthetic peptide derived from the last 50 amino acids of extracellular domain of sortilin protein was selected and conjugated to keyhole limpet hemocyanin and used to immunize mice. The anti-sortilin monoclonal antibody (MAb), clone 2D8, was purified from supernatant of final hybridoma clone using peptide-affinity chromatography column. Reactivity of antibody with the immunizing peptide was assessed in ELISA. Furthermore, flow cytometry and Western blot analyses were used to investigate the reactivity of antibody with its target in a panel of ovarian carcinoma cell lines or tissues. MAb 2D8 was able to recognize the coated immunizing peptide in ELISA and detect its protein target, sortilin, in flow cytometry and Western blot analyses. The achieved data suggest that the developed monoclonal antibody may be applicable as a research tool for detection of sortilin protein in Western blot as well as flow cytometry tests.
Introduction
Ovarian carcinoma is the fifth most-frequent malignancy, and the highest lethal gynecologic cancer in the female population in the United States.(1) In part this high mortality rate is due to the fact that most ovarian carcinoma patients are diagnosed with advanced stage disease, at which point it is more complicated to treat.(1) To better understand tumor cell behavior, as well as to identify effective diagnostic and therapeutic targets, a number of tumor-associated antigens—such as cancer antigen 125 (CA125), glycoprotein 38 kDa (gp38), Mucin 1 (MUC1), tumor-associated glycoprotein 72 (TAG-72), ovarian antigen 3 (OA3), mesothelin, New York esophageal squamous cell carcinoma-1 (NY-ESO-1), and vascular endothelial growth factor (VEGF)—have been investigated.(2)
As a member of mammalian Vps10p-domain proteins, sortilin/NTR3/Gp95 (∼100 kDa) is overexpressed in ovarian carcinoma and may play a main role in ovarian tumor cell survival.(3) Sortilin binds to a growing number of competing ligands, including neuropeptides such as neurotensin (NT) and neurotrophins (e.g., the proforms of nerve growth factor β [NGF-β] and brain-derived neurotrophic factor [BDNF]).(4,5) Accordingly, sortilin serves as a scavenger transmembrane receptor to eliminate NT from the extracellular fluid by endocytosis and triggers its degradation.(4) The expression of sortilin in human prostate, colon, and pancreas cancers implies the role of this receptor in growth response induced by NT in an autocrine manner.(6) Indeed, this protein may be involved in the NT-induced migration of human microglial cells via stimulation of both mitogen-activated protein and phosphoinositide 3-kinase-dependent pathways.(7) Our previous results demonstrated that knocking down of sortilin expression induces apoptosis in Caov-4 ovarian carcinoma cell line.(3) Therefore, further definition of its characteristics in ovarian carcinoma using a novel home-made antibody may provide an improvement in current diagnostic and therapeutic outcomes in patients.
The development of hybridoma technology by Köhler and Milstein in 1975 made possible the production of large quantities of monoclonal antibodies (MAbs) that recognize human tumor-associated antigens.(8) In this study we attempted to develop a MAb against sortilin to be used as a research tool for further investigation of sortilin expression and function in ovarian carcinoma.
Materials and Methods
Specimen collection
Tissue samples from three patients with ovarian carcinoma, pathologically diagnosed as serous adenocarcinoma (n = 1; mean age 33 years), endometrioid carcinoma (n = 1; age 39 years), and mucinous carcinoma (n = 1; age 59 years), were obtained from Imam Khomeini Hospital, Tehran, Iran (Table 1). Each patient signed an informed consent letter and the study was approved by the Avicenna Research Institute local ethics committee. Tissue collection was performed as described previously.(3)
Table 1.
Demographic Data of Ovarian Carcinoma Patients
| Tissue samples | Morphology | Type of differentiation | Age (yr) | Stage | CA125 level (U/mL) | Level of differentiation |
|---|---|---|---|---|---|---|
| OC1 | Epithelial | Serous adenocarcinoma | 33 | I | >1000 | Well |
| OC2 | Epithelial | Endometrioid | 39 | III | 78 | Moderate |
| OC3 | Epithelial | Mucinous | 59 | NA | 205 | NA |
OC, ovarian carcinoma; NA, not assigned.
