Abstract
Background
The molecular mechanisms leading to sporadic medullary thyroid carcinoma (sMTC) and juvenile papillary thyroid carcinoma (PTC), two rare tumours of the thyroid gland, remain poorly understood. Genetic studies on thyroid carcinomas have been conducted, although just a few loci have been systematically associated. Given the difficulties to obtain single-loci associations, this work expands its scope to the study of epistatic interactions that could help to understand the genetic architecture of complex diseases and explain new heritable components of genetic risk.
Methods
We carried out the first screening for epistasis by Multifactor-Dimensionality Reduction (MDR) in genome-wide association study (GWAS) on sMTC and juvenile PTC, to identify the potential simultaneous involvement of pairs of variants in the disease.
Results
We have identified two significant epistatic gene interactions in sMTC (CHFR-AC016582.2 and C8orf37-RNU1-55P) and three in juvenile PTC (RP11-648k4.2-DIO1, RP11-648k4.2-DMGDH and RP11-648k4.2-LOXL1). Interestingly, each interacting gene pair included a non-coding RNA, providing thus support to the relevance that these elements are increasingly gaining to explain carcinoma development and progression.
Conclusions
Overall, this study contributes to the understanding of the genetic basis of thyroid carcinoma susceptibility in two different case scenarios such as sMTC and juvenile PTC.
Electronic supplementary material
The online version of this article (doi:10.1186/s12920-015-0160-7) contains supplementary material, which is available to authorized users.
Keywords: Sporadic medullary thyroid carcinoma, Juvenile papillary thyroid carcinoma, Epistasis, Multifactor-dimensionality reduction, Genome-wide association study
Background
Thyroid carcinoma is the most common endocrine malignancy, which account for more than 1 % of all new malignant tumors [1]. There are several histological types and subtypes according to the endocrine thyroid cells from which thyroid carcinomas are derived. Medullary thyroid carcinoma (MTC) arises from calcitonin-producing parafollicular cells (thyroid C cells) and constitutes around 2–5 % of all thyroid neoplasias [2]. Approximately 25 % of MTC cases present an autosomal dominant inherited disorder named Multiple Endocrine Neoplasia type 2 (MEN 2), which includes three different clinical phenotypes: MEN 2A, MEN 2B, and familial MTC (FMTC) [3]. In >95 % of the cases, the three forms of MEN 2 are caused by specific gain-of-function germline mutations of the RET proto-oncogene [3, 4]. The remaining 75 % of MTC occurs as a non-inherited sporadic lesion without associated endocrinopathy (sporadic MTC, sMTC). Unlike hereditary forms, very little is known about the genetic basis of sMTC. So far, studies have been focused on the analysis of specific single nucleotide polymorphisms (SNPs)/haplotypes and significant associations to sMTC have been described for different susceptibility genes, suggesting that this phenotype may be caused by a combination of multiple common genetic variants [5–7]. However, most of them have failed to be replicated in other populations, maybe due to the difficulty in collect enough patients to reach the necessary statistical power [6].
Nonmedullary thyroid carcinoma (NMTC) comprises thyroid carcinomas of follicular cell origin and among them papillary thyroid carcinoma (PTC) and follicular thyroid carcinoma represent the two most common subtypes (85 and 10 %, respectively) [8]. Regarding PTC, the age is considered as one of the most important prognostic factors. Juvenile PTC (jPCT), a rare disease in children and adolescents, presents with an aggressive initial manifestation, though patients have an excellent overall prognosis, showing longer periods of survival and a lower incidence of recurrence [9, 10]. However, PTC remains under-reported in children and adolescents and there is still disagreement about the standard treatment and optimal type of follow-up needed [9, 11]. The few pathologic studies carried out in jPTC point to exposure to ionizing radiation as the only known environmental risk factor [12, 13]. Although it have reported several SNPs associated with the risk of PTC in the absence of radiation, most of them were carried out with older patients (mean age around 45 years) and/or using somatic rather than germline DNA [6]. As in the case of sMTC, the molecular mechanisms that account for jPTC or explain the development of this tumour remain largely unknown.
Despite the success of GWAS [14] in the identification of hundreds of genetic variants associated to different diseases [15], its application to rare and multifactorial diseases still presents major drawbacks. Conventional GWAS approaches, based on single markers, require of large cohorts, typically unavailable in rare diseases. In fact, the prevalence of sMTC is approximately 7/100,000, according to Orphanet (see http://www.orpha.net/orphacom/cahiers/docs/GB/Prevalence_of_rare_diseases_by_alphabetical_list.pdf) and there are no data available for PTC. Additionally, the complex genetic nature of some diseases make single marker approaches quite inefficient, while suggest taking approaches based on systems biology [16].
We carried out a GWAS in two thyroid carcinoma types (sMTC and jPTC) followed by the detection of epistatic interactions to identify the potential simultaneous involvement of pairs of variants in both diseases. The determination of genome-wide epistasis encompasses statistical and computational challenges [17]. Here, we have used the Multifactor-dimensionality reduction (MDR) method to detect gene-gene interactions (GxG) in GWAS, which has already successfully been used in the detection of multiple and joint genetic factors associated with complex traits [18].
We report here the first study that combines a screening for epistasis by MDR in GWAS on these two rare thyroid carcinoma types.
