Abstract
Orthologous genes involved in the formation of proteins associated with memory acquisition are similarly expressed in forebrain centres that exhibit similar cognitive properties. These proteins include cAMP-dependent protein kinase A catalytic subunit (PKA-Cα) and phosphorylated Ca2+/calmodulin-dependent protein kinase II (pCaMKII), both required for long-term memory formation which is enriched in rodent hippocampus and insect mushroom bodies, both implicated in allocentric memory and both possessing corresponding neuronal architectures. Antibodies against these proteins resolve forebrain centres, or their equivalents, having the same ground pattern of neuronal organization in species across five phyla. The ground pattern is defined by olfactory or chemosensory afferents supplying systems of parallel fibres of intrinsic neurons intersected by orthogonal domains of afferent and efferent arborizations with local interneurons providing feedback loops. The totality of shared characters implies a deep origin in the protostome–deuterostome bilaterian ancestor of elements of a learning and memory circuit. Proxies for such an ancestral taxon are simple extant bilaterians, particularly acoels that express PKA-Cα and pCaMKII in discrete anterior domains that can be properly referred to as brains.
Keywords: evolution, hippocampus, mushroom body, homology, convergence
1. Introduction
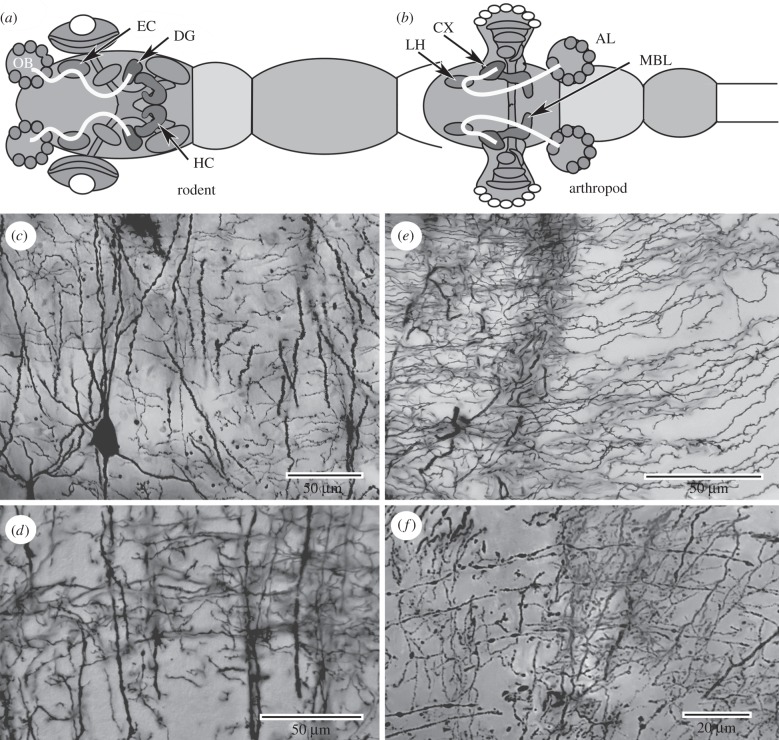
A motif in the brains of invertebrates and vertebrates is that of relay neurons from primary olfactory neuropils projecting to distinctive higher order structures in the forebrain. In insects these structures are the mushroom bodies, characterized by bifurcating lobes composed of many thousands of approximately parallel processes that establish pre- and postsynaptic sites with systems of intersecting afferent and efferent arborizations. A characteristic of these centres is that afferents and efferents define discrete domains through the lobes, from which centrifugal cells provide reafferent feedback to more distal levels (figure 1a). The same ground pattern exists in crustaceans (figure 1b) in dome-like centres referred to as hemiellipsoid bodies, the organization of which genealogically corresponds to mushroom bodies [1,2]. Mushroom bodies, like those of insects, occur in Lophotrochozoa, as in the annelid's asegmental and supraesophageal brain (‘acron’) where paired mushroom bodies are supplied by relays from olfactory glomeruli [3]. In the vertebrate forebrain, the same ground pattern defines layered hippocampi of non-mammalian vertebrates (figure 1c) or the horn-like CA3 region of the mammalian hippocampus whose afferent supply originates in the olfactory bulbs and is relayed to the dentate gyrus via intermediate centres, such as the piriform cortex, amygdala and entorhinal cortex [4].
Figure 1.
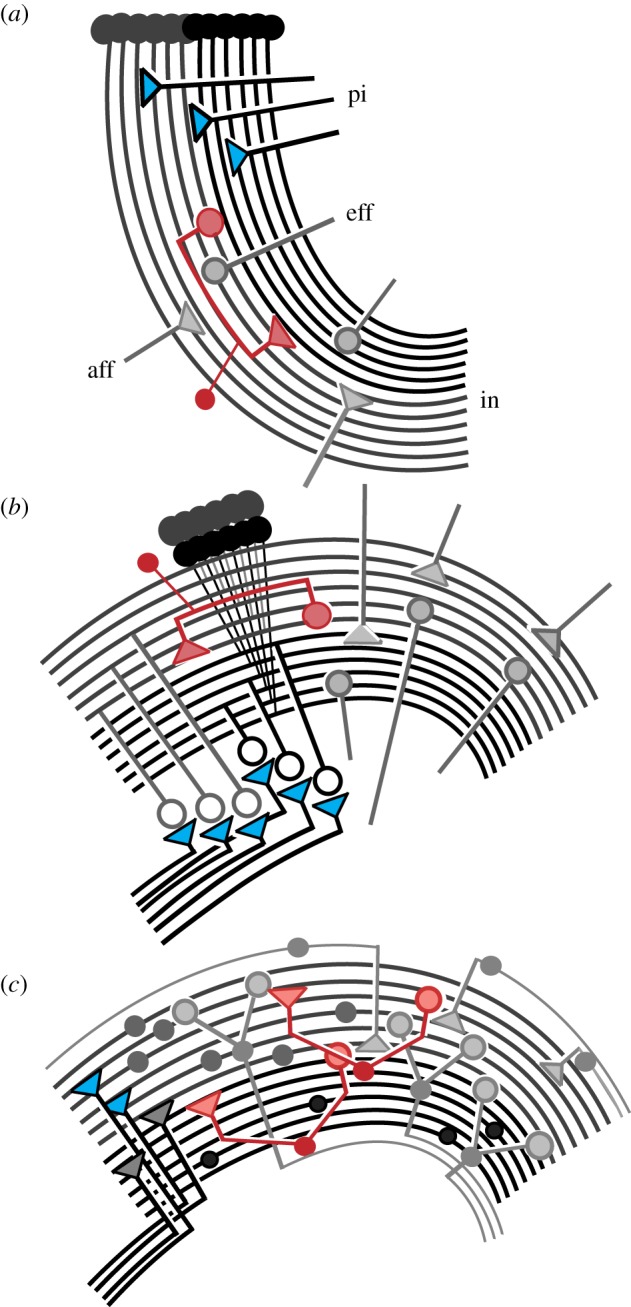
Ground pattern organization: neuronal arrangements correspond in the lobed mushroom bodies of arthropods and annelids (a), the dome-like hemiellipsoid bodies of malacostracan crustaceans (b), and in stratified hippocampus of mammalian and non-mammalian vertebrates (c). Primary inputs (pi; e.g. olfactory) terminate distally on systems of approximately parallel fibres belonging to intrinsic neurons (in) in arthropods and the mossy fibres or equivalent in vertebrates. These are intersected by systems of afferent (aff) and outgoing efferent (eff) neurons, with local interneurons (red) defining sequential domains along the parallel fibre array.
Pivotal roles in learning and memory, including place memory, have been ascribed to insect mushroom bodies and to mammalian hippocampus [5–7]. Disruption of homologous genes in fruit flies and mice results in learning and memory defective phenotypes that relate to defects of those centres (see table 1). For example, null mutations of the gene encoding Drosophila protein kinase A catalytic subunit (DC0), an orthologue of mammalian protein kinase A catalytic subunit alpha (PKA-Cα), result in poor performance of olfactory memory tasks [13]. Protein kinase A is also necessary for the formation of long-term memory in the vertebrate hippocampus and in the Drosophila mushroom bodies [13,14,54,55]. Numerous other orthologous genes have been identified in hippocampus and mushroom bodies that are required for the same functions in each (table 1).
Table 1.
Orthologous gene expression correspondences in hippocampus and mushroom body.
| mouse/rat hippocampus | Drosophila/honey bee mushroom body |
|---|---|
| Neurl1: Encodes ubiquitin ligase protein, Neuralized 1. Promotes protein-synthesis-dependent long-term memory [8] | Neur: Encodes ubiquitin ligase protein, Neuralized. Promotes protein-synthesis-dependent long-term memory [9] |
| Prkar1b: Encodes neuronal isoform of cAMP-dependent protein kinase A type I regulatory subunit. Role in hippocampal long-term depression and depotentiation [10] | PKA-RI: Expressed at high levels in mushroom bodies. Role in associative learning [11] |
| PRKACA: Encodes PKA-Cα, involved in the regulation of hippocampus-dependent memory [12] | DC0: Required for synthesis of cAMP-dependent protein kinase A (PKA) catalytic subunit DCO. Required for cAMP regulation [13–15] |
| Adcyap1: Encodes PACAP (pituitary adenylate cyclase-activating polypeptide), promotes long-term facilitation; NMDA receptor regulation in CA1 pyramidal cells [16–18] | Amn: Encodes PACAP-like neuropeptide required for memory retention, olfactory memory [19–21] |
|
Egr1: Early Growth Response Protein 1 expressed during initial phase of spatial learning [22,23] |
Egr (in honey bee): Early Growth Response Protein. Expressed during initial phase of spatial learning [24] |
| CPEB gene family: Encode cytoplasmic polyadenylation element binding proteins, which regulate synaptic plasticity in the hippocampus [8,25] | Orb/Orb2: Encode Drosophila CPEB proteins necessary in mushroom body neurons for long-term memory formation [26,27] |
| Itga3: Encodes the α3 integrin subunit, which facilitates long-term potentiation (LTP) in Schaffer collateral-CA1 synapses in the hippocampus and is required for working memory behaviour [28] | vol: Encodes αPS3-integrin, preferentially expressed in mushroom bodies. Required for formation of short-term memory [29] |
| EGFR: Encodes epidermal growth factor receptor, which is expressed at relatively high levels in the hippocampus. Epidermal growth factor in the hippocampus facilitates induction of LTP [30,31] | EGFR: Epidermal growth factor receptor required in the mushroom bodies of Drosophila larvae for olfactory learning [32] |
| mDach1: Encodes a nuclear protein with activity in the forebrain and especially CA1 field of the hippocampus [33] | dac: Encodes a transcription factor necessary for proper mushroom body development [34] |
| Pax6: Encodes transcription factor Pax6 necessary during CNS development, for hippocampus-dependent short-term memory, and plays a role in adult hippocampal neurogenesis [35] | toy: Encodes a Pax6 protein necessary for proper mushroom body development [36] |
| Rheb: Encodes small GTP-binding protein Rheb, expressed at high levels in the hippocampus. Rheb expression is up-regulated in hippocampus granule cells following LTP induction [37] | Rheb: Encodes small GTP-ase Rheb, which regulates mushroom body neuron growth and induction of long-term memory [38] |
| Fmr1: Encodes fragile × Mental Retardation Protein (FMRP), necessary for proper hippocampal development and social/cognitive behaviours [39,40] | dfmr1: Encodes the FMRP orthologue dFMRP, highly enriched in the mushroom bodies and necessary for proper development of these structures [41,42] |
| NCAM: Encodes Neural Cell Adhesion Molecule (NCAM), necessary for proper synaptic targeting of hippocampal mossy fibre projections as well as for olfactory learning. Role in LTP [43–45] | Fas2: Encodes cell adhesion molecule fasciclin II, orthologous to NCAM. Necessary for proper mushroom body development, particularly lobe morphology [46] |
| L1CAM: Encodes L1-cell adhesion molecule (L1CAM), necessary for axonal and dendritic bundling of hippocampal neurons. Role in LTP [44,47] | Nrg: Encodes L1CAM orthologue Neuroglian, necessary for mushroom body axon development [48] |
| CaMKII: Encodes a calcium/calmodulin-activated protein kinase. CaMKIIα protein expression is highest in the dentate gyrus, CA1 and CA3 of the hippocampus. Dysfunction implicated in Angelman syndrome [49,50] | CaMKII: Encodes a calcium/calmodulin-activated protein kinase. CaMKII immunoreactivity is most highly concentrated in the mushroom body lobes and calyces [51] |
| 14-3-3ζ: Encodes 14-3-3 proteins necessary for neurodevelopment and social cognition [52] | leo: Encodes 14-3-3ζ orthologue Leonardo, enriched in mushroom bodies and necessary for olfactory learning and memory [53] |
Across insects, mushroom bodies and their crustacean homologues are exceptionally immunoreactive to antibodies raised against DC0 [2,56]. This molecular character also corresponds across all arthropod groups and lophotrochozoan phyla including annelids, Platyhelminthes and nemerteans [3]. Because Drosophila protein DC0 and mammalian PKA-Cα are 82% conserved [57], and because the sequence of protein kinase A is highly conserved across Metazoa [58,59], immunohistological localization of this enzyme should allow the detection of possible corresponding centres even across very distantly related phyla. Additionally, antibodies against phosphorylated Ca2+/calmodulin-dependent protein kinase II (pCaMKII) likewise localize to centres that correspond to the insect mushroom body across Ecdysozoa and Lophotrochozoa, show highly conserved sequence between insects and mammals, and may also reveal structures that correspond genealogically across the Bilateria [3,60].
