Abstract
Many attempts have been made to correlate degrees of both animal and human intelligence with brain properties. With respect to mammals, a much-discussed trait concerns absolute and relative brain size, either uncorrected or corrected for body size. However, the correlation of both with degrees of intelligence yields large inconsistencies, because although they are regarded as the most intelligent mammals, monkeys and apes, including humans, have neither the absolutely nor the relatively largest brains. The best fit between brain traits and degrees of intelligence among mammals is reached by a combination of the number of cortical neurons, neuron packing density, interneuronal distance and axonal conduction velocity—factors that determine general information processing capacity (IPC), as reflected by general intelligence. The highest IPC is found in humans, followed by the great apes, Old World and New World monkeys. The IPC of cetaceans and elephants is much lower because of a thin cortex, low neuron packing density and low axonal conduction velocity. By contrast, corvid and psittacid birds have very small and densely packed pallial neurons and relatively many neurons, which, despite very small brain volumes, might explain their high intelligence. The evolution of a syntactical and grammatical language in humans most probably has served as an additional intelligence amplifier, which may have happened in songbirds and psittacids in a convergent manner.
Keywords: high intelligence, mammals, brain size, cortex size, number of cortical neurons, information processing capacity
1. Introduction
According to the majority of behaviourists and animal psychologists (cf. [1]), ‘intelligence’ can be understood as mental or behavioural flexibility or the ability of an organism to solve problems occurring in its natural and social environment, culminating in the appearance of novel solutions that are not part of the animal's normal repertoire. This includes forms of associative learning and memory formation, behavioural flexibility and innovation rate, as well as abilities requiring abstract thinking, concept formation and insight.
In the past, many attempts have been made to correlate intelligence with brain properties, the most influential work being Harry Jerison's book ‘Evolution of the brain and intelligence’ [2]. A much discussed trait is absolute brain size, because many experts were convinced that absolutely bigger brains mean higher intelligence. Another much discussed trait is relative brain size, i.e. per cent of body size or the relative size of alleged ‘seats’ of intelligence like the cerebral cortex in mammals. As it becomes clear that much of brain size is determined by body size [2], experts have tried to determine the degree of ‘encephalization’, i.e. brain size beyond the mass related to body size, e.g. Jerison's ‘encephalization quotient (EQ)’ (for a critical overview, see [3]). One could also look for neurobiologically more meaningful traits like the number of neurons in the entire brain or in the pallium or cortex, the degree of connectivity, axonal conduction velocity, etc., relevant for ‘information processing capacity (IPC)’ of the brain or of the pallium or cortex, respectively [4]. IPC is coincident with the notion of ‘general intelligence’ as largely defined by the efficiency of working memory and, accordingly, mental manipulation abilities [5–8]. Finally, one could look for ‘unique’ properties that could best explain the observed differences in intelligence in the context of ‘mosaic brain evolution’.
In this article, we will investigate to which degree differences in intelligence can be correlated with brain traits. Because of lack of sufficient comparative data in other taxa, we will restrict our discussion mostly to primates and compare them to other mammals and, where possible, to birds (see also [4]).
2. Brain size and body size
Body and brain weights of vertebrates refer to data from Jerison [2], Haug [9], Russell [10] and van Dongen [11], while those of hominids and australopithecines are based on data from Jerison [2] and Falk [12]. Data for birds were taken from Jerison [2] and Iwaniuk et al. [13].
Mammals, like animals in general, vary enormously in body size (volume or weight, which are directly convertible). The smallest mammal is the Etruscan (or pygmy) shrew Suncus etruscus with a body weight of 2 g, and the largest mammal and animal is the blue whale Balaenoptera musculus with a length of 33 m and a body weight up to 200 tons. The largest living terrestrial animal is the African elephant, Loxodonta africana, with a body weight up to 7.5 tons. Thus, among mammals there is a range in body size or weight of 8 orders of magnitude.
The volumes or weights of brains likewise vary enormously. Among mammals, the smallest brain is found in the bat, Tylonycteris pachypus, which weighs 74 mg in the adult animal, and the largest brains of all animals are found in the sperm whale (Physeter macrocephalus) and ‘killer whale’ (Orcinus orca), with up to 10 kg. African elephant brains weigh up to 6 kg. This is an again enormous range, here roughly 5 orders of magnitude.
Mammals and birds generally have brains that are about 10 times larger than those of bony fishes, amphibians and reptiles of the same body size [3]. Within mammals, primates, with the exception of prosimians, generally have larger brains than the other orders with the same body size. In primates, brain size ranges from 1.67 g in the prosimian mouse lemur Microcebus to 1350 g in Homo sapiens. Generally, prosimians and tarsiers have relatively small brains with a range of 1.67–12.9 g (average 6.7 g), followed by New World monkeys with a range of 9.5–118 g (average 45 g) and Old World monkeys with a range of 36–222 g (average 115 g), with the largest brains found in baboons. Among apes, gibbons have brain sizes of 105–135 g, which lie within the range of Old World monkeys, while the large apes, i.e. orangutans (Pongo pygmaeus), gorillas (Gorilla gorilla) and chimpanzees (Pan troglodytes), have brain weights of between 330 and 570 g (males).
Thus, in extant primates, we recognize six non-overlapping or only slightly overlapping groups with respect to brain size: (i) prosimians and tarsiers, (ii) New World monkeys, (iii) Old World monkeys and hylobatids, (iv) the great apes, and (v) extant humans. The gap between non-human apes and humans is filled by brains of extinct australopithecines (e.g. Australopithecus afarensis and A. africanus), with reconstructed brain sizes of 343–515 g, H. habilis with brains of 600–780 g and H. erectus with brains of 909–1149 g [10]. The largest hominine brain, i.e. that of H. neanderthalensis, had a mean weight of 1487 g.
Within mammals, monkeys have much smaller brains than ungulates, and humans have much smaller brains than whales and elephants, while they are, without any doubt, more intelligent [14]. However, there are groups like primates, where ‘bigger is better’ appears to hold. Nevertheless, there remains the question: why are bigger brains not uniformly smarter?
3. The significance of relative brain size and of ‘encephalization’
One could assume that in vertebrates an increase in body size (volume or weight) is accompanied by a proportional increase in brain size, because the brain is involved in the control of what the body does, and a larger body may require more brain mass. If such a proportional increase happened, we speak of isometric growth. However, with respect to body size, as mentioned above, among mammals we find an increase by 8 orders of magnitude, while brain size increases ‘only’ by 5 orders of magnitude. This means that an increase in brain size dramatically ‘lags behind’ an increase in body size. But the opposite may also happen in the sense that brains or parts of them like the cortex increase faster in volume or weight than the body. In both cases, we speak of allometric brain growth, and in the former case of negative, in the latter of positive allometric brain growth.
