Abstract
Older evolutionary scenarios for the origin of vertebrates often gave nervous systems top billing in accordance with the notion that a big-brained Homo sapiens crowned a tree of life shaped mainly by progressive evolution. Now, however, tree thinking positions all extant organisms equidistant from the tree's root, and molecular phylogenies indicate that regressive evolution is more common than previously suspected. Even so, contemporary theories of vertebrate origin still focus on the nervous system because of its functional importance, its richness in characters for comparative biology, and its central position in the two currently prominent scenarios for the invertebrate-to-vertebrate transition, which grew out of the markedly neurocentric annelid and enteropneust theories of the nineteenth century. Both these scenarios compare phyla with diverse overall body plans. This diversity, exacerbated by the scarcity of relevant fossil data, makes it challenging to establish plausible homologies between component parts (e.g. nervous system regions). In addition, our current understanding of the relation between genotype and phenotype is too preliminary to permit us to convert gene network data into structural features in any simple way. These issues are discussed here with special reference to the evolution of nervous systems during proposed transitions from invertebrates to vertebrates.
Keywords: invertebrate-to-vertebrate transition, annelid scenario, enteropneust scenario
1. Introduction
The first unequivocal scheme for the evolution of an invertebrate into a vertebrate was published by Lamarck in 1809 [1]. In a phylogenetic tree, he showed molluscs transitioning to vertebrates, but included nothing on the structure of the nervous systems (nor any other organs, for that matter). He expected that such details would come to light through the discovery of existing animals with intermediate morphologies. During the next few decades, comparisons were made between the central nervous system (CNS) of arthropods and vertebrates by Geoffroy Saint-Hilaire [2] and Kölliker [3]. These pre-Darwinian authors stressed the inverse dorsoventral relationship between the arthropod and vertebrate nerve cord—not in evolutionary terms, but according to their vision of a deep fundamental unity among animal body plans. In 1859, Darwin published On the origin of species, a book that included nothing about the evolutionary source of the vertebrates and very little about nervous systems. Even so, Darwin's book soon stimulated a spate of publications on the invertebrate-to-vertebrate transition, often with special reference to nervous system evolution; for instance, the earliest of these focused almost entirely on comparing CNS histology between arthropods and vertebrates [4].
By now, about a hundred scenarios for the origin of the vertebrates have been proposed, for which full citations are available in Holland et al. [5]. Almost every animal phylum has been suggested as a key starting point for the invertebrate-to-vertebrate transition. Many of these older ideas have been rendered highly unlikely because they are so out of tune with molecule-based phylogenies and with the wealth of new information at the gene and genomic levels of organization [6]. Currently, only a few of the scenarios are subjects of active research programmes. The less contentious of these consider the evolution of invertebrate chordates (amphioxus-like or tunicate-like ancestors) into vertebrates and do not delve deeper into the tree of animal life. In contrast, the more controversial scenarios now under discussion (namely the revived annelid and enteropneust theories) consider a broader sweep of evolution extending back into more basal regions of the tree. This review concentrates on these two longer-range theories and will cover their nineteenth century origins as well as their resurgence, which began a few decades ago, driven by the advent of evolutionary developmental biology. The contemporary annelid and enteropneust theories propose radically different evolutionary histories for the nervous system. Deciding between them, as will be discussed, is currently hindered by difficulties in elucidating the detailed intermediate steps occurring between widely separated nodes in phylogenetic trees.
2. Original annelid scenario
In 1874 and 1875, Semper published, respectively, preliminary and full versions of his proposal that annelid worms evolved into vertebrates [7,8]. He had found that the shark excretory system included ciliated funnels iterated segmentally along the body to drain the coelom, and these reminded him of the coelomoducts (metanephridia) typical of annelids. He additionally noted that a dorsoventral inversion of the body would be needed to translate the ventral nerve cord of the annelid into the dorsal CNS of the vertebrate, and he suggested an annelid tissue from which the vertebrate notochord might have arisen. Contemporaneously, Dohrn published a short book featuring a more comprehensive scenario for an annelid-to-vertebrate transition [9], causing Semper [10, p. 463 (footnote)] to complain that, ‘it remains doubtful whether Dohrn would have taken an annelid as his starting point if he had not been acquainted with my work before publishing his own’. This complaint was groundless, because Dohrn had established his priority by discussing his plan for ‘homologisation of the nervous chain of Arthropods, Annelids and Vertebrates' in an 1871 letter to Darwin [11, p. 34].
Dohrn [9] started with an annelid (figure 1a) that inverted the body dorsoventrally on its way to becoming a vertebrate. The inversion positioned the old ventral nerve cord as well as the original foregut towards the dorsal side of the body, whereas a new foregut formed ventroanteriorly (figure 1b). The new foregut opened at a new mouth that originated from the most anterior pair of coelomoducts when they fused with one another in the midline of the body. In addition, some of the other coelomoducts established openings with this new gut region on either side to form gill slits. Meanwhile, the original foregut, which had been brought into a dorsal position, atrophied, but persisted in part as the epiphysis and hypophysis of the vertebrate brain. With a little reconsideration, however, Dohrn decided to relocate the gut remnant a little more posteriorly in the vertebrate CNS, as a portion of the rhomboid fossa of the hindbrain (figure 1c). He then briefly covered the origins of: the post-anal tail (resulting from the opening of a new, subterminal anus), the vertebrate kidney (from some of the more posterior coelomoducts) and the notochord (from a connective tissue strand running along the dorsal side of the annelid nerve cord).
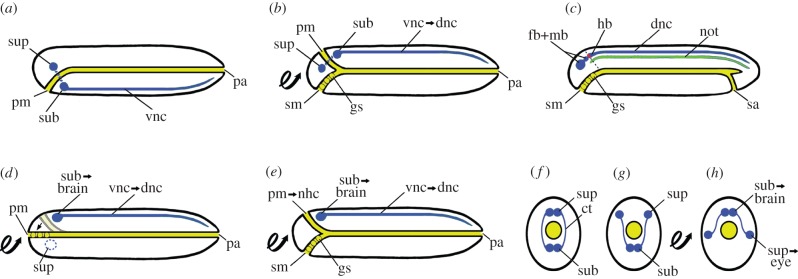
Figure 1.
