Abstract
Background:
Keratoconus is a bilateral non-inflammatory corneal disease. Collagen cross-linking (CXL) is a new treatment option for the disease that uses ultraviolet A light irradiation and riboflavin administration. The aim of this study is to evaluate the effect of CXL on corneal topographic and refractive values in patients with keratoconus younger than 18 years of age.
Materials and Methods:
For the clinical trial study, 37 patients (64 eyes) younger than 18 years of age with progressive keratoconus were included. Age, sex, family history of keratoconus, and history of allergic disorders and eye rubbing were recorded. Refractive, topographic, and topometric indices were evaluated before and 12 months after the CXL with 3mW for 30 minutes.
Results:
Mean age (±SD) of the patients was 15.83 ± 1.53 years; 26 (70.3%) of the 37 patients were male. Fourteen (37.8%) had positive family history of keratoconus, 11 (29.7%) had history of allergic disorders, and 15 (40.5%) had positive history of eye rubbing. Of the refractive values, cylinder value decreased significantly from −4.50 ± 0.29 to −4.11 ± 0.28 (P = 0.001). Also, the logarithm of minimal angle of resolution (logMAR) uncorrected visual acuity (UCVA) and best corrected visual acuity (BCVA) improved significantly 12 months after CXL (P = 0.012 and 0.001, respectively). Maximum keratometry before and after the operation was 53.82 ± 0.72 and 53.33 ± 0.72, respectively (P = 0.018). Differences for simulated K values, the thinnest cornea pachymetry, keratoconus index (KI), index of highest asymmetry (IHA), and index of highest decentration (IHD) before and 12 months after the CXL were statistically significant (P = 0.015, 0.034, <0.001, 0.017, 0.019, and 0.004, respectively).
Conclusion:
CXL improves the refractory, topographic, and topometric indices in patients with keratoconus younger than 18 years of age.
Keywords: Collagen cross-linking, progressive keratoconus, refractive value, topography
INTRODUCTION
Keratoconus is a bilateral non-inflammatory corneal disease that is characterized by thinning of corneal tissue and a cone-shaped protrusion of the central area.[1] The incidence and prevalence of keratoconus are 50–230 and 54.4 per 100,000 in the general population, respectively.[2,3] It usually starts at the age of puberty and progresses till the third to fourth decades of life. The most severe state of the disease occurs at the age of 20–40.[1,4] Positive family history is reported in 6–8% of the patients and the disease is highly associated with eye rubbing, hard contact lenses, and atopy.[5] Keratoconus disease does not make the patients totally blind, but in advanced stages, it leads to significant impairment of vision.[1] Keratoconus progression usually arrests after a period and rigid contact lenses are a successful treatment for most of the patients. Also, corneal transplant is used to restore vision in 10–20% of them.[6]
Collagen cross-linking (CXL) was used for the first time in 2004 for management of keratoconus by using ultraviolet A (UVA) light irradiation and topical riboflavin (vitamin B2) administration.[7] CXL increases the chemical bonds between the fibers of the corneal collagen. Riboflavin acts as a photosensitizer and causes production of free radicals of oxygen on being activated by UVA. This helps in the physical cross-linking of collagen. Also, by absorbing the UVA light, it prevents damage to deeper ocular structure.[8] To increase the penetration of riboflavin into the stroma, the corneal epithelial layer is generally removed.[9]
There are limited numbers of studies about the therapeutic effects of CXL on patients with keratoconus in the age group of less than 18 years. A study conducted on patients with keratoconus revealed that CXL improves the topographic corneal indices and refractive values 1 year after the operation.[10] The aim of the current study is to evaluate the effect of CXL on corneal topographic and refractive values in patients with keratoconus younger than 18 years of age.
