Abstract
Animal pain is defined by a series of expectations or criteria, one of which is that there should be a physiological stress response associated with noxious stimuli. While crustacean stress responses have been demonstrated they are typically preceded by escape behaviour and thus the physiological change might be attributed to the behaviour rather than a pain experience. We found higher levels of stress as measured by lactate in shore crabs exposed to brief electric shock than non-shocked controls. However, shocked crabs showed more vigorous behaviour than controls. We then matched crabs with the same level of behaviour and still found that shocked crabs had stronger stress response compared with controls. The finding of the stress response, coupled with previous findings of long-term motivational change and avoidance learning, fulfils the criteria expected of a pain experience.
Keywords: decapod, crustacean, pain, nociception, lactate
1. Introduction
In the UK, vertebrates are protected in scientific investigations and this has recently been extended to cephalopod molluscs (Directive 2010/63/EU). However, the vast bulk of invertebrates are considered not to experience pain and receive no protection, their responses regarded as purely nociceptive reflexes. Indeed, a recent review dismisses the idea that any invertebrates (or fish) experience pain because they lack the specific brain areas implicated in human pain experience [1]. However, this reasoning ignores the different neuronal structures in widely divergent taxa that have the same function, e.g. visual processing occurs in very different brains of vertebrates and invertebrates [2,3]. Nevertheless, we cannot determine what any animal specifically feels when exposed to noxious stimuli [4]. Thus, to guide investigations, recent definitions of animal pain include criteria that should be fulfilled before we accept possible pain experience (e.g. [5]). In particular, we expect activities that go beyond mere reflex response and instead indicate central processing and long-term motivational change that protects the animal from further damage [3,5], and physiological changes in response to aversive stimuli [5,6]. Cephalopods fulfil such criteria, showing complex and long-lasting motivational change accompanied by physiological changes after tissue damage and increased wariness against subsequent predatory attempts [7–10]. Importantly, these changes confer an advantage to the animals' survival [10].
Here we focus on decapod crustaceans, which also show responses consistent with the idea of pain. For example, shore crabs rapidly learn to avoid particular locations associated with electric shock [11]. Hermit crabs shocked briefly within their gastropod shell show a marked prolonged increase in their motivation to get a new shell [12,13]. Hermit crabs [12] subject to shock on the abdomen, and glass prawns that have acetic acid or sodium hydroxide applied to an antenna [14] show prolonged grooming and rubbing of the specific afflicted abdominal area or antenna, which in the latter is reduced by local anaesthetic. Hermit crabs subject to a shock on the abdomen differ in the tendency to abandon the shell depending on their shell preference, indicating central processing and a motivational trade-off between retaining a desired shell and shock avoidance [13]. Further, crabs that have formalin injected into a cheliped show shaking of the appendage and prolonged changes in brain and thoracic ganglion function [15]. Finally, crayfish subject to a noxious electric field response show a subsequent greater avoidance of light arms of a dark/light plus maze, which has been interpreted as anxiety [16].
Thus, decapods show behavioural responses to noxious stimuli that meet criteria expected for animals that experience pain [5]. However, we also expect to see physiological changes. Certainly, stressed decapods show elevated crustacean hyperglycaemic hormone (CHH), resulting in elevated glucose and lactate analogous to the vertebrate stress response [17]. Effective stressors include emersion [17], hypoxia, elevated temperature, altered salinity and disease [18]. Further, pulling off a cheliped, in the manner of some commercial fisheries, produces a rapid elevation of both glucose and lactate in edible crabs [19]. Also, crayfish repeatedly subject to noxious electric field over a period of 30 min show elevated glucose [16,20]. However, these two findings may not be due to a pain-like state because with the appendage removal there is substantial haemolymph loss and that might cause the stress. Further, crayfish respond to an electric field with repeated escape ‘tail flips’ and this prolonged vigorous activity might initiate glucose mobilization. To overcome these problems we compare animals that vary in the shock they receive but not in haemolymph loss or overt behaviour. We apply electric shock to some shore crabs and monitor behaviour and lactate levels to assess if lactate is elevated in shocked animals and if lactate levels are associated with particular activities during treatment. We then compare lactate levels of animals that show the same level of activity.
2. Material and method
European shore crabs (Carcinus maenas; 5–8 cm carapace width) were collected from Portaferry, Northern Ireland, UK, using baited pots and transported to Queen's University, Belfast, UK. They were housed in plastic tanks (76 × 38 × 17 cm) filled with aerated seawater to a depth of 5.6–6.5 cm and maintained at a 12 L : 12 D regime. Seaweed (Ascophyllum nodosum) was provided as shelter.