When we compare the overall relationship between body size and brain size across all vertebrate classes, we easily see that this relationship is negatively allometrical. In figure 1, the body–brain relationship (BBR) for 200 vertebrates is shown, including data from bony fishes (light grey circles), reptiles (light grey triangles), birds (grey triangles), mammals except primates (dark grey circles) and primates (grey squares) including man (four measurements, encircled four grey squares). The figure shows the data in a double-logarithmic representation, which makes a nonlinear function—here a power function—linear.
Figure 1.
The relationship between brain weight (ordinate, grams) and body weight (abscissa, kilograms) in 200 vertebrate taxa in double-logarithmic presentation. Light grey circles: bony fishes; light grey triangles: reptiles; grey triangles: birds; dark grey circles: mammals except primates; grey squares: primates; and encircled grey squares: Homo sapiens. Adapted from [1]; modified after [15].
The general power function for the BBR in vertebrates is E = kPα, in which E and P are brain and body weights or volumes, respectively, and k and α are constants. k is a proportionality factor for the different vertebrate taxa, and α is the allometric (or scaling) exponent, which indicates how strongly the brain grows compared with body growth [12]. With α = 1, we would have an isometric growth, whereas α > 1 would indicate a positive and α < 1 a negative allometry. In double-logarithmic transformation, we obtain the linear equation log E = log k – α log P, where k is the intercept with the y-axis and α is the slope of the line.
The exact value of α is still a matter of debate. For vertebrates in general, von Bonin [16] found a value of two-thirds. For Jerison [2], this relates to the fact that with an increase in volume the body surface increases by two-thirds, arguing that the most important factors of a brain are the sensory surfaces of the body and the processing of the information coming from them. However, Jerison [2] pointed out that there are differences in α across the different vertebrate classes. For reptiles, one finds a value of 0.53 and for birds and mammals [17] values of 0.68 and 0.74, respectively. In primates, α = 1 was found by Herculano-Houzel [18], which would indicate an isometric growth of brain size. Finally, in extinct hominins plus living Homo sapiens, α amounts to 1.73 [19], which is the steepest increase in size during the entire brain evolution. So far there is no convincing explanation for the differences in α.
Three fundamental statements regarding BBR become evident: (i) small animals have small brains and large animals large brains in absolute terms, (ii) small animals have larger brains and large animals smaller brains relative to body size, (iii) up to 90% of increase in brain size, depending on the taxa under consideration, can be explained by increase in body size. Thus, animals mostly get large brains by becoming large.
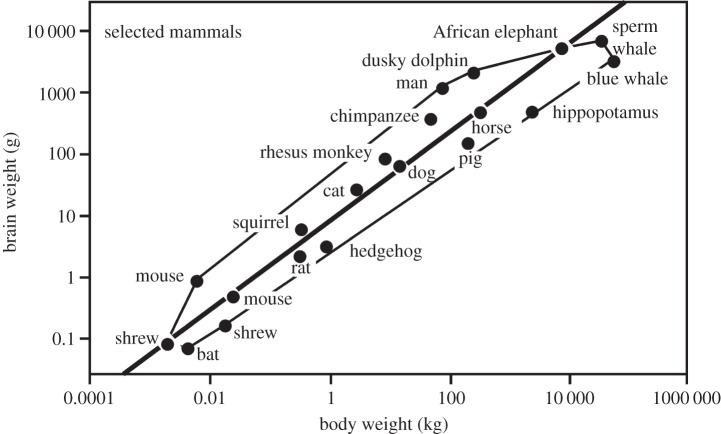
In figure 2, we get a closer look at the situation in mammals, again in a double-logarithmic representation. The bold diagonal (i.e. regression line) drawn through the data polygon has a slope of 0.74, which is typical of the BBR in mammals. We recognize that the values for some shrew and mice species, for dogs, horses and the African elephant lie on the line and therefore represent the average of mammals. The values for another mice species, chimpanzees, humans, but also for dolphins, lie above the line and accordingly represent BBRs above average, while those for another shrew species, bats, hedgehog, pig, hippopotamus, blue whale and sperm whale are found below the line and accordingly represent BBRs below average. The value for humans lies in the upper left edge of the polygon and farthest from the regression line, which means that we humans have a brain size that is the largest relative to the mammalian average.
Figure 2.
The relationship between brain weight and body weight in mammals in double-logarithmic presentation. Some species of shrews, mouse, dog, horse and African elephant have ‘average’ brain weights; accordingly their data points lie exactly on the regression line. Chimpanzees, humans, but also dolphins have brain weights above average; some bat species, hedgehog, pig, hippopotamus, blue whale and sperm whale have brain weights below average. Adapted from [11]; modified after [11].
As a consequence of negative brain allometry, with increasing body weight, the relative brain weight decreases from more than 10% in very small mammals to less than 0.005% in the blue whale [11]. The human brain again ranks relatively high with roughly 2% of body weight, but in close proximity to apes and dolphins. We thus conclude that relative brain size by itself is inappropriate for a correlation with intelligence. If this had any impact, then the tiniest animals should be the smartest ones, which apparently is not the case in any animal phylum, class or order. What we find, however, is more complicated: small animals with absolutely small brains can be surprisingly smart and large animals with absolutely large brains relatively unintelligent.
Within classes (birds, mammals) or orders (e.g. primates), animals of the same body size may exhibit large differences in absolute as well as relative brain size. If we calculate a reference value for that class or order, e.g. average brain–body ratio, then we can assess to which degree brain size of a given species lies above or below that reference value, i.e. is extraordinarily large or small. Jerison [2] tried to illustrate the observed deviations of given brain sizes from average by calculation that he called ‘EQ’, according to the formula EQ = Ea/Ee. This quotient indicates the extent to which relative brain size of a given species Ea deviates from the expected or average relative brain size Ee of the larger taxon (genus, family, order etc.) under consideration [2]. Accordingly, an EQ of 1, as found in the cat, tells us that the cat has a brain with (more or less) average relative size regarding all mammals investigated, while an EQ larger than 1 indicates that a brain is larger, and an EQ below 1 that a brain is smaller than expected given a certain body size.
