Abstract
Antibiotic resistance has been increasing at an alarming rate in the United States and globally for decades, but the problem has only recently gained broad attention at the highest levels of the US government. More and more patients are dying of infections that do not respond to antibiotics that are currently available. Meanwhile, the antibacterial product pipeline remains fragile in part because of a lack of commercial interest from pharmaceutical companies. The Biomedical Advanced Research and Development Authority (BARDA) Broad Spectrum Antimicrobials (BSA) program leads the US government's effort to bridge this gap by advancing new antibacterials through late stages of clinical development. Other commentators have described in detail BARDA's structure, process, and role in antibacterial development. This commentary offers a public policy perspective on the emerging politics of antibiotic resistance in the context of US biosecurity politics and medical countermeasure (MCM) development. It identifies promising developments and difficult challenges that together will ultimately determine whether BARDA can become a global leader for antibiotic development.
Antibiotic resistance has been increasing at an alarming rate, but the problem has only recently gained broad attention at the highest levels of the US government. The antibacterial product pipeline remains fragile in part because of a lack of commercial interest from pharmaceutical companies. The Biomedical Advanced Research and Development Authority (BARDA) Broad Spectrum Antimicrobials (BSA) program leads the US government's effort to bridge this gap by advancing new antibacterials through late stages of clinical development. This article offers a public policy perspective on the emerging politics of antibiotic resistance in the context of US biosecurity policies and medical countermeasure (MCM) development. It identifies promising developments and difficult challenges that will ultimately determine whether BARDA can become a global leader for antibiotic development.
Antibiotic resistance* is increasingly recognized by experts and government leaders as an imminent threat to the foundation of public health and modern medicine.1 Meanwhile, the antibacterial research and development (R&D) pipeline is showing modest signs of recovery but remains fragile in part because of the substantial investments required by pharmaceutical companies relative to expected commercial rewards. In addition, promising nontraditional antibacterial approaches (eg, vaccines, bacteriophages, monoclonal antibodies) also draw insufficient public and private investment.2 The US Biomedical Advanced Research and Development Authority (BARDA), which was created to respond to biological threats (or “biothreats”) like pandemic influenza and bioterrorism-related pathogens like smallpox and anthrax, also leads US civilian efforts to spur advanced antibacterial development. BARDA's achievements to date are cause for optimism. However, the US Congress, which controls BARDA's purse strings, has not universally embraced antibiotic resistance as a biodefense priority. This commentary provides a public policy perspective on antibiotic resistance in the context of contemporary biosecurity politics and contends that the future of BARDA's antibiotic development program ultimately hinges on whether Congress affirmatively expands BARDA's core mission.
Antibiotics and Biosecurity
Estimates of US morbidity and mortality associated with antibiotic-resistant infections are significant and thought to be conservative because of patchy antibiotic resistance surveillance capabilities.3 Global surveillance capacity is also severely limited, where it exists at all,4 but worldwide antibiotic resistance mortality forecasts are nonetheless daunting.5 Because most routine and lifesaving medical procedures rely on antibiotics to reduce the risk of hospital-acquired infections, left unchecked antibiotic resistance could break down even the most advanced health systems.6 The potential for antibiotic resistance to “disrupt the normal functioning of societies,” and therefore elevate beyond an isolated public health concern, is real.7
In addition, antibiotic-resistant bacteria can be both highly transmissible and mobile on a global scale. For example, carbapenem-resistant Enterobacteriaceae (CRE), identified by the US Centers for Disease Control and Prevention (CDC) as one of the top antibiotic-resistant threats in the United States,3 has demonstrated its ability to spread globally, killing patients from New Delhi to New York.8 CRE strains often carry resistance enzymes like Klebsiella pneumoniae carbapenemase (KPC) and New Delhi Metallo-beta-lactamase-1 (NDM-1), which can travel from bacterium A to bacterium B, enhancing CRE's ability to spread. Fatality rates are high among those most often infected—hospitalized patients with serious underlying illnesses, people who are immunocompromised, or those undergoing invasive medical procedures. CREs that enter the bloodstream can kill 50% of those infected.9 CDC conservatively estimates that there are at least 600 deaths from CRE each year in the United States,3 and global mortality is believed to be “considerable.”10