Each crab (N = 40) was individually transferred to a plastic tank (34.4 × 11.5 cm) containing a layer of gravel and approximately 1 cm seawater. Insulated copper wire (0.20 mm diameter), with the insulation removed from each end was placed around the proximal joint, where there is no calcification, of each fifth walking leg. Crabs were randomly assigned to shock (n = 20) or no-shock (control) (n = 20) groups, decided by a roll of a dice. The crabs to be shocked then had the other end of each wire attached to a Grass S9 electric stimulator (West Warwick, RI, USA). The left and right legs had wires randomly attached to the positive and negative terminals of the stimulator and, following Magee & Elwood [11], shock was set to deliver at 10 V and 180 Hz for 200 ms with 10 s intervals for 2 min. The wires for the control group were not attached to the stimulator but the crabs were otherwise treated the same.
Behaviour was observed during the 2 min shock or control period and crabs were categorized into three types, no movement throughout, walking but no extreme response, and extreme response, which included animals that attempted to climb the walls of the tank, showed the threat posture or autotomized a walking appendage. The number of shocked and non-shocked crabs in these categories was compared using a χ2-contingency test.
We waited for 4 min after treatment to allow for thorough circulation of the haemolymph, before a haemolymph sample was taken with a syringe inserted into the base of a fifth walking leg and the lactate level measured using a Lactate pro (Arkray Inc., Arkray Europe). The data for lactate were not normally distributed and were transformed (lactate + 1) log10 and the means compared using a Student's t test. We show untransformed means in the figure.
3. Results
Shocked crabs had higher haemolymph lactate than controls (t38 = 4.97, p < 0.0001). However, the behavioural responses differed between the two groups with controls only showing either no movement (N = 6) or walk (N = 14), whereas shocked crabs showed either walking (N = 16) or the more extreme responses (N = 4) (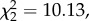 p = 0.0063). The lactate levels of controls did not differ between those that walked and remained still (t18 = 0.79, p = 0.42), but for shocked crabs there was a trend for those that showed the more extreme responses to have higher lactate than those that just walked (t18 = 2.06, p = 0.053). Thus, we focused on crabs that showed walking as their highest activity (non-shock N = 14, shock N = 16). Those that received shock had substantially higher levels of lactate than the non-shocked controls (t28 = 3.71, p < 0.001, figure 1).
p = 0.0063). The lactate levels of controls did not differ between those that walked and remained still (t18 = 0.79, p = 0.42), but for shocked crabs there was a trend for those that showed the more extreme responses to have higher lactate than those that just walked (t18 = 2.06, p = 0.053). Thus, we focused on crabs that showed walking as their highest activity (non-shock N = 14, shock N = 16). Those that received shock had substantially higher levels of lactate than the non-shocked controls (t28 = 3.71, p < 0.001, figure 1).
Figure 1.
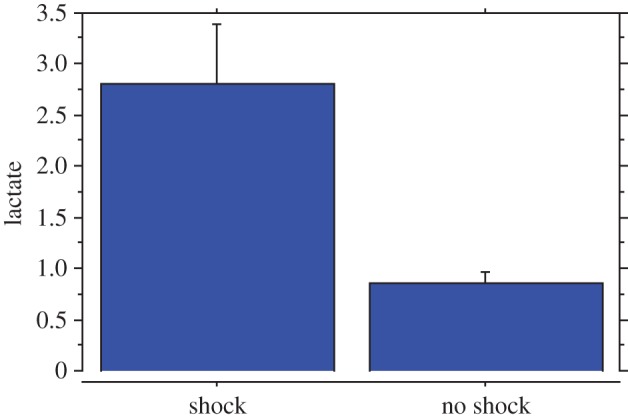
Means and standard errors of lactate (mmol l−1) for shock and control crabs that showed walking as most active response. (Online version in colour.)
4. Discussion
Electric shock as an aversive stimulus is widely used in studies of animal pain/nociception. The genetic basis of the nociceptor response to shock has recently been examined in Drosophila melanogaster [21]. Many identified candidate genes that influence the nociceptive response affect the mechanosensory bristles. Thus, it appears that shock triggers mechanoceptors, which normally respond to tissue damage. Although electric shock has little ecological relevance to decapods, the same could be said of almost any animal. Shock induces pain states in humans although it presumably has played no part in human evolution. Decapods give up key resources to avoid shock [13,22] and show rapid avoidance learning [11], indicating that shock is aversive to these animals.
