Abstract
Methods to mitigate the impacts of emerging infectious diseases affecting wildlife are urgently needed to combat loss of biodiversity. However, the successful mitigation of wildlife pathogens in situ has rarely occurred. Indeed, most strategies for combating wildlife diseases remain theoretical, despite the wealth of information available for combating infections in livestock and crops. Here, we report the outcome of a 5-year effort to eliminate infection with Batrachochytrium dendrobatidis affecting an island system with a single amphibian host. Our initial efforts to eliminate infection in the larval reservoir using a direct application of an antifungal were successful ex situ but infection returned to previous levels when tadpoles with cleared infections were returned to their natal sites. We subsequently combined antifungal treatment of tadpoles with environmental chemical disinfection. Infection at four of the five pools where infection had previously been recorded was eradicated, and remained so for 2 years post-application.
Keywords: chytridiomycosis, Batrachochytrium dendrobatidis, mitigation, Alytes muletensis, Mallorca
1. Introduction
Emerging infections are on the increase, incurring extraordinary economic and health costs and globally degrading our natural capital. In response, several efforts to eradicate animal pathogens are underway, however with few successes reported [1,2]. Research on livestock pathogens predominates and provides insight as to how pure wildlife pathogens may be combated for host conservation purposes [1,2]. Delivery of an efficient and practical intervention is a cornerstone of any scheme to eliminate infectious diseases, and the direct application of antimicrobials to infected hosts or immunization can be used effectively to control pathogen replication within a host and to reduce the likelihood of transmission to susceptible individuals [3]. However, for these types of interventions to be effective, control of environmental reservoirs of (re)infection must also be achieved. Local control of pathogens through the use of environmental chemical treatments has been effectively used to disinfect areas where environmental transmission of parasites can occur, but the impact of chemical treatment on transmission and maintenance of infection in concert with antimicrobial treatments has rarely been examined [4].
Amphibian chytridiomycosis, a disease predominantly caused by the aquatic chytrid fungus Batrachochytrium dendrobatidis (Bd) has driven population declines, local extirpations and species extinctions across five continents [5]. The pathogen is an extreme generalist, infecting over 700 amphibian species (http://www.bd-maps.net). Strategies developed to ameliorate the impacts of chytridiomycosis are predominantly geared towards disease-free maintenance of captive assurance colonies, and multiple methods have been developed to treat captive amphibians against infection with Bd [6–8]; however, most attempts at immunization have failed [9]. The remaining approaches that hold promise for in situ control include bioaugmentation with bacteria, direct application of antifungal drugs and environmental application of anti-Bd chemicals. Although not without promise, research on the application of bioaugmentation so far describes complex interactions between host, beneficial bacteria, the broader microbiota and pathogen that are strongly dependent upon environmental context and amphibian community structure [10,11]. For this reason, bioaugmentation strategies are unlikely to converge on an intervention that can be generalized across amphibian communities and ecosystems. The immediacy of the epizootic of chytriomycosis calls for an intervention that can be applied across systems, so we chose to explore direct application of antifungal drugs to infected hosts and environmental application of chemicals as strategies to eliminate Bd from a simple, single host system [12].
2. Material and methods
Biannual surveys at five permanent ponds (3 × Torrent des Ferrerets, 2 × Cocó de sa Bova; Mallorca, Spain) were undertaken from 2008 and are ongoing. We sampled Mallorcan midwife toad (Alytes muletensis) tadpoles, as terrestrial stages are rarely captured as they take refuge in inaccessible locations. Tadpoles of this and other Alytes sp. are recognized as reservoirs of infection [13,14]. To sample, we swabbed tadpole mouthparts following established protocols [12,13]. All ponds affected by chytridiomycosis on the island were included in the study and none was left as untreated controls owing to conservation requirements. However, chemical disinfection efforts at Torrent de Ferrerets preceded those at Cocó de sa Bova, affording us the opportunity to compare across sites.
Swabs were processed according to standard extraction and quantitative PCR (qPCR) methods [15] in duplicate and run against negative controls and positive controls (0.1, 1, 10 and 100 zoospore genomic equivalents, GE).
For antifungal treatments, tadpoles were collected and transported in plastic bottles containing pond water. We used air pumps and tubes with aeration stones to ensure tadpole survival during the outward hikes. Tadpoles were then transported to the laboratory and kept in several cooled, glass aquaria. All tadpoles were bathed daily for 7 days in aged tapwater containing 1.0 mg l−1 itraconazole (Sporanox, Janssen-Cilag Inc.) and returned to aquaria after each treatment. Aquaria water was replaced every day during the 7 days treatment. After treatment, tadpoles were returned to the collection sites by helicopter, either immediately if ponds were not drained or after ponds were refilled by autumn rain. In these cases, subsets of 40 tadpoles from each aquarium were swab-sampled 15 days post-treatment.
