Abstract
Since the first documentation of climate-warming induced declines in arctic sea-ice, predictions have been made regarding the expected negative consequences for endemic marine mammals. But, several decades later, little hard evidence exists regarding the responses of these animals to the ongoing environmental changes. Herein, we report the first empirical evidence of a dramatic shift in movement patterns and foraging behaviour of the arctic endemic ringed seal (Pusa hispida), before and after a major collapse in sea-ice in Svalbard, Norway. Among other changes to the ice-regime, this collapse shifted the summer position of the marginal ice zone from over the continental shelf, northward to the deep Arctic Ocean Basin. Following this change, which is thought to be a ‘tipping point’, subadult ringed seals swam greater distances, showed less area-restricted search behaviour, dived for longer periods, exhibited shorter surface intervals, rested less on sea-ice and did less diving directly beneath the ice during post-moulting foraging excursions. In combination, these behavioural changes suggest increased foraging effort and thus also likely increases in the energetic costs of finding food. Continued declines in sea-ice are likely to result in distributional changes, range reductions and population declines in this keystone arctic species.
Keywords: climate change, polar, Pusa hispida, ringed seal, sympagic community
1. Introduction
Over the past few decades, the Arctic has warmed at twice the global average and sea-ice extent and volume have declined markedly [1]. Continued declines are expected to result in an ice-free Arctic Ocean as early as 2037 [2], with transformative consequences for arctic ecosystems [3,4]. Endemic arctic animals that rely on sea-ice and the associated sympagic (ice-associated) ecosystem are expected to undergo major ecological shifts or face extinction [3].
Ringed seals are a keystone, endemic arctic species that is the principal prey of polar bears (Ursus maritimus) and an important resource for coastal arctic people. Their circumpolar distribution and high abundance, along with their vital roles both as a high-trophic predator and as prey in this relatively species-poor system, makes them a key link in the arctic food web. This species is intimately associated with sea-ice for most aspects of its existence [5]. Ringed seals are born on sea-ice and moult and rest on it throughout their lives, experiencing low reproductive success in years with poor ice or snow conditions [6–8].
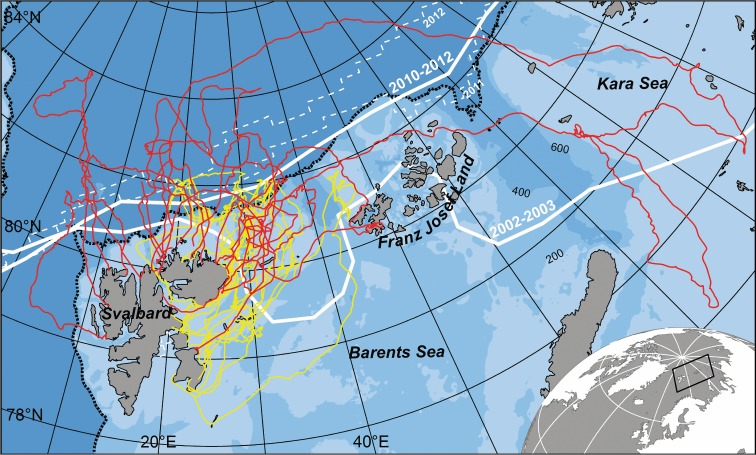
Ringed seal biological investigations have been undertaken in Svalbard, Norway, since the 1980s, providing background data on this population [8]. In 2006, the sea-ice regime in this region underwent an unpredicted collapse that has persisted to the present. Among other changes, the southern edge of the summer arctic icecap (the marginal ice zone, MIZ) has shifted northward from its previous norm over the continental shelf to a location over the deep Arctic Ocean Basin (figure 1). A ringed seal tracking study conducted in Svalbard in 2002–2003 [9] documented extensive offshore summer excursions to the MIZ by subadult seals during the post-moulting period when they acquire most of their annual energy intake [10]. The unpredicted regional sea-ice collapse that occurred several years after this study has created the possibility for a natural experiment exploring the impact of this dramatic environmental change on the behaviour of this species, via repeating tracking efforts on this population. Such knowledge will greatly improve our predictive capacity regarding global warming impacts on this keystone species because the changes occurring currently in Svalbard are predicted to spread throughout the Arctic.
Figure 1.
Offshore trips undertaken by ringed seals in 2002–2003 (yellow) and 2010–2012 (red) in Svalbard, Norway. White lines show the average (solid) and minimum (dashed) September sea-ice extent (National Snow and Ice Data Center, Boulder, CO). The black dotted line denotes the continental shelf.
