Abstract
Maternal antibodies (MatAb) are known to provide passive protection early in life for young vertebrates but their effects on the development of offspring immune response across generations are still unknown. Here, we investigated the effects of antigen exposure (keyhole limpet haemocyanin, KLH) experienced by urban pigeon (Columba livia) females on the amount of antigen-specific antibodies (Abs) transferred into the egg yolk of their daughters and on the humoural immune response towards this same antigen in their grandchildren. We found that chicks from KLH-injected maternal grandmothers had a higher humoural response than chicks from sham-injected grandmothers. However, we did not detect a significant effect of female KLH exposure on the ability of their daughters to transmit anti-KLH Abs into their eggs. These results suggest that antigen exposure at one generation may shape the immune profile of offspring over two next generations, although the underlying mechanisms remain to be investigated.
Keywords: maternal antibodies, immune response, grand-maternal effects
1. Introduction
The transfer of maternal antibodies (MatAb) in vertebrates is a well-documented example of maternal effect [1,2] by which mothers can confer protection against parasites to their offspring [3] and may educate their developing immune system [4,5]. Some recent empirical studies suggest that the effect of MatAb could span over two generations [6]. Indeed, in the developing immune system of the neonate, MatAb could prime the mother's antigen repertoire to which the grandmother has been exposed [6] and enhance the immune system of the mother, which could in turn influence the immune system of their grandchildren through MatAb. For instance, F0 grandparents exposed to parasites would produce Abs towards the parasite. Such specific Abs will be then transferred to the F1 generation and would enhance the immune response towards the same parasite in the F1 generation through educational effects. This F1 MatAb would be transferred to the F2 generation and could trigger a better immune response towards the same antigen in the F2 generation (transgenerational educational effects). We define epigenetic as the inheritable modifications of gene expression without changes in the underlying DNA sequences [7,8]. In the context of immune maternal effects, the phenotypic level of parasite resistance induced by the transfer and the educational properties of MatAb would be a non-genetically inherited phenotype from mothers and therefore could constitute a good example of an epigenetic effect.
Recently, the evolutionary importance of epigenetic effects in inclusive heritability and fitness [8] has been the focus of fascinating debates [9] but most empirical work mainly focused on cultural heritability [10] and on DNA switch-off [11]. Here, we hypothesized that specific MatAb would be a non-genetic indirect messenger of the phenotypic level of parasite resistance over two successive generations. This hypothesis therefore predicted that the humoural immune response of juveniles towards a specific antigen would be positively linked to the level of antigen exposure towards this same antigen of their mothers and grandmothers. Although previous studies on pigeons did not bring clear evidence of educational effects of MatAb on short timescales [12], offspring long-term immune repertoires could be modified by MatAb [13] and may enhance the transfer of MatAb to the next generation. If this hypothesis is true, we predict that daughters of antigen-exposed mothers would transfer more antibodies (Abs) into their eggs than non-exposed mothers. We tested these predictions in urban pigeons (Columba livia) by investigating the effects of antigen exposure experienced by F0 grandmothers on the amount of Abs transferred into the egg yolk of their F1 daughters and on the humoural immune response towards this same antigen in their F2 grandchildren.
2. Material and methods
The experiment was conducted between 2010 and 2012; 120 adult feral pigeons (60 females and 60 males) from three suburban locations near Paris were captured by the SACPA company (France) under the authorization of local authorities and kept in 10 outdoor aviaries located at the CEREEP field station (CEREEP-Ecotron Ile-de-France, UMS 3194, Ecole Normale Supérieure, St-Pierre-les-Nemours). Each aviary contained six males and six females in similar conditions (F0 generation).
The F0 generation was injected in 2010. Sixty birds (three breeding pairs chosen randomly in each aviary) received a subcutaneous injection of a 100-µl solution containing 0.5 mg ml−1 of keyhole limpet haemocyanin (KLH). We chose to use KLH, a novel antigen for pigeons, and not a natural antigen, to ensure that females had no pre-existing specific Abs that could have masked the effects of the experimental treatment. The 60 remaining birds were injected with phosphate-buffered saline (PBS). A second injection was performed two weeks later to ensure that blood anti-KLH Ab levels differed between KLH and sham-injected treatment groups. In a previous study, we found that these injections induced a higher transmission of maternal anti-KLH Ab from the F0 mother to the F1 generation [12].