MATERIALS AND METHODS
Study population
This clinical trial was conducted in 2012 and 2013. Subjects were randomly selected from patients who were referred to a referral eye hospital and had the diagnosis of progressive keratoconus. Inclusion criteria were: a) Age less than 18 years, b) documented progressive keratoconus defined as increase in maximum keratometry (K) of 1.00 diopter (D) in 1 year and deteriorating best corrected visual acuity (BCVA),[9,11] and c) corneal thickness of at least 400 μm. Patients with the following criteria were excluded from the study: History of herpetic keratitis, history of previous eye surgery, severe dry eye, corneal infection, history of autoimmune disorders, and usage of hard contact lens during a month before the study. Sample size was calculated based on Z1 = 1.96, Z2 = 11.28, and d = 0.4. The sample size was calculated to be N = 50 and finally 64 eyes were included in the study. The protocol was described to all the participants and an informed consent was obtained from them. Non-consenting individuals were excluded from the study.
Assessments
Demographic characteristics of all participants including age and sex were collected. Also, family history of keratoconus and history of allergic disorders (atopy and vernal conjunctivitis) and eye rubbing were recorded. Before surgery, all subjects were examined by slit-lamp and also funduscopic examinations. Uncorrected visual acuity (UCVA) and BCVA with a logarithm of minimal angle of resolution (logMAR) scale were measured using Snellen chart. Corneal topographic and pachymetry values were derived from Scheimpflug tomography (Pentacam, Oculus). One year after the surgery, the same examinations were performed.
Surgical technique
All the surgeries were done by one surgeon. After topical anesthesia was administered with tetracaine 0.5% eye drops, the epithelium was removed using a blunt blade and under sterile conditions in a central area of 8.0 mm diameter. This was to ensure the penetration of riboflavin. As a photosensitizer, 0.1% riboflavin solution was applied to the cornea every 3 min for 30 min. Then, examination with slit lamp was performed for ensuring penetration of riboflavin into the cornea. Then, central cornea (of 8.0 mm diameter) was irradiated with UVA light (IROC Innocross, Zürich, Switzerland) at a wavelength of 370 nm and an irradiance of 3 mW/cm2 for 30 min. The device was set at a working distance of 5 cm from the corneal surface.
After the treatment, a soft bandage contact lens placed on cornea and then the subjects received ciprofloxacin 0.30% and betamethasone 0.1% eye drops (four times daily) with an extended-wear CIBA vision (focus night and day) soft bandage contact lens for 7 days. On the first and seventh days after the surgery, examination for epithelial healing and absence of postoperative infection was done.[10,12] Betamethasone drops was continued q8h for another 3 weeks after removal of bandage lens.
Data analysis
Statistical analysis was performed using SPSS for Windows (Version 16.0, 2007; SPSS Inc, Chicago, IL, USA). Paired t-test (for continuous variables) was used to compare the variables. Statistical significance was assessed at 0.05 probability level. All the values are presented as mean ± standard error mean (mean ± SE) or numbers (%).
Ethical approval
The study protocol was approved by the ethical committee of Isfahan University of Medical Sciences.
RESULTS
After 12 months, 37 patients (64 eyes) completed the study. Mean age (±SD) of the patients was 15.83 ± 1.53 years and 26 (70.3%) of them were male. Of the participants, 14 (37.8%) had a positive family history of keratoconus, 11 (29.7%) had history of allergic disorders, and 15 (40.5%) had a positive history of eye rubbing.
Of the refractive values, cylinder value decreased significantly from −4.50 ± 0.29 to −4.11 ± 0.28 (P = 0.001). Also, UCVA and BCVA (logMAR) improved (0.58 ± 0.04 and 0.53 ± 0.04 for UCVA and 0.24 ± 0.02 and 0.19 ± 0.02 for BCVA before and after the operation, respectively) significantly 12 months after CXL (P = 0.012 and 0.001, respectively). Table 1 shows the comparison of refractive values before and after CXL. Mean intraocular pressure (IOP) before CXL was 12.84 ± 0.30 and was not significantly different from postoperative IOP (12.59 ± 0.34, P = 0.375).
Table 1.