Genealogical correspondence would be further supported if neuropils selectively identified by those antibodies shared additional orthologous genes involved in corresponding memory functions. And, crucially, correspondence would be underpinned by shared neural organizations. Such combinations of shared characters would extend the claims of a common ancestry of annelid and insect mushroom bodies, and the murine pallium, proposed by Tomer et al. [61] on the basis of their similar temporal and spatial patterning by orthologous transcription factor genes. The suite of differentiation and regulatory genes expressed by a cell type, or the ‘molecular fingerprint’ [61] provides a more robust search image for comparison of tissues than a single gene and allows for more confident conclusions about common ancestors of phylogenetically distant groups than traditional morphological comparisons alone. Molecular fingerprinting also aids in the identification of evolutionary convergences, when cell types with different gene expression profiles in different taxa arrive at functional similarity via exaptation by upstream signalling pathways.
One condition for common ancestry, hereafter referred to as genealogical correspondence, is that corresponding centres share specific characters recognized not only by shared gene expression but also by other independent traits. Four of these are here: (1) the presence of dense clusters of thousands, sometimes hundreds of thousands, of small basophilic neuron cell bodies, in arthropods referred to as globuli cells [62]; (2) an extension from each cell body of a long, slender process not necessarily providing apical dendrites but always bearing pre- and postsynaptic specializations along its length; (3) the intersection of the ensemble of such processes by sequential domains of afferent terminals and efferent dendrites; and (4) the presence of recurrent interneurons within the ensemble of processes. In arthropods, thousands of such processes are intersected by successive afferent–efferent domains to form an approximately orthogonal matrix [2]. In the mammalian hippocampal formation, intrinsic cell bodies of the dentate gyrus comprise the granule cell layer and those of CA1–CA3 comprise the stratum pyramidale. These send parallel tracts of axons through the cornu ammonis that form an orthogonal matrix, interfacing with local interneurons, afferent inputs from the entorhinal cortex, and projecting to the subiculum and back to the entorhinal cortex [63]. Another trait is that centres morphologically corresponding to mushroom bodies are, at least ancestrally, supplied from primary olfactory centres except in species where there has been an evolved switch of modality or loss of odorant receptors, as in some anosmic insects and odontocete whales [64,65]. Another trait is that the location of any centre that is claimed to genealogically correspond to an insect mushroom body is situated in the most anterior neuromere of the brain or just the rostral brain in those invertebrates that possess asegmental brains, such as nemertean worms, annelids and Platyhelminthes. As demonstrated by species of chelicerates, this rostral location of a mushroom body is independent of the segmental location of the primary olfactory neuropils that supply it [62]. Although previous work has demonstrated that these conditions are met across four invertebrate phyla [3], here we describe that they are also fulfilled across the Chordata. In Xenacoelomorpha, a phylum claimed as a sister group to all deuterostomes [66], or to Bilateria [67], the asegmental brains of the acoel Symsagittifera roscoffensis also contain regions that reveal highly enriched ‘mushroom body-identifying’ proteins.
2. Material and methods
(a). Research animals
Cockroaches (Periplaneta americana), land hermit crabs, various species of myriapods and arachnids were obtained either from breeding colonies in the Department of Neuroscience, University of Arizona, purchased from domestic suppliers, or collected in the wild. Some hundred specimens of the acoel S. roscoffensis were kindly provided by Dr Xavier Bailly, Station Biologique de Roscoff, France. The laboratories of Dr Carol Barnes and Dr Nate McMullen at the University of Arizona provided formaldehyde-fixed rat and murine brains. Formaldehyde-fixed brains of the newt, Plethodon shermani, was generously donated by Dr Sarah Woodley at Duquesne University and similarly fixed brains of the turtle, Trachemys scripta elegans were generously donated by Dr Catherine Carr at the University of Maryland. Brains of the sea lamprey, Petromyzon marinus were processed at the laboratory of Dr Sten Grillner at the Karolinska Institutet's Nobel Institute for Neurophysiology. All procedures were in accordance with National Institutes of Health guidelines and protocols approved by the University of Arizona Institutional Animal Care and Use Committee or equivalent guidelines and protocols at the other aforementioned institutions.
(b). Antibodies
The monoclonal antiserum against alpha-tubulin (12G10) was used at a concentration of 1 : 100 and was developed by Drs J. Frankel and E. M. Nelsen. This antiserum was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biology (Iowa City, IA, USA). The ancient and highly conserved nature of tubulin suggests that antibodies raised against it recognize this protein across a broad range of Metazoa, including taxa used in this study.
Anti-DC0, a generous gift of Dr Daniel Kalderon [68], was used at a concentration of 1 : 250 for immunohistochemistry. Anti-PKA C-alpha antibody (Cell Signaling Technology, Danvers, MA, USA, Cat. no. 4782) was used at a concentration of 1 : 250 for immunohistochemistry and 1 : 2500 for western blot assays. This antibody also recognized a band of the expected molecular weight at approx. 39 kDa on a western blot comparing tissue of P. americana and R. norvegicus (figure 2). Finally, antisera against pCaMKII (Santa Cruz Biotechnology, Dallas, TX, USA) were used at a concentration of 1 : 100 for immunohistochemistry.
Figure 2.
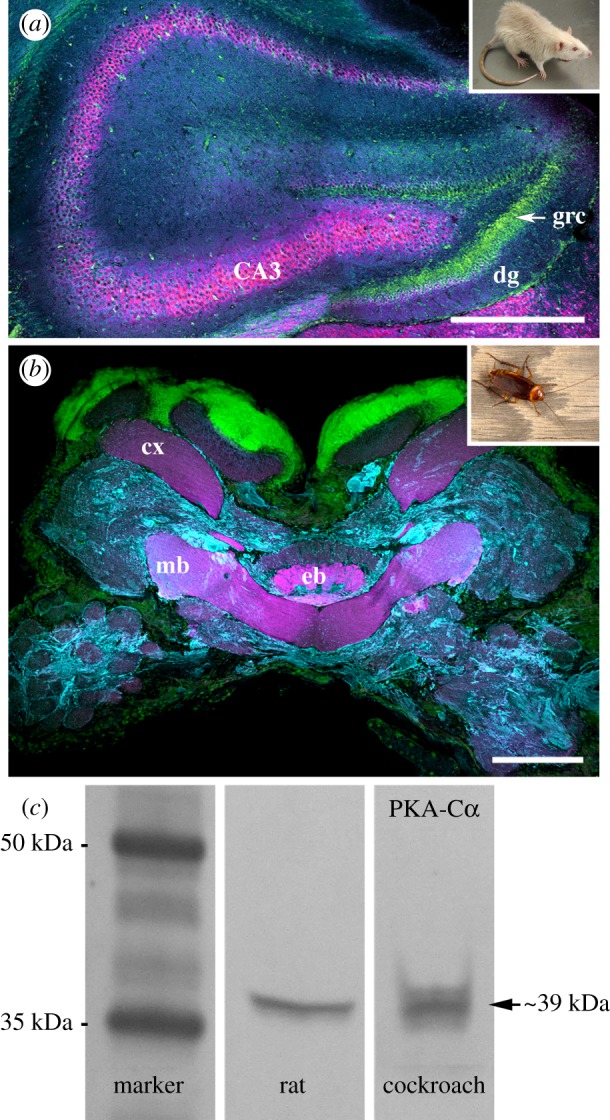
Cross-phyletic correspondence of PKA-Cα immunoreactivity. (a) In the hippocampus of the rat, Rattus norvegicus, mammalian PKA-Cα immunoreactivity (magenta) is strong in the CA fields (CA3). (b) Confocal laser scan of brain of the cockroach, Periplaneta americana, labelled with antibodies against mammalian PKA-Cα (magenta). The concentration of PKA-Cα immunoreactivity in the mushroom body (mb) and ellipsoid body (eb) of the central complex is the same as the pattern of DC0 antibody localization in the cockroach brain [2]. α-Tubulin immunoreactivity (cyan) and nucleic acid stain (green) provide structural reference (a and b). Other abbreviations: dg, dentate gyrus; cx, calyx; grc, granule cells. (c) Western blot assay of mammalian PKA-Cα antibody comparing brain tissue of R. norvegicus (rat) and P. americana reveals a band around 39 kDa, indicating cross-phyletic specificity of this antibody. Scale bars: (a) 600 µm; (b) 200 µm.
(c). Immunohistochemistry
Invertebrate animals were immobilized by refrigeration at 4°C. Brains were dissected free in cold (4°C) fixative (4% paraformaldehyde in phosphate-buffered saline, pH 7.4 [PBS, Sigma, St Louis, MO, USA]). Brains were fixed in a microwave at 18°C for two cycles of 2 min with power and 2 min under vacuum. Brains were left in fresh fixative overnight at 4°C, then washed three times for 10 min in PBS before embedding in albumin gelatine. Mammalian brains were acquired after the stage of fixation in 4% formaldehyde under the abbreviated protocol 15–571 approved by the University of Arizona Institutional Animal Care and Use Committee Program. Other vertebrate brains used here were donated from other laboratories (see acknowledgements) and were prepared according to the same or equivalent protocols. Sixty micrometre serially sectioned vibratome (Leica, Nussloch, Germany) sections were placed in a well plate for further processing.
Sections were washed six times over 20 min in PBS containing 0.5% Triton X-100 (Electron Microscopy Supply, Fort Washington, PA, USA, Cat. no. 22140; PBS-TX). Fifty-microlitre normal serum was added to each well containing 1000 µl PBS-TX. After 1 h, primary antibody was added to each well and the well plate was left on a shaker overnight at room temperature. Sections were washed six times over 3 h in PBS-TX. One thousand microlitre aliquots of PBS-TX were placed in tubes with 2.5 µl of secondary Cy2-, Cy3-, or Cy5-conjugated IgGs (Jackson ImmunoResearch, West Grove, PA, USA) and centrifuged at 13 000 rpm for 15 min at 4°C. A 900-µl aliquot of this solution was added to each well. The well plate was left on a shaker to gently agitate the sections overnight at room temperature. Tissue sections were then washed in PBS six times over 3 h, mounted on glass slides in a medium of 25% polyvinyl alcohol, 25% glycerol and 50% PBS, and imaged using confocal microscopy. Where applicable, sections were incubated in the fluorescent nuclear stain Syto-13 (Life Technologies, Grand Island, NY, USA) at a concentration of 1 : 4000 prior to embedding on glass slides.
(d). Golgi impregnations
Invertebrate animals were cold anaesthetized. The antennae were cut off from the head at their base, mouthparts were removed and the rostrum opened while submerged in the chromation solution (2.5% potassium dichromate containing 1.3% sucrose). The anterior part of the head containing the brain was then cut away from the body and placed in a vial of fresh fixative consisting of five parts of chromation solution and one part 25% glutaraldehyde for 4 days at room temperature. Tissue was cleaned of muscle, fat bodies and trachea. Vertebrate tissue was acquired post-fixation. Subsequently, brain tissue was treated as follows. Brains were washed in several changes of 2.5% potassium dichromate (without sucrose). This was followed by a second chromation (3 days at room temperature) in 2.5% potassium dichromate and 1% osmium tetroxide (99 : 1). Tissue was then swirled a few seconds in distilled water and then immersed in 0.75% silver nitrate. After 3 days of impregnation, brains were dehydrated, embedded in epoxy resin and serial sectioned at 30 µm.
(e). Western blots
Brain tissue for P. americana was homogenized in lithium dodecyl sulfate (LDS) sample buffer with a protease inhibitor cocktail (Sigma) and run under reducing conditions. Cell lysate from the hippocampus of R. norvegicus was purchased from G-Biosciences (no. SLR-024, St Louis, MO, USA) and added to LDS sample buffer containing protease inhibitor cocktail. The Novex electrophoresis system was used for protein separation as described by Gibson and Tolbert [69].