Jerison's findings [2] tell us that—somewhat astonishingly—rats (0.4) have an EQ well below average, followed by mice (0.5). The cat has an average relative brain (1.0), while dogs (1.2), camels (1.2), elephants (1.3) and whales (1.8) have EQs slightly above average. Among primates, Old World monkeys have slightly higher EQs on average (1.7–2.7) than New World monkeys (1.7–2.3), with the exception of the white-fronted capuchin monkey (4.8). Gorillas and chimpanzees have astonishingly low EQs (1.5–1.8 and 2.2–2.5, respectively), and the highest EQs are found in dolphins (5.3) and finally humans (7.4–7.8). This means that the human brain is seven to eight times larger than an average mammal of the same body size. While the high EQs of humans and dolphins are of no surprise, the relatively high EQ of the capuchin monkey is unexpected, as are the relatively low EQs of chimpanzees, whales and gorillas. A survey on studies using the most frequently used paradigms for measuring animal intelligence yielded that intelligent behaviour of primates reveals a more overlapping distribution of intelligent behaviour across non-human primates than previously thought. Nevertheless, New World monkeys possess capacities in various cognitive domains that partially overlap with those of Old World monkeys, while great apes clearly outperform the other non-human primate taxa in most respects [14].
Partly to account for these inconsistencies, Jerison tried to make his calculations again more realistic by distinguishing between brain parts necessary for the maintenance and control of the body (Ev) and those associated with improved cognitive capacities (Ec)—in mammals mostly the cortex—which Jerison called ‘extra neurons’ (Nc) [2]. The idea behind this is that the neuronal expenses for the control of a large body are not nearly as high as the expenses for the processing of complex sensory data and related cognitive functions. Therefore, we expect an increase in behavioural intelligence to be paralleled by an increase in ‘extra neurons’. Calculating the number of such extra neurons removes some striking inconsistencies in the EQ list. For example, while the New World capuchin monkeys Cebus albifrons and C. apella have unusually high EQs compared even with large-brained apes, their Nc is much lower than that of the latter and even lower than that of the Old World monkeys. As with EQ, there is a huge gap between the great apes (3.2) and humans (8.8 for male Homo sapiens), which, however, can be filled by an average Nc of 3.9 in australopithecines [2].
More recently, experts in brain allometry adopted a slightly different method to correct relative brain size for body size, i.e. remove that portion of observed brain size that is simply owing to negative brain allometry (cf. [3,20]). The authors start with a linear regression of brain size against body size in a given taxon (e.g. birds), and then measure the deviation of a data point from the regression line (residuals). However, this method likewise does not solve the main problems of taking relative brain size even after correction for body size. For example, among birds, corvids and psittacids (parrots) have roughly equal values for corrected relative brain size that lie far above average, and both are considered to be comparably intelligent. But parrots on average have larger to much larger brains than corvids. On the other hand, dolphins exhibit much larger corrected relative brain sizes than gorillas and even chimpanzees, but are not considered as intelligent as the former [21,22]. It is clear that it is not the brain in total that counts, but only some parts—above all the cortex in mammals or the meso-nidopallium (MNP) in birds—because those are the parts of the brain that are believed to be most closely related to intelligence [1].
4. The cortex as a dominant contributor to the ‘seat’ of intelligence and mind
Intelligence in the above-defined sense results from the interaction of a large number of forebrain structures (e.g. cortex, basal ganglia, basal forebrain, dorsal thalamus), of which the cerebral cortex plays a dominant role (see below). This neural network structure and its processing algorithms enable higher cognitive, executive and communicative functions including language and vocal learning [2,23].
With increasing brain size in mammals, cortices increase in surface area as well as in volume. The smallest mammals, for example shrews, have a cortical surface (both hemispheres together) of 0.8 cm2 or less, in the rat we find 6 cm2, in the cat 83 cm2, in humans about 2400 cm2, in the elephant 6300 cm2 and in the false killer whale (Pseudorca crassidens) a maximum of 7400 cm2. Thus, from shrews to false killer whale we find a nearly 10 000-fold increase in cortical surface area, following exactly the increase in brain volume at an exponent of two-thirds, as expected [2].
This dramatic increase in brain surface area contrasts with a moderate increase in cortical thickness, i.e. from 0.4 mm in very small shrews and mice to 3–5 mm in humans and the great apes. The large-brained whales and dolphins have surprisingly thin cortices of between 1.2 and 1.6 mm, and even the elephant, again with a very large brain, has an average cortical thickness of ‘only’ 1.9 mm [9]. If we compare cortical volume across mammals and examine its relationship to brain size, then we recognize that the cortex grows faster than the rest of the brain, i.e. in a positive allometric fashion, with an average exponent α of about 1 in primates [24]. This exponent is slightly higher in primates and slightly smaller, but still positive, in ungulates, whereas in whales as well as in the elephant it is below 1. This means that in the latter two animals, cortical volume, while increasing in absolute volume, decreases in relative volume in a negative allometric fashion.
However, while looking for anatomical correlations with intelligence, one might argue that the entire mass of the cortex is not as important as the volume of the associative cortex in the sense of Jerison's [2] concept of ‘extra neurons’. Of special interest in this context is the size of the frontal or prefrontal cortex assumed to be the ‘seat’ of working memory, action planning and intelligence. Therefore, the question is whether primates—and especially humans—have a particularly large frontal–prefrontal cortex. There is a much-cited statement by Deacon [25] that humans have a prefrontal cortex that is three times larger in relative terms than that of the other apes.
Studies by Semendeferi and co-workers [26,27] using structural MRI confirmed, on the one hand, that among primates, humans have the largest frontal including prefrontal cortex (grey plus white matter), with about 280 cm3 compared to 80 cm3 in the other great apes. For gibbons and monkeys, the authors found 14 cm3. The relative size (i.e. per cent of total brain volume) of the human frontal cortex amounted to 38%, and the same value was found in the orangutan. The gorilla had 37%, the chimpanzee 35%, the gibbon 30% and the monkeys 31%. This means that in general, the size of the frontal cortex increases slightly positively with respect to the total brain mass, with an exponent of 1.14, but given the fact that humans have a brain that is more than two times larger than that of a gorilla and three times larger than that of a chimpanzee, the human frontal cortex is even smaller than expected—it should have a relative size of more than 40%. Inside the human frontal cortex, mostly the dorsal part and particularly the frontopolar area (BA 10) have increased and appear to be twice the size of what one would expect. The ventral parts, i.e. the orbitofrontal and ventromedial cortex, have become relatively (although not absolutely) smaller.
In searching for a more direct neurobiological basis of intelligence, the number of neurons, particularly of cortical neurons as well as the effectiveness of their wiring and processing speed, comes to mind quite naturally. Brains and cortices of the same volume may contain very different numbers of neurons depending on their neuron packing density (NPD), which—among others—depends on the size of the neurons, including their dendritic trees. Processing speed depends on interneuronal distance and axonal conduction velocity, which in turn largely depend on the degree of myelination [28].
