Abstract
Both decapentaplegic (dpp) protein and 60A protein have been implicated in pattern formation during Drosophila melanogaster embryogenesis. Within the C-terminal domain, dpp and 60A are similar to human bone morphogenetic protein 2 (75% identity) and human osteogenic protein 1 (70% identity), respectively. Both recombinant human bone morphogenetic protein 2 and recombinant human osteogenic protein 1 have been shown to induce bone formation in vivo and to restore large diaphyseal segmental defects in various animal models. We examined whether the Drosophila proteins, dpp and 60A, have the capacity to induce bone formation in mammals by using the rat subcutaneous bone induction model. Highly purified recombinant dpp and 60A induced the formation of cartilage, bone, and bone marrow in mammals, as determined by histological observations and by measurements of the specific activity of alkaline phosphatase and calcium content of the implants, thereby demonstrating that related proteins from phylogenetically distant species are capable of inducing bone formation in mammals when placed in sites where progenitor cells are available.
Full text
PDF
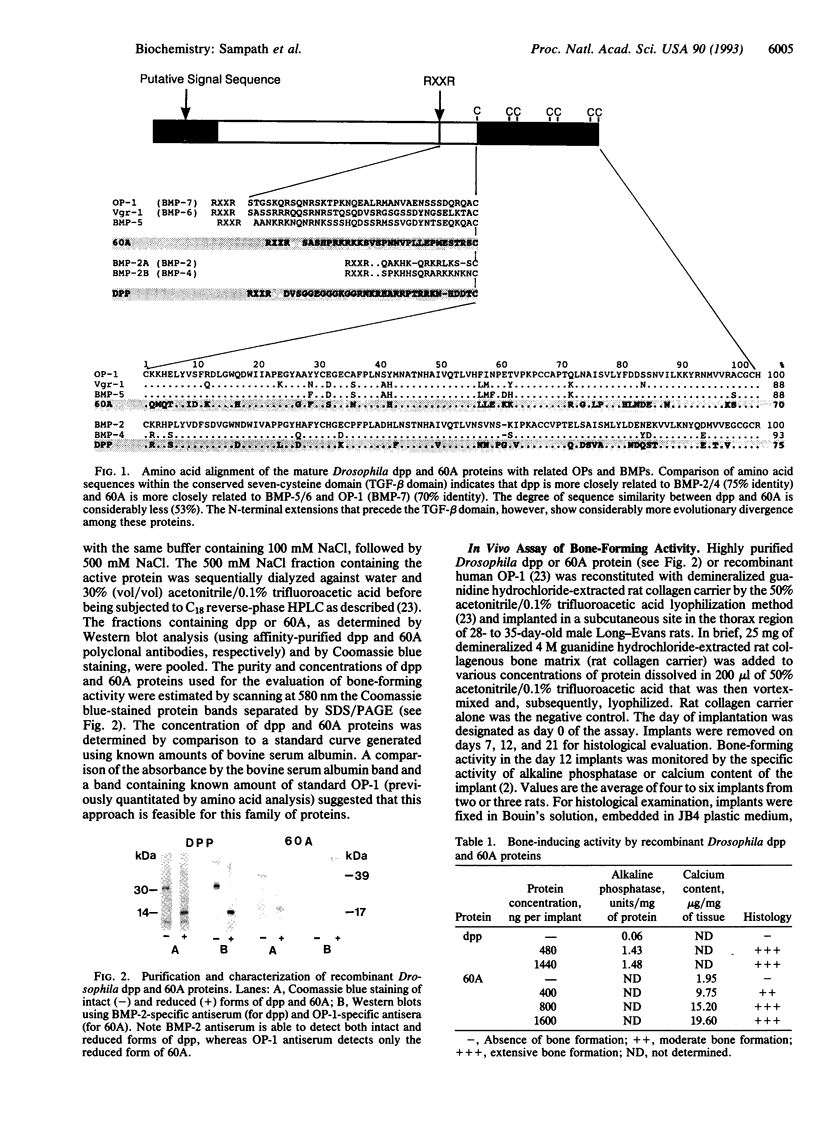
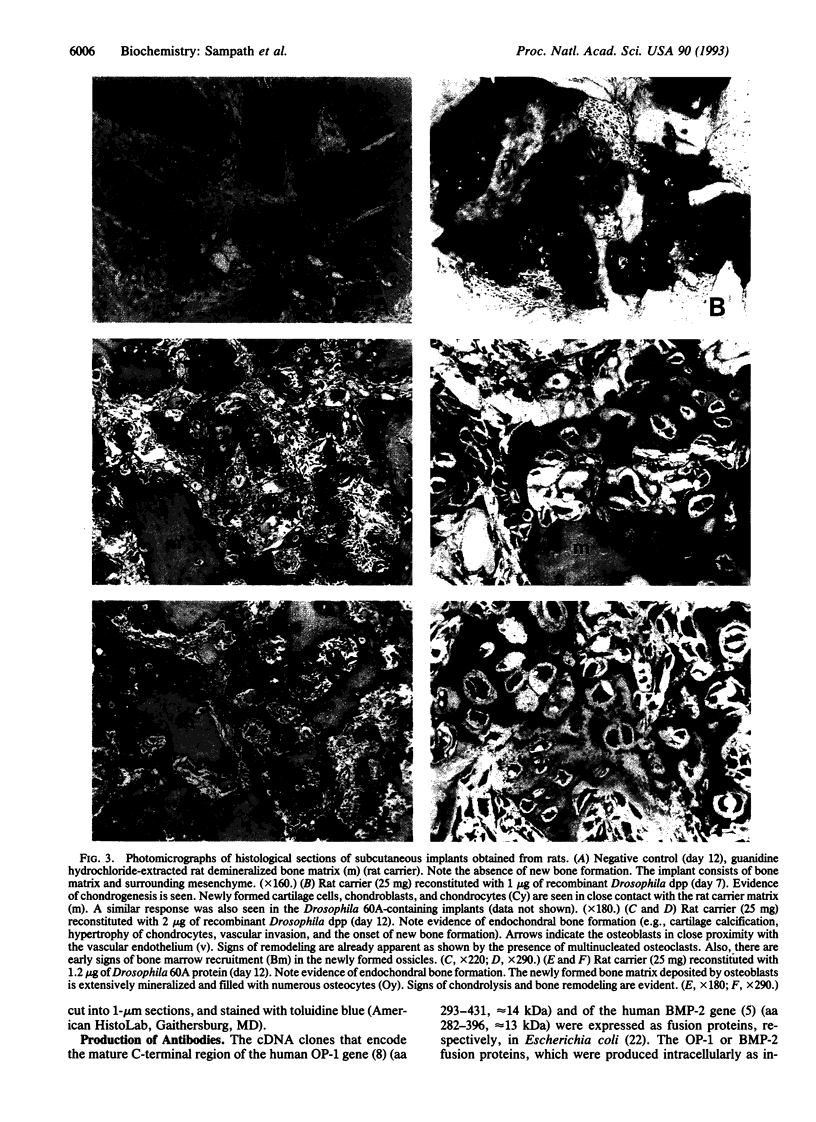


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Celeste A. J., Iannazzi J. A., Taylor R. C., Hewick R. M., Rosen V., Wang E. A., Wozney J. M. Identification of transforming growth factor beta family members present in bone-inductive protein purified from bovine bone. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9843–9847. doi: 10.1073/pnas.87.24.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doctor J. S., Jackson P. D., Rashka K. E., Visalli M., Hoffmann F. M. Sequence, biochemical characterization, and developmental expression of a new member of the TGF-beta superfamily in Drosophila melanogaster. Dev Biol. 1992 Jun;151(2):491–505. doi: 10.1016/0012-1606(92)90188-m. [DOI] [PubMed] [Google Scholar]
- Ferguson E. L., Anderson K. V. Decapentaplegic acts as a morphogen to organize dorsal-ventral pattern in the Drosophila embryo. Cell. 1992 Oct 30;71(3):451–461. doi: 10.1016/0092-8674(92)90514-d. [DOI] [PubMed] [Google Scholar]
- Green J. B., Smith J. C. Graded changes in dose of a Xenopus activin A homologue elicit stepwise transitions in embryonic cell fate. Nature. 1990 Sep 27;347(6291):391–394. doi: 10.1038/347391a0. [DOI] [PubMed] [Google Scholar]
- Irish V. F., Gelbart W. M. The decapentaplegic gene is required for dorsal-ventral patterning of the Drosophila embryo. Genes Dev. 1987 Oct;1(8):868–879. doi: 10.1101/gad.1.8.868. [DOI] [PubMed] [Google Scholar]
- Jones C. M., Lyons K. M., Hogan B. L. Involvement of Bone Morphogenetic Protein-4 (BMP-4) and Vgr-1 in morphogenesis and neurogenesis in the mouse. Development. 1991 Feb;111(2):531–542. doi: 10.1242/dev.111.2.531. [DOI] [PubMed] [Google Scholar]