(f). Imaging and reconstruction
Confocal reconstructions were made with an LSM 3 Pascal confocal microscope (Zeiss, Jena, Germany). From 10 to 30 images of 1024 × 1024 pixel resolution at 12-bit colour depth were scanned by using 10×/0.3 plan Plan-Neofluar objectives. For large brains scanned, overlapping areas were stitched and their intensities adjusted to achieve uniform balance. Light microscopy images were obtained with a Zeiss Axio Imager Z.2. Step focus series (0.5–1.0 µm increments) of stitched images were reconstructed using the software Helicon Focus (Helicon Soft, Kharkov, Ukraine). Selected images were digitally processed and assembled using Adobe Photoshop CS4 (Adobe Systems, San Jose, CA, USA).
3. Results
(a). PKA-C immunoreactivity resolves the insect mushroom body and mammalian hippocampus
Enriched expression of PKA-Cα is a molecular character present in paired forebrain structures in arthropods, annelids, Platyhelminthes and nemerteans [3]. We first tested the affinity of both mammalian and insect brains to antibodies raised against the mammalian PKA-Cα. As expected, in the rat (Rattus norvegicus), areas CA1, CA2 and CA3 were intensely immunoreactive to antibodies against PKA-Cα (figure 2a). Tests for immunoreactivity to mammalian PKA-Cα in the cockroach, Periplaneta americana showed high affinity in the mushroom bodies and the central complex's ellipsoid body (figure 2b). Next, in order to demonstrate specificity of the PKA-Cα antibodies, we performed western blot assays on brain tissue of the cockroach and rat. Antibodies against mammalian PKA-Cα recognized bands of the expected molecular weight around 39 kDa in both the rat and cockroach, revealing cross-phyletic specificity of this antibody (figure 2c).
(b). Morphological correspondences of mushroom bodies and hippocampus
The organization of the hippocampus and mushroom bodies correspond (figure 3a,b). The ancestral condition in vertebrates is the hippocampal afferent supply from the olfactory lobes. In mammals, this is provided by relays via the entorhinal cortex to the dentate gyrus there supplying dendrites of its granule cells, a system of densely packed neurons with extremely small perikarya. A ubiquitous feature of insect mushroom bodies, recognized as long ago as the mid-nineteenth century [70], is a highly circumscribed distal mass of minute basophilic cell bodies, called globuli cells, that cap the peripheral neuropil of mushroom body—the calyces—that contains their dendrites. Afferents from primary olfactory neuropils supply these dendrites. Neurons originating in the dentate gyrus provide long processes (mossy fibres) to CA3. These intrinsic elements of the hippocampus correspond to long pre- and postsynaptic intrinsic processes provided by globuli cells that comprise the mushroom bodies' horn-like lobes. Golgi impregnations of the rat hippocampus and the insect mushroom body resolve these approximately parallel arrangements of fibres intersecting local efferent neuron dendrites at approximately right angles (figure 3c,e). Together, the whole system of neurons comprises a highly ordered orthogonal network.
Figure 3.
Ground pattern organization: correspondence of pathways and orthogonal organization of neurons. (a,b) Congruent circuitry of the rodent hippocampus and insect mushroom body is demonstrated by pathways from olfactory bulb/antennal lobes (OB, AL) targeting, respectively, the dentate gyrus (DG) and calyces (CX), via the entorhinal cortex (EC) and lateral horn (LH). Mossy fibres, whose dendrites are located in the DG, send their parallel processes into CA fields of the hippocampus (HC). Globuli cells, whose dendrites are located in the CX, send their parallel processes in to the mushroom body lobes (MBL). (c,d.) Golgi impregnated rat CA3 (c) or turtle medial cortex (d) resolve orthogonal neuronal arrangement of parallel mossy fibre processes intersected by efferent dendrites of pyramidal cells. (e,f) Golgi-impregnated cockroach mushroom body lobes (e) or layers of Coenobita hemiellipsoid body (f) resolve corresponding orthogonal arrangements of parallel fibres.
Thus the mammalian hippocampus and insect mushroom bodies, both forebrain centres, share key features: a uniquely concentrated population of minute basophilic neurons providing a dense system of dendrites receives inputs from higher olfactory centres. These dendrites provide, respectively, the main elements of the dentate gyrus and mushroom body calyx. In both, they give rise to parallel intrinsic fibres intersected by sequential domains of afferent and efferent arborizations, resulting in an orthogonal organization of neuronal processes. A further characteristic of both hippocampus and mushroom bodies are systems of GABAergic centrifugal cells and other local neurons extending between different levels of the parallel fibre array [71–73].
Discrete pallial regions in non-mammalian vertebrates also reveal the same ground pattern of orthogonal arrangements even though not organized as horn-like centres (figure 3d). The same is observed in malacostracan crustaceans [2]. Evolved dome-like forebrain centres, provided by relays from the olfactory lobes, manifest the same ground pattern of orthogonal arrangements (figure 3f). Such gross morphological departures from a horn-like volume do not imply divergence from the ground pattern but merely a divergence of the pallium's geometry, in which the ground pattern is expressed. Comparable modifications are apparent across Arthropoda. In numerous groups, such as in Myriapoda, mushroom bodies are lobed whereas in other taxa modifications of overall shape nevertheless maintain the mushroom body ground pattern [3]. The most spectacular examples are in chelicerate arthropods: mushroom bodies in Amblypygidae and Xiphosura have both evolved a convoluted pallial-like architecture whose surfaces show sulci and gyri reminiscent of a folded mammalian cortex [74,75].
That the mushroom body ground pattern likely existed in the protostome–deuterostome ancestor is supported by observations of lophotrochozoan brains [3]. In annelid worms, such as the polychaete Nereis virens, paired brain structures that correspond to insect mushroom bodies are similarly characterized by a globuli cell layer that sends parallel processes into the lobes, where they interface with afferent and efferent neurons as well as centrifugal cells that feed back onto intrinsic neurons. Likewise, in Platyhelminthes, such as the polyclad flatworm Notoplana sanguinea, clusters of globuli cells extend parallel processes into stalk-like structures in the brain, which conform to the mushroom body ground pattern of organization, intersecting with other neuronal processes, although the identity of those orthogonal elements have not yet been characterized [3]. Hence, the presence of neuroanatomical characters of the mushroom body ground pattern is observed in all three bilaterian superphyla: Ecdysozoa, Lophotrochozoa and Deuterostomia.
(c). Correspondence of PKA-Cα and pCaMKII immunoreactivity in paired forebrain centres across Chordata
We next investigated whether intense immunoreactivity to mushroom body marker antibodies is a character shared by paired forebrain neuropils across vertebrates. We applied PKA-Cα or pCaMKII antibody to vibratome sections of brains of representative taxa from major vertebrate classes: Petromyzontida (lampreys), Amphibia, Reptilia and Mammalia.
Nuclei of the medial pallium of the lamprey are selectively resolved by PKA-Cα immunoreactivity (figure 4a), which also resolves mammalian hippocampus (figure 4d). Application of antibodies against pCaMKII resolved enrichment of this protein in the lateral pallium of an amphibian newt and the medial cortex of the turtle (figure 4b,c). Although well-resolved brain atlases are not available for these taxa, evidence from goldfish and turtles suggests that the medial cortex of reptiles and lateral pallium of ray-finned fishes share functional correspondence in learning and memory with the mammalian hippocampus [76]. We find that all taxa investigated in this study show regions of concentrated expression of mushroom body marker proteins in brain areas originating from the pallium, a molecular character present in five vertebrate groups, indicating a continuum of genealogical correspondence, and thus inheritance from a common ancestor.
Figure 4.
Anti-PKA-Cα and anti-pCaMKII reveal corresponding immunoreactivity of higher order brain centres across vertebrates. (a–d) Confocal laser scans of brain sections of the lamprey, P. marinus; newt, P. shermani; turtle, T. scripta elegans; and the rat Rattus norvegicus. Circumscribed volumes of the medial pallium of the lamprey (a), and rat hippocampus (d) are highly PKA-Cα immunoreactive (magenta) compared to surrounding tissue. In the newt (b), a circumscribed region of the lateral pallium shows high intensity immunoreactivity to anti-pCaMKII whereas in the turtle (c) the medial cortex is labelled. In all figures, α-tubulin immunoreactivity (cyan) and nucleic acid stain (green) provide structural references. Scale bars: (a–c) 200 µm; (d) 500 µm.
(d). PKA-Cα immunoreactivity resolves delineated brain centres in the acoel S. roscoffensis
If paired mushroom body-like centres and pallial centres in the forebrains of invertebrates and vertebrates correspond genealogically, might members of a phylum claimed as either sister to all bilaterians [77] or sister group to all deuterostomes [66], reveal comparable centres? To answer this question we selected amongst Xenacoelomorpha the acoel S. roscoffensis, which, recent studies demonstrate, possesses a ganglionic area rostrally from which extend caudally directed nerve cords and nerve nets that are resolved using antisera against neural proteins and peptides [78–80].
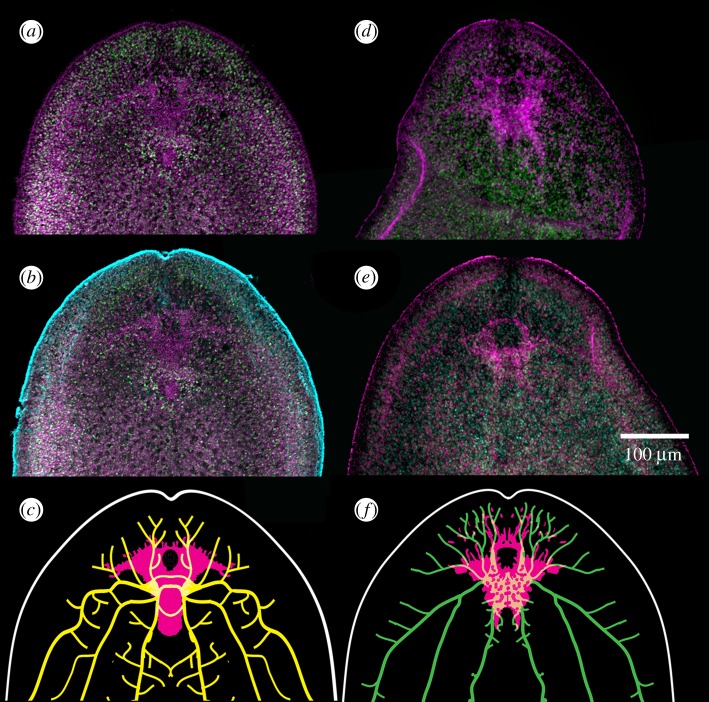
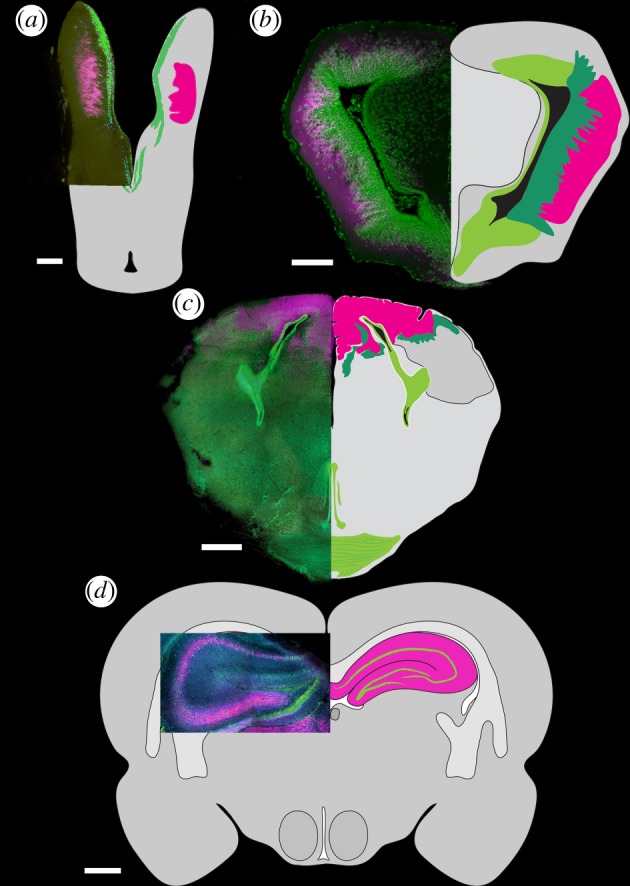
Application of antisera against the Drosophila PKA-Cα orthologue, DC0, as well as phosphorylated Ca2+/calmodulin-dependent protein kinase II (pCaMKII) to S. roscoffensis shows a substantial and bilaterally symmetric domain expressing high levels of these proteins at this anterior neuronal domain and low levels of the proteins distributed through other tissues, as would be expected. The localized elevated expression in a substantial anterior structure (figure 5a–f) suggests that the ‘ganglion’ of S. roscoffensis should be considered a true brain denoted by a discrete integrative centre associated with rostral sensory systems: the gravity-sensing medial statocyst and, rostro-laterally, the paired eye spots (figure 5a–g). Abundant chemosensory receptors on the acoel's epidermis would be disposed to provide numerous axons to the DC0-positive domain, possible via extensive processes revealed by antisera against elav and synaptotagmin that converge there [67]. The DC0 and pCaMKII domains coincide in part with a second bilateral system expressing serotonin that also extends axon bundles caudally [78,79]. However, the DC0/pCaMKII domain is constrained anteriorly and does not extend into rostrally directed nerve cords.