The mammalian cortex comprises at least 70% of pyramidal cells, which are large excitatory neurons possessing axons that descend to the subcortical white matter [29–31]. The rest are different kinds of excitatory and inhibitory interneurons [32]. However, the size or volume of pyramidal cells (measured in cubic micrometres) varies greatly among mammals and roughly increases with an increase in brain size, i.e. larger brains and cortices tend to have larger pyramidal cells [28], which are presumably correlated with the length of the axons they support. The average size in mammals is 2300 µm3. Accordingly, cetaceans and elephants with large to very large brains have large to very large pyramidal cells. The bottlenose dolphin Tursiops truncatus with 5400 µm3 has ‘giant’ pyramidal cells, followed by the elephant with 4100 µm3. Primates generally have small pyramidal cells, with volumes around 1000 µm3 [9].
An increase in volume of pyramidal cells is accompanied by a decrease in packing density with a negative exponent of −1/3 [28]. This is a consequence of several factors. On the one hand, larger neurons have larger dendritic trees, and the arborization of local axon collaterals is wider. This enlarges the entire space occupied by a neuron and its appendages. Additionally, the number of glial cells and blood vessels tends to increase, albeit with large deviations, with increasing neuron size. Glial cells play an important role in nutrition, and the supply of oxygen and glucose as well as other substances increases with increasing cell volume.
According to measurements by Haug [9], NPD is high to very high in primates. Here, the prosimian mouse lemur (Microcebus sp.) and the New World marmoset (Callithrix jacchus) have the highest NPD with about 75 000 neurons mm−3, followed by the New World squirrel monkey (Saimiri sciureus) and baboons (Papio sp.) with about 60 000 neurons mm−3. Macaques (Macaca sp.), talapoins (Miopithecus) and chimpanzees have about 40 000, spider monkeys (Ateles sp.), woolly monkeys (Lagothrix sp.), gorillas and humans 25–30 000 neurons mm−3. By contrast, the cortices of whales and elephants with 6000–7000 neurons mm–3 have a very low NPD. These results are inconsistent with the much-cited statement by Rockel et al. [33] that in all mammals a cortical column with a given cross-sectional area, e.g. 1 mm2, contains the same number of neurons independent of the size of the cortex. Instead, while in monkeys such a cortical column may contain 190 000 and in humans on average 50 000 neurons (ranging between 30 000 and 100 000, depending on cortical areas), in cetaceans and whales we find only 19 000 neurons per column [18,34].
On the basis of these data on cortical volume and NPD, we can calculate the number of cortical neurons in mammals [14]. Owing to their large cortex volumes, their small neurons and high NPD, primates have many more cortical neurons than expected on the basis of absolute brain size. The relatively small New World squirrel monkey has 430 million, and the much larger Old World rhesus monkey about 480 million, the New World white-fronted capuchin 610 million, gorillas 4300 million, chimpanzees about 6200 million and humans about 15 000 million cortical neurons. The largest number of cortical neurons in non-primate mammals is found in the false killer whale with 10 500 million and the African elephant with 11 000 million, which is less than the number found in humans, despite the much larger brains of the former two. Herculano-Houzel et al. [35] reported a much lower number of 5600 million neurons in the African elephant, which is about one-third of the number of neurons found in the human cerebral cortex. The reason for this is that the cortices of whales and elephants, despite their very large surface area, are much thinner, their cortical neurons are much larger and accordingly their NPDs are much lower.
Chimpanzees have brains that represent one-third of the cortical volume of that of humans, while its cortex is as thick as the human one and the size of their pyramidal cells is comparable to that of humans. Because their NPD is higher than in humans, they have roughly half of the cortical neuron number found in humans. Cats have much smaller brains (25 g) than dogs (74 g), but a much higher NPD, and they therefore have almost twice as many cortical neurons (300 million) as dogs (160 million). Particularly impressive are the results from a comparison between horses and chimpanzees: the latter have a smaller brain, but five times more cortical neurons than the former.
Estimates on cell numbers are strongly influenced by the methods applied. Herculano-Houzel [36] reports 1100 million cortical neurons for the rhesus monkey, which appears far too high given the robust data by Haug [9] on cortex volume and NPD in that species. Estimates in humans also vary widely in the literature between 10 000 and 22 000 million, the latter being reported by Pakkenberg & Gundersen [37], which, however, again appear to be too high even when calculated on the basis of the highest measured human NPD. Herculano-Houzel and co-workers [18,38], with their isotropic fractionator method [39], arrive at 16 000 million cortical neurons in humans, which roughly corresponds to the number of 15 000 million neurons calculated by Roth & Dicke [14].
With respect to the IPC of the cortex, the number of synapses may be of importance. However, this topic is controversial. Some authors, like Schüz [40], state that the number of cortical synapses per neuron is constant throughout mammals, while others, like Changizi [28], assume that it increases with cortical volume and neuron size with an exponent of 0.33. Thus, larger cortical neurons should have larger numbers of synapses, but this increase in number of synapses is believed to be compensated by a decrease in NPD, so that in mammals cortical synapse density would remain constant. Unfortunately, exact data on number of synapses are largely lacking. The number of synapses per neuron in the human cortex likewise is highly controversial; Cherniak [41] reports 1000–10 000, and Rockland [42] nearly 30 000 synapses per neuron on average. Thus, the connectivity between neurons may range from loosely to highly connected by at least one order of magnitude. If we, somewhat arbitrarily, assume 20 000 synapses per neuron for the human cortex, this would yield a total number of 3 × 1014 synapses.
Besides the number of cortical neurons and synapses, another factor that is important for cortical IPC is processing speed, which in turn critically depends on (i) interneuronal distance, (ii) axonal conduction velocity and (iii) synaptic transmission speed. Interneuronal distance is determined by NPD: the higher the NPD, the shorter, trivially, is the interneuronal distance. We easily see that large brains with low NPD might have severe problems in this respect. Conduction velocity rather strictly depends on the diameter of mostly myelinated axons, i.e. axons with a thin myelin sheath (or none at all) have low, and those with a thick myelin sheet high conduction velocities. In mammals, axon diameter varies little from 0.5 µm in the mouse to 1 µm in monkeys [40]. Apes are reported to have thicker axons than other mammals, and for fibres connecting cortical and subcortical areas in the brain, velocities of 10 m s−1 are reported, while peripheral nerves (e.g. the sciatic nerve) may reach 150 m s−1. On the other hand, the axons of cetaceans (whales and dolphins) and elephants have thin myelin sheaths and consequently relatively low conduction velocities [28,42,43]. Finally, the speed of synapse transmission is assumed to be constant among mammals and primates, but exact data are lacking.
