Abstract
Significance: Traumatic injury elicits a complex, dynamic, multidimensional inflammatory response that is intertwined with complications such as multiple organ dysfunction and nosocomial infection. The complex interplay between inflammation and physiology in critical illness remains a challenge for translational research, including the extrapolation to human disease from animal models. Recent Advances: Over the past decade, we and others have attempted to decipher the biocomplexity of inflammation in these settings of acute illness, using computational models to improve clinical translation. In silico modeling has been suggested as a computationally based framework for integrating data derived from basic biology experiments as well as preclinical and clinical studies. Critical Issues: Extensive studies in cells, mice, and human blunt trauma patients have led us to suggest (i) that while an adequate level of inflammation is required for healing post-trauma, inflammation can be harmful when it becomes self-sustaining via a damage-associated molecular pattern/Toll-like receptor-driven feed-forward circuit; (ii) that chemokines play a central regulatory role in driving either self-resolving or self-maintaining inflammation that drives the early activation of both classical innate and more recently recognized lymphoid pathways; and (iii) the presence of multiple thresholds and feedback loops, which could significantly affect the propagation of inflammation across multiple body compartments. Future Directions: These insights from data-driven models into the primary drivers and interconnected networks of inflammation have been used to generate mechanistic computational models. Together, these models may be used to gain basic insights as well as serving to help define novel biomarkers and therapeutic targets. Antioxid. Redox Signal. 23, 1370–1387.
Trauma: A Significant Burden
Trauma/hemorrhagic shock remains the leading cause of death in patients younger than 45 years (70). It is the third leading cause of death worldwide, resulting in five million or 10% of all deaths annually and thus considered the fifth leading cause of significant disability (137). Traumatic injury is a pandemic disease, one that affects every nation in the world regardless of the level of socioeconomic development (70, 71).
The disease is acute in onset, but often results in chronic, debilitating health problems that have effects beyond the individual victims. The financial impact of traumatic injuries is staggering: in 2000 in the United States, 10% of hospital discharges were due to injuries, and the direct cost of treating 50 million injury cases was $80.2 billion, with an estimated additional $326 billion in indirect costs (38). Indeed, according to the best available data, trauma will equal or surpass communicable disease in the year 2020 as the number one cause of morbidity and mortality worldwide (96).
Diversity of Immune Regulation
The immune system responds rapidly to traumatic injuries by reacting to tissue damage. Following this initial response to injury, both innate immune (myeloid) and lymphoid-derived cells and mediators drive responses that have been categorized broadly as proinflammatory or counter-inflammatory components. A more nuanced view of these systemic responses has suggested the presence of a systemic inflammatory response syndrome (SIRS), a compensatory anti-inflammatory response, or a mixed antagonist response syndrome (117, 122). Although these descriptive terminologies for this complex host response were first suggested over 20 years ago, they persist and remain useful as terms for delineating the clinical manifestations of trauma (28).
Indeed, these syndromes have become more relevant recently: with recent advances in clinical care, the outcome landscape in trauma has shifted from mortality to multiple organ dysfunction, nosocomial infection, and extended hospital and intensive care unit length of stay (25, 110). Thus, in the current clinical setting, the clinical outcomes of trauma patients who do not achieve full recovery from the initial insult can progress to a state of persistent inflammation, immunosuppression, and catabolism syndrome (57). Rather than return to a functional life, these patients are discharged to long-term acute care facilities, subsequent intensive care unit readmission, or indolent death.
However, as we discuss below, insights from computational modeling suggest a much more intertwined process, where dynamic is not the same as sequential. Rather, the innate and adaptive immune responses overlap temporally, despite regulating aspects of each other. Similarly, we believe that these intertwined changes in typical innate immune cells and T-cell-derived phenotype and function ultimately disrupt immune system homeostasis. It is the loss of homeostasis that predisposes trauma patients to opportunistic infections and other complications of traumatic injury such as multiple organ dysfunction. We also suggest that injuries induce an adaptive type of immune response that may have evolved to protect the injured host from opportunistic infections and excessive reactivity to damaged tissues and cells. This adaptive response can become dysregulated in patients who survive due to modern trauma care advances (110, 156).
The Conundrum of Acute Inflammation
Trauma-induced inflammation, with its manifold manifestations at the molecular, cellular, tissue, organ, and whole-organism levels, is a key driver of outcomes following injury. These dynamic processes involve the activation of signaling pathways at the cellular level, which mobilize inflammatory cells and stimulate the secretion of chemokines, cytokines, and damage-associated molecular pattern (DAMP) molecules (160).
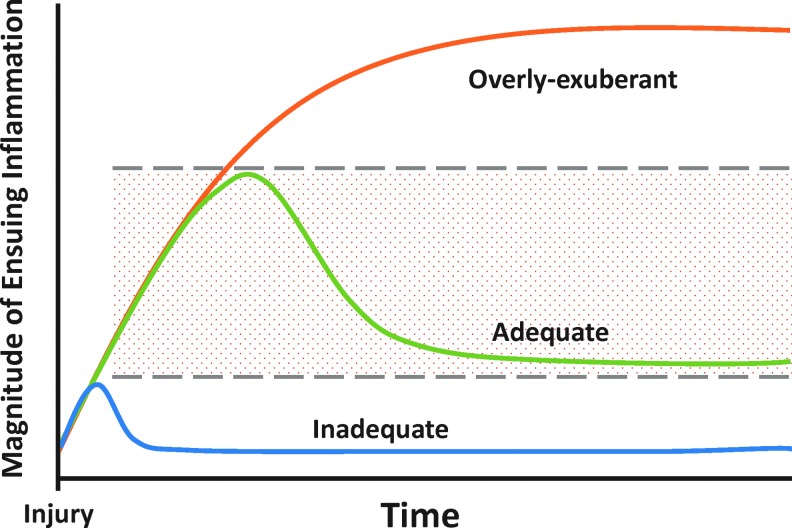
Although properly regulated inflammation allows for timely recognition and effective reaction to injury, the acute immune dysregulation that can accompany trauma/hemorrhage impairs physiological functions and predisposes to late complications such as nosocomial infection (34, 56, 59, 72). It is critical to note that inflammation is not in and of itself detrimental; rather, well-regulated self-resolving inflammation is necessary for the appropriate resolution of injury and for maintenance of proper physiology and homeostasis. Thus, injury-induced inflammation presents the paradox of a robust, evolutionarily conserved network whose very structure may lead to disease (144, 149, 150). Indeed, most evidence suggests that either insufficient (105) or self-sustaining (114) inflammation (Fig. 1) drives the pathobiology of trauma/hemorrhage and subsequent processes such as persistent critical illness.
FIG. 1.
Course of acute inflammation following injury. Properly regulated and self-resolving inflammation allows for effective resolution, while inadequate or overly exuberant inflammation can result in immune dysregulation and subsequent processes such as persistent critical illness. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The complexity of this response has stymied attempts at early diagnosis and therapeutic modulation of trauma-induced inflammation, resulting in a dearth of targeted therapeutic options. The use of animals in inflammation research has been essential for defining specific mechanisms of discovery of possible therapeutic targets (92, 129). However, translating the basic knowledge into clinical intervention has often been unsuccessful. This reflects lack of complete understanding of human inflammatory response, which has led to challenges of the validity of animal models that are used for drug discovery (153). However, as we discuss below, novel approaches from computational studies may help in deciphering this complexity and driving novel translational approaches (11, 60, 113).
We and others have carried out combined experimental, clinical, and computational studies with the explicit goal of advancing the understanding of the immune networks involved in trauma/hemorrhage and sepsis at the clinical level (6, 10, 11, 108, 144, 145, 149, 151). Over a decade of studies in cells, mice, large animals, and humans, we have developed an integrated view of the postinjury acute inflammatory response, which is regulated by initial chemokine-driven cues and propagated by DAMPs. These studies involved multiple paradigms of acute inflammation, including endotoxemia and experimental sepsis in mice (32, 35, 109, 125), rats (39, 107, 112), and swine (43, 50, 112, 116)), experimental trauma/hemorrhagic shock in mice (35, 45, 81, 84, 97, 125), and traumatic injury in humans (3, 31, 84, 110, 136, 158).
We propose that the pathways identified in these studies are associated with defined trajectories and distinct dynamic networks of inflammatory mediators, in turn resulting in a state of immune dysregulation in a large proportion of trauma patients (Fig. 2). Although these mediators have been appreciated as being central to the acute inflammatory response, computational modeling has allowed for both their integration into a system-based process and raising the potential for novel translational applications targeting nonintuitive central mediators as defined by modeling. In this review, we will discuss this framework and suggest how it can lead to the rational discovery of both basic and clinically applicable insights.
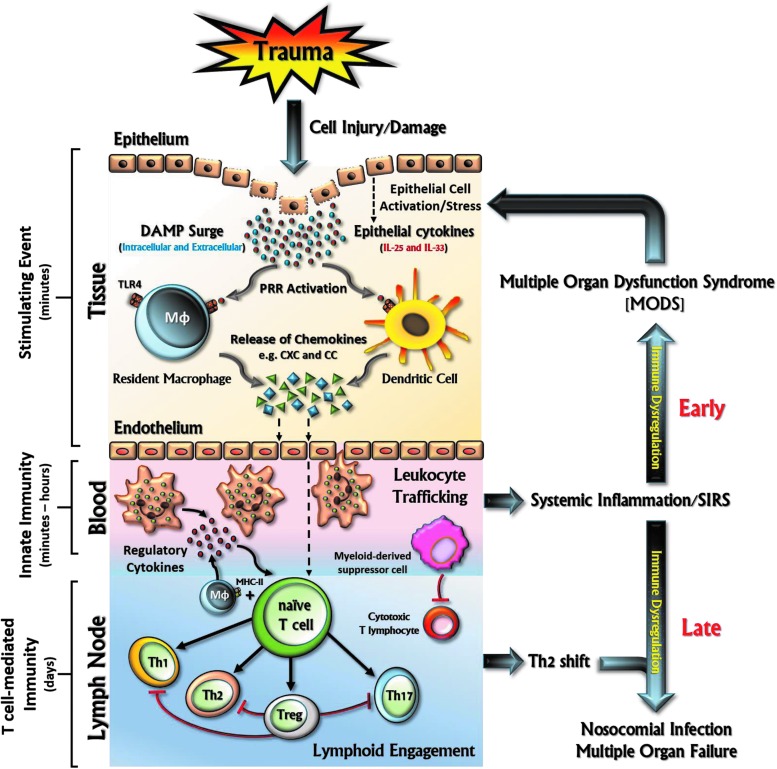
FIG. 2.
Schematic representation of activation of innate and T-cell-mediated responses following traumatic injury. Tissue injury generates damage-associated molecular patterns (DAMPs) from damaged cells, which initiate innate immune pathways by activation of pattern recognition receptors (PRRs) and, at least in part, through Toll-like receptor (TLR) signaling. This results in the release of inflammatory cytokines and chemokines from both structural cells (epithelial and fibroblasts) and antigen-presenting cells, such as resident macrophages (MΦ) and dendritic cells (DCs). These mediators are responsible for the activation of the endothelium (e.g., upregulation of adhesion molecules) and the recruitment and activation of leukocytes critical for innate immune responses (neutrophils, eosinophils, basophils, natural killer [NK] cells, and monocytes) and T-cell-mediated responses. The activation of these immune responses is essential to eliminate the inciting insult and to repair the destroyed tissue, thereby maintaining homeostasis. However, when uncontrolled, sustained, and exaggerated, the immune response becomes dysregulated, resulting in further damage through vicious feed-forward circuits causing further harm. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Acute Inflammation: Can We Extrapolate from Mice to Humans?
Injury-induced acute inflammation has been a conundrum: how can relatively well-studied inflammatory mechanisms that lead to well-defined outcomes in experimental mice models lead to such diverse outcomes in trauma patients? Thus, one key question pursued by investigators is whether or not it is possible to extrapolate directly from studies in cells or mice to define mechanisms operant in humans (5, 11).