Cell lines and culture conditions
Cell lines, including Caov-4 (HTB-76), SKOV-3 (HTB-77), SKBR3 (HTB-30), ACHN (CRL-1611), BT-474 (HTB-20), A549 (CCL-185), SW480 (CCL-228), MCF7 (HTB-22), CHO (CCL-61), HeLa (CCL-2), OVCAR3 (HTB-161), HEK-293 (CRL-1573), PC3 (CRL-1435), A172 (CRL-1620), LNCaP (CRL-1740), A375 (CRL-1619), Calu-6 (HTB-56), NIH/3T3 (CRL-1658), 2008/C13.R, and A2780S (National Cell Bank of Iran, Tehran, Iran), were cultured in their optimal culture conditions in RPMI-1640 (Gibco, Grand Island, NY), containing 10% fetal bovine serum (FBS, Gibco), 100 U/mL penicillin (ICN Biomedicals, Solon, OH), and 100 μg/mL streptomycin (Sigma, St. Louis, MO) at 37°C in a humidified incubator with 5% CO2 atmosphere.
Immunogen and immunization
A synthetic peptide derived from the last 50 amino acids of extracellular domain of sortilin protein was designed. The immunograde peptide (Thermo Electron, Ulm, Germany) was conjugated to keyhole limpet hemocyanin (KLH, Sigma) via glutaraldehyde linker as described previously.(9) Three BALB/c mice were immunized using peptide-KLH, according to the protocol described previously.(10)
Fusion and screening
Mouse myeloma SP2/0 cell line was cultured in RPMI-1640 containing 10% FBS. The feeder cells, obtained from the peritoneal cavity of a normal BALB/c mouse by RPMI-1640 injection, were plated in 96-well plates and cultured for 24 h before fusion. The mouse spleen cells were obtained 3 days after the last booster immunization and fused with SP2/0 cells at a ratio of 5:1 in 50% (W/V) polyethylene glycol 1500 (PEG 1500, Sigma). The cell fusion method was accomplished according to the technique described by Köhler and Milstein.(8) The hybridoma cells were cultured in 96-well plates containing feeder cells with RPMI-1640, 20% FBS, and hypoxanthineaminopterin-thymidine (HAT, Sigma). Hybridoma cells producing anti-sortilin antibodies were screened using indirect ELISA by coating immunizing-sortilin peptide, according to the method described previously.(9) The positive clones were subcloned four times using limiting dilution until the final clone was obtained.
Isotype determination and antibody purification
Subclass of anti-sortilin antibody was identified using IsoStrip mouse monoclonal antibody isotyping kit (Roche, Basel, Switzerland) according to the manufacturer's instruction. The antibody purification using affinity chromatography column was described previously.(9) The affinity column was prepared by coupling the immunizing peptide to CNBr-activated Sepharose-4B (GE Healthcare, Uppsala, Sweden) according to the manufacturer's instructions.
Stable expressing of sortilin gene in NIH/3T3
To stably transfect sortilin gene to NIH/3T3 cells, human sortilin cDNA was extracted from pCMV6-XL5-sortilin construct (OriGene, Rockville, MD) and subcloned into the pCMV6-neo plasmid (OriGene). The sequence and orientation of the fragment in pCMV6-Neo-sortilin were confirmed by enzyme digestion and DNA sequencing. The constructs of pCMV6-neo-sortilin or pCMV6-neo-empty vector were transfecetd in NIH/3T3 cells using the JetPEI™ DNA transfection reagent (Polyplus-transfection, New York, NY) according to the manufacturer's recommendations. After 48 h, cells were analyzed by Western blot analysis for sortilin expression. Transfected cells were subsequently selected using gradually increasing concentrations of G418 (Gibco) to 400 μg/mL. At the end of selection, cells were analyzed by Western blotting.
