Abstract
Dry powder inhalers (DPIs) are commonly used for the delivery of inhaled medications, and should provide consistent, efficient dosing, be easy to use correctly, and be liked by patients; these attributes can all affect patient compliance and therefore treatment efficacy. The ELLIPTA® DPI was developed for the delivery of once-daily therapies for the treatment of asthma and chronic obstructive pulmonary disease. It has moderate resistance to airflow and can hold one or two blister strips, with each blister containing a sealed single dose of medication. Monotherapies can be delivered by the single-strip configuration and, in the two-strip configuration, one dose from each strip can be aerosolized simultaneously to allow combination therapies to be delivered, which enables the formulations for each product to be developed individually, since they are stored separately until the point of administration. There are three principal operating steps to administer a dose: open, inhale, close. This article summarizes the design, functionality, and in vitro dose-delivery characteristics of the ELLIPTA inhaler, and describes the results of human factors validation tests, designed to assess the performance of critical tasks required to use the inhaler. Results from the in vitro studies indicate that the ELLIPTA inhaler performs consistently with respect to in vitro dose delivery characteristics at a range of flow rates that can be achieved by the target population (≥30 L/min) and over its 30-day in-use life. Data from the human factors validation tests demonstrated that almost all participants (≥97%) were able to complete each of the steps required to prepare a dose for inhalation without error. Overall, the ELLIPTA inhaler has a versatile single- or two-strip design that allows it to be used for the delivery of a range of treatment options. It also improves patient ease-of-use when compared with the DISKUS® DPI.
Key words: : asthma, chronic obstructive pulmonary disease, dry powder inhaler, ELLIPTA, respiratory diseases
Introduction
Anumber of therapeutic options exist in asthma and chronic obstructive pulmonary disease (COPD). In asthma, inhaled corticosteroids (ICS) are the mainstay of maintenance therapy, and for patients whose asthma symptoms persist, ICS in combination with a long-acting beta2-adrenergic agonist (LABA)(1) is often prescribed. In COPD, an inhaled long-acting bronchodilator (a LABA or a long-acting muscarinic receptor antagonist [LAMA]) is used as maintenance therapy for patients who are symptomatic using rescue medication alone. As the disease progresses, COPD patients often require a combination of these therapies.(2)
Addition of an ICS to bronchodilator therapy is an option, and this has been shown to improve outcomes for COPD patients with the frequent exacerbator phenotype.(3) ICS can be added to bronchodilator monotherapy, or for patients with more severe disease, to combination bronchodilator therapy (i.e., triple therapy). Combination treatments of different drug classes can help improve symptoms compared with monotherapy in both asthma(1) and COPD.(2)
The medications outlined above are typically delivered via hand-held aerosol inhalers. Pressurized metered-dose inhalers (pMDIs) are popular due to a variety of factors, including their small size and non-obtrusive nature.(4) However, there are issues with pMDIs: they are associated with ballistic dose delivery, which can exacerbate the unpleasant taste in the mouth that is caused by propellants and excipients,(5) and it is well reported that patients (particularly children and the elderly) can have difficulty coordinating actuation of a pMDI with their inhalation.(2)
These issues can be overcome with dry powder inhalers (DPIs). Single-unit dose, multi-unit dose, and multi-dose reservoir DPIs are available, all of which are breath-actuated so that patients do not need to coordinate actuation with inhalation,(2,6) which has been associated with improved disease control.(2,7) Furthermore, DPIs do not produce the unpleasant cold feeling that is commonly associated with pMDIs.(8)
Despite the benefits associated with DPIs, there are still problems to overcome, such as general patient difficulties in preparing a dose, generating the required inspiratory effort, or using the correct inhalation technique.(9,10) Some types of DPI also have more specific problems, including patient errors when loading and preparing the dose in a single-unit dose DPI,(11) and difficulties protecting the formulation from environmental moisture for multi-dose reservoir DPIs.(9)
The DISKUS® DPI (also known as ACCUHALER®1; GSK, Hertfordshire, UK; Everux, France; North Carolina, USA) is an existing inhaler that can hold up to a month's supply of twice-daily medication (60 doses), without the need to replace cartridges or capsules. It is reportedly preferred by patients compared with alternative DPIs and provides consistent and reliable dosing of medication.(9)
The ELLIPTA® DPI (GSK, Hertfordshire, UK; DISKUS, ACCUHALER, and ELLIPTA are trademarks of the GSK group of companies) was designed as a next generation inhaler that improves patient ease-of-use and gives greater versatility in terms of the range of treatment options available, even when compared with the DISKUS inhaler. It is used for the delivery of various once-daily respiratory therapies developed by GSK and can hold up to 1 month's supply (30 doses) of these medications.
The ELLIPTA inhaler is currently used to deliver all of the following therapies, which are approved in a number of countries: the LAMA monotherapy umeclidinium (UMEC) for the treatment of COPD;(12) the LAMA/LABA combination therapy UMEC/vilanterol (VI) for patients with COPD;(13) the ICS monotherapy fluticasone furoate (FF) for the treatment of asthma;(14) and the ICS/LABA combination FF/VI for the treatment of asthma(15) and COPD.(15,16)
In this article, we describe the design of the ELLIPTA inhaler, and the underlying technological features that allow delivery of both mono- and combination therapies. We also present the results of in vitro studies that were used to assess dose delivery characteristics, and an overview of results from human factors validation tests that assessed whether the target population is able to operate the inhaler correctly without previous training or verbal instruction.
Materials and Methods
Design and features of the ELLIPTA inhaler
The ELLIPTA inhaler is a single- or two-strip, single-step activation, multi-dose inhaler that was designed to be used unassisted by patients aged ≥7 years (including patients ≥65 years), and by patients aged ≥4 years with adult supervision; it is currently licensed for use by patients aged ≥12 years, and data for pediatric patients aged <12 years are awaited. The inhaler is slightly smaller in size than the DISKUS inhaler at approximately 8.3 × 6.6 × 3.1 cm, and has a large, centrally located dose counter and a non-detachable mouthpiece cover (Fig. 1).
FIG. 1.

External view of the ELLIPTA inhaler. This figure is copyrighted by GSK, 2014.