Methods
Patients and controls
Two different series of Spanish patients were recruited for this study: one with 66 sMTC patients, with absence of personal or family history suggestive of MEN 2 and absence of traditional germline MEN 2-defining RET mutations and another with 38 jPTC (range from 5 to 24 years of age) with no history of head and/or neck irradiation (see Additional file 1: Table S1) and no familiar aggregation. Additionally, 129 healthy controls comprising unselected, unrelated, race, age, and sex-matched individuals without previous thyroid-related disease history were recruited and used as controls of both carcinoma types.
All subjects underwent peripheral blood extraction for genomic DNA isolation using MagNA Pure LC system (Roche, Indianapolis, IN) according to the manufacturer’s instructions.
A written informed consent was obtained from all the participants for clinical and molecular genetic studies. The study was approved by the Ethics Committee for clinical research in the University Hospital Virgen del Rocío (Seville, Spain) and complies with the tenets of the declaration of Helsinki.
Genome-wide genotyping
DNA derived from peripheral blood was hybridized to Affymetrix Genome-Wide Human SNP 6.0 arrays. CEL files were processed using Affymetrix Power Tools (APT v1.15.0) and genotypes were obtained using the Birdseed (v2) calling algorithm.
Data quality control
Quality control was carried out using PLINK software [19]. Individuals with more than 7 % of missing rate as well as those with excessive or reduced heterozygosity (+/-2 times the standard deviation of the mean) were removed. The data were also checked for duplicates and related individuals. Finally, divergent ancestry was checked using EIGENSTRAT [20] to detect possible population stratification.
Markers with excessive missing rate (>5 %) were discarded. SNPs with minor allele frequency < 1 % and/or not in Hardy-Weinberg equilibrium (in unaffected samples; P < 1 × 10−5) were also excluded.
The data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus [GEO:GSE67047].
Association and gene-gene interaction tests
We carried out a conventional GWAS in our series of patients of jPTC and sMTC. In the analysis strategy followed we have used a Quantile-quantile plot (QQplot) to decide the most appropriate measures of association and relevance of disease models as well as the most suitable test of association [21]. Thus, QQplots and Manhattan plots were generated for each of the models to assess their validity. PLINK permits the GWAS analysis using two different approaches. Models were adjusted by sex and age and no significant differences were found with the unadjusted models. For the best fit model, the final p-value estimations were corrected using the Benjamini and Hochberg’s FDR adjustment [22].
We have reduced the number of SNPs to those representatives of genomic blocks in linkage disequilibrium by searching for tag SNPs using the Tagger software [23]. The study of possible epistatic interactions between pairs of genes was carried out with the Multifactor-dimensionality reduction (MDR) method [18]. MDR allows reducing the dimensionality of SNP data improving the identification of combinations of polymorphisms associated with disease risk. MDR is nonparametric, model-free and can directly be applied to case–control [18]. MDR implements a 10-fold cross-validation value (CVV) which is taken as an indicator of detection of pairs of SNPs showing an association to the disease significantly over the random expectation. Only tag SNPs mapping in genes were used in this study. In addition to coding genes we included in the study any ncRNA and pseudogenes. Despite the dimensionality reduction produced by using tag SNPs, the study of all the interactions still constitutes a challenge. Then, a parallelized version of the MDR method was developed. Our implementation uses all cores in a machine and also distributes the work among the nodes in a cluster. The multi-core part has been implemented using OpenMP directives and SSE instructions. Task distribution across processors is managed by the MPI applications programming interface [24]. The software is open and can be found at https://github.com/opencb/variant.
The main SNPs on the relevant interacting genes detected in sMTC and in jPTC were validated by Taqman technology using 7900HT Fast Real-Time PCR System (Applied Bio- systems, Foster City, California, USA)
Functional interpretation of the results
Functional evidences for the identified epistatic gene interactions were exhaustively analysed using a number of available annotation repositories and functional analysis tools. Gene functionality was assessed using the following resources: DAVID v6.7 [25], canSAR 2.0 [26], and COSMIC [27] databases. Gene-phenotype relationships were explored with VarElect (phenotype assignation tool from GeneCards) [28] and GeneMANIA [29]. Information on gene expression localization was taken from the Human Protein Atlas [30] and the GEO repository [31]. Finally, information about non-coding RNAs was obtained from neXtProt [32], NONCODE v4.0 [33] and Ensembl through the CellBase application [34].
Results and discussion
After the quality control process, a total of 158 samples in the sMTC vs control dataset (49 sMTC and 109 control with 639,289 SNPs) and 149 samples in the PTC vs control dataset (38 PTC and 111 control with 640,597 SNPs) remained (Additional file 1: Tables S2 and S3). After the application of the Tagger software a total of 357,263 tag SNPs in MTC and 344,455 in PTC remained.
In the last decade, many studies on thyroid carcinomas have been conducted but only a few loci have been systematically associated to the disease. The study of epistatic interactions could overcome some of the limitations of the conventional GWAS studies, helping to understand the genetic architecture of the disease and explain new heritable components of genetic risk [16].
Individual SNP associations to the diseases
The results of the conventional GWAS analysis render no significant associations (after multiple testing adjustments) for jPTC and only 21 SNPs with marginally significant associations for MTC (see Additional file 1: Table S4).