But do antibiotic-resistant pathogens like CRE amount to a biosecurity concern? One reason this is a difficult question to answer is because biosecurity policy is a “conceptual and practical minefield.”11 Over time, the definition of biosecurity has expanded beyond its original agricultural and environmental context to encompass human health concerns.12 In the wake of the 2001 anthrax attacks in the United States, biosecurity was predominantly associated with protecting humans from bioterrorism. By 2006, however, the National Academy of Sciences (NAS) offered a broader definition that includes not just potential biological weapons and other dangerous pathogens but also “outbreaks of newly emergent and epidemic disease.”13 This change reflects a general trend toward “securitizing” naturally occurring infectious diseases once left to the public health domain.14 Gostin and Fidler characterized this trend as the convergence of the “high” politics of security policy and the “low” politics of epidemic disease and other public health concerns.11
However, antibiotic resistance is a “slow-burning” crisis compared with potentially pandemic viruses like influenza and coronavirus or bioterror events like anthrax letters or sarin gas attacks.15 With a few exceptions, like methicillin-resistant Staphylococcus aureus (MRSA), urinary tract infections, and some resistant foodborne bacteria, the public and policymakers generally associate antibiotic resistance threats with hospitals rather than the community or the environment, even while clinicians and public health practitioners continue to observe alarming increases in community-acquired antibiotic-resistant pathogens. In addition, although resistant bacteria are prevalent in the food-animal production industry, they pose a minimal threat to business compared to other diseases like H5N2 (“bird flu”) and bovine spongiform encephalopathy (“mad cow disease”). Finally, while nonstate actors could theoretically transfer genetic elements of resistance to biothreat pathogens like anthrax,16 experts today are divided about the feasibility of technologically complex, large-scale biological attacks.17 For these reasons, framing antibiotic resistance as a national security threat remains controversial.18
Antibiotics and the Broad Spectrum Antimicrobials Program
Established by the Pandemic and All Hazards Preparedness Act (PAHPA) in 2006 and organized under the office of the Assistant Secretary for Preparedness and Response (ASPR),19 BARDA's core mission is to “develop and procure medical countermeasures that address the public health and medical consequences of chemical, biological, radiological, and nuclear (CBRN) accidents, incidents and attacks, pandemic influenza, and emerging infectious diseases.”20 BARDA funding and technical assistance helps to bridge the “valley of death” between early and late stages of medical countermeasure (MCM) development, when commercial incentives are otherwise insufficient to draw the substantial R&D investment required from pharmaceutical companies.20 BARDA contracts with MCM manufacturers may include milestone payments, advance market commitments, and other innovative contracting features, including the “portfolio” mechanism, which allows both parties flexibility to discard failed targets in favor of more promising ones.21 Since its inception, BARDA has supported 150 MCM product candidates at some stage of development.22
BARDA's antibiotic initiative, the Broad Spectrum Antimicrobials (BSA) program, was established in 2010. The program focuses on developing antimicrobial products for “treatment or prevention of disease caused by currently defined and future biological threats.”20(p9) Recognizing the urgent public health need for novel antibiotics for clinical use, the program employs a “dual-utility” approach that considers candidate antibiotics for a “commercial, clinically prevalent” indication as long as sponsors “concomitantly support the development of these products for biodefense threat agent indications” consistent with the US Department of Homeland Security (DHS) material threat list.23 The BSA program has already made considerable progress, with at least 7 promising antibacterial compounds in development, 2 portfolio agreements in place with large pharmaceutical companies, and the first BSA-supported antibiotic expected to be submitted to the US Food and Drug Administration (FDA) in early 2016.24
Tellingly, there is no overlap between the top threats identified by CDC in its 2013 report on ABR and the BSA program's priority bacterial pathogens (see Table 1). Although the dual-utility strategy is used in many BARDA programs, it is especially applicable to the BSA; all of the investigational products currently supported by the program target both a “clinically prevalent” and a biodefense indication.21 Indeed, products that are effective against biothreats (eg, Yersinia pestis or Burkholderia spp.) will likely also show activity against their nonbiothreat cousins (eg, CRE and Pseudomonas aeruginosa, respectively). One important limitation of the dual-utility approach, however, is that BARDA must turn down proposals for certain alternative products or research (eg, gut microbiota modulation) that might mitigate ABR but that lack a viable biodefense justification.25
Table 1.