Thus, in large-brained animals like cetaceans and elephants we find an unfavourable combination of high interneuronal distance plus low axonal conduction velocity, which strongly impairs neuronal IPC. In the human brain, by contrast, we find a reasonable interneuronal distance plus very high conduction velocity, and this alone may result in an IPC that is about five times higher compared to that found in cetaceans and elephants.
5. Specialties of the cytoarchitecture of the mammalian cortex
There has been a long debate regarding whether or not, across mammalian taxa, the cortex has to be considered rather homogeneous or heterogeneous with specialties found in different groups. While in previous times authors tended to emphasize the homogeneity, today there is more search for heterogeneity and specialties. Clearly visible are the differences in size and number of sensory, predominantly visual, somatosensory and auditory cortical areas. In ‘insectivores’, the unrelated groups of eulipotyphlans and afrosoricidans, we find the dominance of the olfactory system, while an olfactory cortex is absent in cetaceans, which instead have a large auditory cortex. These animals have a relatively small hippocampus [44], which was the site of olfactory memory in the primitive state of mammals. In addition, the cetacean cortex lacks a prominent layer IV, which is called ‘granular layer’, because of the presence of many small-sized neurons. In most mammals, this layer IV is thick to very thick, particularly in the primary visual cortex, where we find a double layer IV, and it is the input layer of visual afferents from the thalamus. In cetaceans, instead, layer II is relatively thick and contains large pyramidal cells oriented upside down. Layer III is assumed to receive thalamic afferents owing to the high density of neurons positive for the transmitter GABA, a feature that is typical of thalamocortical recipient zones [45]. The reasons for these specialties are unknown, because the cortex of even-toed mammals (artiodactylids), which presumably gave rise to the cetaceans, has a ‘normal’ cortical cytoarchitecture with a well-developed layer IV.
Neuroanatomists like Preuss [46] and Wise [47] argue that only primates have a prefrontal cortex in the strict sense, together with its specific functions like control of attention, action planning and action selection and decision-making. Accordingly, lesions of the granular (pre)frontal area in primates have dramatic consequences for the mentioned functions, which are not the case in rats when the dorsal frontal cortex is lesioned [47]. A specialty of the frontal cortex of primates is the presence of a granural prefrontal area, which is characterized by a layer IV containing many small neurons. The frontal cortex of other mammals (e.g. rodents) lacks such a granular area and is therefore called agranular. In addition, neurons in the prefrontal cortex of humans exhibit a higher degree of branching complexity, an increased number of neurons and number of dendritic spines per neuron, leading to a higher number of spine synapses, and wider cortical columns compared with non-human primates. The authors interpret these findings as proof for a dramatic increase in IPC of the human prefrontal cortex [48].
One alleged peculiarity of the cortex of extant hominid primates (including humans), much discussed in recent times, is the presence of spindle-shaped neurons in layer Vb of the medial frontal and anterior cingulate cortex, which are four times as large as the other pyramid cells and are said to have extraordinarily wide-spread connections with other parts of the brain [49,50]. However, such ‘von Economo cells’ have recently been found in some cetaceans and in elephants as well, but not consistently in all large-brained mammals [44,51]. Whether this mosaic existence of ‘von Economo cells’ is owing to independent evolution or, when absent, to secondary loss, is unclear, as is their specific significance for cognition [52]. Furthermore, it is unlikely that superior mental abilities are based on the presence of a single type of neurons.
6. Bird brains
Corvid birds and parrots are now regarded as the most intelligent birds, and their intelligence has been considered as being equal to that of primates (cf. [1]). Their brains and their telencephala are significantly larger than those of non-passerine birds [13]. In several psittacid species, the telencephalon occupies over 70% of total brain volume and approaches 80% [53]. The MNP, as well as the hyperpallium, are extraordinarily large in a relative sense [54]. However, the brains of both groups are small in an absolute sense, with a range of 8–12 g in corvids and up to 24 g in psittacids, which is equal to the lowest sizes found in monkeys (see above). A capuchin monkey with a roughly comparable degree of intelligence has a brain of 26–80 g.
Birds generally have very small neurons, and these appear to be tightly packed inside the MNP, but unfortunately there are no quantitative data, and the same is true for the diameters of myelinated fibres in that region. Therefore, no direct comparisons between these important parameters in birds and mammals are possible. If we, very speculatively, start from the situation in the cortex of small monkeys characterized by small and densely packed cells and assume an even higher packing density in birds, because their neurons are even smaller, then large-brained corvids and parrots might have around 200 million MNP neurons. In addition, it could well be that owing to an extremely high neuronal packing density, the IPC of these animals is considerably higher than that of monkeys, particularly because the metabolism of birds is higher. However, these speculations must be tested by detailed empirical–experimental studies.
Songbirds, hummingbirds and psittacids exhibit vocal learning, which among mammals is found in bats, elephants, whales and humans. Thus, vocal learning appears to have evolved independently in groups of birds and mammals [55]. The members of the mentioned avian taxa are known to naturally produce sequences of sounds with signs of ‘phonological syntax’, while it is a matter of debate to which degree non-human vocal learners exhibit signs of ‘semantical syntax’ [56]. It is also debated to what degree non-human primates (e.g. gibbons) can be regarded vocal learners (cf. [23]). Recently, Jarvis and collaborators gave evidence that in songbirds and humans very similar networks of cortical–pallial and striatal regions related to vocal learning have evolved, including Broca- and Wernicke-like language centres (cf. [57]).
7. New ideas
Our ideas are in line with two presently much discussed concepts concerning the evolution of superior cognitive abilities such as found in humans. One of these concepts may be termed ‘continuity theory’ in the sense that higher cognitive abilities of humans and their neurobiological basis result from general or ‘conserved’ evolutionary trends found in vertebrates–mammals–primates. These trends result in an increase of absolute and relative brain size, and in a proportional increase of cortical and eventually frontal cortical volume [26,58–60]. Thus, the highest number of cortical neurons (especially those in the frontal lobe), the most efficient connectivity pattern, and consequently, the highest IPC are found in humans. Yet, according to the model developed by Hofman [59], the human brain lies about 20–30% below the optimum, which would be a brain of about 3500 cm3. This would be roughly twice the present human brain volume.
The second concept has been named mosaic or ‘cerebrotype’ brain evolution, referring to the idea that specific rather than general changes took place during human brain evolution, particularly regarding the prefrontal cortex (PFC) [61–63]. The PFC is assumed to have become disproportionally large [25]. According to the authors [61–63], an increase of white matter, i.e. the length of axonal projection and thickness of myelin sheath, between PFC and temporal cortex—including the hippocampal formation on the one hand, and PFC and striatum on the other hand—resulted in higher cognitive and executive/motor abilities. However, these two concepts are not mutually exclusive because both emphasize the strong increase in IPC during human brain evolution.