Environmental disinfection was done using Virkon S (DuPont Inc.) at 1% final concentration and a single application applied ad libitum to the environment. The disinfectant was liberally applied to all rock, gravel, crevice and vegetated areas that surrounded the immediate environs of each breeding site.
3. Results
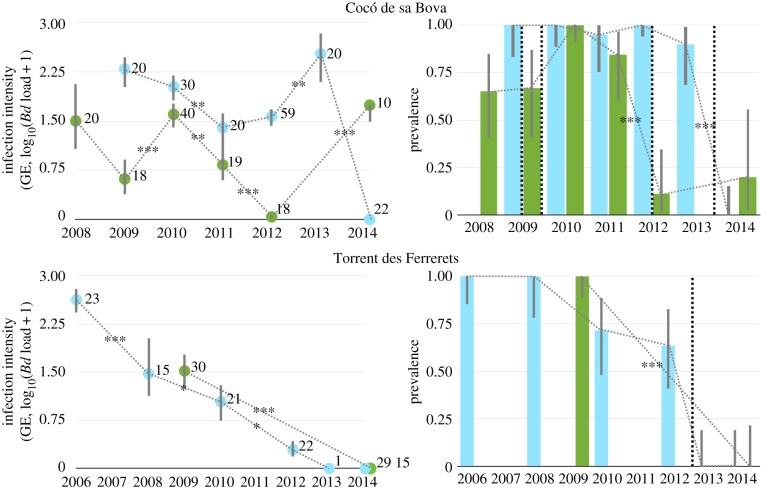
We initially attempted mitigation by treating in 2009 A. muletensis tadpoles inhabiting two permanent pond sites in one of the two infected drainages, Cocó de sa Bova (electronic supplementary material, figure S1), with the antifungal itraconazole. We used a treatment protocol previously shown to eliminate infection in tadpoles [7]. Treatments were applied ex situ, and prior to post-treatment release the two ponds were completely drained of water and naturally dried by the arid environment that typifies Mallorca. We had previously determined that Bd is absent from the other two ephemeral water bodies in this drainage, and environmental Bd is not thought to persist during periods of drying [16]. The two ponds naturally refilled during the autumn rainy season. At no point during this prolonged period of captivity did we detect any evidence of infection in the treated tadpoles. The following spring, qPCR analysis showed that all treated animals had contracted infections not significantly different from what had been recorded at the location before treatment [17] (figure 1). Repeating the protocol in the spring of 2012, this time without draining the breeding sites, and with tadpole release only 7 days after treatment, was again not associated with reduction in the prevalence of infection or reduced burdens of infection in the following spring (figure 1).
Figure 1.
Infection intensity (left panels; mean±95% CI by the bias-corrected and accelerated BCa method with 2000 bootstrap replications) and prevalence (on the right; mean±95% Clopper–Pearson CI) over two pond sites at the Cocó de sa Bova (combined in top panels) and three at the Torrent des Ferrerets (combined in bottom panels), over the course of the study. Blue (light colour) shows values derived from spring sampling, green (darker colour) for summer. Pairwise comparisons (Wilcoxon signed-rank tests for infection intensities and Fisher exact tests for prevalence) are represented by dashed lines and significant differences represented with asterisks (*p < 0.05, **p < 0.01 and ***p < 0.001) after a sequential Bonferroni adjustment. Sample sizes are shown in left panels. Dashed vertical lines in right panels indicate when treatments were implemented. (Online version in colour.)
In contrast, at three breeding sites used by the species in the second drainage, Torrent des Ferrerets (electronic supplementary material, figure S2), we could not detect infection in any animals sampled in 2013 after treatment of tadpoles and whatever terrestrial A. muletensis life stages we could capture with itraconazole, draining the sites and then treating the environment with Virkon S (electronic supplementary material, figures S3 and S4; figure 1). Replication of this protocol at Cocó de sa Bova in 2013 and application of Virkon S solution to the rock crevices located around the ponds where metamorphosed A. muletensis reside again cleared infection in the larger population of tadpoles resident in the larger pond at this location. Residual infection was detected in tadpoles occupying the smaller permanent pond site. Data from samples taken at Torrent des Ferrerets 2 years after chemical disinfection showed that the effect of environmental application of Virkon S twinned with itraconazole treatment of tadpoles carried over across years, as again no evidence of infection was detected in 2014 (figure 1).