Among these SNPs, 6 appear in intergenic regions. The rest of them map in genes or around genes, although, none of them was previously associated to MTC [6]. This is expectable given that most of the known associations are gene or pathway-driven associations and have thus more statistical power, although less reproducibility [6].
Epistastic interactions in sMTC
Epistatic analysis of the sMTC samples genotypes (Table 1; Fig. 1) by MDR revealed three gene-gene interactions (LHFPL3-CHFR, CHFR-AC016582.2 and C8orf37-RNU1-55P) significantly associated with the disease (CVV > 0.5). Here we suggest CHFR- AC016582.2 (rs4758915, adjusted p-value = 0.9812, and rs10402530, adj. p-value = 0.9936, respectively) and C8orf37-RNU1-55P (rs7835921, adj. p-value 00.9669, and rs1287079, adj. p-value = 0.9569, respectively) as interesting potential candidate genes in sMTC. Table 2 contains the p-values as well as ORs and frequencies in cases and controls observed for the SNPs. Next, these SNPs were successfully validated by Taqman technology. It is more unlikely that LHFPL3 plays a relevant role in thyroid gland given that it is not expressed according to the databases of gene expression (GEO ID: GDS1665, corresponding to “Papillary thyroid carcinoma”). This does not mean that this gene cannot be a candidate, given that the expression of cancer-related genes in total thyroid tissue could be minority and hence undetectable in the databases, but here we are focusing on the most likely candidates.
Table 1.
Gene-gene significant interactions in sMTC obtained by MDR
| SNP 1 | SNP 2 | Gene 1 | Gene 2 | Gene name 1 | Gene name 2 | CVV |
|---|---|---|---|---|---|---|
| rs7787988 | rs4758915 | ENSG00000187416 | ENSG00000072609 | LHFPL3 | CHFR | 0.709 |
| rs4758915 | rs10402530 | ENSG00000072609 | ENSG00000225868 | CHFR | AC016582.2 | 0.706 |
| rs7835921 | rs1287079 | ENSG00000156172 | ENSG00000202380 | C8orf37 | RNU1-55P | 0.548 |
Selection based on the significant cross-validation value (CVV)
Fig. 1.
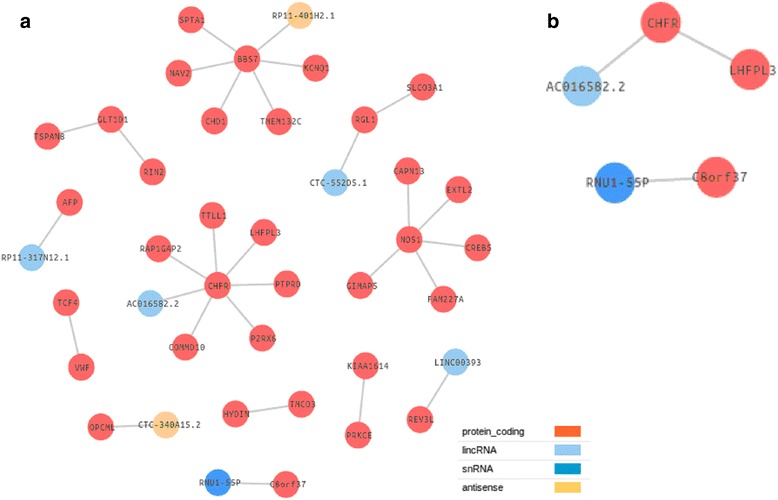
Gene-gene interactions obtained in sMTC patients by MDR analyses. a a total of 29 GxG interactions were obtained, (b) from which three were significant based on cross-validation value (>0.5)
Table 2.
SNPs involved in significant interactions. Columns from left to right are: SNP identifier, region in which the SNP is located, gene, Frequencies in the cases, frequencies in the controls, ORs and 95 % of confidence interval (CI), nominal p-value and adjusted p-value
| Analysis | SNP | Region | Gene | Frequency cases | Frequency controls | OR [95 % CI] | P-value | Adjusted p-value |
|---|---|---|---|---|---|---|---|---|
| sMTC | rs4758915 | Intronic | CHFR | 0.03704 | 0.02586 | 1.449 [0.4003–5.243] | 0.5702 | 0.9812 |
| sMTC | rs10402530 | Downstream | AC016582.2 | 0.1944 | 0.1897 | 1.031 [0.5782–1.84] | 0.9167 | 0.9936 |
| sMTC | rs7835921 | Intronic | C8orf37 | 0.4519 | 0.5086 | 0.7966 [0.5008–1.267] | 0.3365 | 0.9669 |
| sMTC | rs1287079 | Downstream | RNU1-55P | 0.3019 | 0.3664 | 0.7479 [0.4567–1.225] | 0.2476 | 0.9569 |
| jPTC | rs2235544 | Intronic | DIO1 | 0.4667 | 0.5043 | 0.86 [0.487–1.519] | 0.6032 | 0.9696 |
| jPTC | rs16876356 | Intronic | DMGDH | 0.1167 | 0.2308 | 0.4403 [0.1891–1.025] | 0.0518 | 0.9007 |
| jPTC | rs17716031 | Intronic | RP11-648K4.2 | 0.1034 | 0.1336 | 0.7481 [0.2964–1.888] | 0.5379 | 0.9668 |
| jPTC | rs10775207 | Intronic | LOXL1 | 0.0167 | 0.0087 | 1.932 [0.1723–21.67] | 0.5868 | 0.9668 |
In particular, CHFR gene encodes an E3 ubiquitin-protein ligase that acts in the mitotic checkpoint and functions as a tumour suppressor [35]. CHFR gene displays a significant epistatic association with AC016582.2, which is a novel long intergenic non-coding RNA (lincRNA). To date, there are no studies that have linked lincRNAs to sMTC yet.