When we compared all crabs that received electric shock with all the controls, the former had higher lactate, indicating that a stress response had been triggered [17,19]. However, more of the shocked crabs showed active behavioural responses and this activity could have caused the higher lactate. Indeed, in shocked crabs there was a strong tendency for those that showed more extreme responses to have higher lactate than those that just walked. By contrast, in non-shocked crabs those that walked did not differ in lactate from those that did not walk, indicating that walking during this short test did not alter lactate. Nevertheless, we restricted our final analysis to crabs that showed walking as their most active response and found that when the behavioural response was the same the shock nevertheless induced higher lactate. That is, the elevated lactate is not explained by the behavioural response and must be a consequence of the shock. The brief shocks to the base of the legs did not appear to cause substantial muscle contraction, which might have accounted for high lactate, because the crabs walked normally. The data are thus consistent with the idea that shock induces a stressful pain-like state [11,22]. Importantly, this fulfils a key criterion expected of animals that experience pain [5,6].
Fossat et al. [16] found increased glucose levels in crayfish repeatedly exposed to an aversive electric field. These animals showed repeated active tail flick escape responses during the 30-min exposure although the responses declined during this period [20]. By contrast, shore crabs did not show particularly energetically demanding behaviour over the much shorter duration of testing (2 min). That we showed a stress response in crabs that only showed walking as their most vigorous response lends support to the crayfish study [20] in concluding that the stress was induced by the aversive electric field.
The study on crayfish stress/anxiety also noted elevated serotonin, which seems to be responsible for the marked shift in risk taking, i.e. entering a brightly lit arm of a plus maze [16,20]. Serotonin also appears to be involved in the activation of the CHH stress response and subsequent release of glucose and increased lactate. Dopamine is also released after aversive stimuli but its function has yet to be defined [20]. This suggests a complex physiological stress response, the components of which presumably serve different functions.
Although these physiological responses are expected should an animal experience pain [5], they do not prove the feeling of pain in decapods because absolute proof is not possible for any animal [4]. Nevertheless, coupled with the behavioural responses to a variety of aversive stimuli, they provide evidence of both short- and long-term changes similar to those changes found in cephalopods and vertebrates. That is, the criteria suggested to indicate pain in animals [5] are fulfilled for decapods.
Supplementary Material
Acknowledgement
Gillian Riddell provided important help with crab collections and maintenance.
Ethics
No licence was required because crustaceans are excluded from the UK Animals (Scientific Procedures) Act (1986). Nevertheless, we kept the level of shock low to reduce autotomy and the sample size was kept as low as possible.
Data accessibility
All relevant data are in the electronic supplementary material.
Authors' contributions
R.W.E. designed the experiment, L.A. collected the data, and both analysed the data and wrote the manuscript. Both authors approve the final version of the article and agree to be accountable for the content therein.
Competing interests
We have no competing interests.
Funding
Funding was provided by Queen's University.
