Abstract
Overexpression of the epidermal growth factor receptor (EGFR) gene and dysregulation of EGFR signaling are observed in various cancer cells, and EGFR is a validated target for cancer therapy. In the present study, we report on the generation of two rat anti-EGFR antibodies (clones 2C2D3 and 4H7F4) by using the rat lymph node method. Flow cytometric analysis and immunofluorescence showed that both antibodies specifically bound to EGFR on the surface of cancer cells. Competitive analysis demonstrated that the epitope of each antibody had no overlap with that of the therapeutic anti-EGFR antibody cetuximab. These results suggest that 2C2D3 and 4H7F4 are potentially useful in EGFR-targeted cancer therapy.
Introduction
Epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase that belongs to the ErbB family. EGFR plays important roles in multiple cellular processes, including proliferation, migration, and differentiation. These cellular processes are triggered by ligands such as epidermal growth factor (EGF) and transforming growth factor α (TGFα). Upon binding of these ligands to EGFR, the receptor is activated and triggers intracellular signaling pathways. Under normal physiological conditions, EGFR signaling is tightly regulated by the binding of specific ligands.(1) However, in many types of cancer cells, EGFR signaling is dysregulated due to overexpression or mutation of the receptor or excess production of growth factors, or a combination of these, leading to the overgrowth of cancer cells.(2,3) For these reasons, EGFR is an attractive target for cancer therapy.
Anti-EGFR monoclonal antibody-based treatment is a validated strategy for cancer therapy.(4) Cetuximab, a chimeric mouse-human anti-EGFR antibody, is clinically used for the treatment of metastatic colorectal cancer and squamous cell carcinoma of the head and neck. Cetuximab can inhibit cancer cell growth through its antagonistic effect on EGF binding and antibody-dependent cellular cytotoxicity.(5) Nevertheless, it is known that some EGFR mutations and autocrine signaling contribute to poor response or resistance to cetuximab treatment.(6) In fact, Cunningham and colleagues reported that the rate of response to cetuximab in metastatic colorectal cancer patients was 11%.(7) Therefore, development of alternative antibodies against EGFR must be one of the strategies to overcome these therapeutic limitations.
Using the rat lymph node method,(8) we produced two rat monoclonal antibodies against the extracellular domain of EGFR; we describe these antibodies in detail here.
Materials and Methods
Cell culture
Human epithelial carcinoma cell line A431 and mouse fibroblast cell line NIH3T3 were cultured in Dulbecco's Modified Eagle's Medium (DMEM, Wako, Osaka, Japan) supplemented with 10% (v/v) fetal bovine serum (FBS) in a humidified atmosphere of 5% CO2 at 37°C.
Recombinant antigens
Recombinant antigens for immunization and enzyme-linked immunosorbent assay (ELISA) screening were produced using a mammalian expression system. Briefly, DNA encoding the extracellular domain of human EGFR (EGFR-ECD, Swiss-Prot accession no. P00533, amino acids 25-645) was fused with DNA encoding the recognition site (LEVLFQGP) for human rhinovirus 3C protease(9) and human IgG1 Fc (Swiss-Prot accession no. P01857) at the C-terminus. After cloning the DNA fragment into the pCAGGS expression vector,(10) the EGFR/Fc fusion (EGFR-ECD-Fc) gene was transiently expressed in HEK293T cells, and the protein of interest was purified by Protein A column affinity chromatography (rProtein A Sepharose Fast Flow, GE Healthcare, Tokyo, Japan). EGFR-ECD was prepared by treating EGFR-ECD-Fc with human rhinovirus 3C protease (BioVision, Milpitas, CA). After digestion, the protein of interest was eluted in the flow-through fraction of two-step affinity chromatography using rProtein A Sepharose and Ni Sepharose resins (both from GE Healthcare).