Diagrams of variants of the inverted annelid theory of the origin of the vertebrates (a looped arrow indicates that the following stage is inverted). Side views (a–e) are sagittal sections with the head towards the left; cross sections (f–h) are through the brain region. (a) Dohrn's annelid-like ancestor. (b) Transitional stage after body inversion translates the primary mouth to the top of the head, and a new foregut with gill slits forms and opens at the secondary mouth. (c) Succeeding vertebrate stage when fusion of the old sub- and supraesophageal ganglia has produced a fore-, mid- and hindbrain that incorporate a remnant of the old foregut (dashed line) as part of the rhomboid fossa (red); a notochord has originated from the tough coating of the nerve cord, and opening of a secondary anus results in a post-anal tail. (d) Kleinenberg's scenario [12] that, along with inversion of the annelid body, the supraesophageal ganglion of the annelid atrophies and the subesophageal ganglion becomes the vertebrate brain; no new foregut forms, and the original one simply shifts (arrow) to open at the anterior end of the body. (e) Beard's scenario [13] accepting atrophy of the supraesophageal ganglion, body inversion and new foregut production; his innovation was the persistence of the old foregut as a hagfish-like neurohypophyseal canal. (f) Cross sections show Minot's modification of earlier scenarios [14]: instead of proposing atrophy of the supraesophageal ganglia, he gets them out of the way simply by turning them into the lateral eyes of the vertebrate. ct, circumpharyngeal tract; dnc, dorsal nerve cord; gs, gill slit; hb, hindbrain; not, notochord; nhc, neurohypophyseal canal; pa, primary anus; pm, primary mouth; sa, secondary anus; sm, secondary mouth; sub, subesophageal ganglion; sup, supraesophageal ganglion; vnc, ventral nerve cord. (Online version in colour.)
In general, Dohrn's scenario [9] suffered from maladaptive intermediate morphologies. Moreover, vertebrate neuroanatomists did not like his notion that the rhomboid fossa included an endodermal component. He subsequently backed off from neuroanatomy and worked on supporting other aspects of his scenario. In contrast, some of his colleagues continued struggling to explain how the proposed dorsal remnant of the foregut related to the vertebrate brain. Kleinenberg [12] avoided a stage where a foregut would interact with the CNS by assuming that the annelid supraesophageal ganglion (figure 1a) disappears, the body inverts and the subesophageal ganglion becomes homologous to the entire vertebrate brain (figure 1d). An awkward feature of his scheme was the necessity for unplugging the cephalic sensory organs from the old supraesophageal ganglion and reconnecting them to the now-dorsalized subesophageal ganglion. Soon thereafter, Beard [13] mixed Dohrn's idea of a new foregut with Kleinenberg's idea of atrophy of the supraesophageal ganglion and turned the original foregut into a neurohypophyseal canal comparable to that of a hagfish (figure 1e). Next came Minot [14], who modified Beard's modification of Kleinenberg's modification of Dohrn's theory: the two lateral lobes of the supraesophageal ganglion (figure 1f) separated and moved laterally (figure 1g), the body inverted, and the supraesophageal ganglion on either side became a lateral eye (figure 1h).
In Dohrn's scenario [9], ancestral annelids, which he considered to be very similar to living polychaetes, transitioned to vertebrates along an unbranched evolutionary line (anagenesis). Many of Dohrn's contemporaries, however, were already coming to understand that evolution follows a more complicated, branching course [15]. Moreover, by early in the twentieth century, it had been widely accepted that annelids and vertebrates were, respectively, members of the protostome and deuterostome superphyla (figure 2a), and thus only distant relatives [16,18]. These factors rendered the annelid theory unpopular until only a few decades ago, when discovery of developmental genes with highly conserved structures and expression patterns indicated that interesting comparisons could be made between animals over wide stretches of the tree of life.
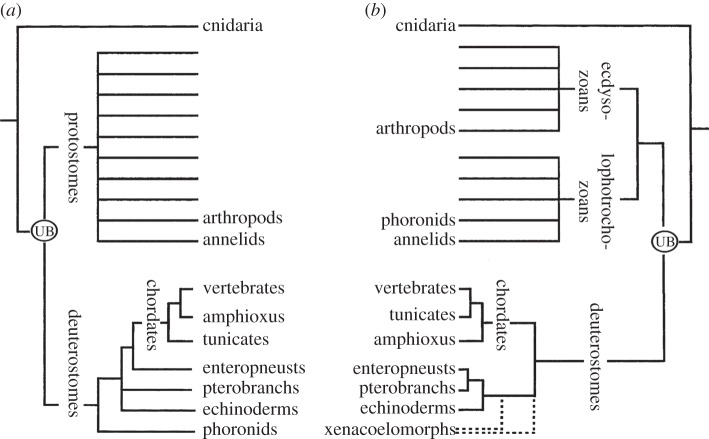
Figure 2.
Animal trees of life. (a) Tree based on morphology [16]. (b) Tree based on molecular sequencing [17]. The unlabelled branches diagrammatically indicate the presence of phyla not mentioned in the present review. The Urbilaterian is abbreviated UB, and the dotted lines indicate the uncertain position of the xenacoelomorphs.
3. Revived annelid scenario—affirming Dohrn
Some of the early studies of developmental genetics revealed parallels in the control of body patterning between invertebrates and vertebrates—including the positioning of the CNS in the dorsoventral axis [19]. These new data stimulated Arendt & Nübler-Jung [20–22] to take a new look at the venerable inversion theories of vertebrate origin [8,9], especially the idea that the ancestor of the bilaterian animals (the Urbilaterian) was a structurally complex organism that could be thought of as either arthropod-like or annelid-like. Eventually, preference shifted towards annelid-like ancestors [23], in part, perhaps, because a heavy cuticle makes arthropods an awkward starting point for evolving vertebrates. Additional reasons for preferring annelids as ancestors were their separation from arthropods (figure 2b) [17] in different superphyletic groupings (respectively, the Lophotrochozoa and Ecdysozoa) combined with the discovery that annelids, in comparison with arthropods, are more vertebrate-like at the genomic level [24]. In recent years, Arendt and co-workers [25–28] have been especially active in applying a wide range of modern techniques to bolster the revived annelid scenario.