2. Material and methods
Ringed seals (22 before (2002–2003) and 38 after (2010–2012) the sea-ice collapse) were equipped with Satellite-Relay Data Loggers (http://www.smru.st-andrews.ac.uk/Instrumentation/Overview/) that provided information on seal movements and behaviour. Nineteen seals in the two periods (nine in 2002–2003 and 10 in 2010–2012) undertook offshore excursions during the post-moulting period (table 1). These excursions are the subject of this study.
Table 1.
Seal and trip metrics for 60 ringed seals tagged in 2002–2003 and 2010–2012 in Svalbard, Norway, with individual details for animals undertaking offshore excursions.
| seal ID | trip | sex | mass (kg) | tagging date | departure date | return date | trip duration (d) | total distance travelled (km) | tracking duration (d) |
|---|---|---|---|---|---|---|---|---|---|
| F31–02 | 1 | F | 31 | 21 July 2002 | 22 July 2002 | 29 Aug. 2002 | 39 | 1587 | 172 |
| F33–02 | 2 | F | 33 | 21 July 2002 | 26 July 2002 | 6 Sept. 2002 | 43 | 1447 | 127 |
| F36–02 | 3 | F | 36 | 21 July 2002 | 29 July 2002 | 2 Sept. 2002 | 36 | 1509 | 126 |
| F37–02 | 4 | F | 37 | 20 July 2002 | 23 Aug. 2002 | 21 Nov. 2002 | 91 | 2203 | 187 |
| F57–02 | 5 | F | 57 | 19 July 2002 | 1 Aug. 2002 | 1 Sept. 2002 | 32 | 1422 | 169 |
| F59–02 | 6 | F | 59 | 21 July 2002 | 1 Aug. 2002 | 25 Aug. 2002 | 25 | 990 | 78 |
| F59–02 | 7 | F | 59 | 21 July 2002 | 15 Sept. 2002 | 30 Sept. 2002 | 16a | 534a | 78 |
| M50–02 | 8 | M | 50 | 19 July 2002 | 23 July 2002 | 20 Oct. 2002 | 90a | 2413a | 96 |
| F34–03 | 9 | F | 34 | 19 July 2003 | 24 July 2003 | 11 Aug. 2003 | 19 | 734 | 129 |
| F34–03 | 10 | F | 34 | 19 July 2003 | 18 Aug. 2003 | 8 Sept. 2003 | 22 | 949 | 129 |
| F37–03 | 11 | F | 37 | 22 July 2003 | 7 Aug. 2003 | 18 Sept. 2003 | 43 | 1391 | 243 |
| mean ± s.d. | 42 ± 11 | 39 ± 21 | 1359 ± 432 | 139 ± 50 | |||||
| F34–10 | 12 | F | 34 | 3 Aug. 2010 | 5 Aug. 2010 | 5 Oct. 2010 | 62 | 2048 | 193 |
| F40–10 | 13 | F | 40 | 25 July 2010 | 2 Aug. 2010 | 30 Sept. 2010 | 60 | 1677 | 235 |
| M36–10 | 14 | M | 36 | 26 July 2010 | 31 July 2010 | 12 Nov. 2010 | 105 | 5393 | 129 |
| F52–10 | 15 | F | 52 | 3 Aug. 2010 | 17 Aug. 2010 | 9 Sept. 2010 | 24 | 1009 | 224 |
| F60–10 | 16 | F | 60 | 29 July 2010 | 31 July 2010 | 2 Sept. 2010 | 34 | 1703 | 202 |
| F60–10 | 17 | F | 60 | 29 July 2010 | 3 Oct. 2010 | 19 Oct. 2010 | 17 | 970 | 202 |
| M62–10 | 18 | M | 62 | 1 Aug. 2010 | 15 Aug. 2010 | 4 Sept. 2010 | 21 | 844 | 197 |
| M90–11 | 19 | M | 90 | 3 Aug. 2011 | 6 Aug. 2011 | 1 Sept. 2011 | 27 | 1540 | 166 |
| F35–12 | 20 | F | 35 | 30 July 2012 | 12 Aug. 2012 | 27 Aug. 2012 | 16 | 677 | 306 |
| F40–12 | 21 | F | 40 | 1 Aug. 2012 | 13 Aug. 2012 | 29 Sept. 2012 | 48 | 2182 | 178 |
| M46–12 | 22 | M | 46 | 1 Aug. 2012 | 4 Aug. 2012 | 6 Aug. 2012 | 3a | 299a | 6 |
| mean ± s.d. | 50 ± 17 | 41 ± 28 | 1804 ± 1362 | 185 ± 74 | |||||
| other tagged seals | |||||||||
| mean ± s.d. | 41 seals | 20 F : 21 M | 62 ± 20 | 165 ± 66 |
aData transmission terminated while offshore (not included in the mean).