Comparison of CDC and BSA Priority Bacterial Threats
| CDC “Urgent” and “Serious” Antibiotic Resistance Threats3 | BSA High-Priority Bacterial Threats23 |
|---|---|
|
Clostridium difficile Carbapenem-resistant Enterobacteriaceae Drug-resistant Neisseria gonorrhoeae Multidrug-resistant Acinetobacter Drug-resistant Campylobacter Fluconazole-resistant Candida (a fungus) Extended spectrum β-lactamase-producing Enterobacteriaceae (ESBLs) Vancomycin-resistant Enterococcus (VRE) Multidrug-resistant Pseudomonas aeruginosa Drug-resistant nontyphoidal Salmonella Drug-resistant Salmonella Typhi Drug-resistant Shigella Methicillin-resistant Staphylococcus aureus (MRSA) Drug-resistant Streptococcus pneumoniae Drug-resistant tuberculosis |
Burkholderia mallei (glanders) Burkholderia pseudomallei (melioidosis), Francisella tularensis (tularemia) Rickettsia prowazekii (typhus) Yersinia pestis (plague) Bacillus anthracis (anthrax) |
Promising Developments, Remaining Challenges
Three Promising Developments
It is too early to tell whether the BSA program will succeed, but it is clear that it has made significant progress despite the limitations inherent in its authorizing statute. Recognizing this potential, the Obama administration recently took 3 actions that could significantly expand the BSA program.
1. Executive order calls for expansion of BSA program scope. In September 2014, President Obama signed an executive order broadly directing the executive branch to coordinate efforts to combat antibiotic resistance.26 The order directs BARDA to develop, “[t]ogether with the countermeasures it develops for biodefense threats, … countermeasures that target antibiotic-resistant bacteria that present a serious or urgent threat to public health [emphasis added].”26 This language appears to open the door for BARDA to dispense with the dual-utility requirement and target CDC's priority pathogens exclusively. Problematically, however, it does not change the underlying statutory language defining BARDA's core mission and qualifying pathogens—only Congress can do that.
2. National action plan sets ambitious BSA goals. The National Action Plan for Combating Antibiotic-Resistant Bacteria (CARB), released in March 2015 at the request of the President, calls on BARDA to “create at least one additional portfolio partnership with a pharmaceutical or biotechnology company to accelerate development of antibacterial drugs” within 1 year.27(p47) It also directs BARDA to identify and develop 12 new candidate antibiotics within 3 years and 2 antibiotic drugs developed through the portfolio approach and submitted for FDA approval within 5 years.27 Finally, the national action plan directs US agencies to explore collaborations with the New Drugs 4 Bad Bugs (ND4BB) program, the European Union's public-private partnership to drive early-stage antibiotic development.28 The CARB plan is encouraging and progress is under way. For example, the administration recently announced a new portfolio partnership with a large pharmaceutical company that will involve collaboration with the ND4BB program.29 However, the White House has acknowledged that meeting the 3- and 5-year goals will require new funding from Congress.
3. President's FY2016 budget calls for new BARDA funding. The President's FY2016 budget proposal to Congress would double federal spending on antibiotic resistance, including an allocation of over $522 million ($108 million over FY2015 funding) for BARDA to “expand development of antibacterial and new rapid diagnostics.”30 Most of this new money is intended to fund BARDA's expanded mission, as directed by the executive order, and to meet the goals outlined in the CARB plan. While a president's budget proposal in no way guarantees federal appropriations from Congress, it does serve as a starting point (though often the high-water mark) for congressional funding negotiations.
Taken together, these actions signal strong support from the Obama administration to expand the BSA program's scope. However, there are at least 3 challenges that must be overcome in order to fulfill this vision.