The modern conception of the annelid theory does not convert annelids directly into vertebrates as envisaged by Semper and Dohrn. Instead, the scenario begins deep in the tree of animal life with the proposal that the Urbilaterian was annelid-like [23]. From that starting point, it is proposed that annelid-like features carried forward in evolution in several lineages, including those leading to modern annelids and to vertebrates. In conformance with modern tree topology, the evolutionary progression from an annelid-like Urbilaterian to the vertebrates would pass through intervening basal groups of deuterostomes. Until recently, one of the more basal groups of deuterostomes (namely, the enteropneust hemichordates) was considered to have a fairly complex CNS, which suggested that enteropneust-like intermediates bridged the gap between annelids and vertebrates [21]; the reputedly simpler nervous system in echinoderms [29] was not perceived as an obstacle to the scenario.
Two recent developments are awkward for the revived annelid theory because they suggest that basal deuterostomes include groups with strikingly simple nervous systems. First is the proposal [30] that the nervous system of enteropneusts lacks a CNS and comprises no more than a nerve net (considered in §6). The second problem stems from the still-uncertain placement of the xenacoelomorphs—as sister group, respectively, to the echinoderm plus enteropneust clade [31] or to the rest of the deuterostomes [32] (figure 2b), or even entirely outside of the deuterostomes [33]. Xenacoelomorphs have, at best, a rudimentary CNS, and if this condition is not derived, it might mean that the Urbilaterian was structurally very simple and that any similarities between the annelid and vertebrate CNS evolved independently. These difficulties for the revived annelid theory could be alleviated by the discovery of structurally complex fossils finding wide acceptance as early deuterostomes. However, although several such fossils have been proposed to be basal deuterostomes (e.g. Herpetogaster and vetulicolians), their affinities remain highly controversial [34].
4. Original enteropneust scenario of Bateson
Enteropneusts, commonly called acorn worms, along with pterobranchs, comprise the phylum Hemichordata. The three main body regions of an enteropneust are the proboscis, collar and trunk (figure 3a). At the proboscis/collar junction, the mouth opens ventrally into a buccal cavity, which gives off a small diverticulum (the stomochord) anteriorly and connects with the pharynx posteriorly. The lumen of the pharynx opens to the exterior via gill slits on either side of the body, and a postpharyngeal gut leads posteriorly to a terminal anus. It was classically accepted that enteropneusts have a diffuse peripheral nervous system plus more condensed regions of neural tissue (proboscis nerve plexus, collar cord, circumenteric nerves and dorsal and ventral trunk nerve cords), some or all of which comprise a CNS.
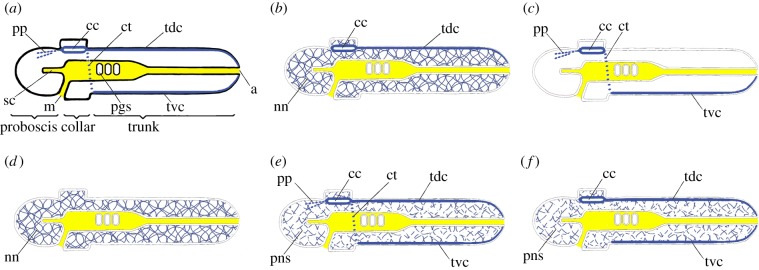
Figure 3.
Mid-sagittal sections of enteropneusts with anterior towards the left (dotted lines are nerves not located in the mid-sagittal plane. (a) Traditional textbook version of the anatomy, assuming that dorsal corresponds to vertebrate dorsal and is up (to accentuate other morphological features, no peripheral nervous system is indicated). a, anus; cc, collar cord; ct, circumpharyngeal tract; m, mouth; nn, nerve net; pgs, pharyngeal gill slit; pp, proboscis plexus; sc, stomochord; tdc, trunk dorsal cord; tvc, trunk ventral cord. (b) Bateson's conception [35] of the nervous system as CNS, comprising the collar cord, on the future vertebrate dorsal side (along with a trunk dorsal cord) plus a peripheral nervous system comprising a diffuse nerve net. (c) Nübler-Jung & Arendt's proposal [21] that the CNS comprises pp + cc + ct + tvc oriented like the uninverted CNS of an annelid (they did not mention a nerve net). (d) Concept of Lowe et al. [30] that the nerve net comprises the entire nervous system and there is no CNS; dorsoventral orientation left unresolved. (e) Nervous system proposed by Nomaksteinsky et al. [36] with a CNS comprising the pp + cc + tdc + ct + tvc; they considered the peripheral nervous system (pns) too sparse to be a nerve net; dorsoventral orientation left unresolved. (f) Concept of Cunningham & Casey [37] of a CNS comprising either the cc + tdc or the tvc alone and a peripheral nervous system of sparsely distributed neurons; dorsoventral orientation left unresolved. (Online version in colour.)
The first clearly enunciated enteropneust theory was by Bateson [35], who is now better remembered as one of the founders of the field of genetics. He began his scenario with the assumption that the dorsoventral orientation of the body was the same in enteropneusts and vertebrates. For him, the enteropneust nervous system consisted of a CNS (the collar cord) plus a peripheral nervous system comprising the trunk dorsal cord plus an extensive nerve net associated with the general epidermis (figure 3b). He also proposed that the stomochord corresponded to the vertebrate notochord and that the pharyngeal gill slits in both groups of animals were homologous; additionally, the trunk was not segmented, except for a vague serial repetition of gonads along its length. Looked at in this way, an enteropneust was rather like a vertebrate without an obviously segmented trunk musculature.
When Bateson proposed his scenario, an obvious weakness was the lack of agreement about plausible ancestors for the enteropneusts themselves. Then, at the end of the nineteenth century, Masterman seemed to alleviate the problem. He proposed [38] that phoronid worms were basal deuterostomes and were ‘the definite meeting-place’ between higher deuterostomes on the one hand, and the annelids and arthropods on the other hand. Masterman's phylogenetic arrangement long appealed to some biologists, although others were strongly opposed to it [39]. By now, this opposition view has been vindicated by molecular phylogenetic data that have unequivocally moved the phoronids from the deuterostomes to the lophotrochozoans [17].
