Abstract
Although exposure to stressors is known to increase disease susceptibility and accelerate ageing, evidence is accumulating that these effects can span more than one generation. Stressors experienced by parents have been reported to negatively influence the longevity of their offspring and even grand offspring. The mechanisms underlying these long-term, cross-generational effects are still poorly understood, but we argue here that telomere dynamics are likely to play an important role. In this review, we begin by surveying the current connections between stress and telomere dynamics. We then lay out the evidence that exposure to stressors in the parental generation influences telomere dynamics in offspring and potentially subsequent generations. We focus on evidence in mammalian and avian studies and highlight several promising areas where our understanding is incomplete and future investigations are critically needed. Understanding the mechanisms that link stress exposure across generations requires interdisciplinary studies and is essential to both the biomedical community seeking to understand how early adversity impacts health span and evolutionary ecologists interested in how changing environmental conditions are likely to influence age-structured population dynamics.
Keywords: ageing, lifespan, parental effects, senescence, telomere dynamics
1. Stress across generations
Understanding the processes that underlie variation in longevity in natural populations remains a central area of biological research. Among vertebrates, one process that is likely to play a key role is the integrated physiological stress response, of which a central component is the release of glucocorticoids [1]. An idea that has permeated gerontology for a century is that prolonged activation of this glucocorticoid stress response can contribute to disease, hasten ageing and increase mortality [2]. Consistent with this, senescent individuals are often characterized by elevated glucocorticoid levels and an inability to properly terminate the stress response [3]. More recently, striking evidence has shown that stress experienced by parents not only affects their own health, but can have long-lasting repercussions on offspring as well [4–6]. Several human and animal studies have established links between stressful conditions during embryonic and fetal development and disease risk later in life [4,6]. For example, during the Dutch Hongerwinter famine of 1944 and 1945, pregnant mothers experienced severe nutritional and psychological stress. The children born during the famine had increased incidence of disease decades after the famine had ended [7]. These cross-generational effects are not limited to mothers and can also be observed through the paternal line, as grandfathers that experienced greater nutritional stress during their own childhood produced grandchildren with reduced lifespans [8].
The mechanisms linking stress exposure to disease progression and ageing either within individuals or across generations are still unclear, but recent work suggests that telomere dynamics (length and loss rate) may play an important role [9,10]. Telomeres are evolutionarily conserved regions of highly structured, non-coding DNA that form protective caps at the ends of linear eukaryotic chromosomes that are necessary for normal cellular function [11]. Telomeres shorten each time a cell divides as a consequence of normal DNA replication [11]. In addition, in vitro work has shown that telomeres are particularly vulnerable to oxidative damage [12], which is also supported by some experimental work in model organisms [13]. Although telomeres can be restored by the enzyme telomerase, if shortened to a critical length, cells stop dividing. These senescent cells can have altered secretory profiles which lead to a decline in tissue function that can contribute to disease and the ageing phenotype [11]. Evidence has begun to accumulate in both the biomedical [14,15] and ecological realms [9,10] that stress may, in part, affect ageing by accelerating telomere loss.
In this review, we begin with an overview of the connections between stress and telomere dynamics and outline some of the most important underlying mechanisms. Then, we discuss and critically appraise the available evidence of how stress in the parental generation can affect the telomere dynamics of subsequent generations. Throughout, we highlight areas where future inquiry is likely to lead to novel insights about how stress across generations may affect the ageing process. Although the majority of work in this field has focused on mammals and birds, the conserved nature of both the glucocorticoid stress response and telomeres suggests that the links found between stress exposure and telomere dynamics are likely to be seen in all vertebrates. Our goal here is to employ an interdisciplinary approach to provide a framework for the emerging field of how stress can shape ageing patterns across generations.
2. Stress and telomeres
Glucocorticoid stress hormones, regulated by the hypothalamic–pituitary–adrenal (HPA) axis, increase survival in the face of stressors by mobilizing energy stores, inhibiting unnecessary physiological functions such as growth and reproduction, and regulating behaviours that control energy intake and expenditure [1]. Once homeostasis has been restored, glucocorticoids quickly return to baseline levels. However, exposure to repeated or prolonged stressors can result in chronic exposure to elevated glucocorticoids, which can increase disease risk and reduce longevity [1,2], in part through effects on telomere dynamics [9].
Accumulating evidence suggests that reduction in telomere length is a component of the ageing phenotype as well as a risk factor in a large number of diseases [11]. There are a growing number of reports that telomere dynamics are associated with longevity in both the human epidemiological [16,17] and the evolutionary ecology literature ([18–20], but see [21]). Given the growing role that telomeres appear to play in ageing and disease, research has shifted to identifying factors that affect telomere dynamics. While these factors are varied, recent work in both human correlative studies [14,22] and experimental models [23–25] has suggested that stress and glucocorticoid exposure hasten telomere shortening and ageing.