4. Discussion and conclusion
We cannot say with certainty why direct treatment of tadpoles with antifungals without environmental disinfection failed to resolve infection at Cocó de sa Bova, but the most likely explanation is that infection reinvaded tadpoles from post-metamorphic animals that we could not access in their terrestrial refuges. We do occasionally discover corpses of juveniles exhibiting a strong molecular signal of infection. Like other amphibian species, Alytes spp. tadpoles scavenge from corpses, and this process is presumed to be a factor in transmission of Bd from corpses to tadpoles in another species [18,19]. Irrespective of this, our application of Virkon S at Torrent des Ferrerets provided proof-of-principle that environmental application of fungicides and other chemical treatments may be a better approach when combined with antimicrobial treatment of infected hosts. This initial conclusion was reinforced when we recapitulated our result by clearing infection in Cocó de sa Bova the following year. In our case, combining chemical disinfection twinned with antifungal treatment of tadpoles proved the better strategy, eliminating infection and preventing spill-back over the short term at four of the five pools where we attempted mitigation.
The development of disinfection strategies alone cannot eliminate the threat of chytridiomycosis, as evidence continues to accumulate that lethal amphibian-associated chytrid fungi are frequently being introduced into Europe and beyond [12,20]. Clearing site-level infection is no guarantee against pathogen reintroduction or the introduction of novel pathogens. However, to cope with the existing, recurring and future threats of chytridiomycosis, rapid response strategies require cheap, simple and transferrable methods for mitigating infection that can be employed as soon as the threat has been identified. We acknowledge that Virkon S is a controversial chemical to use environmentally and our use of it was driven by the urgency of midwife decline on Mallorca [21]. Virkon S is only one of several chemical treatments known to have antifungal properties against chytrid fungi [22,23] and antifungal treatments do not require extensive investment in time and effort. We argue that research informing efforts to combat chytridiomycosis should include in-depth investigations of the impact of antifungals and anti-Bd chemicals on amphibian health without discarding attempts to develop immunization and other methods of disease control. Research on the application of these chemicals for control of wildlife diseases must also include investigation of the potential impacts of chemical application to other biodiversity, the environment and associated ecosystem services.
Supplementary Material
Acknowledgements
We thank S. Pinya, X. Manzano, C. Serrano, A. Díaz-Guerra, E. M. Albert, S. F. Walker, D. Daversa, S. Fernández-Beaskoetxea, J. Vörös, B. R. Schmidt, B. Tapley and J. Bielby for field assistance, the people working at Paratge Natural de la Serra de Tramuntana and Conselleria de Medi Ambient (especially J. Mayol and E. Moragues), and the owner of the Mossa property for field site access.
Ethics
The work was carried out under the Govern de les Illes Balears's permit no. CEP 43/2015.
Data accessibility
Data are available in the electronic supplementary material.
Authors' contributions
J.B., T.W.J.G. and M.C.F. designed and wrote the paper, with contributions from E.S.-T. Data were collected and/or analysed by E.S.-T., A.F.-L. and J.A.O; all authors provided intellectual input and edited/approved the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was funded by Fundación General CSIC, Banco Santander and BiodivERsA project RACE.
References
- 1.Mariner JC, House JA, Mebus CA, Sollod AE, Chibeu D, Jones BA, Roeder PL, Admassu B, van't Klooster GGM. 2012. Rinderpest eradication: appropriate technology and social innovations. Science 337, 1309–1312. ( 10.1126/science.1223805) [DOI] [PubMed] [Google Scholar]
- 2.Wobeser G. 2002. Disease management strategies for wildlife. Rev. Sci. Tech. Off. Int. Epiz. 21, 159–178. [DOI] [PubMed] [Google Scholar]
- 3.Rosatte RC, Power MJ, MacInnes CD, Campbell JD. 1992. Trap-vaccinate: release and oral vaccination for rabies control in urban skunks, raccoons and foxes. J. Wildl. Dis. 28, 562–571. ( 10.7589/0090-3558-28.4.562) [DOI] [PubMed] [Google Scholar]
- 4.Skrjabin KI. 1970. Preventative measures against the spreading of helminthiasis among game animals. Trans. Int. Congr. Game Biol. 9, 54. [Google Scholar]