Comparison of refractive values before CXL and 12 months after surgery
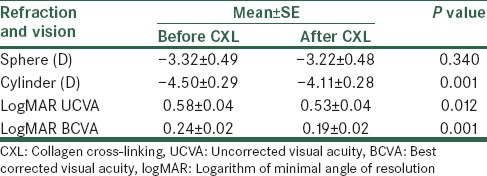
According to Table 2, the maximum keratometry before and after the operation was 53.82 ± 0.72 and 53.33 ± 0.72, respectively, and the difference was statistically significant (P = 0.018). Comparison of topographic and topometric indices before and after CXL is presented in Table 2. According to the table, differences for simulated K values, the thinnest cornea pachymetry, keratoconus index (KI), index of highest asymmetry (IHA), and index of highest decentration (IHD) before and 12 months after the CXL were statistically significant (P = 0.015, 0.034, <0.001, 0.017, 0.019, and 0.004, respectively).
Table 2.
Comparison of topographic and topometric indices before and after CXL
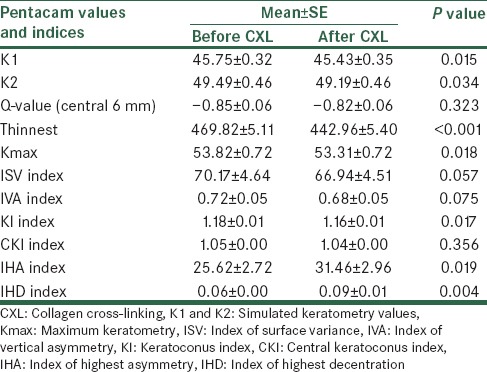
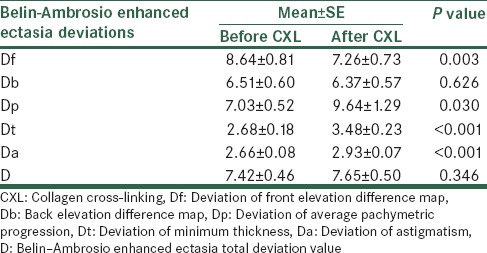
Table 3 shows the comparison of Belin–Ambrosio enhanced ectasia screening indices before and after CXL. According to the table, Belin–Ambrosio enhanced ectasia total deviation value before operation was 7.42 ± 0.46 and after CXL was 7.65 ± 0.50 and the difference was not statistically significant (P = 0.346). Of the other Belin–Ambrosio indices, differences before and after CXL for Df, Dp, Dt, and Da were statistically significant (P = 0.003, 0.030, <0.001, and <0.001, respectively).
Table 3.
Comparison of Belin–Ambrosio enhanced ectasia detection indices before and after CXL
DISCUSSION
From 2004, CXL has been used for the treatment of keratoconus.[7] Various studies have been conducted on the effect of this method on keratoconus. The aim of the current study was to evaluate the therapeutic effect of CXL on keratoconus patients younger than 18 years of age by comparing the refractive values, topographic, topometric, and Belin–Ambrosio indices before and 12 months after the CXL operation. Also, we asked the patients about the history of eye rubbing and atopy and positive family history of keratoconus to compare their prevalence with other studies.
According to earlier studies, keratoconus is highly associated with a positive family history of keratoconus, eye rubbing, and atopy.[5] Our findings showed that 37.5% of patients with keratoconus had a positive family history of the disease. Zandik et al. have reported in their study that only 13.7% of patients with keratoconus had a positive family history of the disease.[13] Another study conducted by Gonzalez et al. revealed that 50% of patients with keratoconus have at least one close relative affected by the disease.[14] Studies conducted by Davies et al. and Rahi et al. have shown that 35% of patients with keratoconus had history of atopy and was significantly higher than in normal controls.[15,16] Although we did not include a normal control group, our results showed similar facts and revealed that 29.7% of patients with keratoconus had a history of atopy. Two surveys have reported the prevalence of eye rubbing as 66% and 73% in patients with keratoconus, respectively.[17,18] Another study has reported that 50.4% of patients with keratoconus had a history of rubbing their eyes.[13] According to our results, 40.5% of patients with keratoconus younger than 18 years had a history of eye rubbing.