Three Challenges Facing BSA Expansion
BARDA's BSA program is currently the US's best bet to bring new antibiotics to the market for patients with highly resistant bacterial infections. But despite high expectations from observers around the world,5 there are at least 3 limiting factors that could impede further expansion of the program.
1. Congress must agree that antibiotics have a place in BARDA's core mission. Because Congress ultimately decides the scope and funding for BARDA activities by statute, congressional leaders must be convinced that an expanded BSA program is consistent with, or at least will not dilute, the BARDA mission. One important drawback of a broad definition of biosecurity is that it can make it less meaningful to policymakers.31 The more biological risks that lawmakers must consider under the biosecurity heading, the harder it is to prioritize them. When there is competition for scarce federal funding, especially during periods of fiscal restraint in Congress, policy goals can become diluted and may “fall victim to lowest-common-denominator solutions.”12 To address this concern, the President's Council of Advisors on Science and Technology (PCAST) proposes a separate fund dedicated to antibiotic-resistant bacteria and other emerging infections, which would thereby reserve established “Bioshield” funds for strictly biothreat indications.32
While there are indications that Congress is warming to the idea of an expanded interpretation of BARDA's role, it is clear that significant reservations remain. For example, at a congressional hearing in February 2015, Senator Richard Burr (called the “Grandpapa of PAHPA” for his lead role in drafting BARDA's authorizing legislation) took the opportunity to “clarify” for BARDA Director Robin Robinson that “BARDA's work in [antibiotics] is tied to its overall work to advance medical countermeasures against CBRN threats, and not outside of this context.”33 Dr. Robinson resolutely assured the senator that BARDA's core mission “was primarily for biothreats [and] it will remain there with the development of these antibiotics.”33
Notwithstanding Senator Burr's concerns, the Senate funding committee responsible for BARDA recently proposed $59 million for FY2016 (significant but still less than the President's FY2016 budget proposal) for BARDA “to spur the development of novel antibiotics and help revitalize the drug development pipeline.”34 However, the report does not specify whether BARDA can use the money to pursue antibiotics that lack a biothreat indication. The parallel funding report in the House of Representatives speaks more directly about the limitations on BARDA's scope of mission, but the ultimate implications for BARDA's antibiotic development activities remain unclear. The report allocates no new money for the President's antibiotic resistance initiative, noting that “funding for BARDA [should remain] focused on its statutory mission to develop CBRN countermeasures.” 35 However, it then directs BARDA to “work closely” with the National Institutes of Health (NIH) and CDC on “government-wide antibiotic resistance activity.35
The BSA program is unlikely to proceed at its current pace—much less expand its scope—unless Congress reaches a spending agreement for FY2016 that includes new funding above current levels. Expanding on Fidler's and Gostin's observation, the program sits at the nexus of the “high” politics of security, the “low” politics of public health, and what might be called the “rising” politics of antibiotic resistance (Figure 1). Unless congressional leaders are convinced that BARDA's mission can and should accommodate antibiotic development, even for indications that lack biothreat indications, it is unlikely that the policy objectives outlined in the President's executive order and CARB plan will come to fruition.
Figure 1.
