Abstract
Psychiatric disorders can affect our ability to successfully and enjoyably interact with others. Conversely, having difficulties in social relations is known to increase the risk of developing a psychiatric disorder. In this article, the assumption that psychiatric disorders can be construed as disorders of social interaction is reviewed from a clinical point of view. Furthermore, it is argued that a psychiatrically motivated focus on the dynamics of social interaction may help to provide new perspectives for the field of social neuroscience. Such progress may be crucial to realize social neuroscience's translational potential and to advance the transdiagnostic investigation of the neurobiology of psychiatric disorders.
Keywords: social interaction, neural mechanisms, psychiatric disorders
1. Introduction: psychiatric disorders as disorders of social interaction rather than social observation
Several—if not all—psychiatric disorders can lead to or are characterized by impairments in social functioning, which serve as an important diagnostic criterion in clinical psychiatry. Having (or having had) difficulties in social interactions, however, is also known to increase the risk of developing a psychiatric disorder (e.g. [1]), thereby pointing towards a reciprocal relationship between social abilities and (mental) health [2–5]. Not having or being able to make use of social resources can lead to a perpetuation or worsening of clinical symptoms [6,7].
An important question for research into social impairments associated with psychiatric disorders has been to ask whether these difficulties are actually subserved by disorder-specific or disorder-general mechanisms. In order to tackle this, a prominent and very productive suggestion has been to reconstruct social difficulties observed in psychiatric disorders as disorders of social cognition (e.g. [8,9]). Social cognition is often broadly defined as the processes by which people make sense of people [10], or processing that is elicited by, about and directed towards other people [11]. More specifically, significant research efforts have gone into understanding the human capacity to understand the mental states of others and how those may help to explain and predict behaviour, often termed having a ‘theory of mind’ [12,13]. This approach has been quite fruitful and has helped, for example, to investigate the neural bases of ‘Theory of Mind’ and its alterations across disorder groups (e.g. [14,15]). In spite of this success, many open questions remain. For instance, it is unclear to what extent and under which circumstances we really rely on an explicit ‘Theory of Mind’ and how helpful this actually is in order to have successful and enjoyable social interactions. Consequently, the underlying conceptual approach has recently been criticized for suggesting that making sense of other people is mainly a matter of detached observation, during which we need to make use of limited information present in others' behaviour in order to infer the hidden, underlying psychological states. By means of observation, we can inform a ‘third-person perspective’ of what it must be like for her (or him) to be in a certain situation [16]. Alternatively, it has been suggested that we may bridge the gap between self and other by projecting our own mental states onto another person, thereby having a ‘first-person grasp’ of what it must be like for her/him (e.g. [17]).
In contrast to these spectatorial accounts of social cognition, which suggest that understanding others is primarily a question of observing, alternatives have been proposed, which emphasize the importance of various aspects of social interactions for social understanding (e.g. [18,19]). These accounts—sometimes contrastively described as the ‘second-person’ approach to other minds—ask whether social cognition from an observer's point of view is really the most pervasive way of knowing other minds and suggest that social cognition may be fundamentally different when we are actively engaged with others in ongoing social interaction, i.e. when we engage in social cognition from an interactor's point of view [3,4]. In such cases of active participation in social interaction, automatically coordinating one's own behaviour with that of the other and being emotionally responsive could complement and/or decrease the need for more cognitive ways of understanding, while the absence of such responses could make social cognition a more effortful process (e.g. [20]). At this point, it is important to point out that a second-person account is not incompatible with first- and third-person accounts, but rather asks how these accounts differ and how they interact with each other (see [3,4] for details). Consequently, a second-person account seeks to systematically investigate the conditions under which individuals tend to use different strategies to understand conspecifics. In this respect, two constituents of second-person engagements have been be distinguished. Firstly, it has been argued [3,4] that awareness of other minds relies on an emotional responsiveness to another person's states or actions as compared to a detached observer's attitude, which does not include such responding. Such emotional responses are assumed to influence action control by a modulation of sensorimotor integration, which, in turn, can solicit activity and observable behaviour (e.g. [21]). Secondly, it has been suggested humans may have an intrinsic motivation to initiated social interaction and engage in the reciprocal relations they entail [22], which may explain why during social interactions perception of the environment appears to be automatically biased towards processing the resources held collectively by both interactors rather than those held by each individual alone [23–25]. Furthermore, social interactions involve a certain historicity, i.e. how one has interacted in the past, which suggests that social phenomena should be understood and investigated in terms of developmental trajectories across different scales of temporal resolution [3,4,26].
As part of the reconstruction of psychiatric disorders as disorders of social cognition, many proponents of such an approach have relied on a characterization of mental disorders being related to disturbances of the ability to consciously think about the mental states of others. In this regard, different patient groups have been thought to fall onto different positions on a spectrum of such mentalizing difficulties (hypo- versus hypermentalizing; cf. [8]). While this approach has been a powerful driving force for research activities, recent evidence demonstrates that important dissociations exist between implicit and explicit levels of social cognition in psychiatric disorders, such that patients may be capable of explicit mental state attribution but show deficits on a more automatic level of processing (e.g. [4,27,28]). But even in cases where surprisingly normal performance on more implicit measures of social cognition has been observed in patients in a laboratory setting (e.g. [29]), this can be completely unrelated to patients' profound impairments in everyday-life social interaction, thereby pointing towards the need for the development of measures with greater ecological validity [30].
Consequently and in contrast to accounts that view psychiatric disorders as disorders of social cognition or social observation, here the idea is advanced that psychiatric disorders constitute in fundamental ways disorders of social interaction. One way to unpack this suggestion is by making reference to the normative dimension of psychopathology, i.e. the necessity to refer to intersubjectively established conventions when defining mental disorders (e.g. [31]). Apart from this important general idea, a focus on social interaction in psychiatry can also help bring into view the importance of developmental trajectories across the lifespan and how experiences in social interaction strongly shape our abilities to relate to one another and can constitute an important risk factor for mental disease [32]. Furthermore, we can think of psychiatric disorders as having a strong impact on our ability to successfully and enjoyably engage in social interaction, thereby pointing towards a reciprocal relationship between social abilities and mental health. In the following sections, the idea of psychiatric disorders as disorders of social interaction rather than social observation will be reviewed from a clinical point of view for different patient groups, while focusing in particular on reward- and motivation-related aspects of social interactions (see also tables 1 and 2). Subsequently, it will be argued that a psychiatrically motivated focus on social interaction may also help to provide new perspectives for the burgeoning field of social neuroscience, thereby promoting the development of a truly social or second-person neuroscience. By focusing on the specific behavioural processes relevant for everyday-life social interaction, it may be possible to investigate those specific neurobiological components that contribute to interaction abilities. Such progress could, therefore, be crucial to realize social neuroscience's translational potential and may help to advance the transdiagnostic investigation of those neurofunctional systems that underlie psychiatric disorders.
Table 1.
Putative neural responses in vSTR across different types of gaze-based social interaction and groups. Cued IA: context of the interaction makes responses of an interaction partner highly predictable. Free IA: context of the interaction does not make responses of an interaction partner predictable. Plus and minus signs are meant to describe interactions, in which the interaction partner responds either in a congruent (+) or incongruent (−) manner.
| interaction types |
||||
|---|---|---|---|---|
| groups | cued IA+ | cued IA− | free IA+ | free IA− |
| neurotypicals | + | − | ++ | − |
| autism | ++ | ++ | −−− | + |
| depression | + | − | − − | − − |
| substance use | − | − | + | − − |
| social anxiety | + | − | − | − − |
| personality disorders | ++ | − − | +++ | −−− |
Table 2.
Putative functional connectivity of vSTR and dorsolateral prefrontal cortex (DLFPC) across different types of gaze-based social interaction and groups.
| interaction types |
||||
|---|---|---|---|---|
| groups | cued IA+ | cued IA− | free IA+ | free IA− |
| neurotypicals | + | ++ | − − | ++ |
| autism | ++ | −−− | +++ | + |
| depression | + | − − | − | − |
| substance use | − − | − | − − | − |
| social anxiety | − | − | ++ | ++ |
| personality disorders | − − | +++ | + | + |
(a). The case of high-functioning autism
Autism spectrum disorders are neurodevelopmental disorders with an estimated global prevalence of approximately 17/10 000, a substantial genetic component and qualitative impairments of social interaction and communication [33]. Autism spectrum disorders are characterized by persistent deficits in social communication and social interaction across multiple contexts and restricted, repetitive patterns of behaviour, interests or activities (DSM5). The impairments of social interaction and communication can only reliably be diagnosed at the age of 2 (cf. [34]), but appear to be preceded by other indicators of social disability in infancy, such as a decline in eye fixations (e.g. [35,36]). So-called high-functioning autism is defined by the clinical triad described above, in the absence of intellectual impairments. Interestingly, individuals with high-functioning autism are not always diagnosed in childhood and adolescence. This might be due to compensatory strategies these individuals can develop, because of their intact intellectual capacities. Often individuals with high-functioning autism are diagnosed in early adulthood when the core autistic symptoms prevent successful professional activities and other forms of social integration.