Figure 5.
Anti-DC0 and anti-pCaMKII resolve a substantial brain in the acoel S. roscoffensis. (a–d) Both antisera (DC0 in a,b; anti-pCaMKII in d,e) resolve a bilaterally symmetric region anterior to the statocyst. The origins of caudally directed nerve cords resolved by antisera against synaptotagmin (c), elav (f) and serotonin domains (see figure 6) (adapted from [66,77]) demonstrate their overlap within restricted regions of the DC0- (c) and anti-pCaMKII (f)-positive domains. Together, these relationships suggest a brain within which there are circumscribed tracts, neuropils and modulatory pathways.
4. Discussion
(a). Selective resolution of learning and memory centres by PKA-Cα and pCaMKII
Anti-PKA-Cα specifically targets the major catalytic subunit of cAMP-dependent protein kinase A (PKA), which is ubiquitous among cell and tissue types. Why then does this antibody selectively reveal the mushroom body with such extraordinarily high affinity compared with surrounding neuropils?
One possible explanation is that affinity might simply be due to the density of neuronal processes in the mushroom body. However, although these are among the densest in the brain, the mid-line fan-shaped body neuropil is as dense, as is synaptic neuropil in antennal lobe glomeruli or in the optic lobe's lobula plate. Yet none of those three centres are differentially resolved by anti-PKA-Cα. Instead, the only other neuropil to reveal high-affinity PKA-Cα labelling is the central body's ellipsoid body, a neuropil also implicated in working memory [81,82]. Observations of the turtle or newt pallium show no obvious differences of neuronal density, yet just one restricted domain, the hippocampus, is demarcated by high affinity to these antibodies. The conclusion is that these paired centres are differentiated by their expression levels of PKA-Cα.
In its inactive form, PKA exists as a holoenzyme consisting of two catalytic subunits negatively modulated by two regulatory subunits. Upon binding cAMP, the holoenzyme dissociates, leaving the catalytic subunits free to phosphorylate various substrates including transcription factors, synaptic vesicle proteins and voltage or ligand-gated ion channels. An explanation for the relatively high immunoreactivity of the PKA-Cα antibody in learning and memory centres is that the epitope might only be available in the dissociated active form of the catalytic subunit and that PKA is persistently active largely in these structures. For example, persistent PKA activation may be achieved by increased production of cAMP during synaptic plasticity as well as ubiquitination of regulatory subunits leading to degradation in the proteosome and an overall higher ratio of catalytic to regulatory subunits [83,84].
High levels of the PKA-Cα orthologue DC0 have previously been reported in the mushroom bodies of Drosophila melanogaster [13] and PKA has long been implicated in a role for synaptic facilitation both in vertebrates and in invertebrates [54,85]. It is significant that centres across arthropods and annelids morphologically corresponding to mushroom bodies share this character of high DC0 immunoreactivity [3]. That high PKA-Cα immunoreactivity reveals the hippocampus of mammals and the corresponding pallial domains in other vertebrate groups (figures 2 and 4) implies that the common ancestor of all Bilateria may have possessed a centre with attributes of the mushroom body ground pattern, including a high ratio of PKA catalytic to regulatory subunits, thereby facilitating integrated sensory information to form associations.
Like PKA, CaMKII is widely expressed in the nervous system; however, immunolocalization of antisera against pCaMKII is concentrated in learning and memory centres across phyla. This antiserum specifically targets CaMKII that is activated via phosphorylation at Thr286 and is implicated in a role for facilitating synaptic plasticity. In the honey bee, pCaMKII immunoreactivity is more widespread in the pupal brain, but the domain of localization becomes more restricted to the mushroom bodies in the adult, possibly due to the involvement of these centres in learning and memory processing that requires plasticity beyond developmental stages [51]. Consequently, high levels of immunoreactivity to pCaMKII may serve as a marker for learning and memory structures.
(b). Correspondence of brain segments and forebrain centres
The current view that the protostome–deuterostome ancestor must have been simple [86] may find an exception with respect to its brain. Molecular studies have determined that the last common ancestor of protostomes and deuterostomes possessed most of the Hox genes present in extant bilaterians [87]. And, more specifically, orthologous genes responsible for the organization of brains into forebrain, midbrain and hindbrain are shared by protostomes and deuterostomes [88]. Conservation of these developmental genes has been verified by cross-phylum rescue of forebrain defective Drosophila mutants lacking the segmental patterning gene orthodenticle (otd) by the murine gene orthologues, Otx1/2 [89,90]. The reverse rescue of forebrain defects in Otx mice is achieved by overexpression of Drosophila otd [91]. Comparisons of two other forebrain centres, the arthropod/annelid central complex and vertebrate basal ganglia, have also resolved suites of orthologous genes that underlie developmental and functional properties shared by these forebrain centres, as well as their neuronal ground patterns [92].
Functional conformity provides another indicator of genealogical correspondence originating in an ancestral network in deep time. In addition to evidence that high levels of PKA-Cα typify mammalian hippocampi and arthropod mushroom bodies, further correspondences are revealed by the specific association in learning and memory processes of specific orthologous genes involved in protein synthesis (table 1). In both mouse and Drosophila, the ubiquitin ligase protein Neuralized, encoded, respectively, by the orthologous genes Neurl1 (Neuralized1) and Neur (Neuralized) promotes protein-synthesis-dependent long-term memory [8,9]. In the murine hippocampus, Neurl1 overexpression results in dendritic growth and enhanced hippocampus-associated long-term memory (LTM) [8]. A corresponding role of Neur has been shown in Drosophila, where Neur expression is specific to one subset of neurons in the vertical and medial (α, β) lobes of the mushroom body. Overexpression of Neur enhances long-term memory, whereas a loss of one Neur gene impairs long-term memory [9]. In, respectively, mammals and in insects the orthologous genes Egr-1 and Egr encode early growth response protein 1. Mice with a 50% reduction of Egr-1 expression suffer highly impaired long-term memory performance in spatial learning and memory tasks, whereas there is no deficit in performance of novelty recognition [22]. In rats there is a significant and transient up-regulation of Egr-1 in the dorsal hippocampus that peaks 30 min after spatial learning tasks, such as water maze navigation [23]. Corresponding relationships of Egr expression are found in insects. Transient up-regulation of Egr expression, also peaking at 30 min, occurs in the mushroom bodies of honey bees learning to orient to novel visual stimuli [24]. In honey bees, as in rats, the absence of visual novelty elicited no Egr up-regulation. These studies demonstrating a highly conserved role for Egr-1/Egr in spatial learning and memory in both hippocampus and mushroom bodies support earlier studies on cockroaches that showed place memory required structural integrity of the mushroom bodies [5].
To date, the enhanced expression or the disruption of at least 16 orthologous protein-encoding genes generate corresponding effects on memory processes in rodents and insects (table 1). Another appealing correspondence is that the hippocampus's dentate gyrus, the mushroom body's calyx and the crustacean hemiellipsoid body all share in common the rare property of a brain centre that generates new neurons during adult life [93–95], suggesting a regenerative programme that may be extremely ancient. In the hippocampus, new granule cells add mossy fibres in parallel to the existing ensemble [94]; in mushroom bodies [95] and hemiellipsoid bodies [93], globuli cells provide new parallel fibres to their ensembles. A shared behaviour in insects and in mammals that promotes such neurogenesis in mushroom bodies and hippocampus is agonistic rivalry where it is in the dominant individual that new neurons are generated [96,97].
The question arises whether such similarities are fortuitous, a result of convergent co-option of genes expressed in structurally corresponding neural networks that, too, have arisen by convergent evolution. Or, does such molecular and structural correspondence reflect an ancient ancestral memory system that has, over hundreds of millions of years, been maintained and elaborated in parallel throughout at least three evolutionary trajectories—vertebrate, lophotrochozoan and arthropod—culminating in present hippocampus and mushroom body circuits and functions? Difficulty arises when comparing characters across phylogenetically distant groups such as across phyla or superphyla because similarities may arise from independent evolution of gene sequences and even functional convergence wherein different gene products may function in the same molecular reaction [98]. Convergent evolution of characters scattered across the phylogeny is concluded when there is no evidence of genealogical correspondence or direct inheritance from a common ancestor.
In this study, we report a multitude of characters that form a pattern that is present in forebrain centres across bilaterian phyla. These characters are both neuroanatomical and molecular, including orthogonal organization of neuronal fibres, immunoreactivity to antisera against PKA-Cα and pCaMKII, as well as expression of a suite of genes, some of which are described here in table 1. Although some characters may be incomplete or missing and some may be shared with other brain structures, it is the pattern that corresponds across mushroom bodies, hippocampi and their equivalents in other taxa. Comparisons across vertebrates and invertebrates all point to the inevitable conclusion that forebrain centres that mediate learning and memory, and are equipped with corresponding neural organization, are unlikely to have evolved by convergence.
(c). Emergence of genealogical correspondence versus convergence of higher brain centres
There is a body of opinion that when brains of very distantly related species show correspondence of structures mediating corresponding functions, these have to be ascribed to convergent evolution because of the phylogenetic distance between those species [99–103]. If only seven of the extant metazoan phyla possess distinctive brains, their evolutionary distance in terms of geological time must imply that their brains have evolved independently at least that many times [101,102]. Those viewpoints relate, first, to assertions that general differences of nervous system organization across phyla are too profound to suggest homology; second, that the protostome–deuterostome ancestor was too simple to have possessed a complex brain, the argument being that the earliest bilateral metazoans possessed a net-like nervous system [101–103].
Fossil evidence resolves brain outlines and disposition of cerebral nerves that already distinguish the two major groups of arthropods, Chelicerata and Mandibulata, 515 million years ago [104]. The even earlier divergence of these two groups was likely close to that of divergence of chordates + vertebrates and the total group Panarthropoda. Thus, in terms of geological time, the evolutionary distance of a housefly and a shrew is comparable to that between a spider and a housefly. Comparisons of spider and fly brains resolve numerous taxon-specific arrangements; yet these differences can be revealed as homologous arrangements when investigated in the context of patterns of gene expression and embryonic development [105]. Evidence for an even broader homology and for a common origin of arthropod and vertebrate brains goes deeper with reference to genetic evidence that corresponding segmental organization of insect and vertebrate brains are defined by the actions of genes that comprise corresponding character identity networks [106], of which the defining examples are those that determine fore-, mid- and hindbrain [107]. Another example of shared character identity networks is revealed by a suite of neural specification and transcription factor genes that determine mediolateral arrangements of neuron identities and patterning along the developing ventral cord of the polychaete annelid Platynereis dumerilii and vertebrate neural tube, revealing an elaborate patterning mechanism, the origins of which must have existed in the common ancestor of these phyla [108].
Despite this support for genealogical correspondence across vertebrate, arthropod and annelid nervous systems, an argument sometimes deployed is that even if such basic organization of neural patterning is an admissible homology, it does not imply that circuits characteristic of, say, the forebrain of a lamprey or mouse are homologous to those in the forebrain of a fly. Yet precisely that implication is suggested from studies that identify corresponding gene expression maps for the developing brain of P. dumerilii and the mouse pallium. Both demonstrate a common suite of regulatory genes underlying an evolutionarily conserved developmental programme for neural tissue patterning leading to the annelid mushroom body and mouse pallium [61]. The inescapable implication is that this developmental programme existed in the protostome–deuterostome ancestor to provide the same fundamental network. Likewise, correspondences of structural, developmental, neurochemical and pathological characters shared by basal ganglia and central complexes, both action selection centres located, respectively, in the forebrains of vertebrates and arthropods, imply the presence of their network ground pattern in the protostome–deuterostome ancestor [92].