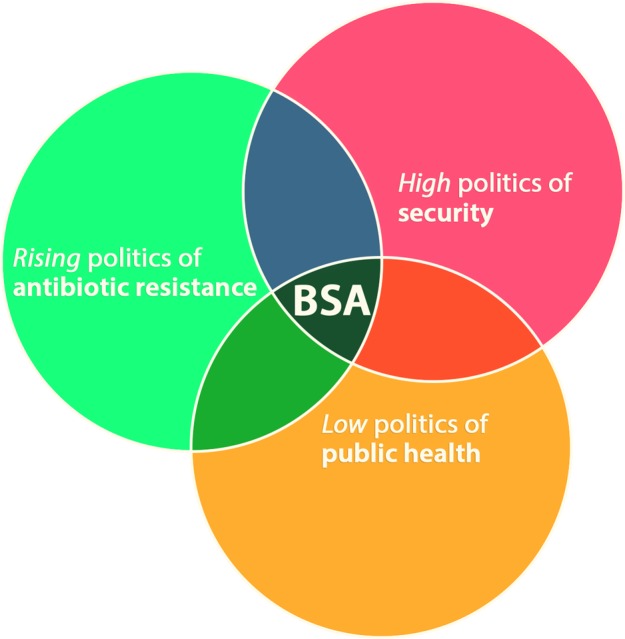
US Broad Spectrum Antimicrobials (BSA) Program at the Nexus of Political Spheres. Adapted from Fidler DP, Gostin LO, ref. 11. Color images available online at www.liebertpub.com/hs
2. Stronger stakeholder support is needed for BARDA's antibiotics role. Stakeholder advocacy and lobbying can help convince Congress that BARDA's core mission should include CDC's antibiotic resistance targets. Since BARDA was created in 2006, MCM companies have become powerful advocates in favor of keeping its core mission undiluted (especially when no new money is on the table). For example, one coalition of biodefense pharmaceutical companies spent over $1 million lobbying Congress in the decade since BARDA's inception.36 Yet, only 1 of the 12 coalition members has a commercial interest in antibiotics not indicated for biothreats (ie, anthrax).37 Most other large pharmaceutical companies have left the antibiotic market in favor of more lucrative targets, and current advocacy efforts by the handful remaining in the market tend to focus on improving the regulatory and reimbursement environment for antibiotics rather than supporting an expanded BSA program.38 Meanwhile, other advocates for antibiotic development, including some public health stakeholders, are less familiar with BARDA's role in antibiotic development compared with the NIH, FDA, and CDC, which they view as the lead health agencies.25 Ultimately, antibiotic resistance stakeholders will need to make a stronger, more unified effort to advocate for an expanded BSA program with dedicated federal funding.
3. BSA contracts should include postlicensure arrangements to ensure both access and conservation. Currently, BSA program contracts end when the sponsor company files a new drug application with the FDA. Other BARDA contracts for biothreat products like smallpox antivirals and anthrax antitoxins include postlicensure procurement agreements that ensure a market for the company while also building a stockpile of MCMs for when they are needed (ie, advance market commitments). However, there are no such contracts requiring any commitments from BSA program sponsors.21,25 Similar commitments are needed for antibiotics to ensure not only that patients needing antibiotics get them, but also that the products are marketed and used appropriately, thereby avoiding further propagation of resistant bacteria. Without these assurances, the medical and public health value (and therefore social utility) of any successful BSA project will be lost.39,40 While BARDA procurement contracts for biodefense products include access considerations, the concept of antibiotic conservation would be new to both the biosecurity paradigm and BARDA's mission. Postmarket requirements may work against efforts to encourage R&D investment from the pharmaceutical industry,21 but this difficult balancing should be done before product registration.
Overcoming each of these challenges will not be easy. Compounding these concerns, there is always a risk that the current political momentum to combat antibiotic resistance will fade or be replaced by other political priorities. President Obama, who led the charge to make antibiotic resistance a top federal government priority, is approaching his last year in office, and there is no guarantee that the next administration will pick up the mantle. And because the congressional appropriations process runs on an annual cycle, congressional support for the BSA (expanded scope or not) can come and go.41 Therefore, continued vigilance is needed to build and sustain political will.
Conclusion
Antibiotic resistance presents an imminent threat to the foundation of public health and modern medicine in the United States and globally. The need for new antibacterial products is urgent. BARDA is the leading driver of late-stage antibiotic development in the United States. Although recent commitments from the Obama administration hold promise for the continued growth of the BSA program, congressional preferences for traditional biothreat targets may jeopardize BSA funding. Given this uncertainty, other antibiotic development models should be carefully considered and pursued in parallel.
Acknowledgments
The author wishes to thank Joe Larsen for providing an interview in connection with this article; the Emerging Leaders in Biosecurity (ELBI) class of 2015 fellows, staff, and advisors for constructive input on an early draft; Nathan Danskey for graphic design; and Jonathan Nurse for lending his expertise on congressional appropriations.