5. First revived enteropneust scenario—repudiating Bateson
In response to the accumulating developmental genetic data, Nübler-Jung & Arendt [21] revived the enteropneust theory. They did not seek to confirm Bateson's ideas, but, on the contrary, sought to strengthen Dohrn's scenario by making enteropneusts a plausible intermediate stage between annelids and vertebrates. Nübler-Jung and Arendt based their argument on older neuroanatomical work reporting giant nerve fibres in the collar cord, circumenteric nerve ring and trunk ventral nerve cord of enteropneusts [40,41]. The CNS was proposed to be coextensive, with all regions characterized by giant fibres (plus the proboscis plexus because of its physiological properties) [21]. Such an enteropneust CNS (figure 3c) would have an overall configuration rather similar to that of an arthropod or annelid CNS. An enteropneust-like ancestor, so conceived, could evolve into a vertebrate by inverting dorsoventrally to convert the former trunk ventral nerve cord into a dorsal nerve cord. In addition, the collar cord and proboscis plexus, after displacement in the new dorsal direction, would become most of the vertebrate brain. As in the classical annelid and arthropod theories, the old mouth atrophies and is replaced by a new one. Inversion also would also convert a thickened ventral mesentery, the pygochord, into a notochord and would convert the epibranchial ridge of the pharynx into an endostyle, a glandular organ for food capture [42]. These transitions are diagrammed in more detail in Holland et al. [5].
6. Second revived enteropneust scenario—repudiating Bateson again
Like Nübler-Jung & Arendt [21], Lowe et al. [30] was stimulated by advances in evolutionary developmental biology to focus on the role of enteropneusts in the invertebrate-to-vertebrate transition—but from the heretofore minority viewpoint [43] that enteropneust morphology is quite simple instead of being relatively complex. To begin testing this idea, the Lowe laboratory determined the expression patterns for several dozen genes known to pattern the anteroposterior axis of the vertebrate CNS or to be general indicators of neurogenesis [30]. Many of the CNS marker genes were expressed in annular bands of ectoderm arranged in the same axial order as their homologues in the developing vertebrate nerve cord. Moreover, neurogenic marker genes were expressed in cells scattered abundantly throughout the ectoderm, instead of being concentrated along the dorsal and ventral midlines; the midlines were where a CNS, if present, might be expected to form. The overall conclusion was that the nervous system consisted exclusively of an epidermal nerve net (figure 3d) that had been inherited from a structurally uncomplicated Urbilaterian ancestor. In this initial publication [30], Lowe and co-workers did raise the possibility that enteropneusts might have lost a CNS originally present in their ancestors, but rejected such a scenario without further discussion (in spite of the realization among contemporary molecular phylogeneticists that regression has been considerably more common during animal evolution than previously suspected).
Lowe and co-workers also sought developmental genetic evidence for a dorsoventral inversion of body orientation during deuterostome evolution. They studied enteropneust homologues of genes involved in establishing the dorsoventral axis in other animals, and found that chordin and BMP (bone morphogenetic protein), respectively, were expressed on sides corresponding to annelid/arthropod dorsal and ventral [44]. Experiments altering chordin/BMP signalling repositioned some non-neural structures in the dorsoventral axis, as expected. Surprisingly, however, these experiments indicated that enteropneusts do not use the chordin/BMP axis to segregate epidermal and neural ectoderm and that this latter function is a novelty that appeared with the advent of the chordates. These results did not resolve the dorsoventral orientation of the enteropneust body, and efforts to answer this question through a comparative study of deuterostome genes involved in left/right asymmetry have yet to supply a firm answer [45].
Recently, several studies of enteropneust neurobiology have questioned the initial idea of Lowe and co-workers that a CNS is entirely lacking in enteropneusts. First, Nomaksteinsky et al. [36] presented cytological, neurochemical and gene expression data indicating that a CNS might be present after all and comprise the proboscis plexus, collar cord, circumenteric nerves, and trunk dorsal and ventral cords (figure 3e). The same authors also questioned the presence of a nerve net associated with the general epidermis because of the sparseness of neurons there. In another gene expression study, Cunningham & Casey [37] similarly proposed that a CNS was present and was composed of the trunk ventral nerve cord, trunk dorsal nerve cord and collar cord (figure 3f); they likewise found that the peripheral nervous system included relatively few neurons at advanced developmental stages. Finally, Miyamoto & Wada [46] focused on the Hedgehog gene in developing enteropneusts and demonstrated its endodermal expression in the stomochord and roof of the buccal cavity, while the dorsal ectoderm was differentiating and invaginating to form the collar nerve cord. The situation was reminiscent of what happens in neurulating embryos of vertebrates when Hedgehog signalling from the notochord plays a key role in the genesis of the neural tube. From these results, one could propose first that enteropneust-like ancestors did not invert dorsoventrally during evolution to vertebrates and second that the collar cord corresponds to at least part of the vertebrate CNS. Such conclusions, although based on limited data, are essentially a return to Bateson's original enteropneust scenario [35]. The situation became even more unsettled when a subsequent study of a wide spectrum of notochordally expressed genes [47] found no clear support for Bateson's homology between the enteropneust stomochord and the vertebrate notochord.
In the light of these more recent developments, Lowe and co-workers have come to accept that enteropneusts have some sort of CNS, albeit a fairly rudimentary one [48]. Even so, this leaves unaddressed a broader question: namely how centralized must a region of the nervous system be to qualify as a CNS? Future progress towards resolving this problem will require thorough comparative studies by neurobiologists working on the widest possible spectrum of invertebrate phyla.
For the enteropneusts, it is likely that some of the current disputes about neurobiology have arisen because of species differences and developmental stage differences between the various studies. Such problems should be largely resolved when more is learned about the developmental genetics, wiring diagrams and physiology of enteropneust nervous systems. A start in this direction has recently been made by Kaul-Strehlow et al. [49], who mapped the distribution of serotonin-containing neurons in two species over several life-history stages. Even so, a better understanding of enteropneust neurobiology will still leave open the broader question of whether the Urbilaterian nervous system was simply a nerve net or also included a CNS. This difficulty is a special case of the broader problem of making long-range comparisons from one phylum to the next, which is discussed in §7.