Some investigators have suggested that there is a disconnection between the responses of mice versus humans, at least at the transcriptomic level (58, 132), and yet, others have suggested that as long as experimental studies were carried out in a manner that reproduces at least some of the features of clinical reality, animal models can be predictive of human outcomes (88, 121, 140). However, while animal models of the human inflammatory response have provided powerful insights into the possible underlying pathologies, these preclinical models are often insufficiently reflective of a particular human disease to be predictive of clinical success (127, 138).
We have taken a computational modeling approach to the question of whether or not studies of trauma/hemorrhage in murine models can be extrapolated to humans. We have postulated that reasonably conserved principal drivers and dynamic networks of inflammation in human blunt trauma patients can be replicated experimentally in mice models as long as the time frame of the experiment (∼24 h) is matched to a similar time frame in patients. We further hypothesize that these computationally identified mechanisms, when superimposed upon distinct initial conditions of injury severity, patient age, and sex, comorbidities that affect inflammation (e.g., obesity, metabolic syndrome) in turn can lead to highly diverse inflammatory trajectories, but only a few outcomes (survival with a low degree of organ dysfunction, survival with high degree of organ dysfunction, and death).
To test these hypotheses, we have carried out studies in both mice and human trauma patients. The former studies were carried out in several strains of mice. The latter studies were carried out in the form of an ∼500-patient observation study of blunt trauma patients (110). Importantly, the early sampling time points in both mice and humans were similar (multiple time points within the first 24 h postinjury). In the clinical study, we followed patients up to 30 days, discharge, or death. From these patients, three blood samples were obtained within the first 24 h postinjury, then daily thereafter for the first week, and then weekly until 30 days (or discharge or death).
A central goal of our studies was to dock our own and others' mouse work to our clinical data, and the criteria for determining this concordance included similarity in dynamics and networks of inflammatory mediators produced over comparable time courses. Our findings suggest that multiple key mediators and dynamic networks (3) (Fig. 3) are similar between mice and humans undergoing trauma/hemorrhage. Clearly, computational studies alone will not end the debate regarding the ability to extrapolate from mice to humans with regard to postinjury inflammation and pathophysiology. Nonetheless, as we discuss below, we suggest that in silico tools can help our understanding of—and connection across—in vitro, in vivo (animal), and clinical studies.
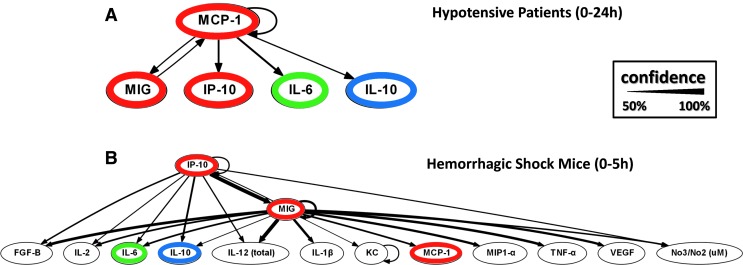
FIG. 3.
Dynamic Bayesian network inference suggests that interleukin (IL)-6 is regulated by monocyte chemotactic protein 1 (MCP-1) and monokine induced by gamma interferon (MIG) following trauma/hemorrhage in both mice and humans. Plasma inflammatory mediators were assessed over 0–24 h postinjury in hypotensive blunt trauma patients (A) or 0–5 h postinjury in C57Bl/6 mice (B) by Luminex™. Dynamic Bayesian network inference was carried out as described previously (3, 18, 19, 50, 158). Red: chemokines. Green: pro-inflammatory cytokine. Blue: anti-inflammatory cytokine. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Translational Systems Biology of Inflammation
As mentioned above, therapies for trauma-induced inflammation and multiple organ dysfunction are lacking. Translational research aims to apply discoveries in basic science into clinical practice to improve healthcare (74). As an adjunct to translational research in the setting of trauma, sepsis, and related inflammatory processes, we have suggested the concept of Translational Systems Biology (7, 8, 11, 145, 150, 151). This concept encompasses the use of computational simulations of clinical trials (4, 36, 79, 147), computational models of individuals to drive personalized medicine (31, 84, 116, 136), streamlined usage of experimental murine animal models (146), and rational device design (106). The cornerstone of Translational Systems Biology is computational modeling (1, 7, 9, 98, 143, 149, 151), which could allow for overcoming translational challenges inherent to complex diseases.
The initial step in the development of these computational models, whether generated using equation- (22, 37, 49, 115, 147, 150, 151), agent- (9, 51, 61, 147, 150, 151), or rule-based (52, 64) computational techniques (see below), involves integrating literature-derived information after a thorough evaluation/survey to determine a consensus on well-vetted mechanisms of inflammation. Thus, Translational Systems Biology involves the use of dynamic mathematical modeling based on mechanistic information generated in basic science research to simulate higher-level behaviors at the organ and organism level, thus affecting a means of translating reductionist experimental data to the level of clinically relevant phenomena.
Computational Models of Inflammation
Computational biology approaches used for the study of inflammation in trauma or sepsis span a broad range of techniques and can be categorized roughly into correlative (data-driven) or causative (mechanistic) approaches, with focus on either learning basic principles of system organization and function (67, 76, 95) or building predictive computational models (17, 95). Although there is an overlap between these areas, most efforts at elucidating biological mechanisms from high-dimensional data have traditionally focused on particular points along this spectrum of computational approaches. We have suggested that gleaning translationally relevant insights into the inflammatory response and its interconnected (patho)physiology will require the successful navigation of this spectrum in a logical progression from data to models to understanding and prediction (11).
Data-driven mathematical models
Correlative, data-driven modeling approaches include regression techniques that build models predictive within the conditions of the data they were trained on (66). Although these methods do not provide detailed mechanistic insight, they can be used to understand abstract features of the response, such as the presence of nonlinearities and the order of the response. The main drawback of this class of models is that they often lack mechanistic insight and can be overfit to the data on which they were trained.
We and others have leveraged data-driven modeling methods to (i) avoid possible bias in selection of variables; (ii) discern principal drivers, key nodes, and positive/negative feedback; and (iii) facilitate the rapid analysis of complex, dynamic, multidimensional datasets with the ultimate goal of generating predictive mechanistic models (11, 18, 145). We carried out an iterative process of evidence-based modeling (146, 148), consisting of biomarker assay, data analysis/data-driven modeling to discern main drivers of a given inflammatory response (67), literature mining to link these principal drivers based on well-vetted and likely mechanisms, calibration to the original data, and then validation using data separate from the calibration data.
With regard to data-driven modeling, various methods such as principal component analysis (PCA) (97, 107, 116) and dynamic Bayesian networks (DyBNs) have been used to discern principal characteristics of inflammation and dynamic networks, respectively, of interaction (3, 19, 50, 97, 110, 158, 161) in multiple inflammatory contexts, including trauma, sepsis, and liver failure. PCA can reduce a high-dimensional dataset into a few principal components that account for much of the observed variance in the data. When applied to time series data, the variables (gene transcripts/protein levels/etc.) that constitute these principal components may be interpreted as the principal contributors of the observed response and can give some mechanistic insights into the underlying process (67).
In the setting of inflammation, correlative approaches such as PCA may facilitate the development of diagnostics by analyzing the inflammatory mediator milieu in the blood, resulting from systemic spillover of local inflammation, to identify the health state of individuals and possibly inform patient-specific interventions (98). While these methods correlate gene transcript/protein levels with the phenotype and can suggest relevant molecular players involved in a given inflammatory process, these methods do not provide much information about how the gene transcripts/proteins interact with each other (67).
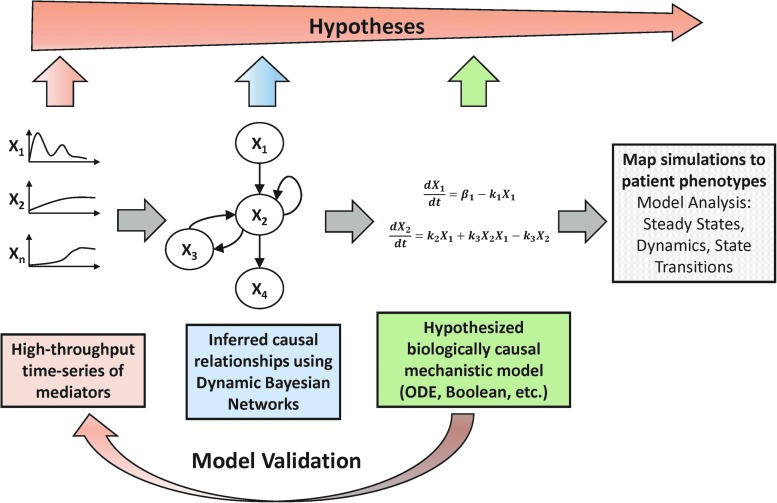
To better discern organizational aspects of interacting networks of mediators, such as coregulation or autoinduction, a variety of methods have been developed. Hierarchical clustering and Bayesian methods use high-throughput genomic or proteomic data of several time points and/or conditions to correlate gene expression patterns with function and infer regulatory networks of correlated genes. Several developments in these methods over the last two decades have yielded more informative networks that can be more easily translated into mechanistic models. Among these methods, DyBNs are particularly suited for inferring directed (causative) networks of interactions based on the probabilistic measure of how well the network can explain observed data. DyBNs can be supplemented by additional experimental evidence and expert knowledge to hypothesize mechanistic models (Fig. 4).
FIG. 4.
Overview of workflow for integrating data-driven and mechanistic modeling. Multiplexed time course data are measured and causal interactions are inferred by dynamic Bayesian networks (DyBNs). Inferred network topology forms the basis of mechanistic equation-based models that can be simulated to compare with experimental/clinical data, suggest diagnostic initial conditions, and analyzed and validated with further experiments. Along this path, more focused hypotheses are generated, from associating dynamic patterns of inflammatory mediators with phenotype to hypothesizing functional roles for particular interactions in the inflammatory network. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Mechanistic mathematical models
Mechanistic computational models, which are based on causative interactions, are derived from more detailed biological and physical descriptions of a system and have a rich set of tools for both analysis and simulation. These models are a core component of Translational Systems Biology and are a class of models in which the (biological) mechanism is abstracted in some manner, leading to insights regarding the way that component pieces interact to form a larger whole (whose properties are usually difficult to infer from the pieces themselves). This class of models is typically also known as dynamic mathematical models because the processes are represented in a way that aims to reproduce the changes in the real-world systems as a function of time.
The primary methods of dynamic mathematical modeling used in translational systems biology that work in acute inflammation are agent-based modeling (ABM) and equation-based modeling (EBM) (13, 14, 80). ABM consists of viewing a system as an aggregation of components (agents), which can be classified into populations or agent classes based on similar intrinsic rules of behavior (51). While a particular population of agents will have the same rules for behavior, the behavior of the individual agents is heterogeneous due to agents implementing their rules based on local conditions that may differ considerably (51). EBM approach begins with assigning variables to various quantities that evolve over time (such as populations of cells or levels of measured mediators) and writing functions or differential equations that describe how those variables change over time (150). The primary EBM used in the inflammation modeling is ordinary differential equation (ODE) (81). In the prominent case of ODEs, the equations are linked to capture the dynamics of the system using time as the sole independent variable (150).
In addition, rule-based models (e.g., BioNetGen) have been used to model inflammatory signaling (7), and Boolean models have been used to study T-cell differentiation and signaling (152). Moreover, mechanistic modeling studies using ODEs, ABMs, and rule-based models have served as a platform for prediction of inflammatory mechanisms and outcomes (9, 145, 151). Table 1 summarizes the main differences between ODE and ABM models.
Table 1.