8. Conclusion
In this article, we asked which brain properties are most closely related to degrees of intelligence as stated by behaviourists and animal psychologists (cf. [4]). We recognized that small vertebrates on average have small brains and large animals large brains in absolute terms, and the reason for this is that brain size is determined roughly 90% by body size. Whales/dolphins and elephants have the largest brains, with weights up to 10 kg; the human brain, with an average weight of 1.35 kg, is of moderately large size. At the same time, brain size relative to body size tends to decrease with an increase in body size, resulting in the fact that small animals have relatively large and large animals relatively small brains. In shrews, brains comprise 10% or more of body volume, while in the largest mammal (and extant animal), the blue whale, the brain occupies less than 0.01% of the body. In this context, the 2% for the human brain is very high given the fact that Homo sapiens belongs to the larger mammals. This becomes evident when we calculate the EQ or residuals of brain–body regression, which, for a given taxon, indicates how much the actual brain size of a species deviates from the average BBR in this taxon. It turns out that humans have a brain that is roughly eight times larger than expected from average mammalian BBR, closely followed by some dolphins, which have a fivefold larger brain than expected.
There is no clear correlation between absolute or relative brain size and intelligence. Assuming that absolute brain size is decisive for intelligence, then whales or elephants should be more intelligent than humans, and horses more intelligent than chimpanzees, which definitely is not the case. If it were relative brain size that counted for intelligence, then shrews should be the most intelligent mammals, which nobody believes. If we take the EQ into account, some inconsistencies are removed; then humans are on top, but many other inconsistencies remain, for example that gorillas have a rather low EQ, but are considered highly intelligent, while capuchin monkeys and dolphins have unusually high EQs, but are not considered to be as intelligent as gorillas. Thus, other factors have to be considered.
The cerebral cortex is considered the ‘seat’ of intelligence and mind in mammals. During their evolution, there was a dramatic increase in cortical surface area with increasing brain size, while the thickness of the cortex increases only slightly. Among large-brained mammals, primates have the thickest cortices of 3–5 mm, while those of cetaceans and the elephant are surprisingly thin (1–1.8 mm). With increasing cortical volume, NPD usually decreases, but primates have unusually high and cetaceans and elephants unusually low packing densities. All this sums up to the fact that the human brain has the largest number of cortical neurons (about 15 billion), despite the fact that the human brain and cortex are much smaller in size than those of cetaceans and elephants (with 10–12 billion or even fewer cortical neurons).
However, this alone cannot explain the superiority of primate—including human—intelligence. Here, differences in the speed of intracortical information processing come into play. We have reason to assume that in primates in general and in apes and humans in particular cortical information processing is much faster than that in the large-brained elephants and cetaceans. Of course, the speed of information processing probably is faster in much smaller brains with still much higher NPDs, but these brains still have much fewer neurons. Thus, it is the combination of very many cortical neurons and a relatively high IPC that appears to make our brains very smart.
Despite intense search, no anatomical or physiological properties have been identified so far that would distinguish qualitatively the human brain from other mammalian or in general animal brains, except perhaps Broca's speech area. All properties mentioned so far are quantitative in nature. However, human language may represent a qualitative step. Certainly, the evolution of a syntactical–grammatical language was a complicated event that included substantial modifications of the vocal apparatus, the evolution or further elaboration of the Broca speech centre as an important cognitive–executive link between dorsal prefrontal regions and motor control of the vocal apparatus, and finally a new pattern of connectivity between the posterior, Wernicke, speech centre and the anterior Broca speech centre [64,65]. One can speculate that the type of intelligence found at the level of the great apes and the direct ancestors of modern humans was strongly amplified by syntactical–grammatical language in modern humans, which is assumed to have evolved 80 000–160 000 years ago paralleling the earliest archeological evidence of symbolic culture [66]. The evolution of bird song may represent a convergent evolutionary event.
The question remains why corvids and parrots, with absolutely small brains compared with those of most mammals including primates, reveal such a high intelligence. Presumably, because of extremely high packing density of neurons, they have an unusually high number of pallial neurons, probably several hundred millions, despite the small size of their brains. This could result in a very high IPC. Most astonishing is the fact that the ‘seat’ of avian intelligence, the nidopallium, exhibits an anatomy and a cytoarchitecture that differ considerably from that of the mammalian isocortex (cf. [4]). This could indicate that high intelligence can be realized by very different neuronal architectures.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Roth G. 2013. The long evolution of brains and minds. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 2.Jerison HJ. 1973. Evolution of the brain and intelligence. Amsterdam, The Netherlands: Academic Press. [Google Scholar]
- 3.Lefebvre L. 2012. Primate encephalization. Prog. Brain Res. 195, 393–412. ( 10.1016/B978-0-444-53860-4.00019-2) [DOI] [PubMed] [Google Scholar]