With regard to autism, a long-standing and prominent suggestion has been that the characteristic social deficits of autism can be construed as a failure of a ‘theory of mind’, i.e. a specific inability to take into account the mental states of others. Interestingly, many individuals with high-functioning autism, however, are actually able to do exactly this in the form of explicit mental state attribution (cf. [27]) and may, in fact, spend much more time thinking about other people's mental states than most non-autistic persons, because they try to make use of this ability in an attempt to compensate for their inability to engage in social interactions. Also, it has been shown that when individuals with high-functioning autism are prompted to use explicit mental state attribution, not only can they do this, but this leads to a recruitment of similar brain regions as in neurotypicals [37]. This emphasizes further that what is characteristically different in individuals with autism is a lack of spontaneous mentalizing and/or behavioural adaptation and that in spite of an intact ability to consciously reflect upon others' mental states, patients still have dramatic difficulties in real-time social interactions.
In light of the discrepancy between intact ‘mind-reading’ abilities and an inability to successfully participate in everyday-life social interactions, it has been suggested that social impairments in autism—rather than being a result of deficits in ‘theory of mind’—could be related to an inability to automatically and quickly integrate social cues when generating actions oneself (e.g. [3,4,25]). Indeed, patients with autism describe that direct social interactions are difficult, because of their immediacy and the necessity to respond quickly, while more structured interactions (e.g. via email or text messaging) or situations of social observation, which require no active engagement at all, are experienced as much less stressful. These clinical insights are consistent with recent data that show intact action perception in autism during observation [29], but an absence of automatic behavioural adaptation when social stimuli are directed towards patients (e.g. [25]). The exact nature and interaction of the subpersonal processes that underlie this observable lack of social responsivity in autism in direct interaction are, however, still a matter of controversy.
A currently prominent theoretical suggestion states that the autistic spectrum might be characterized by deficits of predictive coding or Bayesian inference [38,39], i.e. alterations of the brain's inference on the hidden causes of socially relevant sensory signals. Furthermore, predictive coding formulations of perception propose that expectations in higher brain areas generate top-down predictions that meet bottom-up stimulus-related signals from lower sensory areas. The discrepancy between actual sensory input and predictions of that input is described as a prediction error. With regard to autism, it has been proposed that autistic traits might be related to higher sensory precision, i.e. a stronger reliance on (bottom-up) sensory evidence as compared to (top-down) prior beliefs, which can lead to a failure of contextualizing sensory information in a socially adequate fashion [40]. Furthermore, the reliance on prior beliefs might be particularly important and relevant in situations of high uncertainty such as real-time social interactions with others. Again, this proposition resonates with clinical descriptions of patients with autism having a particular dislike for situations of direct social interaction with others, whereas situations of social observation (when other agents are merely being observed) are described as less difficult [3,4]. Other accounts that have recently been linked to putative alterations of predictive coding in autism are ‘weak central coherence’, which considers autism as a different, detailed-oriented cognitive style [41,42]. More precisely, it claims that people with an autism spectrum disorder tend have difficulties in perceiving information in context, because of difficulties in predicting visual information based on neighbouring and historical information [43]. Also executive dysfunction is observed in autism [44] and could also be related to underlying deficits of predictive coding ([45]; for a review of different cognitive theories of autism and their putative relationship to predictive coding, see [46]).
Another important theoretical account of autism suggests that autistic symptomatology and social impairments might be related to deficits in social reward processing [47]. Importantly, this ‘social motivation theory of autism’ assumes that deficits in social cognition are preceded by and secondary to a diminished social motivation. The emphasis on social reward processing resonates with the here described account of understanding autism (and other psychiatric disorders) as disturbances of social interaction, because human beings are known to be intrinsically motivated to engage in social interactions [22] and different psychiatric disturbances are likely to have an effect on this ([3,4,48]; see tables 1 and 2). So far, however, many studies have focused on extrinsic forms of reward (money, food, etc.), because the rewarding aspects of social interaction have been difficult to investigate (e.g. [49]). Here, the use of ecologically valid, gaze-based social interactions opens a new avenue for neuroimaging research in psychiatry [49], and could help to investigate how contextual cues make an otherwise non-rewarding social interaction more interesting for individuals with autism.
In this respect, and somewhat contrary to the above-described theory of social motivation, it is important to point out that individuals with high-functioning autism appear to develop some level of social motivation. Patients may, for instance, be prepared to establish and engage in social contacts when those can be maintained in a way that is compatible with their own communication requirements: e.g. it has been demonstrated that individuals with autism prefer verbal rather than non-verbal communication [50] and are often keen on using mediated forms of communication, which allow for a structured and sequential exchange of information. Most interestingly, high-functioning individuals with autism describe that social interactions with other autistic individuals tend to be less effortful and more efficient compared to interactions with non-autistic persons. This level of success during within-group interactions does contrast with a general notion of interaction impairments—as suggested by the psychiatric classifications—and could be taken to suggest that greater similarities in communication and interaction styles exist within the patient as compared to non-patient groups. Future research could address similarities and differences in communication and interaction across patient and control groups and investigate how those might be related to similarities and differences in measures of functional and anatomical brain connectivity across autistic brains and non-autistic brains.
(b). The case of personality disorders
Impairments of interpersonal functioning are also central to personality disorders (e.g. [51]). This insight has been explicitly translated into the model of personality disorder as part of the latest version of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5, Section III), which views impairments of interpersonal functioning as a primary manifestation of an impairment of personality functioning. In contrast to neurodevelopmental disorders, differences in interaction styles and impairments of interpersonal functioning associated with personality disorders present later in life after a period of relatively normal social functioning. Nonetheless, personality disorders are thought to be characterized by alterations of developmental trajectories during which relevant experiences (or a lack thereof) made in social interactions may bring about maladaptive patterns of thought and behaviour, which can constrain and increasingly limit flexibility in social interactions later in life [52]. This, in turn, negatively affects experiences of social interaction in individuals with personality disorders and can even lead to derailments thereof. In fact, the clinical presentation of personality disorders is often dominated by severe interpersonal dysfunctions, which constitute an important discriminator for diagnosis. Furthermore, chronic, personality-related difficulties in social interaction can also lead to other forms of transiently present psychopathology (such as states of depression) as evidenced by high rates of psychiatric co-morbidity in personality disorders (e.g. [53]).
While research in the past has attempted to explain interpersonal impairments in personality disorders most often in terms of individual deficits emotional dysregulation, behavioural dyscontrol, and impaired social cognition, the interactionist account of psychiatry advocated here suggests an emphasis on how these individual capacities are tightly interwoven and interdependent with the process of social interaction (cf. [54–56]). This development appears essential as patients often show normal performance in non-interactive social cognitive tasks (cf. [4,27,52]). Therefore, the use of more complex and ecologically valid paradigms is likely to help tap into those processes that underlie social dysfunction in everyday-life social interactions. With regard to emotional regulation, for instance, it can be argued that emotion regulation in ontogeny is primarily an interpersonally constituted process, which may rely on parental abilities of social buffering [57]. Only later in life do children fully develop the capacity to independently regulate their own emotions. Even in adulthood, however, emotions are often regulated by family members, friends and—when emotions become a burden to our health—by professional therapists [58,59]. Consistently, effective psychotherapies for personality disorders include interventions to ameliorate psychopathology by addressing interpersonal difficulties [60,61].
On the interactive view, personality-related differences in interpersonal expectations are likely to have an impact on how rewarding social interactions are experienced and how individuals react to signals of social support and exclusion ([62]; see tables 1 and 2 for suggestions about the underlying neurobiology). In this respect, deficits in empathic abilities are likely to be also relevant and could interact with lower level, reward-based processes. Recent studies have, indeed, produced evidence for personality-associated differences in reward processing (e.g. [62–66]), but this has been restricted to the use of stimuli relatively poor in ecological validity, which has limited the possibility of translating these insights into therapeutic options. In light of the tight association of impairments in personality functioning and impairments of social interaction, future research could, therefore, focus on the study of interaction-based social rewards to search for disorder-specific differences and how those interact with social cognitive abilities, but could also be used to characterize this interplay in a transdiagnostic manner.
(c). The case of depression
Depression is a highly prevalent mental disorder that is characterized by low mood, anhedonia and decreased activity. Furthermore, depression is associated with abnormally increased, self-defeating thoughts and self-referential concerns, which are known to contribute to dysfunctional interpersonal expectations and thereby make successful participation in social interaction difficult [67]. In particular, individuals with depression lose the sense of having an impact on other people, while, in fact, they do. Since social interactions are normally experienced as intrinsically rewarding [22], unsuccessful or reduced social interactions can further contribute to depressive symptomatology and its prolongation [68]. But as in all cases of psychiatric disorders, depressive symptomatology will also affect the interaction partners of patients: from a clinical perspective, it is well recognized that depressive symptoms in a person are likely to trigger support and encouragement from others to which the depressed individual cannot respond adequately. This can, in turn, lead to even greater efforts by others. When those also fail to have an effect, social support is often withdrawn, which may also be accompanied by sentiments of anger and frustration. This development often further accentuates feelings of low self-esteem and self-defeating thoughts in the depressed individual. Breaking this cycle of negative reinforcement by providing information about the disorder and how to treat it to both patients and families constitutes an important therapeutic intervention.
