Abstract
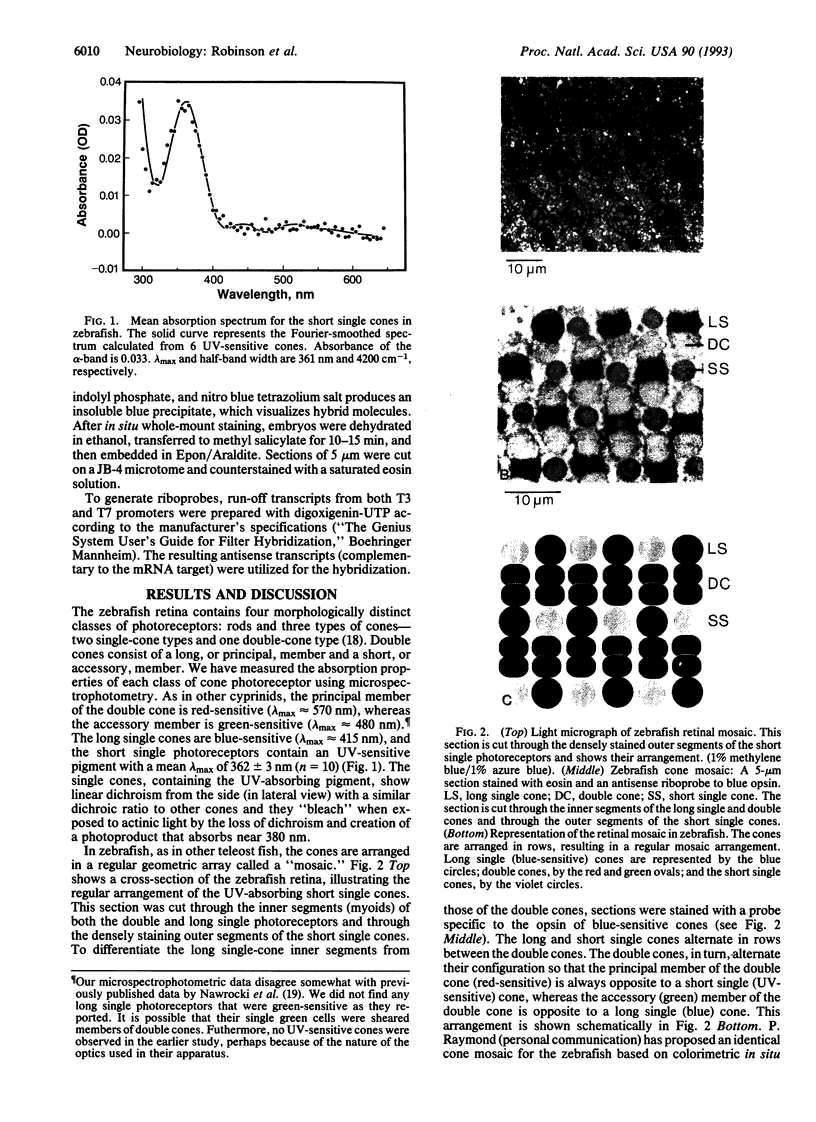
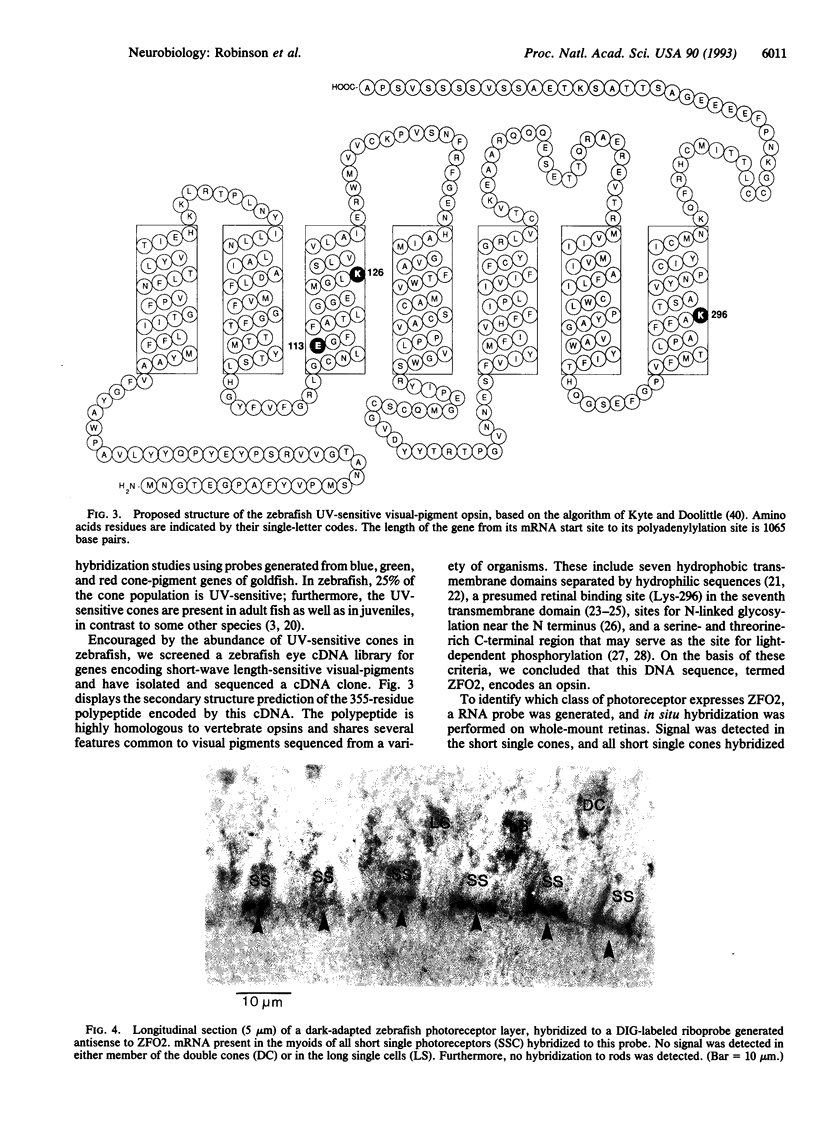
In many vertebrates, UV-sensitive photoreceptors have been identified by microspectrophotometry and UV-visual sensitivity has been identified by behavioral studies, but as yet no vertebrate UV-sensitive pigment gene has been isolated. We have sequenced a cDNA clone that hybridizes to short single cone cells in the zebrafish (Brachydanio rerio). These cells, which make up 25% of the cone population in zebrafish retinae, are UV-sensitive (lambda max approximately 360 nm). The visual pigment encoded by this gene is unusual in that its amino acid sequence is more homologous to the rod pigment rhodopsin (up to 89%) than to other cone pigments (35-83%). Like all other vertebrate visual pigments, it contains a lysine residue at position 296, the presumptive retinal binding site, and a glutamate residue at position 113. However, it is unique in possessing a lysine residue at position 126, which may account for the UV-sensitivity of the pigment.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applebury M. L., Hargrave P. A. Molecular biology of the visual pigments. Vision Res. 1986;26(12):1881–1895. doi: 10.1016/0042-6989(86)90115-x. [DOI] [PubMed] [Google Scholar]
- Archer S. N., Lythgoe J. N., Hall L. Rod opsin cDNA sequence from the sand goby (Pomatoschistus minutus) compared with those of other vertebrates. Proc Biol Sci. 1992 Apr 22;248(1321):19–25. doi: 10.1098/rspb.1992.0037. [DOI] [PubMed] [Google Scholar]
- Bowmaker J. K., Kunz Y. W. Ultraviolet receptors, tetrachromatic colour vision and retinal mosaics in the brown trout (Salmo trutta): age-dependent changes. Vision Res. 1987;27(12):2101–2108. doi: 10.1016/0042-6989(87)90124-6. [DOI] [PubMed] [Google Scholar]
- Branchek T., Bremiller R. The development of photoreceptors in the zebrafish, Brachydanio rerio. I. Structure. J Comp Neurol. 1984 Mar 20;224(1):107–115. doi: 10.1002/cne.902240109. [DOI] [PubMed] [Google Scholar]
- Chen D. M., Collins J. S., Goldsmith T. H. The ultraviolet receptor of bird retinas. Science. 1984 Jul 20;225(4659):337–340. doi: 10.1126/science.6740315. [DOI] [PubMed] [Google Scholar]
