Abstract
The model of accelerated senescence with the prolonged administration of d-galactose is used in anti-aging studies because it mimics several aging-associated alterations such as increase of oxidative stress and decline of cognition. However, there is no standardized protocol for this aging model, and recently some reports have questioned its effectiveness. To clarify this issue, we used a model of high-dose d-galactose on 1-month-old male Wistar rats and studied the hippocampus, one of the most affected brain regions. In one group (n = 10), d-galactose was daily administered intraperitoneally (300 mg/kg) during 8 weeks whereas age-matched controls (n = 10) were injected intraperitoneally with saline. A third group (n = 10) was treated with the same dose of d-galactose and with oral epigallocatechin-3-gallate (EGCG) (2 grams/L), a green tea catechin with anti-oxidant and neuroprotective properties. After treatments, animals were submitted to open-field, elevated plus-maze and Morris water maze tests, and neurogenesis in the dentate gyrus subgranular layer was quantified. There were no significant alterations when the three groups were compared in the number of doublecortin- and Ki-67–immunoreactive cells, and also on anxiety levels, spatial learning, and memory. Therefore, d-galactose was not effective in the induction of accelerated aging, and EGCG administered to d-galactose–treated animals did not improve behavior and had no effects on neurogenesis. We conclude that daily 300 mg/kg of d-galactose administered intraperitoneally may not be a suitable model for inducing age-related neurobehavioral alterations in young male Wistar rats. More studies are necessary to obtain a reliable and reproducible model of accelerated senescence in rodents using d-galactose.
Introduction
Aging leads to a progressive deterioration of tissues and organs, accompanied by an increased incidence and severity of a wide variety of chronic pathological conditions, including neurodegenerative diseases.1–3 The possibility of reducing or alleviating the incidence of these diseases and the improvement of the quality of life in the elderly, the fastest growing part of the world population,4 has renewed interest in the search for effective anti-aging therapies and the emergence of the discipline of medical gerontology. Physicians and researchers are trying to interfere with the risk factors and lifestyle issues that can be altered with prevention strategies.5 Modification of diet and/or the use of nutraceuticals are the most promising pathways, and several experimental paradigms are used.4,6
Mainly for the purpose of testing nutraceuticals and other pharmacological compounds, a model of accelerated senescence in rodents induced by d-galactose was gradually established.7 d-Galactose is a reducing monosaccharide present in very small quantities in organisms. An excessive dose of d-galactose is reported to induce oxidative damage to numerous tissues and organs related to the increased production of advanced glycation end products and reactive oxygen species.8 Because the systemic exposure to d-galactose apparently results in acceleration of biochemical and morphological senescence in several organs, including the central nervous system, it has been established as an experimental model for brain aging.9–11 Shortened life span and behavioral impairments, such as deficits in learning and memory10,11 and decline in other cognitive functions,12–14 have also been described in association with d-galactose exposure. Therefore, it is not surprising that, until April, 2015, and according to a simple query in PubMed Database of the US National Library of Medicine, more than 600 papers have been published using the d-galactose accelerated aging model. This paradigm seems to be established as a rapid and economic aging model and is widely used for anti-aging pharmacology investigations.15 Other advantages are the easy application, low incidence of tumors, and the high survival rate of the animals throughout the experimental period.16,17
It is important to note that, more recently, some dissonant reports concerning the effectiveness of this accelerated aging paradigm have been published.18–20 Indeed, Parameshwaran and collaborators have concluded that the d-galactose treatment (intraperitoneal [i.p] injection of 100 mg/kg per day) in female C57BL/61 mice was not suitable for testing anti-oxidant compounds in mice.18 Moreover, Tikhonova and collaborators found that d-galactose treatment (i.p. injection of 150 mg/kg per day) did not affect sexual incentive motivation, working memory, and object recognition in Wistar male rats.20 Likewise, Salkovic-Petresic and collaborators found that 1-month oral galactose treatment (200 mg/kg per day) failed to induce changes in the spatial memory of male Fisher and female Wistar rats.19 Several factors could be at the base of the apparent lack of congruence of d-galactose as an accelerated aging model. For instance, as in normal aging21,22 gender also appears to be an important factor in accelerated senescence induction.15 Other factors, such as the use of different strains of rats and mice, variable doses of d-galactose (50 mg/kg to 1250 mg/kg), administration type (oral, i.p., subcutaneous [s.c.]), and duration of the treatment, may also explain some conflicting or incongruous results.15,17,23
To shed light on this issue, we have used a model of high-dose d-galactose, administrated intraperitoneally, on young post-weaning male Wistar rats. The majority of d-galactose studies use a dose of 100 mg/kg.15 However, because we intended to have effects on a period of treatment of 2 months on young rats, we opted to use a higher dose, using the d-galactose concentration of 300 mg/kg. Moreover, we used the i.p. administration of d-galactose because substances administered subcutaneously tend to be absorbed at a slower rate24 and because i.p. injection is better tolerated during long periods of time (2 months) and using high doses (300 mg/kg).17,24
We focused the present study on the hippocampal formation (HF), a brain limbic region particularly affected by aging25–29 and presenting the hallmarks of neuronal age-related deterioration.14,16 Furthermore, because d-galactose may interfere with neurogenesis,9,30 we decided to analyze the effects of the high-dose d-galactose on the neurogenesis of the hippocampal dentate granule cells using two neurogenic markers, doublecortin (DCX) and Ki-67, that are easily quantified in young rodents.11,21 The focus on the neurogenic process assumes importance because new neurons can replace those lost through pathological or physiological events in the adult rodent brain,31 but this capacity decreases with aging.14,32 However, this residual neurogenesis can be important in aging, because even small increases in the number of dentate granule cells may have profound influences in learning and memory.33–35
Moreover, knowing that newly born granule cells in the adult HF are very important to spatial learning and memory processes36 and that all these processes could affect functionally the HF, we decided to evaluate also the behavioral performance of the animals, including the spatial learning and memory, anxiety levels, and locomotor activity.1,7,37 Finally, because chronic green tea consumption prevented the cognitive deterioration associated with normal aging and maintained the HF neurogenesis,1,21 we further investigated if its main catechin, epigallocatechin-3-gallate (EGCG) administered orally,38,39 could present these neuroprotective effects in the d-galactose–treated rats.
Materials and Methods
Animals and diets
Male Wistar rats obtained from the colony of the Institute of Molecular and Cell Biology (Porto, Portugal) were maintained under standard laboratory conditions (20–22°C and a 12-hr light/dark cycle) with free access to food and water. At 4 weeks of age, 30 animals weighing 134 ± 9.5 grams were randomly distributed in three groups. The animals of the d-galactose group were treated with an i.p. injection of d-galactose (300 mg/kg), and the ad libitum consumption of water and standard laboratory chow (Mucedola, Italy) was maintained. d-Galactose was purchased from Sigma-Aldrich Co., purity ≥ 99%, dissolved in 0.9% saline, and injected intraperitoneally daily at 14:00 hr during the experimental period of 2 months.
Considering that solubility of d-galactose is 100 mg/mL, every day we prepared a solution of d-galactose (100 mg/mL) and injected a volume of that solution intraperitoneally according to the concentration 300 mg/kg body weight. The animals of the d-galactose + EGCG group were also treated with an i.p. injection of d-galactose (300 mg/kg) every day, but were also treated simultaneously with EGCG in an oral solution (2 grams/L) during 2 months; the ad libitum consumption of the standard laboratory chow was maintained.
EGCG was purchased from Holliday & Company Inc., purity 95%, and dissolved in water (2 grams/L). The animals of the control group received an i.p. injection of 0.9% saline every day for 2 months, and the ad libitum consumption of water and standard laboratory chow was maintained. Rats were weighed weekly, and bedding was changed at the same time to minimize stress due to handling. The handling and care of the animals followed the Principles of Laboratory Animal Care (National Institutes of Health [NIH] Publication No. 86-23, revised 1985) and the European Communities Council Guidelines in Animal Research (86/609/UE). All efforts were made to minimize the number of animals used and their suffering.