Those examples contradict arguments that insist there could not have been a condensed brain in the protostome–deuterostome ancestor that possessed ground patterns from which diverge those more elaborate arrangements recognized as hippocampus/mushroom bodies or basal ganglia/central complex. Even in minute bilaterians there exist rostral arrangements of neurons deservingly described as a brain. Symsagittifera roscoffensis, a minute member of Acoelomorpha, possibly the sister group to other Bilateria [109], possesses a well-defined brain comprising about 700 neurons organized as circumscribed domains [110]. The smallest arthropods, such as Collembola, whose brains comprise about the same number of neurons, possess circuits recognizable as central complexes and mushroom bodies [111]. These centres are composed of just a few dozen neurons yet they reveal the same ground pattern organization that in larger species are manifested by thousands of neurons. Divergent evolution of such centres is one of the many examples demonstrating that elaborations evolve from ancestral ground patterns [112]. Paleontological evidence of fossilized tracks indicative of directions selected and locations revisited by small bilaterians about 550 million years ago [113] further weaken arguments that such organisms could not accommodate circuitry enabling action selection and allocentric memory [114].
Although evidence discussed here advocates genealogical correspondences of forebrain (meaning most rostral) centres across five phyla, there is an ever increasing body of evidence for independent evolution of molluscan brains [101], particularly the brains of cephalopods. These lophotrochozoan animals, whose earliest blastoderm formation shows remarkable convergence with that of teleosts [115], exhibit behaviours and cognitive functions comparable with those of many mammalian and avian species [116]. Yet in possessing elaborate brains that bear hardly any resemblance to those of other phyla they bring into even sharper focus correspondences across vertebrates and arthropods.
Recent studies have confirmed that molluscs share genes encoding transcription factors underlying central nervous system development in common with vertebrates and arthropods [117]. However, the orthologues of Hox genes that in bilaterians pattern the rostral–caudal arrangement of the central nervous system underlie wholly different developmental programmes in cephalopods, and the expansion of certain families of genes unique to cephalopods relate to the formation of brain centres that have no counterparts in vertebrates and arthropods [118–120].
Among the numerous examples of convergent brain evolution, one of the most obvious is the cephalopod visual system, in which a camera eye comparable to the vertebrate eye, but with everted rhabdomeric photoreceptor neurons, supplies afferents to an optic lobe the deeper levels of which directly control motor actions [121]. Yet, apart from retinotopic organization in its outer layers, the cephalopod visual system bears almost no resemblance to parallel processing architectures and sequential neuropils typifying arthropod and vertebrate visual systems [122,123]. And although classical and recent studies have identified the vertical lobes of the octopus brain with elaborate divisions into discrete neuroanatomical domains, as one site (amongst others) of learning and memory [124,125], there is little evidence for network organization corresponding to vertebrate pallium or arthropod mushroom bodies [126]. Thus, for cephalopod molluscs, evidence overwhelmingly points to comparable behaviours driven by computational networks that have wholly independent ancestral origins. Distributed networks of millions of neurons comprising cephalic ganglia reveal a complete absence of somatotopic representation of sensory afferents or circumscribed domains dedicated to motor control [127]. Indeed, sensory maps appear to be constrained to within a parallel brain-like organization provided by the interconnected brachial plexi and ganglia that serve the arms and which prolongate down into the arms where segment-like domains of synaptic neuropil associated with suckers control extraordinarily versatile movements [128]. There may be no analogue of such a control system in any animal with segmented nerve cord.
Whereas evidence for independent origins and convergent evolution of molluscan brains is strongly supported, convergence of brain centres in arthropods with those of vertebrates is easier to assume than to demonstrate. However, one indisputable example is the convergence of acoustic and vestibular circuits in mammals and auditory and gravitational circuits in Drosophila, which in both taxa process sound and gravitational cues in parallel. The cochlear and vestibular nuclei of the medulla in mammals have distinct counterparts in the discrete antennomechanosensory neuropils of the Drosophila hindbrain (tritocerebrum) [129]. That these similarities of organization cannot refer to an ancestral ground pattern is implicit in the relatively recent evolution of auditory systems in insects, which emerged onto land about 400 million years ago [130].
Within vertebrates there is demonstrable convergent evolution of genetic mechanisms underlying the development of specialized acoustic pathways involved in vocal learning that have evolved within homologous brain regions of song-birds and primates [131]. The relatively late appearance of such convergent traits is also demonstrated by the co-evolution of auditory genes underlying the evolution of ultrasonic communication in cetaceans and chiropterans [132]. Such examples may suggest that convergent evolution occurs late rather than early in the evolutionary history of phylogenetically widely separated taxa.
(d). An ancestral bilaterian brain
Mushroom body networks are today resolved in some of the simplest bilaterians, such as polyclad flatworms [3] that might serve as proxies for ancestral metazoans, which at the transition of the Ediacaran and Cambrian 541 million years ago left no fossils other than preserved traces of their foraging behaviour [133,134]. These preserved tracks are not unlike foraging excursions made by polyclads, or tracks excavated by modern leaf borers, or Drosophila larvae foraging on an agar plate. All of these behavioural records suggest some simple memory of place and selection of behavioural actions. Despite their simple morphology, polyclads have elaborate brains containing paired PKA-Cα-positive centres, the shapes and neural arrangements of which correspond to iconic mushroom bodies [3]. The brain even has a PKA-Cα-positive mid-line neuropil comparable to the arthropod ellipsoid body, part of the elaborate central complex, proposed to correspond to vertebrate basal ganglia [92]. Polyclads exhibit a substantial repertoire of motor actions, exploratory behaviour and memory of place [135].
But how deep in time did such ‘memory’ centres appear? Although we can only surmise what the earliest bilaterian might have looked like, evidence from palaeontology suggests that in the Late Ediacaran there already existed a quite elaborate metazoan. This was the molluscan like organism Kimberella, resolved as fossilized traces of a mantel, a foot and an anterior proboscis likely equipped with a radula [136,137]. This species, which lived about 555 million years ago, must have derived from simpler (and earlier) bilaterian antecedents. While nothing from that age persists today, we can identify one further taxon that may serve as a useful proxy of a metazoan existing before the dichotomy to protostomes and deuterostomes. The taxon in question is Xenacoelomorpha, comprising some of the simplest extant metazoans, amongst which the clade Aceola contains various unelaborate bilaterians. Irrespective of the disputed phylogenetic position of Xenacoelomorpha—claimed as sister taxon to all other Bilateria [67,77] or sister to Deuterostomia [66]—an intriguing aspect is that two species suggest what might have composed the earliest true brain. The 2–3 mm long acoel S. roscoffensis, populated by thousands of symbiotic single celled algae, and a related species called Isodiametra pulchra both show clear evidence of an anterior concentration of neurons, from which extend caudally directed nerve cords providing bilateral nerve nets [79,80,138]. Frontally in the body of I. pulchra, just anterior to a prominent midline statocyst diagnostic of all acoels, a cluster of about 700 neurons provides an elaborate system of anterior commissures and neuronal aggregations [80].
As in higher metazoans, where DC0 and pCaMKII are expressed throughout tissues, the same is observed in S. roscoffensis. However, both proteins are expressed at very high levels within a region associated with S. roscoffensis's most important sensory specializations: the gravity-sensing medial statocyst and the rostro-lateral eye spots. That the epidermis of S. roscoffensis is decorated with chemosensory receptors suggests this modality reaches the brain as well. Thus, a DC0- and pCaMKII-immunoreactive centre restricted to the brain of one of the simplest and most basal metazoans possibly integrates sensory information pertaining to at least three modalities: haptic, chemosensory and photic. Irrespective of its phylogenetic status, genetic networks required to build a simple DC0/pCaMKII-positive centre in S. roscoffensis might correspond to components of genetic networks that underlie the development of annelid and arthropod mushroom bodies and the vertebrate archipallium.
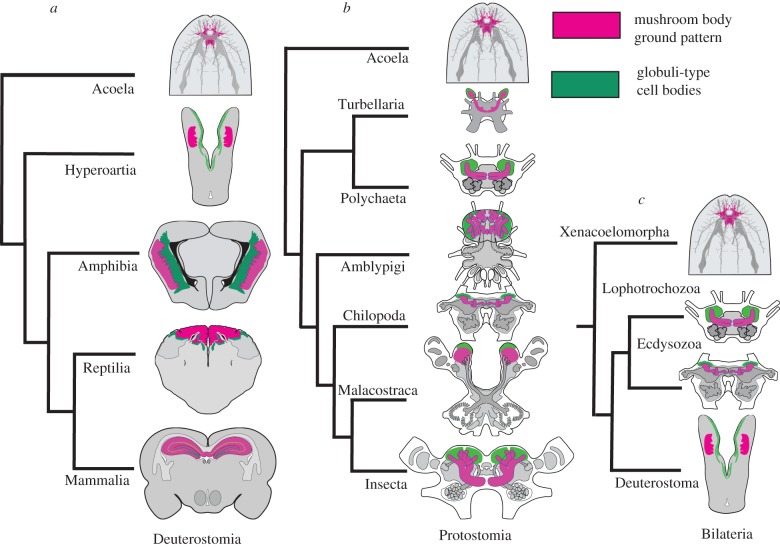
In conclusion, the present data, along with the revolutionary study by Tomer et al. [61] that identified orthologous transcription factor gene patterning in the developing murine pallium and annelid mushroom bodies, provide to date the most parsimonious interpretation for the extensive suite of observed correspondences summarized in figure 6. The sum of evidence suggests that two rostral neural arrangements mediating allocentric learning and memory originated before the beginning of the Palaeozoic era. Corresponding circuit organization, gene expression and function, as well as the expression of proteins crucial to learning and memory, have been maintained and elaborated throughout three major evolutionary trajectories: ecdysozoans, lophotrochozoan and vertebrate deuterostomes.
Figure 6.
Brain anatomy and corresponding ground patterns with three possible relationships to acoels (brains not to scale): (a) sister group to Deuterostomia, (b) sister group to Protostomia, (c) sister group to Bilateria. In (a–c), DC0 immunoreactivity (magenta) in S. roscoffensis is shown superimposed on serotonin, elav and synaptotagmin immunoreactivity (grey). See [66,77]. In (a) regions corresponding to the hippocampus/mushroom body ground pattern (figure 1), and in (b) neuropils corresponding to mushroom bodies are shown in magenta; their globuli cells are shown in green. Data in (b) are from [3]. Magenta for the brains of S. roscoffensis implies putative ground pattern indicated by expression of elevated DC0 and pCaMKII.
Our understanding of the trajectory of forebrain evolution in Deuterostomia would be significantly improved by future studies to further characterize both neuroanatomy and gene expression in more basal deuterostomes such as “invertebrate” chordates, hemichordates and echinoderms where the absence of forebrain or even brain may speak for evolved reduction and loss [139]. Likewise, there are many taxa within Protostoma that lack sufficient characterization. Analysis of these groups will increase confidence in their phylogenetic relationships as well as whether structures correspond genealogically or have evolved convergently. Thus far, the abundance of correspondences in the rostral brain of polyclads, annelids, nemerteans, arthropods and vertebrates, and in simpler forms in acoels, speaks persuasively against their evolution by convergence.
Acknowledgements
We are indebted to Professor Sten Grillner, the Karolinska Institute, Stockholm for generously hosting G.H.W. during preparation of lamprey material and Dr Brita Robertson for her invaluable advice and assistance. We thank Dr Sarah Woodley at Duquesne University and Dr Catherine Carr at the University of Maryland for providing, respectively, newt and turtle material. Dr Monica Chawla at the Evelyn F. McKnight Brain Institute, University of Arizona, kindly provided rat tissue. Dr Nate McMullen provided slides of Golgi impregnated rat brain and Dr Nick Gibson at the Department of Neuroscience, University of Arizona, gave valuable advice regarding western blot analyses. We again thank Dr Daniel Kalderon, Columbia University, for generous donations of anti-DC0 antibody. Drs Frank Hirth, Wulfila Gronenberg and Camilla Strausfeld commented on drafts of the manuscript. N.J.S. thanks Prof. Martin Heisenberg at the Rudolf Virchow Center, Würzburg, for many discussions about learning, memory and what it might mean to have a brain. The constructive advice of the two referees is gratefully acknowledged.
Authors' contributions
G.H.W. carried out the histology and image collections. G.H.W. and N.J.S. participated in the design of the study and drafted the manuscript. Both authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
G.H.W. was funded by a National Science Foundation Graduate Research Fellowship (grant no. DGE-1143953, 7/15/2011-6/30/2016) and a P.E.O. Scholar Award. Other support derived from an Alexander von Humboldt Foundation Senior Research Prize, a Henry and Phyllis Koffler Prize and a University of Arizona Regents' fund to N.J.S.