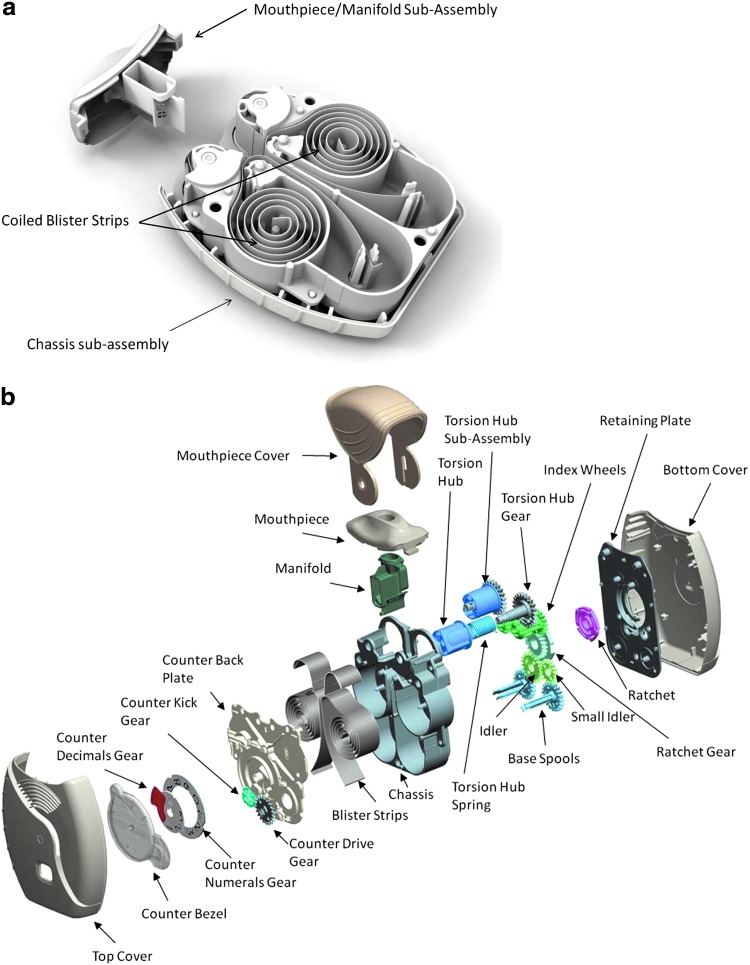
It is assembled using 25 separate plastic components and two stainless steel torsion springs (Fig. 2). The inhaler is pre-loaded with 1 month's supply of pre-filled, foil-laminated, individually sealed blisters containing drug formulation. For a monotherapy product, the ELLIPTA inhaler is supplied in the single-strip configuration with one 30-dose blister strip; a single dose from one blister on this strip is delivered during an inhalation.
FIG. 2.
(a) View of coiled blister strips within the inhaler chassis and mouthpiece/manifold assembly; (b) exploded view of the ELLIPTA inhaler. The images included in this figure are copyrighted by GSK, 2014.
For a combination therapy product, the inhaler is supplied in the two-strip configuration with two 30-dose blisters that contain separate drug formulations; one blister from each strip is delivered simultaneously during a single inhalation to provide a single dose of the combination therapy. Seven and 14 dose blister strips are also available for sample purposes. The blister strips help to protect the drug formulation from environmental moisture and contaminants, and are not accessible to patients. There are no differences between the 7, 14, and 30 dose variants other than length of the blister strip, number of blister pockets, and the starting number displayed in the dose counter window.
Operation of the ELLIPTA inhaler
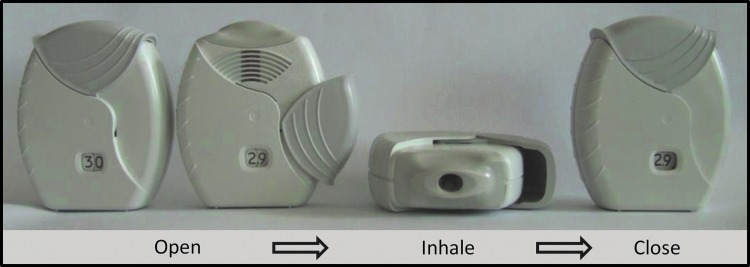
The inhaler is operated through three simple steps: 1) opening the mouthpiece cover fully; 2) inhaling the dose; and 3) closing the mouthpiece cover (Fig. 3). To enable users to identify the mouthpiece cover, it is a distinctly different color from the gray body of the inhaler; the mouthpiece cover color differs for each product that is delivered via the ELLIPTA inhaler and can be used to aid identification of the product. The opening of the mouthpiece cover is guided by a recess in the inhaler body, which provides visual and tactile clues indicating the direction and full extent of travel of the mouthpiece cover. When the mouthpiece cover is fully opened, the mouthpiece is exposed, the inhaler is activated, and the dose is ready to be inhaled.
FIG. 3.
Operation of the ELLIPTA DPI. This figure is copyrighted by GSK, 2014. DPI, dry powder inhaler.
The mechanical function required for activation simultaneously performs five functions: 1) advances one blister per strip, aligning with the mouthpiece manifold airflow path; 2) peels the foil laminate(s) to expose the contents of the blister(s) for inhalation; 3) collects the used portion(s) of foil laminate(s); 4) drives the dose counter gears to move the dose counter display by a one unit decrease; and 5) provides an audible “click” at the end of the actuation stroke of the mouthpiece cover.
If the patient opens the inhaler, prepares a dose, and closes the inhaler without inhaling the dose, that dose will be lost (i.e., not available to the patient) and held securely inside the inhaler, ensuring that next time the patient uses the inhaler a double dose is not administered. As the inhaler is used, sequential layers of peeled foil laminate accumulate around the torsion hub and increase its diameter. The hub assemblies contain torsion springs that compensate for the winding movement used to peel the foil laminate and ensure consistent lid foil peeling throughout the inhaler's lifetime. When the mouthpiece cover is closed, the chassis sub-assembly gear mechanism is reset, ready for the next actuation, and the mouthpiece is protected from contamination by dust or foreign particles.
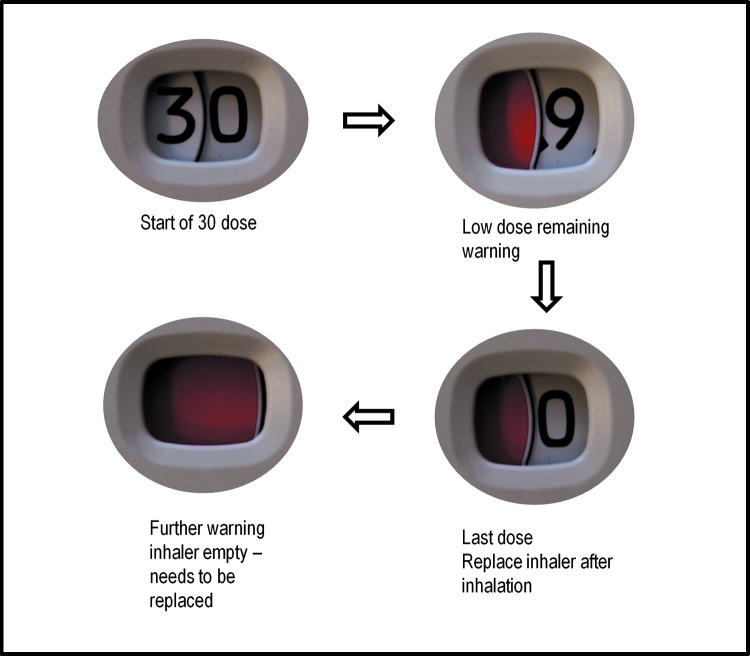
Each complete movement of the mouthpiece cover drives the dose counter display to decrease by one dose. Examples of the numbers and flags shown on the dose counter for the 30-dose inhaler are shown in Figure 4. When nine (or fewer) doses remain, a red flag appears, to highlight to the patient that a replacement will be needed soon; when the last dose has been dispensed, the counter shows a ‘0’ in the display window. As a final reminder, a second red flag fills the display window if the patient tries to operate the inhaler again, and repeated actuation will not change the counter display any further.
