Abstract
Long-distance migratory birds have relatively smaller brains than short-distance migrants or residents. Here, we test whether reduction in brain size with migration distance can be generalized across the different brain regions suggested to play key roles in orientation during migration. Based on 152 bird species, belonging to 61 avian families from six continents, we show that the sizes of both the telencephalon and the whole brain decrease, and the relative size of the optic lobe increases, while cerebellum size does not change with increasing migration distance. Body mass, whole brain size, optic lobe size and wing aspect ratio together account for a remarkable 46% of interspecific variation in average migration distance across bird species. These results indicate that visual acuity might be a primary neural adaptation to the ecological challenge of migration.
Keywords: brain mass, cerebellum, migration distance, neuroecology, optic lobe, telencephalon
1. Introduction
Long-distance migration in birds requires acquisition and processing of information to enable geo-positioning (map), orientation (compass) and the recognition of familiar sites [1,2]. To successfully migrate, birds use a combination of visual cues (i.e. spatial landmarks, sun, stars, colour, luminance, motion), magnetic cues and proprioceptive information [1,2]. Information processing efficiency can be achieved by an increase in the number of neurons, which would result in increased neural structure volumes, structural complexities and/or their increased neuron densities [3]. Therefore, relative enlargement of brain regions responsible for processing this information has been predicted in animals with greater need for orientation, such as migrants [1]. Here, we investigate how migration distance is associated with relative sizes of different brain regions across birds.
The regions of the avian brain that might be relevant for migration include the telencephalon, the cerebellum and the optic lobe [2–4]. Diverse information relevant for migration projects to nuclei of the telencephalon processing spatial cues (hippocampus), magnetoreception and night vision (cluster N), audition (auditory cortex), olfaction (olfactory bulb), visual cues (visual Wulst, entopallium) and putative non-compass magnetic map information (trigeminal nerve recipient hindbrain nuclei) [4–12]. The telencephalon serves various functions, and navigation and sensory information processing constitute only a fraction of these. Therefore, given the high energy demands of large brains, an overall increase in telencephalon or whole brain size can hardly be expected [13], and especially not in species with demanding life-histories, such as migrants. Indeed, the telencephalon is smaller in migratory than resident bats [14], in 15 closely related songbirds [4], as well as in the migratory subspecies of dark-eyed junco (Junco hyemalis) [15].
The cerebellum of birds and mammals is relatively large and well developed compared with other vertebrates [16]. It coordinates skeletal muscles, and hence a well-developed cerebellum would imply fine motor dexterity, higher motion precision, and better coordination and timing during flight [17]. However, increased structural complexity and not cerebellar volume correlates with tool use and nest complexity in birds [3,18]. Additionally, the relative size of the cerebellum does not differ between sedentary and migratory bats [14]. Whether an enlarged cerebellum in birds serves as an evolutionary adaptation to long-distance flight is an open question.
The optic lobe is part of the midbrain and is well developed in birds [16,19]; it processes visual, auditory and somatosensory information. The optic tectum, the elaborately laminated supraventricular part of the lobe, is a mainly retinorecipient brain region (part of the primary visual pathway) and receives up to 90% of visual information in birds [20]. The roles of the optic tectum also include head and eye orientation towards visual and auditory stimuli, visual discrimination, spatial positioning of stimuli and motion processing [16,19,21]. Therefore, the optic lobe may play an important role in navigation, although such an association lacks evidence.
Here, we hypothesize that the size of different avian brain regions has coevolved with migration distance. We predict that longer migration distance will be associated with decreased whole brain and telencephalon size due to energetic limitations [4,22,23] and increased cerebellum and optic lobe size, because the amount of motor, visual and positional information to be processed increases with migration distance. We test these predictions using brain component sizes and migration distance for birds of six continents from a wide taxonomic range.
2. Material and methods
We extracted brain size (whole brain, telencephalon, cerebellum, optic lobe) and body mass data for 152 species of birds (electronic supplementary material, appendix S1). We calculated species-specific migration distances using distribution map shape files [24]. Wing morphology has been suggested to explain variation in migration distance [25], and, therefore, the potential confounding effects of wing area and aspect ratio (available for 91 species) were controlled in multivariate models. We built phylogenetic generalized least squares (PGLS) models with body mass, brain region sizes, and wing morphology as explanatory variables and migration distance as the dependent variable (electronic supplementary material, appendix S1).