3. Cross-generational effects
Stress experienced by parents can broadly impact the physiology and behaviour of their offspring. These effects can happen in a variety of ways, for example, stress experienced by mothers can expose fetuses to maternally derived glucocorticoids through the placenta [26], or through their presence in eggs of oviparous species [5]. Whether the transfer of maternal glucocorticoids to offspring is an inevitable physiological constraint associated with poor environmental conditions, or an adaptive maternally mediated cue that alters offspring phenotype in preparation for a stressful environment is a topic of current debate [5]. However, recent work reports that one long-term cost of stress experienced by the parental generation may not only be to their own telomeres, but also to the telomeres of their offspring, suggesting that stress in one generation can have unintended consequences for future generations as well [24,27].
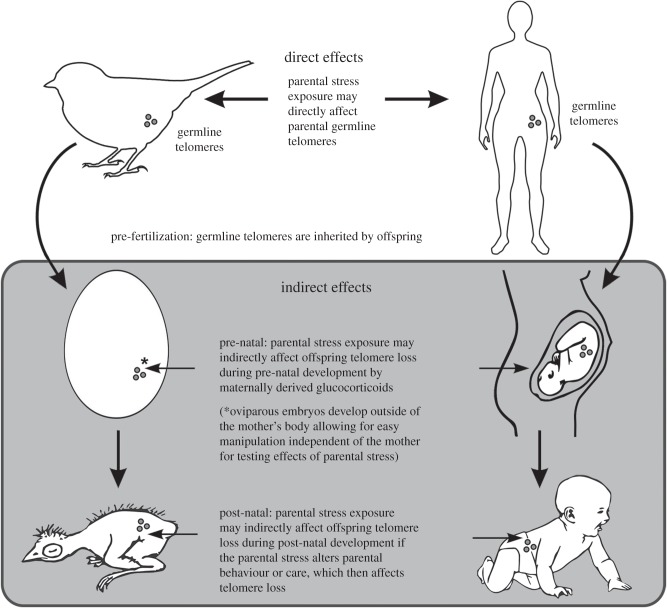
The routes whereby parental stress can affect the telomere dynamics of subsequent generations are varied, and taken together we refer to these as cross-generational effects (figure 1). First, parental stress may directly influence the parental germline telomeres pre-fertilization, affecting the telomere length inherited by offspring. Alternatively, parental stress may affect telomere dynamics indirectly either pre- or post-natally. The physiological mechanisms by which stress elicits changes in telomere length are also diverse, and we briefly outline some of the most important mechanisms (box 1).
Figure 1.
The routes by which parental exposure to stressors can influence the telomere dynamics of subsequent generations. These effects can occur to germline telomere length prior to fertilization (direct effects) and influence the telomeres that offspring inherit. These effects can also occur to telomere loss during both pre- and post-natal development and we refer to these as indirect effects (within the grey box).
Box 1. Mechanisms by which stress affects telomere dynamics.
Parental stress exposure may affect telomere dynamics in their offspring through a number of non-mutually exclusive mechanisms, which may be interconnected. For example, recent literature has shown that nutrient signalling [28], insulin resistance [29], sex steroids [30,31], inflammation [32] and even chronic pain [33] may affect telomere dynamics. However, glucocorticoids have received the most attention thus far, and below we focus on how elevated parental or offspring glucocorticoids may affect offspring telomeres.
Oxidative stress. A recent meta-analysis reported that elevated glucocorticoids are often associated with an increase in oxidative stress levels [34]. Compared with other regions of DNA, telomeres are particularly vulnerable to oxidative damage [35] owing to both a relatively high guanine content and also reduced DNA repair [35]. In addition, oxidative stress exacerbates telomere loss, because the amount of unrepaired oxidative damage to the telomeres influences the magnitude of telomere loss at the next cell division [12]. In vitro work has shown that blocking the glucocorticoid receptors via RU486, a glucocorticoid receptor antagonist, also blocks the glucocorticoid-mediated rise in free radical production, suggesting that glucocorticoids regulate genes involved in free radical generation [36]. Likewise, short-term exposure to glucocorticoids induced a fivefold increase in DNA damage and pre-treatment with RU486 eliminated this increase. These effects of elevated glucocorticoids appear to specifically interfere with DNA repair mechanisms [37]. Glucocorticoids may also promote oxidative stress by disabling either enzymatic antioxidants or dietary antioxidants [38], but these effects may vary by tissue [39].