FIG. 4.
Diagram of the numbers and flags in the viewing window for 30-dose ELLIPTA DPI. This figure is copyrighted by GSK, 2014. DPI, dry powder inhaler.
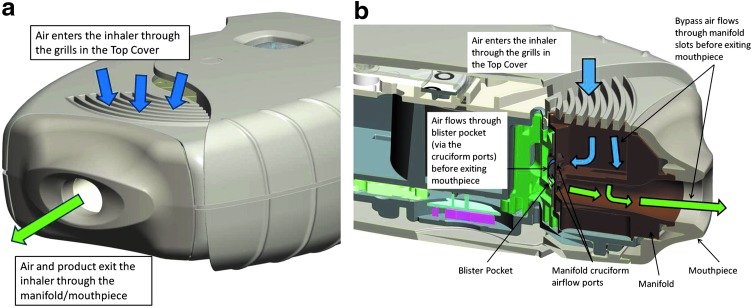
Opening the mouthpiece cover allows the patient to inhale through the exposed mouthpiece. During inhalation, air passes through a grill on the top of the inhaler body that prevents foreign objects from entering. Patients must remember not to block all the grills while using the inhaler, as documented in the patient information leaflet/medication guide. Some of the air inhaled is guided through a cruciform airflow port of the manifold component, which is closely aligned with the exposed blister pocket, enabling the drug formulation to be aerosolized and delivered to the patient (Fig. 5).
FIG. 5.
Diagram of airflow path through the ELLIPTA DPI (a) exterior view and (b) interior view. The images included in this figure are copyrighted by GSK, 2014. DPI, dry powder inhaler.
The remaining air passes through bypass slots within the manifold component and combines with the air that has passed through the pockets to be inhaled by the patient. The airflow path is defined by a minimal number of components (three in total: the mouthpiece, the manifold, and the top cover) and is discrete from the inhaler mechanism.
The airflow geometry, which is defined principally by the manifold component, provides an inhaler with moderate resistance to patient inspiratory effort, with a typical specific resistance of 0.0286 kPa0.5(L/min)–1 when used in the two-strip configuration. The resulting pressure drop when tested under standard conditions (i.e., flow rate of 60 L/min), is typically 3.0 kPa, which is similar to that of the DISKUS inhaler.(6)
Assessing consistency of dose delivery
To demonstrate consistency of dose emission from the ELLIPTA inhaler, the in vitro dose delivery has been tested under a variety of conditions, and results will be presented for the following products: UMEC 62.5 μg (label claim delivered dose: 55 μg); UMEC/VI 62.5/25 μg (label claim delivered dose: 55/22 μg); FF 100 μg (label claim delivered dose: 90 μg); FF/VI 100/25 μg (label claim delivered dose: 92/22 μg); and FF/VI 200/25 μg (label claim delivered dose: 184/22 μg). Evidence generated demonstrated consistent dosing of FF 100 μg, FF/VI 100/25 μg, and 200/25 μg, and therefore a risk-based decision to not conduct analyses for FF 200 μg was taken; no data are reported for this product.
Simulated in-use testing
The consistency of in vitro dose delivery over the inhaler in-use life of 30 days was assessed through life-simulated in-use testing. Delivered dose (defined as the total recovered from all stages ex-device) and fine particle mass ([FPM]; fraction of material with aerodynamic particle size of 0.94–4.46 μm) were assessed separately. To assess the delivered dose, the inhalers had been stored in their secondary packaging at 25°C and 60% relative humidity for 29 months (FF monotherapy), 11 months (UMEC monotherapy), 9 months (UMEC/VI), or 12 months (FF/VI) prior to testing; over the course of the assessments, the inhalers were stored under ambient laboratory conditions.
Using a vacuum pump, critical flow controller (Copley TPK) and a unit dose collector (GSK), individual doses were dispensed at a flow rate of 60 L/min for a duration of 4 s for all doses over the 30-day in-use inhaler lifetime, with single doses dispensed between Tuesday and Thursday, and two doses dispensed (but collected separately) on Mondays and Fridays to cover weekend doses. Delivered dose was assayed by high performance liquid chromatography (HPLC) and all tests were performed in triplicate.
To assess FPM, doses were aerosolized into a Next Generation Impactor at a flow rate of 60 L/min for 4 s duration, using a vacuum pump and critical flow controller. Doses were characterized on days 1, 2, 3, 4, 7, 11, 14, 17, 21, 23, 28, and 30 for UMEC monotherapy; 1, 2, 3, 4, 7, 11, 14, 17, 21, 24, 28, and 30 for UMEC/VI; 1, 2, 3, 4, 7, 11, 14, 17, 20, 24, 27, and 30 for FF monotherapy; and 1, 2, 3, 4, 7, 11, 14, 17, 21, 24, 27, and 30 for FF/VI. Doses were actuated to waste on intermediate days.
Prior to testing, the inhalers were stored in their secondary packaging either at controlled conditions of 25°C and 60% relative humidity for 11 months (UMEC), 9 months (UMEC/VI), 29 months (FF), or 15 months (FF/VI), or under ambient laboratory conditions (typically 20°–22°C/50% relative humidity) for 2 weeks (UMEC/VI), 6 months (FF/VI), or 8 months (UMEC monotherapy). FPM was determined using composite samples of 5 UMEC or UMEC/VI doses or 6 FF or FF/VI doses, each from a separate inhaler. All tests were performed in duplicate (quadruplicate for FF monotherapy).
Performance over a range of flow rates
To assess the dose delivery performance at different flow rates, the delivered dose and fine particle dose (FPD; mass of particles <5 μm, determined using a Next Generation Impactor [NGI]) of each product was tested in triplicate (quadruplicate for FF) at flow rates of 30, 60, and 90 L/min, with an inhaled volume of 4 L (standard test conditions). Five doses of UMEC or UMEC/VI, or six doses of FF or FF/VI, stratified throughout the 30-dose inhaler, were actuated into the NGI to produce 5- or 6-blister composite samples, respectively, which were assessed using a standard protocol assay by HPLC. FPD was calculated through interpolation via a validated template and is reported for this data set to allow comparison of a defined size fraction (mass <5 μm) at all flow rates.
Assessment of performance of critical tasks (Human Factors Validation Tests)
While functionality of the inhaler is clearly critical, it is also important to assess whether it can be operated without error by subjects representative of the target patient population. For this purpose, three human factors validation tests, designed to assess errors in using the inhaler, were performed in Europe (United Kingdom; February 2012), the United States (March 2012), and Japan (April 2012).
To assess all potential users, four groups were identified: 12–17-year-old adolescent asthma patients; 18–55-year-old adult asthma patients; adult COPD patients aged ≥55 years and considered to be at ‘high risk’ of experiencing handling difficulties due to arthritis and/or sight and/or hearing problems; and caregivers (professional and non-professional). In each location, approximately 15 participants from each of the above groups were recruited.