Generation of hybridoma cell lines
A 10-week-old female Wistar-Kyoto rat was injected in the hind footpad with 200 μL of an emulsion containing 170 μg of EGFR-ECD-Fc and Freund's complete adjuvant. Nineteen days after the immunization, the cells from the medial iliac lymph nodes of the rat were fused with mouse myeloma SP2 cells at a ratio of 1:1 in a 50% polyethylene glycol solution (PEG1500, Roche, Basel, Switzerland). The resulting hybridoma cells were plated onto six 96-well plates and cultured in HAT selection medium (Hybridoma-SFM [Life Technologies, Grand Island, CA], 10% FBS, 1 ng/mL recombinant mouse interleukin [IL]-6, 100 mM hypoxanthine [Sigma, St. Louis, MO], 0.4 mM aminopterin [Sigma], and 16 mM thymidine [Wako]). At 7 days post-fusion, the hybridoma supernatants were screened by ELISA with EGFR-ECD as the antigen and by immunofluorescence with A431 cells as target cells. Cells from the positive wells were cloned by limiting dilution and replated onto 96-well plates. Then, the hybridoma supernatants were further screened by flow cytometry with A431 cells as target cells. Positive hybridoma clones were cultured in serum-free media (Hybridoma-SFM), and the rat monoclonal antibodies were purified from the supernatants by using Protein G Sepharose (GE Healthcare). The class and subclass of the rat monoclonal antibodies were determined by using the Rat Monoclonal Antibody Isotyping Test Kit (Serotec, Oxford, United Kingdom).
ELISA
EGFR-ECD (1 μg/mL) in phosphate buffer was adsorbed on the surface of a 96-well plate by overnight incubation at 4°C. The plate was blocked with 1% bovine serum albumin in phosphate-buffered saline (PBS) to avoid non-specific binding. Hybridoma supernatants were added onto the plate and incubated for 1 h at room temperature. The plate was washed three times with PBS and then incubated for 30 min at room temperature with peroxidase-conjugated anti-rat IgG antibody (Sigma). After washing once with TBS-T (10 mM Tris-HCl [pH 7.4], 100 mM NaCl, and 0.1% Tween-20) and twice with PBS; 3,3′,5,5′-tetramethylbenzidine solution (Wako) was added to each well to visualize the immunoreactivity.
Flow cytometry
To investigate specific binding of the rat monoclonal antibodies (clones 2C2D3 and 4H7F4) to the cell surface antigen, 5 × 105 cells (A431 or NIH3T3) were treated with 2 μg/mL of purified 2C2D3 or 4H7F4 and incubated for 30 min on ice. After washing with FACS buffer (PBS containing 0.1% NaN3), cells were treated with Alexa 488-conjugated anti-rat IgG antibody (Life Technologies) and incubated for 30 min on ice. The stained cells were analyzed by flow cytometry (FACSCalibur, Becton Dickinson, Tokyo, Japan).
For the competition assay, 5 × 105 A431 cells were treated with a binder (10 nM biotinylated EGF [Life Technologies], 5 μg/mL of rat anti-EGFR antibody [2C2D3 or 4H7F4], or 5 μg/mL of cetuximab) in the presence or absence of a competitor (100 nM competitor for biotinylated EGF, or 5 μg/mL of competitor for rat anti-EGFR antibodies and cetuximab) and incubated for 30 min on ice. After washing with FACS buffer, cells were treated with Alexa 488-conjugated streptavidin (Jackson ImmunoResearch Laboratories, West Grove, PA), Alexa 488-conjugated anti-rat IgG antibody, or FITC-conjugated anti-human IgG antibody (Sigma) and incubated for 30 min on ice. The stained cells were analyzed by flow cytometry.
Immunofluorescence
A431 cells were plated onto a multi-test slide (MP Biomedicals Europe, Illkirch, France) at 5 × 103 cells/well and grown in culture medium for 24 h. Cells were incubated with hybridoma supernatants or 20 nM purified rat anti-EGFR monoclonal antibody (2C2D3 or 4H7F4) for 60 min at room temperature and then fixed with 3.7% formaldehyde in PBS for 15 min at room temperature. After washing with PBS, cells were incubated with Alexa 488-conjugated anti-rat IgG antibody. The samples were examined by using the IX-71 fluorescent microscope (Olympus, Tokyo, Japan).