- Fukuda M. N., Papermaster D. S., Hargrave P. A. Rhodopsin carbohydrate. Structure of small oligosaccharides attached at two sites near the NH2 terminus. J Biol Chem. 1979 Sep 10;254(17):8201–8207. [PubMed] [Google Scholar]
- Goldsmith T. H. Optimization, constraint, and history in the evolution of eyes. Q Rev Biol. 1990 Sep;65(3):281–322. doi: 10.1086/416840. [DOI] [PubMed] [Google Scholar]
- Hall M. D., Hoon M. A., Ryba N. J., Pottinger J. D., Keen J. N., Saibil H. R., Findlay J. B. Molecular cloning and primary structure of squid (Loligo forbesi) rhodopsin, a phospholipase C-directed G-protein-linked receptor. Biochem J. 1991 Feb 15;274(Pt 1):35–40. doi: 10.1042/bj2740035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrave P. A., McDowell J. H., Curtis D. R., Wang J. K., Juszczak E., Fong S. L., Rao J. K., Argos P. The structure of bovine rhodopsin. Biophys Struct Mech. 1983;9(4):235–244. doi: 10.1007/BF00535659. [DOI] [PubMed] [Google Scholar]
- Hawryshyn C. W., Harosi F. I. Ultraviolet photoreception in carp: microspectrophotometry and behaviorally determined action spectra. Vision Res. 1991;31(3):567–576. doi: 10.1016/0042-6989(91)90107-g. [DOI] [PubMed] [Google Scholar]
- Honig B., Greenberg A. D., Dinur U., Ebrey T. G. Visual-pigment spectra: implications of the protonation of the retinal Schiff base. Biochemistry. 1976 Oct 19;15(21):4593–4599. doi: 10.1021/bi00666a008. [DOI] [PubMed] [Google Scholar]
- Hárosi F. I. Cynomolgus and rhesus monkey visual pigments. Application of Fourier transform smoothing and statistical techniques to the determination of spectral parameters. J Gen Physiol. 1987 May;89(5):717–743. doi: 10.1085/jgp.89.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hárosi F. I., Hashimoto Y. Ultraviolet visual pigment in a vertebrate: a tetrachromatic cone system in the dace. Science. 1983 Dec 2;222(4627):1021–1023. doi: 10.1126/science.6648514. [DOI] [PubMed] [Google Scholar]
- Ibbotson R. E., Hunt D. M., Bowmaker J. K., Mollon J. D. Sequence divergence and copy number of the middle- and long-wave photopigment genes in Old World monkeys. Proc Biol Sci. 1992 Feb 22;247(1319):145–154. doi: 10.1098/rspb.1992.0021. [DOI] [PubMed] [Google Scholar]