Telomerase. Elevated glucocorticoids have also been shown to influence telomerase; however, the results are somewhat conflicting [14,15,40]. In humans, women exposed to long-term, chronic stress had decreased telomerase expression and greater telomere shortening in leucocytes [14], and experimentally elevated glucocorticoids in lymphocytes reduce telomerase expression in vitro [40]. However, in rats, experimental exposure to stressors resulted in elevated telomerase expression in leucocytes [15]. These contrasting results may be due to differences in the severity or duration of exposure to the stressor.
Reprogramming the HPA axis. Given that glucocorticoid levels can affect telomeres through both oxidative stress and telomerase, it is important to note that exposure to maternal glucocorticoid stress hormones during embryonic development can have long-term ‘programming’ effects on the HPA axis [4,5]. The direction of this modification is quite variable and not only depends on the timing, duration and magnitude of glucocorticoid exposure, but also varies by species and sex [41]. Maternal transfer of glucocorticoids can reduce the number of glucocorticoid type I and II receptors in the hippocampus [42], resulting in impaired negative feedback control of glucocorticoid secretion leading to higher baseline levels and a prolonged duration of the stress response [41]. In a manipulative study in chickens, individuals exposed to experimentally elevated glucocorticoids during embryonic development had elevated glucocorticoid exposure following an acute stressor, and shorter telomeres compared with the control birds [24]. In addition, seabird chicks exposed to handling stress during post-natal development secreted higher levels of glucocorticoids and experienced greater telomere loss than unexposed chicks [43].
Epigenetics. Epigenetics, the alteration of DNA expression through histone modifications, methylation or the production of small RNA molecules, may also mediate parental stress exposure on offspring telomeres. Humans that experienced pre-natal exposure to famine had less DNA methylation of the imprinted IGF2 gene 60 years later relative to unexposed siblings [7]. These epigenetic effects can also be mediated through the paternal line. For example, male mice that were exposed to chronic stress prior to breeding produced offspring with altered expression of glucocorticoid-responsive genes that were less responsive to stress than those of control males. Importantly, the treatment also influenced the micro RNA of the males' sperm [44]. It remains unclear whether these epigenetic changes to offspring would influence their telomere dynamics. However, it is also possible that stress exposure in the parental generation could have epigenetic effects on offspring telomere regulation, as there is increasing evidence that epigenetic markers play a role in telomere length homeostasis [11].
(a). Direct effects
Parental exposure to stressors may affect the telomere length of gametes before fertilization, which could influence the telomere length that offspring inherit. The degree to which pre-fertilization effects influence offspring telomere length is complicated by the fact that the inheritance of telomere length remains poorly understood. Reported heritability estimates for telomere length range from 32 to 84% [45,46]. However, recent studies in humans and birds have demonstrated that these heritability estimates are likely to be inflated by parental effects [30,45–47], of which parental stress exposure might be particularly important [48].
Germ cells may be more susceptible to telomere erosion than somatic tissues because they are predicted to be especially vulnerable to oxidative stress [49]. Some evidence suggests that these effects of stress on the germline are transient because unlike most somatic tissues, telomerase in the germline is constitutively expressed [11], and may ‘reset’ the telomere length of developing embryos [50]. Nonetheless, recent papers report that the telomere length of parental gametes is sensitive to environmental conditions [51,52]. In humans, while telomere length tends to decrease with age in somatic tissues [16], telomere length can increase with age in sperm [51], and this telomere length may be passed on to offspring. In support of this, offspring produced by older fathers appear to have longer telomeres [51,53]. Recently, these effects were reported to span multiple generations as the ages of the father and the paternal grandfather at the time of conception both had a positive influence on offspring telomere length [54]. Although these results are intriguing, they are cross-sectional. Consequently, they might have occurred because older fathers represent a distinct subset of the population that always had relatively long telomeres in their sperm rather than age-related changes in sperm telomere length within these fathers.
A recent longitudinal study in birds also found a connection between parental age and offspring telomere length, but this time in mothers [52]. Interestingly, the effect depended on the malarial infection status of the mother. As infected mothers aged, they produced offspring with shorter telomeres, whereas the opposite was true in uninfected mothers [52]. This suggests that the state of the mother, and hence prior exposure to stressors, can influence offspring telomere length. In this study as well as the aforementioned human studies, telomere length in offspring was only measured once, typically at the end of post-natal development or in adulthood. So while suggestive, these effects could also be the result of indirect effects on telomere loss that occurred during both pre- and post-natal development (see below) rather than direct effects on gamete telomere length.
Demonstrating conclusively that parental exposure to stressors influences the telomere lengths of gametes, which are then transmitted to offspring, will require measuring gamete telomere length and experimental designs that employ artificial insemination and cross-fostering techniques to rule out the possibility of changes in maternal and paternal investment [55]. However, if parental exposure to stressors has direct effects on offspring telomere length, this could have large health implications for multiple future generations [54].