7. Nervous system evolution within phyla versus between phyla
In the past few decades, molecular phylogenetic studies have gone far towards establishing a widely agreed-upon framework for much of the tree of extant animal life at the phylum level [33], although some serious difficulties persist—for example, the arrangements near the base of the tree [50] and the placement of the xenacoelomorphs [31]. Even knowing the correct framework relating the terminal taxa, however, does not necessarily lead to an understanding of the detailed events that unfolded along the tree branches connecting the nodes. A gratifyingly detailed time-lapse movie of evolution requires two things above all. The first is that the overall body morphologies of the organisms being compared are similar enough to permit one to make plausible homologies at the level of component parts (for instance, organs, tissues and even cells). The second requirement is the discovery of gap-bridging fossils that can be identified to the general satisfaction. Fossils may be slotted into a crown group (a collection of living species plus their close extinct relatives that trace back to a common ancestor) or a stem group (a collection of extinct species forming a clade just outside the crown group). When crown and stem groups both are included within the bounds of a single phylum, the latter can be invaluable for showing the evolutionary details leading up to the crown group [51]. In contrast, when crown group is at a higher level of analysis, the stem group fossils identified by one palaeontologist are all too often considered to be problematic by another palaeontologist.
As subjects for reconstructing a series of relatively detailed steps during phylogenetic transitions, the vertebrates are by far the most tractable group of animals. The overall body plans from agnathans to mammals are similar enough to permit widely acceptable homologies to be made between their smaller components. For the nervous system, for instance, populations of glutaminergic neurons in forebrains of extant lampreys and mammals are comparable on the basis of neurochemistry and developmental gene expression [52]. Moreover, as already mentioned, an adequate fossil record is a major factor permitting reconstruction of detailed intermediate changes along the branches of the tree. Although vertebrate fossils preserving soft brain tissues are extremely rare [53], endocranial casts of a wide spectrum of vertebrate fossils have revealed the history of the major CNS regions and cranial nerves during evolution from agnathans through gnathostomes [54].
For invertebrates, when compared with vertebrates, the detailed evolutionary changes within each phylum are not as well understood. Even so, different members within a given phylum have sufficiently similar overall body plans, so that homologies can often be proposed between their component parts at both morphological and molecular levels (arthropod examples will be used here) [55,56]. Such homologies, when examined in the framework of a widely acceptable branching pattern within the phylum [57], can give plausible insights into some of the detailed changes that took place along the evolutionary lines connecting the nodes for the extant subgroups. Such insights can be greatly strengthened if fossil evidence becomes available. In the arthropods, for instance, a very good fossil record has supplied a wealth of evidence about changes in external features, including such nervous-system-related structures as eyes and antennae. Until recently, such fossils indicated nothing about the evolution of the internal parts of the nervous system [58], but this has now changed with the discovery of fossilized CNS tissue in the Early Cambrian stem group arthropod, Fuxianhuia protensa. The fossil's brain had a tripartite organization that was unexpected so early in arthropod evolution [59]. This discovery indicated that the CNS became secondarily simplified in several arthropod groups during the subsequent history of the phylum.
Difficulties increase considerably when one attempts to reconstruct the details of evolution from one phylum to the next. As already mentioned, the overall body plan is highly distinctive for each phylum, and this hampers the establishment of plausible body part homologies across such wide evolutionary divides. Although such interphylum homologies are not infrequently proposed at the morphological level, they tend not to gain wide acceptance [60]. A good recent example is the proposed homology between the chordate notochord and an annelid structure called the axochord [28], which was firmly questioned [61] within a few months of its publication.
When the field of evolutionary development was young (in its ‘enthusiastic phase’ [62]), there was some expectation that a knowledge of homologous genes would widen the taxonomic gap over which credible morphological homologies could be made [63]. It was hoped that developmental genes, although conserved, would somehow be parcelled out in phylum-specific patterns that would persist even after divergent evolution had obscured morphological homologies and thus help in the recognition of interphylum homologies. This hope has not been realized for two reasons: first, because developmental genes turned out to be so highly conserved across animals that few obviously phylum-specific signals could be extracted, and second, because of the complex relationship between the genotype and the phenotype. It has long been realized that this relationship can be confounded by genetic piracy—whereby different genes direct development of the same morphological character [64]. However, the converse can also be observed when similar gene regulatory networks direct the development of very different morphologies from one group of animals to the next. This latter phenomenon, which might appropriately be named ‘phenetic piracy’, is discussed by Lowe et al. [48], who believe that the highly conserved gene regulatory networks deployed along the anterior–posterior axis direct the development of a strikingly different set of morphological features from one phylum to the next. The same publication, however, adds that there is a more predictable linkage between conserved gene networks and phenotypic features along the dorsoventral axis across phyla [48]. One consequence of genetic and phenetic piracy is that there is currently no simple formula for translating gene activity into morphological characters. The relationships between the genotype and phenotype are so complex that, barring some unexpected breakthrough, their satisfactory elucidation is probably far in the future [65]. In conclusion, although molecular genetic studies have been highly successful in supporting body part homologies among distant relatives within phyla [66], a similar approach has not brought to light a wide spectrum of plausible homologies between phyla [67].