- Jones C. M., Lyons K. M., Lapan P. M., Wright C. V., Hogan B. L. DVR-4 (bone morphogenetic protein-4) as a posterior-ventralizing factor in Xenopus mesoderm induction. Development. 1992 Jun;115(2):639–647. doi: 10.1242/dev.115.2.639. [DOI] [PubMed] [Google Scholar]
- Jones C. M., Lyons K. M., Lapan P. M., Wright C. V., Hogan B. L. DVR-4 (bone morphogenetic protein-4) as a posterior-ventralizing factor in Xenopus mesoderm induction. Development. 1992 Jun;115(2):639–647. doi: 10.1242/dev.115.2.639. [DOI] [PubMed] [Google Scholar]
- Kingsley D. M., Bland A. E., Grubber J. M., Marker P. C., Russell L. B., Copeland N. G., Jenkins N. A. The mouse short ear skeletal morphogenesis locus is associated with defects in a bone morphogenetic member of the TGF beta superfamily. Cell. 1992 Oct 30;71(3):399–410. doi: 10.1016/0092-8674(92)90510-j. [DOI] [PubMed] [Google Scholar]
- Lyons K. M., Jones C. M., Hogan B. L. The DVR gene family in embryonic development. Trends Genet. 1991 Nov-Dec;7(11-12):408–412. doi: 10.1016/0168-9525(91)90265-r. [DOI] [PubMed] [Google Scholar]
- Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- Ozkaynak E., Rueger D. C., Drier E. A., Corbett C., Ridge R. J., Sampath T. K., Oppermann H. OP-1 cDNA encodes an osteogenic protein in the TGF-beta family. EMBO J. 1990 Jul;9(7):2085–2093. doi: 10.1002/j.1460-2075.1990.tb07376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkaynak E., Schnegelsberg P. N., Jin D. F., Clifford G. M., Warren F. D., Drier E. A., Oppermann H. Osteogenic protein-2. A new member of the transforming growth factor-beta superfamily expressed early in embryogenesis. J Biol Chem. 1992 Dec 15;267(35):25220–25227. [PubMed] [Google Scholar]
- Padgett R. W., St Johnston R. D., Gelbart W. M. A transcript from a Drosophila pattern gene predicts a protein homologous to the transforming growth factor-beta family. Nature. 1987 Jan 1;325(6099):81–84. doi: 10.1038/325081a0. [DOI] [PubMed] [Google Scholar]
- Panganiban G. E., Rashka K. E., Neitzel M. D., Hoffmann F. M. Biochemical characterization of the Drosophila dpp protein, a member of the transforming growth factor beta family of growth factors. Mol Cell Biol. 1990 Jun;10(6):2669–2677. doi: 10.1128/mcb.10.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panganiban G. E., Reuter R., Scott M. P., Hoffmann F. M. A Drosophila growth factor homolog, decapentaplegic, regulates homeotic gene expression within and across germ layers during midgut morphogenesis. Development. 1990 Dec;110(4):1041–1050. doi: 10.1242/dev.110.4.1041. [DOI] [PubMed] [Google Scholar]