References
- 1.Brown S, Wolff G. 2012. Fine structural organization of the hemiellipsoid body of the land hermit crab, Coenobita clypeatus. J. Comp. Neurol. 520, 2847–2863. ( 10.1002/cne.23058) [DOI] [PubMed] [Google Scholar]
- 2.Wolff G, Harzsch S, Hansson BS, Brown S, Strausfeld N. 2012. Neuronal organization of the hemiellipsoid body of the land hermit crab, Coenobita clypeatus: correspondence with the mushroom body ground pattern. J. Comp. Neurol. 520, 2824–2846. ( 10.1002/cne.23059) [DOI] [PubMed] [Google Scholar]
- 3.Wolff GH, Strausfeld NJ. 2015. Genealogical correspondence of mushroom bodies across invertebrate phyla. Curr. Biol. 25, 38–44. ( 10.1016/j.cub.2014.10.049) [DOI] [PubMed] [Google Scholar]
- 4.Insausti R, Marcos P, Arroyo-Jiménez MM, Blaizot X, Martínez-Marcos A. 2002. Comparative aspects of the olfactory portion of the entorhinal cortex and its projection to the hippocampus in rodents, nonhuman primates, and the human brain. Brain Res. Bull. 57, 557–560. ( 10.1016/S0361-9230(01)00684-0) [DOI] [PubMed] [Google Scholar]
- 5.Mizunami M, Weibrecht JM, Strausfeld NJ. 1998. Mushroom bodies of the cockroach: their participation in place memory. J. Comp. Neurol. 402, 520–537. () [DOI] [PubMed] [Google Scholar]
- 6.Kahsai L, Zars T. 2011. Learning and memory in Drosophila: behavior, genetics, and neural systems. Int. Rev. Neurobiol. 99, 139–167. ( 10.1016/B978-0-12-387003-2.00006-9) [DOI] [PubMed] [Google Scholar]
- 7.Morris RGM. 2006. Elements of a neurobiological theory of hippocampal function: the role of synaptic plasticity, synaptic tagging and schemas. Eur. J. Neurosci. 23, 2829–2846. ( 10.1111/j.1460-9568.2006.04888.x) [DOI] [PubMed] [Google Scholar]
- 8.Pavlopoulos E, Trifilieff P, Chevaleyre V, Fioriti L, Zairis S, Pagano A, Malleret G, Kandel ER. 2011. Neuralized1 activates CPEB3: a function for nonproteolytic ubiquitin in synaptic plasticity and memory storage. Cell 147, 1369–1383. ( 10.1016/j.cell.2011.09.056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pavlopoulos E, Anezaki M, Skoulakis EMC. 2008. Neuralized is expressed in the α/β lobes of adult Drosophila mushroom bodies and facilitates olfactory long-term memory formation. Proc. Natl Acad. Sci. USA 105, 14 674–14 679. ( 10.1073/pnas.0801605105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandon EP, Zhuo M, Huang YY, Qi M, Gerhold KA, Burton KA, Kandel ER, McKnight GS, Idzerda RL. 1995. Hippocampal long-term depression and depotentiation are defective in mice carrying a targeted disruption of the gene encoding the RI beta subunit of cAMP-dependent protein kinase. Proc. Natl Acad. Sci. USA 92, 8851–8855. ( 10.1073/pnas.92.19.8851) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodwin SF, Vecchio MD, Velinzon K, Hogel C, Russell SRH, Tully T, Kaiser K. 1997. Defective learning in mutants of the Drosophila gene for a regulatory subunit of cAMP-dependent protein kinase. J. Neurosci. 17, 8817–8827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abel T, Nguyen PV, Barad M, Deuel TAS, Kandel ER, Bourtchouladze R. 1997. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell 88, 615–626. ( 10.1016/S0092-8674(00)81904-2) [DOI] [PubMed] [Google Scholar]
- 13.Skoulakis EMC, Kalderon D, Davis RL. 1993. Preferential expression in mushroom bodies of the catalytic subunit of protein kinase A and its role in learning and memory. Neuron 11, 197–208. ( 10.1016/0896-6273(93)90178-T) [DOI] [PubMed] [Google Scholar]
- 14.Gervasi N, Tchénio P, Preat T. 2010. PKA dynamics in a Drosophila learning center: coincidence detection by rutabaga adenylyl cyclase and spatial regulation by dunce phosphodiesterase. Neuron 65, 516–529. ( 10.1016/j.neuron.2010.01.014) [DOI] [PubMed] [Google Scholar]
- 15.Yamazaki D, Horiuchi J, Nakagami Y, Nagano S, Tamura T, Saitoe M. 2007. The Drosophila DCO mutation suppresses age-related memory impairment without affecting lifespan. Nat. Neurosci. 10, 478–484. ( 10.1038/nn1863) [DOI] [PubMed] [Google Scholar]
- 16.Roberto M, Scuri R, Brunelli M. 2001. Differential effects of PACAP-38 on synaptic responses in rat hippocampal CA1 region. Learn. Mem. 8, 265–271. ( 10.1101/lm.40501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuyama S, Matsumoto A, Hashimoto H, Shintani N, Baba A. 2003. Impaired long-term potentiation in vivo in the dentate gyrus of pituitary adenylate cyclase-activating polypeptide (PACAP) or PACAP type 1 receptor-mutant mice. Neuroreport 14, 2095–2098. ( 10.1097/01.wnr.0000090953.15465.5a) [DOI] [PubMed] [Google Scholar]
- 18.Yang K, Lei G, Jackson MF, MacDonald JF. 2010. The involvement of PACAP/VIP system in the synaptic transmission in the hippocampus. J. Mol. Neurosci. 42, 319–326. ( 10.1007/s12031-010-9372-7) [DOI] [PubMed] [Google Scholar]
- 19.Feany MB, Quinn WG. 1995. A neuropeptide gene defined by the Drosophila memory mutant amnesiac. Science 268, 869–873. ( 10.1126/science.7754370) [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto H, Shintani N, Baba A. 2002. Higher brain functions of PACAP and a homologous Drosophila memory gene amnesiac: insights from knockouts and mutants. Biochem. Biophys. Res. Commun. 297, 427–432. ( 10.1016/S0006-291X(02)02144-7) [DOI] [PubMed] [Google Scholar]
- 21.Keene AC, Stratmann M, Keller A, Perrat PN, Vosshall LB, Waddell S. 2004. Diverse odor-conditioned memories require uniquely timed dorsal paired medial neuron output. Neuron 44, 521–533. ( 10.1016/j.neuron.2004.10.006) [DOI] [PubMed] [Google Scholar]
- 22.Jones MW, et al. 2001. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat. Neurosci. 4, 289–296. ( 10.1038/85138) [DOI] [PubMed] [Google Scholar]
- 23.Guzowski JF, Setlow B, Wagner EK, McGaugh JL. 2001. Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genes Arc, c-fos, and zif268. J. Neurosci. 21, 5089–5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lutz CC, Robinson GE. 2013. Activity-dependent gene expression in honey bee mushroom bodies in response to orientation flight. J. Exp. Biol. 216, 2031–2038. ( 10.1242/jeb.084905) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alarcon JM, Hodgman R, Theis M, Huang Y-S, Kandel ER, Richter JD. 2004. Selective modulation of some forms of schaffer collateral-CA1 synaptic plasticity in mice with a disruption of the CPEB-1 gene. Learn. Mem. 11, 318–327. ( 10.1101/lm.72704) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pai T-P, Chen C-C, Lin H-H, Chin A-L, Lai JS-Y, Lee P-T, Tully T, Chiang A-S. 2013. Drosophila ORB protein in two mushroom body output neurons is necessary for long-term memory formation. Proc. Natl. Acad. Sci. USA 110, 7898–7903. ( 10.1073/pnas.1216336110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keleman K, Krüttner S, Alenius M, Dickson BJ. 2007. Function of the Drosophila CPEB protein Orb2 in long-term courtship memory. Nat. Neurosci. 10, 1587–1593. ( 10.1038/nn1996) [DOI] [PubMed] [Google Scholar]
- 28.Chan C-S, Levenson JM, Mukhopadhyay PS, Zong L, Bradley A, Sweatt JD, Davis RL. 2007. α3-Integrins are required for hippocampal long-term potentiation and working memory. Learn. Mem. 14, 606–615. ( 10.1101/lm.648607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grotewiel MS, Beck CDO, Wu KH, Zhu X-R, Davis RL. 1998. Integrin-mediated short-term memory in Drosophila. Nature 391, 455–460. ( 10.1038/35079) [DOI] [PubMed] [Google Scholar]
- 30.Tucker MS, Khan I, Fuchs-Young R, Price S, Steininger TL, Greene G, Wainer BH, Rosner MR. 1993. Localization of immunoreactive epidermal growth factor receptor in neonatal and adult rat hippocampus. Brain Res. 631, 65–71. ( 10.1016/0006-8993(93)91187-W) [DOI] [PubMed] [Google Scholar]
- 31.Abe K, Xie F, Saito H. 1991. Epidermal growth factor enhances short-term potentiation and facilitates induction of long-term potentiation in rat hippocampal slices. Brain Res. 547, 159–162. ( 10.1016/0006-8993(91)90589-N) [DOI] [PubMed] [Google Scholar]
- 32.Rahn T, Leippe M, Roeder T, Fedders H. 2013. EGFR signaling in the brain is necessary for olfactory learning in Drosophila larvae. Learn. Mem. 20, 194–200. ( 10.1101/lm.029934.112) [DOI] [PubMed] [Google Scholar]
- 33.Machon O, van den Bout CJ, Backman M, Røsok Ø, Caubit X, Fromm SH, Geronimo B, Krauss S. 2002. Forebrain-specific promoter/enhancer D6 derived from the mouse Dach1 gene controls expression in neural stem cells. Neuroscience 112, 951–966. ( 10.1016/S0306-4522(02)00053-2) [DOI] [PubMed] [Google Scholar]
- 34.Martini SR, Davis RL. 2005. The dachshund gene is required for the proper guidance and branching of mushroom body axons in Drosophila melanogaster. J. Neurobiol. 64, 133–144. ( 10.1002/neu.20130) [DOI] [PubMed] [Google Scholar]
- 35.Tuoc TC, Radyushkin K, Tonchev AB, Piñon MC, Ashery-Padan R, Molnár Z, Davidoff MS, Stoykova A. 2009. Selective cortical layering abnormalities and behavioral deficits in cortex-specific pax6 knock-out mice. J. Neurosci. 29, 8335–8349. ( 10.1523/JNEUROSCI.5669-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furukubo-Tokunaga K, Adachi Y, Kurusu M, Walldorf U. 2009. Brain patterning defects caused by mutations of the twin of eyeless gene in Drosophila melanogaster. Fly (Austin) 3, 263–269. ( 10.4161/fly.10385) [DOI] [PubMed] [Google Scholar]
- 37.Yamagata K, Sanders LK, Kaufmann WE, Yee W, Barnes CA, Nathans D, Worley PF. 1994. rheb, a growth factor- and synaptic activity-regulated gene, encodes a novel Ras-related protein. J. Biol. Chem. 269, 16 333–16 339. [PubMed] [Google Scholar]
- 38.Brown HLD, Kaun KR, Edgar BA. 2012. The small GTPase Rheb affects central brain neuronal morphology and memory formation in Drosophila. PLoS ONE 7, e44888 ( 10.1371/journal.pone.0044888) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McNaughton CH, Moon J, Strawderman MS, Maclean KN, Evans J, Strupp BJ. 2008. Evidence for social anxiety and impaired social cognition in a mouse model of fragile X syndrome. Behav. Neurosci. 122, 293–300. ( 10.1037/0735-7044.122.2.293) [DOI] [PubMed] [Google Scholar]
- 40.Levenga J, de Vrij FMS, Buijsen RAM, Li T, Nieuwenhuizen IM, Pop A, Oostra BA, Willemsen R. 2011. Subregion-specific dendritic spine abnormalities in the hippocampus of Fmr1 KO mice. Neurobiol. Learn. Mem. 95, 467–472. ( 10.1016/j.nlm.2011.02.009) [DOI] [PubMed] [Google Scholar]
- 41.Michel CI, Kraft R, Restifo LL. 2004. Defective neuronal development in the mushroom bodies of Drosophila fragile × mental retardation 1 mutants. J. Neurosci. 24, 5798–5809. ( 10.1523/JNEUROSCI.1102-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schenck A, Van de Bor V, Bardoni B, Giangrande A. 2002. Novel features of dFMR1, the Drosophila orthologue of the fragile × mental retardation protein. Neurobiol. Dis. 11, 53–63. ( 10.1006/nbdi.2002.0510) [DOI] [PubMed] [Google Scholar]
- 43.Knafo S, Barkai E, Herrero AI, Libersat F, Sandi C, Venero C. 2005. Olfactory learning-related NCAM expression is state, time, and location specific and is correlated with individual learning capabilities. Hippocampus 15, 316–325. ( 10.1002/hipo.20052) [DOI] [PubMed] [Google Scholar]