(b). Indirect effects
Parental exposure to stressors can also influence offspring telomere loss indirectly during both pre- and post-natal development. In two separate human correlative studies, mothers that reported experiencing higher levels of stress during pregnancy produced offspring with shorter telomeres at birth [27] and in young adulthood [48]. In addition, children exposed to higher levels of parental abuse and neglect during childhood experienced greater telomere loss [22]. While these human studies establish an interesting pattern, they are necessarily correlative and include potential biases associated with self-reporting, which makes it difficult to determine causal relationships. For example, these patterns could also have arisen if certain parental phenotypes are more likely to encounter stressors and to produce offspring with shorter telomeres or faster erosion rates.
A handful of experimental studies in laboratory animals have also explored indirect cross-generational effects of parental stress exposure on offspring telomere dynamics. For example, in zebra finches, glucocorticoids were experimentally elevated in mothers during egg production [23] and in chickens, glucocorticoids were directly injected into eggs [24]. In both cases, the resulting chicks had shorter telomeres than controls. These results could be due to effects that occurred during pre- and post-natal development. In the chickens, these effects are most likely due to exposure while in ovo since offspring received no post-natal care [24]. In birds, recent experimental research during the post-natal period has also demonstrated that exposure to nutritional and social stressors such as being raised in a larger brood or at a lower social rank leads to greater telomere loss [56–58].
More experimental studies at these different life-history stages are critically needed, as the effects of parental stress exposure during pre- and post-natal development might interact to influence offspring telomere dynamics. For example, rat pups that were gestated by mothers on a low protein diet, but weaned by mothers on a control diet had significantly shorter telomeres and reduced longevity compared with pups that were gestated by mothers on a control diet and weaned by mothers on a low protein diet [59]. This suggests that both the timing of parental stress exposure and the interaction between developmental stages can have an important impact on offspring telomere dynamics, possibly even changing the direction of the effect. Future work should prioritize experimental approaches that employ full factorial designs that manipulate parental stress exposure during both pre- and post-natal development.
As the studies above show, parental stress exposure can clearly influence offspring telomere dynamics. But, these effects can differ substantially among individuals based on how they respond to stressors [60] and this has received much less attention. A recent human study suggests that individual differences in stress resilience might depend critically on genetic–environmental interactions [61]. For example, boys with the greatest genetic sensitivity within the serotonergic and dopaminergic pathways experienced greater telomere shortening in stressful social environments, but less telomere shortening in non-stressful social environments, compared with boys with less genetic sensitivity in the same pathways. In other words, both the magnitude and the direction of the influence of stress on telomere attrition depended on the individual's genetic background. Individual variation in how exposure to stressors influences telomere attrition and thus how these traits are related to one another clearly deserves more study. Future work in this area could have important implications for our understanding of the role of stress responsiveness and telomere dynamics in mediating life-history trade-offs [62] and for human health [63].
In addition, although parental stress exposure has been shown to have indirect effects on offspring telomere dynamics in a range of tissues [56,59], whether certain tissues are more buffered than others remains poorly understood. Generally, there is good correspondence in telomere lengths among tissues within individuals in humans [64], as well as in birds [65]. A recent cross-sectional study in humans reported that telomere loss rates are relatively constant across tissue types in adulthood. This suggests that any differences among tissues are likely established during pre- and post-natal development [64] and there is some evidence that the effects of parental stress on offspring telomere dynamics can be tissue specific [59]. For example, rat mothers exposed to stress during gestation produced pups with shorter telomeres in the liver and in the kidney, but not in the brain [59]. This may be because less proliferative tissues are less sensitive to parental stress exposure. Variation in the timing of parental stress exposure, telomerase expression patterns and sensitivity to oxidative stress may also contribute to differences in offspring telomere lengths among tissues (box 1). Whether tissue-specific effects have long-term functional consequences making certain tissues more susceptible to age-related disease is currently unknown and is an important area for future study.
4. Conclusion
Evidence is growing that exposure to stressors in the parental generation can have long-term consequences for offspring health [4–6] and this connection may be mediated in part by telomere dynamics. Currently, there is great interest in whether parents that experience stressful circumstances adaptively ‘programme’ their offspring to be better able to cope with stressful environments at subsequent life-history stages [5,6,66]. Interestingly, recent data also suggest that offspring are unlikely to be passive recipients of maternal glucocorticoids during embryonic development [67] and may temper parental stress effects. Regardless, while most evidence suggests that the effect of parental stress exposure on offspring telomeres is negative, it is important to remember that this is just one trait that can contribute to parental and offspring fitness. For example, in red squirrels, in high-density years, mothers transferred elevated glucocorticoids to their offspring in utero, increasing offspring growth and fitness [68]. Although offspring telomere dynamics were not measured in that study, previous research in mammals and in birds suggests that accelerated growth increases telomere loss [69]. Consequently, investment in traits that increase fitness is expected to be favoured, even if they come at a cost to traits associated with longevity, such as telomere length [62].