Western blot analysis
Tissue and cell samples were lysed in a non-reducing lysis buffer followed by Western blotting, as described in detail previously.(3) The produced anti-sortilin MAb and commercial rabbit anti-sortilin PAb (ab16640, Abcam, Cambridge, United Kingdom), at a concentration of 10 μg/mL and 1μg/mL, respectively, were used as first antibodies. Horseradish peroxidase-conjugated sheep anti-mouse or anti-rabbit immunoglobulins (Avicenna Research Institute, Tehran, Iran) were used to probe the blots.
Flow cytometry
Human ovarian carcinoma cell lines, including Caov-4, A2780S, SKOV-3, 2008/C13.R, and pCMV6-neo transfected NIH/3T3 (NIH/3T3-vector) as negative control, were incubated with the produced anti-sortilin MAb and IgG1 isotype control, anti-HIV protein envelope MAb (Avicenna Research Institute). Both MAbs were incubated at a concentration of 10 μg/mL for 30 min at 4°C. Following three washes, FITC-sheep anti-mouse antibody (1:50; Avicenna Research Institute) was incubated for 30 min at 4°C and washed. The results were examined using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) and analyzed using FlowJo software (v. 7.6.1).
Results
Production of anti-sortilin MAb
Splenocytes of a hyperimmunized mouse were fused with SP2/0 cells and all growing hybridomas were initially screened, based on their reactivity with the immunizing sortilin peptide, by indirect ELISA. All non-reactive clones were excluded after ELISA screening. Among the reactive hybridoma clones, one clone named 2D8 demonstrated higher reactivity with the immunogenic peptide in ELISA assay. Further characterization of the antibody showed that 2D8 was an IgG1 MAb with a kappa light chain.
Antibody purification and ELISA
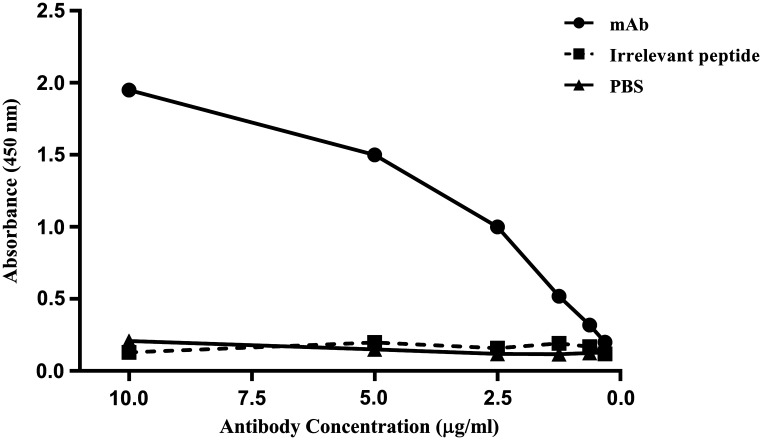
Purification of MAb 2D8 from hybridoma cell culture supernatant was performed using an affinity column. ELISA assay showed that the antibody had excellent immunoreactivity with the immunizing peptide, confirming its functionality after purification (Fig. 1). Furthermore, the lack of MAb reactivity with an irrelevant peptide and PBS alone confirmed its specificity for the immunizing sortilin peptide.
FIG. 1.
Titration of purified 2D8 in ELISA test. 2D8 was purified by peptide affinity column and its reactivity with the immunizing peptide was evaluated by ELISA. Lack of reactivity with an 18-mer irrelevant peptide and PBS alone served as negative controls.
