Abstract
The halteres of flies are mechanosensory organs that provide information about body rotations during flight. We measured haltere movements in a range of fly taxa during free walking and tethered flight. We find a diversity of wing–haltere phase relationships in flight, with higher variability in more ancient families and less in more derived families. Diverse haltere movements were observed during free walking and were correlated with phylogeny. We predicted that haltere removal might decrease behavioural performance in those flies that move them during walking and provide evidence that this is the case. Our comparative approach reveals previously unknown diversity in haltere movements and opens the possibility of multiple functional roles for halteres in different fly behaviours.
Keywords: halteres, kinematics, flight
1. Introduction
Fly flight is enabled in part by halteres, mechanosensory organs that detect body rotations [1,2]. Halteres are homologous to hindwings [3] and are oscillated at wingbeat frequency during flight. Haltere neurons are sensitive to small movements and not specialized for particular frequencies [4]; thus, any movement of the haltere may be detected by the nervous system. Characterizing these movements is essential to understanding the information that halteres provide to the fly's nervous system.
In four-winged insects, hindwings are often coupled to front wings [5], but in the small number of flies that have been observed, the halteres oscillate out of phase with the wings [6]. The wings possess the same type of mechanosensory afferents as the halteres [7,8], and the relative timing of wing and haltere nerve activity may be essential to steering [9,10]. Many insects generate flight manoeuvres by altering the phase of front and hindwing oscillations. It is possible that some fly groups retain this capability, with important consequences for flight control, but this has not been measured.
Still less is known about haltere function when flies are on the ground. There are reports of haltere movements during walking in some flies [11–13], but these observations were not detailed, and were limited to only two families. What are the kinematics of these movements, and are they relevant for fly behaviour?
Here, we observed haltere movements during flying and walking behaviour across several fly families (figure 1a). Our observations show a large diversity of haltere movements in flight and in walking, suggesting that halteres have a role in fly behaviour beyond that known in flight.
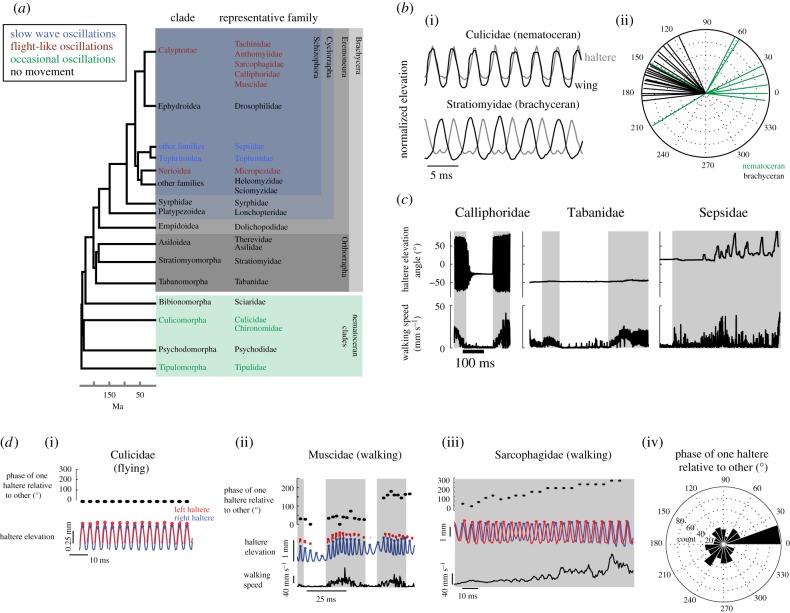
Figure 1.