3. Results
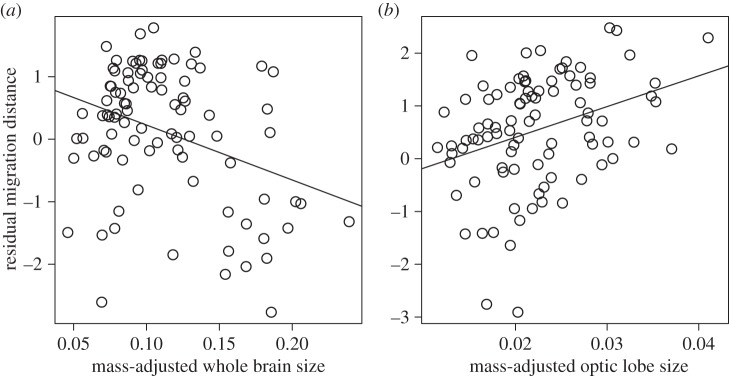
Migration distance in 152 bird species ranged from 0 to 8466 km and was strongly positively correlated with wing aspect ratio across 91 species (table 1). Relative brain mass strongly decreased, while the relative size of the optic lobe increased with increasing migration distance (table 1 and figure 1). Telencephalon size decreased with migration distance in models only containing body mass and the size of telencephalon (PGLS, n = 152, β (s.e.) = –3.67 (0.79), t = –4.62, p < 0.0001), although this association disappeared when mass of the entire brain was included (PGLS, n = 152, β (s.e.) = –3.24 (3.59), t = –0.90, p = 0.3685). The size of the cerebellum was not related to migration distance (table 1; electronic supplementary material, table S1). Aspect ratio, brain mass and size of the optic lobe explained 46% of total variance in average migration distance of 91 species. These results were robust regardless of how we controlled for brain allometry, and whether we controlled for wing architecture (table 1; electronic supplementary material, table S1). Results were very similar when considering passerines only, which represent a phylogenetically, morphologically and behaviourally more uniform taxonomic group than our complete dataset (electronic supplementary material, table S1).
Table 1.
Results of multivariate PGLS models explaining variation in migration distance across 91 species of birds.
| full model |
minimal model |
||||||
|---|---|---|---|---|---|---|---|
| predictor | β (s.e.) | t | p-value | predictor | β (s.e.) | t | p-value |
| (intercept) | –0.50 (4.58) | 0.11 | 0.9138 | (intercept) | –4.89 (2.20) | 2.22 | 0.0290 |
| body mass | 1.02 (0.94) | 1.09 | 0.2780 | body mass | 0.96 (0.80) | 1.20 | 0.2333 |
| aspect ratio | 11.09 (2.05) | 5.42 | <0.0001 | aspect ratio | 11.05 (1.89) | 5.85 | <0.0001 |
| wing area | 0.32 (0.88) | 0.37 | 0.7156 | brain mass | –4.83 (1.15) | 4.21 | 0.0001 |
| brain mass | –15.17 (9.13) | 1.66 | 0.1004 | size of optic lobe | 2.69 (1.25) | 2.15 | 0.0345 |
| size of telencephalon | 7.29 (6.46) | 1.13 | 0.2623 | ||||
| size of optic lobe | 3.69 (1.65) | 2.23 | 0.0285 | ||||
| size of cerebellum | 1.44 (2.03) | 0.71 | 0.4799 | ||||
| Pagel's λ = 0.76, n = 91, R2 = 0.47 | Pagel's λ = 0.80, n = 91, R2 = 0.46 | ||||||
Figure 1.
Residual migration distance extracted from the minimal model presented in table 1 after excluding (a) brain mass or (b) optic lobe, in relation to body mass-adjusted (a) brain mass or (b) optic lobe of different bird species. Slopes were obtained from a linear regression between the plotted variables.
4. Discussion
Using data on 152 bird species from six continents and 61 families, we provide evidence of a positive association between optic lobe size and migration distance. Additionally, migration distance has a non-uniform association with different brain regions with increasing migration distance. Whole brain and telencephalon sizes decreased, while cerebellum size did not change with increasing migration distance.
The importance of high visual abilities in navigation has long been proposed [1,2]. The increase in optic lobe size with longer migration distance suggests that visual cues play a crucial role in migration. Visual cues from the environment are projected to the superficial layers of the optic tectum in the form of a topographic map (retinotopic map; [26]), while the deeper layers are motoric, guiding eye and head movement and spatial attention to salient environmental stimuli, without the need of cortical processing [19,26]. Our result indicates that the ecological challenge imposed by orientation during migration might favour the evolution of an efficient neural substrate responsible for the above capacities. Sun compass, surface reflections, motion relative to flock-mates and stabilizing visual stimuli during flight may all select for larger optic lobe in migrants, for a better visual perception and for quick flight manoeuvres. Alternatively, long-distance migrants encounter a diverse set of habitats during migration, where developed visual processing may allow for faster survey of the new environment and, therefore, better predator avoidance [27]. Note however that (i) the optic lobe is multisensory, also processing auditory and somatosensory information, which might explain the association found and (ii) apart from the tectofugal visual pathway, the thalamofugal and accessory optic pathways may also be relevant for migrants.