Behavioral procedures
Behavioral testing began when animals were aged 3 months and was conducted by experimenters blinded to the treatments. Before testing, animals were handled for 5 consecutive days. Experiments were performed after at least 30-min habituation of animals to the testing room. Testing was done at the same time of day, beginning at 14:00 hr.
Open-field and elevated plus-maze tests
The open-field apparatus consisted of a white acrylic arena measuring 100 × 100 × 40 cm. The rats were placed in a corner of the apparatus and tested during 5-min sessions. Distances traveled in the outer zone of the open field, defined as 20 cm from any wall, and in its inner zone, defined as the 60 × 60 cm square in the center of the arena, were measured using a computerized video-tracking system (EthoVision XT 8.5, Noldus, The Netherlands). At the end of each session, the urine deposited was collected using filter paper. The difference between the weight of the paper in grams before and after collecting the urine was considered as a measure of the amount of urine deposited during the session. The number of fecal boli deposited was also recorded, and the floor of the apparatus was thoroughly cleaned and dried.
The elevated plus-maze apparatus was consisted of black acrylic and was arranged as a cross with two opposite open and two opposite closed arms (50 × 12 cm) joined by a common central square (12 × 12 cm). The closed arms were enclosed by 50-cm-high walls. The rats were placed on the central square facing one of the closed arms and allowed to explore the apparatus for 5 min. The behavior of rats was recorded and analyzed using a computerized video-tracking system (EthoVision XT 8.5, Noldus, The Netherlands). The percentages of time spent and distances traveled by rats in the open arms, closed arms, and in the central square were measured. At the end of each session, the number of fecal boli and the amount of urine deposited were recorded, and the apparatus was thoroughly cleaned and dried.
Morris water maze test
The maze consisted of a black circular pool (180-cm diameter, 50 cm deep) located in a corner of a room containing extra-maze cues. The apparatus was filled with water at room temperature (21 ± 1°C). The maze was virtually divided into four equal-sized quadrants. A black escape platform, 10 cm in diameter, was placed in the center of one of the quadrants 2 cm below the water surface. The swim path was recorded by a computerized video-tracking system (EthoVision XT 8.5, Noldus, The Netherlands). In the place learning task, the animals were trained to find the submerged escape platform and to climb on it. For acquisition, rats were given two trials per day for 14 consecutive days. Each rat was placed in the water facing the pool wall at one of the four starting points that were used in a pseudo-random order so that each position was used once in each block of four trials. If the rats did not find the escape platform within 60 sec, the experimenter guided them to the platform where they were allowed to remain for 15 sec. After the first daily trial, the animals were placed in a clean cage, and a 30-sec interval was imposed before the beginning of the next trial. The platform location was not changed during the acquisition period. The swim path length in each trial was measured.
One day after completion of the acquisition, animals were submitted to a single 60-sec probe trial in which the platform was removed from the pool. The number of times the rats swam through the zone where the platform had been located (platform crossings) provided a measure of accuracy in recalling the former position of the platform. The time spent by the animals swimming on the target quadrant was also recorded. One day after the completion of the probe trial, all animals were tested on the visible platform task during a 2-day period to evaluate their sensorimotor abilities. In this task, the rats were given one block of four trials per day separated by 30-sec inter-trial intervals. The platform, painted in white, was exposed 3 cm above the water surface. The position of the platform was different in each trial. The distances swum to locate the platform were recorded and averaged across eight trials.
Tissue preparation
General procedures
Following completion of the behavioral studies, six animals in each group, selected at random, were deeply anesthetized with sodium pentobarbital and perfused transcardially with 150 mL of 0.1 M phosphate buffer followed by a fixative solution containing 4% paraformaldehyde in phosphate buffer at pH 7.6. The brains were removed from the skulls, coded for blind processing and analysis, and separated by a mid-sagittal cut into right and left halves. The frontal and occipital poles were removed, and the remaining blocks of tissue containing the HF were separated and processed for immunohistochemistry. Because prior studies have shown that the HF of rodents displays right/left asymmetries,40 the blocks were sampled alternately from the right and left hemispheres, so that whatever the procedure performed the HFs from both sides were included.41,42
Immunohistochemistry for doublecortin and Ki-67 and Nissl staining
After perfusion, the blocks destined to immunohistochemistry containing the HF were stored for 1 hr in the fixative solution used in the perfusion and maintained overnight in the 10% sucrose solution at 4°C. Blocks were then mounted on a vibratome, serially sectioned in the coronal plane at 40 μm, and collected in phosphate-buffered saline (PBS). From each brain, two sets of vibratome sections containing the HF were selected using a systematic random sampling procedure to be used for immunostaining of DCX and Ki-67. Sections were washed twice in PBS and treated with 3% hydrogen peroxide (H2O2) for 10 min to inactivate endogenous peroxidase.
For Ki-67 detection, an antigen retrieval step in sodium citrate buffer for 1 hr at 65°C was added following the H2O2 step. Sections were washed twice in PBS and blocked in 5% normal serum (species as appropriate) during 1 hr. Thereafter, the sections were incubated at 4°C overnight with the primary antibody against either DCX (Santa Cruz Biotechnology, 1:500 dilution in PBS) or 48 hr with antibody against Ki-67 (Vector Laboratories; 1:500 dilution in PBS). Thereafter, the sections were washed twice and incubated with the respective biotinylated secondary antibody (Vector Laboratories; 1:400 dilution in PBS). Sections were then treated with avidin–biotin peroxidase complex (Vectastain Elite ABC kit, Vector Laboratories; 1:800 dilution in PBS). In the two last steps, the incubation was carried out for at least 1 hr at room temperature.
Following treatment with the peroxidase complex, sections were incubated for 10 min in 0.05% diaminobenzidine (Sigma-Aldrich Co.) to which 0.01% H2O2 was added. Sections were rinsed with PBS for at least 15 min between each step. To increase the tissue penetration, 0.5% Triton X-100 was added to PBS that was used in all immunoreactions and washes. Specificity of the immune reactions was controlled by omitting the incubation step with primary antisera. All immunochemical reactions and washings described above were carried out in 12-well tissue culture plates, four sections in each well, to assure that staining of the sections from all groups analyzed was performed in parallel and under identical conditions. Following termination of the staining procedures, sections were mounted on gelatin-coated slides and air-dried. They were then dehydrated in a series of ethanol solutions (50%, 70%, 90%, and 100%) and coverslipped using Histomount (National Diagnostics). Another set of sections was mounted and processed to Nissl staining, as previously described.43,44
Morphometric analyses: Estimation of the total number of DCX- and Ki-67–immunoreactive cells in the subgranular layer of the dentate gyrus
Neurons immunoreactive to DCX or to Ki-67 were identified as darkly stained perikarya and on the basis of their location and morphology (Fig. 1). The total number of these neurons was estimated using the optical fractionator method.45 The subgranular layer of the dentate gyrus was defined consistently as an approximately 30-μm-thick ribbon of tissue between the granular layer and the hilus46 at all levels along the septotemporal axis of the HF on the basis of cytoarchitectonic criteria47 and using a rat brain atlas.48 Neuronal counting was carried out using the Olympus C.A.S.T.-Grid System (Denmark), and a mean of 11 systematically sampled sections was used per animal. Beginning at a random starting position, visual fields were sampled systematically along the x and y axes, using a raster pattern procedure. The neuronal nuclei were selected as the counting unit. They were counted in every frame using the optical dissector at a final magnification, at the level of the monitor, of 2000×. The coefficient of error (CE) of the individual estimates was calculated according to Gundersen and collaborators49 and ranged between 0.08 and 0.11.