Participants attended an interview session for approximately 1 hour, in which they received a plain white carton, representative of the package that would be given to patients at a pharmacy. The carton contained a foil tray, in which a fully functioning production ELLIPTA inhaler, containing empty blister strips, was sealed together with a sachet of desiccant, and the patient information leaflet. Participants were asked to remove the inhaler from the packaging (one attempt was allowed), and simulate the administration of a dose of medication, without inserting the inhaler into their mouth (three attempts in the European and Japanese studies, regardless of whether the initial attempt was successful; one attempt in the United States study).
Six pre-defined critical tasks that users must be able to perform to ensure that the ELLIPTA inhaler is made ready for inhalation were assessed during the demonstration: 1) opening the foil tray to ensure the patient can access their medication; 2) not mistaking the desiccant sachet for medication, and not eating or inhaling the desiccant in error; 3) sliding the mouthpiece cover down fully, until a click is heard, thereby ensuring that the dose pocket is opened and aligned and that the correct dose is administered; 4) locating the mouthpiece during a simulated inhalation; 5) understanding that the inhaler is empty (i.e., a replacement should be obtained) when the dose counter displays ‘0’; and 6) closing the mouthpiece cover after simulated inhalation, so that the inhaler is reset and the next dose can be actuated when necessary. After the demonstration, participants' understanding and perceptions of the inhaler were assessed using a series of questions.
Results
Simulated in-use testing at 60 L/min
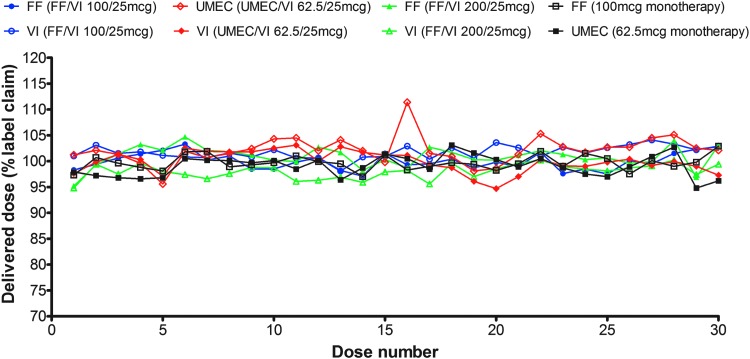
At 60 L/min, the mean delivered dose expressed as % nominal blister content for each product over the 30-dose in-use life of the inhaler was 89.1 (FF monotherapy 100 μg), 81.0 (UMEC monotherapy 62.5 μg), 85.8/85.5 (UMEC 62.5 μg/VI 25 μg), 93.7/89.6 (FF 100 μg/VI 25 μg), and 92.8/87.3 (FF 200 μg/VI 25 μg [Fig. 6)]. Overall, FPM for each product was generally consistent over the inhaler in-use life for both controlled and ambient laboratory conditions (Table 1).
FIG. 6.
In vitro dose delivery performance (delivered dose) over 30-day inhaler lifetime.a Data are mean. aAfter storage at 25°C and 60% relative humidity for 29 months (FF monotherapy), 11 months (UMEC monotherapy), 9 months (UMEC/VI), or 12 months (FF/VI). FF, fluticasone furoate; UMEC, umeclidinium; VI, vilanterol.
Table 1.
Results of Simulated In-Use Testing over Inhaler Lifetime, After Storage in Secondary Packaging in Controlled (25°C and 60% Relative Humidity) or Ambient Laboratory Conditions
| FPM, mean (range) % nominal blister content | |||||||
|---|---|---|---|---|---|---|---|
| Controlled conditions (25°C and 60% relative humidity) | Ambient laboratory conditions | ||||||
| Flow rate (L/min) | Product delivered | UMEC | FF | VI | UMEC | FF | VI |
| 60 | FF monotherapy (100 μg)a | 23.7 (20.9–25.6) | – | ||||
| UMEC monotherapy (62.5 μg) | 35.0 (31.3–38.8) | 34.4 (30.3–36.7) | |||||
| FF/VI (100/25 μg) | 20.6 (18.0–22.2) | 30.7 (27.5–34.2) | 18.5 (16.5–21.0) | 31.0 (28.2–33.8) | |||
| FF/VI (200/25 μg) | 20.1 (18.1–21.2) | 29.5 (25.8–32.8) | 18.6 (16.9–20.4) | 30.8 (28.9–32.9) | |||
| UMEC/VI (62.5/25 μg) | 31.5 (29.4–33.3) | 28.4 (26.0–30.5) | 31.8 (29.0–35.4) | 27.0 (23.2–31.8) | |||
Data are mean (range). aAssessments after storage under ambient conditions were not performed.
FF, fluticasone furoate; FPM, fine particle mass; UMEC, umeclidinium; VI, vilanterol.
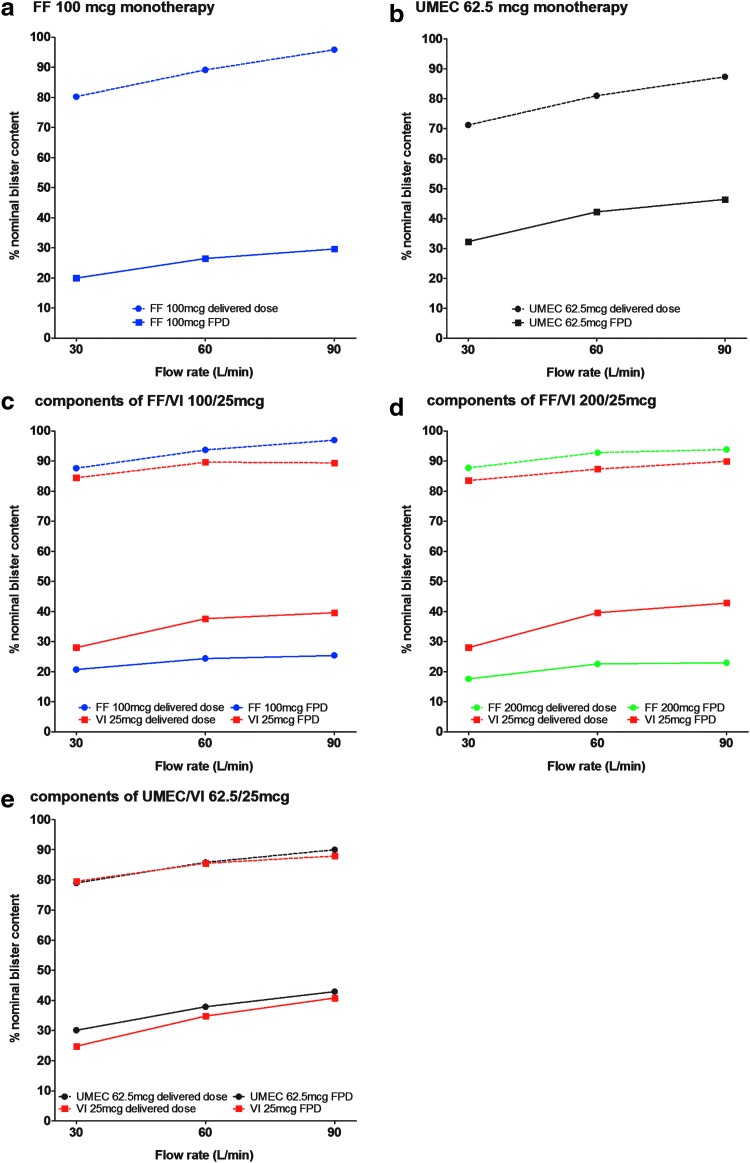
Dose delivery at varying flow rates of 30, 60, and 90 L/min
Across the flow rates of 30, 60, and 90 L/min, the delivered dose ranged from 71.2% to 87.3% of the nominal blister content for UMEC 62.5 μg monotherapy, 79.0%–90.0% for UMEC when delivered as UMEC/VI 62.5/25 μg, 80.2%–95.8% for FF 100 μg monotherapy, 87.6%–96.9% for FF delivered as FF/VI 100/25 μg, 87.7%–93.8% for FF delivered as FF/VI 200/25 μg, and 79.5%–89.9% for VI delivered via any of the combination therapies. The delivered dose tended to increase slightly as the flow rate increased. The FPD range was 32.3%–46.4% of the nominal blister content for UMEC 62.5 μg monotherapy, 30.1%–42.9% for UMEC delivered as UMEC/VI 62.5/25 μg, 19.9%–29.6% for FF 100 μg monotherapy, 20.7%–25.4% for FF delivered as FF/VI 100/25 μg, 17.6%–23.0% for FF delivered as FF/VI 200/25 μg, and 24.8%–42.8% for VI delivered via any of the combination therapies (Fig. 7).