Both whole brain and telencephalon size decreased with migration distance, although the latter effect disappeared when whole brain size was controlled statistically. This result indicates that increasing migration distance selects for decreased whole brain size, and that the decrease in the size of telencephalon accounts for most of this overall brain size reduction. Decrease in brain size and/or telencephalon with migration has repeatedly been shown in diverse taxa [5,14,23,28]. The energy trade-off hypothesis suggests that the energetically demanding brain and migration compete for resources, which leads to a compromise in brain size. The behavioural plasticity hypothesis states that resident species experience selection for large brains because better cognition would help them survive in seasonally changing and capricious environments [23].
Cerebellum size did not change with migration distance, suggesting that migratory flight does not depend on motor dexterity. In fact, motor dexterity has repeatedly been linked to cerebellar structure complexity rather than cerebellar volume [3,18].
Brain compartmentalization reflects the distinct selective pressures to which species are subject [3,29]. Therefore, comparative studies that link complex behaviours to brain size should handle different brain regions separately [13]. Here, we performed a detailed study of migration distance and its association with gross sections of the brain. A more powerful approach would be to study brain subdivisions on finer structural scales (e.g. hippocampus, entopallium). We further emphasize the importance of using continuous rather than categorical measures of migratory behaviours in neuroecological studies for more reliable results.
In conclusion, bird migration is associated with a smaller whole brain, smaller telencephalon and a larger optic lobe, implying that visual information might play a key role in the evolution of this behavioural syndrome.
Supplementary Material
Supplementary Material
Ethics
This study required no ethical permit, since all data were retrieved from the published literature.
Data accessibility
Data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.5q034.
Authors' contribution
A.P.M. designed the study; O.V. carried out statistical analyses; O.V., C.I.V., P.L.P. and A.P.M. wrote the manuscript with input from G.O.; all authors collected data, provided intellectual input and approved the final manuscript.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by TÁMOP 4.2.4. A/2-11-1-2012-0001 ‘National Excellence Program’. Mobility was financed by a bilateral grant (no. RO-HU 679/2013). Four anonymous reviewers provided constructive criticism.
References
- 1.Frost BJ, Mouritsen H. 2006. The neural mechanisms of long distance animal navigation. Curr. Opin. Neurobiol. 16, 481–488. (doi:10.1016/j.conb.2006.06.005) [DOI] [PubMed] [Google Scholar]
- 2.Cochran WW, Mouritsen H, Wikelski M. 2004. Migrating songbirds recalibrate their magnetic compass daily from twilight cues. Science 304, 405–408. (doi:10.1126/science.1095844) [DOI] [PubMed] [Google Scholar]
- 3.Iwaniuk AN, Lefebvre L, Wylie DR. 2009. The comparative approach and brain–behaviour relationships: a tool for understanding tool use. Can. J. Exp. Psychol. 63, 150–159. (doi:10.1037/a0015678) [DOI] [PubMed] [Google Scholar]
- 4.Fuchs R, Winkler H, Bingman VP, Ross JD, Bernroider G. 2014. Brain geometry and its relation to migratory behavior in birds. J. Adv. Neurosci. Res. 1, 1–9. (doi:9.10.15379/2409-3564) [Google Scholar]
- 5.Healy SD, Krebs JR. 1991. Hippocampal volume and migration in passerine birds. Naturwissenschaften 78, 424–426. (doi:10.1007/BF01133419) [Google Scholar]
- 6.Sherry DF. 2006. Neuroecology. Annu. Rev. Psychol. 57, 167–197. (doi:10.1146/annurev.psych.56.091103.070324) [DOI] [PubMed] [Google Scholar]
- 7.Heyers D, Manns M, Luksch H, Güntürkün O, Mouritsen H. 2007. A visual pathway links brain structures active during magnetic compass orientation in migratory birds. PLoS ONE 2, e937 (doi:10.1371/journal.pone.0000937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zapka M, et al. 2009. Visual but not trigeminal mediation of magnetic compass information in a migratory bird. Nature 461, 1274–1278. (doi:10.1038/nature08528) [DOI] [PubMed] [Google Scholar]