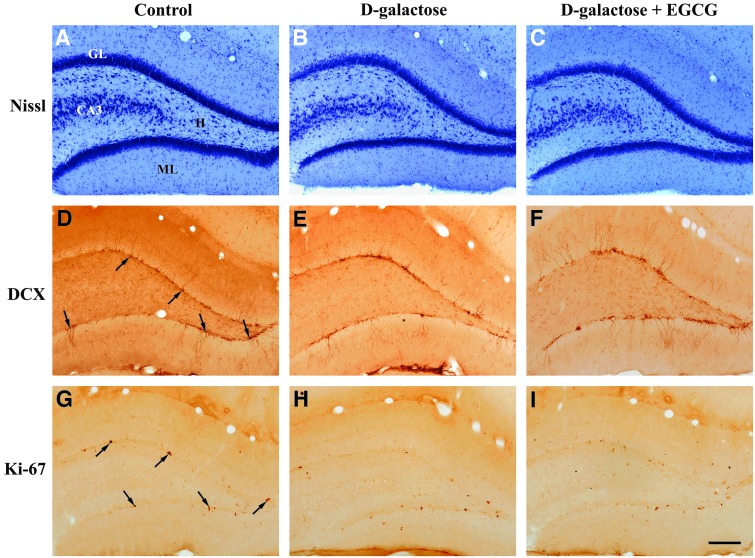
FIG. 1.
Representative photomicrographs of level-matched coronal sections of the dentate gyrus from 3-month control (A, D, G), 3-month d-galactose (B, E, H), and 3-month d-galactose + epigallocatechin-3-gallate (EGCG) (C, F, I) rats. Sections shown in A, B, and C were Nissl-stained, whereas D, E, and F were immunostained for doublecortin (DCX) and those shown in G, H, and I were immunostained for Ki-67. Note that there are no differences in the density of neurons in the dentate gyrus subgranular layer in the Nissl-stained sections of the three groups. Arrows in D show some DCX-immunopositive neurons and arrows in G show some Ki-67–immunopositive cells in the dentate gyrus subgranular layer. Note also that there are no differences in the density of the DCX- and Ki-67–immunoreactive cells between the three groups. ML, dentate gyrus molecular layer; GL, granule cell layer; H, dentate hilus; CA3, pyramidal cell layer of CA3 hippocampal field. Scale bar, 200 μm. Color images available online at www.liebertpub.com/rej
Statistical analysis
Before conducting statistical comparisons, data were tested for normality using the Kolmogorov–Smirnov one-sample test. Data derived from acquisition trials of water maze were analyzed using repeated-measures analysis of variance (ANOVA). The remaining data were analyzed using a one-way ANOVA test. Post hoc analyses were performed whenever appropriate, using the Newman–Keuls test. Differences were considered significant at the p < 0.05 level. All behavioral data are presented as mean ± standard error of the mean (SEM), whereas morphological results are expressed as mean ± standard deviation (SD).
Results
Animals and diets
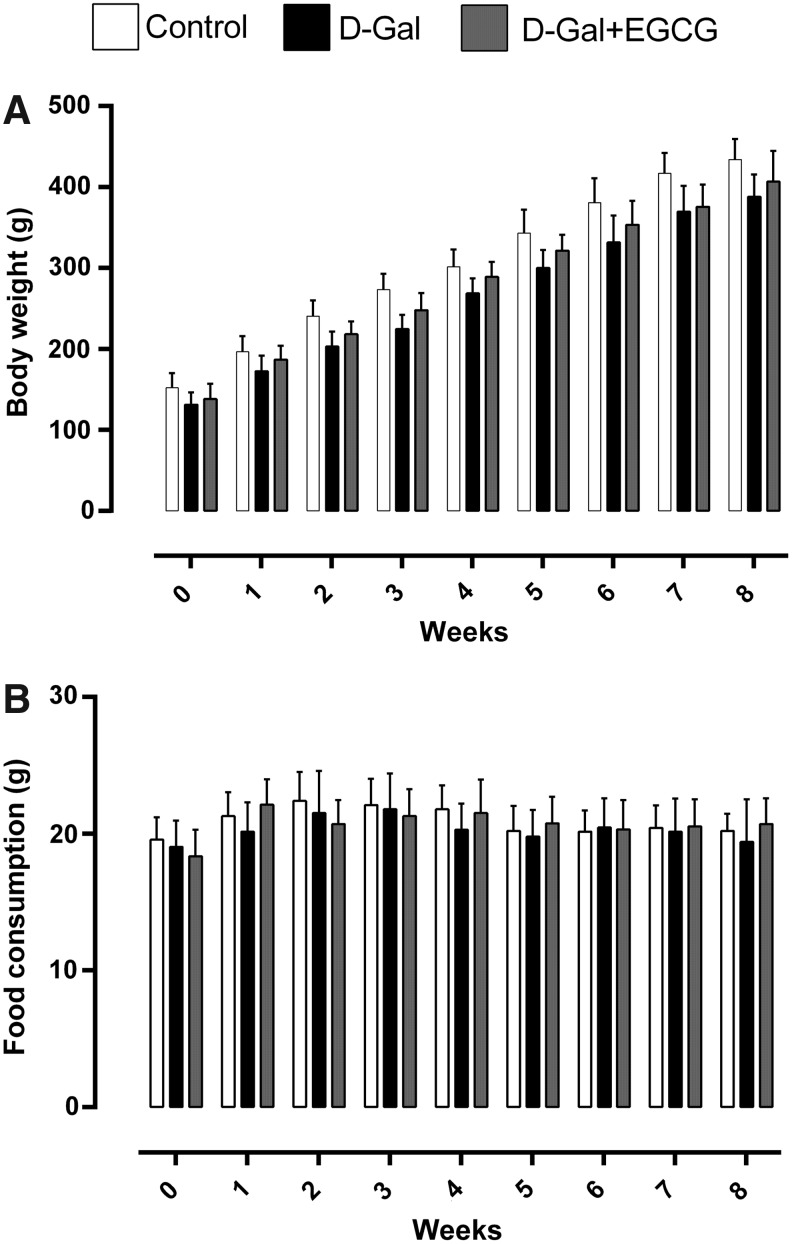
Daily food intake and weights of animals are shown in Fig. 2. Daily food intake, measured at 09:00 hr every day, was on average 20.2 ± 1.25 grams in 3-month control rats, 19.4 ± 3.10 grams in 3-month d-galactose animals, and 20.7 ± 1.90 grams in the 3-month d-galactose + EGCG group. On average, the animals of the d-galactose + EGCG group drank 31.5 ± 3.6 mL/day of the EGCG solution (2 grams/L) per animal, which corresponds to the EGCG consumption of approximately 200 mg/day per kg. By the end of the experiment, the mean body weight of 3-month control rats (434 ± 25.20 grams) was similar to 3-month d-galactose (388 ± 28.26 grams) and to 3-month d-galactose + EGCG rats (406 ± 38.10 grams). No significant difference was detected between the mean brain weights of 3-month controls (1.39 ± 0.03 grams), 3-month d-galactose (1.40 ± 0.05 grams), and 3-month d-galactose + EGCG (1.40 ± 0.04 grams) animals.
FIG. 2.
Body weight (A) and food consumption (B) from control (Control), d-galactose (D-Gal), and d-galactose +epigallocatechin-3-gallate (D-Gal + EGCG) rats during the 8 weeks of treatment. Data are presented as mean ± SD.