Regarding C8orf37 gene, it encodes a ubiquitously expressed protein of unknown function. This gene has found to be over-expressed in astrocytoma, prostate carcinoma and undifferentiated sarcoma, as well as under-expressed in benign prostate hyperplasia (canSAR database). Moreover, copy number variation including this gene has been described in a thyroid carcinoma cell line (Human Protein Atlas database). C8orf37 gene was found in epistasis with RNU1-55P (RNA, U1, small nuclear 55, pseudogene). Small nuclear RNAs (snRNA) are known to act as guide molecules for pseudouridylation and site-specific methylation of other RNAs [36]. For example, a novel snRNA (RN7SK) has been described to interact with HMGA1 gene, which is overexpressed in thyroid carcinoma [37]. RNU1-55P is also a pseudogene, which can play an important role in physiology and disease (e.g. as BRAF pseudogene in different thyroid carcinomas [38]). It is noteworthy that one of the genes implicated in each of the two relevant pairs of gene interactions in sMTC was a type of ncRNA.
Epistastic interactions in jPTC
MDR analysis of jPTC samples (Additional file 1: Table S5) rendered a total of 133 GxG interactions related with the disease (with a CVV >0.5) (Fig. 2). Among all these interactions, we focused on those more likely to be related to the disease, according to their functionalities and gene expression patterns. The application of the DAVID and Babelomics [39] tools resulted in several gene clusters, one of them composed of 10 genes associated with thyroid gland. According to the CVV values obtained by MDR analyses, only five of those 10 genes were significantly associated with the phenotype (CHST8, DIO1, DMGDH, LOXL1 and PXDNL). Among these genes, DIO1, in epistasis with RP11-648k4.2, was directly associated to the disease by the VarElect genotype-phenotype association tool (Tables 3, 4 and Fig. 3). Available information on gene expression in GEO allowed us to discard CHST8 and PXDNL genes because no difference among normal and pathologic tissue expression was reported for them, while both DIO1 and DMGDH genes were underexpressed and LOXL1 gene was overexpressed in thyroid carcinoma [31]. In particular, DIO1 encodes an iodothyronine deiodinase type 1, which is a protein linked to carcinoma risk and specifically with PTC [40]. This link between DIO1 and PTC reinforces the validity of epistasis as an optimal strategy to find candidate genes for rare diseases or, in general, when large sample sizes are not available.
Fig. 2.
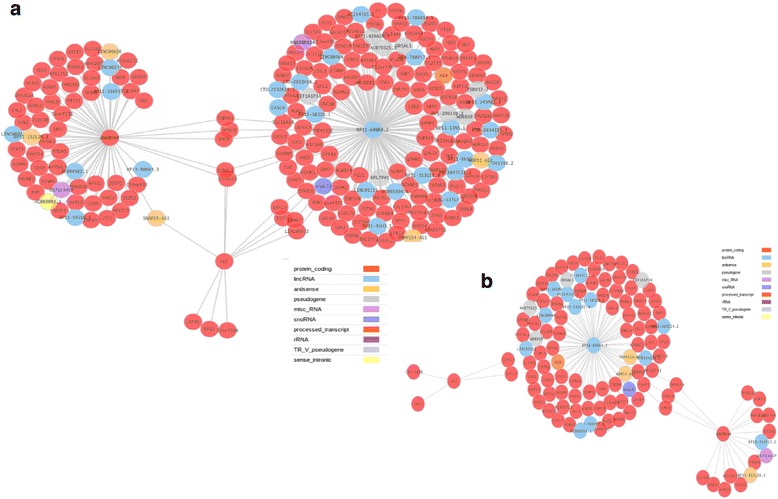
Gene-gene interactions obtained in jPTC patients by MDR analyses. a a total of 259 GxG interactions were obtained, (b) from which 133 were significant based on cross-validation value (>0.5)
Table 3.