References
- 1.Rose JD, Arlinghaus R, Cooke SJ, Diggles BK, Sawynok W, Stevens ED, Wynne CDL. 2014. Can fish really feel pain? Fish Fisheries 15, 97–133. ( 10.1111/faf.12010) [DOI] [Google Scholar]
- 2.Broom DM. 2014. Sentience and animal welfare. Wallingford, UK: CABI. [Google Scholar]
- 3.Elwood RW. 2011. Pain and suffering in invertebrates? ILAR J. 52, 175–184. ( 10.1093/ilar.52.2.175) [DOI] [PubMed] [Google Scholar]
- 4.Dawkins MS. 2012. Why animals matter. Animal consciousness, animal welfare, and human well-being. Oxford, UK: Oxford University Press. [Google Scholar]
- 5.Sneddon LU, Elwood RW, Adamo SA, Leach MC. 2014. Defining and assessing animal pain. Anim. Behav. 97, 202–212. ( 10.1016/j.anbehav.2014.09.007) [DOI] [Google Scholar]
- 6.Bateson P. 1991. Assessment of pain in animals. Anim. Behav. 42, 827–839. ( 10.1016/S0003-3472(05)80127-7) [DOI] [Google Scholar]
- 7.Alupay JS, Hadjisolomou SP, Crook RJ. 2014. Arm injury produces long term behavioural and neural hypersensitivity in octopus. Neurosci. Lett. 558, 137–142. ( 10.1016/j.neulet.2013.11.002) [DOI] [PubMed] [Google Scholar]
- 8.Crook RJ, Lewis T, Roger T, Hanlon RT, Walters ET. 2011. Peripheral injury induces long-term sensitization of defensive responses to visual and tactile stimuli in the squid Loligo pealeii, Lesueur 1821. J. Exp. Biol. 214, 3173–3185. ( 10.1242/jeb.058131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crook RJ, Hanlon RT, Walters ET. 2013. Squid have nociceptors that display widespread long-term sensitization and spontaneous activity after bodily injury. J. Neurosci. 33, 10 021–10 026. ( 10.1523/JNEUROSCI.0646-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crook RJ, Dickson K, Hanlon RT, Walters ET. 2014. Nociceptive sensitization reduces predation risk. Curr. Biol. 24, 1121–1125. ( 10.1016/j.cub.2014.03.043) [DOI] [PubMed] [Google Scholar]
- 11.Magee B, Elwood RW. 2013. Shock avoidance by discrimination learning in the shore crab (Carcinus maenas) is consistent with a key criterion for pain. J. Exp. Biol. 216, 353–358. ( 10.1242/jeb.072041) [DOI] [PubMed] [Google Scholar]
- 12.Appel M, Elwood RW. 2009. Gender differences, responsiveness and memory of a potentially painful event in hermit crabs. Anim. Behav. 78, 1373–1379. ( 10.1016/j.anbehav.2009.09.008) [DOI] [Google Scholar]
- 13.Elwood RW, Appel M. 2009. Pain in hermit crabs? Anim. Behav. 77, 1243–1246. ( 10.1016/j.anbehav.2009.01.028) [DOI] [Google Scholar]
- 14.Barr S, Laming PR, Dick JTA, Elwood RW. 2008. Nociception or pain in a decapod crustacean? Anim. Behav. 75, 745–751. ( 10.1016/j.anbehav.2007.07.004) [DOI] [Google Scholar]
- 15.Dyuizen IV, Kotsyuba EP, Lamash NE. 2012. Changes in the nitric oxide system in the shore crab Hemigrapsus sanguineus (Crustacea, Decapoda) CNS induced by a nociceptive stimulus. J. Exp. Biol. 215, 2668–2676. ( 10.1242/jeb.066845) [DOI] [PubMed] [Google Scholar]
- 16.Fossat P, Bacque-Cazenave J, De Deurwaerdere P, Delbecque J-P, Cattaert D. 2014. Anxiety-like behavior in crayfish is controlled by serotonin. Science 344, 1293–1297. ( 10.1126/science.1248811) [DOI] [PubMed] [Google Scholar]
- 17.Webster SG. 1996. Measurement of crustacean hyperglycaemic hormone levels in the edible crab Cancer pagurus during emersion stress. J. Exp. Biol. 199, 1579–1585. [DOI] [PubMed] [Google Scholar]
- 18.Chang ES. 2005. Stressed-out lobsters: crustacean hyperglycemic hormone and stress proteins. Integr. Comp. Biol. 45, 43–50. ( 10.1093/icb/45.1.43) [DOI] [PubMed] [Google Scholar]
- 19.Patterson L, Dick JTA, Elwood RW. 2007. Physiological stress responses in the edible crab, Cancer pagurus, to the fishery practice of de-clawing. Mar. Biol. 152, 265–272. ( 10.1007/s00227-007-0681-5) [DOI] [Google Scholar]
- 20.Fossat P, Bacque-Cazenave J, De Deurwaerdere P, Cattaert D, Delbecque J-P. 2015. Serotonin, but not dopamine, controls stress response and anxiety-like behavior in crayfish, Procambarus clarkii. J. Exp. Biol. 218, 2745–2752. ( 10.1242/jeb.120550) [DOI] [PubMed] [Google Scholar]
- 21.Appel M, et al. 2015. Genome-wide association analyses point to candidate genes for electric shock avoidance in Drosophila melanogaster. PLoS ONE 10, e0126986 ( 10.1371/journal.pone.0126986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barr S, Elwood RW. 2011. No evidence of morphine analgesia to noxious shock in the shore crab, Carcinus maenas. Behav. Proc. 86, 340–344. ( 10.1016/j.beproc.2011.02.002) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are in the electronic supplementary material.