Apart from alterations of social interaction during the acute illness, it has been recognized that the absence of social support and/or negative interpersonal experiences throughout the lifespan constitute an important risk factor for the development of depression and have, thus, become a key target of psychotherapeutic interventions [69,70]. Apart from being intrinsically rewarding and motivating, social interactions (or their absence) can also constitute an important stressor [71,72], which may be particularly true for individuals for whom positive reinforcement in social interactions is very important and/or for individuals who react strongly to signs of social rejection or inclusion [73,74]. Indeed, neuroimaging studies have found reduced brain activations in reward-related neurocircuitry during depressive episodes ([75–77]; see tables 1 and 2), but social interaction-based rewards have not yet been systematically studied.
Furthermore, recent studies have produced evidence for an emerging ‘hyperconnectivity’ hypothesis of depression, which suggests that higher connectivity is present in individuals with depression as compared to healthy individuals and that normalization of these aberrant patterns of connectivity correlates with reductions of clinical symptoms [78]. Interestingly, the areas of hyperconnectivity in depression overlap largely with areas of the ‘social’ brain, which has been demonstrated by means of a meta-analytically defined network analysis of resting-state fMRI data in depression [79]. The latter study was based on the idea that depression is characterized by affective symptoms and alterations of self-referential cognition and introspection, which together adversely affect social interaction. Importantly, a control analysis in this study, which investigated a language-related neural network, demonstrates that the observed group differences are specific to the social brain and not manifestations of a more general pathology.
(d). The case of schizophrenia
Schizophrenia (SCZ) is a complex mental disorder whose underlying neurobiology is only incompletely understood. Due to its early peak age of onset, severity of symptoms and associated disability, SCZ is one of the costliest mental disorders in terms of human suffering and economic expenditure (cf. [80]). In terms of psychopathology, SCZ is characterized by delusions and hallucinations (positive symptom dimension), but also alterations of drive and volition (negative symptom dimension), cognitive symptoms and affective dysregulation (cf. [81]). Impairments of the ability to engage successfully in social interactions are also well documented in schizophrenia (e.g. [82–85]). Most interestingly, it has been shown that in interaction with a schizophrenic patient, it is the behaviour of the ‘normal’ interaction partner that changes to compensate for the behaviour of the patient [86].
In terms of the underlying cognitive processes, social impairments in schizophrenia have prominently been related to disturbances of self- and other-related processing (e.g. [87]). Here, patients are thought to attribute more meaning to their social surroundings than usual, reflected in so-called positive symptoms such as delusions and paranoia [8]. Alternatively, social impairments in schizophrenia have been described as being related to a ‘loss of natural evidence’ for being in a world that is implicitly and intersubjectively shared with others. Such a state, it has been argued, can easily lead to alienation and social withdrawal, which may culminate in a psychotic crisis, in which the lost intersubjective meanings are replaced by a private world of delusions [88,89]. The latter aspects have been related to the negative symptom dimension of schizophrenia, which is known to be highly relevant for prognosis and socio-economic outcome [90,91]. Interestingly, social cognitive impairments found are not only in schizophrenia but already during prodromal stages of the illness during which, in particular, alterations of attributional biases can already be detected [92]. Differences in attributional style have been thought to play an important role in the formation and maintenance of persecutory ideations and other positive symptoms [93].
In addition, different lines of research implicate dysfunction of striatal neurocircuitry in schizophrenia, which has been associated with impairments of wanting, liking and learning and could be particularly relevant for social interaction, as it has been shown that successful and enjoyable participation in social interaction significantly draws upon these neurocircuits [22,49]. Mesolimbic dopamine circuitry has been shown to play a role in so-called ‘wanting’ responses in animals and humans, i.e. their motivation for a reward as measured by a willingness to work for it (e.g. [94,95]). Consistently, non-invasive neuroimaging has shown differential effects in the striatum during reward anticipation in healthy controls and a reduction thereof across different stages of schizophrenia (e.g. [96–99]; see tables 1 and 2). As for the ‘liking’ component of reward processing, i.e. the actual self-reported pleasure of reward, activations of the nucleus accumbens have been reported across different reward types (e.g. [22,100]). In patients with schizophrenia, studies suggest that they show blunted responses when receiving reward, while a correlation has also been reported with negative symptoms [101,102].
With regard to learning processes, it has been shown that ventral striatal activity is involved in computing prediction errors, which are believed to drive surprised-based reinforcement learning (cf. [103]). With regard to schizophrenia, it has been suggested that deficits in such predictive signals may actually underlie psychotic symptoms [104]. In the context of a framework that assumes that the brain relies on Bayesian inference to predict the causes of sensory signals, it has been proposed that many disorder-associated abnormalities could be the result of a failure to predict sensory input, thereby rendering all input surprising. Specifically, the main problem may not lie with the prediction of sensory input per se, but in the balance of precision or confidence, which is ascribed to prior beliefs about the state of the world and sensory evidence (cf. [105]). Here, patients with schizophrenia have been shown to be abnormally aware of the sensations associated with voluntary movements. Because of this failure to attenuate sensory feedback associated with the movement, voluntary movements may actually feel like involuntary movements for patients [106,107]. Furthermore, it has been suggested that in patients with schizophrenia, the updating of beliefs about the world is disrupted, such that patients base their conclusions on less evidence than healthy controls [108], that they over-attribute contingency to the actions of other agents and that this is related to an over-active mesolimbic dopamine system [109,110].
With regard to the over-attribution of contingency and/or intentionality to the actions of others, the use of truly interactive experimental paradigms which include a closed loop feedback between the behaviour of patients and (virtual) agents might also be particularly promising. In previous research, Pfeiffer et al. [111] were able to demonstrate that the subjective experience of interpersonal contingencies is tightly linked to certain latencies during which the behaviour of another person is perceived as contingent upon one's own or as belonging to another human being (rather than a computer). These kinds of non-verbal Turing tests could be exploited further to investigate aspects of interpersonal behaviour and cognition across the psychotic spectrum.
(e). The case of substance use disorders
Substance use and dependence disorders are chronically relapsing disorders which are defined by uncontrolled and compulsive drug use, and constitute a major social, legal and public health problem. Despite severe negative consequences including disrupted social relationships, loss of employment, and somatic and psychiatric illnesses, an addicted person's life is often centred around the drug of choice and activities related to it. After cannabis, cocaine is the second most prevalent illegal drug in the United States and Europe with a lifetime prevalence among young adults of 6.3% in Europe (15–34 years) and 13.3% in the United States (18–25 years) (EMCDDA 2012; HHS 2011). Apart from the negative consequences of drug use on social relationships, additional evidence exists to suggest that problems in social relationships may also be a contributing factor to the development of substance use (e.g. [112]).
Social cognition and social support in drug users are vital, as they have been reported to influence both the onset of substance use and treatment success in substance use disorders. Previous research suggests that, for example, cocaine users show only relatively subtle impairments in different facets of social cognition, particularly in emotional empathy, mental perspective taking and emotion recognition in prosody [113]. Furthermore, in money distribution games, cocaine users act more self-servingly and less altruistic than stimulant-naive controls, which is also consistent with clinical descriptions of addiction-related behaviour.
Based on these observations, Preller et al. [114] conducted a study to investigate the impact of cocaine use on interpersonal functioning and its relation to real-life social behaviour. In this study, a gaze-based social interaction task established in our laboratory was used in a multimodal, psychophysiological and neuroimaging study. Here, it was demonstrated that cocaine users process social gaze cues during an ongoing interaction differently from healthy controls and that these impairments are, in fact, related to a reduced activation of the brain's reward system (tables 1 and 2). Other studies have found evidence for dysconnectivity between brain areas underlying reward processing and areas involved in cognitive control, which may represent an additional factor that could explain socially inadequate behaviour (e.g. [115]).
(f). The case of social anxiety disorders
Social anxiety disorder (SAD) or social phobia is a highly prevalent psychiatric disorder (lifetime prevalence of about 12%), the most common anxiety disorder and is characterized by anxiety-related symptoms, such as intense fear in one or more social situations, which cause significant distress and disability. As with other psychiatric disorders, social phobia-related difficulties extend beyond the individual: while it has been stressed that social anxiety may arise from the reactivation of relational knowledge based on negative experiences with significant others, it has also been shown that social anxiety, in turn, impairs interpersonal relationships and that it is, therefore, associated with fewer and more negative social relationships at all stages of life. For instance, shy individuals are seen as less intelligent than non-shy people during initial interactions, even though there is no actual association between social anxiety and intelligence [116]. Apart from negative responses by others' towards individuals with social anxiety, it is a matter of some debate whether interpersonal problems on the side of the patient are primarily related to social skill deficits or constitute self-protective strategies [117]. Irrespective of this question, it is well known that interpersonal processes continue to shape and perpetuate social fears across the lifespan, which has led to the proposition of an interpersonal cycle of social anxiety [118].
In parallel with the conceptual emphasis on intra- as compared to interpersonal aspects of SAD, different therapeutic approaches have been developed. Whereas cognitive approaches focus on the modification of intrapersonal mechanisms, interpersonal approaches focus on the modification of patterns of interpersonal relationships and thereby help to alleviate SAD symptoms. While cognitive therapy has been shown to be more effective in reducing social phobia symptoms, it has been shown in a direct comparison study that both forms of therapy lead to considerable clinical improvements maintained 1 year after treatment [119].