Total number of DCX- and Ki-67–immunoreactive neurons
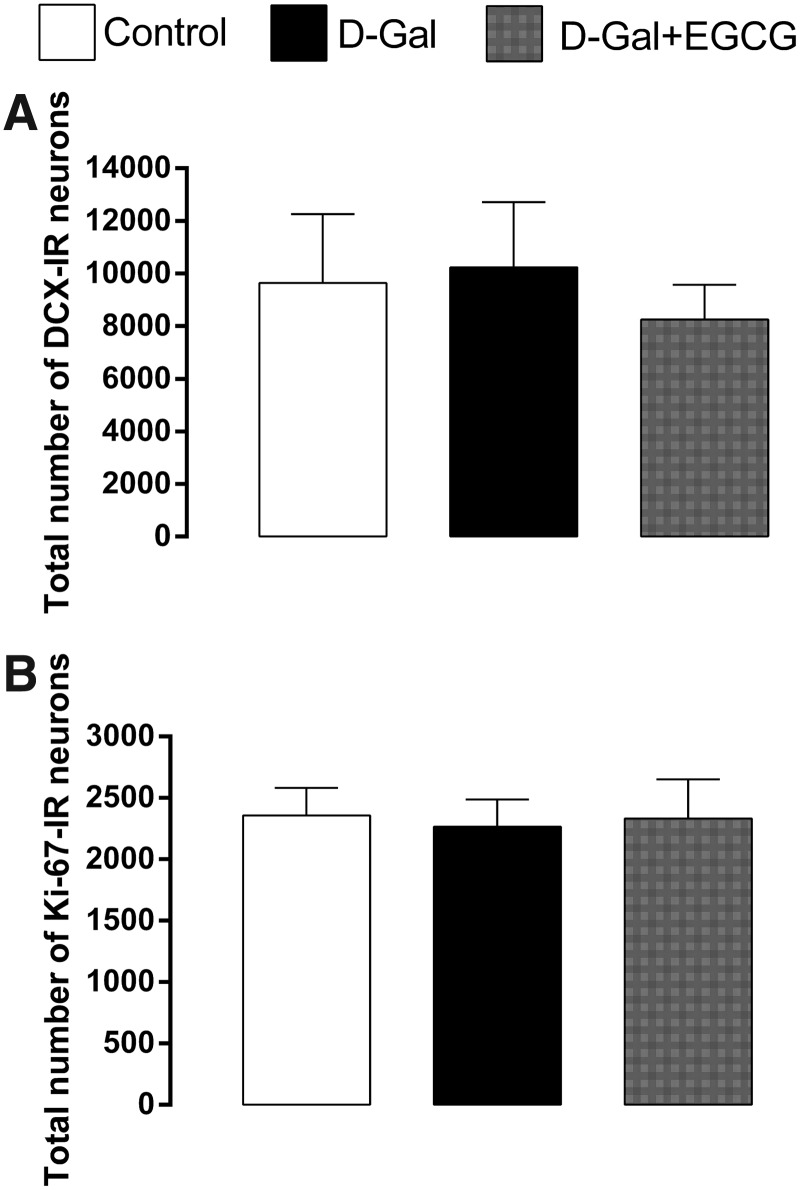
The estimates of the total number of DCX- and Ki-67–immunoreactive neurons in the subgranular layer of the HF are shown in Fig. 3. Analyses of these data have revealed that there was not a significant effect of the treatment on cell numbers immunopositive to DCX (F2, 15 = 1.41, p = 0.27; Fig. 2A) and immunopositive to Ki-67 (F2, 15 = 0.14, p = 0.87; Fig. 2B) in the subgranular layer of the HF dentate gyrus.
FIG. 3.
Total number of doublecortin (DCX)-immunoreactive cells (A) and Ki-67–immunoreactive cells (B) in the subgranular layer of the dentate gyrus from 3-month control (Control), 3-month d-galactose (D-Gal), and 3-month d-galactose + epigallocatechin-3-gallate (D-Gal + EGCG) rats. No significant differences in the total number of DCX- and Ki-67–immunoreactive cells were observed among the three groups. Data are presented as mean ± SD.
Open-field test
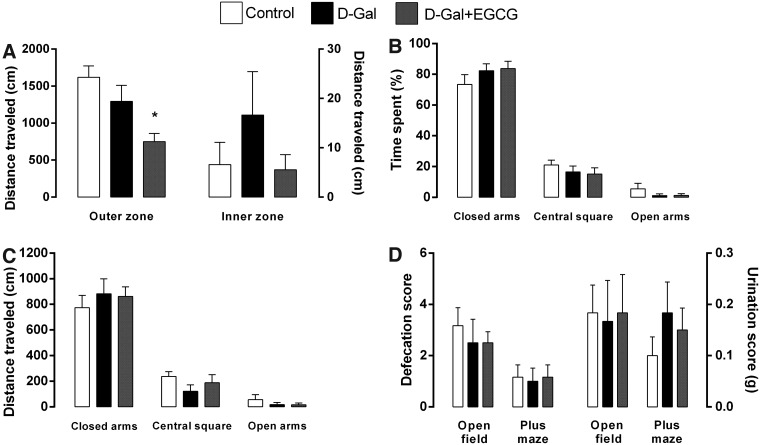
The results of the open-field test are shown in Fig. 4A, D. The animals treated with d-galactose did not differ significantly from the control rats in the locomotion scores in the outer zone of the open field. The locomotion activity in the inner zone was higher in the d-galactose–treated rats when compared with the control animals, although this difference did not achieve the significant level. The animals treated with d-galactose + EGCG traveled less distance in the outer zone when compared with the control group and d-galactose group, although these differences have only achieved the significant level when compared with the control animals. There were no significant differences in the distance traveled in the inner zone of the d-galactose + EGCG-treated animals when compared with the control and d-galactose–treated rats. There were no significant differences in the defecation and urination scores between the three groups (Fig. 4D).
FIG. 4.
Distance traveled (cm) on the open-field (A) and of the percentage of time spent (B) and the distance traveled (C) of elevated plus-maze from 3-month control (Control), 3-month d-galactose (D-Gal), and 3-month d-galactose + epigallocatechin-3-gallate (D-Gal + EGCG) rats. (A) There are no significant differences in the distance traveled on the inner zone between the three groups. The animals treated with d-galactose + EGCG traveled less distance in the outer zone when compared to the control group and d-galactose group, although these differences only have achieved the significant level when compared with the control animals. (B) No significant differences in the percentage of time spent on the central square and open and closed arms of elevated plus-maze between the three groups were found. (C) There are no significant differences in the distance traveled on the central square and open and closed arms of elevated plus-maze between the three groups. (D) Number of fecal boli and amount of urine deposited by rats during the open-field and elevated-plus maze tests. Note that there are no significant differences between the groups. Data are presented as mean ± SEM. (*) p < 0.05 versus control group.
Elevated plus-maze test
The results of the elevated plus-maze test are shown in Fig. 4B–D. The animals of the three groups showed, as expected, preference for protected zones of the plus-maze apparatus, becaused they spent more time in the closed arms and central square areas when compared with the open arms (Fig. 4B). However, the animals treated with d-galactose and treated with d-galactose + EGCG showed more preference to the closed arms when compared with controls, although there were no significant differences between the three groups. Consequently, they spent less time in the central zone and in the open arms, although these differences were not significant. The three groups exhibited similar levels of locomotor activity because there were no significant differences between the three groups in the distance traveled on the three zones of the elevated plus-maze (Fig. 4C). There were also no significant differences in the defecation and urination scores between the three groups (Fig. 4D).