Summary of Main Differences Between Ordinary Differential Equations and Agent-Based Models
| Ordinary differential equation | Agent-based Model |
|---|---|
| Highly aggregate (makes an abstraction from multiple events and individuals) | Highly disaggregate (based on actions of individual agents and their interactions with other agents) |
| Broad boundary | Narrow boundary |
| Perfect mixing assumption | Heterogeneity in agent attributes |
| Small number of parameters | Large number of parameters |
| Computationally efficient | Computationally intensive |
| Continuous time | Discrete time |
| Does not capture spatial dynamics or stochastic effects | Captures spatial dynamics or stochastic effects |
| Evaluation of what-if scenarios | Observing patterns of emerging behavior |
The limitation of ABM is that it is not a direct inferable relationship between the agent rules and the system's behavior, so it can be very difficult to calibrate in a quantitative way (147). ODEs, on the other hand, have limitations as they are completely deterministic with respect to their behavior, given a certain set of initial conditions (128). Increasing evidence of stochastic behavior in critical biological processes, such as gene regulation and cellular behavior, points to the possible need to account for stochasticity in mathematical models (128).
In addition, ODEs require the assumption that spatial aspects can validly be ignored, which allows for mean field approximations and mass action kinetics rather than partial differential equations (150). There is growing concern that the crowded environment and spatial architecture within cells, and at a higher level in tissues and organs, might violate these assumptions to a point where ODEs are no longer valid (128).
While we wish to navigate through the process of data to data-driven model to mechanistic model to prediction and understanding of the innate immune response, we seek to put it in the perspective of translational applications with a focus on clinical and preclinical settings. Both data-driven and mechanistic modeling have helped us link data to the mechanism (116), link in vitro studies to clinical biomarkers (161), and discern novel interactions among biomarkers based on comparisons of multiple clinical datasets (158). Below, we discuss the response to injury and the insights that we have gained from these computational approaches.
From Injury to Initial Decision of Inflammatory Fate
Table 2 summarizes the main inflammatory mediators that propagate the inflammatory and immune responses to injury in mice and humans. These mediators include pathogen-associated molecular patterns (PAMPs), DAMPs, chemokines, cytokines, and nitric oxide (and related redox species).
Table 2.
Summary of Some Known Inflammatory Mediators, Damage-Associated Molecular Patterns, and Reactive Oxygen Species in Mice and Humans
| Inflammatory mediators | Mouse | Human | Major cellular/tissue source |
|---|---|---|---|
| Cytokines | |||
| IL-1α | x | x | Monocyte/macrophages, neutrophils, epithelial cells, endothelial cells, fibroblasts, and dendritic cells |
| IL-1β | x | x | Monocyte/macrophages, neutrophils, epithelial cells, endothelial cells, fibroblasts, and dendritic cells |
| IL-2 | x | x | Th1 cells |
| IL-3 | x | x | Activated T helper cells, mast cells, NK cells, endothelium, and eosinophils |
| IL-4 | x | x | Th2 cells, mast cells, macrophages, and basophils |
| IL-5 | x | x | Th2 cells, mast cells, and eosinophils |
| IL-6 | x | x | Monocyte/macrophages, Th2, B cells, fibroblasts, and epithelial and endothelial cells |
| IL-7 | x | x | Thymus and bone marrow stromal cells, dendritic cells, epithelial cells, and hepatocytes |
| IL-9 | x | x | Th2 cells |
| IL-10 | x | x | Th2 cells, B cells, and monocytes |
| IL-12 p40 | x | x | Macrophages, B cells, and Langerhans cells |
| IL-12 p70 | x | x | Macrophages, B cells, and Langerhans cells |
| IL-13 | x | x | Th2 cells, mast cells, and NK cells |
| IL-15 | x | x | Epithelial cells and monocytes |
| IL-17A | x | x | Neutrophils, mast cells, activated memory T cells, and γδ T cells |
| IL-21 | x | x | Activated T helper cells and NK cells |
| IL-22 | x | x | Th17 cells |
| IL-23 | x | x | Macrophages and dendritic cells |
| IL-24 | x | x | Melanocytes, keratinocytes, monocytes, and T cells |
| IL-25/17E | x | x | T cells, mast cells, eosinophils, macrophages, and mucosal epithelial cells |
| IL-26 | x | x | T cells and monocytes |
| IL-27 | x | x | Macrophages and dendritic cells |
| IL-33 | x | x | Th2 cells, mast cells, and group 2 innate lymphocytes |
| IFN-α | x | x | Macrophages, fibroblasts, lymphoblastoid cells |
| IFN-γ | x | x | Th1 cells, NK cells, and dendritic cells |
| TNF-α | x | x | Monocyte/macrophages, T cells, and fibroblasts |
| GM-CSF | x | x | T cells, fibroblasts, endothelial cells, and macrophages |
| TGF-β1 | x | x | Neutrophils and macrophages |
| Chemokines | |||
| RANTES (CCL5) | x | x | T cells and epithelial cells |
| MCP-1 (CCL2) | x | x | Monocyte/macrophages, dendritic cells (immature), memory T cells, fibroblasts, endothelial cells, hepatocytes, and epithelial cells |
| MIP-1α (CCL3) | x | x | Monocytes, fibroblasts, and activated T cells |
| MIP-1β (CCL4) | x | x | Monocytes, fibroblasts, and activated T cells |
| Eotaxin (CCL11) | x | x | Th2, eosinophils, basophils, and mast cells |
| KC (CXCL1) | x | – | Neutrophils, monocytes, microvascular endothelium |
| IL-8 (CXCL8) | – | x | Neutrophils, monocyte/macrophages, fibroblasts, epithelial cells, and endothelial cells |
| IP-10 (CXCL10) | x | x | Th1, mast cells, monocytes, endothelial cells, fibroblasts, and mesangial cells |
| MIG (CXCL9) | x | x | Th1, mast cells, and mesangial cells |
| IL-8RA (CXCR1) | – | x | Neutrophils |
| PPBP (CXCL7) | – | x | Platelets |
| I-TAC (CXCL11) | – | x | Leucocytes, pancreas, liver, thymus, spleen, and lung |
| MCP-4 (CCL13) | – | x | Monocytes, T cells, eosinophils, and basophils |
| HCC-1 (CCL14) | – | x | Spleen, bone marrow, liver, muscle, and intestine |
| MIP-5 (CCL15) | – | x | Liver, small intestine, colon, and macrophages of lung |
| PARC (CCL18) | – | x | Monocyte/macrophages and dendritic cells |
| MPIF-1 (CCL23) | – | x | Lung, liver, bone marrow, and placenta |
| MRP-1 (CCL6) | x | – | Neutrophil and macrophages |
| MIP-1γ (CCL9) | x | – | Follicle-associated epithelium |
| Lungkine (CXCL15) | x | – | Epithelial cells of lung, mucosa of gastrointestinal and urogenital tract |
| MCP-5 (CCL12) | x | – | Lymph node and thymus |
| DAMPs | Derived from many sources within the cell, including the plasma membrane, nucleus, cytosol, endoplasmic reticulum, and mitochondria | ||
| HMGB1 | x | x | |
| Heat shock proteins | x | x | |
| Hyaluronan | x | x | |
| Heparan sulfate | x | x | |
| Uric acid | x | x | |
| Galectins | x | x | |
| Thioredoxin | x | x | |
| Adenosine | x | x | |
| S100 | x | x | |
| DNA | x | x | |
| PAMPs | Molecules associated with groups of pathogens that are recognized by cells of the innate immune system | ||
| Lipopolysaccharide | Gram-negative bacteria | ||
| Diaminopimelic acid | |||
| Porins | |||
| Peptidoglycan | Gram-positive bacteria | ||
| Lipoteichoic acid | |||
| Lipopeptide | |||
| Lipoarabinomannan | |||
| Chitin | Fungi | ||
| β Glucans | |||
| Zymosan | |||
| Double- and single-stranded RNA | Viruses | ||
| Hemagglutinin | |||
| CpG unmethylated DNA | Bacteria, viruses, protozoa | ||
| Flagellin | Flagellated bacteria | ||
| GPI-mucin | Protozoa | ||
| Glycoinositol phospholipids | |||
| Reactive oxygen species | Chemically reactive molecules formed as a natural by-product of the normal metabolism of oxygen and have important roles in cell signaling, homeostasis, and immune regulation | ||
| Peroxides | x | x | |
| Superoxide | x | x | |
| Hydroxyl radical | x | x | |
| Singlet oxygen | x | x | |
Mediators/molecules that are present in either mice or humans are signified by (x), whereas those that are absent are signified by (–).
DAMP, damage-associated molecular pattern; IL, interleukin; IP-10, interferon gamma-induced protein 10; MCP-1, monocyte chemotactic protein 1; MIG, monokine induced by gamma interferon; NK, natural killer; PAMP, pathogen-associated molecular pattern; TGF-β1, transforming growth factor beta 1; TNF-α, tumor necrosis factor alpha.
PAMPs and DAMPs
The inflammatory response following trauma comprises various systems of the human body, which are cross-linked with each other within a highly complex network of inflammation driven by numerous inflammatory mediators. There is evidence that the immune system has evolved to recognize both key molecular signatures of (dangerous) pathogens (68, 93, 94) and endogenous signals that originate from stressed, injured, or necrotic cells, signifying danger to the host (89). Initially, it was believed that necrotic cells and not apoptotic cells are a source for DAMPs. However, it became evident that DAMPs can also be released during a specific modality of programmed cell death, referred to as immunogenic apoptosis (55, 119, 123).
DAMPs share structural and functional similarities with exogenous, conserved, microbial surface structures released from invading microorganisms, so-called PAMPs. These PAMPs, like DAMPs, are recognized by a set of receptors, termed pattern-recognition receptors (2, 69, 91, 133, 154), which include the Toll-like receptors and NOD-like receptors (2, 24, 134). However, this definition of DAMPs is not always used consistently, and sometimes endogenous alarmins and exogenous PAMPs are collectively classified as DAMPs (26).
In the context of computational modeling of injury-induced inflammation, we have focused much of our mechanistic modeling work on the positive feedback loop of inflammation driving damage or dysfunction (at the cellular, tissue, organ, and whole-organism levels), which in turn stimulates further inflammation (150). In this context, our overarching hypothesis is that DAMPs stimulate and propagate inflammation in both infectious and sterile inflammatory settings using similar signaling pathways (90, 99, 150) and act as integrators of the inflammatory response and surrogates for an individual's health status.
Mechanistic models developed by our group integrate both PAMPs and DAMPs to describe and predict features of acute inflammation, such as priming, desensitization/tolerance, nonlinear dose effects and interactions between trauma and hemorrhage, individual-specific differences in inflammatory responses, the impact of probiotics on local and systemic inflammation in the setting of necrotizing enterocolitis, and the effects of aging on the inflammatory response (15, 16, 23, 35, 36, 39, 41, 79–81, 109, 116, 125, 126, 142). As we describe in the following section, this view of DAMPs as positive feedback elements has become more nuanced and integrated with a chemokine-centered inflammatory architecture (Fig. 5).
FIG. 5.