Today, the prediction of treatment outcome in social anxiety is difficult to do on a behavioural basis. Only very few neuroimaging studies exist that have attempted to measure brain activation in patients with SAD in search for biomarkers. While this approach has shown some success for cognitive therapy (e.g. [120]), no attempts have been made so far for interpersonal therapy. Future research could, therefore, turn towards the use of interaction-based fMRI in order to fill this gap and to further investigate the interpersonal dimension of social anxiety and differences in reward-based neurocircuitry and its interaction with the amygdala at the neural level (e.g. [121,122]; tables 1 and 2).
2. Towards a second-person neuroscience: targeting the neural mechanisms of social interaction rather than social observation
The burgeoning field of social neuroscience has come a long way since its inception [123,124] and has helped to characterize the neurobiological bases of social cognition. The neural mechanisms that underlie actual social interactions in real life, however, are less well understood [3,4,125]. An important avenue for future research in social neuroscience, therefore, consists in systematically investigating the neural basis of active participation in real-time social interaction. This endeavour has been described as the development towards a ‘second-person neuroscience’, which promises to provide new insights into how activity in large-scale neural networks, which are considered as the neural substrates of social cognition and constitute the so-called ‘social brain’, is modulated by social interactions ([3,4]; cf. [126,127]; see figure 1 for a depiction of the relevant brain regions).
Figure 1.
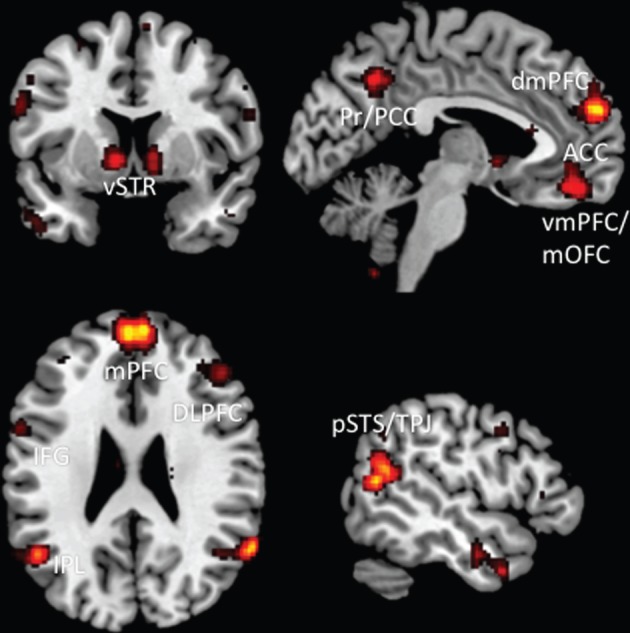
Brain regions involved during social interaction. This figure was created by using the automated meta-analysis functionality provided by NeuroSynth.org and depicts brain regions that are associated with ‘mentalizing’, ‘mirror’, ‘social reward’, ‘intentions' and ‘executive’ (cf. different domains of social cognition as described by Frith & Frith [128]).
Furthermore, this second-person approach suggests that social cognition is fundamentally different when we are in interaction with others rather than merely observing them, thereby pointing towards the importance of experiencing and interacting with others as our primary ways of knowing them [3,4]. On this view, firstly, awareness of other minds is thought to hinge upon emotional engagement and a responsiveness to another person's states or actions as compared to a detached observer's attitude, which does not include such responding (cf. [129]). Here, emotional responses are thought to constitute an important way of perceiving and integrating the state of the other by way of experiencing one's own bodily responses to her (e.g. [21]). Secondly, the process of social interaction is seen as a key constituent of grasping other minds. Social interactions are characterized by reciprocal relations, with the perception of socially relevant information prompting (re-)actions, which are themselves reacted to. In social interaction, we rely upon our practical ‘know-how’ in dealing with others [130]. From this perspective, other accounts of social cognition may overemphasize that social cognition is grounded in explicit inferences about others' minds. In contrast to this, social cognition is thought to occur within and to be motivated by social interaction [3,4].
Recent studies have, indeed, provided evidence that human beings are intrinsically motivated to engage in (gaze-based) social interactions and to coordinate their visual attention with others. Furthermore, engaging in such behaviour activates parts of the ‘reward system’ of the brain (ventral striatum (vSTR)), even in the absence of a common goal or shared intention [22,49]. Interestingly, the vSTR is also closely connected to hubs of other neural networks that are thought to be relevant for social cognition and interaction, such as the so-called ‘mentalizing network’, which comprises medial prefrontal, medial parietal cortex and the temporoparietal junction area (tables 1 and 2 and figure 1). For instance, measures of connectivity between vSTR and medial prefrontal cortex have been related to self-evaluations in comparison to conspecifics [131] and are likely to represent a driving force behind the human motivation to engage in social interaction and to be strongly affected by social comparisons [132]. In recent years, an increasing number of neuroimaging studies have been realized that are targeting the dynamics of social interaction, and these have begun to yield new insights into the neural mechanisms of social behaviour as it unfolds in real life (see [125] for a review).
Taken together, the second-person neuroscience approach suggests that a characterization of the neural networks which allow for active engagement in real-life social encounters has to rely on using ecologically valid and truly interactive paradigms, which can be used to investigate the neural mechanisms of participation in social interaction in the brains of one or both interaction partners. Preliminary results from neuroimaging highlight the involvement of reward-related neurocircuitry in interaction with large-scale neural networks known to subserve social cognition, thereby pointing towards a complex interplay of brain regions that are needed to account for interaction-specific processes.
3. Towards a second-person neuropsychiatry: how a truly social neuroscience can advance the transdiagnostic investigation of the neurobiology of mental illness
Based on the assumption that psychiatric disorders can be reconstructed as disorders of social cognition, attempts have been made to relate social cognitive deficits in patient populations to possible dysfunction of what has become known as the ‘social brain’ [11,133]. While there is great merit to be found in this approach, it should have become clear from the description of psychiatric disorders provided above that this initial formulation may have overemphasized explicit forms of social cognition while neglecting those automatic processes that are most relevant when we actively engage with others during real-time social interactions. Consequently, the knowledge of alterations of neurofunctional systems which are relevant for those specific behavioural processes that are relevant for successful participation in real-life social interaction is still limited, which has impeded translational social neuroscience approaches whose aim it is to understand the neural bases of commonalities and differences in fundamental deficits of interpersonal behaviour associated with different psychiatric disorders (e.g. [9,134]). Advances in this direction could ultimately contribute to the aim of improving therapeutic interventions by means of neuroimaging (e.g. [135]).
The interpersonal dimension of psychopathology has long been recognized in psychiatry, because assessment of individual behaviour and first-person experiences require comparison with an intersubjectively shared consensus and can only take place in communication (e.g. [136,137]). Interestingly, recent developments of the psychiatric diagnostic manuals have begun to re-focus the interpersonal dimension of psychopathology across different diagnostic categories, which parallels—to some extent—the above-described developments within social neuroscience. Also, recently proposed developments of the approach to the scientific study of the neurobiology of mental disorders have focused on transdiagnostic investigations and include social processes as a key target [138].
The approach advocated in this article suggests that making use of the second-person neuroscience toolbox for the investigation of the neurobiology of psychiatric disorders can help to address and study phenomena that are inherently linked to participation in social interaction and may thereby provide more sensitive measures of those social processes that really matter in real-life social interactions. A second-person neuropsychiatry, therefore, promises to interrogate those brain systems that subserve active participation in and understanding of social interactions and may provide novel neuroimaging biomarkers of social interaction-based processes that might be both diagnostically and therapeutically helpful across different disorders. Of note, the second-person approach advocated here draws upon a conceptual framework [3,4] which addresses the importance of being part of a social interaction and the embeddedness in a social environment, but is also open to explore how computational approaches can help to explain the underlying cognitive architecture possessed by individuals in the context of complex social situations [139].
Taking social interaction seriously in psychiatry may mean to consider how social difficulties of patients are not only a result of their own deficits but also tightly linked to interpersonal expectations of putatively normal interaction partners: patients with high-functioning autism, for instance, report that—in spite of their disorder of social interaction and communication—they have very efficient and pleasurable social interactions with other autistic persons. Future research could extend the use of two-person experimental paradigms (e.g. [48,125,140]) for a multimodal investigation of interpersonal matching under more ecologically valid conditions to study how various aspects of neurobiology constrain and interact with external factors to (dis-)allow for interpersonal coordination and communication.
4. Summary and Conclusion
The present review illustrates that a prominent and growing literature exists that reconstructs psychiatric disorders as disturbances of social cognition in an attempt to investigate the neural bases of mental disorders. While this approach has helped to throw some light on the underlying neural substrates and alterations thereof in mental disorders, it is still significantly impeded by a lack of ecological validity. This, in turn, prevents the investigation of those specific behavioural processes and their underlying neural mechanisms that are relevant for everyday-life social interactions. Consequently, a key proposition of this article has been that for this line of research to become clinically and therapeutically relevant, i.e. predictive of real-life social dysfunction and recovery, further steps need to be taken in order to establish experimental paradigms that robustly tap into those biological processes that are fundamental for social interaction. This suggestion is deeply rooted in a clinical viewpoint according to which psychiatric disorders are more commonly characterized by impairments of social interaction rather than social observation. In particular, future research may want to turn towards the study of interaction-based reward processes, as live social interactions are known to be intrinsically rewarding and social isolation and other forms of stress have been recognized as a significant risk factor in the pathogenesis and maintenance of various psychiatric disorders.