FIG. 7.
In vitro dose delivery (delivered dose and FPD) by flow rate FF, VI, and UMEC, when delivered as: Data are mean. FF, fluticasone furoate; FPD, fine particle dose; UMEC, umeclidinium; VI, vilanterol.
Overall, the ELLIPTA inhaler was shown, in vitro, to deliver a delivered dose close to the stated label claim delivered dose at flow rates between 30 and 90 L/min; the 30 L/min flow rate is lower than the minimum >43 L/min peak inspiratory flow rate previously observed in asthma or COPD patients of varying disease severity, when using the two-strip configuration of the inhaler.(17)
Human factors validation tests
The sample size followed the guidance that validation testing activities should include 15 subjects from each major user group.(18) Sixty participants from Europe, 62 from the United States, and 60 from Japan were enrolled, including a mixture of participants from each of the four previously identified groups in each country. All of the critical tasks were completed correctly by almost all (97%–100%) asthma and COPD patients and caregivers (Table 2).
Table 2.
Summary of Results from Human Factors Validation Tests for Critical Tasks
| Europe | United States | Japan | ||||
|---|---|---|---|---|---|---|
| N = 60 | N = 62 | N = 60 | ||||
| Critical task | Number of attempts | Completed without error, n (%) | Number of attempts | Completed without error, n (%) | Number of attempts | Completed without error, n (%) |
| Opens foil tray | 1 | 60 (100) | 1 | 62 (100) | 1 | 60 (100) |
| Understands desiccant should not be ingested or inhaled | 1 | 60 (100) | 1 | 62 (100) | 1 | 60 (100) |
| Slides cover down fully until it clicks | 3 | 178a (99) | 1 | 61b (98) | 3 | 174c (97) |
| Identifies mouthpiece during simulated use | 3 | 180 (100) | 1 | 62 (100) | 3 | 180 (100) |
| Understands inhaler is empty when counter is red or “0” | 1 | 60 (100) | 1 | 62 (100) | 1 | 59d (98) |
| Closes the mouthpiece after use | 3 | 180 (100) | 1 | 62 (100) | 3 | 180 (100) |
Errors encountered were as follows: aparticipant blocked the movement of the cover with a finger on one attempt (n = 1), participant failed to open the cover fully when demonstrating cleaning technique (n = 1); bparticipant partially opened the cover during the observed demonstration, but had performed the step correctly prior to observation (n = 1); cparticipant failed on all three attempts, but had not read the patient information leaflet (n = 1); participant failed on the second attempt, this appeared to be due to lack of attention after succeeding on the first attempt (n = 1); participant failed on second and third attempt, but had not read the patient information leaflet (n = 1); dparticipant thought that ‘0’ on dose counter indicated that the device was empty (which is correct), but could also see how this meant there was still one dose remaining as both of the markers were not red (which is incorrect).
It should be noted that these tests were carried out using a ‘worst-case’ scenario in which participants received the inhaler with no prior verbal instruction or demonstration. In clinical practice, the patient should receive a demonstration and/or explanation of how the inhaler works, and this should further minimize any potential for errors. The sample size in this study was small in relation to the number of variables that could potentially occur and was not appropriate to support statistical technical testing; however, sufficient participants from each group were recruited to establish that there were no trends indicating that users of the inhaler would not be able to operate the device safely and correctly, and no trend indicating any difference in the error rates between the groups was observed.
Discussion
The ideal inhaler should provide a consistent dose that is delivered to the correct part of the respiratory system, be robust, easy to use, liked by patients, and able to deliver a range of therapies.(9) The ELLIPTA DPI is a single- or two-strip inhaler designed to meet these criteria, and can be used by patients across a wide range of ages and disease severities to deliver a number of mono- and combination respiratory therapies for treating asthma and COPD. Despite its complex internal features, the inhaler has been shown to be user friendly and well-liked by patients,(19) with very low error rates observed in the human factors validation tests.
Patient ease-of-use and convenience were key considerations when developing the ELLIPTA inhaler, and information gathered though voice-of-the-customer research and learning from the DISKUS inhaler was utilized during the development process. The mouthpiece of the ELLIPTA inhaler has contouring similar to that found on the DISKUS inhaler to help patients identify how much should be put into the mouth. The pre-filled, non-refillable blister strips ensure that each blister contains a consistent dose of medication, avoiding problems with variable dosing that have been observed with other multi-dose inhalers.(9)
Similarly, as the ELLIPTA inhaler is delivered ready for use, potential sources of error that are encountered with some other inhalers are avoided, including mistakes in assembling or re-filling the inhaler.(20) The red flags that appear on the dose counter when less than 10 doses remain, and when there are no doses remaining, serve as visual reminders for the patient to obtain a replacement before the inhaler is empty.
The capacity to deliver both mono- and combination therapies potentially minimizes the number of inhalers that a patient must learn to use, as the same inhaler could be used to continue maintenance therapy when the disease state changes, or if ‘step-up’ therapy is required. Current international guidelines(1,21) recommend that asthma patients uncontrolled with low-dose ICS alone are stepped up to an ICS/LABA combination therapy; the ELLIPTA inhaler can deliver a therapy from each of these classes of treatment. A similar sequence may also be possible in COPD patients, where stepping up from inhaled bronchodilator monotherapy to a combination therapy(2) is very common.