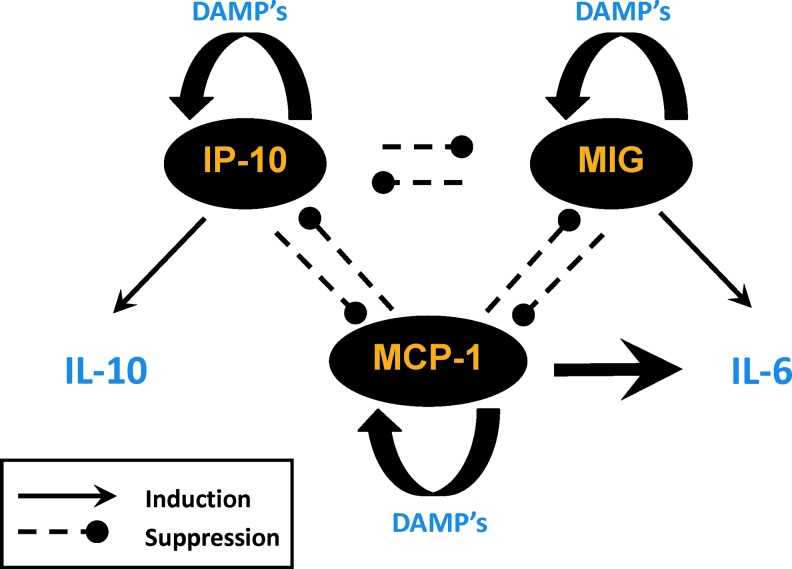
A hypothetical chemokine-switching network. In this hypothetical framework based on protein-level (Luminex) data and DyBN inference from multiple studies in mice and humans, trauma stimulates the release of three key chemokines (interferon gamma-induced protein 10 [IP-10], MIG, and MCP-1). We hypothesize that MIG drives low-level adaptive production of IL-6, while MCP-1 drives high-level detrimental production of IL-6. We further hypothesize that IP-10 drives IL-10 production, which is beneficial within a range, but detrimental when overproduced. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Chemokines
The migration of immune cells into and through tissues is coordinated by chemokines, which are thought to act early following injury or infection. Chemokines are generally considered redundant in their actions. However, an emerging, but not yet fully accepted, notion with regard to early inflammatory fate decision involves a nuanced and central role for chemokines induced as an immediate consequence of cell stress and damage. While chemokines have generally been considered to be redundant in their strictly proinflammatory actions (20, 33), some investigators have hypothesized that different polymorphonuclear neutrophil (PMN) subsets—including those with anti-inflammatory activity—are recruited to sites of injury/inflammation and exhibit different profiles of chemokine and cytokine expression (77).
Although PMNs are critical to host defense against bacterial infection postinjury, pathologic PMN hyperactivity is also implicated in adult respiratory distress syndrome and multiple organ failure (27, 29, 44). Early studies attributed this PMN priming to plasma levels of the CXC chemokine interleukin (IL)-8 through activation of PMN CXC receptors following injury, which may influence the clinical course of trauma patients (30, 48).
Recent studies from our group support this latter notion. Analysis of dynamic networks of inflammation based on multiple experimental and clinical systems (as shown schematically in Fig. 4) suggests that the chemokines, monokine induced by gamma interferon (MIG), monocyte chemotactic protein 1 (MCP-1), and interferon gamma-induced protein 10 (IP-10), form a core control structure—which we have termed a chemokine switch.
Essentially, we hypothesized a three-way switch—much like an electrical three-way switch—comprising the chemokines, MCP-1, MIG, and IP-10. We hypothesize that depending on the chemokine whose levels predominate in any given patient at the time of (or very shortly following) injury, this switching architecture drives the production of cytokines, such as IL-6 and IL-10 (19, 158) (Fig. 5). Indeed, a key aspect of the chemokine switch architecture exhibits this feature of intertwined responses since the activation of this chemokine network appears simultaneous in various aspects.
Multiple lines of evidence suggest the presence and importance of the chemokine switch. Our initial attempt at defining dynamic networks of inflammatory mediators induced by trauma/hemorrhage in mice showed that MIG was the dominant early chemokine in the hemorrhage group, while IP-10 was the dominant chemokine in the control (surgical cannulation) group (97). In recently published studies by our group (Fig. 3), we utilized DyBN inference to analyze time course data from circulating inflammatory mediators obtained within the first 24 h posthemorrhage in blunt trauma patients (3) (Fig. 3A) versus mice (Fig. 3B). We noted that the chemokines, MIG, MCP-1, and IP-10, appeared to play central coordinating roles in the inflammatory response postinjury/hemorrhage, with IL-6 being an output of these networks.
We have seen similar networks when comparing blunt trauma patients without spinal cord injury with patients with spinal cord injury. In that setting, IP-10 was a major node that distinguished spinal cord injury patients from blunt trauma patients. Moreover, we inferred that IP-10 drove the production of the anti-inflammatory cytokine IL-10 in these patients (158). Separately, using mutliple data-driven analyses, including PCA and DyBN, we demonstrated that MCP-1 is a key driver of hepatic inflammation in vitro and a biomarker of clinical outcomes in blunt trauma, likely by controlling IL-6 production (161).
The hypothetical mutually antagonistic and self-reinforcing regulatory motif characteristic of our chemokine switching model was used to generate a mechanistic computational model, in which each chemokine upregulated its own expression while downregulating the expression of the other two in a manner dependent on injury severity. This model predicted key qualitative features of systemic inflammation in patient subgroups as well as the different patterns of hospital discharge of moderately versus severely injured patients.
Switching behavior is prevalent throughout biology. Recently, a similar two-way switch motif was described by other investigators in the control of Th17 differentiation, leading to the production of either Th17-promoting or T regulatory cell (Treg)-promoting gene activation (157). Thus, we suggest that the balance of early chemokine production may regulate the production of key inflammatory mediators such as IL-6 and possibly also clinical outcomes.
Nitric oxide
Nitric oxide is the metabolic by-product of the conversion of L-arginine to L-citrulline by the activity of nitric oxide synthase (NOS). To date, three isoforms of NOS have been identified: neuronal NOS (nNOS or NOS1), endothelial NOS (eNOS or NOS3), and inducible NOS (iNOS or NOS2), which is expressed only in response to certain inflammatory stimuli such as bacterial products and cytokines (100, 103). It has been suggested that low amounts of NO derived from eNOS and nNOS are generally beneficial, whereas the large quantity produced by iNOS is generally harmful and contributes to the injury observed in different experimental models of inflammation. However, other investigators showed that inhibition of iNOS may exacerbate injury in certain situations, suggesting that iNOS-derived NO may be protective in the setting of acute inflammation (62).
In view of this, NO seems to have paradoxical actions in biological systems. One mechanism by which NO may modulate the inflammatory process is via its interaction with the Rel/nuclear transcription factor κB family of transcription factors (82).
In vivo metabolism of NO results in the formation of nitrate and nitrite as stable end products, along with a multitude of other reactive nitrogen species such as S-nitrosothiols; alterations in the plasma concentrations of these stable end products have been demonstrated in critically ill patients (63, 75, 120). Trauma patients were observed to have plasma nitrate levels below the normal range. Interestingly, even when trauma patients became septic, they were apparently still unable to upregulate NO production (120). During shock and sepsis, superoxide is formed. This cytotoxic compound can combine with NO to form peroxynitrite, which is difficult to measure in plasma (78). When peroxynitrite combines with tyrosine residues, it forms nitrotyrosine, which is a detectable marker in human plasma and urine in various inflammatory conditions (65). Nitrotyrosine has been reported to be present in several forms of shock (139) and in patients with acute lung injury post-trauma (141).
The complex biological chemistry of NO has been examined in various mathematical modeling contexts. Computational mechanistic models were recently utilized to evaluate ratios of kinetic expressions for interactions between NO and superoxide based on a number of simplifying cases to gain insight into the protective role of superoxide dismutase and predict the relative rate of peroxynitrite and hydrogen peroxide formation in the setting of acute inflammation (85). This mechanistic model provided insights into complex interactions between reactive oxygen and nitrogen species in blood and tissue. The model inferred that accelerating the removal of oxygen molecules could prevent oxidation of biological targets and that superoxide dismutase should improve the bioavailability of NO, restrict peroxynitrite formation, and reduce its potentially harmful effects. We have also incorporated the impact of NO on the process of apoptosis in hepatocytes, suggesting that reactive NO species can impact various interacting and competing pathways (21).
Furthermore, multiple mechanistic models from our group have incorporated both eNOS- and iNOS-derived NO in the context of multiple other inflammatory mechanisms, ultimately affecting blood pressure and whole-organism health status (31, 35, 36, 79, 81, 109, 116, 125, 142). We suggest that these insights provide the potential to drive a novel generation of diagnostic and therapeutic modalities.
Innate Immune and T-Cell-Mediated Pathways in Trauma-Induced Acute Inflammation
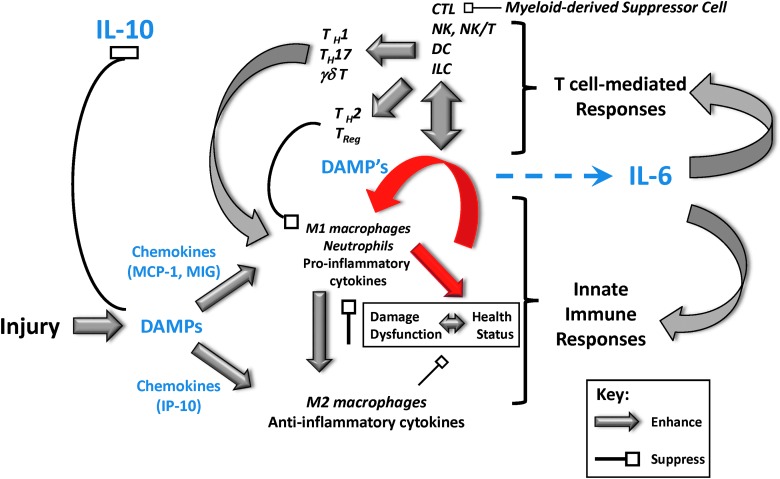
Perturbations of both the innate immune cells (neutrophils, mast cells, monocytes, and macrophages) and lymphoid cells have been described in both injured patients and animals (53, 101, 135, 155) undergoing experimental injury. However, the relative contributions of each of these cell types to SIRS, multiple organ dysfunction, and death postinjury remain speculative. In the classical view, the innate immune system is responsible for the initial inflammatory reaction to traumatic or thermal injury, and the adaptive immune system is activated only by antigen presentation and costimulation. A more current view suggests an intertwined dynamic activation of both typical innate immune cells and lymphoid cells directly following trauma (Fig. 6). This interaction is needed to ultimately drive specific, focused immune responses that better allow innate immune cells to augment antimicrobial immunity and resolve inflammation.
FIG. 6.
A systems view of innate immune and T-cell-mediated acute inflammation. Trauma leads to early release of DAMPs, stimulating either proinflammatory (M1 macrophages, neutrophils, cytokines such as TNF-α) or anti-inflammatory (M2 macrophages, cytokines such as IL-10) pathways via the early production of defined chemokine subsets. This leads to either the resolution of inflammation via chemokines such as IP-10 or exacerbated inflammation via chemokines such as MIG and MCP-1 in concert with secondary release of DAMPs. In the setting of post-trauma infection, proinflammatory agents (e.g., TNF-α) cause further inflammation and tissue damage/dysfunction. When the positive feedback loop of inflammation→damage→inflammation (indicated in red) exceeds certain thresholds (tipping points), T-cell-mediated responses are initiated via activation of dendritic cells, NK, NK-T cells, cytotoxic T lymphocytes (CTL), and innate lymphoid cells (ILC). T-cell-mediated responses include early (min) γδ T cells and later (4–12 h) Th17 cells. This response either resolves via IL-10, with relatively low systemic spillover of mediators and little organ damage, or propagates in a feed-forward manner, with worsening of organ damage and the attendant elevation of IL-6. Thus, chemokines such as IP-10, MCP-1, and MIG, as well as cytokines such as IL-6 and IL-10 (indicated in blue), can be both biomarkers and potential therapeutic targets under the appropriate circumstances. TNF-α, tumor necrosis factor alpha. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Antigen-presenting cells (APCs) provide one direct link between innate and adaptive immune cell activation during infections and vaccinations. On activation by APCs, naive CD4+ T cells differentiate into at least four T helper subsets: Th1, characterized by production of IFN-γ, which mediates cellular immunity; Th2 cells that synthesize IL-4, IL-5, IL-10, and IL-13 and promote humoral immunity and allergic responses; Th17 cells that produce IL-17, IL-21, and IL-22, which are implicated in host defense and autoimmunity (46, 47, 102); and Tregs (131). These CD4+ T-cell subsets produce cytokines, which then provide activating signals to cells of the innate immune system, such as neutrophils, macrophages, and natural killer cells.