We used a generalized linear model with the binomial family and a logistic link to investigate the probability of taking an offshore trip. Possible explanatory variables included mass, sex and time period. We also conducted first-passage time (FPT) analysis on each offshore excursion to identify the spatial scale of area-restricted search (ARS) behaviour, which is associated with intense feeding activity in many species [11], and employed linear-mixed effects models to explore whether diving and movement behaviour varied between the two time periods. Trip number was included as a random effect to account for non-independence of observations within trips. Bayesian information criterion (BIC) was used for backward model selection; p-values ≤0.05 in the BIC-selected model indicated significance. A nested random effect of trip number within year was also considered but was not retained after model selection. See the electronic supplementary material for further details.
3. Results and Discussion
During offshore excursions, 19 seals transmitted over 25 000 dives over 764 tracking days (table 2; electronic supplementary material, figure S1 and S2). Body mass measurements clearly show that smaller animals (i.e. subadults) had a higher probability of undertaking offshore movements (table 1; electronic supplementary material, tables S1 and S2). Time period did not impact the probability of undertaking offshore trips, which are foraging–migratory movements rather than dispersal movements; the seals return to Svalbard in time for the traditional land-fast ice formation.
Table 2.
Offshore-trip metrics for 19 ringed seals tagged in 2002–2003 and 2010–2012 in Svalbard, Norway.
| no. individuals | no. trips | total distance travelled (km) | no. tracking days | no. locations transmitted | no. dives transmitted | |
|---|---|---|---|---|---|---|
| 2002–2003 | 9 | 11 | 12 232 | 350 | 7140 | 13 961 |
| 2010–2012 | 10 | 11 | 18 043 | 414 | 8581 | 12 744 |
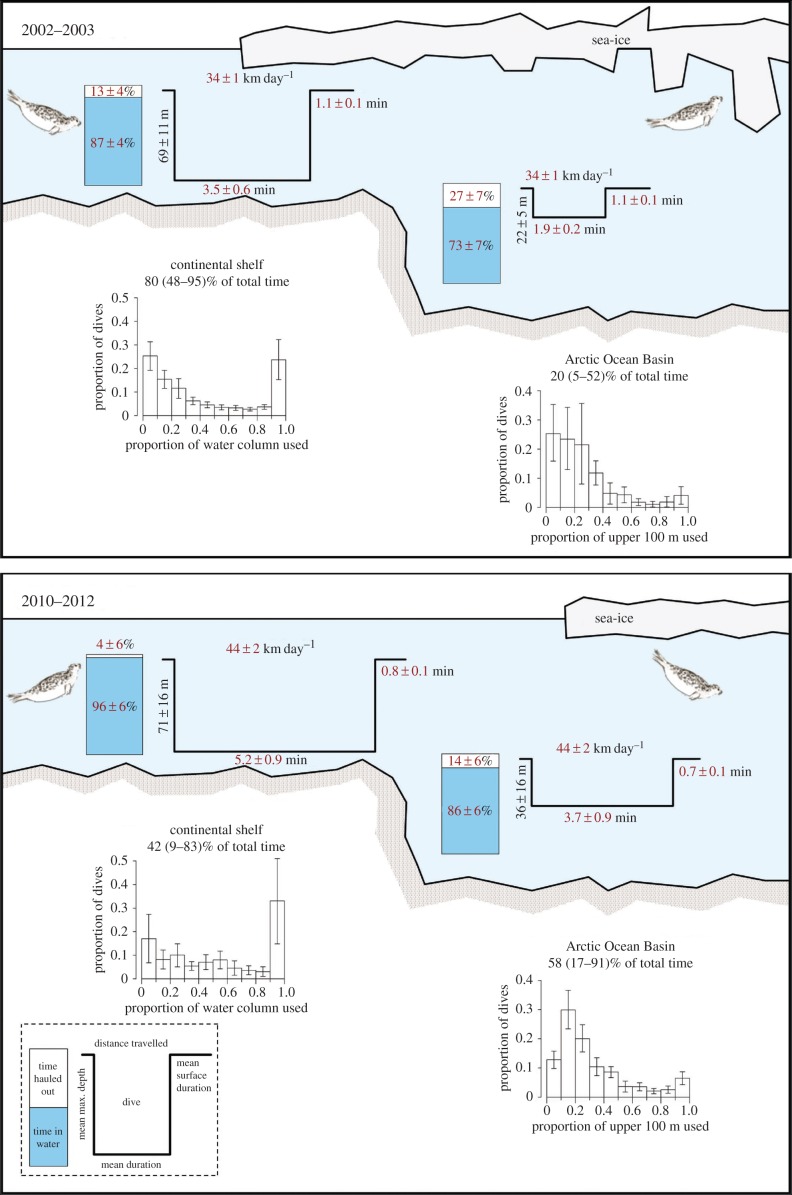
Seals in the two time periods undertook offshore trips of similar duration (p = 0.831, table 1), but they encountered sea-ice for the first time approximately 1° of latitude further north in 2010–2012 than in 2002–2003 (p = 0.004), and thus spent more time further from land (p = 0.003) and a greater proportion of time over the Arctic Ocean Basin (p = 0.072; figures 1 and 2; electronic supplementary material, tables S3 and S4). Seals in both periods occupied areas with similar sea-ice concentrations (p = 0.605) and similar proportions of first-year ice versus multiyear ice (p = 0.943; electronic supplementary material, table S3), suggesting that they sought this particular habitat, despite having to travel farther to reach it and despite its location shifting from being over the productive continental shelf to being over the less-productive Arctic Ocean Basin [12]. However, when they arrived in areas with these ice conditions significant changes in their behaviour were detected. The seals moved greater distances