In addition to convenience, the delivery of two therapeutic agents from a single inhaler has previously been shown to improve compliance when compared with using separate inhalers for each component of a combination therapy.(22) Furthermore, the possibility of using the inhaler to deliver multiple treatments in both asthma and COPD means that the need for healthcare professionals to familiarize themselves with multiple inhalers is reduced, as they have access to a common inhaler for a range of patients.
The ELLIPTA inhaler provides further advantages. The mouthpiece cover is used to actuate the dispensing mechanism within the device, which avoids the requirement for a separate actuation step and therefore eliminates a potential source of user error. It is similar in size to, but slightly smaller than, the DISKUS inhaler, which was cited as a positive aspect by patients who had used the inhaler in Phase III clinical trials.(19) The ELLIPTA inhaler has also been designed to stand in an upright position (the DISKUS inhaler cannot), which allows for easy storage and helps to guide the patient to the proper orientation of the inhaler. The dose counter is centrally positioned and larger than on the DISKUS inhaler, allowing it to be easily located and read by the user. Finally, the ability to hold two separate blister strips allows combination medicines to be formulated and stored separately, until the point of inhalation.
The ease-of-use of the ELLIPTA inhaler is reflected by the results of the human factors validation tests, which demonstrated that patients and caregivers are able to perform critical tasks associated with the correct use of the inhaler to prepare the dose for inhalation without previous instruction, indicating that the inhaler is easy and intuitive to use. This is further supported by previously published patient perception data, which show that patients find the inhaler easy to use.(23,24)
Additionally, patient preference data show that patients prefer the ELLIPTA inhaler to other inhalers, including the DISKUS inhaler, because of the ease of handling, the number of steps required to use the inhaler, size, mouthpiece design, the dose counter display, portability, and convenient storage.(19)
In addition to ease-of-use, DPIs must demonstrate consistent and reliable dose delivery for patients in the target population. The in vitro data presented suggest that the ELLIPTA inhaler should be suitable for patients who can achieve flow rates ≥30 L/min for the delivery of any of the products tested; this is lower than the minimum peak inspiratory flow rate of >43 L/min measured for any asthma or COPD patient (all severities) in a previous study using the two-strip configuration of the inhaler,(17) indicating that the inhaler can be readily and comfortably used even by severe asthma or severe COPD patients.
These findings support those of previously published in vitro studies, which indicate that the ELLIPTA inhaler has the qualities required to consistently deliver UMEC, UMEC/VI, or FF/VI to the lungs in adult patients.(25,26) In addition, dose delivery was consistent over the inhaler in-use life, and was not affected by storage conditions when the DPIs were stored in their secondary packaging. Furthermore, robust performance of the inhaler, and efficacy and tolerability of the therapeutic products when delivered via the ELLIPTA inhaler has been demonstrated across a number of clinical trials of medicines including FF monotherapy for asthma,(27–33) FF/VI combination for asthma and COPD,(34–44) UMEC monotherapy for COPD,(45–49) and UMEC/VI combination for COPD.(50–53)
The ELLIPTA inhaler was also designed to be robust enough to withstand patient use and occasional misuse, as well as being rugged enough to withstand a number of defined patient abuse scenarios. Use and misuse testing was performed to confirm that the robustness and ruggedness of the inhaler was sufficient to meet the demands of expected patient use (e.g., the misuse scenario of dropping the inhaler from 1 M in a number of different orientations and abuse scenarios such as actuating the device without inhaling or shaking before repeating the scenario); this testing was conducted in accordance with the International Standard ISO/FDIS 20072:2009.(54)
In addition, the ELLIPTA inhaler has been designed to withstand extensive travel including shipping and distribution; testing was conducted in accordance with ASTMD-416909 with assurance level 2 test intensity, the standard practice for performance testing of shipping containers and systems.(55)
Conclusion
The ELLIPTA DPI provides a next generation inhaler that improves user experience and versatility, even when compared with the DISKUS DPI, and appears suitable for the majority of COPD and asthma patients. The experiments reported here clearly demonstrate consistent dose delivery across in-use life (30 doses) and flow rates of 30, 60, and 90 L/min under standard impactor conditions. The ELLIPTA DPI has also been used successfully in numerous clinical studies and offers flexibility through the single- or two-strip design enabling the delivery of a range of treatment options.
Acknowledgments
Editorial support in the form of development of a draft outline, the manuscript first draft, editorial comments, assembling tables and figures, collating author comments, copyediting, fact checking, referencing, and graphic services under guidance of the authors was provided by Laura Maguire, MChem at Gardiner-Caldwell Communications (Macclesfield, UK) and was funded by GSK.
All of the authors were involved in the design and development of the ELLIPTA DPI. MH was involved in the acquisition, interpretation and analysis of the in-vitro dosing performance data. All authors contributed to the development of this manuscript and approved the final version.
Funding: GSK.
Source: The ELLIPTA® dry powder inhaler was developed by GSK and the development of this manuscript was funded by GSK.
Author Disclosure Statement
The ELLIPTA DPI was developed by GSK. ACG was employed by GSK at the time that the ELLIPTA DPI was developed and performs contract work for and holds stock in GSK. RW, MH, and KG are employees of, and hold stock in, GSK.