Moving towards a second-person neuropsychiatry, therefore, could be seen as an effort that tries to incorporate both biological and psychosocial aspects of mental disease while considering the brain as the interface at which genetic and environmental influences interact to produce those thoughts, perceptions, beliefs and feelings that are relevant for the joys and sorrows that characterize our everyday life.
Acknowledgements
L.S. thanks the Social Neuroscience Group at the Max Planck Institute of Psychiatry for feedback on previous versions of this manuscript. In addition, L.S. gratefully acknowledges Marie-Luise Brandi's support in generating the figure.
Competing interests
I declare I have no competing interests.
Funding
L.S. was supported by the Max Planck Society via a grant for an independent, free-floating Max Planck Research Group.
References
- 1.Fowler JC, Allen JG, Oldham JM, Frueh BC. 2013. Exposure to interpersonal trauma, attachment insecurity, and depression severity. J. Affect. Disord. 149, 313–318. ( 10.1016/j.jad.2013.01.045) [DOI] [PubMed] [Google Scholar]
- 2.Fredman L, Weissman MM, Leaf PJ, Bruce ML. 1988. Social functioning in community residents with depression and other psychiatric disorders: results of the New Haven Epidemiologic Catchment Area Study. J. Affect. Disord. 15, 103–112. ( 10.1016/0165-0327(88)90077-8) [DOI] [PubMed] [Google Scholar]
- 3.Schilbach L, Timmermans B, Reddy V, Costall A, Bente G, Schlicht T, Vogeley K. 2013. Toward a second-person neuroscience. Behav. Brain Sci. 36, 393–414. ( 10.1017/S0140525X12000660) [DOI] [PubMed] [Google Scholar]
- 4.Schneider D, Slaughter VP, Bayliss AP, Dux PE. 2013. A temporally sustained implicit theory of mind deficit in autism spectrum disorders. Cognition 129, 410–417. ( 10.1016/j.cognition.2013.08.004) [DOI] [PubMed] [Google Scholar]
- 5.Coan JA, Sbarra DA. 2015. Social baseline theory: the social regulation of risk and effort. Curr. Opin. Psychol. 1, 87–91. ( 10.1016/j.copsyc.2014.12.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coyne JC. 1976. Depression and the response of others. J. Abnorm. Psychol. 85, 186–193. ( 10.1037/0021-843X.85.2.186) [DOI] [PubMed] [Google Scholar]
- 7.Hawkley LC, Cacioppo JT. 2010. Loneliness matters: a theoretical and empirical review of consequences and mechanisms. Ann. Behav. Med. 40, 218–227. ( 10.1007/s12160-010-9210-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frith CD. 2004. Schizophrenia and theory of mind. Psychol. Med. 34, 385–389. ( 10.1017/S0033291703001326) [DOI] [PubMed] [Google Scholar]
- 9.Crespi B, Badcock C. 2008. Psychosis and autism as diametrical disorders of the social brain. Behav. Brain Sci. 31, 241–261. ( 10.1017/S0140525X08004214) [DOI] [PubMed] [Google Scholar]
- 10.Ochsner KN, Lieberman MD. 2001. The emergence of social cognitive neuroscience. Am. Psychol. 56, 717–734. ( 10.1037/0003-066X.56.9.717) [DOI] [PubMed] [Google Scholar]
- 11.Kennedy DP, Adolphs R. 2012. The social brain in psychiatric and neurological disorders. Trends Cogn. Sci. 16, 559–572. ( 10.1016/j.tics.2012.09.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baron-Cohen S. 1991. The development of a theory of mind in autism: deviance and delay? Psychiatr. Clin. North Am. 14, 33–51. [PubMed] [Google Scholar]
- 13.Frith CD, Frith U. 2008. Implicit and explicit processes in social cognition. Neuron 60, 503–510. ( 10.1016/j.neuron.2008.10.032) [DOI] [PubMed] [Google Scholar]
- 14.Gallagher HL, Jack AI, Roepstorff A, Frith CD. 2002. Imaging the intentional stance in a competitive game. Neuroimage 16, 814–821. ( 10.1006/nimg.2002.1117) [DOI] [PubMed] [Google Scholar]
- 15.Castelli F, Frith C, Happe F, Frith U. 2002. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain 125, 1839–1849. ( 10.1093/brain/awf189) [DOI] [PubMed] [Google Scholar]
- 16.Vogeley K, May M, Ritzl A, Falkai P, Zilles K, Fink GR. 2004. Neural correlates of first-person perspective as one constituent of human self-consciousness. J. Cogn. Neurosci. 16, 817–827. ( 10.1162/089892904970799) [DOI] [PubMed] [Google Scholar]
- 17.Rizzolatti G, Sinigaglia C. 2010. The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nat. Rev. Neurosci. 11, 264–274. ( 10.1038/nrn2805) [DOI] [PubMed] [Google Scholar]
- 18.Gallagher S, Zahavi D. 2008. The phenomenological mind. Abingdon, UK: Taylor & Francis Ltd. [Google Scholar]
- 19.Reddy V. 2008. How infants know minds. Cambridge, MA: Harvard University Press. [Google Scholar]
- 20.Krueger J, Michael J. 2012. Gestural coupling and social cognition: Mobius Syndrome as a case study. Front. Hum. Neurosci. 6, 81 ( 10.3389/fnhum.2012.00081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schilbach L, Eickhoff SB, Mojzisch A, Vogeley K. 2008. What's in a smile? Neural correlates of facial embodiment during social interaction. Soc. Neurosci. 3, 37–50. ( 10.1080/17470910701563228) [DOI] [PubMed] [Google Scholar]
- 22.Schilbach L, Wilms M, Eickhoff SB, Romanzetti S, Tepest R, Bente G, Shah NJ, Fink GR, Vogeley K. 2010. Minds made for sharing: initiating joint attention recruits reward-related neurocircuitry. J. Cogn. Neurosci. 22, 2702–2715. ( 10.1162/jocn.2009.21401) [DOI] [PubMed] [Google Scholar]
- 23.Sebanz N, Bekkering H, Knoblich G. 2006. Joint action: bodies and minds moving together. Trends Cogn. Sci. 10, 70–76. ( 10.1016/j.tics.2005.12.009) [DOI] [PubMed] [Google Scholar]
- 24.Marsh KL, Richardson MJ, Schmidt RC. 2009. Social connection through joint action and interpersonal coordination. Top. Cogn. Sci. 1, 320–339. ( 10.1111/j.1756-8765.2009.01022.x) [DOI] [PubMed] [Google Scholar]
- 25.Schilbach L, Eickhoff SB, Cieslik EC, Kuzmanovic B, Vogeley K. 2012. Shall we do this together? Social gaze influences action control in a comparison group, but not in individuals with high-functioning autism. Autism 16, 151–162. ( 10.1177/1362361311409258) [DOI] [PubMed] [Google Scholar]
- 26.Behrens TE, Hunt LT, Woolrich MW, Rushworth MF. 2008. Associative learning of social value. Nature 456, 245–249. ( 10.1038/nature07538) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Senju A, Southgate V, White S, Frith U. 2009. Mindblind eyes: an absence of spontaneous theory of mind in Asperger syndrome. Science 325, 883–885. ( 10.1126/science.1176170) [DOI] [PubMed] [Google Scholar]
- 28.Callenmark B, Kjellin L, Ronnqvist L, Bolte S. 2014. Explicit versus implicit social cognition testing in autism spectrum disorder. Autism 18, 684–693. ( 10.1177/1362361313492393) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cusack JP, Williams JH, Neri P. 2015. Action perception is intact in autism spectrum disorder. J. Neurosci. 35, 1849–1857. ( 10.1523/JNEUROSCI.4133-13.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henry JD, Cowan DG, Lee T, Sachdev PS. 2015. Recent trends in testing social cognition. Curr. Opin. Psychiatry 28, 133–140. ( 10.1097/YCO.0000000000000139) [DOI] [PubMed] [Google Scholar]
- 31.Schramme T. 2013. On the autonomy of the concept of disease in psychiatry. Front. Psychol. 4, 457 ( 10.3389/fpsyg.2013.00457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korkmaz B. 2011. Theory of mind and neurodevelopmental disorders of childhood. Pediatr. Res. 69, 101R–108R. ( 10.1203/PDR.0b013e318212c177) [DOI] [PubMed] [Google Scholar]
- 33.Elsabbagh M, et al. 2012. Global prevalence of autism and other pervasive developmental disorders. Autism Res. 5, 160–179. ( 10.1002/aur.239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bolte S, Marschik PB, Falck-Ytter T, Charman T, Roeyers H, Elsabbagh M. 2013. Infants at risk for autism: a European perspective on current status, challenges and opportunities. Eur. Child Adolesc. Psychiatry 22, 341–348. ( 10.1007/s00787-012-0368-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baron-Cohen S, Cox A, Baird G, Swettenham J, Nightingale N, Morgan K, Drew A, Charman T. 1996. Psychological markers in the detection of autism in infancy in a large population. Br. J. Psychiatry 168, 158–163. ( 10.1192/bjp.168.2.158) [DOI] [PubMed] [Google Scholar]