- 44.Lüthi A, Laurent J-P, Figurovt A, Mullert D, Schachnert M. 1994. Hippocampal long-term potentiation and neural cell adhesion molecules L1 and NCAM. Nature 372, 777–779. ( 10.1038/372777a0) [DOI] [PubMed] [Google Scholar]
- 45.Vogt J, et al. 2011. Homeostatic regulation of NCAM polysialylation is critical for correct synaptic targeting. Cell. Mol. Life Sci. 69, 1179–1191. ( 10.1007/s00018-011-0868-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fushima K, Tsujimura H. 2007. Precise control of fasciclin II expression is required for adult mushroom body development in Drosophila. Dev. Growth Differ. 49, 215–227. ( 10.1111/j.1440-169X.2007.00922.x) [DOI] [PubMed] [Google Scholar]
- 47.Barry J, Gu Y, Gu C. 2010. Polarized targeting of L1-CAM regulates axonal and dendritic bundling in vitro. Eur. J. Neurosci. 32, 1618–1631. ( 10.1111/j.1460-9568.2010.07447.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goossens T, Kang YY, Wuytens G, Zimmermann P, Callaerts-Végh Z, Pollarolo G, Islam R, Hortsch M, Callaerts P. 2011. The Drosophila L1CAM homolog Neuroglian signals through distinct pathways to control different aspects of mushroom body axon. Development 138, 1595–1605. ( 10.1242/dev.052787) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steinkellner T, et al. 2012. Ca2+/Calmodulin-dependent protein kinase IIα (αCaMKII) controls the activity of the dopamine transporter implications for Angelman syndrome. J. Biol. Chem. 287, 29 627–29 635. ( 10.1074/jbc.M112.367219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang X, Zhang C, Szábo G, Sun Q-Q. 2013. Distribution of CaMKIIα expression in the brain in vivo, studied by CaMKIIα-GFP mice. Brain Res. 1518, 9–25. ( 10.1016/j.brainres.2013.04.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pasch E, Muenz TS, Rössler W. 2011. CaMKII is differentially localized in synaptic regions of Kenyon cells within the mushroom bodies of the honeybee brain. J. Comp. Neurol. 519, 3700–3712. ( 10.1002/cne.22683) [DOI] [PubMed] [Google Scholar]
- 52.Cheah P-S, et al. 2012. Neurodevelopmental and neuropsychiatric behaviour defects arise from 14-3-3ζ deficiency. Mol. Psychiatry 17, 451–466. ( 10.1038/mp.2011.158) [DOI] [PubMed] [Google Scholar]
- 53.Skoulakis EMC, Davis RL. 1996. Olfactory learning deficits in mutants for leonardo, a Drosophila gene encoding a 14-3-3 protein. Neuron 17, 931–944. ( 10.1016/S0896-6273(00)80224-X) [DOI] [PubMed] [Google Scholar]
- 54.Abel T, Nguyen PV. 2008. Regulation of hippocampus-dependent memory by cyclic AMP-dependent protein kinase (Chapter 6). In Progress in brain research (eds Vincent J-CL, Castellucci F, Belleville S, Sossin WS), pp. 97–115. Amsterdam, The Netherlands: Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim M, Park AJ, Havekes R, Chay A, Guercio LA, Oliveira RF, Abel T, Blackwell KT. 2011. Colocalization of protein kinase a with adenylyl cyclase enhances protein kinase a activity during induction of long-lasting long-term-potentiation. PLoS Comput Biol 7, e1002084 ( 10.1371/journal.pcbi.1002084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Farris SM. 2005. Developmental organization of the mushroom bodies of Thermobia domestica (Zygentoma, Lepismatidae): insights into mushroom body evolution from a basal insect. Evol. Dev. 7, 150–159. ( 10.1111/j.1525-142X.2005.05017.x) [DOI] [PubMed] [Google Scholar]
- 57.Kalderon D, Rubin GM. 1988. Isolation and characterization of Drosophila cAMP-dependent protein kinase genes. Genes Dev. 2, 1539–1556. ( 10.1101/gad.2.12a.1539) [DOI] [PubMed] [Google Scholar]
- 58.Perino A, Ghigo A, Scott JD, Hirsch E. 2012. Anchoring proteins as regulators of signaling pathways. Circ. Res. 111, 482–492. ( 10.1161/CIRCRESAHA.111.262899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li H-L, Song L-S, Qian P-Y. 2008. Cyclic AMP concentration and protein kinase A (PKA) gene expression at different developmental stages of the polychaete Hydroides elegans. J. Exp. Zool. B Mol. Dev. Evol. 310B, 417–427. ( 10.1002/jez.b.21214) [DOI] [PubMed] [Google Scholar]
- 60.Wang Z, Palmer G, Griffith LC. 1998. Regulation of Drosophila Ca2+/calmodulin-dependent protein kinase II by autophosphorylation analyzed by site-directed mutagenesis. J. Neurochem. 71, 378–387. ( 10.1046/j.1471-4159.1998.71010378.x) [DOI] [PubMed] [Google Scholar]
- 61.Tomer R, Denes AS, Tessmar-Raible K, Arendt D. 2010. Profiling by image registration reveals common origin of annelid mushroom bodies and vertebrate pallium. Cell 142, 800–809. ( 10.1016/j.cell.2010.07.043) [DOI] [PubMed] [Google Scholar]
- 62.Strausfeld NJ, Hansen L, Li Y, Gomez RS, Ito K. 1998. Evolution, discovery, and interpretations of arthropod mushroom bodies. Learn. Mem. 5, 11–37. ( 10.1101/lm.5.1.11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Freund TF, Buzsáki G. 1996. Interneurons of the hippocampus. Hippocampus 6, 347–470. () [DOI] [PubMed] [Google Scholar]
- 64.Lin C, Strausfeld NJ. 2013. A precocious adult visual center in the larva defines the unique optic lobe of the split-eyed whirligig beetle Dineutus sublineatus. Front. Zool. 10, 7 ( 10.1186/1742-9994-10-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoch E. 2000. Olfaction in whales: evidence from a young odontocete of the late Oligocene North Sea. Hist. Biol. 14, 67–89. ( 10.1080/10292380009380556) [DOI] [Google Scholar]
- 66.Philippe H, Brinkmann H, Copley RR, Moroz LL, Nakano H, Poustka AJ, Wallberg A, Peterson KJ, Telford MJ. 2011. Acoelomorph flatworms are deuterostomes related to Xenoturbella. Nature 470, 255–258. ( 10.1038/nature09676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Perea-Atienza E, Gavilán B, Chiodin M, Abril JF, Hoff KJ, Poustka AJ, Martinez P. 2015. The nervous system of Xenacoelomorpha: a genomic perspective. J. Exp. Biol. 218, 618–628. ( 10.1242/jeb.110379) [DOI] [PubMed] [Google Scholar]
- 68.Lane ME, Kalderon D. 1993. Genetic investigation of cAMP-dependent protein kinase function in Drosophila development. Genes Dev. 7, 1229–1243. ( 10.1101/gad.7.7a.1229) [DOI] [PubMed] [Google Scholar]
- 69.Gibson NJ, Tolbert LP. 2006. Activation of epidermal growth factor receptor mediates receptor axon sorting and extension in the developing olfactory system of the moth Manduca sexta. J. Comp. Neurol. 495, 554–572. ( 10.1002/cne.20890) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Strausfeld NJ, Seyfarth E-A. 2008. Johann Flögel (1834–1918) and the birth of comparative insect neuroanatomy and brain nomenclature. Arthropod. Struct. Dev. 37, 434–441. ( 10.1016/j.asd.2008.02.003) [DOI] [PubMed] [Google Scholar]
- 71.Woodson W, Nitecka L, Ben-Ari Y. 1989. Organization of the GABAergic system in the rat hippocampal formation: a quantitative immunocytochemical study. J. Comp. Neurol. 280, 254–271. ( 10.1002/cne.902800207) [DOI] [PubMed] [Google Scholar]
- 72.Ascoli GA, Brown KM, Calixto E, Card JP, Galván EJ, Perez-Rosello T, Barrionuevo G. 2009. Quantitative morphometry of electrophysiologically identified CA3b interneurons reveals robust local geometry and distinct cell classes. J. Comp. Neurol. 515, 677–695. ( 10.1002/cne.22082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grünewald B. 1999. Physiological properties and response modulations of mushroom body feedback neurons during olfactory learning in the honeybee, Apis mellifera. J. Comp. Physiol. A 185, 565–576. ( 10.1007/s003590050417) [DOI] [Google Scholar]
- 74.Strausfeld NJ. 2012. Arthropod brains: evolution, functional elegance, and historical significance. Cambridge, MA: Belknap Press. [Google Scholar]
- 75.Fahrenbach WH. 1979. The brain of the horseshoe crab (Limulus polyphemus). III. Cellular and synaptic organization of the corpora pedunculata. Tissue Cell 11, 163–199. ( 10.1016/0040-8166(79)90016-8) [DOI] [PubMed] [Google Scholar]
- 76.Rodríguez F, López JC, Vargas JP, Gómez Y, Broglio C, Salas C. 2002. Conservation of spatial memory function in the pallial forebrain of reptiles and ray-finned fishes. J. Neurosci. 22, 2894–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ruiz-Trillo I, Riutort M, Littlewood DTJ, Herniou EA, Baguñà J. 1999. Acoel flatworms: earliest extant bilaterian metazoans, not members of Platyhelminthes. Science 283, 1919–1923. ( 10.1126/science.283.5409.1919) [DOI] [PubMed] [Google Scholar]
- 78.Semmler H, Chiodin M, Bailly X, Martinez P, Wanninger A. 2010. Steps towards a centralized nervous system in basal bilaterians: insights from neurogenesis of the acoel Symsagittifera roscoffensis. Dev. Growth Differ. 52, 701–713. ( 10.1111/j.1440-169X.2010.01207.x) [DOI] [PubMed] [Google Scholar]
- 79.Bailly X, et al. 2014. The chimerical and multifaceted marine acoel Symsagittifera roscoffensis: from photosymbiosis to brain regeneration. Microb. Symbioses 5, 498 ( 10.3389/fmicb.2014.00498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Achatz JG, Martinez P. 2012. The nervous system of Isodiametra pulchra (Acoela) with a discussion on the neuroanatomy of the Xenacoelomorpha and its evolutionary implications. Front. Zool. 9, 27 ( 10.1186/1742-9994-9-27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang Z, Pan Y, Li W, Jiang H, Chatzimanolis L, Chang J, Gong Z, Liu L. 2008. Visual pattern memory requires foraging function in the central complex of Drosophila. Learn. Mem. 15, 133–142. ( 10.1101/lm.873008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pfeiffer K, Homberg U. 2014. Organization and functional roles of the central complex in the insect brain. Annu. Rev. Entomol. 59, 165–184. ( 10.1146/annurev-ento-011613-162031) [DOI] [PubMed] [Google Scholar]
- 83.Nguyen PV, Woo NH. 2003. Regulation of hippocampal synaptic plasticity by cyclic AMP-dependent protein kinases. Prog. Neurobiol. 71, 401–437. ( 10.1016/j.pneurobio.2003.12.003) [DOI] [PubMed] [Google Scholar]
- 84.Tai H-C, Schuman EM. 2008. Ubiquitin, the proteasome and protein degradation in neuronal function and dysfunction. Nat. Rev. Neurosci. 9, 826–838. ( 10.1038/nrn2499) [DOI] [PubMed] [Google Scholar]
- 85.Burrell BD, Sahley CL. 2001. Learning in simple systems. Curr. Opin. Neurobiol. 11, 757–764. ( 10.1016/S0959-4388(01)00281-1) [DOI] [PubMed] [Google Scholar]
- 86.Erwin DH, Davidson EH. 2002. The last common bilaterian ancestor. Development 129, 3021–3032. [DOI] [PubMed] [Google Scholar]
- 87.De Rosa R, Grenier JK, Andreeva T, Cook CE, Adoutte A, Akam M, Carroll SB, Balavoine G. 1999. Hox genes in brachiopods and priapulids and protostome evolution. Nature 399, 772–776. ( 10.1038/21631) [DOI] [PubMed] [Google Scholar]
- 88.Hirth F, Kammermeier L, Frei E, Walldorf U, Noll M, Reichert H. 2003. An urbilaterian origin of the tripartite brain: developmental genetic insights from Drosophila. Development 130, 2365–2373. ( 10.1242/dev.00438) [DOI] [PubMed] [Google Scholar]