- 4.Roth G. 2015. Convergent evolution of complex brains and high intelligence. Phil. Trans. R. Soc. B 370, 20150049 ( 10.1098/rstb.2015.0049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hofman MA. 2003. Of brains and minds. A neurobiological treatise on the nature of intelligence. Evol. Cogn. 9, 178–188. [Google Scholar]
- 6.Marois R, Ivanoff J. 2005. Capacity limits of information processing in the brain. Trends Cogn. Sci. 9, 296–305. ( 10.1016/j.tics.2005.04.010) [DOI] [PubMed] [Google Scholar]
- 7.Lefebvre L, Sol D. 2008. Brains, lifestyles and cognition: are there general trends? Brain Behav. Evol. 72, 135–144. ( 10.1159/000151473) [DOI] [PubMed] [Google Scholar]
- 8.Hofman MA. 2012. Design principles of the human brain: an evolutionary perspective. Prog. Brain Res. 195, 373–390. ( 10.1016/B978-0-444-53860-4.00018-0) [DOI] [PubMed] [Google Scholar]
- 9.Haug H. 1987. Brain sizes, surfaces, and neuronal sizes of the cortex cerebri: a stereological investigation of man and his variability and a comparison with some mammals (primates, whales, marsupials, insectivores, and one elephant). Amer. J. Anat. 180, 126–142. ( 10.1002/aja.1001800203) [DOI] [PubMed] [Google Scholar]
- 10.Russell S. 1979. Brain size and intelligence: a comparative perspective. In Brain, behavior and evolution (eds Oakley DA, Plotkin HC), pp. 126–153. London, UK: Methuen. [Google Scholar]
- 11.van Dongen PAM. 1998. Brain size in vertebrates. In The central nervous system of vertebrates (eds Nieuwenhuys R, ten Donkelaar HJ, Nicholson C), pp. 2099–2134. Berlin, Germany: Springer. [Google Scholar]
- 12.Falk D. 2007. Evolution of the primate brain. In Handbook of palaeanthropology. Primate evolution and human origins, vol. 2 (eds Henke W, Tattersall I), pp. 1133–1162. Berlin, Germany: Springer. [Google Scholar]
- 13.Iwaniuk AN, Dean KM, Nelson JE. 2005. Interspecific allometry of the brain and brain regions in parrots (psittaciformes): comparisons with other birds and primates. Brain Behav. Evol. 65, 40–59. ( 10.1159/000081110) [DOI] [PubMed] [Google Scholar]
- 14.Roth G, Dicke U. 2012. Evolution of the brain and intelligence in primates. Prog. Brain Res. 195, 413–430. ( 10.1016/B978-0-444-53860-4.00020-9) [DOI] [PubMed] [Google Scholar]
- 15.Jerison HJ. 1969. Brain evolution and dinosaur brains. Am. Nat. 103, 575–588. ( 10.1086/282627) [DOI] [Google Scholar]
- 16.von Bonin G. 1937. Brain weight and body weight in mammals. J. Gen. Psychol. 16, 379–389. ( 10.1080/00221309.1937.9917959) [DOI] [Google Scholar]
- 17.Jerison HJ. 2001. The study of primate brain evolution: where do we go from here? In Evolutionary anatomy of the primate cerebral cortex (eds Falk D, Gibson K), pp. 305–337. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 18.Herculano-Houzel S. 2012. Neuronal scaling rules for primate brains: the primate advantage. Prog. Brain Res. 195, 325–340. ( 10.1016/B978-0-444-53860-4.00015-5) [DOI] [PubMed] [Google Scholar]
- 19.Pilbeam D, Gould SJ. 1974. Size and scaling in human evolution. Science 186, 892–901. ( 10.1126/science.186.4167.892) [DOI] [PubMed] [Google Scholar]
- 20.Lefebvre L, Reader SM, Sol D. 2004. Brains, innovations and evolution in birds and primates. Brain Behav. Evol. 63, 233–246. ( 10.1159/000076784) [DOI] [PubMed] [Google Scholar]
- 21.Manger PR. 2013. Questioning the interpretations of behavioral observations of cetaceans: is there really support for a special intellectual status for this mammalian order? Neuroscience 250, 664–696. ( 10.1016/j.neuroscience.2013.07.041) [DOI] [PubMed] [Google Scholar]
- 22.Güntürkün O. 2014. Is dolphin cognition special? Brain Behav. Evol. 83, 177–180. ( 10.1159/000357551) [DOI] [PubMed] [Google Scholar]
- 23.Petkov CI, Jarvis ED. 2012. Birds, primates, and spoken language origins: behavioral phenotypes and neurobiological substrates. Front. Evol. Neurosci. 4, 1–24. ( 10.3389/fnevo.2012.00012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herculano-Houzel S, Collins CE, Wong P, Kaas JH. 2007. Cellular scaling rules for primate brains. Proc. Natl Acad. Sci. USA 104, 3562–3567. ( 10.1073/pnas.0611396104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deacon TW. 1990. Rethinking mammalian brain evolution. Amer. Zool. 30, 629–705. ( 10.1093/icb/30.3.629) [DOI] [Google Scholar]
- 26.Semendeferi K, Lu A, Schenker N, Damasio H. 2002. Humans and great apes share a large frontal cortex. Nat. Neurosci. 5, 272–276. ( 10.1038/nn814) [DOI] [PubMed] [Google Scholar]
- 27.Teffer K, Semendeferi K. 2012. Human prefrontal cortex: evolution, development, and pathology. Prog. Brain Res. 195, 191–218. ( 10.1016/B978-0-444-53860-4.00009-X) [DOI] [PubMed] [Google Scholar]
- 28.Changizi MA. 2001. Principles underlying mammalian neocortical scaling. Biol. Cybern. 84, 207–215. ( 10.1007/s004220000205) [DOI] [PubMed] [Google Scholar]
- 29.Creutzfeldt OD. 1983. Cortex Cerebri. Leistung, strukturelle und funktionelle Organisation der Hirnrinde. Berlin, Germany: Springer. [Google Scholar]
- 30.Nieuwenhuys R. 1994. The neocortex. An overview of its evolutionary development, structural organization and synaptology. Anat. Embryol. (Berl) 190, 307–337. ( 10.1007/BF00187291) [DOI] [PubMed] [Google Scholar]
- 31.Schüz A. 2002. Introduction: homogeneity and heterogeneity of cortical structure: a theme and its variations. In Cortical areas: unity and diversity (eds Schüz A, Miller R), pp. 1–11. London, UK: Taylor & Francis. [Google Scholar]
- 32.Rudy B, Fishell G, Lee S, Hjerling-Leffler J. 2011. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev. Neurobiol. 71, 45–61. ( 10.1002/dneu.20853) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rockel AJ, Hiorns W, Powell TPS. 1980. The basic uniformity in structure of the neocortex. Brain 103, 221–244. ( 10.1093/brain/103.2.221) [DOI] [PubMed] [Google Scholar]
- 34.Cherniak C. 2012. Neuronal wiring optimization. Prog. Brain Res. 195, 361–371. ( 10.1016/B978-0-444-53860-4.00017-9) [DOI] [PubMed] [Google Scholar]