Morris water maze test
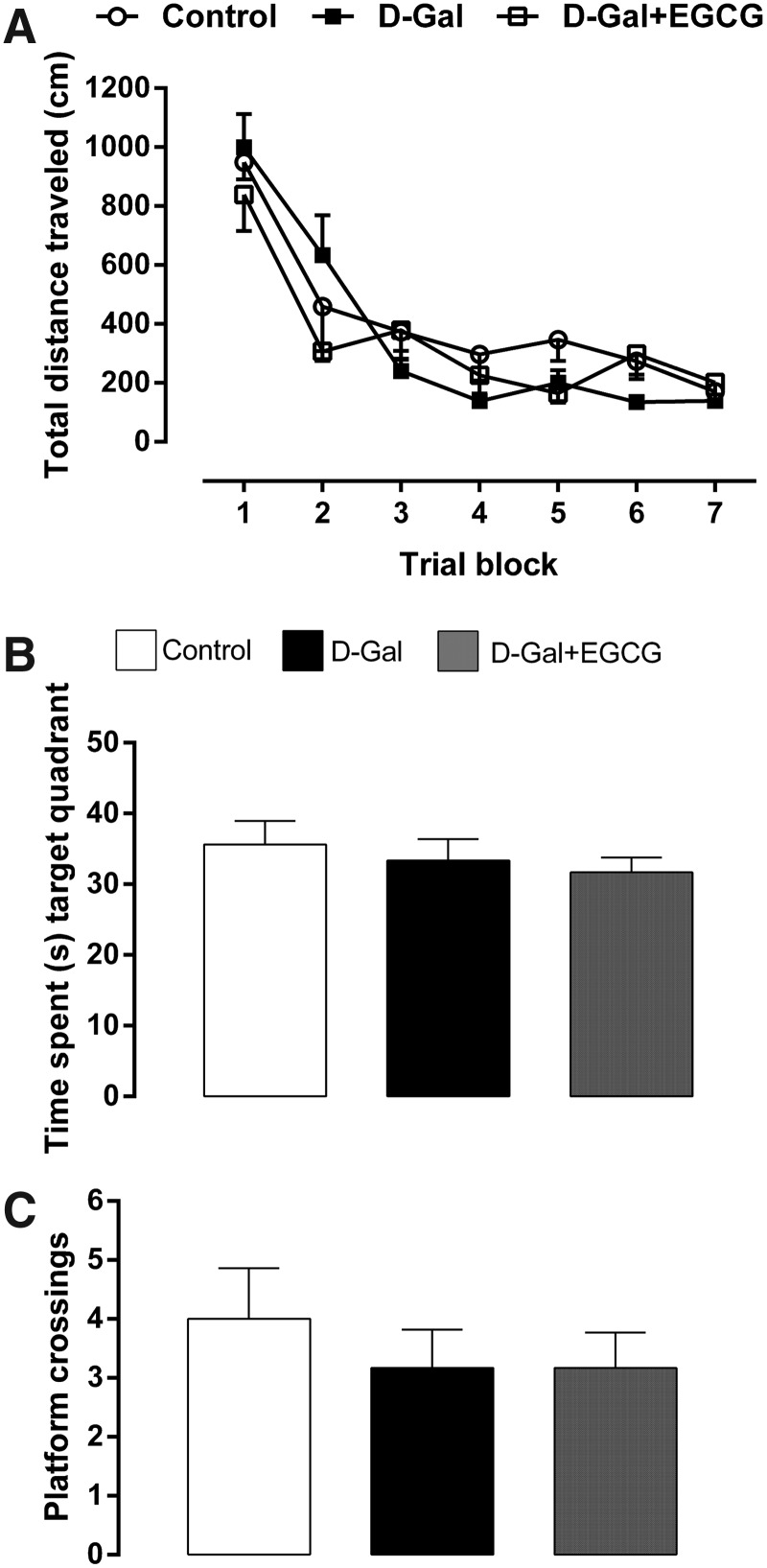
The mean distances traveled by control, d-galactose–treated, and d-galactose + EGCG-treated rats to find the hidden platform in the reference memory task are presented in Fig. 5A. The statistical analysis repeated-measures ANOVA revealed that the animals of the three groups progressively improved their capacity to locate the hidden platform during the 14 days of acquisition (F[6,162] = 51.90, p < 0.000001). However, although there were slight differences in the distance traveled in the first two trial blocks between the three groups, the differences were not significant (no significant effect of the treatment and no significant treatment × trial block interaction).
FIG. 5.
Distance traveled (A), time spent (sec) on the target quadrant (B), and platform crossings (C) of 3-month control (Control), 3-month d-galactose (D-Gal), and 3-month d-galactose + epigallocatechin-3-gallate (D-Gal + EGCG) rats on the Morris water maze test. (A) It is shown the total distance traveled (mean ± SEM, cm) to achieve the hidden platform for each block of four consecutive trials during the acquisition task. Note that there are no significant differences in performance of the test between the three groups. (B) There are no significant differences in the time spent on the target quadrant between the three groups. (C) No significant differences in the number of passages on the former position of the platform between the three groups were found. Data are presented as mean ± SEM.
Behavioral analyses derived from the probe trial are shown in Fig. 5B, C. Statistical analyses showed that animals of the three groups spend similar time on the target quadrant (Fig. 5B), revealing that there were no significant differences between the groups on the spatial strategy to search the escape platform during the probe trial. Although the d-galactose–treated rats and d-galactose + EGCG-treated animals crossed the former position of the platform less frequently compared with the control animals, the results revealed that there were no significant differences between the three groups (Fig. 5C).
Animals of the three groups quickly learned to find the visible platform. The distances swum to locate the platform position, averaged over eight trials, were 247 ± 93 cm in the control group, 235 ± 81 cm in the d-galactose–treated group, and 283 ± 87 cm in the d-galactose + EGCG-treated group. No significant differences among groups were found in this test, showing that all animals had similar sensorimotor abilities.
Discussion
The main goal of the present study was to evaluate the actions of the d-galactose accelerated aging model in young male Wistar rats. The results showed that there were no significant changes in neurogenesis, exploratory behavior, anxiety, and spatial learning and memory in the d-galactose–treated animals. Consequently, the secondary objective of the experiment, i.e., to verify if EGCG administration prevents the morphological and functional deterioration, associated with d-galactose administration, was not achieved because there were no significant differences when all groups were compared.
The d-galactose model of accelerated aging is widely used in the fields of age-related brain damage and pharmacological research.17 Nevertheless, the fine molecular mechanisms underlying the accelerated senescence remain unclear. However, most of the authors accept the main role of oxidative stress,13,18,23,50 due to the increase of pro-oxidant reactive species with disruption of the anti-oxidant defenses in a dose-dependent manner.11,14,23,51,52 Because the accumulation of free radicals progressively damages the brain structure and function, d-galactose administration mimics many characteristics of natural brain aging.23
Still, a review of published papers shows that there is no consensus concerning the dosage, age, strain of mice and rats, and time interval.15,17,19 For example, different strains of mice and rats are used, the injection dose varies between 50 mg/kg to 1250 mg/kg and the period of injection varies from 6 to 12 weeks or more.15,17 The administration can be i.p., s.c., or oral. When administered subcutaneously, substances tend to be absorbed at a slower rate, compared to i.p. administration,24 but it appears to be very effective as shown by the reduced levels of anti-oxidant activity and high levels of oxidative damage.15,23 The i.p. injection appears to be better tolerated17 during long periods of time (2 months) and using high doses (300 mg/kg) as those used in the present experiment. Gender is equally relevant because young male mice were more vulnerable to the effects of d-galactose.15 Moreover, the adult female brain was more sensitive to the oxidant effects of d-galactose than adolescent female brain.15 Taking altogether, it seems clear that the procedure of the d-galactose model of accelerated aging in rodents lacks standardization.