Western blot analysis
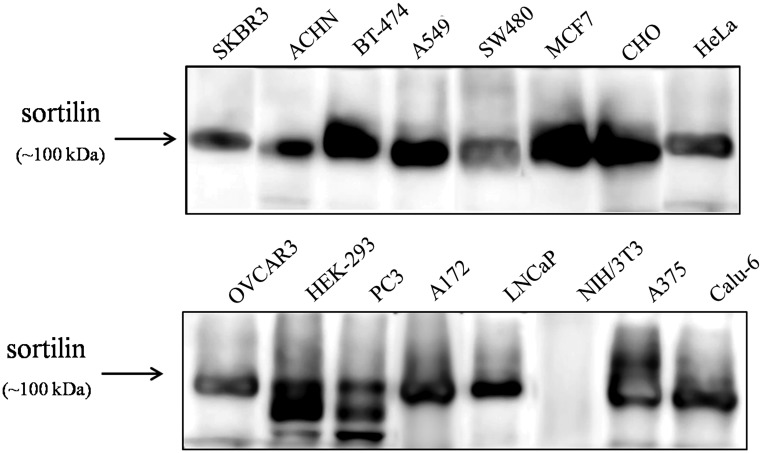
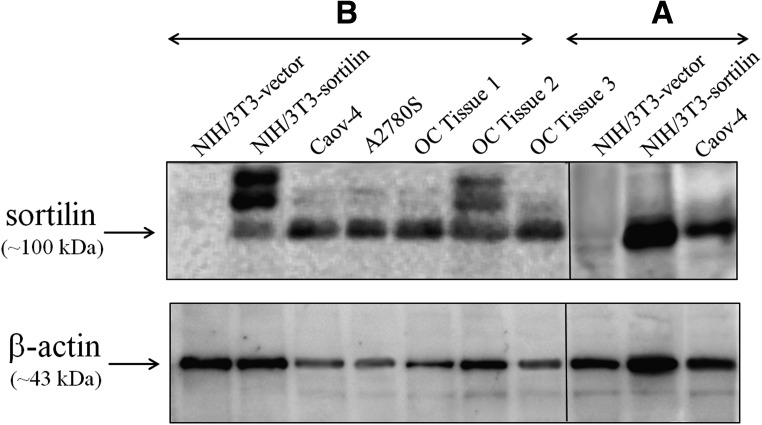
To provide a “negative cell control” in analysis of reactivity and specificity of 2D8, the expression of sortilin was explored in a number of cell lines with various origins using non-reducing Western blot analysis (Fig. 2). Results showed that all examined cells expressed various amounts of sortilin protein except the mouse embryonic fibroblast cell line, NIH/3T3, which was negative for sortilin expression in Western blot analysis (Fig. 2). Furthermore, to possess a “consistent positive control,” the sortilin-pCMV6-Neo construct was stably transfected into NIH/3T3 cells. Then, the cells were harvested, lysed, and analyzed by Western blot. As shown in Figure 3A, commercial anti-sortilin antibody recognized the 100 kDa band of sortilin in lysates of Caov-4 (as positive control) and NIH/3T3-sortilin (stably sortilin-transfected NIH/3T3 cells) but not in NIH/3T3-vector (stably pCMV6-Neo-transfected NIH/3T3 cells). Indeed, Figure 3B shows that 2D8 could detect sortilin at approximately 100 kDa in lysates from sortilin-transfected NIH/3T3, A2780S, SKOV-3 cell lines and three ovarian carcinoma tissues, confirming its application in Western blot analysis. The expression of β-actin as internal protein loading control was detected in all samples.
FIG. 2.
Investigation of sortilin expression in a number of cell lines using commercial rabbit anti-sortilin antibody in Western blot analysis. All cells expressed the main isoform (NP_002950.3, 833 aa, ∼100 kDa) of sortilin protein except the mouse embryonic fibroblast cell line, NIH/3T3, which was negative for sortilin expression in the Western blot. Presence of some low-molecular-weight bands in HEK-293 and PC3 cell lines may represent the expression of smaller sortilin protein isoforms such as NP_001192157 (694 aa, ∼80 kDa).
FIG. 3.
(A) Detection of sortilin expression in NIH/3T3-sortilin (stably sortilin-transfected NIH/3T3) using reactivity with commercial rabbit anti-sortilin antibody in Western blot analysis. Commercial antibody recognized 100 kDa band of sortilin in lysates of Caov-4 (positive control) and NIH/3T3-sortilin but not in NIH/3T3-vector (stably pCMV6-Neo-transfected NIH/3T3). (B) Reactivity of 2D8 to human sortilin molecule by Western blot analysis. 2D8 recognized human sortilin as a 100 kDa band in the lysates of NIH/3T3-sortilin, ovarian carcinoma tissues, and cell lines but not in NIH/3T3-vector. NIH/3T3-sortilin and NIH/3T3-vector provided positive and negative controls, respectively. β-actin protein was detected as internal control in all samples. OC, ovarian carcinoma.