Results
Isolation of rat anti-EGFR antibodies
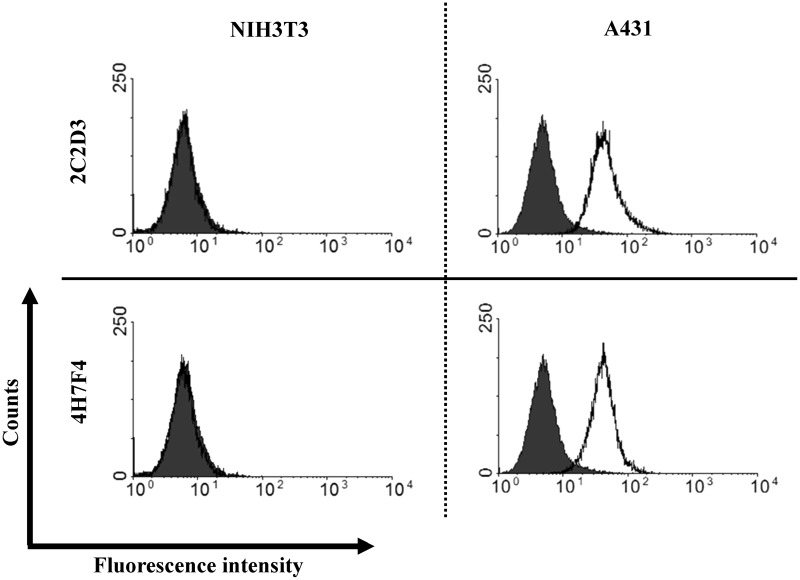
As an antigen for immunization, we used a fusion protein of the extracellular domain of human EGFR and the human IgG1 Fc region, which was designated as EGFR-ECD-Fc. A Wistar-Kyoto rat was immunized via hind footpad injection. Nineteen days after the immunization, the lymphocytes were collected from the enlarged medial iliac lymph nodes of the rat and then fused with mouse myeloma cells. The hybridoma lines were subcloned by dilution of the cells, plated onto 96-well plates, and screened by ELISA and immunofluorescence. Because there could be more than one clone in the original well at this point, the cells from the positive wells (designated as 1A11, 2C2, 2F8, 3C8, 4G9, 4H7, 5H4, and 6E8) were re-plated onto 96-well plates to ensure a completely monoclonal colony. Then, the hybridoma supernatants were further screened by using flow cytometry. Flow cytometric analysis showed that two rat anti-EGFR antibodies, 2C2D3 (IgG1/κ) and 4H7F4 (IgG2a/κ), produced strong fluorescence signal for A431 cells (EGFR-positive) but did not cross-react with NIH3T3 cells (EGFR-negative) (Fig. 1).
FIG. 1.
Flow cytometric analysis of the binding of 2C2D3 and 4H7F4. NIH3T3 cells (EGFR-negative) and A431 cells (EGFR-positive) were incubated with a rat control antibody (shaded area) or rat anti-EGFR antibody (2C2D3 or 4H7F4; open area). Bound antibodies were detected with Alexa 488-conjugated anti-rat IgG antibody.