It was previously reported that d-galactose administration leads to morphological and functional neuronal lesions accompanied by the increase of apoptosis and loss of neuronal density of gamma-aminobutyric acid–producing (GABAergic) neurons of the cortex.10,11,53–56 In the present study, we did not find, from the qualitative point of view, signs of neuronal degeneration such as gross decrease of perikarya and nuclear volumes in the dentate gyrus when all groups were compared (data not shown). More importantly, we did not find alterations in the neurogenesis in the dentate gyrus both in d-galactose–treated animals and in d-galactose + EGCG-treated rats. In particular, we did not find alterations in the number of DCX-immunopositive cells, widely accepted as a neurogenesis marker.11,21,46,57,58 Also, no changes were found in the number of Ki-67–immunopositive cells, a marker of cell proliferation,46,58 in the subgranular zone of the dentate gyrus. Interestingly, the present results are in disagreement with previous studies, which found that even smaller doses of subcutaneously administrated d-galactose (100 mg/kg per day) in mice induced a reduction of cell proliferation and neuroblast differentiation using DCX and Ki-67 markers.9
There are other studies describing a decrease of cell proliferation or increase of apoptosis with several protocols of accelerated aging using d-galactose.10,11,53–56 The reduction in neurogenesis in 7-week-old mice treated with d-galactose subcutaneous injection for 3 weeks was related to the decrease in the levels of phosphorylated cyclic adenosine monophosphate (cAMP)-response element binding protein (pCREB).55 Furthermore, subcutaneous administration of d-galactose (120 mg/kg) for 42 days in 3-month-old Sprague–Dawley rats also decreased neurogenesis.23 The discrepancy between the present results and the data from previous studies could reside in the usage of different doses of d-galactose, different manner of administration, several strains of rats and mice used in the experiments, and age or other factors, as referred to above.
We have also found that EGCG administration during d-galactose treatment did not improve the behavioral performance and did not affect the neurogenic process because there were no significant differences in the total number of DCX- and Ki-67–immunopositive cells between the control, d-galactose, and d-galactose + EGCG-treated animals.
One caveat is that we did not analyze the potential effects of the EGCG treatment on young animals in the present study. We did not include that group because the main goal was to analyze the effectiveness of d-galactose as an accelerated aging model and to analyze the potential preventive effects of EGCG on this aging model. Furthermore, in other studies, it was found that EGCG and β-alanine supplementation in 19-month-old BALB/cJ mice during a short period (28 days) reduced brain oxidative stress but failed to increase neurogenesis or improve behavioral performance.30
The same research group also found recently that oral administration of EGCG (approximately 250 mg/day) in 2-month-old male BALB/cJ mice did not improve the number of new cells in the dentate gyrus and did not ameliorate behavioral performance.59 On the contrary, it was reported that 25 mg/kg per day EGCG gavage administration during 4 weeks enhanced cell proliferation and increased the number of neuroblasts in 2-month-old C57BL/6J mice dentate gyrus.25 Also, oral administration of 270 mg/kg per day EGCG in male Wistar rats for 26 weeks enhanced neurogenesis and presented anti-oxidative activity.60 The same neurogenic effect was observed in 3-month-old male C57BL/6J mice injected intraperitoneally with EGCG (20 mg/kg) once daily for 60 days.61 The different methods for measuring neurogenesis, different strains of rodents used, and the different doses and administration modes of EGCG can justify these differing results, but additional research is needed to identify the main parameters explaining these discrepancies.59 Unfortunately, we could not advance further information about the effects of EGCG oral administration (approximately 200 mg/kg per day) on neurogenesis on accelerated aged rats due to the lack of aging effects of d-galactose in the current experiment.
Concerning the behavioral data, we have found that the d-galactose treatment did not interfere with exploratory activity and anxiety levels, because significant alterations were not observed either in the distance traveled in the outer and inner zones of the open-field test or in the three zones of the elevated plus-maze. Also, defecation and urination in the open field and plus-maze was similar in all groups of rats, showing similar emotionality during the experiment. These results corroborate previous studies12,18,20 that did not find alterations in the exploratory locomotor activity and anxiety levels in the open-field test associated with d-galactose treatment.
In contrast to these results, other studies showed alterations in the open-field test with deficits in general ambulatory and exploratory activities in the d-galactose–treated animals mimicking the results of naturally aged animals.8,54,56 Once again, these discrepancies among the data could be explained by several factors, including the strain, age or administration and dosage of d-galactose. Interestingly, we observed that animals treated with d-galactose and EGCG moved less in the outer zone when compared with controls, suggesting alterations in the locomotor activity. However, this result was not accompanied by alterations in the distance traveled in the inner zone nor corroborated by the results of the plus maze, as significant alterations of the distance traveled in the three zones of the apparatus were not observed.
The present results also showed that d-galactose chronic treatment did not impair spatial learning and memory, because significant differences in the performance of the Morris water maze test between control and d-galactose–treated rats were not observed. Furthermore, it was observed that the rats treated with d-galactose and EGCG did not perform better in the Morris water maze test than control or d-galactose–treated animals, meaning that the treatment with EGCG did not improve the spatial learning and memory of the d-galactose–treated animals.
These results confirm data from other studies where it was also observed that the d-galactose treatment did not impair spatial learning and memory.18,20 On the contrary, there are several studies12,14,23,62 that reported impairments of the spatial learning and memory induced by d-galactose treatment. Indeed, in a study where C57BL/6 mice were used, there were deficits in spatial learning and memory but curiously, non-spatial cognitive ability such as novelty-seeking behavior was not affected.14 Trying to shed some light on these conflicting results, Parameshwaran and collaborators concluded that d-galactose administration may not be indicated to evaluate accelerated aging memory deficits, particularly in paradigms that do not involve reward/penalty stimuli as verified in the forced swimming in Morris water maze.18 It is important to stress that the present behavioral results observed during the Morris water maze test are consistent with the results of the neurogenesis in the HF. In fact, increasing evidence suggests that newly born granule cells in adult HF are very important to spatial learning and memory processes.36 Thus, the absence of spatial learning and memory impairment in the d-galactose and d-galactose and EGCG-treated animals was indeed underlined by the absence of alterations in the HF neurogenesis.