Flow cytometry
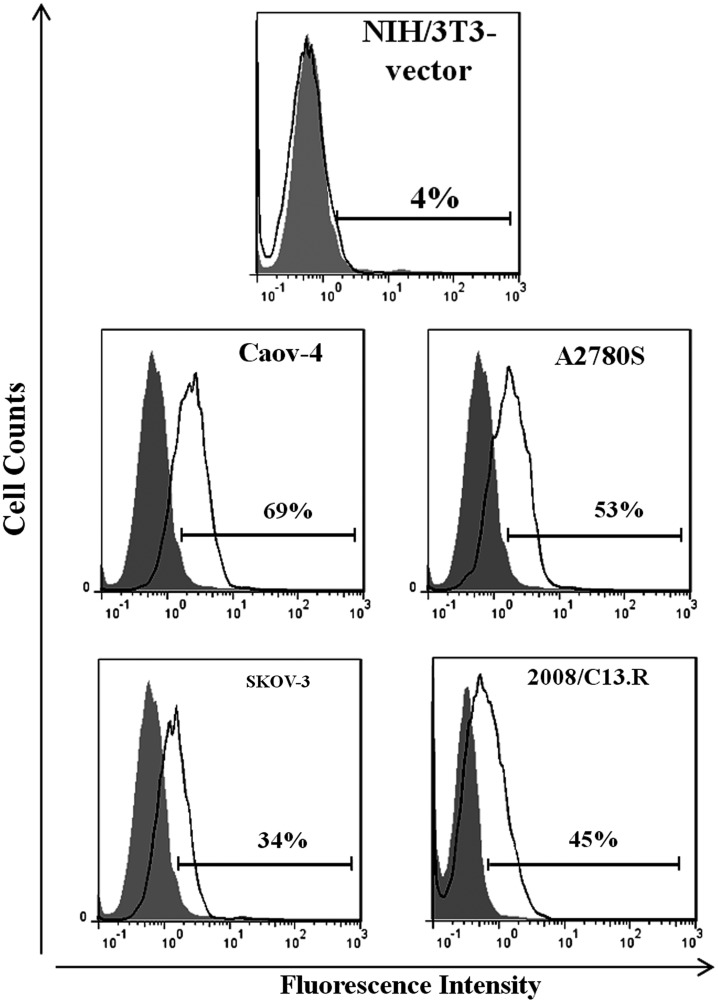
Cell surface staining of a number of ovarian carcinoma cell lines expressing sortilin on their surfaces, including Caov-4, A2780S, SKOV-3, and 2008/C13.R, demonstrated that 2D8 could react with an extracellular epitope of sortilin protein (Fig. 4). MAb 2D8 did not react with NIH/3T3-vector cells (negative control), which confirmed its specificity for sortilin in flow cytometry.
FIG. 4.
Reactivity of 2D8 with cell surface sortilin on membranes of ovarian cancer cell lines using flow cytometry analysis. Values demonstrate the percentages of positive cells. 2D8 detected transmembrane sortilin on Caov-4, A2780S, SKOV-3, and 2008/C13.R surfaces but not on NIH/3T3- vector membrane as negative control.
Discussion
Our previous results(3) demonstrating overexpression of sortilin in ovarian carcinoma encouraged us to produce a lab-generated MAb against sortilin for further study of sortilin molecule characteristics in this malignancy.
Antibodies have provided and will continue to provide scientists with a powerful and important research tool.(6) They are also becoming a significant diagnostic and therapeutic tool in the clinic.(6) To our knowledge, the majority of anti-sortilin MAbs has been developed against the intracellular domain of molecule, perhaps due to the fact that the majority of sortilin localizes in cytoplasmic organelles, like trans-Golgi-network and vesicles, while only about 10% transfers to the cell surface.(11) Consequently, most investigations have been focused on intracellular functions of sortilin. On the other hand, it was obviously demonstrated that the expression of sortilin on cell surface is increased after NT-induced sequestration of NTR1 and it plays a key role in internalization of NT and consequently cell proliferation.(12) Therefore, developing a MAb able to be applied in flow cytometry analysis and targeting cell surface expressed sortilin would be advantageous.