Direct and indirect gene-gene interactions obtained by GWAS-epistasis analyses in jPTC patients by VarElect
| Direct GxG interactions | |||||
|---|---|---|---|---|---|
| Significant interaction | Not significant interaction | ||||
| Gene | Gene | CVV | Gene | Gene | CVV |
| RP11-648k4,2 | DIO1 | 0.559 | ANKRD44 | STAT3 | 0.280 |
| RP11-648k4,2 | TPO | 0.371 | |||
| Indirect and significant interactions | |||||
| Gene | Gene | CVV | Gene | Gene | CVV |
| RP11-648K4.2 | ARHGAP22 | 0.559 | RP11-648k4,2 | PCYT1B | 0.650 |
| ARHGEF11 | 0.555 | PLAUR | 0.851 | ||
| ATP8B4 | 0.748 | PRICKLE1 | 0.557 | ||
| CA6 | 0.744 | PRICKLE2 | 0.744 | ||
| CHST8 | 0.555 | PRKAG2 | 0.556 | ||
| CNTN2 | 0.557 | RNF14 | 0.650 | ||
| COL19A1 | 0.651/0.555 | ROBO2 | 0.646 | ||
| COL24A1 | 0.747 | RYR3 | 0.559 | ||
| CRMP1 | 0.744 | SEMA6D | 0.558 | ||
| DMGDH | 0.747 | SGCG | 0.557 | ||
| EXTL3 | 0.747 | SKI | 0.557 | ||
| FLI1 | 0.649 | SLC5A1 | 0.654 | ||
| GALNS | 0.650 | SLC5A4 | 0.652 | ||
| GALNT16 | 0.555/0.555 | SYNE1 | 0.650 | ||
| GCN1L1 | 0.650/0.650 | TICRR | 0.844 | ||
| GPR45 | 0.555 | UNC5B | 0.744 | ||
| GRM7 | 0.740 | ZNF529 | 0.650 | ||
| H19 | 0.556 | ZNF662 | 0.653 | ||
| HDAC9 | 0.649 | ZNF793 | 0.653 | ||
| KIFC3 | 0.937 | ANKRD44 | DOCK1 | 0.652 | |
| KIR3DL1 | 0.744 | HDAC9 | 0.652 | ||
| LOXL1 | 0.743 | PPP2R2A | 0.652 | ||
| MAGED1 | 0.649 | PRKCB | 0.558 | ||
| MAGI1 | 0.650 | SESN1 | 0.652 | ||
| NCAM1 | 0.650 | SLC1A1 | 0.558 | ||
| NPAS3 | 0.652 | WIPI1 | 0.942 | ||
| NUP107 | 0.649 | DCC | C6orf100 | 0.651 | |
| OPCML | 0.743 | ERI3 | 0.937 | ||
| OR5AL1 | 0.744 | EXTL3 | 0.748 | ||
| PARP1 | 0.950/0.650/0.650 | GCN1L1 | 0.558 | ||
More than one value in cross-validation value (CVV) cells means more than one interaction between these genes
Table 4.
Analyses by DAVID and GeneMANIA of the five significant genes found in jPTC by MDR
| CHST8 | DIO1 | DMGDH | LOXL1 | PXDNL | ||
|---|---|---|---|---|---|---|
| MDR Analyses | RP11-648k4.2 | RP11-648k4.2 | RP11-648k4.2 | RP11-648k4.2 | RP11-648k4.2 | |
| Interacting genes according to scientific literature | - | DIO2 | - | - | - | |
| Interacting genes according to DAVID | HS2ST1, TMEM132C, TMEM182 | - | - | - | - | |
| Interactions Analyses by GeneMANIA | Co-expression | - | TPO, PXDNL, DMGDH | DIO1 | - | DIO1 |
| Co-localization | - | TPO, DIO2 | - | - | - | |
| Genetic interactions | ANKRD44, NNT | NNT | - | ANKRD44, TPO | - | |
| Shared protein domain | HS2ST1 | DIO2 | - | - | TPO | |
Fig. 3.
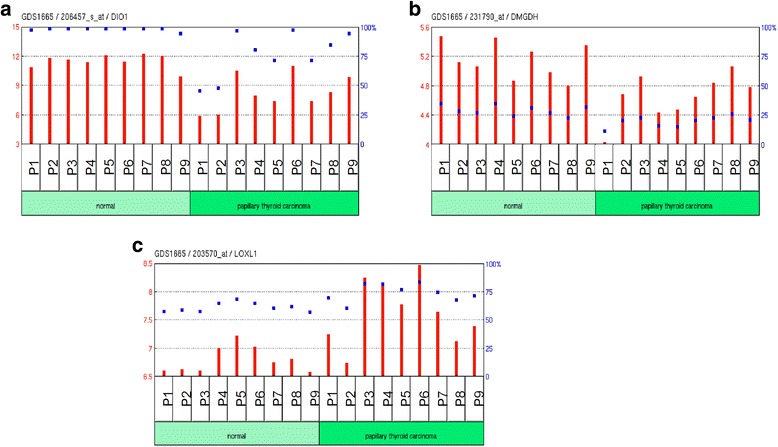
Expression profiling for arrays in 18 PTC samples (a) DIO1, (b) DMGD and (c) LOXL1 genes. Figures adapted from GeoDataSets
Regarding DMGDH gene, it encodes a mitochondrial dimethylglycine dehydrogenase related with oxidative demethylation of dimethylglycine in vitro with the formation of sarcosine, hydrogen peroxide and formaldehyde [41]. LOXL1 gene encodes a lysyl oxidase-like 1 enzyme involved in the connective tissue biogenesis. It has been related with different tumour progression and metastasis [42]. LOXL1 gene is expressed in thyroid tissue and one registered mutation has been reported in this gene for PTC, although it remains to be validated (COSMIC database).
Thereby, we suggest DIO1 (rs2235544, adj. p-value = 0.9696), DMGDH (rs16876356, adj. p-value = 0.9007) and LOXL1 (rs10775207, adj. p-value = 0.9668) genes, all of them in epistasis with RP11-648k4.2 (rs17716031, adj. p-value = 0.9668), a lincRNA which has been scarcely studied, as candidate genes for jPTC. See also ORs and frequencies in cases and controls observed for the SNPs in Table 2. Again, these SNPs were validated using Taqman technology. It is worth noting that different lincRNA have recently been linked to PTC. For example, a novel lincRNA gene (PTCSC2) has been found down-regulated in PTC tumours [43]. Also, up-regulation of a BRAF-activated lincRNA, previously associated with melanoma, has been related to PTC by increasing cell proliferation and autophagy activation [44]. It is important to highlight that these three genes, with a clear relationship with the PTC phenotype, found in jPTC were in epistasis with the same lincRNA RP11-648k4.2, which was also present in 181 additional genetic interactions, suggesting the potential involvement of this LincRNA in PTC. The large number of interactions displayed by RP11-648k4.2 suggests a regulatory role for this lincRNA. Our observation reinforces the increasingly important role of ncRNAs in carcinoma.