We conclude that the available data are not enough to achieve a firm conclusion of the effectiveness of the d-galactose model of accelerated senescence. Indeed, in the present experiment, the d-galactose treatment failed to induce the expected effects to neurogenesis, cognition, and anxiety in post-weaning male Wistar rats. It is mandatory to standardize dose, administration, time period, gender, and strain of mice or rats to obtain a reliable and reproducible model and to avoid inconsistent and/or conflicting results.15,17–19
Acknowledgments
This work was supported by Projectos IJUP (PP_IJUP2011 73) and National Funds through FCT–Fundação para a Ciência e a Tecnologia within the scope of the Strategic Project Centro de Morfologia Experimental (CME/FM/UP) 2011-2012 and Project PEst-OE/SAU/UI0121/2011.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Andrade JP, Assuncão M. Protective effects of chronic green tea consumption on age-related neurodegeneration. Curr Pharm Des 2012;18:4–14 [DOI] [PubMed] [Google Scholar]
- 2.Chen D, Milacic V, Chen MS, Wan SB, Lam WH, Huo C, Landis-Piwowar KR, Cui QC, Wali A, Chan TH, Dou QP. Tea polyphenols, their biological effects and potential molecular targets. Histol Histopathol 2008;23:487–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halliwell B. Oxidative stress and neurodegeneration: Where are we now? J Neurochem 2006;97:1634–1658 [DOI] [PubMed] [Google Scholar]
- 4.Macready AL, Kennedy OB, Ellis JA, Williams CM, Spencer JP, Butler LT. Flavonoids and cognitive function: A review of human randomized controlled trial studies and recommendations for future studies. Genes Nutr 2009;4:227–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol 2011;10:819–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKay DL, Blumberg JB. The role of tea in human health: An update. J Am Coll Nutr 2002;21:1–13 [DOI] [PubMed] [Google Scholar]
- 7.Wei H, Li L, Song Q, Ai H, Chu J, Li W. Behavioural study of the d-galactose induced aging model in C57BL/6J mice. Behav Brain Res 2005;157:245–251 [DOI] [PubMed] [Google Scholar]
- 8.Lu J, Wu DM, Zheng YL, Hu B, Zhang ZF, Ye Q, Liu CM, Shan Q, Wang YJ. Ursolic acid attenuates D-galactose-induced inflammatory response in mouse prefrontal cortex through inhibiting AGEs/RAGE/NF-kappaB pathway activation. Cereb Cortex 2010;20:2540–2548 [DOI] [PubMed] [Google Scholar]
- 9.Nam SM, Choi JH, Yoo DY, Kim W, Jung HY, Kim JW, Yoo M, Lee S, Kim CJ, Yoon YS, Hwang IK. Effects of curcumin (Curcuma longa) on learning and spatial memory as well as cell proliferation and neuroblast differentiation in adult and aged mice by upregulating brain-derived neurotrophic factor and CREB signaling. J Med Food 2014;17:641–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen CF, Lang SY, Zuo PP, Yang N, Wang XQ, Xia C. Effects of d-galactose on the expression of hippocampal peripheral-type benzodiazepine receptor and spatial memory performances in rats. Psychoneuroendocrinology 2006;31:805–811 [DOI] [PubMed] [Google Scholar]
- 11.Cui X, Zuo P, Zhang Q, Li X, Hu Y, Long J, Packer L, Liu J. Chronic systemic d-galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice: Protective effects of R-alpha-lipoic acid. J Neurosci Res 2006;83:1584–1590 [DOI] [PubMed] [Google Scholar]
- 12.Kumar A, Prakash A, Dogra S. Naringin alleviates cognitive impairment, mitochondrial dysfunction and oxidative stress induced by d-galactose in mice. Food Chem Toxicol 2010;48:626–632 [DOI] [PubMed] [Google Scholar]
- 13.Kumar A, Prakash A, Dogra S. Centella asiatica attenuates d-galactose-induced cognitive impairment, oxidative and mitochondrial dysfunction in mice. Int J Alzheimers Dis 2011;2011:347569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W, Li S, Dong HP, Lv S, Tang YY. Differential impairment of spatial and nonspatial cognition in a mouse model of brain aging. Life Sci 2009;85:127–135 [DOI] [PubMed] [Google Scholar]
- 15.Hao L, Huang H, Gao J, Marshall C, Chen Y, Xiao M. The influence of gender, age and treatment time on brain oxidative stress and memory impairment induced by d-galactose in mice. Neurosci Lett 2014;571:45–49 [DOI] [PubMed] [Google Scholar]
- 16.Yanar K, Aydin S, Cakatay U, Mengi M, Buyukpinarbasili N, Atukeren P, Sitar ME, Sonmez A, Uslu E. Protein and DNA oxidation in different anatomic regions of rat brain in a mimetic ageing model. Basic Clin Pharmacol Toxicol 2011;109:423–433 [DOI] [PubMed] [Google Scholar]
- 17.Cebe T, Atukeren P, Yanar K, Kuruc AI, Ozan T, Kunbaz A, Sitar ME, Mirmaroufizibandeh R, Aydin S, Cakatay U. Oxidation scrutiny in persuaded aging and chronological aging at systemic redox homeostasis level. Exp Gerontol 2014;57:132–140 [DOI] [PubMed] [Google Scholar]
- 18.Parameshwaran K, Irwin MH, Steliou K, Pinkert CA. d-galactoseeffectiveness in modeling aging and therapeutic antioxidant treatment in mice. Rejuvenation Res 2010;13:729–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salkovic-Petrisic M, Osmanovic-Barilar J, Knezovic A, Hoyer S, Mosetter K, Reutter W. Long-term oral galactose treatment prevents cognitive deficits in male Wistar rats treated intracerebroventricularly with streptozotocin. Neuropharmacology 2014;77:68–80 [DOI] [PubMed] [Google Scholar]
- 20.Tikhonova MA, Yu CH, Kolosova NG, Gerlinskaya LA, Maslennikova SO, Yudina AV, Amstislavskaya TG, Ho YJ. Comparison of behavioral and biochemical deficits in rats with hereditary defined or d-galactose-induced accelerated senescence: Evaluating the protective effects of diosgenin. Pharmacol Biochem Behav 2014;120:7–16 [DOI] [PubMed] [Google Scholar]
- 21.Rodrigues J, Assuncão M, Lukoyanov N, Cardoso A, Carvalho F, Andrade JP. Protective effects of a catechin-rich extract on the hippocampal formation and spatial memory in aging rats. Behav Brain Res 2013;246:94–102 [DOI] [PubMed] [Google Scholar]
- 22.Yanar K, Atukeren P, Cebe T, Kunbaz A, Tuna O, Kansu AD, Durmaz S, Gulec V, Belce A, Aydin S, Cakatay U, Rizvi SI. Ameliorative effects of testosterone administration on renal redox homeostasis in naturally aged rats. Rejuvenation Res 2015;18:299–312 [DOI] [PubMed] [Google Scholar]
- 23.Zhu J, Mu X, Zeng J, Xu C, Liu J, Zhang M, Li C, Chen J, Li T, Wang Y. Ginsenoside Rg1 prevents cognitive impairment and hippocampus senescence in a rat model of d-galactose-induced aging. PLoS One 2014; 9:e101291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner PV, Pekow C, Vasbinder MA, Brabb T. Administration of substances to laboratory animals: Equipment considerations, vehicle selection, and solute preparation. J Am Assoc Lab Anim Sci 2011; 50:614–627 [PMC free article] [PubMed] [Google Scholar]
- 25.Yoo KY, Choi JH, Hwang IK, Lee CH, Lee SO, Han SM, Shin HC, Kang IJ, Won MH. (−)-Epigallocatechin-3-gallate increases cell proliferation and neuroblasts in the subgranular zone of the dentate gyrus in adult mice. Phytother Res 2010;24:1065–1070 [DOI] [PubMed] [Google Scholar]
- 26.Vauzour D. Dietary polyphenols as modulators of brain functions: Biological actions and molecular mechanisms underpinning their beneficial effects. Oxid Med Cell Longev 2012;2012:914273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q, Zhao HF, Zhang ZF, Liu ZG, Pei XR, Wang JB, Li Y. Long-term green tea catechin administration prevents spatial learning and memory impairment in senescence-accelerated mouse prone-8 mice by decreasing Abeta1-42 oligomers and upregulating synaptic plasticity-related proteins in the hippocampus. Neuroscience 2009;163:741–749 [DOI] [PubMed] [Google Scholar]
- 28.Assunção M, Santos-Marques MJ, Carvalho F, Andrade JP. Green tea averts age-dependent decline of hippocampal signaling systems related to antioxidant defenses and survival. Free Radic Biol Med 2010; 48:831–838 [DOI] [PubMed] [Google Scholar]