It is generally agreed that trauma suppresses CD4+ T-cell responses (54, 83, 124, 159). For example, Th1-type immune responses have been shown to be reduced markedly following trauma in patients and in mouse injury models (42, 73, 104). Trauma can also promote Th2-type immune responses and T-cell anergy (a tolerance mechanism, in which the lymphocyte is intrinsically functionally inactivated following an antigen encounter, but remains alive for an extended period of time in a hyporesponsive state) in human trauma patients and in mice (87, 118). These observations underlie the idea that major injury triggers the development of a counter-inflammatory adaptive immune response that may help control excessive innate immune inflammatory reactivity.
We and others (86, 111) have focused significant efforts on identifying how trauma might suppress CD4+ T-cell activation and push the adaptive immune response toward a counter-inflammatory phenotype. Early studies suggested that the counter-inflammatory behavior of that adaptive immune system may be a result of a shift toward high Th2 and low Th1 responses in mice and in patients.
Other investigators (42) argued that as Th1- and Th2-type immune responses were found in trauma patients, T-cell anergy may be another mechanism contributing to suppressed CD4+ T-cell responses following trauma. However, we now propose that trauma-induced changes in adaptive immune responses might occur to protect the injured host from DAMP-induced innate responses and to prevent possible self-antigen reactivity to injury antigens released by tissue damage. In addition, CD4+ Th2 cells can produce typical anti-inflammatory cytokines such as IL-10. Therefore, a persistent Th2 response predisposes trauma and septic patients to subsequent infection.
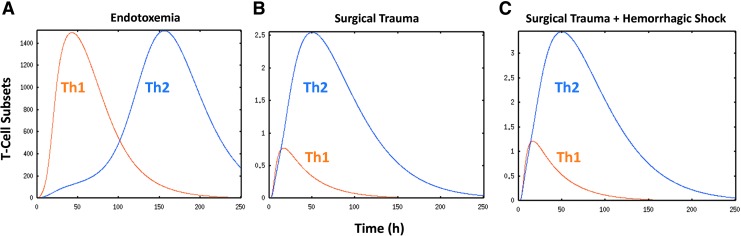
To help define the potential role of T-cell subsets in trauma, we created a mechanistic, differential equation-based model of acute inflammation, in which, in addition to the innate components previously considered in our models of acute inflammation (35, 81, 125, 142), we included dendritic cells as well as Th1 and Th2 cells in this augmented model (40). The model was partially calibrated to circulating levels of tumor necrosis factor alpha (TNF-α), IL-6, IL-10, and NO2−/NO3− over a 24-h time course from experimental data of mice subjected to endotoxemia, surgical trauma, or surgical trauma+hemorrhagic shock. The model reproduced the kinetics of cytokine and NO production following endotoxemia, surgical trauma, or surgery+hemorrhage over 24 h (Fig. 7).
FIG. 7.
Predicted dynamics of Th1 and Th2 cells in mouse models of acute inflammation. A differential equation model of acute inflammation was modified to include DC, Th1 cells, and Th2 cells. The model was partially calibrated against trajectories of TNF-α, IL-6, IL-10, and NO2−/NO3− obtained from C57Bl/6 mice subjected to endotoxemia (A), surgical cannulation trauma (B), or surgical cannulation+hemorrhagic shock (C). Predicted trajectories of Th1 and Th2 cells are shown for the three inflammatory scenarios. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Importantly, to simulate these accurate time courses of inflammation under these diverse inflammatory challenges, the model invoked differential Th1 versus Th2 activation in endotoxemia versus surgical trauma/hemorrhagic shock. Figure 7 shows the Th1 and Th2 time courses produced by the model simulations for each of these three experimental scenarios, respectively. This figure illustrates that all the scenarios are predicted to elicit both Th1 and Th2 responses. In line with the general dogma, the model predicted an initial Th1 response, followed by a Th2 response, with some overlap in the setting of experimental endotoxemia. However, the mathematical model predicted that in trauma±hemorrhage, both Th1 and Th2 responses are reduced compared with endotoxemia, and that the Th1 and Th2 responses are initiated early postinjury and evolve simultaneously. These results support the concept of an early T-cell-mediated response as well as a dominance of a Th2 response postinjury.
From Data to Knowledge in Acute Inflammation: A Computational Modeling Framework
As can be readily discerned from the foregoing discussion, a large number of cells and the mediators they produce are induced following traumatic injury. The complexity of this response can be daunting, and it may be argued that this has presented a major barrier to clinical translation. We have suggested that appropriately obtained data on the dynamics of inflammation in cells, experimental murine animal models, and patients could be integrated via data-driven modeling; that key inferences from these modeling approaches could be encoded into explicitly mechanistic computational models; and that those computational models could be used to make testable predictions regarding emergent properties that would not otherwise be readily discerned from the data or from standard statistical analyses (6, 10–12, 18, 98, 108, 144, 145, 151).
We hypothesize that both parenchymal and inflammatory cells (resident and infiltrating) sense cues regarding injury and, in response, elaborate chemokines that form defined networks. In these networks, we hypothesize the presence of negative feedback among chemokines that results in their cross-regulation, along with DAMP-mediated positive feedback that amplifies the expression of a given chemokine. As the presence of signals regarding the original stress/injury persists, along with the development and actions of these chemokine/DAMP networks, early regulatory cytokines such as IL-6, trasforming growth factor beta 1 (TGF-β1), TNF-α, and IL-1β begin to be secreted. Interestingly, some of these mediators (e.g., TNF-α and IL-1β) are present at low levels, often with high variance, and thus may be considered insignificant using standard statistical analyses. However, their presence and effect may be inferred using computational techniques such as PCA.
Indeed, IL-1β was identified as a principal mediator in a recent study from our group on endotoxemia in swine (116) as well as in trauma patients (unpublished observations). We hypothesize that the dynamic chemokine networks and initial cytokines together overcome thresholds of activation for later innate and lymphoid mediators such as IL-4 and IL-13. These mediators are usually sufficiently significantly elevated as defined by typical statistical analyses and thus are typically defined as biomarkers of elevated inflammation. We and others have suggested that by using techniques such as DyBN and PCA, other mediators or networks thereof can be considered novel biomarkers (11, 12, 145).
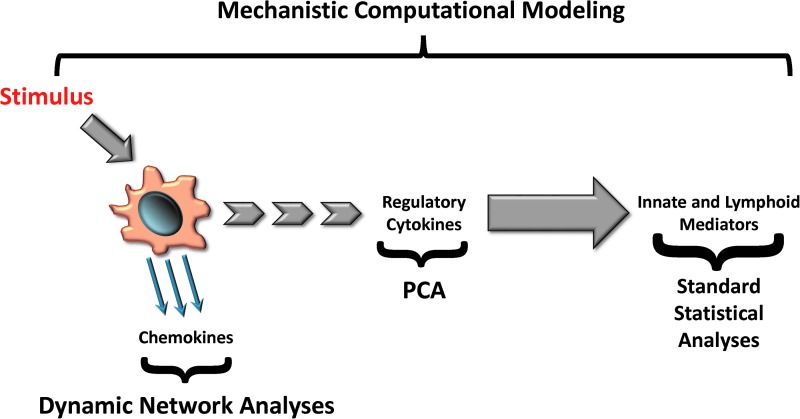
As summarized conceptually in Figure 8, this process involves obtaining highly granular, time series data on relevant inflammatory mediators or biomarkers (at any level from the transcriptomic through the ultimate metabolic response), discerning dynamic networks of interaction using tools such as DyBN (possibly with PCA as an initial filter) (130), and then focusing on central nodes (e.g., nodes that exhibit self-feedback and connectivity to other nodes) as potential novel biomarkers or therapeutic targets.
FIG. 8.
From data to models: a roadmap. Cells respond to cues regarding injury by elaborating chemokines that form defined networks, which can be detected using dynamic network analysis techniques. As the presence of signals and networks persists, early regulatory cytokines such as TNF-α and IL-1β begin to be secreted. These mediators are present at low levels, often with high variance, and their presence and effect may be inferred using techniques such as principal component analysis (PCA). Dynamic chemokine networks and initial cytokines together overcome thresholds of activation for later innate and lymphoid mediators such as IL-4 and IL-13, which would then be significantly elevated as defined by standard statistical analyses. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
In conclusion, although we have learned much from combined in vitro, in vivo, clinical, and in silico studies, there is much more yet to be elucidated regarding the acute inflammatory response to injury and infection. We hope that the methodology and approach we have described will help drive a rational framework for gaining clinically actionable knowledge from data.
Abbreviations Used
- ABM
agent-based modeling
- APCs
antigen-presenting cells
- CTL
cytotoxic T lymphocytes
- DAMP
damage-associated molecular pattern
- DC
dendritic cells
- DyBN
dynamic Bayesian network
- EBM
equation-based modeling
- eNOS
endothelial NOS
- IL
interleukin
- ILC
innate lymphoid cells
- iNOS
inducible NOS
- IP-10
interferon gamma-induced protein 10
- MCP-1
monocyte chemotactic protein 1
- MIG
monokine induced by gamma interferon
- NK
natural killer
- nNOS
neuronal NOS
- NOS
nitric oxide synthase
- ODE
ordinary differential equation
- PAMP
pathogen-associated molecular pattern
- PCA
principal component analysis
- PMN
polymorphonuclear neutrophil
- PRRs
pattern recognition receptors
- SIRS
systemic inflammatory response syndrome
- TGF-β1
transforming growth factor beta 1
- TLRs
Toll-like receptors
- TNFα
tumor necrosis factor alpha
- Treg
T regulatory cell
Acknowledgments
This work was supported, in part, by the National Institutes of Health grants R01GM67240, P50GM53789, R33HL089082, R01HL080926, R01AI080799, R01HL76157, R01DC008290, and UO1DK072146; the National Institute on Disability and Rehabilitation Research grant H133E070024; a Shared University Research Award from IBM, Inc.; and grants from the Commonwealth of Pennsylvania, the Pittsburgh Life Sciences Greenhouse, and the Pittsburgh Tissue Engineering Initiative/Department of Defense.