The open reading frame of sortilin cDNA encodes a protein of 833 amino acids containing N-terminal signal peptide, putative furin cleavage site, long luminal domain, single transmembrane part, and short cytoplasmic tail containing 53 amino acids.(13) We selected our immunizing peptide from the last 50 amino acids in extracellular domain of sortilin. In our attempt to produce antibodies against sortilin, a number of monoclonal antibodies were produced; however, 2D8 was found more effective in ELISA, Western blot, and flow cytometry.
A previous study by Peterson and colleagues showed that sortilin mRNA is expressed in many human tissues including brain, heart, placenta, skeletal muscle, pancreas, colon, stomach, thyroid, and spinal cord.(14) Others showed that sortilin protein is expressed in human prostate, colon, and pancreatic cancer cells in which this receptor is involved in the growth response induced by NT.(12) Chung and colleagues demonstrated that sortilin is involved directly in the growth response to NT in LoVo cells and in normal intestinal epithelial cells.(15) Furthermore, the expression of sortilin in vascular smooth muscle cells was recently reported.(16) We also analyzed many different cell lines to find a sortilin–non-expressing cell line to serve as negative control in our experiments.
Similar to other investigators, our results obtained from Western blot analysis (Fig. 2) demonstrated that the main sortilin protein isoform (NP_002950.3, 833 aa) is expressed by cell lines derived from various human tissues including breast (SKBR3, BT-474, MCF7), kidney (ACHN, HEK-293), lung (A549, Calu-6), colon (SW480), cervix (HeLa), prostate (PC3, LNCaP), skin (A375), brain (A172), and Chinese hamster ovary (CHO). Furthermore, presence of some low-molecular-weight bands in HEK-293 and PC3 cell lines may represent the expression of smaller sortilin protein isoforms (e.g., NP_001192157, 694 aa). Among studied cells, human embryonic kidney NIH/3T3 cell line did not express sortilin protein, and therefore, pCMV6-Neo-transfected NIH/3T3 and sortilin-transfected NIH/3T3 provided us with negative and positive cell controls, respectively.
The aim of this study was to develop a MAb against sortilin to be used as a research tool for further study of sortilin characteristics in ovarian carcinoma malignancy. With this end in mind, we investigated the potency of 2D8 in reactivity with lysates of ovarian carcinoma tissues and cell lines. The detected blots demonstrate that 2D8 is suitable for Western blot analysis under non-reducing conditions. Comparably, when reducing conditions were applied, no band was detected in the lysates (data not shown), which might imply that 2D8 recognizes a denatured non-reduced epitope.
We also investigated the application of 2D8 in cell surface staining by flow cytometry. The antibody was able to recognize the extracellular part of sortilin. In addition, we had previously shown that the majority of sortilin molecules are localized on membranes of ovarian carcinoma tissues and cell lines.(17) As it was expected, 2D8 did not react with sortilin non-expressing pCMV6-transfected NIH/3T3 cells that served as negative control.
In conclusion, 2D8 is an anti-sortilin MAb appropriate for use in Western blot and flow cytometry analyses.
Acknowledgment
The authors would like to thank Mrs. Zohreh Sadeghian (Imam Khomeini Hospital, Tehran, Iran) for her assistance in collecting tissue samples. This article was derived in part from a doctoral dissertation entitled, “Studying the role of SORT1 in survival of ovarian cancer cell line.”
Author Disclosure Statement
The authors have no financial interests to disclose.