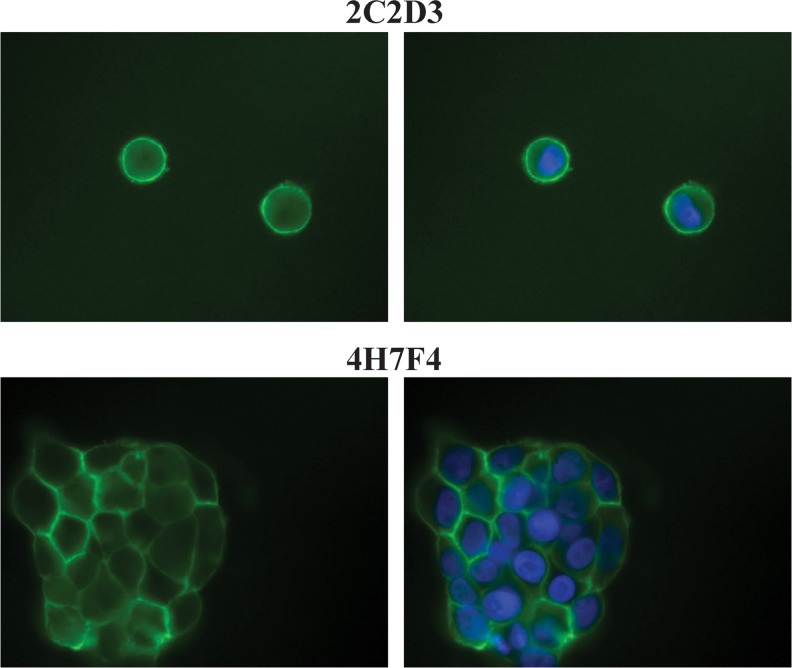
To further evaluate the binding specificities of 2C2D3 and 4H7F4, we used them to perform immunofluorescence staining of A431 cells. The two antibodies were applied to the cells prior to fixation to confirm that they bound to the extracellular domain of EGFR. The fluorescence signal for both 2C2D3 and 4H7F4 was detected mainly on the cell surface (Fig. 2). Although the fluorescence signal from 2C2D3 was weaker than that from 4H7F4, these results indicate that the two anti-EGFR antibodies can bind to cell surface antigens.
FIG. 2.
Indirect immunofluorescence of 2C2D3 and 4H7F4. A431 cells were incubated with rat anti-EGFR antibody (2C2D3 or 4H7F4) prior to fixation. Bound antibodies were detected by Alexa 488-conjugated anti-rat IgG antibody (green). The nuclei were counterstained with Hoechst dye (blue).
Epitope analysis of rat anti-EGFR antibodies
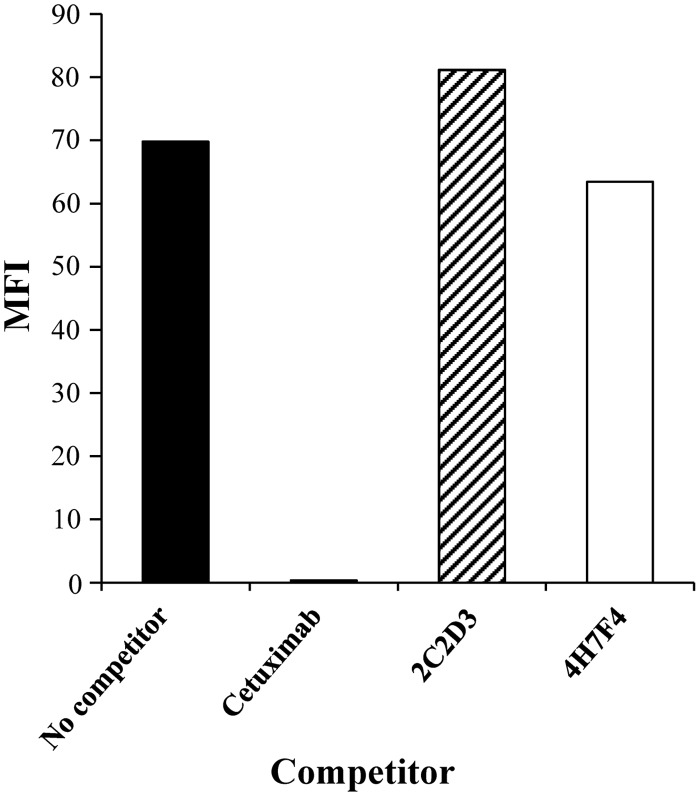
We first investigated whether the rat anti-EGFR antibodies compete with EGF for binding to EGFR using the method of flow cytometry. A431 cells were incubated with biotinylated EGF in the presence or absence of cetuximab, 2C2D3, or 4H7F4, and then bound EGF was detected by Alexa 488-conjugated streptavidin. In this analysis, cetuximab, which competes with EGF for binding to EGFR, was used as a positive control.(11) While cetuximab inhibited the binding of EGF to A431 cells, 2C2D3 and 4H7F4 displayed no inhibition (Fig. 3). Thus, both 2C2D3 and 4H7F4 exhibited no antagonistic activity against EGFR.