daily (even in areas with high sea-ice concentrations) and spent less time resting on sea-ice in 2010–2012 (figure 2; electronic supplementary material, table S4). Resting time is likely kept to a minimum if the animals are not finding sufficient food to be satiated. Seals in 2010–2012 also had longer dive durations and shorter surface intervals compared with seals in 2002–2003 (figure 2; electronic supplementary material, table S4). These findings suggest that energetic costs associated with finding food have increased in the latter period. Time-at-depth also suggests that sympagic feeding was replaced by pelagic and benthic feeding modes in 2010–2012.
Figure 2.
Behaviour metrics for ringed seals in Svalbard, Norway, during offshore trips in 2002–2003 and 2010–2012. Values in red indicate significant differences between the two time periods. Barplots over the Arctic Ocean Basin only consider the upper 100 m of the water column. Error bars in the graphs indicate 95% confidence intervals.
FPT analyses easily defined a common spatial scale for ARS during offshore excursions in 2002–2003 [9]. But, fewer seals performed clear ARS behaviour in 2010–2012 (electronic supplementary material, figure S3, p = 0.016), suggesting that the sea-ice extent retreating north of the continental shelf resulted in fewer areas where the seals could feed intensively. Two seals tagged in 2010–2012 employed strategies not seen in 2002–2003. One travelled to a glacier front in Franz Josef Land, Russia, and the other swam to the eastern Kara Sea in the Russian Arctic (total trip length of approx. 5400 km; figure 1; electronic supplementary material, figure S2). Both seals travelled to these areas via routes over the Arctic Ocean Basin. Given it is unlikely that ringed seals bypass productive foraging areas, it is probable that they did not find sufficient food along their tracks, closer to Svalbard, and thus continued to search further afield.
Ringed seals are opportunistic predators but they do show a preference for polar cod (Boreogadus saida) and lipid-rich pelagic crustaceans [5,13]. Polar cod are found in cold water masses in the Barents Sea, occupying sympagic, pelagic and benthic environments [14]. Larval and juvenile polar cod are directly affiliated with sea-ice, occupying small cracks where they are afforded some predator-protection [14]. Regionally, polar cod abundance has decreased and the 0-age class has shifted its distribution northeastward over the last decade [15], part of the rapid ‘borealization’ of the fish community of this region [16]. Polar cod occur at lower densities in the Arctic Ocean Basin compared with the arctic shelf seas [17] and the more than 40% decrease in arctic multiyear sea-ice from 2005 to 2008 [18] has likely decreased habitat quality for these fish and other ice-associated lower trophic organisms. These factors are almost certainly related to the increased foraging effort and less spatially concentrated foraging activity we document herein for ringed seals during 2010–2012.