Integrative studies of the fitness consequences of parental stressor exposure on offspring telomere dynamics and the proximate mechanisms that underlie them will be critical for predicting the long-term consequences of stress exposure in the parental generation on future generations. As highlighted above, we need more information about how these effects vary between developmental stages, among individuals, and within tissues of individuals. This knowledge will be essential for both evolutionary ecologists interested in predicting the effects of increasing human perturbations and environmental stochasticity on population dynamics, and the biomedical community, which is interested in the best ways to mitigate the effects of early life adversity on human health.
Acknowledgements
We are grateful to Pat Monaghan, Sue Anne Zollinger, Tim Greives, Benjamin Melby, Elissa Epel, Michael Sheriff and anonymous reviewers for valuable feedback on an earlier draft. We also thank Sue Anne Zollinger for artistic assistance with figure 1.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by an NSF grant no. IOS-1145625 and a Bucknell Swanson Fellowship in Science and Engineering to M.F.H. and by an ND EPSCoR FAR0022429 award to B.J.H.
References
- 1.Sapolsky RM, Romero LM, Munck AU. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89. ( 10.1210/er.21.1.55) [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS. 1998. Stress, adaptation, and disease. Allostasis and allostatic load. Ann. NY Acad. Sci. 840, 33–44. ( 10.1111/j.1749-6632.1998.tb09546.x) [DOI] [PubMed] [Google Scholar]
- 3.Stein-Behrens BA, Sapolsky RM. 1992. Stress, glucocorticoids, and aging. Aging Clin. Exp. Res. 4, 197–210. ( 10.1007/BF03324092) [DOI] [PubMed] [Google Scholar]
- 4.Seckl JR, Meaney MJ. 2004. Glucocorticoid programming. Ann. NY Acad. Sci. 1032, 63–84. ( 10.1196/annals.1314.006) [DOI] [PubMed] [Google Scholar]
- 5.Sheriff MJ, Love OP. 2012. Determining the adaptive potential of maternal stress. Ecol. Lett. 16, 271–280. ( 10.1111/ele.12042) [DOI] [PubMed] [Google Scholar]
- 6.Burton T, Metcalfe NB. 2014. Can environmental conditions experienced in early life influence future generations? Proc. R. Soc. B 281, 20140311 ( 10.1098/rspb.2014.0311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lumey LH, Stein AD, Susser E. 2011. Prenatal famine and adult health. Annu. Rev. Public Health 32, 237–262. ( 10.1146/annurev-publhealth-031210-101230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaati G, Bygren LO, Pembrey M, Sjöström M. 2007. Transgenerational response to nutrition, early life circumstances and longevity. Eur. J. Hum. Genet. 15, 784–790. ( 10.1038/sj.ejhg.5201832) [DOI] [PubMed] [Google Scholar]
- 9.Haussmann MF, Marchetto NM. 2010. Telomeres: linking stress and survival, ecology and evolution. Curr. Zool. 56, 714–727. [Google Scholar]
- 10.Monaghan P. 2013. Organismal stress, telomeres and life histories. J. Exp. Biol. 217, 57–66. ( 10.1242/jeb.090043) [DOI] [PubMed] [Google Scholar]
- 11.Aubert G, Lansdorp PM. 2008. Telomeres and aging. Physiol. Rev. 88, 557–579. ( 10.1152/physrev.00026.2007) [DOI] [PubMed] [Google Scholar]
- 12.Richter T, Zglinicki TV. 2007. A continuous correlation between oxidative stress and telomere shortening in fibroblasts. Exp. Gerontol. 42, 1039–1042. ( 10.1016/j.exger.2007.08.005) [DOI] [PubMed] [Google Scholar]
- 13.Cattan V, et al. 2008. Chronic oxidative stress induces a tissue-specific reduction in telomere length in CAST/Ei mice. Free Radic. Biol. Med. 44, 1592–1598. ( 10.1016/j.freeradbiomed.2008.01.007) [DOI] [PubMed] [Google Scholar]
- 14.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. 2004. Accelerated telomere shortening in response to life stress. Proc. Natl Acad. Sci. USA 101, 17 312–17 315. ( 10.1073/pnas.0407162101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beery AK, Lin J, Biddle JS, Francis DD, Blackburn EH, Epel ES. 2012. Chronic stress elevates telomerase activity in rats. Biol. Lett. 8, 1063–1066. ( 10.1006/jmcc.1999.1084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. 2003. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 361, 393–395. ( 10.1016/S0140-6736(03)12384-7) [DOI] [PubMed] [Google Scholar]