References
- 1.Coward JI, Middleton K, and Murphy F: New perspectives on targeted therapy in ovarian cancer. Int J Womens Health 2015;7:189–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu B, Nash J, Runowicz C, Swede H, Stevens R, and Li Z: Ovarian cancer immunotherapy: opportunities, progresses and challenges. J Hematol Oncol 2010;3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghaemimanesh F, Ahmadian G, Talebi S, Zarnani AH, Behmanesh M, Hemmati S, Hadavi R, Jeddi-Tehrani M, Farzi M, Akhondi MM, and Rabbani H: The effect of sortilin silencing on ovarian carcinoma cells. Avicenna J Med Biotechnol 2014;6:169–177 [PMC free article] [PubMed] [Google Scholar]
- 4.Morinville A, Martin S, Lavallee M, Vincent JP, Beaudet A, and Mazella J: Internalization and trafficking of neurotensin via NTS3 receptors in HT29 cells. Int J Biochem Cell Biol 2004;36:2153–2168 [DOI] [PubMed] [Google Scholar]
- 5.Chen ZY, Ieraci A, Teng H, Dall H, Meng CX, Herrera DG, Nykjaer A, Hempstead BL, and Lee FS: Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. J Neurosci 2005;25:6156–6166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazella J, and Vincent JP: Functional roles of the NTS2 and NTS3 receptors. Peptides 2006;27:2469–2475 [DOI] [PubMed] [Google Scholar]
- 7.Martin S, Vincent JP, and Mazella J: Involvement of the neurotensin receptor-3 in the neurotensin-induced migration of human microglia. J Neurosci 2003;23:1198–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohler G, and Milstein C: Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975;256:495–497 [DOI] [PubMed] [Google Scholar]
- 9.Hadavi R, Zarnani AH, Ahmadvand N, Mahmoudi AR, Bayat AA, Mahmoudian J, Sadeghi MR, Soltanghoraee H, Akhondi MM, Tarahomi M, Jeddi-Tehrani M, and Rabbani H: Production of monoclonal antibody against human nestin. Avicenna J Med Biotechnol 2010;2:69–77 [PMC free article] [PubMed] [Google Scholar]
- 10.Zarei S, Bayat AA, Hadavi R, Mahmoudi AR, Tavangar B, Vojgani Y, Jeddi-Tehrani M, and Amirghofran Z. Production and characterization of a peptide-based monoclonal antibody against CD44 variant 6. Monoclon Antib Immunodiagn Immunother 2015;34:36–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ni X, Canuel M, and Morales CR: The sorting and trafficking of lysosomal proteins. Histol Histopathol 2006;21:899–913 [DOI] [PubMed] [Google Scholar]
- 12.Dal Farra C, Sarret P, Navarro V, Botto JM, Mazella J, and Vincent JP: Involvement of the neurotensin receptor subtype NTR3 in the growth effect of neurotensin on cancer cell lines. Intl J Cancer 2001;92:503–509 [DOI] [PubMed] [Google Scholar]
- 13.Vincent JP, Mazella J, and Kitabgi P: Neurotensin and neurotensin receptors. Trends Pharmacol Sci 1999;20:302–309 [DOI] [PubMed] [Google Scholar]
- 14.Petersen CM, Nielsen MS, Nykjaer A, Jacobsen L, Tommerup N, Rasmussen HH, Roigaard H, Gliemann J, Madsen P, and Moestrup SK: Molecular identification of a novel candidate sorting receptor purified from human brain by receptor-associated protein affinity chromatography. J Biol Chem 1997;272:3599–3605 [DOI] [PubMed] [Google Scholar]
- 15.Chung DH, Evers BM, Shimoda I, Townsend CM, Jr, Rajaraman S, and Thompson JC: Effect of neurotensin on gut mucosal growth in rats with jejunal and ileal Thiry-Vella fistulas. Gastroenterology 1992;103:1254–1259 [DOI] [PubMed] [Google Scholar]
- 16.Campagnolo L, Costanza G, Francesconi A, Arcuri G, Moscatelli I, and Orlandi A: Sortilin expression is essential for pro-nerve growth factor-induced apoptosis of rat vascular smooth muscle cells. PLoS One 2014;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hemmati S, Zarnani AH, Mahmoudi AR, Sadeghi MR, Soltanghoraee H, Akhondi MM, Tarahomi M, Jeddi-Tehrani M, and Rabbani H: Ectopic expression of sortilin 1 (NTR-3) in patients with ovarian carcinoma. Avicenna J Med Biotechnol 2009;1:125–131 [PMC free article] [PubMed] [Google Scholar]