In our previous study that was conducted in Isfahan on patients with keratoconus, the results showed that CXL causes a significant improvement of topographic and refractive values 1 year after the operation. The study was suggestive of the efficacy of CXL in the treatment of keratoconus in Iranian patients.[10] The current study revealed the same results for patients with keratoconus younger than 18 years of age. The CXL method improved the visual acuity in those patients after 1 year and also improved most of the topographic and topometric indices. For the indices not showing any significant changes, the results showed that no worsening had happened after 1 year. A study by Saffarian et al. revealed that 1 year after CXL in patients with keratoconus, significant decrease in cylindrical power had occurred, but no significant changes were found in spherical power.[19] Our results were similar to the results of this study. Also, similar to our results, Saffarian et al. have reported that both UCVA and BCVA were statistically higher after the operation.[19] Another study by Raiskup-Wolf et al. reported that 12 months after CXL, BCVA and Kmax improved significantly. Also, the effect of CXL was maintained 6 years after the operation.[20] In our study, we followed up the patients for 12 months and found similar results. More studies for evaluating the long-term effect of CXL are recommended. Various studies have reported that CXL improves different indices of keratoconus. Arbelaez et al. have mentioned that CXL is a safe and effective therapeutic method of treating keratoconus in their 1-year follow-up study.[8] Hoyer et al. have reported the same results and claimed that CXL has several clinical, economic, and psychosocial benefits.[21] In an Indian study with 1 year follow-up period, the results showed that CXL causes stabilization and improvement of keratoconus.[22] Some other studies have reported that CXL has a stabilizing effect on progressive keratoconus after the operation.[23,24,25]
Transplantation, another method of treating keratoconus, has the risk of infection, rejection, cataract, glaucoma, etc., and young patients with keratoconus may need one or more repeated grafts in their life.[26] According to our results, CXL improves refractory, topographic, and topometric indices in patients with keratoconus younger than 18 years of age. As CXL is much safer than some previous methods such as corneal graft and gas-permeable contact lens[26] and based on the positive results obtained in various studies conducted on the efficacy of this method, including the current study, and also considering the fact that CXL is an outpatient and cost-effective treatment, this method is recommended for treating progressive keratoconus, especially in young patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 2.Kim H, Joo CK. Measure of keratoconus progression using Orbscan II. J Refract Surg. 2008;24:600–5. doi: 10.3928/1081597X-20080601-08. [DOI] [PubMed] [Google Scholar]
- 3.Bechrakis N, Blom ML, Stark WJ, Green WR. Recurrent keratoconus. Cornea. 1994;13:73–7. doi: 10.1097/00003226-199401000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Pantanelli S, MacRae S, Jeong TM, Yoon G. Characterizing the wave aberration in eyes with keratoconus or penetrating keratoplasty using a high-dynamic range wavefront sensor. Ophthalmology. 2007;114:2013–21. doi: 10.1016/j.ophtha.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Krachmer JH, Feder RS, Belin MW. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol. 1984;28:293–322. doi: 10.1016/0039-6257(84)90094-8. [DOI] [PubMed] [Google Scholar]
- 6.Tuft SJ, Moodaley LC, Gregory WM, Davison CR, Buckley RJ. Prognostic factors for the progression of keratoconus. Ophthalmology. 1994;101:439–47. doi: 10.1016/s0161-6420(94)31313-3. [DOI] [PubMed] [Google Scholar]