- Jacobs G. H., Neitz J., Deegan J. F., 2nd Retinal receptors in rodents maximally sensitive to ultraviolet light. Nature. 1991 Oct 17;353(6345):655–656. doi: 10.1038/353655a0. [DOI] [PubMed] [Google Scholar]
- Johnson R. L., Grant K. B., Zankel T. C., Boehm M. F., Merbs S. L., Nathans J., Nakanishi K. Cloning and expression of goldfish opsin sequences. Biochemistry. 1993 Jan 12;32(1):208–214. doi: 10.1021/bi00052a027. [DOI] [PubMed] [Google Scholar]
- KROPF A., HUBBARD R. The mechanism of bleaching rhodopsin. Ann N Y Acad Sci. 1959 Nov 12;74(2):266–280. doi: 10.1111/j.1749-6632.1958.tb39550.x. [DOI] [PubMed] [Google Scholar]
- Koike S., Nabeshima Y., Ogata K., Fukui T., Ohtsuka E., Ikehara M., Tokunaga F. Isolation and nucleotide sequence of a partial cDNA clone for bovine opsin. Biochem Biophys Res Commun. 1983 Oct 31;116(2):563–567. doi: 10.1016/0006-291x(83)90560-0. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Loew E. R., Wahl C. M. A short-wavelength sensitive cone mechanism in juvenile yellow perch, Perca flavescens. Vision Res. 1991;31(3):353–360. doi: 10.1016/0042-6989(91)90088-m. [DOI] [PubMed] [Google Scholar]
- Montell C., Jones K., Zuker C., Rubin G. A second opsin gene expressed in the ultraviolet-sensitive R7 photoreceptor cells of Drosophila melanogaster. J Neurosci. 1987 May;7(5):1558–1566. doi: 10.1523/JNEUROSCI.07-05-01558.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans J. Determinants of visual pigment absorbance: role of charged amino acids in the putative transmembrane segments. Biochemistry. 1990 Jan 30;29(4):937–942. doi: 10.1021/bi00456a013. [DOI] [PubMed] [Google Scholar]
- Nathans J., Thomas D., Hogness D. S. Molecular genetics of human color vision: the genes encoding blue, green, and red pigments. Science. 1986 Apr 11;232(4747):193–202. doi: 10.1126/science.2937147. [DOI] [PubMed] [Google Scholar]
- Nathans J., Thomas D., Hogness D. S. Molecular genetics of human color vision: the genes encoding blue, green, and red pigments. Science. 1986 Apr 11;232(4747):193–202. doi: 10.1126/science.2937147. [DOI] [PubMed] [Google Scholar]
- Nawrocki L., BreMiller R., Streisinger G., Kaplan M. Larval and adult visual pigments of the zebrafish, Brachydanio rerio. Vision Res. 1985;25(11):1569–1576. doi: 10.1016/0042-6989(85)90127-0. [DOI] [PubMed] [Google Scholar]