- 36.Jones W, Klin A. 2013. Attention to eyes is present but in decline in 2–6-month-old infants later diagnosed with autism. Nature 504, 427–431. ( 10.1038/nature12715) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dufour N, Redcay E, Young L, Mavros PL, Moran JM, Triantafyllou C, Gabrieli JDE, Saxe R. 2013. Similar brain activation during false belief tasks in a large sample of adults with and without autism. PLoS ONE 8, e75468 ( 10.1371/journal.pone.0075468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pellicano E, Burr D. 2012. When the world becomes ‘too real’: a Bayesian explanation of autistic perception. Trends Cogn. Sci. 16, 504–510. ( 10.1016/j.tics.2012.08.009) [DOI] [PubMed] [Google Scholar]
- 39.Sinha P, Kjelgaard MM, Gandhi TK, Tsourides K, Cardinaux AL, Pantazis D, Diamond SP, Held RM. 2014. Autism as a disorder of prediction. Proc. Natl Acad. Sci. USA 111, 15 220–15 225. ( 10.1073/pnas.1416797111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friston KJ, Lawson R, Frith CD. 2013. On hyperpriors and hypopriors: comment on Pellicano and Burr. Trends Cogn. Sci. 17, 1 ( 10.1016/j.tics.2012.11.003) [DOI] [PubMed] [Google Scholar]
- 41.Frith U. 1996. Cognitive explanations of autism. Acta Paediatr. Suppl. 416, 63–68. ( 10.1111/j.1651-2227.1996.tb14280.x) [DOI] [PubMed] [Google Scholar]
- 42.Happe F, Frith U. 2006. The weak coherence account: detail-focused cognitive style in autism spectrum disorders. J. Autism Dev. Disord. 36, 5–25. ( 10.1007/s10803-005-0039-0) [DOI] [PubMed] [Google Scholar]
- 43.Rao RP, Ballard DH. 1999. Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nat. Neurosci. 2, 79–87. ( 10.1038/4580) [DOI] [PubMed] [Google Scholar]
- 44.Ozonoff S, Pennington BF, Rogers SJ. 1991. Executive function deficits in high-functioning autistic individuals: relationship to theory of mind. J. Child Psychol. Psychiatry 32, 1081–1105. ( 10.1111/j.1469-7610.1991.tb00351.x) [DOI] [PubMed] [Google Scholar]
- 45.Kopp B. 2012. A simple hypothesis of executive function. Front. Hum. Neurosci. 6, 159 ( 10.3389/fnhum.2012.00159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bolis D, Stephan KE, Wenderoth N, Balsters J, Becchio C, Schilbach L. In preparation A synthesis of the autism space: toward a pluralistic account grounded in an intersubjective predictive coding framework. [Google Scholar]
- 47.Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. 2012. The social motivation theory of autism. Trends Cogn. Sci. 16, 231–239. ( 10.1016/j.tics.2012.02.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Timmermans B, Schilbach L. 2014. Investigating alterations of social interaction in psychiatric disorders with dual interactive eye tracking and virtual faces. Front. Hum. Neurosci. 8, 758 ( 10.3389/fnhum.2014.00758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pfeiffer UJ, Schilbach L, Timmermans B, Kuzmanovic B, Georgescu AL, Bente G, Vogeley K. 2014. Why we interact: on the functional role of the striatum in the subjective experience of social interaction. Neuroimage 101, 124–137. ( 10.1016/j.neuroimage.2014.06.061) [DOI] [PubMed] [Google Scholar]
- 50.Kuzmanovic B, Bente G, von Cramon DY, Schilbach L, Tittgemeyer M, Vogeley K. 2012. Imaging first impressions: distinct neural processing of verbal and nonverbal social information. Neuroimage 60, 179–188. ( 10.1016/j.neuroimage.2011.12.046) [DOI] [PubMed] [Google Scholar]
- 51.Hopwood CJ, Wright AG, Ansell EB, Pincus AL. 2013. The interpersonal core of personality pathology. J. Pers. Disord. 27, 270–295. ( 10.1521/pedi.2013.27.3.270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jeung H, Herpertz SC. 2014. Impairments of interpersonal functioning: empathy and intimacy in borderline personality disorder. Psychopathology 47, 220–234. ( 10.1159/000357191) [DOI] [PubMed] [Google Scholar]
- 53.Gremaud-Heitz D, Riemenschneider A, Walter M, Sollberger D, Kuchenhoff J, Dammann G. 2014. Comorbid atypical depression in borderline personality disorder is common and correlated with anxiety-related psychopathology. Compr. Psychiatry 55, 650–656. ( 10.1016/j.comppsych.2013.11.021) [DOI] [PubMed] [Google Scholar]
- 54.Zaki J, Williams WC. 2013. Interpersonal emotion regulation. Emotion 13, 803–810. ( 10.1037/a0033839) [DOI] [PubMed] [Google Scholar]
- 55.Thompson RA. 1994. Emotion regulation: a theme in search of definition. Monogr. Soc. Res. Child Dev. 59, 25–52. ( 10.2307/1166137) [DOI] [PubMed] [Google Scholar]
- 56.King-Casas B, Sharp C, Lomax-Bream L, Lohrenz T, Fonagy P, Montague PR. 2008. The rupture and repair of cooperation in borderline personality disorder. Science 321, 806–810. ( 10.1126/science.1156902) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ostfeld-Etzion S, Golan O, Hirschler-Guttenberg Y, Zagoory-Sharon O, Feldman R. 2015. Neuroendocrine and behavioral response to social rupture and repair in preschoolers with autism spectrum disorders interacting with mother and father. Mol. Autism 6, 11 ( 10.1186/s13229-015-0007-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marroquin B. 2011. Interpersonal emotion regulation as a mechanism of social support in depression. Clin. Psychol. Rev. 31, 1276–1290. ( 10.1016/j.cpr.2011.09.005) [DOI] [PubMed] [Google Scholar]
- 59.Eisenberger NI, Cole SW. 2012. Social neuroscience and health: neurophysiological mechanisms linking social ties with physical health. Nat. Neurosci. 15, 669–674. ( 10.1038/nn.3086) [DOI] [PubMed] [Google Scholar]
- 60.Bamelis LL, Evers SM, Spinhoven P, Arntz A. 2014. Results of a multicenter randomized controlled trial of the clinical effectiveness of schema therapy for personality disorders. Am. J. Psychiatry 171, 305–322. ( 10.1176/appi.ajp.2013.12040518) [DOI] [PubMed] [Google Scholar]
- 61.Skewes SA, Samson RA, Simpson SG, van Vreeswijk M. 2014. Short-term group schema therapy for mixed personality disorders: a pilot study. Front. Psychol. 5, 1592 ( 10.3389/fpsyg.2014.01592) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vrticka P, Andersson F, Grandjean D, Sander D, Vuilleumier P. 2008. Individual attachment style modulates human amygdala and striatum activation during social appraisal. PLoS ONE 3, e2868 ( 10.1371/journal.pone.0002868) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Enzi B, Doering S, Faber C, Hinrichs J, Bahmer J, Northoff G. 2013. Reduced deactivation in reward circuitry and midline structures during emotion processing in borderline personality disorder. World J. Biol. Psychiatry 14, 45–56. ( 10.3109/15622975.2011.579162) [DOI] [PubMed] [Google Scholar]
- 64.Glenn AL, Yang Y. 2012. The potential role of the striatum in antisocial behavior and psychopathy. Biol. Psychiatry 72, 817–822. ( 10.1016/j.biopsych.2012.04.027) [DOI] [PubMed] [Google Scholar]
- 65.Vollm B, Richardson P, McKie S, Elliott R, Dolan M, Deakin B. 2007. Neuronal correlates of reward and loss in Cluster B personality disorders: a functional magnetic resonance imaging study. Psychiatry Res. 156, 151–167. ( 10.1016/j.pscychresns.2007.04.008) [DOI] [PubMed] [Google Scholar]
- 66.Vollm B, Richardson P, McKie S, Reniers R, Elliott R, Anderson IM, Williams S, Dolan M, Deakin B. 2010. Neuronal correlates and serotonergic modulation of behavioural inhibition and reward in healthy and antisocial individuals. J. Psychiatr. Res. 44, 123–131. ( 10.1016/j.jpsychires.2009.07.005) [DOI] [PubMed] [Google Scholar]
- 67.Kashdan TB, Roberts JE. 2011. Comorbid social anxiety disorder in clients with depressive disorders: predicting changes in depressive symptoms, therapeutic relationships, and focus of attention in group treatment. Behav. Res. Ther. 49, 875–884. ( 10.1016/j.brat.2011.10.002) [DOI] [PubMed] [Google Scholar]
- 68.Cusi AM, Macqueen GM, Spreng RN, McKinnon MC. 2011. Altered empathic responding in major depressive disorder: relation to symptom severity, illness burden, and psychosocial outcome. Psychiatry Res. 188, 231–236. ( 10.1016/j.psychres.2011.04.013) [DOI] [PubMed] [Google Scholar]
- 69.McCullough JP. 1984. Cognitive-behavioral analysis system of psychotherapy: an interactional treatment approach for dysthymic disorder. Psychiatry 47, 234–250. [DOI] [PubMed] [Google Scholar]