Diversity of haltere movement during flight and walking. (a) Phylogeny of Diptera after Wiegmann et al. [14]. Families are colour-coded according to haltere movements during walking. (b) (i) Traces of haltere and wing elevations in flight for a nematoceran (top) and brachyceran fly (bottom). (ii) Haltere oscillation phases relative to wingbeat for flies shown in table 1. The phase is represented by the angle of each line and the length of each line represents vector strength. (c) Raw data traces from videos of walking flies. Top trace: haltere elevation angle. Bottom trace: horizontal body velocity. (d) Phase relationships between the two halteres are different during walking and flying. (i) The two halteres of flying flies are consistently in phase (0°) with each other. Top: Phases of the left haltere (bottom, red) relative to the right (bottom, blue). Circles denote elevation peaks. (ii) The oscillation phases can change rapidly during walking. A housefly (Muscidae) shows a switch between in-phase haltere oscillations and out-of-phase haltere oscillations. Data were captured with a single camera and thus, both haltere bases were not visible in all frames. Grey boxes designate walking bouts. (iii) A two-camera video capturing both halteres shows a gradual shift in phase. (iv) Rose plot of the distribution of relative phases of one haltere to the other includes the entire oscillation cycle, n = 7 trials in four flies.
2. Results and discussion
(a). During flight, the phase of the haltere stroke relative to the wing stroke varies across species
We filmed 41 flies (26 families) in tethered flight (table 1). All flies in the brachyceran (short-antennaed) suborder oscillated their halteres near 180° with respect to the wings, but nematoceran (long-antennaed) flies showed a variety of phase relationships (figure 1b). Mosquitoes and midges oscillated their halteres nearly in phase with the wings (table 1; figure 1b). The sensilla on both halteres and wings fire phase-locked spikes in each oscillation [4,7]. If the phases of haltere movement vary, relative phases of spiking in wing and haltere sensilla will also vary, requiring different decoding strategies in the central nervous system. Nematoceran families are ancient relative to brachyceran families, suggesting that mechanisms for wing–haltere coordination evolved after the halteres themselves.
Table 1.
Measurements of various parameters of flight motion for 41 flies in 26 families. Each row represents a unique individual fly performing a single bout of flight.
| suborder | family | phase of haltere relative to wing (deg) | vector strength | wingbeat frequency (Hz) | no. wingbeats analysed |
|---|---|---|---|---|---|
| Brachycera | Asilidae | 157 | 0.99 | 137 ± 0.8 | 22 |
| Brachycera | Calliphoridae | 141 | 0.99 | 185.6 ± 1.1 | 21 |
| Brachycera | Calliphoridae | 146 | 1 | 160.6 ± 1.6 | 21 |
| Brachycera | Calliphoridae | 159 | 0.98 | 199.3 ± 1.5 | 21 |
| Brachycera | Calliphoridae | 130 | 0.98 | 200.8 ± 1.8 | 21 |
| Brachycera | Chamaemyidae | 160 | 0.96 | 262.9 ± 2.6 | 24 |
| Brachycera | Chloropidae | 175 | 0.98 | 202.8 ± 2.1 | 22 |
| Brachycera | Dolichopodidae | 127 | 0.98 | 167.8 ± 1.2 | 22 |
| Brachycera | Dolichopodidae | 148 | 0.95 | 152.3 ± 1.4 | 23 |
| Brachycera | Dolichopodidae | 154 | 0.97 | 244.2 ± 3 | 21 |
| Brachycera | Dolichopodidae | 115 | 0.99 | 166.8 ± 1.9 | 22 |
| Brachycera | Drosophilidae | 152 | 0.99 | 207.7 ± 0.9 | 26 |
| Brachycera | Heleomyzidae | 180 | 0.99 | 92.5 ± 0.8 | 21 |