The F1 generation contained 88 chicks born in 2010 (42 females and 46 males). All individuals of this F1 generation were injected with KLH (0.5 mg ml−1) at 21 and 35 days of age. Reproduction of the F1 generation was then monitored during 1 year after these early injections. From April to September 2011, we collected 142 eggs from 25 females of the F1 generation. Ninety-two eggs were laid by 14 F1 daughters from 10 F0 sham-injected females and 50 eggs were laid by 11 F1 daughters from eight F0 KLH-injected females. There was no significant effect of F0 treatment on the number of eggs laid by F1 daughters (F1,16 = 0.90, p = 0.36). Egg yolks were isolated and diluted 1 : 1 in PBS, homogenized for 1 min with a homogenizer (ULTRA-TURRAX, T10 Basic Disperser, Science Lab, Houston, USA). Chloroform was then added 1 : 1 and homogenized for 1 min with a vortex. After centrifugation (6 min at 13 r.p.m.), the supernatant was used for Ab assays.
In 2012, eggs laid by F1 birds were left in the nests to hatch. Thirty-three chicks were born and constituted the F2 generation. We injected all F2 chicks at 21 and 35 days subcutaneously with 100 µl of a solution containing 0.5 mg ml−1 of KLH. To monitor the dynamics of the humoural immune response towards KLH across time, we collected blood samples from chicks from the brachial vein at 3, 7, 14, 21, 28, 35, 42, 49 and 56 days. Fourteen chicks had KLH-injected maternal grandmothers (N = 6 grandmothers) and 19 chicks had sham-injected maternal grandmothers (N = 7 grandmothers). Plasma were then extracted and stored at −20°C. The ELISA technique was used to quantify the amount of anti-KLH Abs in the plasma as described by Jacquin et al. [13].
All statistical analyses were performed using SAS (v. 9.4). We first used a linear mixed model with the anti-KLH Ab concentration in eggs of the F1 generation as the dependent variable and the immune treatment of the F0 females as an explanatory variable. We added the F0 and F1 female identities as random effects to take into account for the non-independence of eggs laid by the same mothers. Second, we used a generalized linear mixed model with the anti-KLH Ab concentration of the F2 chicks as the dependent variable and the immune treatment of the F0 grandmothers and F2 chick age as a fixed effect. Chick, F0 and F1 female identities were added as random effects for similar reasons as previously described. Anti-KLH Ab concentrations were log transformed to achieve normality.
3. Results
The concentration of anti-KLH Abs in eggs laid by F1 daughters was not significantly affected by the immune treatment of the F0 grandmothers (figure 1, F1,117 = 0.76, p = 0.38). The dynamics of the humoural immune response towards KLH injections of the F2 chicks was affected by the immune treatment of their F0 maternal grandmother over time as shown by the significant interaction between chick age and immune treatment of the F0 maternal grandmother (table 1 and figure 2). At 49 days, anti-KLH Ab concentrations was higher in F2 chicks from KLH-injected maternal grandmothers compared to chicks from sham-injected maternal grandmothers (F1,20 = 5.09, p = 0.04). No significant differences were detected at the other ages (all p-values > 0.08).
Figure 1.
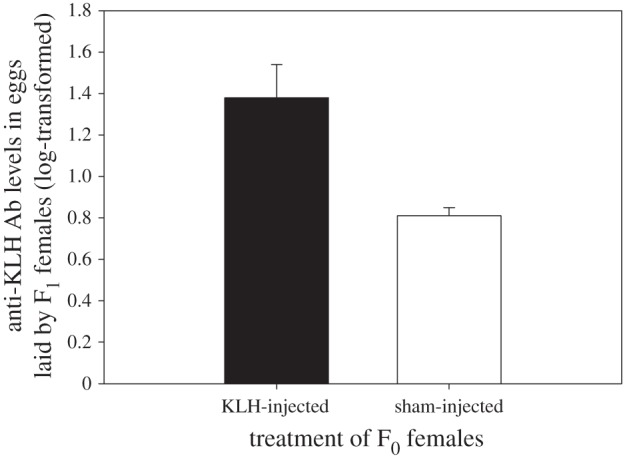
Means ± s.e. of anti-KLH Ab level in egg yolk laid by F1 daughters of KLH-injected (black bar, N = 50) or sham-injected (open bar, N = 92) F0 females. The apparent difference between the two bars is not significant when taking into account the pseudo-replication (F1,117 = 0.76, p = 0.38).
Table 1.
Output of the best-fitted generalized mixed models explaining variations in anti-KLH Ab levels over time of F2 chicks (F2 chick age) following two injections (at 21 and 35 days old) in interaction with the treatment of their F0 maternal grandmothers. d.f. represents the degrees of freedom, the F and p represent, respectively, the F statistics and the p-values.
| F2 anti-KLH humoural response | |||
|---|---|---|---|
| effects | d.f. | F | p |
| F2 chick age | 8, 241 | 99.65 | <0.0001 |
| F0 grandmother treatment | 1, 241 | 1.96 | 0.16 |
| F2 chick age × F0 grandmother treatment | 8, 241 | 2.83 | 0.005 |
Figure 2.