- 70.McCullough JPJ, Lord BD, Martin AM, Conley KA, Schramm E, Klein DN. 2011. The significant other history: an interpersonal-emotional history procedure used with the early-onset chronically depressed patient. Am. J. Psychother. 65, 225–248. [DOI] [PubMed] [Google Scholar]
- 71.Admon R, Holsen LM, Aizley H, Remington A, Whitfield-Gabrieli S, Goldstein JM, Pizzagalli DA. 2015. Striatal hypersensitivity during stress in remitted individuals with recurrent depression. Biol. Psychiatry 78, 67–76. ( 10.1016/j.biopsych.2014.09.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cacioppo JT, Cacioppo S, Capitanio JP, Cole SW. 2015. The neuroendocrinology of social isolation. Annu. Rev. Psychol. 66, 733–767. ( 10.1146/annurev-psych-010814-015240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.DeWall CN, Masten CL, Powell C, Combs D, Schurtz DR, Eisenberger NI. 2012. Do neural responses to rejection depend on attachment style? An fMRI study. Soc. Cogn. Affect. Neurosci. 7, 184–192. ( 10.1093/scan/nsq107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Donges US, et al. 2012. Adult attachment anxiety is associated with enhanced automatic neural response to positive facial expression. Neuroscience 220, 149–157. ( 10.1016/j.neuroscience.2012.06.036) [DOI] [PubMed] [Google Scholar]
- 75.Satterthwaite TD, et al. 2015. Common and dissociable dysfunction of the reward system in bipolar and unipolar depression. Neuropsychopharmacology 40, 2258–2268. ( 10.1038/npp.2015.75) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Redlich R, Dohm K, Grotegerd D, Opel N, Zwitserlood P, Heindel W, Arolt V, Kugel H, Dannlowski U. 2015. Reward processing in unipolar and bipolar depression: a functional MRI study. Neuropsychopharmacology 40, 2623–2631. ( 10.1038/npp.2015.110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hanson JL, Hariri AR, Williamson DE. 2015. Blunted ventral striatum development in adolescence reflects emotional neglect and predicts depressive symptoms. Biol. Psychiatry 78, 598–605. ( 10.1016/j.biopsych.2015.05.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Perrin JS, Merz S, Bennett DM, Currie J, Steele DJ, Reid IC, Schwarzbauer C. 2012. Electroconvulsive therapy reduces frontal cortical connectivity in severe depressive disorder. Proc. Natl Acad. Sci. USA 109, 5464–5468. ( 10.1073/pnas.1117206109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schilbach L, Mulle VI, Hoffstaedter F, Clos M, Goya-Maldonado R, Grude O, Eickhoff SB. 2014. Meta-analytically informed network analysis of resting state FMRI reveals hyperconnectivity in an introspective socio-affective network in depression. PLoS ONE 9, e94973 ( 10.1371/journal.pone.0094973) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van Os J, Kapur S. 2009. Schizophrenia. Lancet 374, 635–645. ( 10.1016/S0140-6736(09)60995-8) [DOI] [PubMed] [Google Scholar]
- 81.Eaton WW, Thara R, Federman B, Melton B, Liang KY. 1995. Structure and course of positive and negative symptoms in schizophrenia. Arch. Gen. Psychiatry 52, 127–134. ( 10.1001/archpsyc.1995.03950140045005) [DOI] [PubMed] [Google Scholar]
- 82.Fiszdon JM, Fanning JR, Johannesen JK, Bell MD. 2013. Social cognitive deficits in schizophrenia and their relationship to clinical and functional status. Psychiatry Res. 205, 25–29. ( 10.1016/j.psychres.2012.08.041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Savla GN, Vella L, Armstrong CC, Penn DL, Twamley EW. 2013. Deficits in domains of social cognition in schizophrenia: a meta-analysis of the empirical evidence. Schizophr. Bull. 39, 979–992. ( 10.1093/schbul/sbs080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee J, Altshuler L, Glahn DC, Miklowitz DJ, Ochsner K, Green MF. 2013. Social and nonsocial cognition in bipolar disorder and schizophrenia: relative levels of impairment. Am. J. Psychiatry 170, 334–341. ( 10.1176/appi.ajp.2012.12040490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Billeke P, Aboitiz F. 2013. Social cognition in schizophrenia: from social stimuli processing to social engagement. Front. Psychiatry 4, 4 ( 10.3389/fpsyt.2013.00004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lavelle M, Healey PG, McCabe R. 2013. Is nonverbal communication disrupted in interactions involving patients with schizophrenia? Schizophr. Bull. 39, 1150–1158. ( 10.1093/schbul/sbs091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Frith CD, Corcoran R. 1996. Exploring ‘theory of mind’ in people with schizophrenia. Psychol. Med. 26, 521–530. ( 10.1017/S0033291700035601) [DOI] [PubMed] [Google Scholar]
- 88.Blankenburg W. 1971. The loss of natural self-evidence: a contribution to the study of sypmtom-poor schizophrenias. Berlin, Germany: Parados. [Google Scholar]
- 89.Klosterkotter J, Hellmich M, Steinmeyer EM, Schultze-Lutter F. 2001. Diagnosing schizophrenia in the initial prodromal phase. Arch. Gen. Psychiatry 58, 158–164. ( 10.1001/archpsyc.58.2.158) [DOI] [PubMed] [Google Scholar]
- 90.Salvatore G, Dimaggio G, Lysaker PH. 2007. An intersubjective perspective on negative symptoms of schizophrenia: implications of simulation theory. Cogn. Neuropsychiatry 12, 144–164. [DOI] [PubMed] [Google Scholar]
- 91.Fulford D, Niendam TA, Floyd EG, Carter CS, Mathalon DH, Vinogradov S, Stuart BK, Loewy RL. 2013. Symptom dimensions and functional impairment in early psychosis: more to the story than just negative symptoms. Schizophr. Res. 147, 125–131. ( 10.1016/j.schres.2013.03.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee TY, Hong SB, Shin NY, Kwon JS. 2015. Social cognitive functioning in prodromal psychosis: a meta-analysis. Schizophr. Res. 164, 28–34. ( 10.1016/j.schres.2015.02.008) [DOI] [PubMed] [Google Scholar]
- 93.Mehl S, et al. 2014. Why do bad things happen to me? Attributional style, depressed mood, and persecutory delusions in patients with schizophrenia. Schizophr. Bull. 40, 1338–1346. ( 10.1093/schbul/sbu040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wardle MC, Treadway MT, Mayo LM, Zald DH, de Wit H. 2011. Amping up effort: effects of d-amphetamine on human effort-based decision-making. J. Neurosci. 31, 16597–16602. ( 10.1523/JNEUROSCI.4387-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Treadway MT, et al. 2012. Dopaminergic mechanisms of individual differences in human effort-based decision-making. J. Neurosci. 32, 6170–6176. ( 10.1523/JNEUROSCI.6459-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Juckel G, Schlagenhauf F, Koslowski M, Wustenberg T, Villringer A, Knutson B, Wrase J, Heinz A. 2006. Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage 29, 409–416. ( 10.1016/j.neuroimage.2005.07.051) [DOI] [PubMed] [Google Scholar]
- 97.Barch DM, Dowd EC. 2010. Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophr. Bull. 36, 919–934. ( 10.1093/schbul/sbq068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Grimm O, Vollstadt-Klein S, Krebs L, Zink M, Smolka MN. 2012. Reduced striatal activation during reward anticipation due to appetite-provoking cues in chronic schizophrenia: a fMRI study. Schizophr. Res. 134, 151–157. ( 10.1016/j.schres.2011.11.027) [DOI] [PubMed] [Google Scholar]
- 99.Grimm O, et al. 2014. Striatal response to reward anticipation: evidence for a systems-level intermediate phenotype for schizophrenia. JAMA Psychiatry 71, 531–539. ( 10.1001/jamapsychiatry.2014.9) [DOI] [PubMed] [Google Scholar]
- 100.Miller EM, Shankar MU, Knutson B, McClure SM. 2014. Dissociating motivation from reward in human striatal activity. J. Cogn. Neurosci. 26, 1075–1084. ( 10.1162/jocn_a_00535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Simon JJ, Biller A, Walther S, Roesch-Ely D, Stippich C, Weisbrod M, Kaiser S. 2010. Neural correlates of reward processing in schizophrenia: relationship to apathy and depression. Schizophr. Res. 118, 154–161. ( 10.1016/j.schres.2009.11.007) [DOI] [PubMed] [Google Scholar]