8. Possible ways forward
The general difficulties reviewed in §7 are exactly those that make it difficult to evaluate the relative merits of the revived annelid and enteropneust theories, the two currently contending long-range scenarios for the origin of the vertebrates. For the future, progress would be facilitated by advances in at least five different areas. First is the need for additional competent work in all aspects of neurobiology in all the animal phyla [68], and, just as important, the weeding out of the misinformation that has all too often been perpetuated by a succession of appeals to authority from one uncritical author to the next [69]. Second, the contentious phylogenetic relationships that remain in regions of the tree of animal life (for instance the position of the xenacoelomorphs) need to be resolved to the general satisfaction. Third, much more needs to be learned about whether genotype can predict phenotype in vivo—during embryogenesis and the rest of the life history; advances here will probably require a combination of experimental data and computational biology [65]. Moreover, much more needs to be discovered about the genomes and gene regulatory networks for the broadest possible range of animal phyla. Fourth, new approaches for reconstructing the details of big-picture evolution should be sought. One possibility might be phylostratigraphy [70], which examines the accretion of novel genes as organisms progressively evolve. It has been speculated that, periodically during evolution, there have been bouts of conversion of non-genic DNA into functional genes [71], such that constellations of these new genes may provide a ‘snapshot’ of a marked evolutionary change [72]. Fifth, there is always the chance that discovery of a living representative of a new phylum or some superb fossil might allow one to decide between the revived annelid and arthropod theories—or, alternatively, suggest some quite different invertebrate source for the vertebrates. Certainly, the discovery of fossilized nerve tissue in the Early Cambrian arthropod, Fuxianhuia protensa [58] serves as a reminder that fossils of preternatural importance are waiting to be discovered and are worth the trouble and expense to search out.
Acknowledgement
This manuscript was much improved by the criticisms of Linda Z. Holland.
Competing interests
I declare I have no competing interests.
Funding
I received no funding for this study.
References
- 1.Lamarck JBPA. 1809. Philosophie zoologique, vol. 2 Paris, France: Dentu. [Google Scholar]
- 2.Geoffroy Saint-Hilaire E. 1822. Considérations générales sur la vertèbre. Mém. Mus. Natl. Hist. Nat. 9, 89–119. [Google Scholar]
- 3.Kölliker A. 1842. Observationes de prima insectorum genesi: adiecta articulatorum evolutionis cum vertebratorum comparatione. Zürich, Switzerland: Meyer and Zeller. [Google Scholar]
- 4.Leydig F. 1864. Vom Bau des thierischen Korpers, Handbuch der vergleichenden Anatomie, vol. 1 Tübingen, Germany: Laupp and Siebeck. [Google Scholar]
- 5.Holland ND, Holland LZ, Holland PWH. 2015. Scenarios for the making of vertebrates. Nature 520, 450–455. ( 10.1038/nature14433) [DOI] [PubMed] [Google Scholar]
- 6.Leroi AM. 1997. A duck folded in half. Lond. Rev. Books 19, 19–20. [Google Scholar]
- 7.Semper C. 1874. Ueber die Stammverwandtschaft der Wirbelthiere und Anneliden. Centralbl. Med. Wissensch. 12, 545–547. [Google Scholar]
- 8.Semper C. 1875. Die Stammesverwandschaft der Wirbelthiere und Wirbellosen. Arb. Zool.-zootom. Inst. Würzburg 2, 25–76. [Google Scholar]
- 9.Dohrn A. 1875. Der Ursprung der Wirbelthiere und das Princip des Functionswechsels: Geneologische Skizzen. Leipzig, Germany: Engelmann; [English translation and analysis in Ghiselin MT. 1994 Hist. Phil. Life Sci. 16, 3–39.] [DOI] [PubMed] [Google Scholar]
- 10.Semper C. 1876. On the identity in type of the annelids and vertebrates. A preliminary communication. Ann. Mag. Nat. Hist. (Ser. 4) 17, 462–473. [Google Scholar]
- 11.Groeben C. 1982. Charles Darwin 1808–1882 Anton Dohrn 1840–1909, Correspondence. Naples, Italy: Macchiaroli. [Google Scholar]
- 12.Kleinenberg N. 1886. Die Entstehung des Annelids aus der Larve von Lopadorhynchus, nebst Bemerkungen über die Entwicklung anderer Polychaeten. Z. Wiss Zool. 44, 1–127. [Google Scholar]
- 13.Beard J. 1888. The old mouth and the new: a study in vertebrate morphology. Nature 32, 224–227. ( 10.1038/037224d0) [DOI] [Google Scholar]
- 14.Minot CS. 1897. Cephalic homologies. A contribution to the determination of the ancestry of the verebrates. Am. Nat. 31, 927–943. ( 10.1086/276733) [DOI] [Google Scholar]
- 15.Haeckel E. 1866. Generelle Morphologie der Organismen. Band 2. Allgemeine Entwickelungsgeschichte der Organismen. Berlin, Germany: Reimer. [Google Scholar]
- 16.Nielsen C. 1995. Animal evolution; interrelationships of the living phyla, 1st edn Oxford, UK: Oxford University Press. [Google Scholar]
- 17.Halanych KM, Bacheller JD, Aguinaldo AMA, Liva SM, Hillis DM, Lake JA. 1995. Evidence from 18S ribosomal DNA that the lophophorates are protostome animals. Science 267, 1641–1643. ( 10.1126/science.7886451) [DOI] [PubMed] [Google Scholar]
- 18.Grobben K. 1908. Die systematische Einteilung des Tierreiches. Verh. Zool.-Bot. Ges. Wien 58, 491–511. [Google Scholar]
- 19.Holley SA, De Robertis EM, Sasai Y, Jackson PD, Liu B, Hoffman FM, Ferguson EL. 1995. A conserved system for dorso-ventral patterning in insects and vertebrates involving sog and chordin. Nature 376, 249–253. ( 10.1038/376249a0) [DOI] [PubMed] [Google Scholar]