References
- 1.An G. and Vodovotz Y. Complex Systems and Computational Biology Approaches to Acute Inflammation. New York: Springer, 2013 [Google Scholar]
- 2.Akira S, Takeda K, and Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol 2: 675–680, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Almahmoud K, Namas RA, Zaaqoq AM, Abdul-Malak O, Namas R, Zamora R, Sperry J, Billiar TR, and Vodovotz Y. Prehospital hypotension is associated with altered inflammation dynamics and worse outcomes following blunt trauma in humans. Crit Care Med 43: 1395–1404, 2015 [DOI] [PubMed] [Google Scholar]
- 4.An G. In-silico experiments of existing and hypothetical cytokine-directed clinical trials using agent based modeling. Crit Care Med 32: 2050–2060, 2004 [DOI] [PubMed] [Google Scholar]
- 5.An G. Small to large, lots to some, many to few: in silico navigation of the translational dilemma. Crit Care Med 40: 1334–1335, 2012 [DOI] [PubMed] [Google Scholar]
- 6.An G, Bartels J, and Vodovotz Y. In silico augmentation of the drug development pipeline: examples from the study of acute inflammation. Drug Dev Res 72: 1–14, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.An G, Faeder J, and Vodovotz Y. Translational systems biology: introduction of an engineering approach to the pathophysiology of the burn patient. J Burn Care Res 29: 277–285, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.An G, Hunt CA, Clermont G, Neugebauer E, and Vodovotz Y. Challenges and rewards on the road to translational systems biology in acute illness: four case reports from interdisciplinary teams. J Crit Care 22: 169–175, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.An G, Mi Q, Dutta-Moscato J, Solovyev A, and Vodovotz Y. Agent-based models in translational systems biology. WIRES 1: 159–171, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.An G, Namas R, and Vodovotz Y. Sepsis: from pattern to mechanism and back. Crit Rev Biomed Eng 40: 341–351, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.An G, Nieman G, and Vodovotz Y. Computational and systems biology in trauma and sepsis: current state and future perspectives. Int J Burns Trauma 2: 1–10, 2012 [PMC free article] [PubMed] [Google Scholar]
- 12.An G, Nieman G, and Vodovotz Y. Toward computational identification of multiscale tipping points in multiple organ failure. Ann Biomed Eng 40: 2412–2424, 2012 [DOI] [PubMed] [Google Scholar]
- 13.An G. Introduction of an agent-based multi-scale modular architecture for dynamic knowledge representation of acute inflammation. Theor Biol Med Model 5: 11, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.An G. and Vodovotz Y. Translational Systems Biology: concepts and Practice for the Future of Biomedical Research. New York: Elsevier, 2014 [Google Scholar]
- 15.Arciero J, Ermentrout GB, Siggers R, Afrazi A, Hackam D, Vodovotz Y, and Rubin J. Modeling the interactions of bacteria and toll-like receptor-mediated inflammation in necrotizing enterocolitis. J Theor Biol 321: 83–99, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arciero J, Rubin J, Upperman J, Vodovotz Y, and Ermentrout GB. Using a mathematical model to analyze the role of probiotics and inflammation in necrotizing enterocolitis. PLoS One 5: e10066, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arkin AP. and Schaffer DV. Network news: innovations in 21st century systems biology. Cell 144: 844–849, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Azhar N, Mi Q, Ziraldo C, Buliga M, Constantine G, and Vodovotz Y. Integrating data driven and mechanistic models of the inflammatory response in sepsis and trauma. In: Complex Systems and Computational Biology Approaches to Acute Inflammation, edited by Vodovotz Y. and An G. New York: Springer, 2013, pp. 143–147 [Google Scholar]
- 19.Azhar N, Ziraldo C, Barclay D, Rudnick D, Squires R, and Vodovotz Y. Analysis of serum inflammatory mediators identifies unique dynamic networks associated with death and spontaneous survival in pediatric acute liver failure. PLoS One 8: e78202, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aziz M, Jacob A, Yang WL, Matsuda A, and Wang P. Current trends in inflammatory and immunomodulatory mediators in sepsis. J Leukoc Biol 93: 329–342, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bagci EZ, Vodovotz Y, Billiar TR, Ermentrout B, and Bahar I. Computational insights on the competing effects of nitric oxide in regulating apoptosis. PLoS One 3: e2249, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailey JE. Mathematical modeling and analysis in biochemical engineering: past accomplishments and future opportunities. Biotechnol Prog 14: 8–20, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Barber J, Tronzo M, Harold HC, Clermont G, Upperman J, Vodovotz Y, and Yotov I. A three-dimensional mathematical and computational model of necrotizing enterocolitis. J Theor Biol 322: 17–32, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benko S, Philpott DJ, and Girardin SE. The microbial and danger signals that activate Nod-like receptors. Cytokine 43: 368–373, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Berg RJ, Okoye O, Teixeira PG, Inaba K, and Demetriades D. The double jeopardy of blunt thoracoabdominal trauma. Arch Surg 147: 498–504, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81: 1–5, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Bone RC. Toward an epidemiology and natural history of SIRS (systemic inflammatory response syndrome). JAMA 268: 3452–3455, 1992 [PubMed] [Google Scholar]
- 28.Bone RC. Sir Isaac Newton, sepsis, SIRS, and CARS. Crit Care Med 24: 1125–1128, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Botha AJ, Moore FA, Moore EE, Kim FJ, Banerjee A, and Peterson VM. Postinjury neutrophil priming and activation: an early vulnerable window. Surgery 118: 358–364; discussion 36 4–365, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Botha AJ, Moore FA, Moore EE, Peterson VM, Silliman CC, and Goode AW. Sequential systemic platelet-activating factor and interleukin 8 primes neutrophils in patients with trauma at risk of multiple organ failure. Br J Surg 83: 1407–1412, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown D, Namas RA, Almahmoud K, Zaaqoq A, Sarkar J, Barclay DA, Yin J, Ghuma A, Abboud A, Constantine G, Nieman G, Zamora R, Chang SC, Billiar TR, and Vodovotz Y. Trauma in silico: individual-specific mathematical models and virtual clinical populations. Sci Transl Med 7: 285ra61, 2015 [DOI] [PubMed] [Google Scholar]
- 32.Buras JA, Holzmann B, and Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov 4: 854–865, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Cardona SM, Garcia JA, and Cardona AE. The fine balance of chemokines during disease: trafficking, inflammation, and homeostasis. Methods Mol Biol 1013: 1–16, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaudry IH. and Ayala A. Mechanism of increased susceptibility to infection following hemorrhage. Am J Surg 165: 59S–67S, 1993 [DOI] [PubMed] [Google Scholar]
- 35.Chow CC, Clermont G, Kumar R, Lagoa C, Tawadrous Z, Gallo D, Betten B, Bartels J, Constantine G, Fink MP, Billiar TR, and Vodovotz Y. The acute inflammatory response in diverse shock states. Shock 24: 74–84, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Clermont G, Bartels J, Kumar R, Constantine G, Vodovotz Y, and Chow C. In silico design of clinical trials: a method coming of age. Crit Care Med 32: 2061–2070, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Clermont G, Vodovotz Y, and Rubin J. Equation-based models of dynamic biological systems. In: Endothelial Biomedicine, edited by Aird WC. Cambridge, MA: Cambridge University Press, 2007, pp. 1780–1785 [Google Scholar]
- 38.Corso P, Finkelstein E, Miller T, Fiebelkorn I, and Zaloshnja E. Incidence and lifetime costs of injuries in the United States. Inj Prev 12: 212–218, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daun S, Rubin J, Vodovotz Y, Roy A, Parker R, and Clermont G. An ensemble of models of the acute inflammatory response to bacterial lipopolysaccharide in rats: results from parameter space reduction. J Theor Biol 253: 843–853, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Day J, Hoffman R, Kumar R, Chow CC, Clermont G, and Vodovotz Y. A mathematical model of innate and adaptive immune responses to shock. J Leukoc Biol Supplement: 43, 2003 [Google Scholar]
- 41.Day J, Rubin J, Vodovotz Y, Chow CC, Reynolds A, and Clermont G. A reduced mathematical model of the acute inflammatory response: II. Capturing scenarios of repeated endotoxin administration. J Theor Biol 242: 237–256, 2006 [DOI] [PubMed] [Google Scholar]
- 42.De AK, Kodys KM, Pellegrini J, Yeh B, Furse RK, Bankey P, and Miller-Graziano CL. Induction of global anergy rather than inhibitory Th2 lymphokines mediates posttrauma T cell immunodepression. Clin Immunol 96: 52–66, 2000 [DOI] [PubMed] [Google Scholar]
- 43.de Azevedo LC, Park M, Noritomi DT, Maciel AT, Brunialti MK, and Salomao R. Characterization of an animal model of severe sepsis associated with respiratory dysfunction. Clinics (Sao Paulo) 62: 491–498, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Deitch EA. Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg 216: 117–134, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deitch EA. Animal models of sepsis and shock: a review and lessons learned. Shock 9: 1–11, 1998 [DOI] [PubMed] [Google Scholar]
- 46.Dong C. Differentiation and function of pro-inflammatory Th17 cells. Microbes Infect 11: 584–588, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dong C. and Flavell RA. Th1 and Th2 cells. Curr Opin Hematol 8: 47–51, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Donnelly SC, Strieter RM, Kunkel SL, Walz A, Robertson CR, Carter DC, Grant IS, Pollok AJ, and Haslett C. Interleukin-8 and development of adult respiratory distress syndrome in at-risk patient groups. Lancet 341: 643–647, 1993 [DOI] [PubMed] [Google Scholar]
- 49.Edelstein-Keshet L. Mathematical Models in Biology. New York: Random House, 1988 [Google Scholar]
- 50.Emr B, Sadowsky D, Azhar N, Gatto L, An G, Nieman G, and Vodovotz Y. Removal of inflammatory ascites is associated with dynamic modification of local and systemic inflammation along with prevention of acute lung injury: in vivo and in silico studies. Shock 41: 317–323, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ermentrout GB. and Edelstein-Keshet L. Cellular automata approaches to biological modeling. J Theor Biol 160: 97–133, 1993 [DOI] [PubMed] [Google Scholar]
- 52.Faeder JR, Blinov ML, Goldstein B, and Hlavacek WS. Rule-based modeling of biochemical networks. Complexity 10: 22–41, 2005 [Google Scholar]
- 53.Faist E, Kupper TS, Baker CC, Chaudry IH, Dwyer J, and Baue AE. Depression of cellular immunity after major injury. Its association with posttraumatic complications and its reversal with immunomodulation. Arch Surg 121: 1000–1005, 1986 [DOI] [PubMed] [Google Scholar]