- 102.Simon JJ, Cordeiro SA, Weber MA, Friederich HC, Wolf RC, Weisbrod M, Kaiser S. 2015. Reward system dysfunction as a neural substrate of symptom expression across the general population and patients with schizophrenia. Schizophr. Bull. 41, 1370–1378. ( 10.1093/schbul/sbv067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Garrison J, Erdeniz B, Done J. 2013. Prediction error in reinforcement learning: a meta-analysis of neuroimaging studies. Neurosci. Biobehav. Rev. 37, 1297–1310. ( 10.1016/j.neubiorev.2013.03.023) [DOI] [PubMed] [Google Scholar]
- 104.Adams RA, Stephan KE, Brown HR, Frith CD, Friston KJ. 2013. The computational anatomy of psychosis. Front. Psychiatry 4, 47 ( 10.3389/fpsyt.2013.00047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Friston K. 2005. A theory of cortical responses. Phil. Trans. R. Soc. B 360, 815–836. ( 10.1098/rstb.2005.1622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Blakemore SJ, Smith J, Steel R, Johnstone CE, Frith CD. 2000. The perception of self-produced sensory stimuli in patients with auditory hallucinations and passivity experiences: evidence for a breakdown in self-monitoring. Psychol. Med. 30, 1131–1139. ( 10.1017/S0033291799002676) [DOI] [PubMed] [Google Scholar]
- 107.Shergill SS, White TP, Joyce DW, Bays PM, Wolpert DM, Frith CD. 2014. Functional magnetic resonance imaging of impaired sensory prediction in schizophrenia. JAMA Psychiatry 71, 28–35. ( 10.1001/jamapsychiatry.2013.2974) [DOI] [PubMed] [Google Scholar]
- 108.Garety PA, Freeman D. 2013. The past and future of delusions research: from the inexplicable to the treatable. Br. J. Psychiatry 203, 327–333. ( 10.1192/bjp.bp.113.126953) [DOI] [PubMed] [Google Scholar]
- 109.Kapur S. 2003. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am. J. Psychiatry 160, 13–23. ( 10.1176/appi.ajp.160.1.13) [DOI] [PubMed] [Google Scholar]
- 110.Stephan KE, Friston KJ, Frith CD. 2009. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr. Bull. 35, 509–527. ( 10.1093/schbul/sbn176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pfeiffer UJ, Timmermans B, Bente G, Vogeley K, Schilbach L. 2011. A non-verbal Turing test: differentiating mind from machine in gaze-based social interaction. PLoS ONE 6, e27591 ( 10.1371/journal.pone.0027591) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Galea S, Nandi A, Vlahov D. 2004. The social epidemiology of substance use. Epidemiol. Rev. 26, 36–52. ( 10.1093/epirev/mxh007) [DOI] [PubMed] [Google Scholar]
- 113.Hulka LM, Preller KH, Vonmoos M, Broicher SD, Quednow BB. 2013. Cocaine users manifest impaired prosodic and cross-modal emotion processing. Front. Psychiatry 4, 98 ( 10.3389/fpsyt.2013.00098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Preller KH, et al. 2014. Functional changes of the reward system underlie blunted response to social gaze in cocaine users. Proc. Natl Acad. Sci. USA 111, 2842–2847. ( 10.1073/pnas.1317090111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Motzkin JC, Baskin-Sommers A, Newman JP, Kiehl KA, Koenigs M. 2014. Neural correlates of substance abuse: reduced functional connectivity between areas underlying reward and cognitive control. Hum. Brain Mapp. 35, 4282–4292. ( 10.1002/hbm.22474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Paulhus DL, Morgan KL. 1997. Perceptions of intelligence in leaderless groups: the dynamic effects of shyness and acquaintance. J. Pers. Soc. Psychol. 72, 581–591. ( 10.1037/0022-3514.72.3.581) [DOI] [PubMed] [Google Scholar]
- 117.Meleshko KG, Alden LE. 1993. Anxiety and self-disclosure: toward a motivational model. J. Pers. Soc. Psychol. 64, 1000–1009. ( 10.1037/0022-3514.64.6.1000) [DOI] [PubMed] [Google Scholar]
- 118.Alden LE, Taylor CT. 2004. Interpersonal processes in social phobia. Clin. Psychol. Rev. 24, 857–882. ( 10.1016/j.cpr.2004.07.006) [DOI] [PubMed] [Google Scholar]
- 119.Stangier U, Schramm E, Heidenreich T, Berger M, Clark DM. 2011. Cognitive therapy vs interpersonal psychotherapy in social anxiety disorder: a randomized controlled trial. Arch. Gen. Psychiatry 68, 692–700. ( 10.1001/archgenpsychiatry.2011.67) [DOI] [PubMed] [Google Scholar]
- 120.Doehrmann O, et al. 2013. Predicting treatment response in social anxiety disorder from functional magnetic resonance imaging. JAMA Psychiatry 70, 87–97. ( 10.1001/2013.jamapsychiatry.5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Richey JA, Rittenberg A, Hughes L, Damiano CR, Sabatino A, Miller S, Hanna E, Bodfish JW, Dichter GS. 2014. Common and distinct neural features of social and non-social reward processing in autism and social anxiety disorder. Soc. Cogn. Affect. Neurosci. 9, 367–377. ( 10.1093/scan/nss146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cremers HR, Veer IM, Spinhoven P, Rombouts SA, Roelofs K. 2014. Neural sensitivity to social reward and punishment anticipation in social anxiety disorder. Front. Behav. Neurosci. 8, 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ochsner KN, Lieberman MD. 2001. The emergence of social cognitive neuroscience. Am. Psychol. 56, 717–734. ( 10.1037/0003-066X.56.9.717) [DOI] [PubMed] [Google Scholar]
- 124.Frith CD, Frith U. 1999. Interacting minds--a biological basis. Science 286, 1692–1695. ( 10.1126/science.286.5445.1692) [DOI] [PubMed] [Google Scholar]
- 125.Schilbach L. 2015. Eye to eye, face to face and brain to brain: Novel approaches to study the behavioral dynamics and neural mechanisms of social interactions. Curr. Opin. Behav. Sci. 3, 130–135. ( 10.1016/j.cobeha.2015.03.006) [DOI] [Google Scholar]
- 126.Heyes C. 2010. Where do mirror neurons come from? Neurosci. Biobehav. Rev. 34, 575–583. ( 10.1016/j.neubiorev.2009.11.007) [DOI] [PubMed] [Google Scholar]
- 127.Cook R, Bird G, Catmur C, Press C, Heyes C. 2014. Mirror neurons: from origin to function. Behav. Brain Sci. 37, 177–192. ( 10.1017/S0140525X13000903) [DOI] [PubMed] [Google Scholar]
- 128.Frith CD, Frith U. 2012. Mechanisms of social cognition. Annu. Rev. Psychol. 63, 287–313. ( 10.1146/annurev-psych-120710-100449) [DOI] [PubMed] [Google Scholar]
- 129.Reddy V. 2003. On being the object of attention: implications for self-other consciousness. Trends Cogn. Sci. 7, 397–402. ( 10.1016/S1364-6613(03)00191-8) [DOI] [PubMed] [Google Scholar]
- 130.Klin A, Jones W, Schultz R, Volkmar F. 2003. The enactive mind, or from actions to cognition: lessons from autism. Phil. Trans. R. Soc. Lond. B 358, 345–360. ( 10.1098/rstb.2002.1202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yamada H, Inokawa H, Matsumoto N, Ueda Y, Enomoto K, Kimura M. 2013. Coding of the long-term value of multiple future rewards in the primate striatum. J. Neurophysiol. 109, 1140–1151. ( 10.1152/jn.00289.2012) [DOI] [PubMed] [Google Scholar]
- 132.Lebreton M, et al. 2009. The brain structural disposition to social interaction. Eur. J. Neurosci. 29, 2247–2252. ( 10.1111/j.1460-9568.2009.06782.x) [DOI] [PubMed] [Google Scholar]
- 133.Burns J. 2006. The social brain hypothesis of schizophrenia. World Psychiatry 5, 77–81. [PMC free article] [PubMed] [Google Scholar]
- 134.Tost H, Meyer-Lindenberg A. 2012. Puzzling over schizophrenia: schizophrenia, social environment and the brain. Nat. Med. 18, 211–213. ( 10.1038/nm.2671) [DOI] [PubMed] [Google Scholar]
- 135.Linden DE. 2012. The challenges and promise of neuroimaging in psychiatry. Neuron 73, 8–22. ( 10.1016/j.neuron.2011.12.014) [DOI] [PubMed] [Google Scholar]
- 136.Glatzel J, Yang Y. 1982. Das psychisch Abnorme. Kritische Ansätze zu einer Psychopathologie. München, Germany: Urban & Fischer. [Google Scholar]
- 137.Jaspers K. 1958. Philosophische Logik, Bd. 1, Von der Wahrheit. München, Germany: Piper. [Google Scholar]
- 138.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. 2010. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry 167, 748–751. ( 10.1176/appi.ajp.2010.09091379) [DOI] [PubMed] [Google Scholar]
- 139.Sevgi M, Diaconescu AO, Tittgemeyer M, Schilbach L. Submitted. Social Bayes: Using Bayesian modeling to study autistic trait-related differences in social cognition. [DOI] [PubMed] [Google Scholar]
- 140.Montague PR, et al. 2002. Hyperscanning: simultaneous fMRI during linked social interactions. Neuroimage 16, 1159–1164. ( 10.1006/nimg.2002.1150) [DOI] [PubMed] [Google Scholar]