- 9.Heyers D, Zapka M, Hoffmeister M, Wild JM, Mouritsen H. 2010. Magnetic field changes activate the trigeminal brainstem complex in a migratory bird. Proc. Natl Acad. Sci. USA 107, 9394–9399. (doi:10.1073/pnas.0907068107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lefeldt N, Heyers D, Schneider NL, Engels S, Elbers D, Mouritsen H. 2014. Magnetic field-driven induction of ZENK in the trigeminal system of pigeons (Columba livia). J. R. Soc. Interface 11, 20140777 (doi:10.1098/rsif.2014.0777) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mouritsen H, Feenders G, Liedvogel M, Wada K, Jarvis ED. 2005. Night-vision brain area in migratory songbirds. Proc. Natl Acad. Sci. USA 102, 8339–8344. (doi:10.1073/pnas.0409575102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kishkinev D, Chernetsov N, Heyers D, Mouritsen H. 2013. Migratory reed warblers need intact trigeminal nerves to correct for a 1,000 km eastward displacement. PLoS ONE 8, e65847 (doi:10.1371/journal.pone.0065847) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Healy SD, Rowe C. 2007. A critique of comparative studies of brain size. Proc. R. Soc. B 274, 453–464. (doi:10.1098/rspb.2006.3748) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGuire LP, Ratcliffe JM. 2011. Light enough to travel: migratory bats have smaller brains, but not larger hippocampi, than sedentary species. Biol. Lett. 7, 233–236. (doi:10.1098/rsbl.2010.0744) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cristol DA, Reynolds EB, Leclerc JE, Donner AH, Farabaugh CS, Ziegenfus WS. 2003. Migratory dark-eyed juncos, Junco hyemalis, have better spatial memory and denser hippocampal neurons than nonmigratory conspecifics. Anim. Behav. 66, 317–328. (doi:10.1006/anbe.2003.2194) [Google Scholar]
- 16.Butler AB, Hodos W. 2005. Comparative vertebrate neuroanatomy: evolution and adaptation, 2nd edn New York, NY: Wiley-Liss. [Google Scholar]
- 17.Kaas JH (ed.). 2009. Evolutionary neuroscience. Oxford, UK: Academic Press. [Google Scholar]
- 18.Hall ZJ, Street SE, Healy SD. 2013. The evolution of cerebellum structure correlates with nest complexity. Biol. Lett. 9, 20130687 (doi:10.1098/rsbl.2013.0687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wylie DRW, Gutiérrez-Ibáñez C, Iwaniuk AN, Pakan JMP. 2009. The optic tectum of birds: mapping our way to understanding visual processing. Can. J. Exp. Psychol. 63, 328–338. (doi:10.1037/a0016826) [DOI] [PubMed] [Google Scholar]
- 20.Wylie DR, Gutiérrez-Ibáñez C, Iwaniuk AN. 2015. Integrating brain, behavior, and phylogeny to understand the evolution of sensory systems in birds. Front. Neurosci. 9, 281 (doi:10.3389/fnins.2015.00281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimizu T, Bowers AN. 1999. Visual circuits of the avian telencephalon: evolutionary implications. Behav. Brain Res. 98, 183–191. (doi:10.1016/S0166-4328(98)00083-7) [DOI] [PubMed] [Google Scholar]
- 22.Bennett PM, Harvey PH. 1985. Relative brain size and ecology in birds. J. Zool. 207, 151–169. (doi:10.1111/j.1469-7998.1985.tb04920.x) [Google Scholar]
- 23.Sol D, Lefebvre L, Rodríguez-Teijeiro FD. 2005. Brain size, innovative propensity and migratory behaviour in temperate Palaearctic birds. Proc. R. Soc. B 272, 1433–1441. (doi:10.1098/rspb.2005.3099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.BirdLife International, NatureServe. 2012. Bird species distribution maps of the world, version 2.0. Cambridge, UK: BirdLife International and NatureServe. [Google Scholar]
- 25.Vágási CI, Pap PL, Vincze O, Osváth G, Erritzøe J, Møller AP. In press. Morphological adaptations to migration in birds. Evol. Biol. (doi:10.1007/s11692-015-9349-0) [Google Scholar]
- 26.Knudsen EI. 2011. Control from below: the role of a midbrain network in spatial attention. Eur. J. Neurosci. 33, 1961–1972. (doi:10.1111/j.1460-9568.2011.07696.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mettke-Hofmann C, Gwinner E. 2003. Long-term memory for a life on the move. Proc. Natl Acad. Sci. USA 100, 5863–5866. (doi:10.1073/pnas.1037505100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winkler H, Leisler B, Bernroider G. 2004. Ecological constraints on the evolution of avian brains. J. Ornithol. 145, 238–244. (doi:10.1007/s10336-004-0040-y) [Google Scholar]
- 29.Iwaniuk AN. 2004. A mosaic pattern characterizes the evolution of the avian brain. Proc. R. Soc. Lond. B 271, S148–S150. (doi:10.1098/rsbl.2003.0127) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.5q034.