- 17.Bakaysa SL, Mucci LA, Slagboom PE, Boomsma DI, Mcclearn GE, Johansson B, Pedersen NL. 2007. Telomere length predicts survival independent of genetic influences. Aging Cell 6, 769–774. ( 10.1111/j.1474-9726.2007.00340.x) [DOI] [PubMed] [Google Scholar]
- 18.Haussmann MF, Winkler DW, Vleck CM. 2005. Longer telomeres associated with higher survival in birds. Biol. Lett. 1, 212–214. ( 10.1098/rsbl.2005.0301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salomons HM, Mulder GA, van de Zande L, Haussmann MF, Linskens MHK, Verhulst S. 2009. Telomere shortening and survival in free-living corvids. Proc. R. Soc. B 276, 3157–3165. ( 10.1098/rspb.2009.0517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P. 2012. Telomere length in early life predicts lifespan. Proc. Natl Acad. Sci. USA 109, 1743–1748. ( 10.1073/pnas.1113306109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bischoff C, Petersen HC, Graakjaer J, Andersen-Ranberg K, Vaupel JW, Bohr VA, Kølvraa S, Christensen K. 2006. No association between telomere length and survival among the elderly and oldest old. Epidemiology 17, 190–194. ( 10.1097/01.ede.0000199436.55248.10) [DOI] [PubMed] [Google Scholar]
- 22.Shalev I, Moffitt TE, Sugden K, Williams B, Houts RM, Danese A, Mill J, Arseneault L, Caspi A. 2012. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Mol. Psychiatry 18, 576–581. ( 10.1038/mp.2012.32) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tissier ML, Williams TD, Criscuolo F. 2014. Maternal effects underlie ageing costs of growth in the zebra finch (Taeniopygia guttata). PLoS ONE 9, e97705 ( 10.1371/journal.pone.0097705.g003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haussmann MF, Longenecker AS, Marchetto NM, Juliano SA, Bowden RM. 2012. Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proc. R. Soc. B 279, 1447–1456. ( 10.1098/rspb.2011.1913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hau M. 2015. Repeated stressors in adulthood increase the rate of biological ageing. Front. Zool. 12, 4 ( 10.1186/s12983-015-0095-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinstock M. 2005. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav. Immunol. 19, 296–308. ( 10.1016/j.bbi.2004.09.006) [DOI] [PubMed] [Google Scholar]
- 27.Entringer S, Epel ES, Kumsta R, Lin J, Hellhammer DH, Balckburn EH, Wust S, Wadhwa PD. 2011. Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proc. Natl Acad. Sci. USA 108, E513–E518. ( 10.1073/pnas.1107759108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ungar L, Harari Y, Toren A, Kupiec M. 2011. Tor complex 1 controls telomere length by affecting the level of Ku. Curr. Biol. 21, 2115–2120. ( 10.1016/j.cub.2011.11.024) [DOI] [PubMed] [Google Scholar]
- 29.Demissie S, et al. 2006. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham heart study. Aging Cell 5, 325–330. ( 10.1111/j.1474-9726.2006.00224.x) [DOI] [PubMed] [Google Scholar]
- 30.Entringer S, Epel ES, Lin J, Blackburn EH, Buss C, Simhan HN, Wadhwa PD. 2015. Maternal estriol concentrations in early gestation predict infant telomere length. J. Clin. Endocrinol. Metab. 100, 267–273. ( 10.1210/jc.2014-2744) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin J, Kroenke CH, Epel E, Kenna HA, Wolkowitz OM, Blackburn E, Rasgon NL. 2011. Greater endogenous estrogen exposure is associated with longer telomeres in postmenopausal women at risk for cognitive decline. Brain Res. 1379, 224–231. ( 10.1016/j.brainres.2010.10.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Donovan A, et al. 2011. Cumulative inflammatory load is associated with short leukocyte telomere length in the health, aging and body composition study. PLoS ONE 6, e19687 ( 10.1371/journal.pone.0019687.t002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sibille KT, et al. 2012. Chronic pain, perceived stress, and cellular aging: an exploratory study. Mol. Pain 8, 12 ( 10.1186/1744-8069-8-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Costantini D, Marasco V, Møller AP. 2011. A meta-analysis of glucocorticoids as modulators of oxidative stress in vertebrates. J. Comp. Physiol. B 181, 447–456. ( 10.1007/s00360-011-0566-2) [DOI] [PubMed] [Google Scholar]
- 35.Houben JMJ, Moonen HJJ, van Schooten FJ, Hageman GJ. 2008. Telomere length assessment: biomarker of chronic oxidative stress? Free Radic. Biol. Med. 44, 235–246. ( 10.1016/j.freeradbiomed.2007.10.001) [DOI] [PubMed] [Google Scholar]