- 35.Herculano-Houzel S, Avelino-de-Souza K, Neves K, Porfírio J, Messeder D, Mattos Feijó L, Maldonado J, Manger PR. 2014. The elephant brain in numbers. Front Neuroanat. 8, 46 ( 10.3389/fnana.2014.00046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herculano-Houzel S. 2009. The human brain in numbers: a linearly scaled-up primate brain. Front. Hum. Neurosci. 3, 31 ( 10.3389/neuro.09.031.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pakkenberg B, Gundersen HJG. 1997. Neocortical neuron number in humans: effect of sex and age. J. Comp. Neurol. 384, 312–320. () [DOI] [PubMed] [Google Scholar]
- 38.Azevedo FAC, et al. 2009. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J. Comp. Neurol. 512, 532–541. ( 10.1002/cne.21974) [DOI] [PubMed] [Google Scholar]
- 39.Herculano-Houzel S. 2005. Isotropic fractionator: a simple, rapid method for the quantification of total cell and neuron numbers in the brain. J. Neurosci. 25, 2518–2521. ( 10.1523/JNEUROSCI.4526-04.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schüz A. 2001. What can the cerebral cortex do better than other parts of the brain? In Brain evolution and cognition (eds Roth G, Wullimann M), pp. 491–500. New York, NY: Wiley and Sons. [Google Scholar]
- 41.Cherniak C. 1990. The bounded brain: toward quantitative neuroanatomy. J. Cogn. Neurosci. 2, 58–66. ( 10.1162/jocn.1990.2.1.58) [DOI] [PubMed] [Google Scholar]
- 42.Rockland KS. 2002. Non-uniformity of extrinsic connections and columnar organization. J. Neurocytol. 31, 247–253. ( 10.1023/A:1024169925377) [DOI] [PubMed] [Google Scholar]
- 43.Zhang K, Sejnowski TJ. 2000. A universal scaling law between gray matter and white matter of cerebral cortex. Proc. Natl Acad. Sci. USA 97, 5621–5626. ( 10.1073/pnas.090504197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hof PR, van der Gucht E. 2007. Structure of the cerebral cortex of the humpback whale, Megaptera novaeangliae (Cetacea, Mysticeti, Balaenopteridae). Anat. Record A 290, 1–31. ( 10.1002/ar.20407) [DOI] [PubMed] [Google Scholar]
- 45.Revishchin AV, Garey LJ. 1991. Laminar distribution of cytochrome oxidase staining in cetacean isocortex. Brain Behav. Evol. 37, 355–367. ( 10.1159/000114370) [DOI] [PubMed] [Google Scholar]
- 46.Preuss TM. 1995. Do rats have a prefrontal cortex? The Rose–Woolsey–Akert program reconsidered. J. Cogn. Neurosci. 7, 1–24. ( 10.1162/jocn.1995.7.1.1) [DOI] [PubMed] [Google Scholar]
- 47.Wise SP. 2008. Forward frontal fields: phylogeny and fundamental function. Trends Neurosci. 31, 599–608. ( 10.1016/j.tins.2008.08.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elston GN, Benatives-Piccione R, Elston A, Zietsch B, Defelipe J, Manger P, Casagrands V, Kass JH. 2006. Specializations of the granular prefrontal cortex of primates: implication for cognitive processing. Anat. Rec. A: Discov. Mol. Cell. Evol. Biol. 288A, 26–35. ( 10.1002/ar.a.20278) [DOI] [PubMed] [Google Scholar]
- 49.Nimchinsky EA, Gilissen E, Allman JM, Perl DP, Erwin JM, Hof PR. 1999. A neuronal morphologic type unique to humans and great apes. Proc. Natl Acad. Sci. USA 96, 5268–5273. ( 10.1073/pnas.96.9.5268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elston GN. 2002. Cortical heterogeneity: implications for visual processing and polysensory integration. J. Neurocytol. 31, 317–335. ( 10.1023/A:1024182228103) [DOI] [PubMed] [Google Scholar]
- 51.Hakeem AY, Sherwood CC, Bonar CJ, Butti C, Hof PR, Allman JM. 2009. Von Economo neurons in the elephant brain. Anat. Rec. 292, 242–248. ( 10.1002/ar.20829) [DOI] [PubMed] [Google Scholar]
- 52.Sherwood CC, Subiaul F, Zawidzki TW. 2008. A natural history of the human mind: tracing evolutionary changes in brain and cognition. J. Anat. 212, 426–454. ( 10.1111/j.1469-7580.2008.00868.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iwaniuk AN, Hurd PL. 2005. The evolution of cerebrotypes in birds. Brain Behav. Evol. 65, 215–230. ( 10.1159/000084313) [DOI] [PubMed] [Google Scholar]
- 54.Mehlhorn J, Hunt GR, Gray RD, Rehkämper G, Güntürkün O. 2010. Tool-making New Caledonian crows have large associative brain areas. Brain Behav. Evol. 75, 63–70. ( 10.1159/000295151) [DOI] [PubMed] [Google Scholar]
- 55.Jarvis ED. 2009. Evolution. In Encyclopedia of neuroscience, vol. 2 (ed Squire LR.), pp. 209–215. Oxford, UK: Academic Press. [Google Scholar]
- 56.Berwick RC, Okanoya K, Beckers GJL, Bolhuis JJ. 2011. Songs to syntax: the linguistics of bird song. Trends Cogn. Sci. 15, 113–121. ( 10.1016/j.tics.2011.01.002) [DOI] [PubMed] [Google Scholar]
- 57.Pfenning AR, et al. 2014. Convergent transcriptional specialization in the brains of humans and song-learning birds. Science 346, 1333 ( 10.1126/science.1256846) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aboitiz F, Montiel JF. 2012. From tetrapods to primates: conserved developmental mechanisms in diverging ecological adaptations. Prog. Brain Res. 195, 3–24. ( 10.1016/B978-0-444-53860-4.00001-5) [DOI] [PubMed] [Google Scholar]
- 59.Hofman MA. 2014. Evolution of the human brain: when bigger is better. Front. Neuroanat. 8, 15 ( 10.3389/fnana.2014.00015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Finlay BL, Uchiyama R. 2015. Developmental mechanisms channeling cortical evolution. Trends Neurosci. 38, 69–76. ( 10.1016/j.tins.2014.11.004) [DOI] [PubMed] [Google Scholar]
- 61.Smears JB, Soligo C. 2013. Brain reorganization, not relative brain size, primarily characterizes anthropoid brain evolution. Proc. R. Soc. B 280, 20130269 ( 10.1098/rspb.2013.0269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Willemet R. 2013. Reconsidering the evolution of brain, cognition, and behavior in birds and mammals. Front. Psychol. 4, 396 ( 10.3389/fpsyg.2013.00396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rilling JK. 2014. Comparative primate neuroimaging: insights into human brain evolution. Trends Cogn Sci. 18, 46–55. ( 10.1016/j.tics.2013.09.013) [DOI] [PubMed] [Google Scholar]
- 64.Corballis MC. 2010. Mirror neurons and the evolution of language. Brain Lang. 112, 25–35. ( 10.1016/j.bandl.2009.02.002) [DOI] [PubMed] [Google Scholar]
- 65.Friederici A. 2011. The brain basis of language processing: from structure to function. Physiol. Rev. 9, 1357–1392. ( 10.1152/physrev.00006.2011) [DOI] [PubMed] [Google Scholar]
- 66.Atkinson QD. 2011. Phonemic diversity supports a serial founder effect model of language expansion from Africa. Science 332, 346–349. ( 10.1126/science.1199295) [DOI] [PubMed] [Google Scholar]