- Perides G., Safran R. M., Rueger D. C., Charness M. E. Induction of the neural cell adhesion molecule and neuronal aggregation by osteogenic protein 1. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10326–10330. doi: 10.1073/pnas.89.21.10326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddi A. H., Huggins C. Biochemical sequences in the transformation of normal fibroblasts in adolescent rats. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1601–1605. doi: 10.1073/pnas.69.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath T. K., Coughlin J. E., Whetstone R. M., Banach D., Corbett C., Ridge R. J., Ozkaynak E., Oppermann H., Rueger D. C. Bovine osteogenic protein is composed of dimers of OP-1 and BMP-2A, two members of the transforming growth factor-beta superfamily. J Biol Chem. 1990 Aug 5;265(22):13198–13205. [PubMed] [Google Scholar]
- Sampath T. K., Maliakal J. C., Hauschka P. V., Jones W. K., Sasak H., Tucker R. F., White K. H., Coughlin J. E., Tucker M. M., Pang R. H. Recombinant human osteogenic protein-1 (hOP-1) induces new bone formation in vivo with a specific activity comparable with natural bovine osteogenic protein and stimulates osteoblast proliferation and differentiation in vitro. J Biol Chem. 1992 Oct 5;267(28):20352–20362. [PubMed] [Google Scholar]
- Sampath T. K., Reddi A. H. Dissociative extraction and reconstitution of extracellular matrix components involved in local bone differentiation. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7599–7603. doi: 10.1073/pnas.78.12.7599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath T. K., Reddi A. H. Homology of bone-inductive proteins from human, monkey, bovine, and rat extracellular matrix. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6591–6595. doi: 10.1073/pnas.80.21.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urist M. R. Bone: formation by autoinduction. Science. 1965 Nov 12;150(3698):893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- Wang E. A., Rosen V., D'Alessandro J. S., Bauduy M., Cordes P., Harada T., Israel D. I., Hewick R. M., Kerns K. M., LaPan P. Recombinant human bone morphogenetic protein induces bone formation. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2220–2224. doi: 10.1073/pnas.87.6.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton K. A., Thomsen G. H., Gelbart W. M. Drosophila 60A gene, another transforming growth factor beta family member, is closely related to human bone morphogenetic proteins. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9214–9218. doi: 10.1073/pnas.88.20.9214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozney J. M., Rosen V., Celeste A. J., Mitsock L. M., Whitters M. J., Kriz R. W., Hewick R. M., Wang E. A. Novel regulators of bone formation: molecular clones and activities. Science. 1988 Dec 16;242(4885):1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- Yasko A. W., Lane J. M., Fellinger E. J., Rosen V., Wozney J. M., Wang E. A. The healing of segmental bone defects, induced by recombinant human bone morphogenetic protein (rhBMP-2). A radiographic, histological, and biomechanical study in rats. J Bone Joint Surg Am. 1992 Jun;74(5):659–670. [PubMed] [Google Scholar]