- 36.You J-M, Yun S-J, Nam KN, Kang C, Won R, Lee EH. 2009. Mechanism of glucocorticoid-induced oxidative stress in rat hippocampal slice cultures. Can. J. Physiol. Pharmacol. 87, 440–447. ( 10.1139/y09-027) [DOI] [PubMed] [Google Scholar]
- 37.Flint MS, Baum A, Chambers WH, Jenkins FJ. 2007. Induction of DNA damage, alteration of DNA repair and transcriptional activation by stress hormones. Psychoneuroendocrinology 32, 470–479. ( 10.1016/j.psyneuen.2007.02.013) [DOI] [PubMed] [Google Scholar]
- 38.Liu J, Mori A. 1999. Stress, aging, and brain oxidative damage. Neurochem. Res. 24, 1479–1497. ( 10.1023/A:1022597010078) [DOI] [PubMed] [Google Scholar]
- 39.McIntosh LJ, Sapolsky RM. 1996. Glucocorticoids increase the accumulation of reactive oxygen species and enhance adriamycin-induced toxicity in neuronal culture. Exp. Neurol. 141, 201–206. ( 10.1006/exnr.1996.0154) [DOI] [PubMed] [Google Scholar]
- 40.Choi J, Fauce SR, Effros RB. 2008. Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain Behav Immunol. 22, 600–605. ( 10.1016/j.bbi.2007.12.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. 2006. Fetal programming of hypothalamo–pituitary–adrenal function: prenatal stress and glucocorticoids. J. Physiol. 572, 31–44. ( 10.1113/jphysiol.2006.105254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maccari S, Darnaudery M, Morley-Fletcher S, Zuena AR, Cinque C, Van Reeth O. 2003. Prenatal stress and long-term consequences: implications of glucocorticoid hormones. Neurosci. Biobehav. Rev. 27, 119–127. ( 10.1016/S0149-7634(03)00014-9) [DOI] [PubMed] [Google Scholar]
- 43.Herborn KA, Heidinger BJ, Boner W, Noguera JC, Adam A, Daunt F, Monaghan P. 2014. Stress exposure in early post-natal life reduces telomere length: an experimental demonstration in a long-lived seabird. Proc. R. Soc. B 281, 20133151 ( 10.1098/rspb.2013.3151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. 2013. Paternal stress exposure alters sperm microrna content and reprograms offspring HPA stress axis regulation. J. Neurosci. 33, 9003–9012. ( 10.1523/JNEUROSCI.0914-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Broer L, et al. 2013. Meta-analysis of telomere length in 19 713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. Eur. J. Human Genet. 21, 1163–1168. ( 10.1038/ejhg.2012.303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Asghar M, Bensch S, Tarka M, Hansson B, Hasselquist D. 2014. Maternal and genetic factors determine early life telomere length. Proc. R. Soc. B 282, 20142263 ( 10.1098/rspb.2014.2263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Becker PJJ, Reichert S, Zahn S, Hegelbach J, Massemin S, Keller LF, Postma E, Criscuolo F. 2015. Mother–offspring and nest-mate resemblance but no heritability in early-life telomere length in white-throated dippers. Proc. R Soc B 282, 20142924 ( 10.1098/rspb.2014.2924) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Entringer S, Epel ES, Lin J, Buss C, Shahbaba B, Blackburn EH, Simhan HN, Wadhwa PD. 2013. Maternal psychosocial stress during pregnancy is associated with newborn leukocyte telomere length. Am. J. Obstet. Gyn. 208, 134e1–134.e7. ( 10.1016/j.ajog.2012.11.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Metcalfe NB, Alonso-Alvarez C. 2010. Oxidative stress as a life-history constraint: the role of reactive oxygen species in shaping phenotypes from conception to death. Funct. Ecol. 24, 984–996. ( 10.1111/j.1365-2435.2010.01750.x) [DOI] [Google Scholar]
- 50.Vizlin-Hodzic D, Ryme J, Simonsson S, Simonsson T. 2009. Developmental studies of Xenopus shelterin complexes: the message to reset telomere length is already present in the egg. FASEB J. 23, 2587–2594. ( 10.1096/fj.09-129619) [DOI] [PubMed] [Google Scholar]
- 51.Kimura M, et al. 2008. Offspring's leukocyte telomere length, paternal age, and telomere elongation in sperm. PLoS Genet. 4, e37 ( 10.1371/journal.pgen.0040037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Asghar M, Hasselquist D, Hansson B, Zehtindjiev P, Westerdahl H, Bensch S. 2015. Chronic infection. Hidden costs of infection: chronic malaria accelerates telomere degradation and senescence in wild birds. Science 347, 436–438. ( 10.1126/science.1261121) [DOI] [PubMed] [Google Scholar]