- 7.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–7. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 8.Arbelaez MC, Sekito MB, Vidal C, Choudhury SR. Collagen cross-linking with riboflavin and ultraviolet-A light in keratoconus: One-year results. Oman J Ophthalmol. 2009;2:33–8. doi: 10.4103/0974-620X.48420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohlhaas M, Spoerl E, Schilde T, Unger G, Wittig C, Pillunat LE. Biomechanical evidence of the distribution of cross-links in corneastreated with riboflavin and ultraviolet A light. J Cataract Refract Surg. 2006;32:279–83. doi: 10.1016/j.jcrs.2005.12.092. [DOI] [PubMed] [Google Scholar]
- 10.Razmjoo H, Nasrollahi AP, Salam H, Karbasi N, Najarzadegan MR. Topographic corneal changes after collagen cross-linking in patients with corneal keratoconus. J Res Med Sci. 2013;18:882–6. [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki M, Amano S, Honda N, Usui T, Yamagami S, Oshika T. Longitudinal changes in corneal irregular astigmatism and visual acuity in eyes with keratoconus. Jpn J Ophthalmol. 2007;51:265–9. doi: 10.1007/s10384-007-0453-2. [DOI] [PubMed] [Google Scholar]
- 12.Saini JS, Saroha V, Singh P, Sukhija JS, Jain AK. Keratoconus in Asian eyes at a tertiary eye care facility. Clin Exp Optom. 2004;87:97–101. doi: 10.1111/j.1444-0938.2004.tb03155.x. [DOI] [PubMed] [Google Scholar]
- 13.Zadnik K, Barr JT, Edrington TB, Everett DF, Jameson M, McMahon TT, et al. Baseline findings in the collaborative longitudinal evaluation of keratoconus (CLEK) study. Invest Ophthalmol Vis Sci. 1998;39:2537–46. [PubMed] [Google Scholar]
- 14.Gonzalez V, McDonnell PJ. Computer-assisted corneal topography in parents of patients with keratoconus. Arch Ophthalmol. 1992;110:1413–4. doi: 10.1001/archopht.1992.01080220074024. [DOI] [PubMed] [Google Scholar]
- 15.Davies PD, Lobascher D, Menon JA, Rahi AH, Ruben M. Immunological studies in keratoconus. Trans Ophthalmol Soc U K. 1976;96:173–8. [PubMed] [Google Scholar]
- 16.Rahi A, Davies P, Ruben M, Lobascher D, Menon J. Keratoconus and coexisting atopic disease. Br J Ophthalmol. 1977;61:761–4. doi: 10.1136/bjo.61.12.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Copeman PW. Eczema and keratoconus. Br Med J. 1965;2:977–9. doi: 10.1136/bmj.2.5468.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karseras AG, Ruben M. Aetiology of keratoconus. Br J Ophthalmol. 1976;60:522–5. doi: 10.1136/bjo.60.7.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saffarian L, Khakshoor H, Zarei-Ghanavati M, Esmaily H. Corneal crosslinking for keratoconus in iranian patients: Outcomes at 1 year following treatment. Middle East Afr J Ophthalmol. 2010;17:365–8. doi: 10.4103/0974-9233.71600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: Long-term results. J Cataract Refract Surg. 2008;34:796–801. doi: 10.1016/j.jcrs.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 21.Hoyer A, Raiskup-Wolf F, Spörl E, Pillunat LE. Collagen cross-linking with riboflavin and UVA light in keratoconus. Results from Dresden. Ophthalmologe. 2009;106:133–40. doi: 10.1007/s00347-008-1783-2. [DOI] [PubMed] [Google Scholar]
- 22.Agrawal VB. Corneal collagen cross-linking with riboflavin and ultraviolet - A light for keratoconus: Results in Indian eyes. Indian J Ophthalmol. 2009;57:111–4. doi: 10.4103/0301-4738.44515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wittig-Silva C, Whiting M, Lamoureux E, Lindsay RG, Sullivan LJ, Snibson GR. A randomized controlled trial of corneal collagen cross-linking in progressive keratoconus: Preliminary results. J Refract Surg. 2008;24:S720–5. doi: 10.3928/1081597X-20080901-15. [DOI] [PubMed] [Google Scholar]
- 24.Caporossi A, Mazzotta C, Baiocchi S, Caporossi T. Long-term results of riboflavin ultraviolet a corneal collagen cross-linking for keratoconus in Italy: The Siena eye cross study. Am J Ophthalmol. 2010;149:585–93. doi: 10.1016/j.ajo.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 25.Wollensak G, Spörl E, Seiler T. Treatment of keratoconus by collagen cross linking. Ophthalmologe. 2003;100:44–9. doi: 10.1007/s00347-002-0700-3. [DOI] [PubMed] [Google Scholar]
- 26.Snibson GR. Collagen cross-linking: A new treatment paradigm in corneal disease - A review. Clin Experiment Ophthalmol. 2010;38:141–53. doi: 10.1111/j.1442-9071.2010.02228.x. [DOI] [PubMed] [Google Scholar]