The term antibiotic resistance here generally refers to bacterial pathogens that have developed resistance to one or more commonly used antibiotics. The related term, antimicrobial resistance, encompasses other pathogens such as viruses, parasites, and mycobacteria and is beyond the scope of this commentary.
References
- 1.Toner E, Adalja A, Gronvall GK, Cicero A, Inglesby TV. Antimicrobial resistance is a global health emergency. Health Secur 2015;13(3):153-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen HK, Trachsel J, Looft T, Casey TA. Finding alternatives to antibiotics. Ann N Y Acad Sci 2014;1323:91-100 [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013. April 2013. http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf Accessed August8, 2015
- 4.World Health Organization. Antimicrobial Resistance: Global Report on Surveillance 2014. April 2014. http://www.who.int/drugresistance/documents/surveillancereport/en/ Accessed August8, 2015
- 5.Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. Review on Antimicrobial Resistance, chaired by Jim O'Neill. December 2014. http://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf Accessed August8, 2015
- 6.Builder M. Antimicrobial Resistance as an Emerging Threat to National Security. Washington, DC: Atlantic Council; 2014. http://www.atlanticcouncil.org/images/files/Antimicrobial_Resistance_as_an_Emerging_Threat_to_National_Security.pdf Accessed October7, 2015 [Google Scholar]
- 7.Gostin LO, Fidler DP. Biosecurity under the rule of law. Case W Res J Int'l L 2007;38(3/4):437-478 [Google Scholar]
- 8.Perez F, Van Duin D. Carbapenem-resistant Enterobacteriaceae: a menace to our most vulnerable patients. Cleve Clin J Med 2013;80(4):225-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morb Mortal Wkly Rep 2013;62(9):165-170 [PMC free article] [PubMed] [Google Scholar]
- 10.Falagas ME, Tansarli GS, Karageorgopoulos DE, Vardakas KZ. Deaths attributable to carbapenem-resistant Enterobacteriaceae infections. Emerg Infect Dis 2014;20(7):1170-1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fidler DP, Gostin LO. Biosecurity in the Global Age: Biological Weapons, Public Health, and the Rule of Law. Palo Alto, CA: Stanford University Press; 2008 [Google Scholar]
- 12.Koblentz GD. Biosecurity reconsidered: calibrating biological threats and responses. Int Secur 2010;34(4):96-132 [Google Scholar]
- 13.Committee on Advances in Technology and the Prevention of Their Application to Next Generation Biowarfare Threats, National Research Council. Globalization, Biosecurity, and the Future of the Life Sciences. Washington, DC: National Academies Press; 2006 [Google Scholar]
- 14.Elbe S, Roemer-Mahler A, Long C. Medical countermeasures for national security: a new government role in the pharmaceuticalization of society. Soc Sci Med 2015;131:263-271 [DOI] [PubMed] [Google Scholar]
- 15.Monaco L. Opening session at the White House Forum on Antibiotic Stewardship; June 2, 2015
- 16.Rambhia KJ, Gronvall GK. Antibiotic resistance. Biosecur Bioterror 2009;7(4):371-377 [DOI] [PubMed] [Google Scholar]
- 17.Boddie C, Watson M, Ackerman G, Gronvall GK. Assessing the bioweapons threat: is there a foundation of agreement among experts about risk? Science 2015;349(6250):792-793 [DOI] [PubMed] [Google Scholar]
- 18.Peterson S. Epidemic disease and national security. Security Studies 2002;12(2):43-81 [Google Scholar]
- 19.42 U.S.C. § 247d-7e (2012) (enacted via Title IV of the Pandemic and All-Hazards Preparedness Act, Pub. L. No. 109-417)
- 20.US Department of Health and Human Services. BARDA Strategic Plan 2011-2016. 2011. http://www.phe.gov/about/barda/Documents/barda-strategic-plan.pdf Accessed August9, 2015
- 21.Eichberg MJ. Public funding of clinical-stage antibiotic development in the United States and European Union. Health Secur 2015;13(3):156-165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larsen J. BARDA's Broad Spectrum Antimicrobial (BSA) Program. Presentation to the President's Council of Advisors on Science and Technology (PCAST); March2015 https://www.whitehouse.gov/sites/default/files/microsites/ostp/PCAST/Larsen,%20Joe.pdf Accessed August9, 2015 [Google Scholar]
- 23.US Department of Health and Human Services. 2014 Public Health Emergency Medical Countermeasures Enterprise (PHEMCE) Strategy and Implementation Plan. 2014. http://www.phe.gov/Preparedness/mcm/phemce/Documents/2014-phemce-sip.pdf Accessed August12, 2015
- 24.Larsen J. Presentation to U.S. Stakeholder Forum on Antimicrobial Resistance (S-FAR), convened by the Infectious Diseases Society of America (IDSA). August 2015. (Webinar archived and on file with author.)