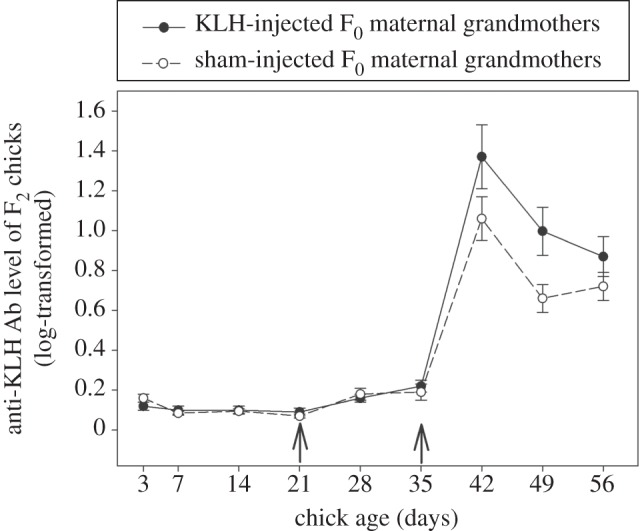
Humoural immune response of F2 chicks over time following KLH injection at 21 and 35 days old, measured by anti-KLH Ab level in relation to age for F2 chicks that had KLH-injected maternal grandmothers (black dots, N = 14) and chicks that had sham-injected maternal grandmothers (white dots; N = 19). Arrows represent KLH injections.
4. Discussion
As predicted, F2 chicks from KLH-injected F0 maternal grandmothers had a higher humoural immune response against KLH, than F2 chicks from F0 sham-injected grandmothers. Our study therefore shows that immune factors transferred from mothers to offspring can influence the immune response of the next generations.
In a previous study, we showed that F0 antigen exposure triggered a higher transmission of MatAb to the F1 generation [12]. These MatAb were found to decrease the F1 humoural response of nestlings injected at 21 and 35 days of age, though a classic blocking effect of MatAb [1]. Here, we hypothesized that F0 MatAb would however increase the long-term immune response of F1 birds through an educational effect and allow them a higher transmission of anti-KLH Abs into F1 eggs transferred to the F2 chicks. However, our results do not support this hypothesis because eggs from F1 daughters from KLH-injected F0 females did not contain a significantly higher amount of MatAb than eggs of F1 daughters from sham-injected F0 females. Therefore, we cannot explain why KLH-injected grandmothers produced F2 grandchildren with a better humoural response against KLH. Three hypotheses can however be proposed to explain this intriguing result. First, our statistical power might not be high enough to detect the difference observed even if specific MatAb are the transgenerational messenger. Second, the Ab and their educational properties might not be the transgenerational messenger and another physiological mechanism may play this role. For instance, there is growing evidence that nutrients and hormones packed into the egg could affect several physiological traits such as the immune system [14]. Prenatal immune activation of F1 generations could positively affect the investment of nutrients and/or hormones into the egg yolk which could affect the development of the immune system over the next generation. Third, the anti-KLH MatAb transferred from F0 to F1 could prime the F1 immune repertoire that could then be transmitted to F2 chicks and shape their immune memory, without involving the transmission of a higher amount of Abs into F1 eggs [15]. Our results are in favour of this last explanation, because only the secondary immune response (involving the formation of immune memory) was affected by F0 injection in F2 chicks but not the primary response (figure 2). This calls for further studies to test this interesting hypothesis, for instance through the direct manipulation of immune factors in the egg. In addition, here we used an artificial antigen to which pigeons were never exposed (KLH), and further works are now needed to test the fitness consequences of grand-maternal exposure to natural parasites.
In conclusion, this study shows that the grandmother's immunological memory can be transferred to the egg yolk and affect the immune quality of the second generation, therefore impacting the ontogenic trajectory of individuals across at least two generations. This suggests the existence of a transgenerational epigenetic effect of maternal parasite exposure on offspring immune profile over two generations which can play a major role in evolutionary ecology. More knowledge is now needed to decipher the physiological messengers of such an epigenetic effect of the antigen repertoire to which the mother has been exposed over her lifetime.
Acknowledgements
We are very grateful to Jade Dauvillers, Anne-Caroline Prévot, Adrien Frantz, Gérard Leboucher, Hélène Corbel, Philippe Lenouvel and the CEREEP station for the help they provided at different stages of the study.
Ethics
All experiments were conducted under the approbation of the French Veterinary Services of the DSV77 (authorization N°77-05).
Data accessibility
Data available from the Dryad Digital Repository: http://datadryad.org/review?doi=doi:10.5061/dryad.nc862.
Authors' contributions
A.I. designed the study, collected field data, carried out the statistical analyses and drafted the manuscript; L.J. participated in the design and conceived the study, helped to collect field data and helped draft the manuscript; C.H. carried out immunological analyses and helped draft the manuscript; S.P. helped to collect field data and helped draft the manuscript; J.G. conceived, designed and coordinated the study, participated in data analysis and helped draft the manuscript. All authors gave final approval for publication. All the authors agree to be held accountable for all aspects of the work.