| Brachycera | Heleomyzidae | 159 | 0.98 | 185.8 ± 1.2 | 22 |
| Brachycera | Lauxaniidae | 168 | 0.96 | 170.5 ± 1.8 | 23 |
| Brachycera | Lonchopteridae | 163 | 0.99 | 102.3 ± 0.9 | 21 |
| Brachycera | Muscidae | 132 | 0.99 | 177 ± 1.6 | 22 |
| Brachycera | Phoridae | 160 | 0.98 | 90.1 ± 0.9 | 21 |
| Brachycera | Pipunculidae | 171 | 0.98 | 269.6 ± 2.3 | 21 |
| Brachycera | Sarcophagidae | 168 | 0.98 | 168.9 ± 2.1 | 21 |
| Brachycera | Sciomyzidae | 167 | 0.96 | 151.5 ± 2.4 | 21 |
| Brachycera | Sepsidae | 174 | 0.99 | 215.1 ± 2.1 | 22 |
| Brachycera | Stratiomyidae | 182 | 0.99 | 109.7 ± 0.9 | 22 |
| Brachycera | Stratiomyidae | 157 | 0.99 | 131.6 ± 1 | 21 |
| Brachycera | Stratiomyidae | 157 | 0.99 | 136.7 ± 0.8 | 21 |
| Brachycera | Syrphidae | 164 | 0.98 | 185.3 ± 1.6 | 24 |
| Brachycera | Syrphidae | 141 | 0.96 | 202.8 ± 3 | 22 |
| Brachycera | Tabanidae | 187 | 0.99 | 148.9 ± 1.2 | 21 |
| Brachycera | Tabanidae | 129 | 0.99 | 102.7 ± 0.9 | 21 |
| Brachycera | Tachinidae | 156 | 0.97 | 179.5 ± 2.3 | 22 |
| Brachycera | Tephritidae | 163 | 0.99 | 157.9 ± 1.5 | 21 |
| Brachycera | Therevidae | 138 | 0.99 | 106.3 ± 0.7 | 17 |
| Nematocera | Chironomidae | 351 | 0.94 | 218.4 ± 1.8 | 47 |
| Nematocera | Chironomidae | 0 | 0.97 | 445.4 ± 4.5 | 51 |
| Nematocera | Culicidae | 24 | 0.98 | 286.1 ± 2.3 | 22 |
| Nematocera | Culicidae | 18 | 0.97 | 298.4 ± 3.9 | 27 |
| Nematocera | Culicidae | 26 | 0.98 | 339.1 ± 2.7 | 21 |
| Nematocera | Psychodidae | 152 | 0.99 | 114.3 ± 0.8 | 21 |
| Nematocera | Sciaridae | 211 | 0.97 | 161.7 ± 1.1 | 21 |
| Nematocera | Tipulidae | 58 | 0.99 | 57 ± 0.4 | 20 |
| Nematocera | Tipulidae | 62 | 0.98 | 48.3 ± 0.5 | 18 |
(b). Haltere movements during walking are correlated with phylogeny
Patterns of haltere movement in walking flies were dependent on phylogeny (figure 1a; [14]). In most families, halteres do not move during standing or walking. These families include flies that diverged from their ancestor over 200 Ma (Sciaridae) to more recent families diverging less than 100 Ma (Drosophilidae). The absence of haltere movements suggests that for diverse flies, the haltere nerve is silent during walking. In sharp contrast, flies in the calyptrate families (Muscidae, Anthomyiidae, Calliphoridae, Sarcophagidae and Tachinidae) and one acalyptrate fly (Micropedizae) always oscillated their halteres during walking (figure 1c). These walking oscillations were similar in frequency and amplitude to the oscillations in tethered flight in the same individuals.
Flies in the families Tephritidae and Sepsidae moved their halteres while walking and standing. These movements are much slower than wingbeat frequency, and not sinusoidal (figure 1c). Tephritid flies have patterned wings that they wave slowly in communication to conspecifics and predators [15]. We find that the halteres move in similarly non-rhythmic ways, probably providing unique patterns of sensory information.
(c). Flies change the phase relationship between the two halteres during walking
In flight, halteres always oscillate in phase with each other, even at high wingbeat frequencies (figure 1d(i)). However, in walking, calyptrate flies can change the phase of the two halteres rapidly from stroke to stroke (figure 1d(ii,iii)). We show the distribution of relative haltere phases for 545 oscillations in calyptrate flies (figure 1d(iv)). The relative phases between the two halteres span the entire cycle, showing that they can take on all possible phase relationships and are not limited to in-phase or out-of-phase oscillations.