- 29.Cardoso A, Silva D, Magano S, Pereira PA, Andrade JP. Old-onset caloric restriction effects on neuropeptide Y- and somatostatin-containing neurons and on cholinergic varicosities in the rat hippocampal formation. Age (Dordr) 2014;36:9737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gibbons TE, Pence BD, Petr G, Ossyra JM, Mach HC, Bhattacharya TK, Perez S, Martin SA, McCusker RH, Kelley KW, Rhodes JS, Johnson RW, Woods JA. Voluntary wheel running, but not a diet containing (−)-epigallocatechin-3-gallate and beta-alanine, improves learning, memory and hippocampal neurogenesis in aged mice. Behav Brain Res 2014; 272:131–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 1999;2:266–270 [DOI] [PubMed] [Google Scholar]
- 32.Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: Sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol 2002;52:135–143 [DOI] [PubMed] [Google Scholar]
- 33.Rothman SM, Griffioen KJ, Wan R, Mattson MP. Brain-derived neurotrophic factor as a regulator of systemic and brain energy metabolism and cardiovascular health. Ann NY Acad Sci 2012;1264:49–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Intlekofer KA, Cotman CW. Exercise counteracts declining hippocampal function in aging and Alzheimer's disease. Neurobiol Dis 2013;57:47–55 [DOI] [PubMed] [Google Scholar]
- 35.van Praag H, Lucero MJ, Yeo GW, Stecker K, Heivand N, Zhao C, Yip E, Afanador M, Schroeter H, Hammerstone J, Gage FH. Plant-derived flavanol (−)-epicatechin enhances angiogenesis and retention of spatial memory in mice. J Neurosci 2007;27:5869–5878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy T, Dias GP, Thuret S. Effects of diet on brain plasticity in animal and human studies: Mind the gap. Neural Plast 2014;2014:563160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murase T, Haramizu S, Ota N, Hase T. Tea catechin ingestion combined with habitual exercise suppresses the aging-associated decline in physical performance in senescence-accelerated mice. Am J Physiol Regul Integr Comp Physiol 2008;295:R281–R289 [DOI] [PubMed] [Google Scholar]
- 38.Higdon JV, Frei B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr 2003;43:89–143 [DOI] [PubMed] [Google Scholar]
- 39.Mähler A MS, Lorenz M, Ruegg U, Wanker EE, Boschmann M, Paul F. Epigallocatechin-3-gallate: A useful, effective and safe clinical approach for targeted prevention and individualised treatment of neurological diseases? EPMA J 2013;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slomianka L, West MJ. Asymmetry in the hippocampal region specific for one of two closely related species of wild mice. Brain Res 1987;436:69–75 [DOI] [PubMed] [Google Scholar]
- 41.Cardoso A, Castro JP, Pereira PA, Andrade JP. Prolonged protein deprivation, but not food restriction, affects parvalbumin-containing interneurons in the dentate gyrus of adult rats. Brain Res 2013;1522:22–30 [DOI] [PubMed] [Google Scholar]
- 42.Hipólito-Reis J, Pereira PA, Andrade JP, Cardoso A. Prolonged protein deprivation differentially affects calretinin- and parvalbumin-containing interneurons in the hippocampal dentate gyrus of adult rats. Neurosci Lett 2013;555:154–158 [DOI] [PubMed] [Google Scholar]
- 43.Cardoso A, Paula-Barbosa MM, Lukoyanov NV. Reduced density of neuropeptide Y neurons in the somatosensory cortex of old male and female rats: Relation to cholinergic depletion and recovery after nerve growth factor treatment. Neuroscience 2006;137:937–948 [DOI] [PubMed] [Google Scholar]
- 44.Cardoso A, Freitas-da-Costa P, Carvalho LS, Lukoyanov NV. Seizure-induced changes in neuropeptide Y-containing cortical neurons: Potential role for seizure threshold and epileptogenesis. Epilepsy Behav 2010;19:559–567 [DOI] [PubMed] [Google Scholar]
- 45.West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec 1991;231:482–497 [DOI] [PubMed] [Google Scholar]
- 46.McClain JA, Hayes DM, Morris SA, Nixon K. Adolescent binge alcohol exposure alters hippocampal progenitor cell proliferation in rats: Effects on cell cycle kinetics. J Comp Neurol 2011;519:2697–2710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amaral DG, Witter MP. The hippocampal formation. In: Paxinos G, ed. The Rat Nervous System, 2nd ed. Academic Press, San Diego,v1995, pp. 443–493 [Google Scholar]
- 48.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, 4th ed. Academic Press, San Diego, 1998 [Google Scholar]
- 49.Gundersen HJ, Jensen EB, Kiêu K, Nielsen J. The efficiency of systematic sampling in stereology-reconsidered. J Microsc 1999;193:199–211 [DOI] [PubMed] [Google Scholar]
- 50.Hsieh HM, Wu WM, Hu ML. Genistein attenuates d-galactose-induced oxidative damage through decreased reactive oxygen species and NF-kappaB binding activity in neuronal PC12 cells. Life Sci 2011;88:82–88 [DOI] [PubMed] [Google Scholar]
- 51.Tian Y, Zou B, Yang L, Xu SF, Yang J, Yao P, Li CM. High molecular weight persimmon tannin ameliorates cognition deficits and attenuates oxidative damage in senescent mice induced by d-galactose. Food Chem Toxicol 2011;49:1728–1736 [DOI] [PubMed] [Google Scholar]
- 52.Wang D, Liu M, Cao J, Cheng Y, Zhuo C, Xu H, Tian S, Zhang Y, Zhang J, Wang F. Effect of Colla corii asini (E'jiao) on d-galactose induced aging mice. Biol Pharm Bull 2012;35:2128–2132 [DOI] [PubMed] [Google Scholar]
- 53.Chen B, Zhong Y, Peng W, Sun Y, Kong WJ. Age-related changes in the central auditory system: Comparison of d-galactose-induced aging rats and naturally aging rats. Brain Res 2010;1344:43–53 [DOI] [PubMed] [Google Scholar]
- 54.Lu J, Zheng YL, Luo L, Wu DM, Sun DX, Feng YJ. Quercetin reverses d-galactose induced neurotoxicity in mouse brain. Behav Brain Res 2006;171:251–260 [DOI] [PubMed] [Google Scholar]
- 55.Yoo DY, Kim W, Lee CH, Shin BN, Nam SM, Choi JH, Won MH, Yoon YS, Hwang IK. Melatonin improves d-galactose-induced aging effects on behavior, neurogenesis, and lipid peroxidation in the mouse dentate gyrus via increasing pCREB expression. J Pineal Res 2012;52:21–28 [DOI] [PubMed] [Google Scholar]
- 56.Gu X, Zhou Y, Hu X, Gu Q, Wu X, Cao M, Ke K, Liu C. Reduced numbers of cortical GABA-immunoreactive neurons in the chronic d-galactose treatment model of brain aging. Neurosci Lett 2013;549:82–86 [DOI] [PubMed] [Google Scholar]
- 57.Mandyam CD, Harburg GC, Eisch AJ. Determination of key aspects of precursor cell proliferation, cell cycle length and kinetics in the adult mouse subgranular zone. Neuroscience 2007;146:108–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morris SA, Eaves DW, Smith AR, Nixon K. Alcohol inhibition of neurogenesis: A mechanism of hippocampal neurodegeneration in an adolescent alcohol abuse model. Hippocampus 2010;20:596–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bhattacharya TK, Pence BD, Ossyra JM, Gibbons TE, Perez S, McCusker RH, Kelley KW, Johnson RW, Woods JA, Rhodes JS. Exercise but not (−)-epigallocatechin-3-gallate or beta-alanine enhances physical fitness, brain plasticity, and behavioral performance in mice. Physiol Behav 2015;145:29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Himi KB, Hashimoto M, Katakura M, Haque AM, Hara Y, Shido O. Long-term administration of green tea catechins increases antioxidative actions and enhances neurogenesis in the hippocampus of rats. Curr Top Nutraceutical Res 2009;7:131–140 [Google Scholar]
- 61.Wang Y, Li M, Xu X, Song M, Tao H, Bai Y. Green tea epigallocatechin-3-gallate (EGCG) promotes neural progenitor cell proliferation and sonic hedgehog pathway activation during adult hippocampal neurogenesis. Mol Nutr Food Res 2012;56:1292–1303 [DOI] [PubMed] [Google Scholar]
- 62.Huang Y, Su Z, Li Y, Zhang Q, Cui L, Su Y, Ding C, Zhang M, Feng C, Tan Y, Feng W, Li X, Cai L. Expression and purification of glutathione transferase-small ubiquitin-related modifier-metallothionein fusion protein and its neuronal and hepatic protection against d-galactose-induced oxidative damage in mouse model. J Pharmacol Exp Ther 2009;329:469–478 [DOI] [PubMed] [Google Scholar]