Competing interests
We have no competing interests.
Funding
This work was financed by grants from the local government (Ile-de-France: Sustainable Development Network R2DS, no. 2008-07). L.J. was supported by a grant from the French Ministry of Research. A.I. was supported by a scholarship from Damascus University in Syria.
References
- 1.Boulinier T, Staszewski V. 2008. Maternal transfer of antibodies: raising immuno-ecology issues. Trends Ecol. Evol. 23, 282–288. ( 10.1016/j.tree.2007.12.006) [DOI] [PubMed] [Google Scholar]
- 2.Hasselquist D, Nilsson J-A. 2009. Maternal transfer of antibodies in vertebrates: trans-generational effects on offspring immunity. Phil. Trans. R. Soc. B 364, 51–60. ( 10.1098/rstb.2008.0137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gasparini J, McCoy KD, Tveraa T, Boulinier T. 2002. Related concentrations of specific immunoglobulins against the Lyme disease agent Borrelia burgdorferi sensu lato in eggs, young and adults of the kittiwake (Rissa tridactyla). Ecol. Lett. 5, 519–524. ( 10.1046/j.1461-0248.2002.00345.x) [DOI] [Google Scholar]
- 4.Gasparini J, McCoy KD, Staszewski V, Haussy C, Boulinier T. 2006. Dynamics of anti-Borrelia antibodies in black-legged Kittiwake (Rissa tridactyla) chicks suggest a maternal educational effect. Can. J. Zool. 84, 623–627. ( 10.1139/z06-024). [DOI] [Google Scholar]
- 5.Reid JM, Arcese P, Keller LF, Hasselquist D. 2006. Long-term maternal effect on offspring immune response in song sparrows Melospiza melodia. Biol. Lett. 2, 573–576. ( 10.1098/rsbl.2006.0544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lemke H, Coutinho A, Lange H. 2004. Lamarckian inheritance by somatically acquired maternal IgG phenotypes. Trends Immunol. 25, 180–186. ( 10.1016/j.it.2004.02.007) [DOI] [PubMed] [Google Scholar]
- 7.Poulin R, Thomas F. 2008. Epigenetic effects of infection on the phenotype of host offspring: parasites reaching across host generations. Oikos 117, 331–335. ( 10.1111/j.2007.0030-1299.16435.x) [DOI] [Google Scholar]
- 8.Danchin E, Charmantier A, Champagne FA, Mesoudi A, Pujol B, Blanchet S. 2011. Beyond DNA: integrating inclusive inheritance into an extended theory of evolution. Nat. Rev. Genet. 12, 475–486. ( 10.1038/nrg3028) [DOI] [PubMed] [Google Scholar]
- 9.Laland K, Uller T, Feldman M, Sterelny K, Mueller GB, Moczek A, Jablonka E, Odling-Smee J. 2014. Does evolutionary theory need a rethink? Nature 514, 161–164. ( 10.1038/514161a) [DOI] [PubMed] [Google Scholar]
- 10.Danchin E, Wagner RH. 2010. Inclusive heritability: combining genetic and non-genetic information to study animal behavior and culture. Oikos 119, 210–218. ( 10.1111/j.1600-0706.2009.17640.x) [DOI] [Google Scholar]
- 11.Bossdorf O, Richards CL, Pigliucci M. 2008. Epigenetics for ecologists. Ecol. Lett. 11, 106–115. ( 10.1111/j.1461-0248.2007.01130.x) [DOI] [PubMed] [Google Scholar]
- 12.Ismail A, Jacquin L, Haussy C, Legoupi J, Perret S, Gasparini J. 2013. Food availability and maternal immunization affect transfer and persistence of maternal antibodies in nestling pigeons. PLoS ONE 8, e79942 ( 10.1371/journal.pone.0079942) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacquin L, Haussy C, Bertin C, Laroucau K, Gasparini J. 2013. Darker female pigeons transmit more specific antibodies to their eggs than do paler ones. Biol. J. Linn. Soc. 108, 647–657. ( 10.1111/bij.12001) [DOI] [Google Scholar]
- 14.Muller W, Groothuis TGG, Kasprzik A, Dijkstra C, Alatalo RV, Siitari H. 2005. Prenatal androgen exposure modulates cellular and humoral immune function of black-headed gull chicks. Proc. R. Soc. B 272, 1971–1977. ( 10.1098/rspb.2005.3178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemke H, Lange H. 1999. Is there a maternally induced immunological imprinting phase à la Konrad Lorenz? Scand. J. Immunol. 50, 348–354. ( 10.1046/j.1365-3083.1999.00620.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available from the Dryad Digital Repository: http://datadryad.org/review?doi=doi:10.5061/dryad.nc862.