References
- 1.Global Initiative for Asthma (GINA): Global strategy for asthma management and prevention. Updated May 2014. Available at http://www.ginasthma.com Accessed March19, 2015
- 2.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of COPD. Updated January 2015. Available at www.goldcopd.org Accessed March19, 2015
- 3.Miravitlles M, Soler-Cataluña JJ, Calle M, and Soriano JB: Treatment of COPD by clinical phenotypes: Putting old evidence into clinical practice. Eur Respir J. 2013;41:1252–1256 [DOI] [PubMed] [Google Scholar]
- 4.Boyd G: The continued need for MDIs. J Aerosol Med. 1995;8:S9–S12 [DOI] [PubMed] [Google Scholar]
- 5.Dalby RN, Eicher J, and Zierenberg B: Development of Respimat® Soft Mist™ Inhaler and its clinical utility in respiratory disorders. Med Devices (Auckl). 2011;4:145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laube BL, Janssens HM, de Jongh FHC, Devadason SG, Dhand R, Diot P, Everard ML, Horvath I, Navalesi P, Voshaar T, and Chrystyn H: What the pulmonary specialist should know about the new inhalation therapies. ERS/ISAM Task Force Report. Eur Respir J. 2011;37:1308–1331 [DOI] [PubMed] [Google Scholar]
- 7.Cochrane MG, Bala MV, Downs KE, Mauskopf J, and Ben-Joseph RH: Inhaled corticosteroids for asthma therapy: Patient compliance, devices, and inhalation technique. Chest. 2000;117:542–750 [DOI] [PubMed] [Google Scholar]
- 8.Gabrio BJ, Stein SW, and Velasquez DJ: A new method to evaluate plume characteristics of hydrofluoroalkane and chlorofluorocarbon metered dose inhalers. Int J Pharm. 1999;186:3–12 [DOI] [PubMed] [Google Scholar]
- 9.Chrystyn H: The Diskus™: A review of its position among dry powder inhaler devices. Int J Clin Pract. 2007;61:1022–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoppentocht M, Hagedoorn P, Frijlink HW, and de Boer AH: Technological and practical challenges of dry powder inhalers and formulations. Adv Drug Deliv Rev. 2014;17:18–31 [DOI] [PubMed] [Google Scholar]
- 11.Rau JL: Practical problems with aerosol therapy in COPD. Respir Care. 2006;51:158–172 [PubMed] [Google Scholar]
- 12.European Medicines Agency: INCRUSE® ELLIPTA®, European public assessment report (EPAR) summary for the public. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/002809/WC500167432.pdf Accessed March19, 2015 (INCRUSE™ is a trademark of the GSK group of companies)
- 13.FDA Medication Guide: ANORO® ELLIPTA® 2013: Available at http://www.fda.gov/downloads/drugs/drugsafety/ucm380278.pdf Accessed March19, 2015 (ANORO™ is a trademark of the GSK group of companies)
- 14.FDA Medication Guide: ARNUITY™ ELLIPTA® 2014. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205625s000lbl.pdf Accessed: 13January2015 (ARNUITY™ is a trademark of the GSK group of companies)
- 15.European Medicines Agency: RELVAR® ELLIPTA® European public assessment report (EPAR) summary for the public. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/002673/WC500157636.pdf Accessed March19, 2015 (RELVAR™ is a trademark of the GSK group of companies)
- 16.FDA Medication Guide: BREO® ELLIPTA® 2013. Available at http://www.fda.gov/downloads/drugs/drugsafety/ucm352347.pdf Accessed March19, 2015 (BREO™ is a trademark of the GSK group of companies)
- 17.Prime D, De Backer W, Hamilton M, Cahn A, Preece A, Kelleher D, and Lindo E: Comparison of inhalation profiles through a novel dry powder inhaler (nDPI) and lung function measurements for healthy subjects, asthma and chronic obstructive pulmonary disease (COPD) patients. Am J Respir Crit Care Med. 2012;185(Meetings Abstracts):A2941 [Google Scholar]
- 18.Draft Guidance for Industry and Food and Drug Administration Staff - Applying Human Factors and Usability Engineering to Optimize Medical Device Design. June 22, 2011. Available at http://www.fda.gov/RegulatoryInformation/Guidances/ucm259748.htm
- 19.Svedsater H, Dale P, Garrill K, Walker R, and Woepse MW: Qualitative assessment of attributes and ease of use of the ELLIPTA™ dry powder inhaler for delivery of maintenance therapy for asthma and COPD. BMC Pulm Med. 2013;13:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Inhaler Error Steering Committee, Price D, Bosnic-Anticevich S, Briggs A, Chrystyn H, Rand C, Scheuch G, and Bousquet J: Inhaler competence in asthma: Common errors, barriers to use and recommended solutions. Respir Med. 2013;107:37–46 [DOI] [PubMed] [Google Scholar]
- 21.National Heart, Lung, and Blood Institute: National Asthma Education and Prevention Program; Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Full Report 2007. Available at http://www.nhlbi.nih.gov/files/docs/guidelines/asthgdln.pdf Accessed March19, 2015
- 22.Chrischilles E, Gilden D, Kubisiak J, Rubenstein L, and Shah H: Delivery of ipratropium and albuterol combination therapy for chronic obstructive pulmonary disease: effectiveness of a two-in-one inhaler versus separate inhalers. Am J Manag Care. 2002;8:902–911 [PubMed] [Google Scholar]
- 23.Kirby SY, Zhu C-Q, Kerwin EM, Stanford RH, and Georges G: A randomized controlled trial comparing two dry powder inhalers: More patients with COPD prefer ELLIPTA compared to DISKUS based on device-specific attributes. Am J Respir Crit Care Med. 2014;189:A3037 [Google Scholar]
- 24.Svedsater H, Jacques L, Goldfrad C, Bleecker ER, O'Byrne PM, and Woodcock A: Ease of use of a two-strip dry powder inhaler (DPI) to deliver fluticasone furoate/vilanterol (FF/VI) and FF alone in asthma. Eur Respir J. 2013b;42:128s–129s [Google Scholar]
- 25.Hamilton M, Prime D, and Leggett R: Ex-vivo product performance of fluticasone furoate/vilanterol delivered from a novel dry powder inhaler, using the Electronic Lung to replicate asthma and COPD patient inhalation profile. Am J Respir Crit Care Med. 2012;185(Meeting Abstracts):A2940 [Google Scholar]
- 26.Hamilton M, Prime D, Bogalo Huescar M, Pang C, Charles S, and Gillett B: In-vitro delivery performance of umeclidinium and umeclidinium/vilanterol from a dry powder inhaler using the Electronic Lung breathing simulator to replicate inhalation profiles from patients with varying COPD severity. Am J Respir Crit Care Med. 2013;187:A4281 [Google Scholar]
- 27.Bateman ED, Bleecker ER, Lötvall J, Woodcock A, Forth R, Medley H, Davis AM, Jacques L, Haumann B, and Busse WW: Dose effect of once-daily fluticasone furoate in persistent asthma: A randomized trial. Respir Med. 2012;106:642–650 [DOI] [PubMed] [Google Scholar]
- 28.O'Byrne PM, Woodcock A, Bleecker ER, Bateman ED, Lötvall J, Forth R, Medley H, Jacques L, and Busse WW: Efficacy and safety of once-daily fluticasone furoate 50 mcg in adults with persistent asthma: A 12-week randomized trial. Respir Res. 2014;15:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Busse W, Bateman E, O'Byrne P, Lötvall J, Woodcock A, Medley H, Forth R, and Jacques L: Once-daily fluticasone furoate 50 mcg in mild to moderate asthma: A 24-week placebo-controlled randomized trial. Allergy. 2014;69:1522–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lötvall J, Bleecker ER, Busse WW, O'Byrne PM, Woodcock A, Kerwin EM, Stone S, Forth R, Jacques L, and Bateman ED: Efficacy and safety of fluticasone furoate 100 μg once-daily in patients with persistent asthma: A 24-week placebo and active-controlled randomised trial. Respir Med. 2014;108:41–49 [DOI] [PubMed] [Google Scholar]