- 54.Faunce DE, Gamelli RL, Choudhry MA, and Kovacs EJ. A role for CD1d-restricted NKT cells in injury-associated T cell suppression. J Leukoc Biol 73: 747–755, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Garg AD, Krysko DV, Verfaillie T, Kaczmarek A, Ferreira GB, Marysael T, Rubio N, Firczuk M, Mathieu C, Roebroek AJ, Annaert W, Golab J, de Witte P, Vandenabeele P, and Agostinis P. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J 31: 1062–1079, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gebhard F. and Huber-Lang M. Polytrauma—pathophysiology and management principles. Langenbecks Arch Surg 393: 825–831, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Gentile LF, Cuenca AG, Efron PA, Ang D, Bihorac A, McKinley BA, Moldawer LL, and Moore FA. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg 72: 1491–1501, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gentile LF, Nacionales DC, Lopez MC, Vanzant E, Cuenca A, Cuenca AG, Ungaro R, Baslanti TO, McKinley BA, Bihorac A, Cuschieri J, Maier RV, Moore FA, Leeuwenburgh C, Baker HV, Moldawer LL, and Efron PA. A better understanding of why murine models of trauma do not recapitulate the human syndrome. Crit Care Med 42: 1406–1413, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Glance LG, Stone PW, Mukamel DB, and Dick AW. Increases in mortality, length of stay, and cost associated with hospital-acquired infections in trauma patients. Arch Surg 146: 794–801, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Godin PJ. and Buchman TG. Uncoupling of biological oscillators: a complementary hypothesis concerning the pathogenesis of multiple organ dysfunction syndrome. Crit Care Med 24: 1107–1116, 1996 [DOI] [PubMed] [Google Scholar]
- 61.Grimm V, Revilla E, Berger U, Jeltsch F, Mooij WM, Railsback SF, Thulke HH, Weiner J, Wiegand T, and DeAngelis DL. Pattern-oriented modeling of agent-based complex systems: lessons from ecology. Science 310: 987–991, 2005 [DOI] [PubMed] [Google Scholar]
- 62.Grisham MB, Jourd'Heuil D, and Wink DA. Nitric oxide. I. Physiological chemistry of nitric oxide and its metabolites: implications in inflammation. Am J Physiol 276: G315–G321, 1999 [DOI] [PubMed] [Google Scholar]
- 63.Hibbs JB, Jr., Westenfelder C, Taintor R, Vavrin Z, Kablitz C, Baranowski RL, Ward JH, Menlove RL, McMurry MP, Kushner JP, et al. Evidence for cytokine-inducible nitric oxide synthesis from L-arginine in patients receiving interleukin-2 therapy. J Clin Invest 89: 867–877, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hlavacek WS, Faeder JR, Blinov ML, Posner RG, Hucka M, and Fontana W. Rules for modeling signal-transduction systems. Sci STKE 2006: re6, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Ischiropoulos H, Zhu L, Chen J, Tsai M, Martin JC, Smith CD, and Beckman JS. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys 298: 431–437, 1992 [DOI] [PubMed] [Google Scholar]
- 66.Janes KA. and Lauffenburger DA. A biological approach to computational models of proteomic networks. Curr Opin Chem Biol 10: 73–80, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Janes KA. and Yaffe MB. Data-driven modelling of signal-transduction networks. Nat Rev Mol Cell Biol 7: 820–828, 2006 [DOI] [PubMed] [Google Scholar]
- 68.Janeway CA., Jr The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today 13: 11–16, 1992 [DOI] [PubMed] [Google Scholar]
- 69.Janeway CA, Jr., and Medzhitov R. Innate immune recognition. Annu Rev Immunol 20: 197–216, 2002 [DOI] [PubMed] [Google Scholar]
- 70.Kauvar DS, Lefering R, and Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma 60: S3–S11, 2006 [DOI] [PubMed] [Google Scholar]
- 71.Kauvar DS. and Wade CE. The epidemiology and modern management of traumatic hemorrhage: US and international perspectives. Crit Care 9 Suppl 5: S1–S9, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Keel M. and Trentz O. Pathophysiology of polytrauma. Injury 36: 691–709, 2005 [DOI] [PubMed] [Google Scholar]
- 73.Kelly JL, O'Suilleabhain CB, Soberg CC, Mannick JA, and Lederer JA. Severe injury triggers antigen-specific T-helper cell dysfunction. Shock 12: 39–45, 1999 [DOI] [PubMed] [Google Scholar]
- 74.Keramaris NC, Kanakaris NK, Tzioupis C, Kontakis G, and Giannoudis PV. Translational research: from benchside to bedside. Injury 39: 643–650, 2008 [DOI] [PubMed] [Google Scholar]
- 75.Kilbourn RG. and Belloni P. Endothelial cell production of nitrogen oxides in response to interferon gamma in combination with tumor necrosis factor, interleukin-1, or endotoxin. J Natl Cancer Inst 82: 772–776, 1990 [DOI] [PubMed] [Google Scholar]
- 76.Kitano H. Systems biology: a brief overview. Science 295: 1662–1664, 2002 [DOI] [PubMed] [Google Scholar]
- 77.Kolaczkowska E. and Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 13: 159–175, 2013 [DOI] [PubMed] [Google Scholar]
- 78.Koppenol WH, Moreno JJ, Pryor WA, Ischiropoulos H, and Beckman JS. Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem Res Toxicol 5: 834–842, 1992 [DOI] [PubMed] [Google Scholar]
- 79.Kumar R, Chow CC, Bartels J, Clermont G, and Vodovotz Y. A mathematical simulation of the inflammatory response to anthrax infection. Shock 29: 104–111, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kumar R, Clermont G, Vodovotz Y, and Chow CC. The dynamics of acute inflammation. J Theor Biol 230: 145–155, 2004 [DOI] [PubMed] [Google Scholar]
- 81.Lagoa CE, Bartels J, Baratt A, Tseng G, Clermont G, Fink MP, Billiar TR, and Vodovotz Y. The role of initial trauma in the host's response to injury and hemorrhage: insights from a comparison of mathematical simulations and hepatic transcriptomic analysis. Shock 26: 592–600, 2006 [DOI] [PubMed] [Google Scholar]
- 82.Laroux FS, Pavlick KP, Hines IN, Kawachi S, Harada H, Bharwani S, Hoffman JM, and Grisham MB. Role of nitric oxide in inflammation. Acta Physiol Scand 173: 113–118, 2001 [DOI] [PubMed] [Google Scholar]
- 83.Lederer JA, Rodrick ML, and Mannick JA. The effects of injury on the adaptive immune response. Shock 11: 153–159, 1999 [DOI] [PubMed] [Google Scholar]
- 84.Li NYK, Verdolini K, Clermont G, Mi Q, Hebda PA, and Vodovotz Y. A patient-specific in silico model of inflammation and healing tested in acute vocal fold injury. PLoS One 3: e2789, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liochev SI. and Fridovich I. Superoxide and nitric oxide: consequences of varying rates of production and consumption: a theoretical treatment. Free Radic Biol Med 33: 137–141, 2002 [DOI] [PubMed] [Google Scholar]
- 86.Mack VE, McCarter MD, Naama HA, Calvano SE, and Daly JM. Dominance of T-helper 2-type cytokines after severe injury. Arch Surg 131: 1303–1308; discussion 130 8–1309, 1996 [DOI] [PubMed] [Google Scholar]
- 87.Mack VE, McCarter MD, Naama HA, Calvano SE, and Daly JM. Candida infection following severe trauma exacerbates Th2 cytokines and increases mortality. J Surg Res 69: 399–407, 1997 [DOI] [PubMed] [Google Scholar]
- 88.Marshall JC, Deitch E, Moldawer LL, Opal S, Redl H, and Poll TV. Preclinical models of shock and sepsis: what can they tell us? Shock 24 Suppl 1: 1–6, 2005 [DOI] [PubMed] [Google Scholar]
- 89.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol 12: 991–1045, 1994 [DOI] [PubMed] [Google Scholar]
- 90.Matzinger P. The danger model: a renewed sense of self. Science 296: 301–305, 2002 [DOI] [PubMed] [Google Scholar]
- 91.Matzinger P. An innate sense of danger. Ann N Y Acad Sci 961: 341–342, 2002 [DOI] [PubMed] [Google Scholar]
- 92.McGonigle P. and Ruggeri B. Animal models of human disease: challenges in enabling translation. Biochem Pharmacol 87: 162–171, 2014 [DOI] [PubMed] [Google Scholar]
- 93.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature 449: 819–826, 2007 [DOI] [PubMed] [Google Scholar]
- 94.Medzhitov R. Approaching the asymptote: 20 years later. Immunity 30: 766–775, 2009 [DOI] [PubMed] [Google Scholar]
- 95.Mesarovic MD, Sreenath SN, and Keene JD. Search for organising principles: understanding in systems biology. Syst Biol (Stevenage) 1: 19–27, 2004 [DOI] [PubMed] [Google Scholar]
- 96.Meyer AA. Death and disability from injury: a global challenge. J Trauma 44: 1–12, 1998 [DOI] [PubMed] [Google Scholar]
- 97.Mi Q, Constantine G, Ziraldo C, Solovyev A, Torres A, Namas R, Bentley T, Billiar TR, Zamora R, Puyana JC, and Vodovotz Y. A dynamic view of trauma/hemorrhage-induced inflammation in mice: principal drivers and networks. PLoS One 6: e19424, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mi Q, Li NYK, Ziraldo C, Ghuma A, Mikheev M, Squires R, Okonkwo DO, Verdolini Abbott K, Constantine G, An G, and Vodovotz Y. Translational systems biology of inflammation: potential applications to personalized medicine. Per Med 7: 549–559, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mollen KP, Anand RJ, Tsung A, Prince JM, Levy RM, and Billiar TR. Emerging paradigm: toll-like receptor 4-sentinel for the detection of tissue damage. Shock 26: 430–437, 2006 [DOI] [PubMed] [Google Scholar]
- 100.Moncada S. Nitric oxide in the vasculature: physiology and pathophysiology. Ann N Y Acad Sci 811: 60–67; discussion 67–69, 1997 [DOI] [PubMed] [Google Scholar]
- 101.Moore FD, Jr., Davis C, Rodrick M, Mannick JA, and Fearon DT. Neutrophil activation in thermal injury as assessed by increased expression of complement receptors. N Engl J Med 314: 948–953, 1986 [DOI] [PubMed] [Google Scholar]
- 102.Mosmann TR. and Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 7: 145–173, 1989 [DOI] [PubMed] [Google Scholar]
- 103.Murad F. Nitric oxide signaling: would you believe that a simple free radical could be a second messenger, autacoid, paracrine substance, neurotransmitter, and hormone? Recent Prog Horm Res 53: 43–59; discussion 59–60, 1998 [PubMed] [Google Scholar]
- 104.Murphy T, Paterson H, Rogers S, Mannick JA, and Lederer JA. Use of intracellular cytokine staining and bacterial superantigen to document suppression of the adaptive immune system in injured patients. Ann Surg 238: 401–410; discussion 410–411, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Namas R, Ghuma A, Torres A, Polanco P, Gomez H, Barclay D, Gordon L, Zenker S, Kim HK, Hermus L, Zamora R, Rosengart MR, Clermont G, Peitzman A, Billiar TR, Ochoa J, Pinsky MR, Puyana JC, and Vodovotz Y. An adequately robust early TNF-α response is a hallmark of survival following trauma/hemorrhage. PLoS One 4: e8406, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Namas R, Mikheev M, Yin J, Over P, Young M, Constantine G, Zamora R, Gerlach J, and Vodovotz Y. A biohybrid device for the systemic control of acute inflammation. Disrupt Sci Technol 1: 20–27, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Namas R, Namas R, Lagoa C, Barclay D, Mi Q, Zamora R, Peng Z, Wen X, Fedorchak M, Valenti IE, Federspiel W, Kellum JA, and Vodovotz Y. Hemoadsorption reprograms inflammation in experimental Gram-negative septic fibrin peritonitis: insights from in vivo and in silico studies. Mol Med 18: 1366–1374, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Namas R, Zamora R, Namas R, An G, Doyle J, Dick TE, Jacono FJ, Androulakis IP, Chang S, Billiar TR, Kellum JA, Angus DC, and Vodovotz Y. Sepsis: something old, something new, and a systems view. J Crit Care 27: 314e1–314e11, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Namas RA, Bartels J, Hoffman R, Barclay D, Billiar TR, Zamora R, and Vodovotz Y. Combined in silico, in vivo, and in vitro studies shed insights into the acute inflammatory response in middle-aged mice. PLoS One 8: e67419, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Namas RA, Vodovotz Y, Almahmoud K, Abdul-Malak O, Zaaqoq A, Namas R, Mi Q, Barclay D, Zuckerbraun B, Peitzman AB, Sperry J, and Billiar TR. Temporal patterns of circulating inflammation biomarker networks differentiate susceptibility to nosocomial infection following blunt trauma in humans. Ann Surg 2014. [Epub ahead of print]; DOI: 10.1097/SLA.0000000000001001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Navarro-Zorraquino M, Lozano R, Deus J, Pastor C, Larrad L, Tejero E, Roman J, Palacios MJ, Torcal J, and Salinas JC. Determination of the immunoglobulin E postoperative variation as a measure of surgical injury. World J Surg 25: 585–591, 2001 [DOI] [PubMed] [Google Scholar]
- 112.Nemzek JA, Hugunin KM, and Opp MR. Modeling sepsis in the laboratory: merging sound science with animal well-being. Comp Med 58: 120–128, 2008 [PMC free article] [PubMed] [Google Scholar]