Ringed seals in other arctic areas also undertake late-summer foraging trips [19]. But, in these other regions sea-ice changes have been much less dramatic compared with Svalbard. The Barents Sea region has experienced the largest declines in seasonal duration of arctic sea-ice cover, with more than 20 weeks fewer with ice cover from 1979 to 2013 (two to four times the reduction in other arctic areas [20]). Future retractions of summer sea-ice extent are expected [1,2]; in these conditions, ringed seals will have to travel further into the Arctic Ocean Basin to find sea-ice, further increasing energetic costs of their important post-moult foraging trips. Alternatively, they could remain in near-shore habitats, but in Svalbard this would increase competition with the older animals that remain coastal, associated with tidewater glaciers [9,21]. Such areas are spatially restricted compared to the MIZ and glaciers in Svalbard are also in negative mass-balance, with the total length of tidewater glacier fronts having already markedly decreased in recent decades [22]. A further decrease in the number of these coastal ‘hot-spots' would be critical for ringed seals in Svalbard.
Our study has shown that past predictions [3] regarding climate change impacts on ringed seals were correct—sea-ice extent declines are affecting their movement patterns and foraging behaviour. The northward retreat of the sea-ice edge has markedly increased foraging effort for subadult ringed seals. If these costs are not compensated for by increased energetic returns, this will affect growth, reproduction and mortality rates. Data documenting changes in these parameters would give additional scope to our findings; however, such data are difficult to obtain in areas such as Svalbard where little hunting takes place. But condition declines concomitant with sea-ice declines elsewhere in the Arctic strongly suggest that plasticity is limited. The behavioural changes we document all suggest that energetic costs have increased, and as sea-ice continues to retreat ringed seals will be further challenged. Shifts in the distribution and abundance of this keystone species are likely to have repercussions throughout the arctic marine ecosystem.
Supplementary Material
Acknowledgements
We thank Magnus Andersen, Lars Boehme, Heinrich Eggenfellner, Mike Fedak, Carla Freitas, Nils Christian Ravnaas Heen, Hans Lund, Benjamin Merkel and Bobben Severinsen for assistance in the field.
Ethics
Animal handling protocols were approved by the Norwegian Animal Research Authority (permit no. 10/45416) and the Governor of Svalbard (permit no. 2010/00093-19).
Data accessibility
The data are available at the Norwegian National Polar Data Centre (data.npolar.no). Contact person Kit M. Kovacs (kit.kovacs@npolar.no).
Authors' contributions
K.M.K. and C.L. conceived the study. K.M.K., C.L. and C.D.H. conducted the fieldwork. All authors analysed and interpreted the data, wrote the manuscript, approved the final version and agree to be held accountable for the manuscripts contents.
Competing interests
We have no competing interests.
Funding
This work was supported by the Norwegian Polar Institute's Centre for Ice, Climate and Ecosystems (ICE) and the Norwegian Research Council (MARE programme). C.D.H. was funded by the VISTA Scholar's programme.
References
- 1.IPCC. 2013. Climate change 2013: the physical science basis. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Wang M, Overland JE. 2009. A sea ice free summer Arctic within 30 years? Geophys. Res. Lett. 36, L07502 ( 10.1029/2009GL037820) [DOI] [Google Scholar]
- 3.Kovacs KM, Lydersen C, Overland JE, Moore SE. 2011. Impacts of changing sea-ice conditions on Arctic marine mammals. Mar. Biodivers. 41, 181–194. ( 10.1007/s12526-010-0061-0) [DOI] [Google Scholar]
- 4.Post E. et al. 2013. Ecological consequences of sea-ice decline. Science 341, 519–524. ( 10.1126/science.1235225) [DOI] [PubMed] [Google Scholar]