- 89.Nagao T, Leuzinger S, Acampora D, Simeone A, Finkelstein R, Reichert H, Furukubo-Tokunaga K. 1998. Developmental rescue of Drosophila cephalic defects by the human Otx genes. Proc. Natl Acad. Sci. USA 95, 3737–3742. ( 10.1073/pnas.95.7.3737) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hirth F, Therianos S, Loop T, Gehring WJ, Reichert H, Furukubo-Tokunaga K. 1995. Developmental defects in brain segmentation caused by mutations of the homeobox genes orthodenticle and empty spiracles in Drosophila. Neuron 15, 769–778. ( 10.1016/0896-6273(95)90169-8) [DOI] [PubMed] [Google Scholar]
- 91.Acampora D, Avantaggiato V, Tuorto F, Barone P, Reichert H, Finkelstein R, Simeone A. 1998. Murine Otx1 and Drosophila otd genes share conserved genetic functions required in invertebrate and vertebrate brain. Development 125, 1691–1702. [DOI] [PubMed] [Google Scholar]
- 92.Strausfeld NJ, Hirth F. 2013. Deep homology of arthropod central complex and vertebrate basal ganglia. Science 340, 157–161. ( 10.1126/science.1231828) [DOI] [PubMed] [Google Scholar]
- 93.Schmidt M. 1997. Continuous neurogenesis in the olfactory brain of adult shore crabs, Carcinus maenas. Brain Res. 762, 131–143. ( 10.1016/S0006-8993(97)00376-4) [DOI] [PubMed] [Google Scholar]
- 94.Gage FH. 2000. Mammalian neural stem cells. Science 287, 1433–1438. ( 10.1126/science.287.5457.1433) [DOI] [PubMed] [Google Scholar]
- 95.Cayre M, Malaterre J, Charpin P, Strambi C, Strambi A. 2000. Fate of neuroblast progeny during postembryonic development of mushroom bodies in the house cricket, Acheta domesticus. J. Insect Physiol. 46, 313–319. ( 10.1016/S0022-1910(99)00184-5) [DOI] [PubMed] [Google Scholar]
- 96.Kozorovitskiy Y, Gould E. 2004. Dominance hierarchy influences adult neurogenesis in the dentate gyrus. J. Neurosci. 24, 6755–6759. ( 10.1523/JNEUROSCI.0345-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ghosal K, Gupta M, Killian KA. 2009. Agonistic behavior enhances adult neurogenesis in male Acheta domesticus crickets. J. Exp. Biol. 212, 2045–2056. ( 10.1242/jeb.026682) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maeso I, Roy SW, Irimia M. 2012. Widespread recurrent evolution of genomic features. Genome Biol. Evol. 4, 486–500. ( 10.1093/gbe/evs022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Farris SM. 2008. Structural, functional and developmental convergence of the insect mushroom bodies with higher brain centers of vertebrates. Brain Behav. Evol. 72, 1–15. ( 10.1159/000139457) [DOI] [PubMed] [Google Scholar]
- 100.Farris SM. 2008. Evolutionary convergence of higher brain centers spanning the protostome-deuterostome boundary. Brain Behav. Evol. 72, 106–122. ( 10.1159/000151471) [DOI] [PubMed] [Google Scholar]
- 101.Moroz LL. 2009. On the independent origins of complex brains and neurons. Brain Behav. Evol. 74, 177–190. ( 10.1159/000258665) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Northcutt RG. 2012. Evolution of centralized nervous systems: two schools of evolutionary thought. Proc. Natl Acad. Sci. USA 109, 10 626–10 633. ( 10.1073/pnas.1201889109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Farries MA. 2013. How ‘basal’ are the basal ganglia? Brain Behav. Evol. 82, 211–214. ( 10.1159/000356101) [DOI] [PubMed] [Google Scholar]
- 104.Edgecombe GD, Ma X, Strausfeld NJ. 2015. Unlocking the early fossil record of the arthropod central nervous system. Phil. Trans. R. Soc. B 370, 20150038 ( 10.1098/rstb.2015.0038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Doeffinger C, Hartenstein V, Stollewerk A. 2010. Compartmentalization of the precheliceral neuroectoderm in the spider Cupiennius salei: development of the arcuate body, optic ganglia, and mushroom body. J. Comp. Neurol. 518, 2612–2632. ( 10.1002/cne.22355) [DOI] [PubMed] [Google Scholar]
- 106.Wagner GP. 2007. The developmental genetics of homology. Nat. Rev. Genet. 8, 473–479. ( 10.1038/nrg2099) [DOI] [PubMed] [Google Scholar]
- 107.Hirth F. 2010. On the origin and evolution of the tripartite brain. Brain. Behav. Evol. 76, 3–10. ( 10.1159/000320218) [DOI] [PubMed] [Google Scholar]
- 108.Denes AS, Jékely G, Steinmetz PRH, Raible F, Snyman H, Prud'homme B, Ferrier DEK, Balavoine G, Arendt D. 2007. Molecular architecture of annelid nerve cord supports common origin of nervous system centralization in Bilateria. Cell 129, 277–288. ( 10.1016/j.cell.2007.02.040) [DOI] [PubMed] [Google Scholar]
- 109.Hejnol A, et al. 2009. Assessing the root of bilaterian animals with scalable phylogenomic methods. Proc. R. Soc. B. 276, 4261–4270. ( 10.1098/rspb.2009.0896) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bailly X, Reichert H, Hartenstein V. 2013. The urbilaterian brain revisited: novel insights into old questions from new flatworm clades. Dev. Genes Evol. 223, 149–157. ( 10.1007/s00427-012-0423-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kollmann M, Huetteroth W, Schachtner J. 2011. Brain organization in Collembola (springtails). Arthropod. Struct. Dev. 40, 304–316. ( 10.1016/j.asd.2011.02.003) [DOI] [PubMed] [Google Scholar]
- 112.Scholtz G. 2004. Baupläne versus ground patterns, phyla versus monophyla: aspects of patterns and processes in evolutionary developmental biology. In Evolutionary developmental biology of Crustacea (ed. Scholtz G.), pp. 3–16. Lisse: A. A. Balkema. [Google Scholar]
- 113.Chen Z, Zhou C, Meyer M, Xiang K, Schiffbauer JD, Yuan X, Xiao S. 2013. Trace fossil evidence for Ediacaran bilaterian animals with complex behaviors. Precambrian Res. 224, 690–701. ( 10.1016/j.precamres.2012.11.004) [DOI] [Google Scholar]
- 114.Strausfeld NJ, Hirth F. 2013. Homology versus convergence in resolving transphyletic correspondences of brain organization. Brain Behav. Evol. 82, 215–219. ( 10.1159/000356102) [DOI] [PubMed] [Google Scholar]
- 115.Wadeson PH, Crawford K. 2003. Formation of the blastoderm and yolk syncytial layer in early squid development. Biol. Bull. 205, 179–180. ( 10.2307/1543240) [DOI] [PubMed] [Google Scholar]
- 116.Hanlon RT, Messenger JB. 1996. Cephalopod behavior. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 117.Wollesen T, McDougall C, Degnan BM, Wanninger A. 2014. POU genes are expressed during the formation of individual ganglia of the cephalopod central nervous system. EvoDevo 5, 41 ( 10.1186/2041-9139-5-41) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee PN, Callaerts P, de Couet HG, Martindale MQ. 2003. Cephalopod Hox genes and the origin of morphological novelties. Nature 424, 1061–1065. ( 10.1038/nature01872) [DOI] [PubMed] [Google Scholar]
- 119.Albertin CB, Simakov O, Mitros T, Wang ZY, Pungor JT, Edsinger-Gonzales E, Brenner S, Ragsdale CW, Rokhsar DS. 2015. The octopus genome and the evolution of cephalopod neural and morphological novelties. Nature 524, 220–224. ( 10.1038/nature14668) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Focareta L, Sesso S, Cole AG. 2014. Characterization of homeobox genes reveals sophisticated regionalization of the central nervous system in the European cuttlefish Sepia officinalis. PLoS ONE 9, e109627 ( 10.1371/journal.pone.0109627) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Boycott BB. 1961. The functional organization of the brain of the cuttlefish Sepia officinalis. Proc. R. Soc. Lond. B 153, 503–534. ( 10.1098/rspb.1961.0015) [DOI] [Google Scholar]
- 122.Cajal SR. 1917. Contribución al conocimiento de la retina y centros ópticos de los cefalópodos. Trab. Lab. Inv. Biol. Univ. Madr. 15, 1–82. [Google Scholar]
- 123.Williamson R, Chrachri A. 2004. Cephalopod neural networks. Neurosignals 13, 87–98. ( 10.1159/000076160) [DOI] [PubMed] [Google Scholar]
- 124.Boycott BB, Young JZ. 1955. A Memory system in Octopus vulgaris Lamarck. Proc. R. Soc. Lond. B 143, 449–480. ( 10.1098/rspb.1955.0024) [DOI] [PubMed] [Google Scholar]
- 125.Hochner B, Brown ER, Langella M, Shomrat T, Fiorito G. 2003. A learning and memory area in the octopus brain manifests a vertebrate-like long-term potentiation. J. Neurophysiol. 90, 3547–3554. ( 10.1152/jn.00645.2003) [DOI] [PubMed] [Google Scholar]
- 126.Shigeno S, Ragsdale CW. 2015. The gyri of the octopus vertical lobe have distinct neurochemical identities. J. Comp. Neurol. 523, 1297–1317. ( 10.1002/cne.23755) [DOI] [PubMed] [Google Scholar]
- 127.Budelmann BU, Young JZ. 1985. Central pathways of the nerves of the arms and mantle of octopus. Phil. Trans. R. Soc. Lond. B 310, 109–122. ( 10.1098/rstb.1985.0101) [DOI] [Google Scholar]
- 128.Grasso FW. 2014. The octopus with two brains: how are distributed and central representations integrated in the octopus central nervous system? In Cephalopod cognition (eds Darmaillacq A-S, Dickel L, Mather J), pp. 94–122. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 129.Kamikouchi A, Inagaki HK, Effertz T, Hendrich O, Fiala A, Gopfert MC, Ito K. 2009. The neural basis of Drosophila gravity-sensing and hearing. Nature 458, 165–171. ( 10.1038/nature07810). [DOI] [PubMed] [Google Scholar]
- 130.Grimaldi DA. 2010. 400 million years on six legs: on the origin and early evolution of Hexapoda. Arthropod. Struct. Dev. 39, 191–203. ( 10.1016/j.asd.2009.10.008) [DOI] [PubMed] [Google Scholar]
- 131.Pfenning AR, et al. 2014. Convergent transcriptional specializations in the brains of humans and song-learning birds. Science 346, 1256846 ( 10.1126/science.1256846) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shen Y-Y, Liang L, Li G-S, Murphy RW, Zhang Y-P. 2012. Parallel evolution of auditory genes for echolocation in bats and toothed whales. PLoS Genet. 8, e1002788 ( 10.1371/journal.pgen.1002788) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Seilacher A, Buatois LA, Gabriela Mángano M. 2005. Trace fossils in the Ediacaran–Cambrian transition: behavioral diversification, ecological turnover and environmental shift. Palaeogeogr. Palaeoclimatol. Palaeoecol. 227, 323–356. ( 10.1016/j.palaeo.2005.06.003) [DOI] [Google Scholar]
- 134.Erwin DH. 2005. The origin of animal body plans. New Haven, CT: Peabody Museum of Natural History, Yale University. [Google Scholar]
- 135.Shomrat T, Levin M. 2013. An automated training paradigm reveals long-term memory in planarians and its persistence through head regeneration. J. Exp. Biol. 216, 3799–3810. ( 10.1242/jeb.087809) [DOI] [PubMed] [Google Scholar]
- 136.Fedonkin MA, Simonetta A, Ivantsov AY. 2007. New data on Kimberella, the Vendian mollusc-like organism (White Sea region, Russia): palaeoecological and evolutionary implications. Geol. Soc. Lond. Spec. Publ. 286, 157–179. ( 10.1144/SP286.12) [DOI] [Google Scholar]
- 137.Vinther J. 2015. The origins of molluscs. Palaeontology 58, 19–34. ( 10.1111/pala.12140) [DOI] [Google Scholar]
- 138.Bery A, Cardona A, Martinez P, Hartenstein V. 2010. Structure of the central nervous system of a juvenile acoel, Symsagittifera roscoffensis. Dev. Genes Evol. 220, 61–76. ( 10.1007/s00427-010-0328-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Holland LZ. 2015. Evolution of basal deuterostome nervous systems. J. Exp. Biol. 218, 637–645. ( 10.1242/jeb.109108) [DOI] [PubMed] [Google Scholar]