FIG. 3.
Antibody and EGF competition assay. A431 cells were treated with 10 nM biotinylated EGF in the presence or absence of 100 nM rat anti-EGFR antibody (2C2D3 or 4H7F4) or 100 nM cetuximab. After washing, cells were treated with Alexa 488-conjugated streptavidin, and stained cells were analyzed by flow cytometry. Mean fluorescence intensity (MFI) was calculated by subtracting the background MFI (no treatment with biotinylated EGF) from experimental MFI.
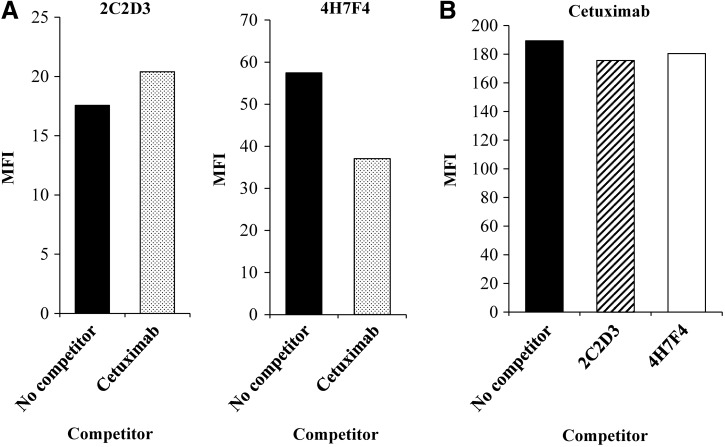
Next, we investigated whether the rat anti-EGFR antibodies compete with cetuximab. This assay was performed in two ways: each rat anti-EGFR antibody and cetuximab were used as (1) a binder and a competitor, respectively (Fig. 4A); and (2) a competitor and a binder, respectively (Fig. 4B). In both cases, a marked decrease in signal after adding a competitor was not observed. These results indicate that the epitope of each rat antibody does not overlap with that of cetuximab.
FIG. 4.
Rat anti-EGFR antibody and cetuximab competition assay. (A) A431 cells were treated with 5 μg/mL of rat anti-EGFR antibody (2C2D3 or 4H7F4) in the presence or absence of 5 μg/mL of cetuximab. After washing, cells were treated with Alexa 488-conjugated anti-rat IgG antibody. (B) A431 cells were treated with 5 μg/mL of cetuximab in the presence or absence of 5 μg/mL of 2C2D3 or 4H7F4. After washing, cells were treated with FITC-conjugated anti-human IgG antibody. All stained cells were analyzed by flow cytometry. Mean fluorescence intensity (MFI) was calculated by subtracting the background MFI (no treatment with primary antibody) from the experimental MFI.
Discussion
Here we describe the development of two rat anti-EGFR antibodies, 2C2D3 and 4H7F4. These antibodies, which were generated by immunization with the extracellular domain of EGFR, can specifically bind to the recombinant antigen and, additionally, to the native antigen on cancer cells. Our next step is chimerization(12) or humanization(13) of these antibodies for therapeutic applications.
Epitope analysis showed that each epitope of 2C2D3 and 4H7F4 does not overlap with that of the therapeutic anti-EGFR antibody cetuximab. It has recently been reported that biparatopic constructs composed of two noncompetitive anti-EGFR molecules can synergistically down-regulate the receptor on the cell surface without activating the receptor.(14) Other reports have also shown the in vitro and in vivo anti-cancer effect of biparatopic antibodies.(15,16) Notably, one of the biparatopic constructs generated by Boersma and colleagues exhibits an anti-cancer effect although the EGFR-targeting molecules that comprise the biparatopic construct have no antagonistic activity.(16) These findings support the notion that 2C2D3 and 4H7F4 have potential not only as candidate therapeutic agents but also as building blocks of next-generation engineered antibodies.