This behaviour is very different from the in-phase oscillations observed in all flying flies (figure 1d(i)). Because haltere afferent neurons are exquisitely sensitive to phase [16], the change in phase indicates that spike arrival times will be more variable during walking than during flight.
(d). Haltere movements during perturbations are similar to haltere movements during walking
To determine if flies actively oscillate their halteres when the substrate becomes unstable, we gently vibrated the surface on which they stood. We tested one species that does not move its halteres while walking (Drosophila melanogaster) and one species that does (Sarcophaga bullata). When challenged with a vibrating substrate, Sarcophaga oscillated its halteres, at approximately the same frequency as in flight, in eight of 12 trials. Drosophila never moved its halteres (5 of 5 trials; figure 2a).
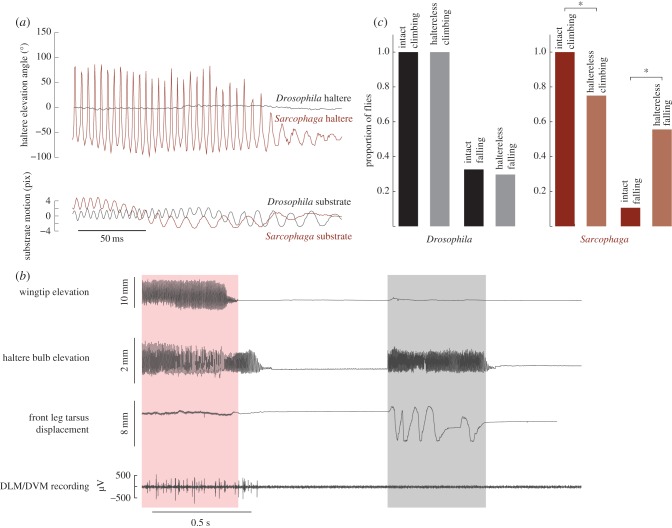
Figure 2.
Haltere movements while walking and standing on unstable surfaces are similar, and they do not require direct flight muscle activity. (a) Haltere movements during perturbations. Top: Sarcophaga shows large haltere oscillations during perturbations; Drosophila does not. Bottom: movement of the substrate. (b) Behaviour and dorsal longitudinal muscle/dorsal–ventral muscle (DLM/DVM) activity in a Sarcophaga fly during flying (red-shaded box, first) and walking (grey-shaded box, second). Top trace: the wing is oscillated during flight and stationary during walking. Second trace: the haltere moves during both walking and flying, with similar amplitude in each. Third trace: the front leg makes large movements only during walking. Bottom trace: DLM/DVM are only active during flight. (c) Halteres influence the proportion of flies climbing a vertical wall and falling from the wall after a perturbation, but only in species that oscillate their halteres while walking. Left: Drosophila does not oscillate its halteres while walking, and haltere ablation has no effect on the proportion of flies climbing or falling. Right: Sarcophaga oscillates its halteres during walking. Haltere ablation decreases the proportion of flies climbing and increases the proportion falling. (Online version in colour.)
(e). Haltere movements do not require activation of indirect flight muscles
Are haltere movements in wing-clipped, walking flies the result of a frustrated take-off attempt? We show that they are not. First, we observed no thoracic movement during haltere movements in walking. Second, recordings of indirect flight muscles (dorsal longitudinal muscle and dorsal–ventral muscle) showed that they are active during wing movement only, and that haltere movements during walking occur without this activation (figure 2b). The halteres of walking flies are thus not moved by thoracic movements, as in flight [6,9], but rather by muscles of the halteres themselves [9,17].