Given that both thyroid carcinomas are extremely rare, all the samples were used in this study and, consequently an independent population to validate the findings is not available. Future research with other cohorts will reveal whether our findings are generalizable to other populations or, on the contrary, different populations will display different susceptibility variants associated, that probably represent different facets of the mechanism of the disease, as it has been observed in other diseases [45].
Conclusions
Here we present the first genome-wide screening to detect epistasis in two thyroid carcinoma entities as sMTC and jPTC. Among our results, we remark the significance of two epistatic interactions in sMTC and three in jPTC. In addition, it is worth mentioning the presence of ncRNAs, and especially lincRNAs, among the epistasis found. Such elements are acquiring an increasingly relevance in carcinoma research in recent years. Although further studies would be needed to corroborate the interactions found, our methodological approach has demonstrated to be a promising complementary tool for finding new susceptibility genes in these thyroid carcinomas.
Acknowledgments
We would like to thank the patients that have participated in this study.
This work was supported by Spanish Ministry of Economy and Competitiveness (BIO2014-57291-R, Institute of Health Carlos III –ISCIII-, PI13/01560 and CDTI, FEDER-Innterconecta EXP00052887/ITC-20111037), co-funded with European Regional Development Funds (ERDF), the Regional Ministry of Innovation, Science and Enterprise of the Autonomous Government of Andalusia (CTS-7447), Regional Ministry of Education of the Valencia Community (PROMETEOII/2014/025), la Marató Foundation TV3 (151/C/2013) and was also co-funded with European Regional Development Funds (ERDF). The CIBER of Rare Diseases (CIBERER) is an initiative of the ISCIII, Spanish Ministry of Economy and Competitiveness. MM-S is supported by a predoctoral fellowship from CIBERER.
Abbreviations
- CVV
Cross-validation value
- FDR
False discovery rate
- GWAS
Genome-wide association study
- jPTC
Juvenile papillary thyroid carcinoma
- MDR
Multifactor-dimensionality reduction
- MEN 2
Multiple endocrine neoplasia type 2
- NMTC
Nonmedullary thyroid carcinoma
- OR
Odd ratio
- PTC
Papillary thyroid carcinoma
- sMTC
Sporadic medullary thyroid carcinoma
- SNP
Single nucleotide polymorphism
Additional file
(DOC 257 kb)
Footnotes
Joaquin Dopazo and Salud Borrego contributed equally to this work.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
BL-T, JD and SB drafted the manuscript. MB, LGA carried out the analysis of the data. BL-T and MM-S carried out the functional interpretation of the results. EN recruited all the patients included in this study. MR-F, RMF and AT carried out the molecular analyses. IM, CYG developed the software used. RMF, MR-F and GA collaborated with valuable contributions to the manuscript. SB and JD conceived and designed the study. JD coordinated the data analyses and SB coordinated all the laboratory tasks. All authors read and approved the final manuscript.
Contributor Information
Berta Luzón-Toro, Email: berta.luzon.exts@juntadeandalucia.es.
Marta Bleda, Email: mb2033@cam.ac.uk.
Elena Navarro, Email: elena.navarro.sspa@juntadeandalucia.es.
Luz García-Alonso, Email: luzgaral@ebi.ac.uk.
Macarena Ruiz-Ferrer, Email: mm.ruiz.exts@juntadeandalucia.es.
Ignacio Medina, Email: im411@cam.ac.uk.
Marta Martín-Sánchez, Email: martamartin-ibis@us.es.
Cristina Y. Gonzalez, Email: cyenyxe@ebi.ac.uk
Raquel M. Fernández, Email: raquelm.fernandez.sspa@juntadeandalucia.es
Ana Torroglosa, Email: ana.torroglosa.exts@juntadeandalucia.es.
Guillermo Antiñolo, Email: guillermo.antinolo.sspa@juntadeandalucia.es.
Joaquin Dopazo, Email: jdopazo@cipf.es.
Salud Borrego, Phone: +34955012780/76, Email: salud.borrego.sspa@juntadeandalucia.es.
References
- 1.Figge JJ. Epidemiology of thyroid cancer. In: Wartofsky LVND, editor. Thyroid Cancer: A Comprehensive Guide to Clinical Management. Totowa: Human Press; 1999. [Google Scholar]
- 2.Randolph GW, Maniar D. Medullary carcinoma of the thyroid. Cancer Control. 2000;7(3):253–261. doi: 10.1177/107327480000700305. [DOI] [PubMed] [Google Scholar]
- 3.Moline J EC: Multiple Endocrine Neoplasia Type 2. In: SourceGeneReviews® [Internet]. Edited by Pagon RA AM, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K, editors. Seattle: University of Washington, Seattle; 2005.