- 5.Fisher MC, Garner TWJ, Walke SF. 2009. The global emergence of Batrachochytrium dendrobatidis in space, time and host. Annu. Rev. Micro. 63, 291–310. ( 10.1146/annurev.micro.091208.073435) [DOI] [PubMed] [Google Scholar]
- 6.Scheele BC, Hunter DA, Grogan LF, Berger L, Kolby JE, McFadden MS, Marantelli G, Skerratt LF, Driscoll DA. 2014. Interventions for reducing extinction risk in chytridiomycosis-threatened amphibians. Conserv. Biol. 28, 1195–1205. ( 10.1111/cobi.12322) [DOI] [PubMed] [Google Scholar]
- 7.Garner TWJ, Garcia G, Carroll B, Fisher MC. 2009. Using itraconazole to clear Batrachochytrium dendrobatidis infection and subsequent depigmentation of Alytes muletensis tadpoles. Dis. Aquat. Org. 83, 257–260. ( 10.3354/dao02008) [DOI] [PubMed] [Google Scholar]
- 8.Martel A, et al. 2011. Developing a safe antifungal treatment protocol to eliminate Batrachochytrium dendrobatidis from amphibians. Med. Mycol. 49, 143–149. ( 10.3109/13693786.2010.508185) [DOI] [PubMed] [Google Scholar]
- 9.Stice MJ, Briggs CJ. 2010. Immunization is ineffective at preventing infection and mortality due to the amphibian chytrid fungus Batrachochytrium dendrobatidis. J. Wildl. Dis. 46, 70–77. ( 10.7589/0090-3558-46.1.70) [DOI] [PubMed] [Google Scholar]
- 10.Michaels CJ, Antwis RE, Preziosi RF. 2014. Impact of plant cover on fitness and behavioural traits of captive red-eyed tree frogs (Agalychnis callidryas). PLoS ONE 9, e95207 ( 10.1371/journal.pone.0095207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kueneman JG, Parfrey LW, Woodhams DC, Archer HM, Knight R, McKenzie VJ. 2014. The amphibian skin-associated microbiome across species, space and life history stages. Mol. Ecol. 23, 1238–1250. ( 10.1111/mec.12510) [DOI] [PubMed] [Google Scholar]
- 12.Walker SF, et al. 2008. Invasive pathogens threaten species recovery programs. Curr. Biol. 18, R853–R854. ( 10.1016/j.cub.2008.07.033) [DOI] [PubMed] [Google Scholar]
- 13.Walker SF, et al. 2010. Factors driving pathogenicity versus prevalence of the amphibian pathogen Batrachochytrium dendrobatidis and chytridiomycosis in Iberia. Ecol. Lett. 13, 372–382. ( 10.1111/j.1461-0248.2009.01434.x) [DOI] [PubMed] [Google Scholar]
- 14.Baláž V, et al. 2014. Assessing risk and guidance on monitoring of Batrachochytrium dendrobatidis in Europe through identification of taxonomic selectivity of infection. Conserv. Biol. 28, 213–223. ( 10.1111/cobi.12128) [DOI] [PubMed] [Google Scholar]
- 15.Boyle DG, Boyle DB, Olsen V, Morgan JAT, Hyatt AD. 2004. Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis. Aquat. Org. 60, 141–148. ( 10.3354/dao060141) [DOI] [PubMed] [Google Scholar]
- 16.Johnson M, Berger L, Philips L, Speare R. 2003. Fungicidal effects of chemical disinfectants, UV light, desiccation and heat on the amphibian chytrid, Batrachochytrium dendrobatidis. Dis. Aquat. Org. 57, 255–260. ( 10.3354/dao057255) [DOI] [PubMed] [Google Scholar]
- 17.Lubick N. 2010. Emergency medicine for frogs. Nature 465, 680–681. ( 10.1038/465680a) [DOI] [PubMed] [Google Scholar]
- 18.Pearman PB, Garner TWJ, Straub M, Greber UF. 2004. Response of the Italian agile frog Rana latastei to a Ranavirus, frog virus 3: a model for viral emergence in a naïve population. J. Wildl. Dis. 40, 600–609. ( 10.7589/0090-3558-40.4.660) [DOI] [PubMed] [Google Scholar]
- 19.Bielby J, et al. 2009. Fatal chytridiomycosis in the Tyrrhenian painted frog. Ecohealth 6, 27–32. ( 10.1007/s10393-009-0232-2) [DOI] [PubMed] [Google Scholar]
- 20.Martel A, et al. 2014. Recent introduction of a chytrid fungus endangers Western Palearctic salamanders. Science 346, 630–631. ( 10.1126/science.1258268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doddington BJ, Bosch J, Oliver JA, Grassly NC, García G, Benedikt RS, Garner TWJ, Fisher MC. 2013. Context-dependent amphibian host population response to an invading pathogen. Ecology 98, 1795–1804. ( 10.1890/12-1270.1) [DOI] [PubMed] [Google Scholar]
- 22.Hanlon SM, Kerby JL, Parris MJ. 2012. Unlikely remedy: fungicide clears infection from pathogenic fungus in larval southern leopard frogs (Lithobates sphenocephalus). PLoS ONE 7, e43573 ( 10.1371/journal.pone.0043573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt BR, Geiser C, Peyer N, Keller N, von Rütte M. 2009. Assessing whether disinfectants against the fungus Batrachochytrium dendrobatidis have negative effects on tadpoles and zooplankton. Amphibia-Reptilia 30, 313–319. ( 10.1163/156853809788795245) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in the electronic supplementary material.