(f). Haltere input aids vertical walking behaviour
Do haltere movements during walking provide useful input to the nervous system, or are they an epiphenomenon associated with locomotion? We ablated both halteres of Sarcophaga and Drosophila and observed walking behaviour. We did not observe any differences in walking on a horizontal surface, but noted that haltere-ablated Sarcophaga were less likely to exhibit the negative geotaxis (climbing a vertical wall) observed in their intact counterparts (figure 2c; Fisher's exact test, 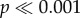 ). Similarly, a significantly higher proportion of haltere-ablated Sarcophaga fell off of the wall when gently perturbed as compared to intact flies (Fisher's exact test,
). Similarly, a significantly higher proportion of haltere-ablated Sarcophaga fell off of the wall when gently perturbed as compared to intact flies (Fisher's exact test, 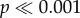 ). No differences were observed between intact and haltere-ablated Drosophila (which only moves the halteres in flight) in climbing or falling (figure 2c). These results suggest that haltere input is behaviourally relevant in behavioural contexts experienced during the fly's natural life.
). No differences were observed between intact and haltere-ablated Drosophila (which only moves the halteres in flight) in climbing or falling (figure 2c). These results suggest that haltere input is behaviourally relevant in behavioural contexts experienced during the fly's natural life.
3. Material and methods
(a). Animals
Flies were collected in Ohio, USA. D. melanogaster and S. bullata were taken from laboratory colonies. All flies were identified to family level using a dichotomous key [18].
(b). Flying flies
Flies were glued to pins and filmed in flight at 4000 frames s−1 (Fastec Imaging, San Diego, CA). The positions of the leading edge of the wing and haltere tip, as well as wing and haltere bases, were digitized using DLTDataViewer [19]. We calculated phase of each haltere stroke relative to the wing stroke, as well as vector strength (see the electronic supplementary material).
(c). Walking flies
Flies walked freely in front of a camera capturing 2000 frames s−1. Wings were removed to prevent flight and ensure haltere visibility. Positions of the haltere tip and base were digitized. In some trials, we observed both halteres using two synchronized cameras. We surveyed 23 fly families (figure 1a).
(d). Perturbation experiments
We glued a glass slide to a small vibration motor and applied a thin layer of Tanglefoot (Contech Inc., Vancouver, Canada) to the slide. We placed all tarsi of a cold-anesthetized fly (Sarcophaga or Drosophila) onto the slide. When the fly assumed a normal posture, the platform was gently vibrated for 0.5 s via microcontroller (Arduino Uno, Sparkfun Electronics, Niwot, CO, USA). Responses were filmed and digitized as above.
(e). Electrophysiological recordings from indirect flight muscles during walking and flight
Sarcophaga bullata were tethered to a pin and implanted with silver wires (one recording and one ground) in the indirect flight muscles. See the electronic supplementary material for details.
(f). Vertical walking behaviour
Six individuals of Sarcophaga or Drosophila were simultaneously placed in a small plastic cup (Reditainer, 5.5 oz) and permitted to walk freely. The number of flies that climbed the wall of the cup was scored. The cup was then manually lifted a small distance (6.35 mm for Sarcophaga, 2 mm for Drosophila) and gently dropped. The number of flies that fell off the wall was scored for 22 repetitions of the experiment.
Supplementary Material
Acknowledgements
We thank Mark Willis and Cole Gilbert for valuable feedback, and Nicole Arnold for experimental assistance.
Ethics
Work complied with local ethical requirements.
Data accessibility
Data are available on Dryad: http://dx.doi.org/10.5061/dryad.0sj1q.
Authors' contributions
J.L.F. and A.M.Y. designed experiments. J.M.H., D.P.M., A.M.Y., N.D.K. and S.M. collected data. J.L.F. wrote the manuscript with input from all authors. All authors approved the final version of the paper and are accountable for its contents.
Competing interests
We declare that we have no competing interests.
Funding
This work was funded by AFOSR grants nos. FA2386-13-1-3007 and FA9550-14-0398.