- 25.Interview with Joe Larsen, Deputy Director, Division of CBRN Medical Countermeasures, BARDA, Arlington, VA; June 25, 2015 [Google Scholar]
- 26.Exec. Order No. 13676, 184 C.F.R. 56931 (2014)
- 27.The White House. National Action Plan for Combating Antibiotic-Resistant Bacteria. March 2015. https://www.whitehouse.gov/sites/default/files/docs/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf Accessed August9, 2015
- 28.Innovative Medicines Initiative. New Drugs 4 Bad Bugs. http://www.imi.europa.eu/sites/default/files/uploads/documents/projects/ND4BB_overview.pdf Accessed August9, 2015
- 29.HHS enters into strategic alliance to accelerate new antibiotic development [press release]. US Department of Health and Human Services; September 16, 2015. http://www.hhs.gov/news/press/2015pres/09/20150916a.html Accessed October7, 2015 [Google Scholar]
- 30.The White House. Fact Sheet: President's 2016 budget proposes historic investment to combat antibiotic-resistant bacteria to protect public health [press release]. January 27, 2015. https://www.whitehouse.gov/the-press-office/2015/01/27/fact-sheet-president-s-2016-budget-proposes-historic-investment-combat-a Accessed August9, 2015
- 31.Owens H, Arneil B. The human security paradigm shift: a new lens on Canadian foreign policy? Report of the University of British Columbia symposium on human security. Canadian Foreign Policy Journal 1999;7(1):1-12 [Google Scholar]
- 32.Executive Office of the President, President's Council of Advisors on Science and Technology. Report to the President on Combating Antibiotic Resistance. September 2014. http://www.whitehouse.gov/sites/default/files/microsites/ostp/PCAST/pcast_carb_report_sept2014.pdf Accessed August10, 2015
- 33.Medical and Public Health Preparedness and Response: Are We Ready for Future Threats? Before the S. Health Ed. Labor & Pens. Comm., 114th Cong. (2015) [Google Scholar]
- 34.S. Rep. No. 114-74, at 14(2015)
- 35.H.R. Rep. No. 114-195, at 113 (2015)
- 36.Center for Responsive Politics. Alliance for Biosecurity. http://www.opensecrets.org/lobby/clientsum.php?id=D000046672&year=2014 Accessed August9, 2015
- 37.Alliance for Biosecurity. Our members. http://www.allianceforbiosecurity.org/#!contact/c1d94 Accessed August9, 2015
- 38.Antimicrobial Innovation Alliance. Key Issues: Incentives. http://www.antimicrobialalliance.com/incentives/ Accessed August9, 2015
- 39.Kesselheim AS, Outterson K. Improving antibiotic markets for long-term sustainability. Yale J Health Policy Law Ethics 2011;11(1):101-167 [PubMed] [Google Scholar]
- 40.Hoffman SJ, Outterson K. Introduction: what will it take to address the global threat of antibiotic resistance? J Law Med Ethics 2015;43(Suppl 3):6-11 [DOI] [PubMed] [Google Scholar]
- 41.Six Years After Anthrax: Are We Better Prepared to Respond to Bioterrorism?: Hearing Before the S. Comm. on Homeland Sec. and Gov. Aff's., 110th Cong. (2007) (statement of Tara O'Toole, Director, Center for Biosecurity of UPMC)