Acknowledgments
The human epidermoid carcinoma cell line A431 (RCB0202) was provided by the RIKEN BioResource Center through the National Bio-Resource Project of MEXT, Japan. This work was supported in part by a Grant-in-Aid for Scientific Research (C) (no. 25420836) from the Japan Society for the Promotion of Science.
Author Disclosure Statement
The authors have no financial interests to disclose.
References
- 1.Ferguson KM, Berger MB, Mendrola JM, Cho HS, Leahy DJ, and Lemmon MA: EGF activates its receptor by removing interactions that autoinhibit ectodomain dimerization. Mol Cell 2003;11:507–517 [DOI] [PubMed] [Google Scholar]
- 2.Yarden Y, and Sliwkowski MX: Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2001;2:127–137 [DOI] [PubMed] [Google Scholar]
- 3.Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F, and Salomon DS: Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 2006;366:2–16 [DOI] [PubMed] [Google Scholar]
- 4.Martinelli E, De Palma R, Orditura M, De Vita F, and Ciardiello F: Anti-epidermal growth factor receptor monoclonal antibodies in cancer therapy. Clin Exp Immunol 2009;158:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferris RL, Jaffee EM, and Ferrone S: Tumor antigen-targeted, monoclonal antibody-based immunotherapy: clinical response, cellular immunity, and immune escape. J Clin Oncol 2010;28:4390–4399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vlacich G, and Coffey RJ: Resistance to EGFR-targeted therapy: a family affair. Cancer Cell 2011;20:423–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, and Van Cutsem E: Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004;22:337–345 [DOI] [PubMed] [Google Scholar]
- 8.Kishiro Y, Kagawa M, Naito I, and Sado Y: A novel method of preparing rat-monoclonal antibody producing hybridomas by using rat medial iliac lymph node cells. Cell Struct Funct 1995;20:151–156 [DOI] [PubMed] [Google Scholar]
- 9.Asano R, Ikoma K, Kawaguchi H, Ishiyama Y, Nakanishi T, Umetsu M, Hayashi H, Katayose Y, Unno M, Kudo T, and Kumagai I: Application of the Fc fusion format to generate tag-free bi-specific diabodies. FEBS J 2010;277:477–487 [DOI] [PubMed] [Google Scholar]
- 10.Miyazaki J, Takaki S, Araki K, Tashiro F, Tominaga A, Takatsu K, and Yamamura K: Expression vector system based on the chicken beta-actin promoter directs efficient production of interleukin-5. Gene 1989;79:269–277 [DOI] [PubMed] [Google Scholar]
- 11.Gill GN, Kawamoto T, Cochet C, Le A, Sato JD, Masui H, McLeod C, and Mendelsohn J: Monoclonal anti-epidermal growth factor receptor antibodies which are inhibitors of epidermal growth factor binding and antagonists of epidermal growth factor binding and antagonists of epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem 1984;259:7755–7760 [PubMed] [Google Scholar]
- 12.Boulianne GL, Hozumi N, and Shulman MJ: Production of functional chimaeric mouse/human antibody. Nature 1984;312:643–646 [DOI] [PubMed] [Google Scholar]
- 13.Jones PT, Dear PH, Foote J, Neuberger MS, and Winter G: Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature 1986;321:522–555 [DOI] [PubMed] [Google Scholar]
- 14.Hackel BJ, Neil JR, White FM, and Wittrup KD: Epidermal growth factor receptor downregulation by small heterodimeric binding proteins. Protein Eng Des Sel 2012;25:47–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roovers RC, Vosjan MJ, Laeremans T, el Khoulati R, de Bruin RC, Ferguson KM, Verkleij AJ, van Dongen GA, and van Bergen en Henegouwen PM: A biparatopic anti-EGFR nanobody efficiently inhibits solid tumour growth. Int J Cancer 2011;129:2013–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boersma YL, Chao G, Steiner D, Wittrup KD, and Plückthun A: Bispecific designed ankyrin repeat proteins (DARPins) targeting epidermal growth factor receptor inhibit A431 cell proliferation and receptor recycling. J Biol Chem 2011;286:41273–41285 [DOI] [PMC free article] [PubMed] [Google Scholar]