References
- 1.Dickinson MH. 1999. Haltere-mediated equilibrium reflexes of the fruit fly, Drosophila melanogaster. Phil. Trans. R. Soc. Lond. B 354, 903–916. ( 10.1098/rstb.1999.0442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nalbach G. 1993. The halteres of the blowfly Calliphora. J. Comp. Physiol. A 173, 293–300. ( 10.1007/BF00212693) [DOI] [Google Scholar]
- 3.De Navas LF, Garaulet DL, Sánchez-Herrero E. 2006. The ultrabithorax hox gene of Drosophila controls haltere size by regulating the Dpp pathway. Development 133, 4495–4506. ( 10.1242/dev.02609) [DOI] [PubMed] [Google Scholar]
- 4.Fox JL, Daniel TL. 2008. A neural basis for gyroscopic force measurement in the halteres of Holorusia. J. Comp. Physiol. A 194, 887–897. ( 10.1007/s00359-008-0361-z) [DOI] [PubMed] [Google Scholar]
- 5.Brodsky AK. 1994. The evolution of insect flight. Oxford, UK: Oxford University Press. [Google Scholar]
- 6.Deora T, Singh AK, Sane SP. 2015. Biomechanical basis of wing and haltere coordination in flies. Proc. Natl Acad. Sci. USA 112, 1481–1486. ( 10.1073/pnas.1412279112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickinson MH. 1990. Linear and nonlinear encoding properties of an identified mechanoreceptor on the fly wing measured with mechanical noise stimuli. J. Exp. Biol. 151, 219–244. [Google Scholar]
- 8.Chan WP, Dickinson MH. 1996. Position-specific central projections of mechanosensory neurons on the haltere of the blow fly, Calliphora vicina. J. Comp. Neurol. 369, 405–418. () [DOI] [PubMed] [Google Scholar]
- 9.Pringle JWS. 1948. The gyroscopic mechanism of the halteres of Diptera. Phil. Trans. R. Soc. Lond. B 233, 347–384. ( 10.1098/rstb.1948.0007) [DOI] [Google Scholar]
- 10.Fayyazuddin A, Dickinson MH. 1999. Convergent mechanosensory input structures the firing phase of a steering motor neuron in the blowfly, Calliphora. J. Neurophysiol. 82, 1916–1926. [DOI] [PubMed] [Google Scholar]
- 11.Fraenkel G. 1939. The function of the halteres of flies (Diptera). Proc. Zool. Soc. Lond. A109, 69–78. ( 10.1111/j.1096-3642.1939.tb00049.x) [DOI] [Google Scholar]
- 12.Sandeman DC, Markl H. 1980. Head movements in flies (Calliphora) produced by deflexion of the halteres. J. Exp. Biol. 85, 43–60. [Google Scholar]
- 13.Miller PL. 1977. Haltere activity in a flightless hippoboscid fly, Crataerina pallida. J. Insect Physiol. 23, 855–860. ( 10.1016/0022-1910(77)90010-5) [DOI] [Google Scholar]
- 14.Wiegmann BM, et al. 2011. Episodic radiations in the fly tree of life. Proc. Natl Acad. Sci. USA 108, 5690–5695. ( 10.1073/pnas.1012675108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greene E, Orsak LJ, Whitman DW. 1987. A tephritid fly mimics the territorial displays of its jumping spider predators. Science 236, 310–312. ( 10.1126/science.236.4799.310) [DOI] [PubMed] [Google Scholar]
- 16.Fox JL, Fairhall AL, Daniel TL. 2010. Encoding properties of haltere neurons enable motion feature detection in a biological gyroscope. Proc. Natl Acad. Sci. USA 107, 3840–3845. ( 10.1073/pnas.0912548107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan WP, Prete F, Dickinson MH. 1998. Visual input to the efferent control system of a fly's ‘gyroscope’. Science 280, 289–292. ( 10.1126/science.280.5361.289) [DOI] [PubMed] [Google Scholar]
- 18.Borror DJ, Triplehorn CA, Johnson NF. 1992. An introduction to the study of insects. Montréal, Canada: Saunders College. [Google Scholar]
- 19.Hedrick TL. 2008. Software techniques for two- and three-dimensional kinematic measurements of biological and biomimetic systems. Bioinspiration Biomimetics 3, 34001 ( 10.1088/1748-3182/3/3/034001) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on Dryad: http://dx.doi.org/10.5061/dryad.0sj1q.