- 5.Reeves RR. 1998. Distribution, abundance and biology of ringed seals (Phoca hispida). NAMMCO Sci. Publ. 1, 9–45. ( 10.7557/3.2979) [DOI] [Google Scholar]
- 6.Hammill MO, Smith TG. 1991. The role of predation in the ecology of the ringed seal in Barrow Strait, NWT, Canada. Mar. Mamm. Sci. 7, 123–135. ( 10.1111/j.1748-7692.1991.tb00559.x) [DOI] [Google Scholar]
- 7.Harwood LA, Smith TG, Melling H. 2000. Variation in reproduction and body condition of the ringed seal (Phoca hispida) in western Prince Albert Sound, NT, Canada. Arctic 53, 422–431. ( 10.14430/arctic872) [DOI] [Google Scholar]
- 8.Lydersen C. 1998. Status and biology of ringed seals (Phoca hispida) in Svalbard. NAMMCO Sci. Publ. 1, 46–62. ( 10.7557/3.2980) [DOI] [Google Scholar]
- 9.Freitas C, Kovacs KM, Ims RA, Fedak MA, Lydersen C. 2008. Ringed seal post-moulting movement tactics and habitat selection. Oecologia 155, 193–204. ( 10.1007/s00442-007-0894-9) [DOI] [PubMed] [Google Scholar]
- 10.Young BG, Ferguson SH. 2013. Seasons of the ringed seal: pelagic open-water hyperphagy, benthic feeding over winter and spring fasting during molt. Wildl. Res. 40, 52–60. ( 10.1071/WR12168) [DOI] [Google Scholar]
- 11.Fauchald P, Tveraa T. 2003. Using first-passage time in the analysis of area-restricted search and habitat selection. Ecology 84, 282–288. ( 10.1890/0012-9658(2003)084[0282:UFPTIT]2.0.CO;2) [DOI] [Google Scholar]
- 12.Sakshaug E, Johnsen G, Kristiansen S, von Quillfeldt C, Rey F, Slagstad D, Thingstad F. 2013. Phytoplankton and primary production. In Ecosystem Barents Sea (eds Sakshaug E, Johnsen G, Kovacs KM), pp. 167–208. Trondheim, Norway: Tapir Academic Press. [Google Scholar]
- 13.Labansen AL, Lydersen C, Haug T, Kovacs KM. 2007. Spring diet of ringed seals (Phoca hispida) from northwestern Spitsbergen, Norway. ICES J. Mar. Sci. 64, 1246–1256. ( 10.1093/icesjms/fsm090) [DOI] [Google Scholar]
- 14.Hop H, Gjøsæter H. 2013. Polar cod (Boreogadus saida) and capelin (Mallotus villosus) as key species in marine food webs of the Arctic and the Barents Sea. Mar. Biol. Res. 9, 878–894. ( 10.1080/17451000.2013.775458) [DOI] [Google Scholar]
- 15.McBride MM, Filin A, Titov O, Stiansen JE (eds). 2014. Status report on the Barents Sea Ecosystem. IMR/PINRO Joint Report Series2014, no.1. Bergen, Norway: Institute for Marine Research.
- 16.Fossheim M, Primercerio R, Johannesen E, Ingvaldsen RB, Aschan MM, Dolgov AV. 2015. Recent warming leads to a rapid borealization of fish communities in the Arctic. Nat. Clim. Change 5, 673–677. ( 10.1038/nclimate2647) [DOI] [Google Scholar]
- 17.David C, Lange B, Krumpen T, Schaafsma F, van Franeker JA, Flores H. In press Under-ice distribution of polar cod Boreogadus saida in the central Arctic Ocean and their association with sea-ice habitat properties. Polar Biol. ( 10.1007/s00300-015-1774-0) [DOI] [Google Scholar]
- 18.Kwok R, Cunningham GF, Wensnahan M, Rigor I, Zwally HJ, Yi D. 2009. Thinning and volume loss of the Arctic Ocean sea ice cover: 2003–2008. J. Geophys. Res. 114, C07005 ( 10.1029/2009JC005312) [DOI] [Google Scholar]
- 19.Harwood LA, Smith TG, Auld JC. 2012. Fall migration of ringed seals (Phoca hispida) through the Beaufort and Chukchi Seas, 2001–02. Arctic 65, 35–44. ( 10.14430/arctic4163) [DOI] [Google Scholar]
- 20.Laidre KL, et al. 2015. A circumpolar assessment of Arctic marine mammals and sea ice loss, with conservation recommendations for the 21st century. Conserv. Biol. 29, 724–737. ( 10.1111/cobi.12474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lydersen C, et al. 2014. The importance of tidewater glaciers for marine mammals and seabirds in Svalbard, Norway. J. Mar. Syst. 129, 452–471. ( 10.1016/j.jmarsys.2013.09.006) [DOI] [Google Scholar]
- 22.Błaszczyk M, Jania JA, Hagen JO. 2009. Tidewater glaciers of Svalbard: recent changes and estimates of calving fluxes. Pol. Polar Res. 30, 85–142. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available at the Norwegian National Polar Data Centre (data.npolar.no). Contact person Kit M. Kovacs (kit.kovacs@npolar.no).