- 20.Arendt D, Nübler-Jung K. 1994. Inversion of dorsoventral axis? Nature 371, 26 ( 10.1038/371026a0) [DOI] [PubMed] [Google Scholar]
- 21.Nübler-Jung K, Arendt D. 1996. Enteropneusts and chordate evolution. Curr. Biol. 6, 352–353. ( 10.1016/S0960-9822(02)00491-8) [DOI] [PubMed] [Google Scholar]
- 22.Arendt D, Nübler-Jung K. 1999. Comparison of early nerve cord development in insects and vertebrates. Development 126, 2309–2325. [DOI] [PubMed] [Google Scholar]
- 23.Balavoine T, Adoutte A. 2003. The segmented Urbilateria: a testable scenario. Int. Comp. Biol. 43, 137–147. ( 10.1093/icb/43.1.137) [DOI] [PubMed] [Google Scholar]
- 24.Simakov O, et al. 2012. Insights into bilaterian evolution from three spiralian genomes. Nature 493, 526–531. ( 10.1038/nature11696) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denes AS, Jékely G, Steinmez PRH, Raible F, Snyman H, Prud'homme B, Ferrier DEK, Balavoine G, Arendt D. 2007. Molecular architecture of annelid nerve cord supports common origin of nervous system centralization in Bilateria. Cell 129, 277–288. ( 10.1016/j.cell.2007.02.040) [DOI] [PubMed] [Google Scholar]
- 26.Kulakova M, et al. 2007. Hox gene expression in larval development of the polychaetes Nereis virens and Platynereis dumerilii (Annelida, Lophotrochozoa). Dev. Genes Evol. 217, 39–54. ( 10.1007/s00427-006-0119-y) [DOI] [PubMed] [Google Scholar]
- 27.Tormer R, Denes AS, Tessmar-Raible K, Arendt D. 2010. Profiling by image registration reveals common origin of annelid mushroom bodies and vertebrate pallium. Cell 142, 800–809. ( 10.1016/j.cell.2010.07.043) [DOI] [PubMed] [Google Scholar]
- 28.Lauri A, Brunet T, Handberg-Thorsager M, Fischer AHL, Simakov O, Steinmetz PRH, Tomer R, Keller PJ, Arendt D. 2014. Development of the annelid axochord: insights into notochord evolution. Science 345, 1365–1368. ( 10.1126/science.1253396) [DOI] [PubMed] [Google Scholar]
- 29.Cobb JLS. 1995. The nervous systems of Echinodermata: recent results and new approaches. In Nervous systems of invertebrates: an evolutionary and comparative analysis (eds Briedbach O, Kutsch W), pp. 407–424. Berlin, Germany: Springer. [Google Scholar]
- 30.Lowe CJ, Wu M, Salic A, Evans L, Lander E, Stange-Thomann N, Gruber CE, Gerhart J, Kirschner M. 2003. Anteroposterior patterning in hemichordates and the origins of the chordate nervous system. Cell 113, 853–865. ( 10.1016/S0092-8674(03)00469-0) [DOI] [PubMed] [Google Scholar]
- 31.Philippe H, Brinkmann H, Copley RR, Moroz LL, Poustka AJ, Wallberg A, Peterson KJ, Telford MJ. 2011. Acoelomorph flatworms are deuterostomes related to Xenoturbella. Nature 470, 255–258. ( 10.1038/nature09676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrier DEK. 2007. Evolution of Hox gene clusters. In Hox gene expression, edn 1 (ed Papageorgiou S.), pp. 53–67. New York, NY: Springer. [Google Scholar]
- 33.Dunn CW, Giribet G, Edgecombe GD, Hejnol A. 2014. Animal phylogeny and its evolutionary implications. Annu. Rev. Ecol. Evol. Syst. 45, 371–395. ( 10.1146/annurev-ecolsys-120213-091627) [DOI] [Google Scholar]
- 34.Swalla BJ, Smith AB. 2008. Deciphering deuterostome phylogeny: molecular, morphological and palaeontological perspectives. Phil. Trans. R. Soc. B 363, 1557–1568. ( 10.1098/rstb.2007.2246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bateson W. 1886. The ancestry of the chordata. Q. J. Micros. Sci. 26, 535–571. [Google Scholar]
- 36.Nomaksteinsky M, Röttinger E, Dufour HD, Chettouh Z, Lowe CJ, Martindale MQ, Brunet JF. 2009. Centralizaton of the deuterostome nervous system predates chordates. Curr. Biol. 19, 1264–1269. ( 10.1016/j.cub.2009.05.063) [DOI] [PubMed] [Google Scholar]
- 37.Cunningham D, Casey ES. 2014. Spatiotemporal development of the nervous system of Saccoglossus kowalevskii. Dev. Biol. 386, 252–263. ( 10.1016/j.ydbio.2013.12.001) [DOI] [PubMed] [Google Scholar]
- 38.Masterman AT. 1897. On the Diplochorda, I. The structure of Actinotrocha. Q. J. Micros. Sci. 40, 281–338. [Google Scholar]
- 39.Meek A. 1917. On the Phoronidea. Rept. Dove Mar. Lab. Cullercoats 6, 33–48. [Google Scholar]
- 40.Bullock TH. 1944. The giant nerve fiber system in balanoglossids. J. Comp. Neurol. 80, 355–367. ( 10.1002/cne.900800305) [DOI] [Google Scholar]
- 41.Knight-Jones EW. 1952. On the nervous system of Saccoglossus cambrensis (Enteropneusta). Phil. Trans. R. Soc. Lond. B 236, 315–354. ( 10.1098/rstb.1952.0004) [DOI] [Google Scholar]
- 42.Ruppert EE, Cameron CB, Frick JE. 1999. Endostyle-like features of the dorsal epibranchial ridge in an enteropneust and the hypothesis of dorsal-ventral inversion in chordates. Invert. Biol. 118, 202–212. ( 10.2307/3227061) [DOI] [Google Scholar]
- 43.Tyler S. 2001. The early worm: origin and relationships of the lower flatworms. In Interrelationships of the platyhelminthes (eds Littlewood DTJ, Bray RA), pp. 3–12. London, UK: Taylor and Francis. [Google Scholar]
- 44.Lowe CJ. 2008. Molecular genetic insights into deuterostome evolution from the direct-developing hemichordate Saccoglossus kowalevskii. Phil. Trans. R. Soc. B 363, 1569–1578. ( 10.1098/rstb.2007.2247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Röttinger E, Duboc T, Martindale MQ. 2010. Investigating the role of the Nodal signaling pathway in a indirect developing hemichordate, Ptychodera flava. Integ. Comp. Biol. 50(Suppl 1), e144. [Google Scholar]
- 46.Miyamoto N, Wada H. 2013. Hemichordate neurulation and the origin of the neural tube. Nat. Commun. 4, 2713 ( 10.1038/ncomms3713) [DOI] [PubMed] [Google Scholar]