- Palczewski K., McDowell J. H., Hargrave P. A. Purification and characterization of rhodopsin kinase. J Biol Chem. 1988 Oct 5;263(28):14067–14073. [PubMed] [Google Scholar]
- Sakmar T. P., Franke R. R., Khorana H. G. Glutamic acid-113 serves as the retinylidene Schiff base counterion in bovine rhodopsin. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8309–8313. doi: 10.1073/pnas.86.21.8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga F., Iwasa T., Miyagishi M., Kayada S. Cloning of cDNA and amino acid sequence of one of chicken cone visual pigments. Biochem Biophys Res Commun. 1990 Dec 31;173(3):1212–1217. doi: 10.1016/s0006-291x(05)80915-5. [DOI] [PubMed] [Google Scholar]
- Wang S. Z., Adler R., Nathans J. A visual pigment from chicken that resembles rhodopsin: amino acid sequence, gene structure, and functional expression. Biochemistry. 1992 Apr 7;31(13):3309–3315. doi: 10.1021/bi00128a002. [DOI] [PubMed] [Google Scholar]
- Weitz C. J., Nathans J. Histidine residues regulate the transition of photoexcited rhodopsin to its active conformation, metarhodopsin II. Neuron. 1992 Mar;8(3):465–472. doi: 10.1016/0896-6273(92)90274-h. [DOI] [PubMed] [Google Scholar]
- Yokoyama R., Yokoyama S. Convergent evolution of the red- and green-like visual pigment genes in fish, Astyanax fasciatus, and human. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9315–9318. doi: 10.1073/pnas.87.23.9315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama R., Yokoyama S. Isolation, DNA sequence and evolution of a color visual pigment gene of the blind cave fish Astyanax fasciatus. Vision Res. 1990;30(6):807–816. doi: 10.1016/0042-6989(90)90049-q. [DOI] [PubMed] [Google Scholar]
- Zhukovsky E. A., Oprian D. D. Effect of carboxylic acid side chains on the absorption maximum of visual pigments. Science. 1989 Nov 17;246(4932):928–930. doi: 10.1126/science.2573154. [DOI] [PubMed] [Google Scholar]
- Zuker C. S., Montell C., Jones K., Laverty T., Rubin G. M. A rhodopsin gene expressed in photoreceptor cell R7 of the Drosophila eye: homologies with other signal-transducing molecules. J Neurosci. 1987 May;7(5):1550–1557. doi: 10.1523/JNEUROSCI.07-05-01550.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]