- 113.Neugebauer EA, Willy C, and Sauerland S. Complexity and non-linearity in shock research: reductionism or synthesis? Shock 16: 252–258, 2001 [DOI] [PubMed] [Google Scholar]
- 114.Neunaber C, Zeckey C, Andruszkow H, Frink M, Mommsen P, Krettek C, and Hildebrand F. Immunomodulation in polytrauma and polymicrobial sepsis—where do we stand? Recent Pat Inflamm Allergy Drug Discov 5: 17–25, 2011 [DOI] [PubMed] [Google Scholar]
- 115.Neves SR. and Iyengar R. Modeling of signaling networks. Bioessays 24: 1110–1117, 2002 [DOI] [PubMed] [Google Scholar]
- 116.Nieman K, Brown D, Sarkar J, Kubiak B, Ziraldo C, Vieau C, Barclay D, Gatto L, Maier K, Zamora R, Mi Q, Chang S, and Vodovotz Y. A two-compartment mathematical model of endotoxin-induced inflammatory and physiologic alterations in swine. Crit Care Med 40: 1052–1063, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Novotny AR, Reim D, Assfalg V, Altmayr F, Friess HM, Emmanuel K, and Holzmann B. Mixed antagonist response and sepsis severity-dependent dysbalance of pro- and anti-inflammatory responses at the onset of postoperative sepsis. Immunobiology 217: 616–621, 2012 [DOI] [PubMed] [Google Scholar]
- 118.O'Sullivan ST, Lederer JA, Horgan AF, Chin DH, Mannick JA, and Rodrick ML. Major injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann Surg 222: 482–490; discussion 490–492, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, Metivier D, Larochette N, van Endert P, Ciccosanti F, Piacentini M, Zitvogel L, and Kroemer G. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 13: 54–61, 2007 [DOI] [PubMed] [Google Scholar]
- 120.Ochoa JB, Udekwu AO, Billiar TR, Curran RD, Cerra FB, Simmons RL, and Peitzman AB. Nitrogen oxide levels in patients after trauma and during sepsis. Ann Surg 214: 621–626, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Osuchowski MF, Remick DG, Lederer JA, Lang CH, Aasen AO, Aibiki M, Azevedo LC, Bahrami S, Boros M, Cooney R, Cuzzocrea S, Jiang Y, Junger WG, Hirasawa H, Hotchkiss RS, Li XA, Radermacher P, Redl H, Salomao R, Soebandrio A, Thiemermann C, Vincent JL, Ward P, Yao YM, Yu HP, Zingarelli B, and Chaudry IH. Abandon the mouse research ship? Not just yet! Shock 41: 463–475, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Osuchowski MF, Welch K, Siddiqui J, and Remick DG. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol 177: 1967–1974, 2006 [DOI] [PubMed] [Google Scholar]
- 123.Panaretakis T, Kepp O, Brockmeier U, Tesniere A, Bjorklund AC, Chapman DC, Durchschlag M, Joza N, Pierron G, van Endert P, Yuan J, Zitvogel L, Madeo F, Williams DB, and Kroemer G. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J 28: 578–590, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pinheiro HS, Camara NO, Noronha IL, Maugeri IL, Franco MF, Medina JO, and Pacheco-Silva A. Contribution of CD4+ T cells to the early mechanisms of ischemia-reperfusion injury in a mouse model of acute renal failure. Braz J Med Biol Res 40: 557–568, 2007 [DOI] [PubMed] [Google Scholar]
- 125.Prince JM, Levy RM, Bartels J, Baratt A, Kane JM, III, Lagoa C, Rubin J, Day J, Wei J, Fink MP, Goyert SM, Clermont G, Billiar TR, and Vodovotz Y. In silico and in vivo approach to elucidate the inflammatory complexity of CD14-deficient mice. Mol Med 12: 88–96, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Reynolds A, Rubin J, Clermont G, Day J, Vodovotz Y, and Ermentrout GB. A reduced mathematical model of the acute inflammatory response: I. Derivation of model and analysis of anti-inflammation. J Theor Biol 242: 220–236, 2006 [DOI] [PubMed] [Google Scholar]
- 127.Rice J. Animal models: not close enough. Nature 484: S9, 2012 [DOI] [PubMed] [Google Scholar]
- 128.Ridgway D, Broderick G, Lopez-Campistrous A, Ru'aini M, Winter P, Hamilton M, Boulanger P, Kovalenko A, and Ellison MJ. Coarse-grained molecular simulation of diffusion and reaction kinetics in a crowded virtual cytoplasm. Biophys J 94: 3748–3759, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ruggeri BA, Camp F, and Miknyoczki S. Animal models of disease: pre-clinical animal models of cancer and their applications and utility in drug discovery. Biochem Pharmacol 87: 150–161, 2014 [DOI] [PubMed] [Google Scholar]
- 130.Sadowsky D, Nieman G, Barclay D, Mi Q, Zamora R, Constantine G, Golub L, Lee HM, Roy S, Gatto LA, and Vodovotz Y. Impact of chemically-modified tetracycline 3 on intertwined physiological, biochemical, and inflammatory networks in porcine sepsis/ARDS. Int J Burns Trauma 5: 22–35, 2015 [PMC free article] [PubMed] [Google Scholar]
- 131.Sakaguchi S, Yamaguchi T, Nomura T, and Ono M. Regulatory T cells and immune tolerance. Cell 133: 775–787, 2008 [DOI] [PubMed] [Google Scholar]
- 132.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, Lopez CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, and Tompkins RG. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A 110: 3507–3512, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Seong SY. and Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol 4: 469–478, 2004 [DOI] [PubMed] [Google Scholar]
- 134.Shaw MH, Reimer T, Kim YG, and Nunez G. NOD-like receptors (NLRs): bona fide intracellular microbial sensors. Curr Opin Immunol 20: 377–382, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Solomkin JS, Cotta LA, Satoh PS, Hurst JM, and Nelson RD. Complement activation and clearance in acute illness and injury: evidence for C5a as a cell-directed mediator of the adult respiratory distress syndrome in man. Surgery 97: 668–678, 1985 [PubMed] [Google Scholar]
- 136.Solovyev A, Mi Q, Tzen Y-T, Brienza D, and Vodovotz Y. Hybrid equation-/agent-based model of ischemia-induced hyperemia and pressure ulcer formation predicts greater propensity to ulcerate in subjects with spinal cord injury. PLoS Comp Biol 9: e1003070, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Soreide K. Epidemiology of major trauma. Br J Surg 96: 697–698, 2009 [DOI] [PubMed] [Google Scholar]
- 138.Sundberg JP, Hogenesch H, Nikitin AY, Treuting PM, and Ward JM. Training mouse pathologists: ten years of workshops on the Pathology of Mouse Models of Human Disease. Toxicol Pathol 40: 823–825, 2012 [DOI] [PubMed] [Google Scholar]
- 139.Szabo C. The pathophysiological role of peroxynitrite in shock, inflammation, and ischemia-reperfusion injury. Shock 6: 79–88, 1996 [DOI] [PubMed] [Google Scholar]
- 140.Takao K. and Miyakawa T. Genomic responses in mouse models greatly mimic human inflammatory diseases. Proc Natl Acad Sci U S A 112: 1167–1172, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Thomson L, Christie J, Vadseth C, Lanken PN, Fu X, Hazen SL, and Ischiropoulos H. Identification of immunoglobulins that recognize 3-nitrotyrosine in patients with acute lung injury after major trauma. Am J Respir Cell Mol Biol 36: 152–157, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Torres A, Bentley T, Bartels J, Sarkar J, Barclay D, Namas R, Constantine G, Zamora R, Puyana JC, and Vodovotz Y. Mathematical modeling of post-hemorrhage inflammation in mice: studies using a novel, computer-controlled, closed-loop hemorrhage apparatus. Shock 32: 172–178, 2009 [DOI] [PubMed] [Google Scholar]
- 143.Vodovotz Y. Translational systems biology of inflammation and healing. Wound Repair Regen 18: 3–7, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vodovotz Y. and An G. Systems biology and inflammation. In: Systems Biology in Drug Discovery and Development: Methods and Protocols, edited by Yan Q. Totowa, NJ: Springer Science & Business Media, 2009, pp. 181–201 [Google Scholar]
- 145.Vodovotz Y. and Billiar TR. In silico modeling: methods and applications to trauma and sepsis. Crit Care Med 41: 2008–2014, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vodovotz Y, Chow CC, Bartels J, Lagoa C, Prince J, Levy R, Kumar R, Day J, Rubin J, Constantine G, Billiar TR, Fink MP, and Clermont G. In silico models of acute inflammation in animals. Shock 26: 235–244, 2006 [DOI] [PubMed] [Google Scholar]
- 147.Vodovotz Y, Clermont G, Chow C, and An G. Mathematical models of the acute inflammatory response. Curr Opin Crit Care 10: 383–390, 2004 [DOI] [PubMed] [Google Scholar]
- 148.Vodovotz Y, Clermont G, Hunt CA, Lefering R, Bartels J, Seydel R, Hotchkiss J, Ta'asan S, Neugebauer E, and An G. Evidence-based modeling of critical illness: an initial consensus from the Society for Complexity in Acute Illness. J Crit Care 22: 77–84, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vodovotz Y, Constantine G, Faeder J, Mi Q, Rubin J, Sarkar J, Squires R, Okonkwo DO, Gerlach J, Zamora R, Luckhart S, Ermentrout B, and An G. Translational systems approaches to the biology of inflammation and healing. Immunopharmacol Immunotoxicol 32: 181–195, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vodovotz Y, Constantine G, Rubin J, Csete M, Voit EO, and An G. Mechanistic simulations of inflammation: current state and future prospects. Math Biosci 217: 1–10, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vodovotz Y, Csete M, Bartels J, Chang S, and An G. Translational systems biology of inflammation. PLoS Comput Biol 4: 1–6, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang RS, Saadatpour A, and Albert R. Boolean modeling in systems biology: an overview of methodology and applications. Phys Biol 9: 055001, 2012 [DOI] [PubMed] [Google Scholar]
- 153.Webb DR. Animal models of human disease: inflammation. Biochem Pharmacol 87: 121–130, 2014 [DOI] [PubMed] [Google Scholar]
- 154.West AP, Shadel GS, and Ghosh S. Mitochondria in innate immune responses. Nat Rev Immunol 11: 389–402, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wood JJ, Rodrick ML, O'Mahony JB, Palder SB, Saporoschetz I, D'Eon P, and Mannick JA. Inadequate interleukin 2 production. A fundamental immunological deficiency in patients with major burns. Ann Surg 200: 311–320, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Brownstein BH, Mason PH, Baker HV, Finnerty CC, Jeschke MG, Lopez MC, Klein MB, Gamelli RL, Gibran NS, Arnoldo B, Xu W, Zhang Y, Calvano SE, McDonald-Smith GP, Schoenfeld DA, Storey JD, Cobb JP, Warren HS, Moldawer LL, Herndon DN, Lowry SF, Maier RV, Davis RW, Tompkins RG; and Inflammation, Host Response to Injury Large-Scale Collaborative Research Program. A genomic storm in critically injured humans. J Exp Med 208: 2581–2590, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yosef N, Shalek AK, Gaublomme JT, Jin H, Lee Y, Awasthi A, Wu C, Karwacz K, Xiao S, Jorgolli M, Gennert D, Satija R, Shakya A, Lu DY, Trombetta JJ, Pillai MR, Ratcliffe PJ, Coleman ML, Bix M, Tantin D, Park H, Kuchroo VK, and Regev A. Dynamic regulatory network controlling TH17 cell differentiation. Nature 496: 461–468, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zaaqoq AM, Namas R, Almahmoud K, Krishnan S, Azhar N, Mi Q, Zamora R, Brienza DM, Billiar TR, and Vodovotz Y. IP-10, a potential driver of neurally-controlled IL-10 and morbidity in human blunt trauma. Crit Care Med 42: 1487–1497, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zedler S, Bone RC, Baue AE, von Donnersmarck GH, and Faist E. T-cell reactivity and its predictive role in immunosuppression after burns. Crit Care Med 27: 66–72, 1999 [DOI] [PubMed] [Google Scholar]
- 160.Zettel KR., II and Billiar TR. Disorder of systemic inflammation in sepsis and trauma: a systems perspective. In: Complex Systems and Computational Biology Approaches to Acute Inflammation, edited by Vodovotz Y. and An G. New York: Springer, 2013, pp. 103–124 [Google Scholar]
- 161.Ziraldo C, Vodovotz Y, Namas RA, Almahmoud K, Tapias V, Mi Q, Barclay D, Jefferson BS, Chen G, Billiar TR, and Zamora R. Central role for MCP-1/CCL2 in injury-induced inflammation revealed by in vitro, in silico, and clinical studies. PLoS One 8: e79804, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]