- 47.Satoh N, et al. 2014. On a possible evolutionary link of the stomochord of hemichordates to pharyngeal organs of chordates. Genesis 52, 925–934. ( 10.1002/dvg.22831) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lowe CJ, Clarke DN, Medeiros DM, Rokhsar DS, Gerhart J. 2015. The deuterostome context of chordate origins. Nature 520, 456–465. ( 10.1038/nature14434) [DOI] [PubMed] [Google Scholar]
- 49.Kaul-Strehlow S, Urata M, Minokawa T, Stach T, Wanninger A. 2015. Neurogenesis in directly and indirectly developing enteropneusts: of nets and cords. Organ. Divers. Evol. 15, 405–422. ( 10.1007/s13127-015-0201-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jákely G, Paps J, Nielsen C. 2015. The phylogenetic position of ctenophores and the origin(s) of nervous systems. EvoDevo 6, article 1. ( 10.1186/2041-9139-6-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Angielczyk K. 2009. Dimetrodon is not a dinosaur: using tree thinking to understand the ancient relatives of mammals and their evolution. Evol. Educ. Outreach 2, 257–271. ( 10.1007/s12052-009-0117-4) [DOI] [Google Scholar]
- 52.Villar-Cerviño V, Barreiro-Iglesias A, Mazan S, Rodicio MC, Anadón R. 2011. Glutaminergic populations in the forebrain of the sea lamprey, Petromyzon marinus: an in situ hybridization and immunological study. J. Comp. Neurol. 519, 1712–1735. ( 10.1002/cne.22597) [DOI] [PubMed] [Google Scholar]
- 53.Pradel A, Langer M, Maisey JG, Geffard-Kuriyama D, Cloetens P, Janvier P, Tafforeau P. 2009. Skull and brain of a 300-million-year-old chimaeroid fish revealed by synchrotron holotomography. Proc. Natl Acad. Sci. USA 106, 5224–5228. ( 10.1073/pnas.0807047106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Janvier P. 2008. The brain in the early fossil jawless vertebrates: evolutionary information from an empty nutshell. Brain Res. Bull. 75, 314–318. ( 10.1016/j.brainresbull.2007.10.024) [DOI] [PubMed] [Google Scholar]
- 55.Strausfeld NJ. 2012. Arthropod brains. Cambridge, MA: Harvard University Press. [Google Scholar]
- 56.Ziegler E, Bräunig P, Harzsch S. 2013. A developmental study of serotonin immunoreactive neurons in the embryonic brain of the marbled crayfish and migratory locust: evidence for a homologous protocerebral group of neurons. Arth. Struct. Funct. 42, 507–520. ( 10.1016/j.asd.2013.08.004) [DOI] [PubMed] [Google Scholar]
- 57.GIGA Community of Scientists. 2014. The global invertebrate geonomics alliance (GIGA): developing community resources to study diverse invertebrate genomes. J. Hered. 105, 1–18. ( 10.1093/jhered/est084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eriksson ME, Terfelt F. 2012. Exceptionally preserved Cambrian trilobite digestive system revealed in 3D by synchrotron-radiation X-ray tomographic microscopy. PLoS ONE 7, e35625 ( 10.1371/journal.pone.0035625) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma XY, Hou XG, Edgecomb GD, Strausfeld NJ. 2012. Complex brain and optic lobes in an early Cambrian arthropod. Nature 490, 258–262. ( 10.1038/nature11495) [DOI] [PubMed] [Google Scholar]
- 60.Jenner R. 2014. Macroevolution of animal body plans: is there science after the tree? Bioscience 64, 653–664. ( 10.1093/biosci/biu099) [DOI] [Google Scholar]
- 61.Henjol A, Lowe CP. 2014. Animal evolution: stiff or squashy notochord origins? Curr. Biol. 24, R1131–R1133. ( 10.1016/j.cub.2014.10.059) [DOI] [PubMed] [Google Scholar]
- 62.Laubichler MD. 2007. Does history recapitulate itself? Epistemological reflections on the origins of evolutionary developmental biology. In From embryology to evo devo (eds Laublichler MD, Maienschein J), pp. 53–67. Cambridge, MA: MIT Press. [Google Scholar]
- 63.Gould SJ. 1997. As the worm turns. Nat. Hist. 106, 24–27; 68–73. [Google Scholar]
- 64.Roth VL. 1988. The biological basis of homology. In Ontogeny and systematics (ed. Humphries CJ.), pp. 1–26. New York, NY: Columbia University Press. [Google Scholar]
- 65.Karr JR, Sanaghvi JC, Macklin DN, Gutschow MV, Jacobs JN, Bloival B, Assad-Garcia N, Glass JI, Covert MW. 2012. A whole-cell computational model predicts phenotype from genotype. Cell 150, 389–401. ( 10.1016/j.cell.2012.05.044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Holland ND, Holland LZ. 1999. Amphioxus and the utility of molecular genetic data for hypothesizing body part homologies between distantly related animals. Am. Zool. 39, 630–640. ( 10.1093/icb/39.3.630) [DOI] [Google Scholar]
- 67.Telford M, Littlewood DTJ. 2009. Reassembling animal evolution: a four-dimensional puzzle. In Animal evolution: genomes, fossils, and trees (eds Telford MJ, Littlewood DTJ), pp. 191–196. Oxford, UK: Oxford University Press. [Google Scholar]
- 68.Northcutt RG. 2012. Evolution of centralized nervous systems: two schools of evolutionary thought. Proc. Natl Acad. Sci. USA 109, 10 626–10 633. ( 10.1073/pnas.1201889109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jenner R. 2001. Bilaterian phylogeny and uncritical recycling of morphological data sets. Syst. Biol. 50, 730–742. ( 10.1080/106351501753328857) [DOI] [PubMed] [Google Scholar]
- 70.Tautz D, Domazet-Lošo T. 2011. The evolutionary origin of orphan genes. Nat. Rev. Genet. 12, 692–702. ( 10.1038/nrg3053) [DOI] [PubMed] [Google Scholar]
- 71.Carvunis AR, et al. 2012. Proto-genes and de novo gene birth. Nature 487, 370–374. ( 10.1038/nature11184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen S, Krinsky BH, Long MY. 2013. New genes as drivers of phenotypic evolution. Nat. Rev. Genet. 14, 645–660. ( 10.1038/nrg3521) [DOI] [PMC free article] [PubMed] [Google Scholar]