- 4.Eng C, Clayton D, Schuffenecker I, Lenoir G, Cote G, Gagel RF, et al. The relationship between specific RET proto-oncogene mutations and disease phenotype in multiple endocrine neoplasia type 2. International RET mutation consortium analysis. Jama. 1996;276(19):1575–1579. doi: 10.1001/jama.1996.03540190047028. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez RM, Pecina A, Antinolo G, Navarro E, Borrego S. Analysis of RET polymorphisms and haplotypes in the context of sporadic medullary thyroid carcinoma. Thyroid. 2006;16(4):411–417. doi: 10.1089/thy.2006.16.411. [DOI] [PubMed] [Google Scholar]
- 6.Landa I, Robledo M. Association studies in thyroid cancer susceptibility: are we on the right track? J Mol Endocrinol. 2011;47(1):R43–58. doi: 10.1530/JME-11-0005. [DOI] [PubMed] [Google Scholar]
- 7.Ruiz-Llorente S, Montero-Conde C, Milne RL, Moya CM, Cebrian A, Leton R, et al. Association study of 69 genes in the ret pathway identifies low-penetrance loci in sporadic medullary thyroid carcinoma. Cancer Res. 2007;67(19):9561–9567. doi: 10.1158/0008-5472.CAN-07-1638. [DOI] [PubMed] [Google Scholar]
- 8.Kondo T, Ezzat S, Asa SL. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer. 2006;6(4):292–306. doi: 10.1038/nrc1836. [DOI] [PubMed] [Google Scholar]
- 9.Dottorini ME, Vignati A, Mazzucchelli L, Lomuscio G, Colombo L. Differentiated thyroid carcinoma in children and adolescents: a 37-year experience in 85 patients. J Nucl Med. 1997;38(5):669–675. [PubMed] [Google Scholar]
- 10.Sassolas G, Hafdi-Nejjari Z, Ferraro A, Decaussin-Petrucci M, Rousset B, Borson-Chazot F, et al. Oncogenic alterations in papillary thyroid cancers of young patients. Thyroid. 2012;22(1):17–26. doi: 10.1089/thy.2011.0215. [DOI] [PubMed] [Google Scholar]
- 11.Hod N, Hagag P, Baumer M, Sandbank J, Horne T. Differentiated thyroid carcinoma in children and young adults: evaluation of response to treatment. Clin Nucl Med. 2005;30(6):387–390. doi: 10.1097/01.rlu.0000162602.48653.54. [DOI] [PubMed] [Google Scholar]
- 12.Schonfeld SJ, Lee C, Berrington de Gonzalez A. Medical exposure to radiation and thyroid cancer. Clin Oncol (R Coll Radiol) 2011;23(4):244–250. doi: 10.1016/j.clon.2011.01.159. [DOI] [PubMed] [Google Scholar]
- 13.Fridman MV, Savva NN, Krasko OV, Zborovskaya AA, Mankovskaya SV, Schmid KW, et al. Clinical and pathologic features of ‘sporadic’ papillary thyroid carcinoma registered in 2005-2008 years in children and adolescents of Belarus. Thyroid. 2012;22(10):1016-24. [DOI] [PubMed]
- 14.Manolio TA, Collins FS. The HapMap and genome-wide association studies in diagnosis and therapy. Annu Rev Med. 2009;60:443–456. doi: 10.1146/annurev.med.60.061907.093117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hindorff LA, Sethupathy P, Junkins HA, Ramos EM, Mehta JP, Collins FS, et al. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc Natl Acad Sci U S A. 2009;106(23):9362–9367. doi: 10.1073/pnas.0903103106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackay TF, Moore JH. Why epistasis is important for tackling complex human disease genetics. Genome Med. 2014;6(6):42. doi: 10.1186/gm561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Musani SK, Shriner D, Liu N, Feng R, Coffey CS, Yi N, et al. Detection of gene x gene interactions in genome-wide association studies of human population data. Hum Hered. 2007;63(2):67–84. doi: 10.1159/000099179. [DOI] [PubMed] [Google Scholar]
- 18.Ritchie MD, Hahn LW, Roodi N, Bailey LR, Dupont WD, Parl FF, et al. Multifactor-dimensionality reduction reveals high-order interactions among estrogen-metabolism genes in sporadic breast cancer. Am J Hum Genet. 2001;69(1):138–147. doi: 10.1086/321276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38(8):904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 21.Clarke GM, Anderson CA, Pettersson FH, Cardon LR, Morris AP, Zondervan KT. Basic statistical analysis in genetic case–control studies. Nat Protoc. 2011;6(2):121–133. doi: 10.1038/nprot.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125(1-2):279–284. doi: 10.1016/S0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 23.de Bakker PI, Yelensky R, Pe’er I, Gabriel SB, Daly MJ, Altshuler D. Efficiency and power in genetic association studies. Nat Genet. 2005;37(11):1217–1223. doi: 10.1038/ng1669. [DOI] [PubMed] [Google Scholar]
- 24.González CYB M, F Salavert, R, Sánchez, J, Dopazo, Medina I. Multicore and Cloud-Based Solutions for Genomic Variant Analysis. In: Euro-Par 2012: Parallel Processing Workshops Lecture Notes in Computer Science vol. 7640; Springer, Berlin 2013: 273–284.