- 53.De Meyer T, et al. 2007. Paternal age at birth is an important determinant of offspring telomere length. Hum. Mol. Genet. 16, 3097–3102. ( 10.1093/hmg/ddm271) [DOI] [PubMed] [Google Scholar]
- 54.Eisenberg DTA, Hayes MG, Kuzawa CW. 2012. Delayed paternal age of reproduction in humans is associated with longer telomeres across two generations of descendants. Proc. Natl Acad. Sci. USA 109, 10 251–10 256. ( 10.1073/pnas.1202092109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saint Jalme M, Hingrat Y, Lacroix F, Sorci G, Preston BT. 2015. The sperm of aging male bustards retards their offspring's development. Nat. Commun. 6, 1–9. ( 10.1038/ncomms7146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nettle D, Monaghan P, Gillespie R, Brilot B, Bedford T, Bateson M. 2014. An experimental demonstration that early-life competitive disadvantage accelerates telomere loss. Proc. R. Soc. B 282, 20141610 ( 10.1098/rspb.2014.1610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lewin N, Treidel LA, Holekamp KE, Place NJ, Haussmann MF. 2015. Socioecological variables predict telomere length in wild spotted hyenas. Biol. Lett. 11, 20140991 ( 10.1098/rsbl.2014.0991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boonekamp JJ, Mulder GA, Salomons HM, Dijkstra C, Verhulst S. 2014. Nestling telomere shortening, but not telomere length, reflects developmental stress and predicts survival in wild birds. Proc. R. Soc. B 281, 20133287 ( 10.1098/rspb.2013.3287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jennings BJ, Ozanne SE, Dorling MW, Hales CN. 1999. Early growth determines longevity in male rats and may be related to telomere shortening in the kidney. FEBS Lett. 448, 4–8. ( 10.1016/S0014-5793(99)00336-1) [DOI] [PubMed] [Google Scholar]
- 60.Zalli A, Carvalho LA, Lin J, Hamer M, Erusalimsky JD, Blackburn EH, Steptoe A. 2014. Shorter telomeres with high telomerase activity are associated with raised allostatic load and impoverished psychosocial resources. Proc. Natl Acad. Sci. USA 111, 4519–4524. ( 10.1073/pnas.1322145111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mitchell C, Hobcraft J, McLanahan SS, Siegel SR, Berg A, Brooks-Gunn J, Garfinkel I, Notterman D. 2014. Social disadvantage, genetic sensitivity, and children's telomere length. Proc. Natl Acad. Sci. USA 111, 5944–5949. ( 10.1073/pnas.1404293111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Monaghan P, Haussmann MF. 2006. Do telomere dynamics link lifestyle and lifespan? Trends Ecol. Evol. 21, 47–53. ( 10.1016/j.tree.2005.11.007) [DOI] [PubMed] [Google Scholar]
- 63.Nestler EJ. 2012. Epigenetics: stress makes its molecular mark. Nature 490, 171–172. ( 10.1038/490171a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Daniali L, Benetos A, Susser E, Kark JD, Labat C, Kimura M, Desai K, Granick M, Aviv A. 2013. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat. Commun. 4, 1597 ( 10.1038/ncomms2602) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reichert S, Criscuolo F, Verinaud E, Zahn S, Massemin S. 2013. Telomere length correlations among somatic tissues in adult zebra finches. PLoS ONE 8, e81496 ( 10.1371/journal.pone.0081496.t004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Monaghan P, Spencer KA. 2014. Stress and life history. Curr. Biol. 24, R408–R412. ( 10.1016/j.cub.2014.04.017) [DOI] [PubMed] [Google Scholar]
- 67.Vassallo BG, Paitz RT, Fasanello VJ, Haussmann MF. 2014. Glucocorticoid metabolism in the in ovo environment modulates exposure to maternal corticosterone in Japanese quail embryos (Coturnix japonica). Biol. Lett. 10, 20140502 ( 10.1038/ncpendmet0515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dantzer B, Newman AEM, Boonstra R, Palme R, Boutin S, Humphries MM, McAdam AG. 2013. Density triggers maternal hormones that increase adaptive offspring growth in a wild mammal. Science 340, 1215–1217. ( 10.1126/science.1235765) [DOI] [PubMed] [Google Scholar]
- 69.Tarry-Adkins JL, Chen J-H, Smith NS, Jones RH, Cherif H, Ozanne SE. 2009. Poor maternal nutrition followed by accelerated postnatal growth leads to telomere shortening and increased markers of cell senescence in rat islets. FASEB J. 23, 1521–1528. ( 10.1096/fj.08-122796) [DOI] [PubMed] [Google Scholar]