- 31.Woodcock A, Bleecker ER, Busse WW, Lötvall J, Snowise NG, Frith L, Jacques L, Haumann B, and Bateman ED: Fluticasone furoate: Once-daily evening treatment versus twice-daily treatment in moderate asthma. Respir Res. 2011;12:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woodcock A, Bateman ED, Busse WW, Lötvall J, Snowise NG, Forth F, Jacques L, Haumann B, and Bleecker ER: Efficacy in asthma of once-daily treatment with fluticasone furoate: A randomized, placebo-controlled trial. Respir Res. 2011;12:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woodcock A, Lötvall J, Busse WW, Bateman ED, Stone S, Ellsworth A, and Jacques L: Efficacy and safety of fluticasone furoate 100 μg and 200 μg once daily in the treatment of moderate-severe asthma in adults and adolescents: A 24-week randomised study. BMC Pulm Med. 2014;14:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agustí A, de Teresa L, De Backer W, Zvarich MT, Locantore N, Barnes N, Bourbeau J, and Crim C: A comparison of the efficacy and safety of once-daily fluticasone furoate/vilanterol with twice-daily fluticasone propionate/salmeterol in moderate to very severe COPD. Eur Respir J. 2014;43:763–772 [DOI] [PubMed] [Google Scholar]
- 35.Bleecker ER, Lötvall J, O'Byrne PM, Woodcock A, Busse WW, Kerwin EM, Forth R, Medley HV, Nunn C, Jacques L, and Bateman ED: Fluticasone furoate-vilanterol 100–25 mcg compared with fluticasone furoate 100 mcg in asthma: A randomized trial. J Allergy Clin Immunol Pract. 2014;2:553–561 [DOI] [PubMed] [Google Scholar]
- 36.Boscia JA, Pudi KK, Zvarich MT, Sanford L, Siederer SK, and Crim C: Effect of once-daily fluticasone furoate/vilanterol on 24-hour pulmonary function in patients with COPD: A randomized, three-way, incomplete block, crossover study. Clin Ther. 2012;34:1655–1666.e5 [DOI] [PubMed] [Google Scholar]
- 37.Busse WW, O'Byrne PM, Bleecker ER, Lötvall J, Woodcock A, Andersen L, Hicks W, Crawford J, Jacques L, Apoux L, and Bateman ED: Safety and tolerability of the novel inhaled corticosteroid fluticasone furoate in combination with the β2 agonist vilanterol administered once daily for 52 weeks in patients ≥12 years old with asthma: A randomised trial. Thorax. 2013;68:513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dransfield MT, Bourbeau J, Jones PW, Hanania NA, Mahler DA, Vestbo J, Wachtel A, Martinez FJ, Barnhart F, Sanford L, Lettis S, Crim C, and Calverley PMA: A once–daily inhaled corticosteroid, long-acting beta2-agonist combination, fluticasone furoate (FF) / vilanterol (VI), for the prevention of COPD exacerbations. Lancet Respir Med. 2013;1:210–223 [DOI] [PubMed] [Google Scholar]
- 39.Kerwin EM, Scott-Wilson C, Sanford L, Rennard S, Agusti A, Barnes N, and Crim C: A randomised trial of fluticasone furoate/vilanterol (50/25 μg; 100/25 μg) on lung function in COPD. Respir Med. 2013;107:560–569 [DOI] [PubMed] [Google Scholar]
- 40.Lötvall J, Bakke PS, Bjermer L, Steinshamn S, Scott-Wilson C, Crim C, Sanford L, and Haumann B: Efficacy and safety of 4 weeks' treatment with combined fluticasone furoate/vilanterol in a single inhaler given once daily in COPD: A placebo-controlled randomised trial. BMJ Open. 2012;2:e000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinez FJ, Boscia J, Feldman G, Scott-Wilson C, Kilbride S, Fabbri L, Crim C, and Calverley PM: Fluticasone furoate/vilanterol (100/25; 200/25 μg) improves lung function in COPD: A randomised trial. Respir Med. 2013;107:550–559 [DOI] [PubMed] [Google Scholar]
- 42.O'Byrne PM, Bleecker ER, Bateman ED, Busse WW, Woodcock A, Forth R, Toler WT, Jacques L, and Lötvall J: Once-daily fluticasone furoate alone or combined with vilanterol in persistent asthma. Eur Respir J 2014;43:773–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oliver A, Quinn D, Goldfrad C, van Hecke B, Ayer J, and Boyce M: Combined fluticasone furoate/vilanterol reduces decline in lung function following inhaled allergen 23 h after dosing in adult asthma: A randomised, controlled trial. Clin Transl Allergy. 2012;2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woodcock A, Bleecker ER, Lötvall J, O'Byrne PM, Bateman ED, Medley H, Ellsworth A, Jacques L, and Busse WW: Efficacy and safety of fluticasone furoate/vilanterol compared with fluticasone propionate/salmeterol combination in adult and adolescent patients with persistent asthma: A randomized trial. Chest. 2013;144:1222–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Church A, Beerahee M, Brooks J, Mehta R, and Shah P: Dose response of umeclidinium administered once or twice daily in patients with COPD: A randomised cross-over study. BMC Pulm Med. 2014;14:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Donohue JF, Anzueto A, Brooks J, Mehta R, Kalberg C, and Crater G: A randomized, double-blind, dose-ranging study of the novel LAMA GSK573719 in patients with COPD. Respir Med. 2012;106:970–979 [DOI] [PubMed] [Google Scholar]
- 47.Decramer M, Maltais F, Feldman G, Brooks J, Harris S, Mehta R, and Crater G: Bronchodilation of umeclidinium, a new long-acting muscarinic antagonist, in COPD patients. Respir Physiol Neurobiol. 2013;185:393–399 [DOI] [PubMed] [Google Scholar]
- 48.Trivedi R, Richard N, Mehta R, and Church A: Umeclidinium in patients with COPD: A randomised, placebo-controlled study. Eur Respir J. 2014;43:72–81 [DOI] [PubMed] [Google Scholar]
- 49.Cahn A, Tal-Singer R, Pouliquen IJ, Mehta R, Preece A, Hardes K, Crater G, and Deans A: Safety, tolerability, pharmacokinetics and pharmacodynamics of single and repeat inhaled doses of umeclidinium in healthy subjects: Two randomized studies. Clin Drug Invest. 2013;33:477. [DOI] [PubMed] [Google Scholar]
- 50.Celli B, Crater G, Kilbride S, Mehta R, Tabberer M, Kalberg C, and Church A: Once-daily umeclidinium/vilanterol 125/25 mcg in COPD: A randomized, controlled study. Chest. 2014;145:981–991 [DOI] [PubMed] [Google Scholar]
- 51.Donohue J, Maleki-Yazdi MR, Kilbride S, Mehta R, Kalberg C, and Church A: Efficacy and safety of umeclidinium/vilanterol 62.5/25 mcg in COPD. Respir Med. 2013;107:1538–1546 [DOI] [PubMed] [Google Scholar]
- 52.Feldman G, Walker RR, Brooks J, Mehta R, Crater G: 28-day safety and tolerability of umeclidinium in combination with vilanterol in COPD: S randomized placebo-controlled trial. Pulm Pharmacol Ther. 2012;25:465–471 [DOI] [PubMed] [Google Scholar]
- 53.Kelleher DL, Mehta RS, Jean-Francois BM, Preece AF, Blowers J, Crater GD, and Thomas P: Safety, tolerability, pharmacodynamics and pharmacokinetics of umeclidinium and vilanterol alone and in combination: A randomized crossover trial. PLoS One. 2012;7:e50716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.ISO 20072:2009: Aerosol drug delivery device design verification – Requirements and test methods. Available at http://www.iso.org/iso/iso_catalogue/catalogue_tc/catalogue_detail.htm?csnumber=41989 Accessed March19, 2015
- 55.ASTM D4169-09, Standard Practice for Performance Testing of Shipping Containers and Systems, ASTM International, West Conshohocken, PA, 2009, www.astm.org Last access: July2015 [Google Scholar]