- 25.da Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 26.Bulusu KC, Tym JE, Coker EA, Schierz AC, Al-Lazikani B. canSAR: updated cancer research and drug discovery knowledgebase. Nucleic Acids Res. 2014;42(Database issue):D1040–1047. doi: 10.1093/nar/gkt1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forbes SA, Bindal N, Bamford S, Cole C, Kok CY, Beare D, et al. COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2011;39(Database issue):D945–950. doi: 10.1093/nar/gkq929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stelzer G, Dalah I, Stein TI, Satanower Y, Rosen N, Nativ N, et al. In-silico human genomics with GeneCards. Hum Genomics. 2011;5(6):709–717. doi: 10.1186/1479-7364-5-6-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warde-Farley D, Donaldson SL, Comes O, Zuberi K, Badrawi R, Chao P, et al. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010;38(Web Server issue):W214–220. doi: 10.1093/nar/gkq537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 31.Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, et al. NCBI GEO: archive for functional genomics data sets--update. Nucleic Acids Res. 2013;41(Database issue):D991–995. doi: 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaudet P, Michel PA, Zahn-Zabal M, Cusin I, Duek PD, Evalet O, et al. The neXtProt knowledgebase on human proteins: current status. Nucleic Acids Res. 2015;43(Database issue):D764–770. doi: 10.1093/nar/gku1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie C, Yuan J, Li H, Li M, Zhao G, Bu D, et al. NONCODEv4: exploring the world of long non-coding RNA genes. Nucleic Acids Res. 2014;42(Database issue):D98–103. doi: 10.1093/nar/gkt1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bleda M, Tarraga J, de Maria A, Salavert F, Garcia-Alonso L, Celma M, et al. CellBase, a comprehensive collection of RESTful web services for retrieving relevant biological information from heterogeneous sources. Nucleic Acids Res. 2012;40(Web Server issue):W609–614. doi: 10.1093/nar/gks575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Derks S, Cleven AH, Melotte V, Smits KM, Brandes JC, Azad N, van Criekinge W, de Bruine AP, Herman JG, van Engeland M: Emerging evidence for CHFR as a cancer biomarker: from tumor biology to precision medicine. Cancer Metastasis Rev 2013. [DOI] [PMC free article] [PubMed]
- 36.Holley CL, Topkara VK. An introduction to small non-coding RNAs: miRNA and snoRNA. Cardiovasc Drugs Ther. 2011;25(2):151–159. doi: 10.1007/s10557-011-6290-z. [DOI] [PubMed] [Google Scholar]
- 37.Frasca F, Rustighi A, Malaguarnera R, Altamura S, Vigneri P, Del Sal G, et al. HMGA1 inhibits the function of p53 family members in thyroid cancer cells. Cancer Res. 2006;66(6):2980–2989. doi: 10.1158/0008-5472.CAN-05-2637. [DOI] [PubMed] [Google Scholar]
- 38.Zou M, Baitei EY, Alzahrani AS, Al-Mohanna F, Farid NR, Meyer B, et al. Oncogenic activation of MAP kinase by BRAF pseudogene in thyroid tumors. Neoplasia. 2009;11(1):57–65. doi: 10.1593/neo.81044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alonso R, Salavert F, Garcia-Garcia F, Carbonell-Caballero J, Bleda M, Garcia-Alonso L, et al. Babelomics 5.0: functional interpretation for new generations of genomic data. Nucleic Acids Res. 2015;43(W1):W117–121. doi: 10.1093/nar/gkv384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arnaldi LA, Borra RC, Maciel RM, Cerutti JM. Gene expression profiles reveal that DCN, DIO1, and DIO2 are underexpressed in benign and malignant thyroid tumors. Thyroid. 2005;15(3):210–221. doi: 10.1089/thy.2005.15.210. [DOI] [PubMed] [Google Scholar]
- 41.Binzak BA, Wevers RA, Moolenaar SH, Lee YM, Hwu WL, Poggi-Bach J, et al. Cloning of dimethylglycine dehydrogenase and a new human inborn error of metabolism, dimethylglycine dehydrogenase deficiency. Am J Hum Genet. 2001;68(4):839–847. doi: 10.1086/319520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bell A, Bell D, Weber RS, El-Naggar AK. CpG island methylation profiling in human salivary gland adenoid cystic carcinoma. Cancer. 2011;117(13):2898–2909. doi: 10.1002/cncr.25818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He H, Li W, Liyanarachchi S, Jendrzejewski J, Srinivas M, Davuluri RV, et al. Genetic predisposition to papillary thyroid carcinoma: involvement of FOXE1, TSHR and a novel lincRNA gene, PTCSC2. J Clin Endocrinol Metab. 2014;100(1):E164–72. doi: 10.1210/jc.2014-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Guo Q, Zhao Y, Chen J, Wang S, Hu J, et al. BRAF-activated long non-coding RNA contributes to cell proliferation and activates autophagy in papillary thyroid carcinoma. Oncol Lett. 2014;8(5):1947–1952. doi: 10.3892/ol.2014.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fernandez RM, Bleda M, Luzon-Toro B, Garcia-Alonso L, Arnold S, Sribudiani Y, et al. Pathways systematically associated to Hirschsprung’s disease. Orphanet J Rare Dis. 2013;8(1):187. doi: 10.1186/1750-1172-8-187. [DOI] [PMC free article] [PubMed] [Google Scholar]


